Abstract
People of the littoral region of cameroon live in fragile ecosystem, which undergo a rise in pressure associated with agricultural exploitation, farming and the increasing use of pesticides in agro-industrial crops. This study aimed to evaluate chronic pesticide exposure effects on liver, renal, and neurological function among banana workers in the Moungo Division, Cameroon. A two-phase approach was employed. Firstly, a survey of pesticide distributors, retailers, and local farmers was conducted using a structured questionnaire to identify the different types of pesticides commonly used in the study area and their reported health effects. Secondly, a case-control study was performed comparing banana farm workers with a control group. Blood samples were used to detect pesticides residues and to quantify key markers involved in the liver, kidney and brain function. The most active ingredients used by farmers were paraquat (59.78%) and glyphosate (34.87%) for herbicides, organophosphates (32.92%), followed by Fipronil (13.42%) among insecticides and Mancozeb (34.04%) for fungicides. The most common symptoms reported were eye irritation (26.77%), skins irritation (42.52%) and respiratory problems (8.66%). Pesticide residues analysis revealed that 46.66% blood samples of farm workers contain more than three pesticide residues. ASAT, ALAT, ALP, LDH activities and plasma urea were significantly elevated in farm workers compared to the control group. Moreover, a significant impact in brain health was observed as shown by the decrease in both erythrocyte acetylcholinesterase and plasma butyrylcholinesterase activities of banana farm workers compared to control. The use of pesticide may be associated with liver, kidney and brain dysfunction. This finding suggests that the use of mixture pesticide is a health risk for banana plantation workers.
Keywords: Banana farm workers, Pesticides, Blood, Biological markers, Occupational health
Subject terms: Biomarkers, Diseases, Health occupations, Risk factors, Signs and symptoms
Introduction
Pesticides are widely used in agriculture to control pests and diseases, improving crop production and playing a role in ensuring global food security1. However, their widespread use raise major concerns about possible harmful effects on human health, particularly for people working in the agricultural sector2. These harmful effects are all the more pronounced in developing countries such as Cameroon, where protective measures and the application of regulations are often inadequate. Chronic occupational exposure to pesticides has been associated with a range of health problems, including oxidative stress, immunotoxicity and impaired neurological, liver and kidney function3.
Many pesticides are recognized as neurotoxins, capable of damaging the nervous system, leading to various neurological problems such as impaired memory, attention and learning ability4. Common symptoms of pesticide neurotoxicity include headaches, dizziness, confusion, tremors and difficulty with concentration5,6. Cholinesterases are a group of enzymes that are essential for the proper functioning of the nervous system. They play a central role in the breakdown of acetylcholine, a neurotransmitter important for communication between nerve cells. Pesticides (organophosphates and carbamates) are known to inhibit cholinesterase activity. Plasma cholinesterase levels are a valuable marker of exposure to these compounds7.
The liver, the vital organ responsible for detoxification and metabolism, is particularly vulnerable to damage caused by pesticides. Acute and chronic exposure to pesticides can induce hepatotoxicity, manifested by liver inflammation, fibrosis, cirrhosis and eventually hepatocellular carcinoma. Elevated plasma levels of liver enzymes, such as aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP) and gamma-glutamyltransferase (GGT), are commonly used as indicators of liver damage8,9. Alongside the liver, the kidneys, which perform the essential function of blood filtration, are also sensitive to the deleterious effects of pesticide exposure. Studies suggest that occupational exposure to pesticides is associated with biomarkers of renal failure, in particular elevated levels of plasma creatinine and blood urea, which may be a signal for early renal dysfunction and progression to chronic renal failure (CKD)10,11. However, little is yet known about the mechanisms by which pesticide exposure causes liver and kidney damage, especially in population with prolonged exposure to low doses. In addition, existing studies have focused mainly on cases of acute poisoning or on individual pesticides, leaving gaps in our understanding of the cumulative effects of mixtures commonly used in agriculture.
In Cameroon’s Moungo Department, banana growing relies heavily on the use of pesticides. Unfortunately, protective measures are not systematically applied. As a result, these farm workers, who form a high-risk but little-studied group, are exposed to sub-clinical and long-term effects on their health, particularly as regards their liver and kidneys. This highlight the urgent need to study pesticide-induced hepatoxicity and nephrotoxicity in real-life occupational settings. Such research is essential if we have to develop safety measures tailored to this region, with a view to reduce the incidence of chronic liver and kidney disease in these vulnerable communities.
