Abstract
Frontotemporal lobar degeneration with tauopathy (FTLD-Tau) can present clinically similar to Alzheimer’s disease but lacks a biomarker. Alzheimer’s disease has been associated with choroidal thinning compared to controls. We compared the choroid of 25 probable FTLD-Tau (pFTLD-Tau) patients (42 eyes), 26 biomarker-determined probable Alzheimer’s disease neuropathologic change (pADNC) patients (49 eyes), and 53 normal controls (80 eyes). Cerebrospinal fluid biomarkers determined presence of ADNC. All pFTLD-Tau patients had a syndrome highly associated with FTLD-Tau. Optical coherence tomography was performed with masked manual choroidal thickness (CT) measurements. With Image J, binarized images determined the choroidal vascularity index (CVI). Linear regression with generalized estimating equations to account for inter-eye correlation was performed. For pFTLD-Tau, pADNC, and controls, the subfoveal CT was 308.9, 286.0, and 301.5 μm, and CVI was 0.72, 0.72, and 0.73, respectively (all p > 0.05 for each group comparison). Adjusting for demographics, the CT and CVI were not significantly different between groups, including 13 CT measurement locations (all p > 0.05). Among pADNC patients, an exploratory analysis found a correlation between CVI and disease duration (Pearson r = 0.32, p = 0.04). We found no significant difference of CT or CVI between pFTLD-Tau, pADNC, and controls. Additional studies are warranted to evaluate how CVI relates to ADNC.
Subject terms: Alzheimer's disease, Dementia, Diagnostic markers
Introduction
The neuropathology of frontotemporal dementia (FTD) is referred to as FTLD (frontotemporal lobar degeneration), and there remains an urgent need for a biomarker for the neuropathologic diagnosis of frontotemporal lobar degeneration from a tauopathy (FTLD-Tau). FTLD-Tau patients can be difficult to clinically distinguish from patients that have Alzheimer’s disease neuropathologic change (ADNC), especially in the young-onset age range where non-amnestic syndromes are more common1–5. While advancements have been made for validated biomarkers for ADNC6–11, cerebrospinal fluid requires an invasive lumbar puncture and the cost of positron emission tomography (PET) limits widespread use. Plasma biomarkers for ADNC hold promise but currently are not able to specifically identify FTLD-Tau6,7. Furthermore, the clinical diagnosis of FTLD-Tau is complicated by the difficulty in clinically distinguishing tauopathies from FTD patients with neuropathology from TAR DNA-binding Protein of 43 kDa (FTLD-TDP), as about half of FTLD neuropathology cases are FTLD-Tau and half are FTLD-TDP1,2. Thus, the absence of biomarkers specific for FTLD-Tau presents challenges for the clinical management of these patients. There is no approved drug therapy for patients with FTLD-Tau, and a biomarker could facilitate clinical trial patient categorization as mechanism-based therapies are emerging12.
As an extension of the central nervous system that can be imaged at high-resolution, the retina is an attractive candidate biomarker tissue for dementia. Inner retina thinning has been the predominant retinal finding for clinical Alzheimer’s disease (AD) patients compared to normal controls13,14. Interestingly, our group and others have found thinning of the photoreceptor outer nuclear layer (ONL), an outer retina layer, in patients with FTLD-Tau15–20. Given the ability to readily image the posterior segment of the eye, studies have also evaluated the choroid, the vascular layer between the sclera and neurosensory retina that provides nourishment for the outer third of the retina. Several studies have found thinning of the choroid among clinical AD patients compared to normal controls; this has been hypothesized to be related to an amyloid-beta 42 (Aβ42) vasculopathy21–25. On the other hand, studies comparing patients with ADNC determined by biomarkers (cerebrospinal fluid or amyloid beta PET) to normal controls have not found differences in choroidal thickness26.
FTLD-Tau patients are not characterized by amyloid beta abnormalities, and there is an absence of studies evaluating the choroid of FTLD-Tau patients. We sought to evaluate the choroid of probable FTLD-Tau patients compared to biomarker-determined ADNC patients and normal controls.
Methods
Subjects
Patients were enrolled consecutively by the Penn Frontotemporal Degeneration Center (FTDC) of the University of Pennsylvania from 2014 to 2021. Each patient was discussed at a FTDC consensus conference to arrive at their diagnosis using current clinical criteria as previously described1,15,27,28. Patients enrolled in this study were co-enrolled into an ongoing FTDC observational study (AG066597) that includes deep phenotyping with cerebrospinal fluid (CSF) biomarkers, genetic analysis, and brain donation when possible. All clinical evaluations were performed masked to ophthalmic imaging data, and clinical data was obtained from the Penn Integrated Neurodegenerative Disease Database (INDD). Disease duration was defined by the number of years from the first reported symptom to the time of the Spectral-domain optical coherence tomography (OCT) imaging.
All probable FTLD-Tau (pFTLD-Tau) patients were negative for a CSF biomarker for ADNC (see below) and also met one of the following criteria: a) diagnosis highly associated with FTLD-Tau including progressive supranuclear palsy, nonfluent primary progressive aphasia (PPA), corticobasal syndrome (with negative CSF for ADNC and no GRN pathogenic variant), b) presence of a pathogenic MAPT (OMIM 157,140) variant for FTLD-Tau, c) neuropathologic data confirming FTLD-Tau3,28–32. The pFTLD-Tau patients had no neuropathologic data indicating significant FTLD-TDP or ADNC (i.e. intermediate or high level) co-pathology33.
Probable ADNC (pADNC) patients were positive for a CSF biomarker for ADNC and had a diagnosis consistent with ADNC: amnestic mild cognitive impairment (MCI), AD, or an FTD spectrum clinical diagnosis often associated with ADNC (posterior cortical atrophy, logopenic variant of PPA, corticobasal syndrome). The pADNC patients also had no genetic (i.e. MAPT, C9orf72, GRN pathogenic variant) or neuropathologic data indicating FTLD-Tau or FTLD-TDP copathology.
As previously described, CSF was obtained with standard lumbar puncture15,19. In more recently enrolled patients (N = 28), CSF was analyzed using the second generation Fujirebio Lumipulse assay10 (Fujirebio, Malvern, PA, USA) or, for legacy data, the Luminex xMAP assay (Luminex, Austin, TX, USA) was used. To categorize patients with pADNC, established cutpoints were used (Fujirebio, Aβ42: Aβ40 ratio ≤ 0.072; Luminex, phosphorylated-tau: Aβ42 ratio > 0.08)34,35.
