Abstract
Postzygotic mosaicism (PZM) has been increasingly recognized in Cornelia de Lange syndrome (CdLS). However, it has been scarcely described when the genetic cause resides in SMC1A, an X-linked cohesin member implicated in approximately 5% of cases. Here, we report the first female patient with classic CdLS harboring a PZM variant in SMC1A and provide a comprehensive characterization of mosaicism in this gene. The identified PZM variant [SMC1A:NM_006306.3:c.2369 = /G > A; p.(Arg790Gln)], was detected in blood and buccal swab-derived DNA at around 30% allele frequency. Molecular dynamics simulations suggest that this mutation disrupts the elbow domain of SMC1A, potentially impairing cohesin function. Further delineation of phenotypes and genotypes associated with PZM variants in SMC1A reveals that, similar to constitutive variants, mosaic mutations are predominantly missense and distributed throughout the gene. Moreover, the phenotype of patients carrying mosaic variants is clinically indistinguishable from those with constitutive mutations, and purifying selection of the pathogenic variant in blood is suggested, as previously observed in NIPBL-related CdLS. However, this process may be more complex in SMC1A due to its X-linked nature. Our findings underscore the importance of high-sensitivity sequencing techniques for detecting mosaicism and highlight the complexity of X-linked mosaic variants in disease expressivity. Further functional studies and larger cohorts are crucial to improve genotype–phenotype correlations and diagnostics in CdLS.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-07268-z.
Keywords: Cornelia de Lange syndrome, SMC1A, Postzygotic mosaicism, Deep-sequencing, X-linked inheritance
Subject terms: Disease genetics, Clinical genetics, Medical genetics, Neurodevelopmental disorders
Introduction
Cornelia de Lange syndrome (CdLS, OMIM #122470, #300590, #610759, #614701, #300882) is a rare, dominant genetic disorder that comprises a broad spectrum of physical and neurodevelopmental abnormalities, whose incidence has been estimated within 1:10,000–1:30,000 live births. Classic CdLS is characterized by global developmental delay, distinctive facial dysmorphia, growth restriction, microcephaly, hirsutism, intellectual disability and limb reduction defects1.
Molecularly, the spectrum has been largely associated with pathogenic variants in genes involved in cohesin functioning, primarily in the cohesin loading factor NIPBL, in which a heterozygous loss-of-function mutation is located in the 60–70% of clinically diagnosed individuals. However, increasing cases are being accredited for other eight genes coding subunits (SMC1A, SMC3, and RAD21) or regulators (HDAC8, MAU2, BRD4 and ANKRD11) of the cohesin complex1,2, as well as transcriptional regulators independent of cohesin (AFF2)3.
SMC1A (Structural Maintenance of Chromosomes 1A, MIM 300,040), which encodes one of the core proteins of the cohesin ring, accounts for approximately 5% of CdLS cases4–6. This X-linked gene produces an evolutionarily conserved protein consisting of a hinge domain flanked by two large coiled-coil motifs, with N- and C-terminal NTPase-binding domains at its ends7,8 as well as an internal region that forms an elbow domain, according to several dynamic models describing the behavior of the cohesin complex as a Brownian ratchet9,10. As a structural component of the cohesin complex, SMC1A not only plays a pivotal role in chromosome segregation11, but is also involved in non-canonical cohesin functions, including 3D genome organization, gene expression, DNA repair processes and genome stability maintenance12–14.
Pathogenic variants in this gene are associated with phenotypes usually milder than those observed in NIPBL-related CdLS15–20. Most of these mutations are missense and result in individuals presenting with a non-classic phenotype characterized by slight cognitive impairment, the absence of major structural anomalies, and fuller eyebrows, a less pronounced shortening of the nasal bridge and a rounder face compared to patients with NIPBL affected8,21. However, reported cases to date comprise a spectrum of phenotypes ranging from classic and severe to moderate CdLS, as well as a non-resembling CdLS phenotype similar to Rett syndrome’s (OMIM #312750), recently referred to as SMC1A epilepsy syndrome or developmental and epileptic encephalopathy-85 with or without midline brain defects (DEE85, OMIM #301044)4–6,22–25. Notably, the heterogenous clinical presentation among affected individuals is apparent and even sharper in the context of SMC1A gene, where expressivity varies depending on sex and, in females, is further influenced by X-inactivation18,26.
In addition to the foregoing, the complexity of this scenario may be heightened by mosaicism, a condition characterized by the presence of genetically distinct cell lineages within the same individual as a result of postzygotic mutational events27. Classically, the source of CdLS was thought to be a de novo mutational event in one of the causative genes leading to a constitutive condition1. However, with the enhanced sensitivity of molecular diagnostic techniques developed over the last few years, which employ high-throughput genomic technologies such as quantitative polymerase chain reaction (qPCR), deep sequencing, or droplet digital PCR (ddPCR), numerous cases of postzygotic mosaicism have been identified in many dominant genetic diseases28. Particularly, in CdLS, several studies demonstrate a high prevalence of genetic mosaicism (around 10%)29–33 compared to other neurodevelopmental disorders34. Even though, the vast majority of the cases described consist of pathogenic mosaic variants in the NIPBL gene, whose delayed finding is due to either the low alternative allele frequency (AAF) of the mutation or the widespread absence of the variant in blood. Regarding SMC1A gene, as far as we are concerned, there are only two mosaic cases reported in male CdLS patients with mild phenotype32,35. Parental mosaicism in SMC1A-related CdLS has also been described in three independent familial cases to date, with no clear evidence that the parent of origin or the sex of the affected offspring influences disease expressivity19,36.
Here, we perform a systematic review and characterization of mosaicism in SMC1A gene, and report the first case of a female affected with classic CdLS due to a causal mosaic variant in SMC1A, identified in blood and buccal swab derived DNA with a similar AAF of around 30% of mosaicism.