The relationship between farmers’ exposure to pesticides and organ dysfunction has been highlighted in the literature. Leonel et al.12 showed that farmers in Cameroon and Pakistan exposed to organophosphates suffered considerable damage to organs such as the liver and kidneys. In addition, Leonel et al.13 showed that chronic exposure to a mixture of organophosphates have harmful effects on the pancreas in Cameroonian and Pakistani populations. It should be noted that this research focused on a single class of pesticides, namely organophosphates. However, in the majority of low-income countries, several classes of pesticides are used simultaneously in agricultural activities. This raises some questions about the effects of farmers’ exposure to a combination of several classes of pesticides. To fill these gaps, this study examines the effects of chronic pesticide exposure on the liver, kidney and neurological functions of agricultural workers in the banana sector by analyzing the main plasma biomarkers: Liver enzymes activities (AST, ALT, ALP, LDH) to assess hepatocyte integrity and metabolic disturbances, and kidney markers (creatinine, urea) to assess glomerular filtration efficiency, cholinesterase activity (AChE, BChE) known as a sensitive indicator of organophosphate induced neurotoxicity.
By integrating these biomarkers, this study provides a holistic assessment of systemic toxicity in a high-risk and understudied population, offering insight into the mechanistic links between exposure to several pesticides and subclinical organ damage. The results are intended to inform evidence-based safety guidelines, thereby reducing the burden of preventable occupational diseases in agrarian communities.
Materials and methods
Study population and design
This study was conducted as part of a research project on the health-related effects of pesticide exposure in Cameroon started in 2018. Data collection was conducted in two phases from October to November 2023 in the Moungo Division, Littoral Region, Cameroon. The first phase involved interviews with local farmers to ascertain their practices and the use and sold of pesticides in the study area. The second phase employed a case-control design to evaluate the health outcomes among banana farm workers compared to a control group. Study participants were men and women aged at least 20, recruited from banana plantations where pesticides are used (cases) and from areas where agriculture is not practised, where the risk of exposure to pesticides is lower (control). The cases were selected on the basis of their potential exposure to pesticides. Thus, people who had pesticide-related activities (mixing pesticides, spraying crops, handling treated crops) were recruited. In addition, only people who had been working for at least one year in these activities were included in the study. The control group consisted of participants with no history of agricultural work or occupational exposure to pesticides. These participants were recruited from the same geographical area to reduce the environmental influence and lifestyle differences associated with pesticide exposure. Control participants were matched to farm workers based on age and gender to reduce potential confounding variables and strengthen comparability between both groups.
Ethics statement
The study was approved by the National Ethics Committee for Human Health Research under registration number 2023/08/348/CE/CNERSH/SP, and complied with the ethical principles set out in the Declaration of Helsinki. Informed consent was obtained from all participants, with an emphasis on voluntary participation. Throughout the study, the protection of participants’ data was ensured. All identifiable information was anonymized and stored in secure, password-protected files. All methods were applied in accordance with current guidelines and regulations for medical and biological research involving human subjects. Clinical data from all patients were collected and analyzed in accordance with standard ethical guidelines. The careful selection of participants and ethical rigor underscore the methodological robustness of the study and its potential contribution to understanding the health effects of pesticide exposure.
Administration of the questionnaire
A structured questionnaire was administered during face-to-face interviews conducted by trained interviewers. Translation services were provided to ensure participant comprehension. The questionnaire was specifically designed to collect information on demographic characteristics, work history, pesticide use practices, and reported health symptoms. Participants underwent medical examinations to assess their overall health status, including blood pressure, respiratory function, and any observed signs or symptoms related to pesticide exposure. These tests have provided valuable data to correlate physiological health outcomes with exposure level.
Blood sampling and biochemical analyses
Blood sampling
Blood samples were collected from all study participants, both banana farm workers and control individuals after obtaining informed consent. Standardized conditions were maintained during sample collection to ensure integrity and minimize potential contamination. Ten milliliters (ml) of venous blood were drawn from each participant 30 days after the last pesticide spraying event to assess effects associated with chronic exposure rather than acute effects. Blood was collected in two tubes (one EDTA tube and one dry tube). The blood was centrifuged at 3500 rpm for 10 min at 25 °C. The plasma obtained from the EDTA was stored at -80 °C for analysis.
Biochemical analyses and assessment of pesticide residues in blood
RBC-AChE activity was measured from whole blood and BChE, AST, ALT, LDH, ALP activities and creatinine and urea levels were determined in plasma.
RBC-AChE and BChE activities were measured using the Ellamn method modified by Worek et al.14. Whole blood was used to determine RBC-AChE while plasma was used for BChE. Blood and plasma dilutions were prepared according to the technique described by Worek et al.14. Spectrophotometric measurement was performed at 436 nm for AChE and BChE.