All FTD patients were tested for pathogenic variants in FTD-associated genes by massively parallel sequencing (whole exome or a custom targeted sequencing panel of > 22 neurodegenerative disease associated genes including GRN, MAPT, TBK1, and VCP) and by repeat-primed PCR for C9orf72 hexanucleotide repeat expansions as previously described36,37. Family history was classified as sporadic or familial using a three-generation pedigree history, as previously reported38.
Normal controls were recruited from patients of the Scheie Eye Institute of the University of Pennsylvania as previously described15. Normal controls had no history of diabetes, neurodegenerative disease, or ocular disease that could affect the retina or choroid.
This study was approved by the University of Pennsylvania Institutional Review Board (protocol #819894, initial approval date 4/7/2014), and it followed the tenets of the Declaration of Helsinki. All subjects (or caregivers when appropriate) provided written informed consent.
Imaging protocol and image analysis
All subjects were seen at the Scheie Eye Institute and received a full dilated eye exam (BJK or TSA) to determine if there was any eye disease that may affect the choroid measurements. As pre-specified criteria, eyes were excluded if there was evidence or history of ocular disease that would affect the choroid (e.g. diabetic retinopathy, hypertensive retinopathy, central serous chorioretinopathy), high refractive error (≥ 6.00 or ≤ − 6.00 diopter spherical equivalent, or known axial length of > 26.5 mm), significant media opacity, intraocular eye surgery within 90 days of study enrollment, or poor OCT image quality. As a pre-specified image quality metric, graders also determined the percent of the choroid-sclera interface that was well-visualized. If the mean percent of well-visualized choroid-sclera interface across the scan was less than 60%, then the eye was excluded from analysis. One patient in the pFTLD-Tau group and one patient in the pADNC group had a history of diabetes with no visible diabetic retinopathy on exam.
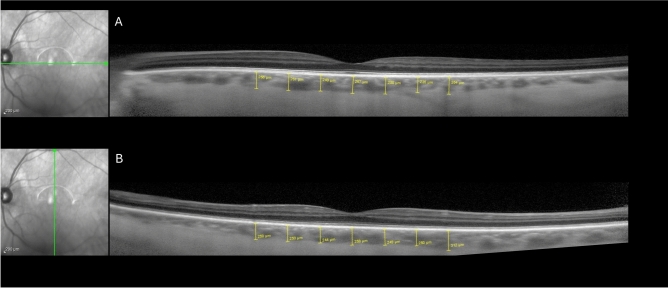
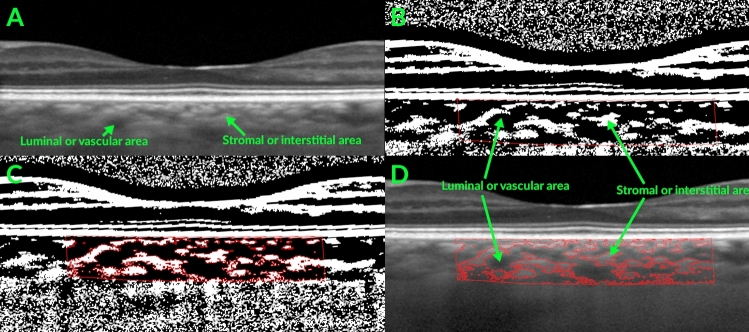
Subjects had Spectral-domain OCT (Heidelberg Engineering, Carlsbad, CA, USA) using enhanced depth imaging (EDI) of the choroid with high-resolution (1024 A-scans per B scan) vertical and horizontal 30 degree cross-sectional images centered on the fovea39. The automatic real-time (ART) was set at 100, and we required analyzed images to have an ART of at least 50, ensuring good image quality. Two masked image graders at the Scheie Image Reading Center independently measured the choroidal thickness using the Heidelberg Eye Explorer software (version 6.12.1) and then measured the choroidal vascularity index (CVI) as described below. Choroidal thickness measurements were made manually for the subfoveal choroidal thickness as well as at 500, 1000, and 1500 μm from the fovea nasally and temporally along the horizontal axis and superiorly and inferiorly along the vertical axis (Fig. 1). The choroidal thickness was measured from the outer (basal) border of the retinal pigment epithelium/Bruch’s membrane interface to the choroidal–scleral interface. If a suprachoroidal space was visible, then the measurement included the suprachoroidal space, meaning that the reference point for the measurement was where the outer boundary of the suprachoroidal space meets the inner boundary of the sclera40. To determine the CVI, Image J (version 1.52n, National Institutes of Health) was used to determine the choroidal vessel lumen area and the total choroidal area of the horizontal B-scans as described by Agrawal et al. and Robbins et al.41,42. Briefly, the polygon tool was used to outline the total fovea-centered choroid area that was 1500 microns in length (Fig. 2), and this was added to the region of interest manager. The image was converted into an 8-bit image, and Niblack was used with auto local threshold. Next, the color threshold function was used to delineate the vessel lumen area, and then this area was merged with the total choroid area with the “AND” function in the region of interest manager. This allowed determination of the lumen area within the total choroid area. The CVI was defined as the vessel lumen area divided by the total choroidal area.
Fig. 1.
Representative optical coherence tomography (OCT) enhanced depth imaging scan with choroidal thickness measurements. Point measurements of the choroidal thickness at the fovea and at 500, 1000, and 1500 microns from the fovea along horizontal (A) and vertical (B) fovea-centered scans.
Fig. 2.
Representative optical coherence tomography (OCT) enhanced depth imaging scan with image binarization. (A) Fovea centered OCT scan with arrows showing luminal and stromal areas within the choroid. (B) Image binarization with segmentation of a fovea-centered choroid area that is 1500 microns wide (outlined in red). (C) Segmentation of the luminal vs. stromal areas of the binarized image41. (D) Overlay of segmented luminal and stromal areas on the OCT scan.
For the choroidal measurements, the mean of the grader measurements for choroidal thickness was the final value used for analysis. However, if the measurements from two independent graders differed by > 20 percent of their mean, then the measurements were adjudicated by the Reading Center Director (ED) and this adjudicated value was used for analysis.