Results
First case of postzygotic mosaicism in a female affected with SMC1A-based CdLS
Clinical report
Our case describes 43-year-old female patient, first child of a non-consanguineous healthy couple, delivered at term by caesarean section due to breech presentation, with no complications during pregnancy. Her birth length, body weight and head circumference were all within normal ranges for sex and age. At one and a half months of age she was hospitalized for vomiting and diagnosed with polymalformative syndrome, congenital heart disease that did not require surgical intervention and hypertrophic pyloric stenosis later, for which she underwent surgery at 3 months of age. When she was 2 and a half years-old the clinical diagnosis of Cornelia de Lange syndrome (CdLS) was suggested by physicians due to the presence of associated treats, namely coarse face features (HP:0000280) synophrys (HP:0000664), hirsutism (HP:0001007), single transverse palmar crease (HP:0000954) and notable intellectual disability (HP:0001249) with zero/no language at this age.
At the age of 16, she began a symptomatologic course of bipolar disorder (HP:0007302), with obsessive traits (HP:0008770), ritualization behaviours (HP:0031432), atypical gestures (HP:0034481) and constant repetition of questions. She frequently exhibits disruptive, non-collaborative behavior, sometimes accompanied by self-injuring (HP:0100716). Over time, obsessive–compulsive symptoms (HP:0008770), hyperphagia (HP:0002591), anxiety (HP:0000739), depression (HP:0000716), and hypomania (HP:0100754) emerged, requiring psychopharmacological adjustments.
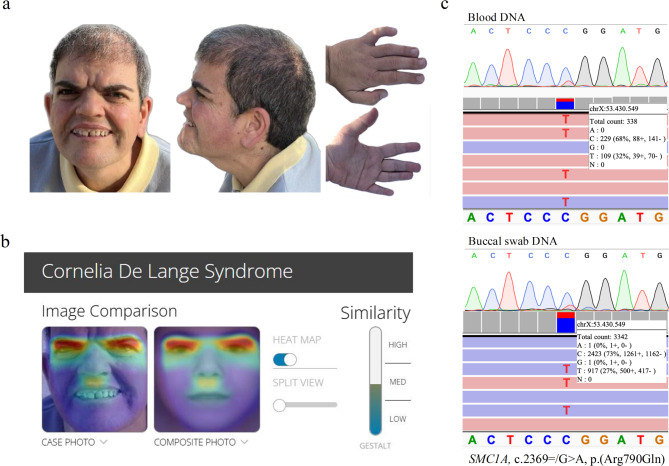
More than 20 years later, when the patient is 42 and still lacking a genetic diagnosis, she visits our Reference Centre in Zaragoza (Spain), where she undergoes a thorough re-evaluation by a clinical geneticist. Physical examination reveals some cardinal features associated with CdLS, namely thick eyebrows (HP:0000574), short nose (HP:0003196) with slightly concave nasal ridge (HP:0011120), smooth philtrum (HP:0000319), thin upper lip vermilion (HP:0000219) and downturned corners of mouth (HP:0002714). Additionally, some suggestive features of CdLS were observed: global developmental delay (HP:0001263), intellectual disability (HP:0001249) and small hands (HP:0200055). Patient’s clinical picture is shown in Fig. 1a.
Fig. 1.
Clinical presentation, Face2Gene evaluation, and sequencing results of the patient’s blood and buccal swab DNA. (a) Facial features, palmar and dorsal views of the left hand of the affected patient. (b) Facial heatmap from the Face2Gene application of a patient’s facial photograph at the age of 41 years. (c) Sanger chromatogram and Integrative Genomics Viewer (IGV) view of sequencing results of the targeted SMC1A variant NM_006306.3:c.2369 = /G > A; p.(Arg790Gln) in the patient’s blood (top) and buccal swab (bottom) derived DNA.
All these findings, together with the above mentioned, confirmed the clinical diagnosis of classic CdLS with a clinical score of 11 according to CdLS diagnosis statement criteria established by Kline et al.1 (Table 1). An additional computer-aided analysis of a patient’s portrait was subjected with the Face2Gene application37,38. Despite being a medical photograph of an adult patient, CdLS was the first-choice syndrome suggested with a high gestalt level (Fig. 1b).
Table 1.
Clinical findings and score calculation of the patient.
| Clinical findings of the patient | |
|---|---|
| Sex/Age | F/43 |
| Cardinal features (2 points each if present) | |
| Synophrys (HP:0000664) and/or thick eyebrows (HP:0000574) | + |
| Short nose (HP:0003196), concave nasal ridge (HP:0011120) and/or upturned nasal tip (HP:0000463) | + |
| Long (HP:0000343) and/or smooth philtrum (HP:0000319) | + |
| Thin upper lip vermilion (HP:0000219) and/or downturned corners of mouth (HP:0002714) | + |
| Hand oligodactyly (HP:0001180) and/or adactyly (HP:0009776) | − |
| Congenital diaphragmatic hernia (HP:0000776) | − |
| Suggestive features (1 point each if present) | |
| Global developmental delay (HP:0001263) and/or intellectual disability (HP:0001249) | + |
| Prenatal growth retardation (< 2 sD) (HP:0001511) | n.d. |
| Postnatal growth retardation (< 2 sD) (HP:0008897) | − |
| Microcephaly (prenatally and/or postnatally) (HP:0000252) | − |
| Small hands (HP:0200055) and/or feet (HP:0001773) | + |
| Short fifth finger (HP:0009237) | − |
| Hirsutism (HP:0001007) | + |
| Clinical score | 11 (Classic) |
| Phenotype | Moderate |
F female, HP Human Phenotype, + positive, − negative, n.d. not determined.
Genetic analyses
A custom gene panel designed specifically for the genetic diagnosis of CdLS was performed to analyse patient’s DNA isolated from blood, identifying a potential disease-causing variant in SMC1A gene [SMC1A:NM_006306.3:c.2369 = /G > A; p.(Arg790Gln)], detected with an allele frequency (AAF) of 32% and 338X sequencing coverage. The same analysis was performed in buccal swab-derived DNA, and the variant was identified in the 27% of the reads (3342X). The presence of the variant was confirmed by Sanger sequencing in both tissues (Fig. 1c).
This de novo variant (PS2) is not registered in gnomAD population frequency database39 (PM2), and all in silico algorithms applied (REVEL40: 0.806, MetaRNN41: 0.7958) (dbNSFP42 version 4.9) predicted it as deleterious (PP3). It is reported in ClinVar43, LOVD44 and UniProtKB45 as pathogenic (PP5) and has been previously described in literature as a constitutive variation in two female patients with moderate7 and mild19 CdLS (PS4). Additionally, a different pathogenic amino acid change occurring at the same position has been described (c.2368C > T; p.(Arg790Trp))32 (PM5). All of this leads to its classification as pathogenic under the ACMG Standards and Guidelines46.