The activity of AST and ALT was determined using the enzymatic method described by Henry et al.15 and modified according to IFCC recommendations16. AST and ALT catalyze the transfer of the amine group from L-Aspartate and L-Alanine to 2-Oxoglutarate to form Oxaloacetate + L-glutamate and Pyruvate + L-glutamate respectively. The decrease in absorbance, proportional to ASAT and ALAT activity, is measured at 340 nm.
ALP activity was measured using the enzymatic method described by Tietz17. In an alkaline medium, alkaline phosphatases catalyze the hydrolysis of p-nitrophenylphosphate to p-nitrophenol and phosphate. The rate of appearance of p-nitrophenol, monitored by the variation in absorbance at 405 nm, is proportional to ALP activity.
LDH activity was assessed using the kinetic method described by Pesce18. The decrease in absorbance due to the conversion of NADH to NAD+, directly proportional to LDH activity, is measured at 340 nm.
Urea levels were assessed using an enzymatic and colorimetric method described by Searcy et al.19. This is based on the specific action of urease, which hydrolyzes urea into ammonium and carbonate ions. The intensity of coloration, proportional to the quantity of urea, is measured at 600 nm.
Creatinine was determined using the method of Fabiny and Ertingshausen20.
Gas chromatography directly coupled to high-resolution mass spectrometry (GC-MS System 5975 C Agilent, UK) was used to assess the pesticide residues in blood. For screening, many types of pesticides were chosen based on data collected from farmers regarding the quality of pesticides used in agricultural farm, stock, and examination of fields and dumping points of impetuous pesticides packages in the study area. The column was initially calibrated at 120 °C for one minute before gradually increasing to 290 °C. The injection orifice and interface temperatures were stabilized at 250 °C, while the detector was maintained at 290 °C. An undivided injection mode was employed, with helium flowing at a rate of 1.0 mL/min as the carrier gas. Calibration and internal quality control were conducted by introducing known concentrations of commercial solutions and internal standard into drug-free whole blood samples. The internal standard concentration was maintained at 0.25 mg/L. Electron impact (EI) mass spectra of the pesticides and the internal standard were recorded using total ion monitoring. For quantification, the surface-to-peak ratios of the target ions corresponding to the insecticides and azobenzene (m/z 182) were calculated based on the substance concentration. The limits of detection (LOD) and quantification (LOQ) were assessed to validate this method. These limits were determined by analyzing blood samples without drugs, which were then fortified with known concentrations of the target compounds. Each concentration was measured in five replicates. The LOD was defined as the lowest concentration producing a signal three times higher than the average baseline noise observed in five unfortified samples. The LOQ, on the other hand, was established as the lowest concentration within 10% of the theoretical value, maintaining acceptable qualifier ion ratios across all five replicates. To ensure accuracy, ten blank blood samples were examined for potential chromatographic interference from each analytes.
Statistical analyses
Descriptive statistics were employed to characterize the variables. Continuous variables (RBC-AChE, BChE, ALT, AST, LDH, ALP activities and creatinine and urea levels) were presented as mean ± standard error, while categorical variables (symptoms observed and number of pesticides detected) were presented as frequencies and percentages. The T-test was used to compare the average levels of markers. Groups were matched for age and gender to ensure that the observed differences are directly related to pesticide exposure. The statistical analyzed results were presented using tables and figures, with p-values and confidence intervals to indicate the statistical significance of the findings. The statistical analysis aimed to identify any statistically significant associations between pesticide exposure and changes in kidney, liver and neurological function markers.
Results
Identification of pesticides used in the study area
Table 1 shows the frequency of use of the different classes of pesticides used by farmers in the study area. Herbicides were the most commonly used class of pesticides in the study (41.82%), followed by insecticides with a frequency of use of 37.27%. Among the insecticides, organophosphates were the most prevalent active molecules (37.80%), followed by Phenylpyrazole (13.42%). However, organochlorine was the least used insecticide active molecule (3.65%). For the herbicides, Bipyridiliums (59.78%) and Aminophosphonates (34.87%) were the most frequently identified active ingredients. Within fungicides, Dithiocarbamates were the most used with a frequency of 34.04%, followed by Dithiocarbamates at 34.04%. The toxicological classification of these pesticides according to the WHO revealed that the majority of the active molecules in insecticides are classified as moderately hazardous (63.80%) and slightly hazardous (33.30%). In the case of fungicides, all the active molecules were classified as slightly dangerous. For the herbicides, 52.40% of active molecules were moderately hazardous, while 46.60% were slightly hazardous. The health effects reported among pesticide-using participants highlighted skin problems, visual problems, and respiratory issues as the most frequent symptoms (Table 1).
Table 1.