Statistical analysis
We pre-specified the statistical comparison of choroidal thickness and each of the choroidal vascularity index parameters (choroid luminal area, choroid area, choroidal vascularity index) for: (1) pFTLD-Tau vs. pADNC; (2) pFTLD-Tau vs. normal controls; (3) pADNC vs. normal controls. Univariable and multivariable linear regression analyses were performed without and with adjustment by age, sex, and race, which can potentially affect choroidal thickness43,44. We also performed these comparisons by restricting to those 10 pADNC patients with a clinical diagnosis of “probable AD” as a sensitivity analyses. The presentation of these 10 patients was consistent with clinical AD as opposed to FTD. For exploratory analyses of choroid measurements and clinical data (disease duration and Aβ42/ Aβ40 ratio), the Pearson correlation coefficient was calculated to evaluate the correlation between choroid measurements and clinical data. Since most patients contributed two study eyes, the inter-eye correlation was accounted for by using generalized estimating equations45,46. All the statistical analyses were performed in SAS v9.4 (SAS Institute Inc., Cary, NC, USA), and two-sided p < 0.05 (without correcting for multiple comparisons) was considered statistically significant.
The study sample size of 42 eyes from 25 patients with pFTLD-Tau and 49 eyes from 26 patients with pADNC provided 80% power to detect a mean difference of 70 microns in subfoveal choroidal thickness, assuming a standard deviation of 89 microns. A difference between these groups of about 70 microns or more can be expected based on other publications of choroidal thinning observed in clinical AD patients compared to normal controls24,25.
Results
Subject characteristics
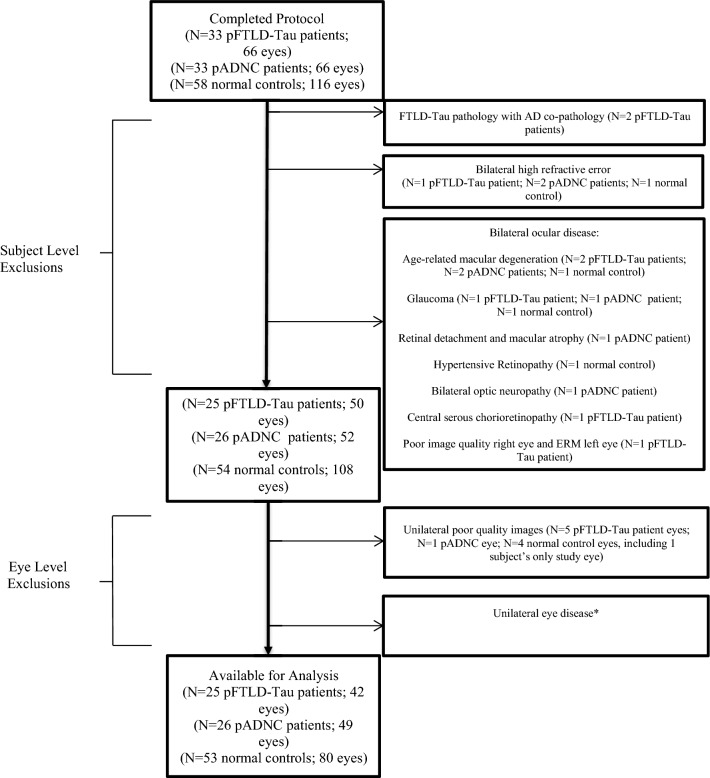
Thirty-three pFTLD-Tau patients, 33 pADNC patients, and 58 normal controls were enrolled. After pre-specified exclusion criteria were applied, there were 25 (42 eyes) pFTLD-Tau patients, 26 (49 eyes) pADNC patients, and 53 (80 eyes) normal controls (Fig. 3). The demographics for these subjects are shown in Table 1. The pFTLD-Tau patients were older than the pADNC and normal control groups with mean ages of 66.8, 61.9, and 58.1 years for the pFTLD-Tau, pADNC, and normal controls, respectively. While the race of the pFTLD-Tau and pADNC groups was similar, there was a significant race difference for the normal controls vs. the other groups, as thirty-eight percent of the controls were African-American. There was no significant difference in sex between the groups. The median disease duration (interquartile range [IQR]) was 3.0 (2.0–4.0) years for the pFTLD-Tau patients and 4.0 (3.0–6.0) years for the pADNC patients. The median (IQR) mini-mental state exam (MMSE) was 27.5 (24.0–29.0) for the pFTLD-Tau patients and 21.5 (16.0–25.0) for the pADNC patients. The median (IQR) clinical dementia rating for FTD (CDR-FTD) sum of boxes score was 6.5 (3.5–8.5) for the pFTLD-Tau patients and 5.0 (3.3–7.5) for the pADNC patients. While eyes with high refractive error were excluded, axial lengths were also available for 16 pFTLD-Tau eyes, 30 pADNC eyes, and 15 normal control eyes. For these eyes, there was no significant difference in the mean axial length (23.7, 24.2, and 24.3 mm for the pFTLD-Tau, pADNC, and normal control eyes, respectively, p = 0.42).
Fig. 3.
Flow Diagram of Patients and Eyes Excluded. Pre-specified exclusion criteria were followed for each of the subject groups. *Unilateral eye disease included: Glaucoma (N = 2 pADNC patient eyes; N = 2 normal control eyes), Epiretinal membrane (N = 5 normal control eyes), Age-related macular degeneration (N = 3 pFTLD-Tau patient eyes; N = 3 normal control eyes), Amblyopia (N = 3 normal control eyes), Retinal tear or hole/retinal detachment (N = 4 normal control eyes), Central retinal artery occlusion (N = 1 normal control eye), Unilateral high refractive error (N = 2 normal control eyes), Cataract (N = 1 normal control eye), Subject only wanted one study eye due to time constraints (N = 3 normal control eyes). Abbreviations: AD = Alzheimer’s disease; AMD = age-related macular degeneration; CRAO = central retinal artery occlusion; ERM = epiretinal membrane; pADNC = probable Alzheimer’s disease neuropathologic change; pFTLD-Tau = probable frontotemporal lobar degeneration tauopathy.
Table 1.