Effect of the Arg790Gln variant in the 3D structure of the elbow domain of SMC1A
Spatial arrangement of the human SMC1A and SMC3 protein model with localization of amino acid Arg790 (R790) is shown in Fig. 2a. The models of the elbow domain of the coiled-coil region of human SMC1A (residues 341 to 415 and 744 to 814), both wild-type and Arg790Gln variant proteins, were simulated for 200 ns using molecular dynamics techniques continuously monitoring changes in the structure. In the case of the wild-type protein domain, the distance between the alpha carbons of amino acids Arg790 and Glu767 remains unchanged throughout the trajectory (Fig. 2b, wt), suggesting their role in local stability. Consistently, the structure shows that the positively charged side chain of amino acid Arg790 interacts with the negatively charged side chains of amino acids Asp774 and Glu767, which are located in the adjacent alpha-helix (Fig. 2c, left). In contrast, in the case of the SMC1A Arg790Gln variant protein, the distance between the alpha carbons of amino acids Gln790 and Glu767 increases rapidly during the first 20–40 ns of the simulation and remains at high values with large fluctuations along the trajectory (Fig. 2b, R790Q). After 200 ns of molecular dynamics (Fig. 2c, right), the interaction between the side chains of amino acids Gln790, Asp774 and Glu767 is lost, causing local disorganization of the elbow domain.
Fig. 2.
Effect of the Arg790Gln variant in the 3D structure of the elbow domain of the human SMC1A protein. (a) Spatial arrangement of the human SMC1A and SMC3 protein model. The SMC1A protein is colored according to its secondary structure elements. The positions of the head, hinge, and elbow domains are indicated, with amino acid Arg790 (R790) marked as a magenta sphere. (b) Distance (in Angstroms) between the alpha carbons of amino acids Arg790 (or Gln790) and Glu767 throughout the trajectory of an unrestricted molecular dynamics simulation of the elbow domain in the wild-type SMC1A protein (wt) and the Arg790Gln variant (R790Q). (c) Structure of the SMC1A elbow domain model before (left, t = 0 ns) and after 200 ns of molecular dynamics simulation, for both the wild-type protein (middle, wt (t = 200 ns)) and the Arg790Gln variant (right, R790Q (t = 200 ns)). The positions of the amino acids Arg790 (R790), Asp774 (D774) and Glu767 (E767), as well as the position of Gln790 (Q790) in the Arg790Gln variant, are indicated.
Systematic review of postzygotic mosaicism in SMC1A
SMC1A-related PZM cases
We conducted a literature review of all disease-causing PZM variants in SMC1A gene reported up to this manuscript was prepared, the results of which are summarized in Table 2. Besides the novel case described in this study, only two other CdLS patients harboring mosaic variants in SMC1A have been described, both males present with mild phenotype. The first case, described within a comprehensive mutation analysis in a cohort of typical and atypical CdLS by Ansari et al.32, involved a patient with an atypical phenotype and moderate growth retardation. The second reported case describes a 6-year-old male presenting with typical CdLS features, including microcephaly (HP:0000252), synophrys (HP:0000664), long eyelashes (HP:0000527), low-set ears (HP:0000369), anteverted nares (HP:0000463), long philtrum (HP:0000343), smooth philtrum (HP:0000319), downturned corners of the mouth (HP:0002714), micrognathia (HP:0000347), growth failure (HP:0001510), developmental delay (HP:0001263), autistic behavior (HP:0000729), hirsutism (HP:0001007) and external genital hypoplasia (HP:0003241). Based on this information, the clinical score was determined, reaching 12 points, consistent with a classic CdLS phenotype. Moreover, his gestalt score strongly matched CdLS when analyzed using the Face2Gene application35.
Table 2.
SMC1A PZM variants in affected individuals.
| PZM variant | Variant detection | Affected individual | Ref | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Nucleotide change | Type | Exon | Protein change | Domain | Method | AAF Blood | AAF Buccal Cells |
Sex/Age | Condition | Phenotype | |
| c.2369G > A | Missense | 15 | p.(Arg790Gln) |
Elbow (C-ter coiled-coil) |
NGS | 32% (338X) | 27% (3342X) | F/43 | CdLS | Moderate | This work |
| c.1585_1587del | In-frame deletion | 10 | p.(Lys529del) | N-ter coiled-coil | NGS | n.a. |
53% (431X) and 10% (450X) |
M/14.3 and 18.3 |
CdLS | Atypical with moderate growth retardation | 32 |
| c.793_795del | In-frame deletion | 2 | p.(Glu265del) |
Hinge (close to N-ter coiled-coil) |
NGS | 2% (199X) | 60% (56X) | M/6 | CdLS | Mild | 35 |
| c.1900C > T | Nonsense | 11 | p.(Gln634Ter) |
C-ter coiled-coil (close to hinge) |
n.a. | n.a. | n.a. | F/n.a. | DEE85 | n.a. | 5 |
Molecular properties of the PZM variants, diagnosis method used, biological sample analyzed, allele frequency and clinical data for each individual are indicated. SMC1A RefSeq NM_006306.4
M male, F female, NGS next-generation sequencing, AAF alternative allele frequency, CdLS Cornelia de Lange syndrome, DEE85 developmental and epileptic encephalopathy-85 with or without midline brain defects, n.d. not determined, n.a. not available.
Notably, the two mosaic SMC1A variants described thus far are de novo 3 bp in-frame deletions. These pathogenic variants, [c.793_795 = /del; p.(Glu265del)] and [c.1585_1587 = /del; p.(Lys529del)], are set into the N-terminal coiled-coil and at the junction between the N-terminal coiled coil and the hinge domain of SMC1A, respectively (Fig. 3). Both were identified in buccal swab-derived DNA. In one case, the presence of the variant in blood is not specified, while in the other, it was detected at a very low AAF. More precisely, the first patient described harbored the causal variant at 53% (431X) and 10% (450X) of mosaicism in two separate saliva samples taken when he was 14.3 and 18.3 years of age, respectively, proving that mosaicism levels can be variable even on the same tissue32. In the second case, a novel hemizygous variant in SMC1A was detected in 60% of sequencing reads (56X) on buccal swab-derived DNA, while the analyses using NGS panel revealed the presence of the variant at 2% (199X) on blood DNA35 (Table 2).