Frequency of use of different classes of pesticides, active molecules, their toxicological classification according to the WHO and the signs of discomfort associated with the use of pesticides.
| Types of pesticides | Trade name | Chemical family | Active ingredients | WHO acute toxicity class |
|---|---|---|---|---|
| Insecticides (37.27%) | Pyriforce | Organophosphorus | Chlorpyrifos (31.70%) | II |
| Pyrifos | ||||
| Dursban | ||||
| Counter | Terbufos (4.88%) | II | ||
| Heron | Phosphorodithioate (1.22%) | II | ||
| Capsidor | Phenylpyrazole | Fipronil (13.42%) | III | |
| Plantima | Neonicotinoids | Imidacloprid (10.98%) | II | |
| Iron | ||||
| Actara | Thiamethoxam (9.76%) | II | ||
| Tara | ||||
| Force | ||||
| Super | Pyrethroids | lambda-cylholothrin (8.54%) | II | |
| Cigogne | Cypermethrin (8.54%) | II | ||
| Cypercal | ||||
| Difender | ||||
| Mocap | Ethoprophos (3.65%) | II | ||
| Gammalin | Organochlorines | Lindane (3.65%) | II | |
| Gloden blue | Sulfure | Copper sulphate pentahydrate (3.66%) | U | |
| Fungicides (20.91%) | Azoxys | Dithiocarbamates | Mancozeb (34.04%) | U |
| Mancozeb | ||||
| Pancozeb | ||||
| Callomil | Phenylamic acid | Metalaxy 8% + Mancozebe 64 (34.04%) | II | |
| Okamil | II | |||
| Okamine | II | |||
| Ridomil | III | |||
| Calomil | Pyrethroids | Copper oxide 600 g/Kg + Melataxyl 120 g/kg (17%) | U | |
| Plantomil | ||||
| Nordox | ||||
| Greecop | ||||
| Hydroxy | ||||
| Parastar | Pyrethroids | lambda- cylholothrine (10.70%) | U | |
| Ecana | Triazolinthiones | Prothioconazole 480 g/l (2.11%) | U | |
| Coxy 50% | Oxyde copper | Copper oxychloride (2.11%) | U | |
| Herbicides (41.82%) | Actavia | Bipyridiliums | 1,1‘-diméthyl-4,4’-bipyridinium (59.78%) | II |
| Almoxone | ||||
| Bastion | ||||
| Calloxone | ||||
| Gramoxone | ||||
| Paraquat | ||||
| Supraxone | ||||
| Paracot | Aminophosphonates | Glyphosate (34.87%) | III | |
| Plantoxone | ||||
| Glyphader | ||||
| Round Up | ||||
| Glucote | ||||
| Glycot | ||||
| Glyfosate | ||||
| Duiron | Substituted urea | Hologenophenylurea (4.26%) | III | |
| Linuron | Phenylureas | 3-(3,4-dichlorophenyl)-1-methoxy-1-methylurea (1.09%) | IB |
SC soluble concentrate, EC emulsifiable concentrate, SL soluble solution, SC2 suspension concentrate, WP wettable powders, GC granules concentrate, Ia extremely dangerous, Ib very dangerous, II moderately dangerous, III slightly hazardous, U unlikely to present an acute.
Self-report health effects by respondents in the study area
The self-report health effects experienced by banana farm workers during or after pesticide use are presented in Table 2. It shows that these health effects impaired several physiological systems, including the central nervous system, respiratory system, digestive system, cardiovascular system, and the skin. Regarding the nervous system, eye irritation (26.77%) was the most frequently reported symptom by workers. With regard to the respiratory system, 8.66% of workers reported respiratory problems. With regard to the digestive system, nausea (3.94%) was the most frequently reported symptom. Skin irritation (42.2%) was the symptom most frequently reported by workers for skin effects.
Table 2.
Health effects experienced during and after farmers’ exposure to pesticides.
| Systems | Effectives | Proportion (%) | |
|---|---|---|---|
| Nervous systems (35.43%) | Headbaches | 3 | 2.36 |
| Dizziness | 2 | 1.57 | |
| Fatigue | 3 | 3.15 | |
| Fever | 2 | 1.58 | |
| Eye irritation | 34 | 26.77 | |
| Respiratory systems (12.6%) | Respiratory problems | 11 | 8.66 |
| Sneezing | 1 | 0.79 | |
| Cough | 3 | 2.36 | |
| Allergy | 1 | 0.79 | |
| Skin effects (42.52%) | Skin irritation | 54 | 42.52 |
| Digestive systems (8.66%) | Vomiting | 4 | 3.15 |
| Nausea | 5 | 3.94 | |
| Abdominal pain | 2 | 1.57 | |
| Cardiovascular systems (0.79%) | Bleeding | 1 | 0.79 |
Frequency of detection of pesticides residues in blood samples of banana farm workers
The Fig. 1 presents the number of pesticide residues detected in blood samples of banana farm workers and controls. The results indicate that 21.25% of blood samples contained one pesticide, 32.08% of samples contained at least two pesticides, 8.75% samples contained at least three pesticide residues and 8.33% of samples contained four or more of 05 pesticides.