Demographics and clinical diagnoses for pFTLD-Tau and pADNC patients.
| pFTLD-Tau* (N = 25 subjects) | pADNC** (N = 26 subjects) | Normal Controls (N = 53 subjects) | P-Value pFTLD-Tau vs. pADNC | P-Value FTLD-Tau vs. Normal Controls | P-Value pADNC vs. Normal Controls | |
|---|---|---|---|---|---|---|
| Age (years) | 0.02 | 0.001 | 0.33 | |||
| Mean (SD) | 66.8 (6.4) | 61.9 (7.3) | 58.1(12.1) | |||
| Q1, Q3 | 63.0, 71.0 | 58.0, 66.0 | 53.0, 66.0 | |||
| Range | 54.0–79.0 | 49.0–76.0 | 26.0–82.0 | |||
| Sex | 0.12 | 0.74 | 0.13 | |||
| F | 17 (68.0%) | 12 (46.2%) | 34 (64.2%) | |||
| M | 8 (32.0%) | 14 (53.8%) | 19 (35.8%) | |||
| Race | 0.23 | 0.004 | 0.003 | |||
| Asian | 0 | 1 (3.8%) | 1 (1.9%) | |||
| Black | 1 (4%) | 0 | 20 (37.7%) | |||
| Other | 2 (8%) | 0 | 2 (3.8%) | |||
| Unknown | 3 (12%) | 1 (3.8%) | 0 | |||
| White | 19 (76%) | 24 (92.3%) | 30 (56.6%) | |||
| Clinical diagnosis | PSP = 14 | AD = 10 | ||||
| CBS = 4 | CBS = 3 | |||||
| bvFTD = 2*** | lvPPA = 2 | |||||
| naPPA = 5 | bvFTD = 4 | |||||
| PCA = 5 | ||||||
| MCI (non-amnestic) = 2 |
* Includes 8 patients with neuropathologic confirmation of FTLD-Tau.
** Includes 4 patients with a neuropathologic confirmation of Alzheimer’s disease.
***One bvFTD had MAPT E10 + 16 C > T and the other bvFTD patient had MAPT c.1216C > T, p.R406W.
5 pFTLD-Tau patients were unable to have CSF collection for logistical reasons: 1 had both MAPT pathogenic variant and FTLD-Tau neuropathology, 1 had FTLD-Tau neuropathology, and the other 3 patients had PSP, which is highly associated with FTLD-Tau1,28.
AD Alzheimer’s disease, pADNC probable Alzheimer’s disease neuropathologic change, bvFTD behavioral variant of frontotemporal dementia, CBS corticobasal syndrome, pFTLD-Tau probable frontotemporal lobar degeneration tauopathy, lvPPA logopenic variant of primary progressive aphasia, MCI mild cognitive impairment, naPPA nonfluent/agrammatic primary progressive aphasia, PCA posterior cortical atrophy, PSP progressive supranuclear palsy.
The 25 pFTLD-Tau patients included 8 patients with neuropathologic confirmation of FTLD-Tau. Among the patients with autopsy data, there was one patient with a MAPT c.1216C > T, p.R406W pathogenic variant and one patient with a MAPT E10 + 16 C > T pathogenic variant. The clinical diagnoses for the pFTLD-Tau patients are shown in Table 1 and included 14 patients with a diagnosis of progressive supranuclear palsy.
The 26 pADNC patients included a range of clinical syndromes often associated with underlying ADNC (Table 1). All pADNC patients had a positive CSF biomarker for ADNC, and 4 patients had neuropathologic confirmation of ADNC.
Choroidal thickness analyses
The unadjusted mean subfoveal choroidal thickness for the pFTLD-Tau, pADNC, and normal control groups was 308.9, 286.0, and 301.5 μm, respectively (all p > 0.05 for each group comparison, Tables 2, 3, 4). After adjusting for age, sex, and race, there was still no significant difference in the subfoveal choroidal thickness between each of the groups. Similarly, the choroidal thickness at twelve other locations along fovea-centered horizontal and vertical scans also showed no significant differences between the groups (all p > 0.05, Tables 2, 3, 4). There was still no significant difference for pFTLD-Tau vs. pADNC and pFTLD-Tau vs. normal controls after limiting the pFTLD-Tau patients to only those patients with a neuropathologic confirmation of tauopathy or PSP, which is highly associated with FTLD-Tau (all p > 0.05, data not shown).
Table 2.
Choroidal evaluation of pFTLD-Tau and pADNC patients.
| Unadjusted | Adjusted* | ||||||
|---|---|---|---|---|---|---|---|
| pFTLD-Tau (n = 25; 42 eyes) Mean(SE) | pADNC (n = 26; 49 eyes) Mean(SE) | P-Value | pFTLD-Tau (n = 25; 42 eyes) Mean(SE) | pADNC (n = 26; 49 eyes) Mean(SE) | Difference (95% CI) | P-Value | |
| Choroidal Thickness (microns) | |||||||
| Subfoveal | 308.9 (15.6) | 286.0 (19.1) | 0.35 | 315.7 (16.6) | 279.3 (22.7) | 36.4 (− 17.0, 89.7) | 0.18 |
| 500 microns (Inferior) | 292.6 (17.2) | 286.5 (19.2) | 0.81 | 308.2 (19.9) | 286.8 (21.8) | 21.5 (− 27.4, 70.3) | 0.39 |
| 500 microns (Nasal) | 294.0 (14.8) | 277.9 (17.7) | 0.49 | 304.3 (16.3) | 275.2 (20.8) | 29.1 (− 18.3, 76.4) | 0.23 |
| 500 microns (Superior) | 295.1 (16.3) | 292.5 (19.6) | 0.92 | 310.0 (20.3) | 290.4 (22.1) | 19.6 (− 30.1, 69.2) | 0.44 |
| 500 microns (Temporal) | 289.9 (13.2) | 278.4 (17.4) | 0.60 | 301.6 (14.1) | 276.2 (19.5) | 25.4 (− 21.4, 72.1) | 0.29 |