Fig. 3.
SMC1A PZM variants distribution. Schematic representation of the SMC1A gene, showing its location on the X chromosome (p11.22) and the localization across exons and protein domains of PZM variants described in the literature, along with the newly identified variant (in bold). PZM variants identified in CdLS-affected individuals are shown in black, the PZM variant associated with DEE85 is shown in orange, and parental mosaicism-related variants are colored in blue.
An additional SMC1A mosaic variant has been reported in a patient with intractable epilepsy, who had no clinical suspicion of CdLS before genetic testing. This variant consists of a nonsense mutation [c.1900 = /C > T; p.(Gln634Ter)] located in the C-terminal coiled-coil of SMC1A, close to the hinge domain5 (Fig. 3, Table 2).
Parental mosaicism in SMC1A
Regarding parental mosaicism, three unrelated familial cases of SMC1A-based CdLS have been documented so far, although additional variants have been reported in clinical and research cohorts47. In one case, gonadosomatic mosaicism was revealed in an unaffected father, who transmitted the causal variant to his daughters [c. 1487 = /G > A; p.(Arg496His)]19. The same variant was reported in another unrelated familial case, and although it could not be directly confirmed in either parent, its presence in two daughters was consistent with germline mosaicism (not included in Table 3)19. The last case of CdLS-related parental PZM describes an unaffected mother carrier of a pathogenic SMC1A variant in very low allele frequencies in blood and buccal swab DNA, and had a son with CdLS [c.2078 = /G > A; p.(Arg693Gln)]36. A further case of parental mosaicism was identified in a patient with early-onset epileptic encephalopathy (EOEE), whose mother harbored the causal nonsense SMC1A variant in a mosaic state [c.511 = /C > T; p.(Arg171Ter)] and exhibited epilepsy (HP:0001250)48. All this information is summarized in Table 3.
Table 3.
Parental mosaicism cases related to SMC1A.
| PZM variant | Variant detection | Parental transmission | Ref | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Nucleotide change | Type | Exon | Protein change | Domain | Method | AAF Blood | AAF Buccal Cells |
Carrier parent | Phenotype | Offspring condition | |
| c. 1487G > A | Missense | 9 | p.(Arg496His) | N-ter coiled-coil | n.a. | n.a. | n.a. | Father | Unaffected | CdLS | 19 |
| c. 2078G > A | Missense | 13 | p.(Arg693Gln) | C-ter coiled-coil | NGS and Sanger | 9% (942X) | n.d. (Sanger) | Mother | Unaffected | CdLS | 36 |
| c. 511C > T | Nonsense | 4 | p.(Arg171Ter) | N-ter coiled-coil | NGS | 32% (9793X) | n.a. | Mother | Epilepsy | EOEE | 48 |
Molecular properties of the PZM variants, diagnosis method used, biological sample analyzed, allele frequency and clinical information for each case are indicated. SMC1A RefSeq NM_006306.4
Sanger Sanger sequencing, NGS next-generation sequencing, AAF alternative allele frequency, CdLS Cornelia de Lange syndrome, EOEE early-onset epileptic encephalopathy, n.d. not determined, n.a. not available.
Discussion
The genetic and clinical heterogeneity of Cornelia de Lange syndrome (CdLS) is well-documented, to the extent that it is currently considered a spectrum. This complexity increases with the presence of mosaicism and is compounded when the causal variant affects the X-linked SMC1A gene, as clinical expressivity varies depending on sex and, among females, is further influenced by X-inactivation. Here, we report the first female patient with classic Cornelia de Lange syndrome (CdLS) harboring a postzygotic mosaic (PZM) variant in SMC1A, and provide a comprehensive characterization of mosaicism in this gene. Although PZM has been extensively described in CdLS, the vast majority of cases involve NIPBL, and to date, only two male patients with mosaic causal variants in SMC1A have been reported.
The patient described herein harbors a PZM variant [SMC1A:NM_006306.3:c.2369 = /G > A; p.(Arg790Gln)], detected in blood and buccal swab-derived DNA with a similar AAF of approximately 30% mosaicism. Although missense mutations in this gene have traditionally been associated with a milder, non-classic presentation of CdLS than the NIPBL-based4,7,19,20, she exhibits clinical features consistent with the classic phenotype, reaching a clinical score of 111. Uncertainty remains regarding the full phenotypic spectrum linked to SMC1A, with cases ranging from severe to mild manifestations of the disorder8. Additionally, a distinct phenotype that recalls Rett syndrome (OMIM #312750) has been recognized, characterized by epileptic encephalopathy, profound intellectual disability, and stereotypic movements. This SMC1A-related disorder has been described exclusively in females, mostly carrying truncating variants, and has been designated as SMC1A epilepsy syndrome or developmental and epileptic encephalopathy-85 with or without midline brain defects (DEE85, OMIM #301044)4–6,22–25.
The PZM variant identified has been previously reported as a de novo constitutive mutation in two female patients with moderate7 and mild19 CdLS. All three individuals share similar craniofacial features, vision impairments and limb anomalies, including small hands and fifth-finger clinodactyly, without hearing or feeding problems. These findings suggest that, as observed with NIPBL and unlike what is typically seen in other conditions, disease severity in patients harbouring mosaic variants is indistinguishable from that of individuals with a constitutive mutation29,32,49. However, considerable clinical variability persists among the three affected females, highlighting the influence of additional factors beyond the disease-causing variant itself, such as the genotype at other loci, environmental modifiers, and/or X-inactivation patterns7. This last aspect is particularly relevant for SMC1A. Given its X-linkage, females might be less affected than males, who carry only the mutant X allele. Compensatory mechanisms such as preferential expression of the wild-type allele19,50 or X-inactivation skewed against the mutant X chromosome26 have been proposed for females. However, SMC1A lies in a region that partially escapes X-inactivation. This leads to variable expression from the inactive X chromosome26,51,52, complicating interpretations of inactivation skewing patterns. Additionally, since total SMC1A transcript levels in affected females are comparable to those in the general population, the phenotypic outcome is unlikely to be driven by an overall expression imbalance. Instead, a dominant-negative effect of the mutant protein has been suggested as a potential explanation for severe cases18.