Fig. 1.
The number of analyzed blood samples, free, with one, two and with more two pesticides.
Long-term effect of pesticides on brain, liver and kidney health among banana farm workers
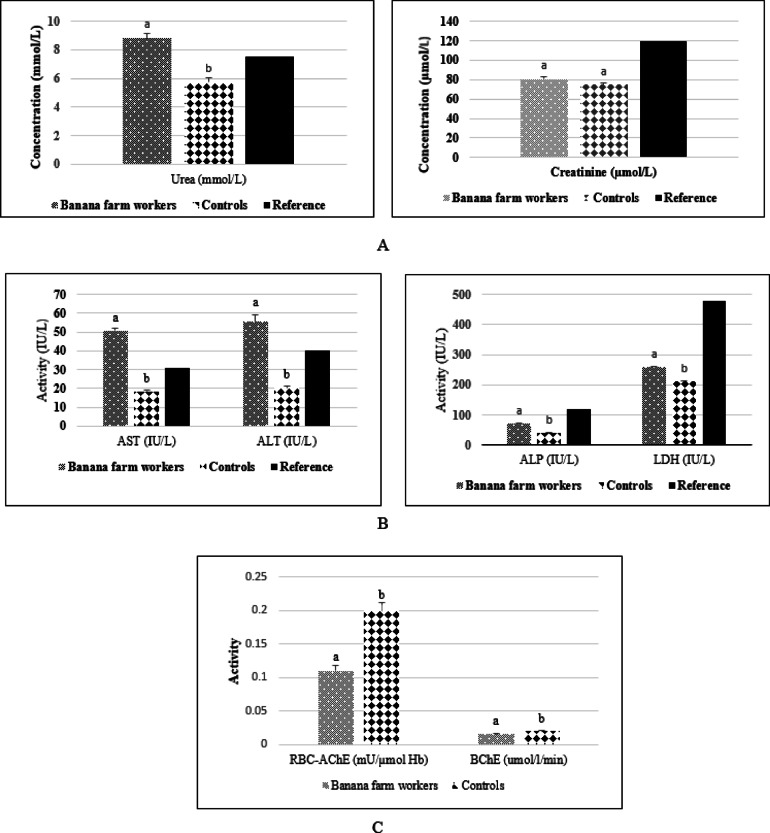
The study revealed a significant decrease in erythrocyte acetylcholinesterase (0.11 ± 0.07 mU/µmol Hb) and plasma butyrylcholinesterase (0.016 ± 0.001µmol/l/min) activities in banana farm workers compared with the control group, 0.20 ± 0.10 mU/µmol Hb; 0.020 ± 0.010 µmol/l/min respectively. In addition, the results revealed statistically significant differences in the plasma levels of some markers of liver function between the two groups. Workers exposed to pesticides exhibited significantly higher levels of AST (50.69 ± 18.28 vs. 18.40 ± 9.00 IU/L), ALT (55.30 ± 10.80 vs. 20.49 ± 4.34 IU/L), and these values were higher than the reference values. However, although LDH (258.10 ± 31.50 vs. 210. 10 ± 8.66 IU/L), and ALP (70.24 ± 31.20 vs. 38.96 ± 17.56 IU/L) levels were significantly higher in the exposed than in the controls, these values were below the reference values. We also observed that banana farm workers had significantly higher plasma urea levels (9.10 ± 3.53 mmol/L) compared to the control group (5.20 ± 1.65 mmol/L) (p < 0.05). Moreover, these values were higher than the reference values. However, there was no statistically significant difference in plasma creatinine concentration between workers (74.30 µmol/L) and controls (59.40 µmol/L) (Fig. 2).
Fig. 2.
(A) Blood urea and creatinine levels in banana farm workers and controls; (B) AST, ALT, ALP and LDH activity in banana farm workers and Controls; (C) RBC-AChE and BChE activity in banana farm workers and controls. RBC-AChE red blood cell acetylcholinesterase, BChE buturylcholinesterase, AST spartate transaminase, ALT alanine transaminase, LDH lactate deshydrogenase, GGT gamma-glutamyl transferase, ALP alkalin phosphatase, Results are mean ± SEM; Histograms with the same letter are not statistically different at p < 0.05.
Discussion
The widespread use of pesticides in agriculture, while contributing to increased crop yields and food security, raises significant concerns regarding occupational health risks, particularly in developing countries like Cameroon where regulations and safety practices may be less rigorous1,3. The aim of this study was to assess the effects of chronic pesticide exposure on the liver, kidney and neurological functions of banana farm workers.