| 1000 microns (Inferior) | 291.8 (16.8) | 284.9 (19.9) | 0.79 | 307.5 (19.7) | 282.7 (21.0) | 24.8 (− 23.0, 72.6) | 0.31 |
| 1000 microns (Nasal) | 278.5 (13.9) | 268.9 (17.3) | 0.66 | 288.1 (15.3) | 263.6 (19.4) | 24.5 (− 22.1, 71.2) | 0.30 |
| 1000 microns (Superior) | 291.9 (14.7) | 294.7 (19.0) | 0.91 | 309.3 (18.9) | 296.0 (20.9) | 13.3 (− 34.8, 61.4) | 0.59 |
| 1000 microns (Temporal) | 291.3 (13.8) | 272.1 (16.3) | 0.37 | 295.9 (13.4) | 267.6 (18.8) | 28.3 (− 17.8, 74.4) | 0.23 |
| 1500 microns (Inferior) | 293.0 (16.8) | 280.3 (20.6) | 0.63 | 308.8 (19.1) | 276.1 (20.3) | 32.7 (− 18.1, 83.4) | 0.21 |
| 1500 microns (Nasal) | 255.0 (14.9) | 248.0 (16.8) | 0.76 | 264.1 (14.7) | 244.3 (18.8) | 19.8 (− 27.3, 66.9) | 0.41 |
| 1500 microns (Superior) | 285.6 (12.1) | 293.2 (18.5) | 0.73 | 304.4 (16.5) | 298.9 (20.3) | 5.5 (− 39.6, 50.6) | 0.81 |
| 1500 microns (Temporal) | 285.2 (14.5) | 267.1 (16.3) | 0.41 | 290.3 (12.6) | 266.7 (18.1) | 23.6 (− 24.2, 71.4) | 0.33 |
| Average of each of the point measurements of choroidal thickness | 292.5 (14.3) | 286.6 (18.7) | 0.80 | 304.7 (17.0) | 287.7 (20.3) | 17.0 (− 30.8, 64.8) | 0.49 |
| Choroidal Vascularity Index Parameters | |||||||
| Choroid luminal area (square pixels) | 337,184.8 (17,443.9) | 328,446.3 (21,829.7) | 0.75 | 348,633.4 (20,771.3) | 324,246.9 (25,441.4) | 24,386.5 (− 33,176.5, 81,949.5) | 0.41 |
| Choroid area (square pixels) | 465,682.6 (23,574.9) | 458,840.7 (30,606.4) | 0.86 | 488,878.2 (28,229.0) | 460,562.7 (33,245.2) | 28,315.5 (− 50,543.6, 107,175.0) | 0.48 |
| Choroidal vascularity index | 0.72 (0.01) | 0.72 (0.01) | 0.55 | 0.71 (0.01) | 0.71 (0.01) | 0.01 (− 0.02, 0.04) | 0.53 |
*Adjusted by age, sex, and race.
pADNC probable Alzheimer’s disease neuropathologic change, pFTLD-Tau probable frontotemporal lobar degeneration tauopathy, SE standard error.
Table 3.
Choroidal evaluation of pFTLD-Tau and normal controls.
| Unadjusted | Adjusted* | ||||||
|---|---|---|---|---|---|---|---|
| pFTLD-Tau (n = 25; 42 eyes) Mean(SE) | Normal Controls (n = 53; 80 eyes) Mean(SE) | P-Value | pFTLD-Tau (n = 25; 42 eyes) Mean(SE) | Normal Controls (n = 53; 80 eyes) Mean(SE) | Difference (95% CI) | P-Value | |
| Choroidal thickness (microns) | |||||||
| Subfoveal | 308.9 (15.6) | 301.5 (12.6) | 0.71 | 314.5 (17.1) | 291.6 (11.8) | 22.8 (− 16.7, 62.4) | 0.26 |
| 500 microns (Inferior) | 292.6 (17.2) | 283.0 (11.8) | 0.64 | 299.0 (16.7) | 275.1 (11.7) | 23.8 (− 14.0, 61.7) | 0.22 |
| 500 microns (Nasal) | 294.0 (14.8) | 299.7 (13.3) | 0.77 | 300.4 (16.2) | 289.7 (12.5) | 10.7 (− 28.3, 49.7) | 0.59 |
| 500 microns (Superior) | 295.1 (16.3) | 280.8 (11.3) | 0.47 | 299.6 (16.0) | 272.5 (11.0) | 27.1 (− 9.8, 64.0) | 0.15 |
| 500 microns (Temporal) | 289.9 (13.2) | 298.2 (12.5) | 0.65 | 301.1 (14.9) | 289.0 (11.3) | 12.1 (− 23.9, 48.1) | 0.51 |
| 1000 microns (Inferior) | 291.8 (16.8) | 284.8 (12.0) | 0.74 | 303.1 (16.9) | 278.0 (11.8) | 25.1 (− 13.3, 63.4) | 0.20 |
| 1000 microns (Nasal) | 278.5 (13.9) | 290.4 (13.4) | 0.54 | 288.0 (15.5) | 281.3 (12.6) | 6.7 (− 31.8, 45.2) | 0.73 |
| 1000 microns (Superior) | 291.9 (14.7) | 289.2 (11.7) | 0.89 | 299.3 (14.9) | 281.4 (10.9) | 17.9 (− 17.2, 53.0) | 0.32 |
| 1000 microns (Temporal) | 291.3 (13.8) | 294.8 (12.3) | 0.85 | 300.6 (15.6) | 285.6 (11.0) | 15.1 (− 20.6, 50.7) | 0.41 |
| 1500 microns (Inferior) | 293.0 (16.8) | 288.9 (11.8) | 0.84 | 304.3 (16.8) | 282.4 (11.6) | 22.0 (− 15.8, 59.7) | 0.25 |
| 1500 microns (Nasal) | 255.0 (14.9) | 263.1 (13.0) | 0.68 | 262.4 (15.7) | 254.6 (12.2) | 7.8 (− 31.1, 46.7) | 0.69 |
| 1500 microns (Superior) | 285.6 (12.1) | 290.6 (11.9) | 0.77 | 299.2 (13.2) | 283.2 (10.5) | 16.0 (− 15.9, 48.0) | 0.33 |
| 1500 microns (Temporal) | 285.2 (14.5) | 283.0 (11.0) | 0.91 | 291.0 (15.8) | 274.8 (10.3) | 16.2 (− 19.3, 51.7) | 0.37 |
| Average of each of the point measurements of choroidal thickness | 292.5 (14.3) | 293.1 (11.8) | 0.97 | 300.8 (15.4) | 284.2 (10.8) | 16.6 (− 19.3, 52.5) | 0.36 |
| Choroidal vascularity index parameters | |||||||
| Choroid luminal Area (square pixels) | 337,184.8 (17,443.9) | 338,560.5 (14,247.8) | 0.95 | 350,322.8 (19,321.7) | 325,122.8 (12,489.1) | 25,200.0 (− 18,841.2, 69,241.2) | 0.26 |
| Choroid area (square pixels) | 465,682.6 (23,574.9) | 466,322.7 (19,534.8) | 0.98 | 486,511.7 (25,832.2) | 448,035.2 (17,009.6) | 38,476.4 (− 21,677.2, 98,630.1) | 0.21 |
| Choroidal vascularity index | 0.72 (0.01) | 0.73 (0.01) | 0.86 | 0.72 (0.01) | 0.73 (0.01) | − 0.01 (− 0.03, 0.02) | 0.66 |
*Adjusted by age, sex, and race.