To date, only two additional SMC1A CdLS-causing variants have been identified, both in male individuals with mild phenotype. Sharing some features with the patient described here, the individual reported by Garcia et al.35 reached a clinical score of 12 points, consistent with a classic CdLS phenotype1. More specifically, both individuals exhibit the four cardinal CdLS features related to facial dysmorphia, as well as some suggestive features, namely global developmental delay (HP:0001263), postnatal growth retardation (< 2 sD) (HP:0008897), microcephaly (HP:0000252) and hirsutism (HP:0001007). Regarding molecular characteristics, the two PZM variants previously reported, p.(Glu265del)35 and p.(Lys529del)32, are located in the N-terminal coiled-coil and in the hinge domain, close to the N-terminal coiled-coil of SMC1A, respectively. Distinctly, the variant described in this study, p.(Arg790Gln), is set into the elbow domain of the C-terminal coiled-coil of SMC1A. Despite these differences, all align with the generalities observed in constitutive CdLS-causing variants: they are either missense or in-frame deletions and are distributed across all gene domains without any specific mutational hotspots8,49.
To gain further insights into how the variant identified might affect SMC1A functionality, molecular dynamics simulations were performed on the structures of the elbow domain of the human SMC1A protein, both wild-type and the Arg790Gln variant. The analysis revealed that the Arg790Gln substitution could induce local structural destabilization of the domain, which is thought to be crucial in several dynamic models proposed for the behavior of the cohesin complex as a Brownian ratchet9,10. In these models, the mechanical tension sustained by the electrostatic interaction between Arg790 and two neighboring negatively charged amino acids would be key in regulating complex motions. The loss of this interaction in the Arg790Gln variant could lead to inhibition or, in any case, deregulation of the movement associated with the Brownian ratchet model. The functionality of the cohesin complex would therefore be compromised.
Regarding tissue distribution of SMC1A PZM variants, it is noteworthy that the two previously reported were identified in buccal swab DNA. While one case lacks blood-derived DNA data32, the other showed a markedly lower AAF in blood (2%) compared to buccal swab (60%)35. According to this, SMC1A could follow a similar pattern to NIPBL undergoing purifying selection in blood29,53. In our patient, the causal variant was detected in both blood and buccal swab-derived DNA at ~ 30% mosaicism. However, unlike prior cases involving males, this study describes a female, and the potential role of sex in this phenomenon should be considered. As males have only one X chromosome in every cell, the absence of the mutation in blood could be driven by mechanisms similar to those observed for NIPBL variants. In contrast, in females, the inactivation of the mutated X chromosome could potentially result in affected cells going unnoticed during this selection process. This would explain why we detect the mutation when analyzing genomic DNA, as the inactive X chromosome, though transcriptionally silenced, remains present in the cells. Another instance of this occurrence involves a mosaic mother who transmitted a causal SMC1A variant to her son with CdLS. She exhibited no clinical manifestations and carried the mutation in very low AAF in both blood and buccal swab DNA36. Only two other cases of parental mosaicism in SMC1A-related CdLS have been described 19, though details on tissue distribution in the carrier parent are lacking. Ultimately, although uncertainty remains regarding the most representative tissues for accurately studying mosaicism in SMC1A, as it occurs with other CdLS-related genes, blood-derived DNA may not be the optimal sample choice.
Once again, this case highlights the importance of employing high-throughput techniques in genetic testing for CdLS, and demonstrates that mosaicism could be more frequent than previously thought not just in the main causal gene, NIPBL. Notably, although the patient was clinically diagnosed at the age of four, her molecular diagnosis was not confirmed until she was 41, when a deep-sequencing panel with very high sequencing depth was performed. Indeed, mosaicism entails a significant challenge in the diagnostic process and routine clinical diagnostic approaches, such as exome sequencing in patients followed by Sanger sequencing for parental validation, are often insufficient for detecting low-level mosaicism. Moreover, as parental mosaic cases are being increasingly detected, the analysis of additional samples with highly sensitive technologies should be recommended for both CdLS diagnosis and co-segregation studies.
This study further expands the clinical and molecular spectrum of SMC1A-related mosaicism, underscoring the challenges in establishing genotype–phenotype correlations when SMC1A is the affected gene. Future studies incorporating functional assays and larger cohorts of individuals with PZM variants will be essential to elucidate the molecular mechanisms underlying CdLS etiopathogenia and refine diagnostic strategies.
Methods
Patient consultation and data collection
The patient of study is registered in the database of our National Center for Cornelia de Lange syndrome (COL: C.0005514, CEICA: COL19/001). Clinical data were collected by a clinical geneticist using a standard restricted-term questionnaire, and deep phenotyping was performed using the Human Phenotype Ontology (HPO) nomenclature. Clinical scores for CdLS were calculated according to the published international consensus guidelines1. All the procedures carried out in this study were performed in accordance with relevant guidelines and regulations, and were reviewed and approved by the Ethics Committee of Clinical Research from the Government of Aragón (CEICA: PI24/288). Written informed consent was obtained from the family before manuscript publication. Additionally, a separate informed consent form was signed for the release of the subjects’ photographs.
Molecular diagnosis
The new mosaic patient described in this article was molecularly diagnosed in the Spanish CdLS Reference Center in Zaragoza.
DNA isolation
Genomic DNA was isolated from peripheral blood using NZY Tissue gDNA Isolation kit (NZYtech), and from oral mucosa epithelial cells using prepIT.L2P (DNA Genotek Inc.) according to the manufacture’s protocols. Quality and concentration of resultant gDNA were determined using the Qubit Fluorometric Quantification (Thermo Fisher Scientific) and Nanodrop 2000 (Thermo Fisher Scientific).