The results revealed that herbicides (41.82%), insecticides (37.27%), and fungicides (20.91%) were the most commonly used pesticide classes in the study area. This result could be explained by climate change and the exponential growth of new pest species or the increase in populations of existing species. Added to this, the phenomenon of resistance of pests to common pesticides used, leads farmers to use more powerful products or combinations of products, justifying the large number of active molecules identified in the site of study (Table 1). These practices also expose farmers to a diversity of molecules21. These multiple exposures could rise the health effects known as ‘mixture effects’, involving the neurological, respiratory, cardiovascular, and ocular systems (Table 2). The results showed that several of the active molecules identified at the site were classified as toxicants of class II and Ia. Substances of class II are moderately dangerous according to the WHO classification, and those from class Ia are very dangerous, which could explain the health effects observed from banana farm workers22. Previous studies have shown that pesticides, particularly insecticides, cause toxic effects with acute neurological, digestive, respiratory, cardiovascular, cutaneous, and ocular signs9. These results corroborate similar studies carried out in African contexts23.
The significant reduction in erythrocyte AChE and plasma BCHE activities observed in exposed workers compared to controls clearly shows that they were exposed to insecticide mixtures. AChE and BCHE activity are key features and essential biomarkers of exposure to some insecticides such as organophosphates. Organophosphates and carbamates, which were identified among the pesticides used, inhibit cholinesterases, leading to the accumulation of acetylcholine at synapses and subsequent cholinergic overstimulation7. The manifestation of neurological problems reported by the workers were headaches, dizziness, confusion, and tremors5,6. These results are consistent with previous studies demonstrating the utility of cholinesterase activity as a biomarker of exposure to organophosphate and carbamate pesticides24. In fact, these compounds can potentially bind to acetylcholine and butyrylcholine and block their hydrolysis, leading to their rapid accumulation and causing neurological symptoms (loss of coordination, seizures, paralysis, and eventually death)25. The findings of this study support the growing evidence suggesting that exposure to pesticides can have detrimental effects on the human nervous system. While the results demonstrated a clear association between pesticide exposure and cholinesterase levels, it is important to note that this study did not establish a causal link.
The study’s findings align with previous research highlighting the potential detrimental effects of pesticides on liver health. The increased levels of biomarkers associated with liver function of banana farm workers suggest that pesticide exposure may cause liver damage. The specific pesticides used in banana farm, their mode of action, and the workers’ individual susceptibility to pesticide-induced liver damage may contribute to the observed differences in liver function markers. These imbalances can be explained by the fact that the liver is the primary detoxification organ of the body, acting through enzymatic catalysis mediated by cytochromes P45026. However, P450-mediated biocatalysis of insecticides was likely inhibited due to liver damage and cell death induced by insecticides9. Therefore, these metabolic imbalances may be attributed to chronic exposure to insecticide mixtures, which can lead to endocrine disruptions, deregulating enzymes of several metabolic pathways, and cause renal failure27,28. These findings are consistent with a growing number of studies indicating that insecticide may lead to an increase in transaminases (ALT, AST) and enzymes of metabolic pathways that are precursors of liver profile disorders, although the results are sometimes contradictory29. The findings of this study provide evidence for a potential link between pesticide exposure and liver function abnormalities of banana farm workers. The increased levels of AST, ALT, observed in workers suggest possible liver damage or inflammation due to pesticide exposure. This finding is consistent with previous studies that have linked pesticide exposure to liver dysfunction30 .
Pesticide exposure is associated with alterations in plasma levels of blood urea, a waste product normally filtered by the kidneys, indicating potential kidney dysfunction31. Several studies have reported associations between pesticide exposure and kidney dysfunction. These studies have found that pesticide exposure can cause a range of kidney problems, including acute kidney injury, chronic kidney disease, and an increased risk of kidney failure. However, the evidence is not conclusive, and further research is needed to understand the specific mechanisms by which pesticides may affect kidney function and the extent of these effects in different populations32.
This study has some limitations. The cross-sectional design precludes establishing a definitive causal relationship between pesticide exposure and the observed health effects. Recall bias may have affected the accuracy of self-reported symptoms and pesticide use practices. Although participants with known pre-existing liver or kidney diseases were excluded and controlled for age and sex, unmeasured confounding factors, such as alcohol consumption, smoking, dietary habits, genetic predisposition, and co-exposure to other environmental toxins, could have influenced the results. Furthermore, the study did not quantify individual pesticide exposure levels, relying instead on self-reported exposure history and job tasks.