pFTLD-Tau probable frontotemporal lobar degeneration tauopathy, SE standard error.
Table 4.
Choroidal evaluation of pADNC and normal controls.
| Unadjusted | Adjusted* | ||||||
|---|---|---|---|---|---|---|---|
| pADNC (n = 26; 49 eyes) Mean(SE) | Normal Controls (n = 53; 80 eyes) Mean(SE) | P-Value | pADNC (n = 26; 49 eyes) Mean(SE) | Normal Controls (n = 53; 80 eyes) Mean(SE) | Difference (95% CI) | P-Value | |
| Choroidal thickness (microns) | |||||||
| Subfoveal | 286.0 (19.1) | 301.5 (12.6) | 0.50 | 301.3 (19.1) | 294.4 (11.6) | 6.8 (− 37.2, 50.8) | 0.76 |
| 500 microns (Inferior) | 286.5 (19.2) | 283.0 (11.8) | 0.88 | 305.2 (17.6) | 280.4 (10.9) | 24.9 (− 14.4, 64.1) | 0.21 |
| 500 microns (Nasal) | 277.9 (17.7) | 299.7 (13.3) | 0.33 | 293.2 (18.5) | 292.9 (12.3) | 0.4 (− 42.8, 43.5) | 0.99 |
| 500 microns (Superior) | 292.5 (19.6) | 280.8 (11.3) | 0.60 | 312.2 (17.9) | 277.0 (10.4) | 35.2 (− 4.5, 74.9) | 0.08 |
| 500 microns (Temporal) | 278.4 (17.4) | 298.2 (12.5) | 0.36 | 295.7 (17.3) | 291.4 (11.2) | 4.3 (− 35.4, 44.0) | 0.83 |
| 1000 microns (Inferior) | 284.9 (19.9) | 284.8 (12.0) | 1.00 | 307.0 (17.7) | 282.6 (10.9) | 24.3 (− 16.0, 64.6) | 0.24 |
| 1000 microns (Nasal) | 268.9 (17.3) | 290.4 (13.4) | 0.33 | 287.1 (17.8) | 284.0 (12.4) | 3.1 (− 40.6, 46.8) | 0.89 |
| 1000 microns (Superior) | 294.7 (19.0) | 289.2 (11.7) | 0.81 | 317.7 (17.5) | 285.6 (10.3) | 32.1 (− 6.6, 70.9) | 0.10 |
| 1000 microns (Temporal) | 272.1 (16.3) | 294.8 (12.3) | 0.27 | 291.5 (16.6) | 287.8 (10.9) | 3.7 (− 33.0, 40.4) | 0.84 |
| 1500 microns (Inferior) | 280.3 (20.6) | 288.9 (11.8) | 0.72 | 299.8 (18.4) | 287.7 (10.8) | 12.1 (− 29.5 53.7) | 0.57 |
| 1500 microns (Nasal) | 248.0 (16.8) | 263.1 (13.0) | 0.48 | 266.5 (17.5) | 257.5 (11.8) | 9.0 (− 31.6, 49.7) | 0.66 |
| 1500 microns (Superior) | 293.2 (18.5) | 290.6 (11.9) | 0.91 | 321.8 (16.8) | 287.3 (9.9) | 34.5 (− 2.4, 71.4) | 0.07 |
| 1500 microns (Temporal) | 267.1 (16.3) | 283.0 (11.0) | 0.42 | 282.7 (16.5) | 276.6 (10.2) | 6.2 (− 29.1, 41.5) | 0.73 |
| Average of each of the point measurements of choroidal thickness | 286.6 (18.7) | 293.1 (11.8) | 0.77 | 306.6 (17.6) | 287.2 (10.5) | 19.4 (− 20.2, 58.9) | 0.34 |
| Choroidal vascularity index parameters | |||||||
| Choroid luminal Area (square pixels) | 328,446.3 (21,829.7) | 338,560.5 (14,247.8) | 0.70 | 349,300.9 (20,702.8) | 330,866.4 (12,285.1) | 18,434.4 (− 28,677.4, 65,546.2) | 0.44 |
| Choroid area (square pixels) | 458,840.7 (30,606.4) | 466,322.7 (19,534.8) | 0.84 | 491,156.4 (28,160.3) | 454,932.7 (16,681.9) | 36,223.7 (− 28,614.5, 101,062.0) | 0.27 |
| Choroidal vascularity index | 0.72 (0.01) | 0.73 (0.01) | 0.39 | 0.71 (0.01) | 0.73 (0.01) | − 0.02 (− 0.04, 0.01) | 0.17 |
*Adjusted by age, sex, and race.
pADNC probable Alzheimer’s disease neuropathologic change, SE standard error.
Choroidal vascularity index analyses
The unadjusted mean CVI for the pFTLD-Tau, pADNC, and normal control groups was 0.72, 0.72, and 0.73, respectively (all p > 0.05 for each group comparison, Tables 2, 3, 4). After adjusting for age, sex, and race, there was still no significant difference in the CVI between each of the groups (Tables 2, 3, 4).
Sensitivity analysis and exploratory correlation analyses
Given the different clinical syndromes composing the pADNC group, we also evaluated the choroid of those pADNC patients with a clinical diagnosis of “probable AD” (10 patients, 18 eyes) with a sensitivity analysis. These 10 patients had no significant differences in the choroidal thickness measurements or CVI compared to normal controls or the pFTLD-Tau patients (all p > 0.05, Supplementary Tables 1–4).