Next-generation sequencing
Both blood and buccal swab derived DNA samples were analyzed using a custom NGS panel targeting 35 CdLS-related genes using the Ion AmpliSeqTM Designer tool (https://ampliseq.com/login/login.action). The panel covered a total of 249.25 kb of selected genomic regions, including NIPBL (NM_133433.3), SMC1A (NM_006306.3), SMC3 (NM_058243.2), RAD21 (NM_006265.2), HDAC8 (NM_018486.2), BRD4 (NM_058243.2), ANKRD11 (NM_001256183.1), MAU2 (NM_015329.3) and AFF2 (NM_002025.4). Library preparation, emulsion PCR, bead enrichment, and chip loading were performed automatically on an Ion Chef™ instrument (Thermo Fisher Scientific) using the Ion AmpliSeq™ Kit for Chef DL8 and 530™ Kits, following the manufacturer’s protocols. Sequencing was conducted on an Ion S5™ XL sequencer (Thermo Fisher Scientific) with read lengths set at 200 bp and eight samples per chip. Sequencing data were analyzed using Ion Torrent Suite™, Ion Reporter™, and IGV (Broad Institute) software. Variants were annotated according to the Human Genome Variation Society (HGVS) nomenclature guidelines54,55 and classified following ACMG recommendations46. Detailed variant information was retrieved from public databases, including gnomAD39, OMIM (https://omim.org/)56, ClinVar43, dbSNP57, LOVD44, and relevant scientific literature. In silico predictions were performed using REVEL40 and MetaRNN41 (dbNSFP version 4.9 (https://www.dbnsfp.org/)42), and Franklin by Genoox (https://franklin.genoox.com) and VarSome58 platforms were used to asses variant interpretation.
Sanger sequencing
To validate the pathogenic variant detected by NGS in the patient and confirm its absence in the parents, an independent PCR amplification followed by Sanger sequencing was conducted. Primers were designed using the Primer-BLAST in silico tool (https://www.ncbi.nlm.nih.gov/tools/primer-blast/)59 and verified with the UCSC In-Silico PCR tool (https://genome.ucsc.edu/cgi-bin/hgPcr)60. Primer sequences and annealing temperatures are provided in Supplementary Table S1. PCR products were sequenced on an ABI3730xl Capillary Electrophoresis Sequencing System (Applied Biosystems) following manufacturer’s instructions. Sequence data were analyzed and compared to reference sequences using the Analysis Module Variant Analysis (VA) software (Applied Biosystems) and Ensembl and NCBI publicly available databases.
Bioinformatic studies
Structural modeling of the SMC1A elbow domain
Structural modeling of the elbow domain of the coiled-coil region of human SMC1A (residues 341 to 415 and 744 to 814; UniprotKB id: Q14683) was performed by homology modeling using as template the crystal structure of the folded elbow of the yeast SMC1 protein (residues 360 to 424 and 757 to 822; UniprotKB id: P32908; Protein Data Bank id: 7OGT)61. The structure of the wild-type domain was modeled by combining residue positions obtained using Phyre262 and SwissModel63. The structure of the SMC1A Arg790Gln variant was modeled using the wild-type elbow domain model as template.
Molecular dynamics simulation of wild-type and mutant proteins
Structure models were subjected to 200 ns of unrestrained Molecular Dynamics (MD) simulation using the Amber18 package (https://ambermd.org; University of California-San Francisco, CA) to analyze the effect of the Arg790Gln variant on the structure of the SMC1A elbow domain, essentially as previously described64. Briefly, after solvation, initial wild-type and variant model structures were subjected to 10,000 cycles of energy minimization, followed by a 1 ns restrained equilibration phase in which the temperature was smoothly raised to 297 K, after which the restraints were gradually removed over 10 ns. Each system was then subjected to a 200 ns free MD production phase. Trajectories were continuously monitored and analyzed using cpptraj65 and VMD66. Plots were generated using Pymol (The PyMOL Molecular Graphics System, Version 3.0 Schrödinger, LLC).
Systematic review
A comprehensive literature search was performed in PubMed covering the period from 2005 to 2025. The search terms included “de Lange Syndrome”, “SMC1A”, “mosaicism”, “parental mosaicism”, and “postzygotic mutation”. Eligible studies comprised case reports, cohort studies and reviews that reported pathogenic mosaic variants in SMC1A. We specifically analyzed: (i) the clinical phenotypes of affected individuals, (ii) the type and tissue distribution of the mosaic variants, and (iii) cases where CdLS was transmitted through parental mosaicism. Additionally, we referred to SMC1A pathogenic and likely pathogenic variants deposited in ClinVar43, LOVD44 and DECIPHER47 databases to search for reported mosaic mutations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank Rebeca Antoñanzas for her valuable technical support. We also gratefully acknowledge the computational support of the “Centro de Computación Científica CCC-UAM”. This work is supported by the Spanish Ministry of Health - ISCIII Fondo de Investigación Sanitaria (FIS) [Ref. PI23/01370, to F.J.R. and J.P.], and co-funded by the European Union.
Author contributions
M.G.-S., A.L.-P., C.L.-C., M.A., B.P. and J.P.: molecular analyses. L.T., P.P.-V., J.R. and F.J.R.: patient’s recruitment and clinical score calculations. I.M.-A. and P.G.-P.: bioinformatic studies. M.G.-S., A.L.-P. and J.P.: manuscript writing, collection, and assembly of data. A.L.-P., F.J.R. and J.P.: manuscript editing and approval of the manuscript. All authors reviewed the submitted version of the manuscript.
Funding
The funding was provided by Spanish Ministry of Health-ISCIII, PI23/01370; Spanish Government grant, PID2021-126625OB-I00; Diputación General de Aragón-FEDER: European Social Fund, B32_20R; University of Zaragoza, JIUZ2023-SAL-06.
Data availability
The data that support the findings of this study are available from the corresponding authors F.J.R., J.P or A.L.-P. upon request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Feliciano J. Ramos, Email: framos@unizar.es
Juan Pié, Email: juanpie@unizar.es.
Ana Latorre-Pellicer, Email: alatorre@unizar.es.