Conclusion
In summary, the study provided evidence of the use of pesticides containing known active molecules in the study area, including herbicides (glyphosate), insecticides (organophosphates) and fungicides (dithiocarbamates). Banana farm workers reported effects on various systems (nervous, respiratory, digestive, cardiovascular and cutaneous), suggesting a link between pesticide exposure and dysfunction of liver, kidney and neurological parameters. The study’s findings add up to a unique challenge for banana farm workers, as they are not exposed to a single pesticide, but to a complex mixture of these harmful chemicals. This can have more serious consequences, as the synergistic effects of these chemical compounds can amplify their individual toxicities, leading to an increased risk of cardiovascular problems.
Acknowledgements
The data for this study come from pesticide-using banana plantation workers in Moungo Division, Cameroon.
Abbreviations
- AST
Aspartate aminotransferase
- ALT
Alanine aminotransferase
- ALP
Alkaline phosphatase
- GGT
Gamma glutamyl transferase
- LDH
Lactate dehydrogenase
- RBC-AChE
Red blood cell acetylcholinesterase
- BChE
Butyrylcholine esterase
- SC
Soluble concentrate
- EC
Emulsifiable concentrate
- SL
Soluble solution
- SC2
Suspension concentrates
- WP
Wettable powder
- GC
Granules concentrate
- Ia
Extremely dangerous
- Ib
Very dangerous
- II
Moderately dangerous
- III
Slightly hazardous
- U
Unlikely to present an acute hazard
- WHO
Word health organization
Author contributions
VJKN, conceptualization, data collection, formal analysis, methodology, writing—original draft. DEM, data validation, formal analysis, methodology, visualization, review—editing. PVH, DG and BRTT, data collection, formal analysis, review—editing. FAE and GRTN, methodology, validation, visualization, review—editing. LJMN, conceptualization, project administration, methodology, investigation. JLN supervision, conceptualization methodology, writing—Review—editing. All authors review the manuscript.
Data availability
The data that support the findings of this study may be available on request from the corresponding author.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Marican, A. & Durán-Lara E. F. A review on pesticide removal through different processes. Environ. Sci. Pollut. Res. Int.25, 2051–2064 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Zhang, N. et al. Evaluation of toxicological effects of organophosphorus pesticide metabolites on human HepG2 cells. Environ. Toxicol. Pharmacol.88, 103741 (2021). [DOI] [PubMed] [Google Scholar]
- 3.Muñoz-Quezada, M. et al. Chronic exposure to organophosphate (OP) pesticides and neuropsychological functioning in farm workers: a review. Int. J. Occup. Environ. Health. 22, 68–79 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee, I., Eriksson, P., Fredriksson, A., Buratovic, S. & Viberg, H. Developmental neurotoxic effects of two pesticides: behavior and biomolecular studies on Chlorpyrifos and Carbaryl. Toxicol. Appl. Pharmcol.288, 429–438 (2015). [DOI] [PubMed] [Google Scholar]
- 5.Karami-Mohajeri, S. & Abdollahi, M. Toxic influence of organophosphate, carbamate, and organochlorine pesticides on cellular metabolism of lipids, proteins, and carbohydrates: a systematic review. Hum. Exp. Toxicol.30, 1119–1140 (2011). [DOI] [PubMed] [Google Scholar]
- 6.Flaskos, J. The developmental neurotoxicity of organophosphorus insecticides: a direct role for the oxon metabolites. Toxicol. Lett.209, 86–93 (2012). [DOI] [PubMed] [Google Scholar]
- 7.Richardson, J. Assessment of the neurotoxic potential of Chlorpyrifos relative to other organophosphorus compounds: a critical review of the literature. J. Toxicol. Environ. Health44, 135–165 (1995). [DOI] [PubMed]
- 8.Li, W., Xiao, H., Wu, H., Xu, X. & Zhang, Y. Organophosphate pesticide exposure and biomarkers of liver injury/liver function. Liver International: Official J. Int. Association Study Liver. 42, 2713–2723 (2022). [DOI] [PubMed] [Google Scholar]
- 9.Mehendale, M. Chlordecone-induced hepatic dysfunction. J. Toxicol. Environ. Health 8, 743–755 (1981). [DOI] [PubMed] [Google Scholar]
- 10.Xing, H. et al. Effects of atrazine and Chlorpyrifos on cytochrome P450 in common carp liver. Chemosphere104, 244–250 (2014). [DOI] [PubMed] [Google Scholar]
- 11.VoPham, T. et al. Pesticide exposure and liver cancer: a review. Cancer Causes Control: CCC. 28, 177–190 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leonel Javeres, M. N., Rabia Habib, N., Judith, L., Syed Tahir, A. & Valis, M. Kamil Kuca, Syed Muhammad N. Chronic exposure to organophosphates pesticides and risk of metabolic disorder in cohort from Pakistan and Cameroon. Int. J. Environ. Res. Public. Health. 18, 2310 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leonel Javeres, M. N. et al. Mixture of organophosphates chronic exposure and pancreatic dysregulations in two different population samples. Front. Public. Health. 8, 534902 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Worek, F., Bäcker, R. & Thiermann, H. Determination of butyrylcholinesterase activity in human serum and plasma using a modified Ellman method. Clinic. Chem. Lab. Medicine. 37(10), 963–970 (1999).10616750 [Google Scholar]
- 15.Henry, R. J., Chiamori, N., Villalobos, O. T. & Berkman, S. Revised spectrophotometric methods for the determination of serum glutamic oxalacetic transaminase, glutamic pyruvic transaminase, and lactic dehydrogenase. Am. J. Clinic.Pathology. 34 (4), 381–398 (1960). [DOI] [PubMed] [Google Scholar]
- 16.IFCC. Methods for the measurement of catalytic concentration of enzymes. Part 2. IFCC method for aspartate aminotransferase (L-aspartate : 2-oxoglutarate aminotransferase, EC 2.6.1.1). J. Clinic. Chem. Clinic. Biochem. 24(7), 497–510 (1985). [PubMed] [Google Scholar]
- 17.Tietz, N. W. Textbook of Clinical Chemistry. (W.B. Saunders, 1999).