The majority of pADNC patients had the Fujirebio Aβ42/ Aβ40 ratio (Fujirebio) CSF biomarker (N = 14 pADNC patients). Among the pADNC patients, there was no correlation of choroidal thickness with the Aβ42/ Aβ40 ratio (all p > 0.05, Supplementary Table 5). However, the CVI showed a mild negative correlation that was borderline significant (Pearson correlation coefficient = − 0.36; p = 0.06).
The choroidal thickness measurements did not correlate with disease duration for the pFTLD-Tau (all p > 0.05, Supplementary Table 6) or pADNC patients (all p > 0.05, Table 5). The CVI also did not correlate with disease duration for the pFTLD-Tau patients (Pearson correlation coefficient = − 0.03, p = 0.81). However, the CVI had a positive correlation with disease duration among the pADNC patients (Pearson correlation coefficient = 0.32, p = 0.04, Table 5, Supplementary Fig. 1).
Table 5.
Correlation of choroidal thickness and choroidal vascularity index with disease duration among pADNC patients.
| pADNC (n = 26 patients; 49 eyes) | |||
|---|---|---|---|
| N of eyes | Pearson correlation coefficient | P-Value * | |
| Choroidal thickness | |||
| Subfoveal | 46 | − 0.11 | 0.64 |
| 500 microns (Inferior) | 46 | − 0.19 | 0.39 |
| 500 microns (Nasal) | 45 | − 0.15 | 0.53 |
| 500 microns (Superior) | 46 | − 0.23 | 0.28 |
| 500 microns (Temporal) | 45 | − 0.08 | 0.70 |
| 1000 microns (Inferior) | 46 | − 0.26 | 0.21 |
| 1000 microns (Nasal) | 45 | − 0.16 | 0.46 |
| 1000 microns (Superior) | 46 | − 0.20 | 0.34 |
| 1000 microns (Temporal) | 45 | − 0.09 | 0.66 |
| 1500 microns (Inferior) | 46 | − 0.22 | 0.27 |
| 1500 microns (Nasal) | 45 | − 0.16 | 0.46 |
| 1500 microns (Superior) | 46 | − 0.20 | 0.32 |
| 1500 microns (Temporal) | 45 | − 0.04 | 0.85 |
| Average of each of the point measurements of choroidal thickness | 49 | − 0.22 | 0.28 |
| Choroidal vascularity index parameters | |||
| Choroid luminal area | 49 | − 0.15 | 0.47 |
| Choroid area | 49 | − 0.20 | 0.32 |
| Choroidal vascularity index | 49 | 0.32 | 0.04 |
*Generalized estimating equations account for the inter-eye correlation.
pADNC probable Alzheimer’s disease neuropathologic change.
Discussion
Biomarkers to distinguish FTLD-Tau pathology from ADNC and other age-associated neurodegenerative pathologies are a high priority for proper enrollment of FTLD-Tau patients into clinical trials leading to drug development. Since some studies have found choroidal thinning in clinical AD compared to normal controls potentially related to an amyloid beta vasculopathy21,22,24,25,47, it is important to determine if the choroid could be a biomarker to distinguish FTLD-Tau from ADNC or from normal controls. Using deeply phenotyped patients including a CSF biomarker for all patients and neuropathologic diagnosis confirmation for almost one third of our FTLD-Tau patients, we were unable to find significant differences of the choroid in our comparison of pFTLD-Tau, pADNC, and normal controls. To our knowledge, this is the first study to evaluate the choroid of pFTLD-Tau patients and directly compare to pADNC patients. One study has evaluated the choroid of progressive supranuclear palsy patients compared to normal controls and also did not find a significant difference48.
We hypothesized that a thinner choroid among pADNC patients may differentiate them from FTLD-Tau and normal controls. There are several reasons to explain why we did not find choroidal thinning in our pADNC group compared to these other groups. Our pADNC group was composed of atypical and younger patients that are more likely to be diagnostically confused with FTD, rather than more typical amnestic late-onset disease. It is possible that choroidal thinning may not be associated with ADNC at a younger age and that some subtypes of ADNC may not have abnormal choroids. Additionally, although our study was powered appropriately, ADNC may be associated with choroidal thinning with an effect size that may have been too small for detection. However, it is worth noting that there is no clearly elucidated mechanism for why human choroidal thinning might be associated with ADNC or for why Aβ42 would be in the choroid. Although animal models of ADNC have shown amyloid beta within the choroid and some investigators have suggested an Aβ42 vasculopathy mechanism49,50, we point out that the presence of Aβ42 has not been shown in the choroid of human tissue to our knowledge. Animal models may not always accurately represent the complex pathophysiology of ADNC. It is quite possible that ADNC does not uniformly associate with abnormal choroidal thinning. While some groups have found choroidal thinning in clinical AD, a significantly thin choroid or decreased CVI has not been a consistent OCT finding26,51,52. Further, those finding choroidal thinning have not consistently seen a correlation with disease severity measured by psychometric scores25. Regarding choroidal thickness studies of human tissue, Asanad et al. evaluated the choroid of 8 patients with ADNC and 11 normal controls. They found choroidal thinning nasal to the optic nerve, but there actually was a thicker choroid at the macula and the temporal periphery53. This contrasted with an earlier report of choroidal tissue showing thinning of the choroid in 6 patients with ADNC compared to 6 normal controls49. Lastly, it may be worth distinguishing OCT imaging studies with ADNC determined by biomarkers (CSF, amyloid beta PET, or genetic pathogenic variants) in contrast to studies of clinical AD. There are a very limited number of other choroid studies with biomarker determined ADNC groups, and these have not consistently found choroidal thinning compared to controls23,26. Using amyloid-positive patients (positive CSF biomarker or amyloid PET indicating ADNC) compared to normal controls, Den Haan et al. did not observe choroidal thickness abnormalities among their patients with ADNC and suggested that the choroidal thinning seen in other studies without biomarker-determined ADNC may have represented dementia cases with a primary vascular etiology or cases with vascular co-pathology26. Another study compared a group of MCI patients and ADNC patients with a positive amyloid PET scan to healthy controls. They found mild choroidal thinning at 5 of 14 choroid locations when comparing the MCI and ADNC patients together vs. healthy controls, but did not see significant choroidal thinning when comparing the ADNC patients vs. healthy controls23. Further work with biomarker-determined populations and human tissue analysis is critical and could clarify these ongoing questions.