References
- 1.Kline, A. D. et al. Diagnosis and management of Cornelia de Lange syndrome: first international consensus statement. Nat. Rev. Genet.19, 649–666 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaur, M. et al. Genomic analyses in Cornelia de Lange Syndrome and related diagnoses: Novel candidate genes, genotype-phenotype correlations and common mechanisms. Am. J. Med. Genet. A191, 2113–2131 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lucia-Campos, C. et al. An intragenic duplication in the AFF2 gene associated with Cornelia de Lange syndrome phenotype. Front. Genet.15, 1472543 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huisman, S. et al. Phenotypes and genotypes in individuals with SMC1A variants. Am. J. Med. Genet. A173, 2108–2125 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Barañano, K. W. et al. Further characterization of SMC1A loss of function epilepsy distinct from Cornelia de Lange syndrome. J. Child Neurol.37, 390–396 (2022). [DOI] [PubMed] [Google Scholar]
- 6.Gibellato, E. et al. SMC1A epilepsy syndrome: Clinical data from a large international cohort. Am. J. Med. Genet. A194, e63577 (2024). [DOI] [PubMed] [Google Scholar]
- 7.Gervasini, C. et al. Cornelia de Lange individuals with new and recurrent SMC1A mutations enhance delineation of mutation repertoire and phenotypic spectrum. Am. J. Med. Genet. A161A, 2909–2919 (2013). [DOI] [PubMed] [Google Scholar]
- 8.Musio, A. The multiple facets of the SMC1A gene. Gene743, 144612 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Higashi, T. L., Pobegalov, G., Tang, M., Molodtsov, M. I. & Uhlmann, F. A Brownian ratchet model for DNA loop extrusion by the cohesin complex. Elife10, e67530 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ros-Pardo, D., Gómez-Puertas, P. & Marcos-Alcalde, Í. STAG2-RAD21 complex: A unidirectional DNA ratchet mechanism in loop extrusion. Int. J. Biol. Macromol.276, 133822 (2024). [DOI] [PubMed] [Google Scholar]
- 11.Yatskevich, S., Rhodes, J. & Nasmyth, K. Organization of chromosomal DNA by SMC complexes. Annu. Rev. Genet.53, 445–482 (2019). [DOI] [PubMed] [Google Scholar]
- 12.Rao, S. S. P. et al. A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping. Cell159, 1665–1680 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwarzer, W. et al. Two independent modes of chromatin organization revealed by cohesin removal. Nature551, 51–56 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Musio, A. et al. SMC1 involvement in fragile site expression. Hum. Mol. Genet.14, 525–533 (2005). [DOI] [PubMed] [Google Scholar]
- 15.Borck, G. et al. Incidence and clinical features of X-linked Cornelia de Lange syndrome due to SMC1L1 mutations. Hum. Mutat.28, 205–206 (2007). [DOI] [PubMed] [Google Scholar]
- 16.Rohatgi, S. et al. Facial diagnosis of mild and variant CdLS: Insights from a dysmorphologist survey. Am. J. Med. Genet.152(7), 1641–1653 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoppman-Chaney, N., Jang, J. S., Jen, J., Babovic-Vuksanovic, D. & Hodge, J. C. In-frame multi-exon deletion of SMC1A in a severely affected female with Cornelia de Lange syndrome. Am. J. Med. Genet. A158A, 193–198 (2012). [DOI] [PubMed] [Google Scholar]
- 18.Liu, J. et al. SMC1A expression and mechanism of pathogenicity in probands with X-linked Cornelia de Lange syndrome. Hum. Mutat.30, 1535 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deardorff, M. A. et al. Mutations in cohesin complex members SMC3 and SMC1A cause a mild variant of Cornelia de Lange syndrome with predominant mental retardation. Am. J. Hum. Genet.80, 485–494 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pié, J. et al. Mutations and variants in the cohesion factor genes NIPBL, SMC1A and SMC3 in a cohort of 30 unrelated patients with Cornelia de Lange syndrome. Am. J. Med. Genet. A152A, 924 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mannini, L., Cucco, F., Quarantotti, V., Krantz, I. D. & Musio, A. Mutation spectrum and genotype-phenotype correlation in Cornelia de Lange syndrome. Hum. Mutat.34, 1589–1596 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Symonds, J. D. et al. Heterozygous truncation mutations of the SMC1A gene cause a severe early onset epilepsy with cluster seizures in females: Detailed phenotyping of 10 new cases. Epilepsia58, 565–575 (2017). [DOI] [PubMed] [Google Scholar]
- 23.Bozarth, X. L. et al. Phenotypes and genotypes in patients with SMC1A-related developmental and epileptic encephalopathy. Genes (Basel)14, 852 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldstein, J. H. R. et al. Novel SMC1A frameshift mutations in children with developmental delay and epilepsy. Eur. J. Med. Genet.58, 562–568 (2015). [DOI] [PubMed] [Google Scholar]
- 25.Jansen, S. et al. De novo loss-of-function mutations in X-linked SMC1A cause severe ID and therapy-resistant epilepsy in females: expanding the phenotypic spectrum. Clin. Genet.90, 413–419 (2016). [DOI] [PubMed] [Google Scholar]
- 26.Fieremans, N. et al. Identification of intellectual disability genes in female patients with a skewed X-inactivation pattern. Hum. Mutat.37, 804–811 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Biesecker, L. G. & Spinner, N. B. A genomic view of mosaicism and human disease. Nat. Rev. Genet.14, 307–320 (2013). [DOI] [PubMed] [Google Scholar]
- 28.Zemet, R., Van den Veyver, I. B. & Stankiewicz, P. Parental mosaicism for apparent de novo genetic variants: Scope, detection, and counseling challenges. Prenat. Diagn.42, 811 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Latorre-Pellicer, A. et al. Clinical relevance of postzygotic mosaicism in Cornelia de Lange syndrome and purifying selection of NIPBL variants in blood. Sci. Rep.11, 15459 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krawczynska, N., Wierzba, J. & Wasag, B. Genetic mosaicism in a group of patients with Cornelia de Lange syndrome. Front. Pediatr.7, 203 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nizon, M. et al. A series of 38 novel germline and somatic mutations of NIPBL in Cornelia de Lange syndrome. Clin. Genet.89, 584–589 (2016). [DOI] [PubMed] [Google Scholar]