- 18.Pesce, A. Lactate dehydrogenase. Clinic. Chem.438, 1124–1127 (2000).
- 19.Searcy, R. L., Reardon, J. E. & Foreman, J. A. A new photometric method for serum urea nitrogen determination. Am J. Medical Technol. 33 (1), 15–20 (1967). [PubMed] [Google Scholar]
- 20.Fabiny, D. L. & Ertingshausen, G. Automated reaction-rate method for determination of serum creatinine with the CentrifiChem. Clinic. Chem. 17(7), 696–700 (1971). [PubMed]
- 21.Xu, S. et al. Analysis of serum levels of organochlorine pesticides and related factors in Parkinson’s disease. Neurotoxicology88, 216–223 (2022). [DOI] [PubMed] [Google Scholar]
- 22.Jia, C. et al. Association between serum pyrethroid insecticide levels and incident type 2 diabetes risk: A nested case-control study in Dongfeng-Tongji cohort. Eur. J. Epidemiol.37, 959–970 (2022). [DOI] [PubMed] [Google Scholar]
- 23.Sopkoutie, K. G. N., Fovo, J. D., Wumbei, A., Spanoghe, P., Galani, Y. J. H. Assessment of pesticide usage, phytosanitary practices and risks associated with pesticide use by farmers in Cameroon: A comprehensive literature review. Cameroon J. Biologic. Biochem. Sci. 32, 82–109 (2024). [Google Scholar]
- 24.Mathuramat, S. et al. Association between Organophosphate Pesticide Exposure and Insulin Resistance in Pesticide Sprayers and Nonfarm workers. Int. J. Environ. Res. Public Health 17–21 (2020). [DOI] [PMC free article] [PubMed]
- 25.Chang, C., Chen, L. & Wang, S. Cytochrome P450 enzymes and pesticide metabolism. J. Agri. Food Chem. 65 (32), 6931–6940 (2017). [Google Scholar]
- 26.Mansour, S.A. Pesticide exposure—Egyptian scene. Toxicology198, 91–115 (2004). [DOI] [PubMed]
- 27.Gamache, L. et al. Exposure to pesticides and welding hastens the age-at-onset of Parkinson’s disease. Can. J. Neurol. Sci.46, 711–716 (2019). [DOI] [PubMed]
- 28.Farkhondeh, T. et al. A systematic review on the metabolic effects of Chlorpyrifos. Rev. Environ. Health. 37, 137–151 (2022). [DOI] [PubMed] [Google Scholar]
- 29.Cassereau, J. et al. Neurotoxicity of insecticides. Curr. Med. Chem.24, 2988–3001 (2017). [DOI] [PubMed] [Google Scholar]
- 30.Casale, G., Perazzo, N. & Bottino, L. Impact of repeated pesticide exposure on liver function and chronic liver diseases. J. Environ. Health. 45 (2), 123–130 (2020). [Google Scholar]
- 31.Hoppin, J. A., Shorter, C. & Davis, D. M. Serum creatinine levels in US agricultural workers: A cross-sectional study. Environ. Health Perspectives. 124 (7), 987–994 (2016). [Google Scholar]
- 32.Blessy George, J., Neetha, M. & Neethu, K. Pesticides and their impact on renal function. Int. J. Res. Med. Sci. 5 (6), 2320–2326 (2017). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study may be available on request from the corresponding author.