- 32.Ansari, M. et al. Genetic heterogeneity in Cornelia de Lange syndrome (CdLS) and CdLS-like phenotypes with observed and predicted levels of mosaicism. J. Med. Genet.51, 659–668 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huisman, S. A., Redeker, E. J. W., Maas, S. M., Mannens, M. M. & Hennekam, R. C. M. High rate of mosaicism in individuals with Cornelia de Lange syndrome. J. Med. Genet.50, 339–344 (2013). [DOI] [PubMed] [Google Scholar]
- 34.Wright, C. F. et al. Clinically-relevant postzygotic mosaicism in parents and children with developmental disorders in trio exome sequencing data. Nat. Commun.10, 2985 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garcia, A. G., Malone, J. & Li, H. A novel mosaic variant on SMC1A reported in buccal mucosa cells, albeit not in blood, of a patient with Cornelia de Lange-like presentation. Cold Spring Harb. Mol. Case Stud.6, a005322 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gil-Salvador, M. et al. Case report: A novel case of parental mosaicism in SMC1A gene causes inherited Cornelia de Lange syndrome. Front. Genet.13, 993064 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Latorre-Pellicer, A. et al. Evaluating Face2Gene as a tool to identify Cornelia de Lange syndrome by facial phenotypes. Int. J. Mol. Sci.21, 1042 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gurovich, Y. et al. Identifying facial phenotypes of genetic disorders using deep learning. Nat. Med.25, 60–64 (2019). [DOI] [PubMed] [Google Scholar]
- 39.Chen, S. et al. A genomic mutational constraint map using variation in 76,156 human genomes. Nature625, 92–100 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ioannidis, N. M. et al. REVEL: An ensemble method for predicting the pathogenicity of rare missense variants. Am. J. Hum. Genet.99, 877–885 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li, C., Zhi, D., Wang, K. & Liu, X. MetaRNN: differentiating rare pathogenic and rare benign missense SNVs and InDels using deep learning. Genome Med.14, 115 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu, X., Li, C., Mou, C., Dong, Y. & Tu, Y. dbNSFP v4: a comprehensive database of transcript-specific functional predictions and annotations for human nonsynonymous and splice-site SNVs. Genome Med.12, 1–8 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Landrum, M. J. et al. ClinVar: public archive of relationships among sequence variation and human phenotype. Nucleic Acids Res.42, D980–D985 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fokkema, I. F. A. C. et al. LOVD v.2.0: The next generation in gene variant databases. Hum. Mutat.32, 557–563 (2011). [DOI] [PubMed] [Google Scholar]
- 45.Consortium T. U. et al. UniProt: The universal protein knowledgebase in 2025. Nucleic Acids Res.53, D609–D617 (2025). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Richards, S. et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of medical genetics and Genomics and the Association for molecular pathology. Genet. Med.17, 405–423 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Firth, H. V. et al. DECIPHER: Database of chromosomal imbalance and phenotype in humans using ensembl resources. Am. J. Hum. Genet.84, 524–533 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shu, L. et al. Parental mosaicism in de novo neurodevelopmental diseases. Am. J. Med. Genet. A185, 2119–2125 (2021). [DOI] [PubMed] [Google Scholar]
- 49.Gervasini, C. et al. Molecular characterization of a mosaic NIPBL deletion in a Cornelia de Lange patient with severe phenotype. Eur. J. Med. Genet.56, 138–143 (2013). [DOI] [PubMed] [Google Scholar]
- 50.Musio, A. et al. X-linked Cornelia de Lange syndrome owing to SMC1L1 mutations. Nat. Genet.38, 528–530 (2006). [DOI] [PubMed] [Google Scholar]
- 51.Brown, C. J. et al. The DXS423E gene in Xp11.21 escapes X chromosome inactivation. Hum. Mol. Genet.4, 251–255 (1995). [DOI] [PubMed] [Google Scholar]
- 52.Carrel, L. & Willard, H. F. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature434, 400–404 (2005). [DOI] [PubMed] [Google Scholar]
- 53.Krawczynska, N. et al. Mosaic intronic NIPBL variant in a family with Cornelia de Lange syndrome. Front. Genet.9, 255 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.den Dunnen, J. T. et al. HGVS recommendations for the description of sequence variants: 2016 Update. Hum. Mutat.37, 564–569 (2016). [DOI] [PubMed] [Google Scholar]
- 55.Hart, R. K. et al. HGVS Nomenclature 2024: Improvements to community engagement, usability, and computability. Genome Med.16, 1–12 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hamosh, A., Scott, A. F., Amberger, J. S., Bocchini, C. A. & McKusick, V. A. Online Mendelian inheritance in Man (OMIM), a knowledgebase of human genes and genetic disorders. Nucleic Acids Res.33, D514–D517 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sherry, S. T. et al. dbSNP: the NCBI database of genetic variation. Nucleic Acids Res.29, 308–311 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kopanos, C. et al. VarSome: The human genomic variant search engine. Bioinformatics35, 1978–1980 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ye, J. et al. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinform.13, 134 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Perez, G. et al. The UCSC genome browser database: 2025 update. Nucleic Acids Res.53, D1243–D1249 (2025). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Petela, N. J. et al. Folding of cohesin’s coiled coil is important for Scc2/4-induced association with chromosomes. Elife10, e67268 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kelley, L. A., Mezulis, S., Yates, C. M., Wass, M. N. & Sternberg, M. J. E. The Phyre2 web portal for protein modelling, prediction and analysis. Nat. Protoc.10, 845 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Waterhouse, A. et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res.46, W296 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ros-Pardo, D., Gómez-Puertas, P. & Marcos-Alcalde, Í. STAG2: Computational analysis of missense variants involved in disease. Int. J. Mol. Sci.25, 1280 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Roe, D. R. & Cheatham, T. E. PTRAJ and CPPTRAJ: Software for processing and analysis of molecular dynamics trajectory data. J. Chem. Theory Comput.9, 3084–3095 (2013). [DOI] [PubMed] [Google Scholar]
- 66.Humphrey, W., Dalke, A. & Schulten, K. V. M. D. Visual molecular dynamics. J. Mol. Graph.14, 33–38 (1996). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors F.J.R., J.P or A.L.-P. upon request.