Importantly, our study also found no significant difference between the choroid of pFTLD-Tau patients and normal controls. While other studies have evaluated the neurosensory retina in detail15,16,18–20, there has been a need for investigations of the choroid of FTLD-Tau. Although some literature suggests that a tauopathy could cause a vasculopathy54,55, phosphorylated tau has not been shown in the choroid of FTLD-Tau patients to our knowledge. Additional studies are needed to determine if FTLD-TDP has choroidal abnormalities, and comparisons of the choroid of FTLD-Tau and FTLD-TDP patients may be worthwhile. There is limited choroidal thickness data for amyotrophic lateral sclerosis (ALS), which is a TDP-43 proteinopathy related to FTLD-TDP; one study showed subfoveal choroidal thickening in ALS compared to healthy controls56.
Interestingly, with exploratory analyses, our data show a mild positive correlation of CVI with disease duration among our pADNC patients (p = 0.04), and consistent with this, there also was a negative correlation of CVI with the CSF Abeta42/Abeta40 ratio that approached statistical significance (p = 0.06). The correlation of CVI with disease duration suggests that there was increased choroid vessel luminal area as the disease duration increased. This positive correlation was unexpected, but it is noteworthy that some other studies have suggested an amyloid beta vasculopathy may be more complex over time than simply a progressive loss of vasculature when patients are at an earlier stage of disease52,57. Data from others have shown that earlier, subclinical stages of AD actually have increasing cerebral blood flow over time that later switches to a hypoperfused state of decreasing blood flow as the disease progresses58. This is relevant in light of the young age of our pADNC patients. As noted above, there is limited data with tissue analysis, with one study demonstrating choroidal thickening with increased vascularity at the macula in advanced cases that had brain pathology showing frequent neuritic plaques and Braak stage V or VI53. We believe our finding should be interpreted with caution as there were multiple comparisons, but along with these other studies, it suggests that longitudinal investigations are needed to help elucidate the relationship of CVI with ADNC.
Our study has both strengths and limitations. Of significant value is the deep phenotyping of patients conducted by our study, including dilated eye exams, the use of CSF biomarkers for ADNC to differentiate the pADNC and pFTLD-Tau groups, genetic analysis, and autopsy data for one third of the pFTLD-Tau group. Further, we performed rigorous analysis with masked manual grading of choroidal images by two independent, trained graders, followed by adjudication with the reading center director if there was a significant difference of measurements. We also measured the CVI, which is an automated image binarization technique that complements the manual choroidal thickness measurements and may have advantages over specific point measurements52. Several study limitations should be noted. First, p-values were not corrected for multiple comparisons, and the correlation of disease duration with CVI among pADNC patients was an exploratory analysis that is hypothesis generating. Additional investigations would be needed before making conclusions about CVI and disease duration of pADNC patients. Second, we did not have axial length data for all eyes. However, among eyes with axial lengths, there was no difference between groups, and eyes with high refractive error were excluded. Third, our normal controls did not have cognitive or genetic testing, and thus they were not phenotyped to the extent of our neurodegenerative cases. Although unlikely, we cannot exclude the possibility of a small number of preclinical neurodegenerative cases within our normal control group that could hinder our ability to see a difference between cases and controls. Fourth, our study had a modest sample size. However, FTLD-Tau patients are uncommon and our study was well-powered to find a choroid difference among the compared groups based on other studies24,25. We would argue that a clinically useful choroid biomarker to distinguish FTLD-Tau from ADNC would likely be observed with this sample size.
Our study did not identify a choroidal measurement to distinguish FTLD-Tau or ADNC from each other or from normal controls. However, our data are consistent with other studies using biomarker determined ADNC groups. It may be worthwhile for future studies to compare the choroid of atypical ADNC groups with typical late-onset ADNC and to compare FTLD-Tau with FTLD-TDP. Finally, longitudinal choroidal thickness and CVI data, investigation of autopsy-defined groups, and ocular tissue analysis could improve our understanding of whether the choroid has abnormalities in these patients.
Supplementary Information
Funding
Supported by NIH (Bethesda, MD) grants including RF1AG083774, AG017586, NS053488, AG052943, 2-P30-EY01583-26, NS109260 (to D.J.I.), AG054519, 5P30AG072979, P01-AG066597 Penn Institute on Aging. Funding was also provided in the form of block grants for general research purposes to the Scheie Eye Institute by Research to Prevent Blindness (New York, NY) and the Paul and Evanina Mackall Foundation Trust (Chicago, IL).
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request. Raw data can be made available on request.
Declarations
Competing interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-02020-z.
References
- 1.Irwin, D. J. et al. Frontotemporal lobar degeneration: Defining phenotypic diversity through personalized medicine. Acta Neuropathol.129, 469–491. 10.1007/s00401-014-1380-1 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grossman, M. et al. Frontotemporal lobar degeneration. Nat. Rev. Dis. Primers9, 40. 10.1038/s41572-023-00447-0 (2023). [DOI] [PubMed] [Google Scholar]
- 3.Irwin, D. J., Trojanowski, J. Q. & Grossman, M. Cerebrospinal fluid biomarkers for differentiation of frontotemporal lobar degeneration from Alzheimer’s disease. Front. Aging Neurosci.5, 6. 10.3389/fnagi.2013.00006 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kertesz, A., McMonagle, P., Blair, M., Davidson, W. & Munoz, D. G. The evolution and pathology of frontotemporal dementia. Brain128, 1996–2005. 10.1093/brain/awh598 (2005). [DOI] [PubMed] [Google Scholar]
- 5.Knibb, J. A., Xuereb, J. H., Patterson, K. & Hodges, J. R. Clinical and pathological characterization of progressive aphasia. Ann. Neurol.59, 156–165. 10.1002/ana.20700 (2006). [DOI] [PubMed] [Google Scholar]
- 6.Keshavan, A. et al. Population-based blood screening for preclinical Alzheimer’s disease in a British birth cohort at age 70. Brain144, 434–449. 10.1093/brain/awaa403 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ashton, N. J. et al. Plasma p-tau231: A new biomarker for incipient Alzheimer’s disease pathology. Acta Neuropathol.141, 709–724. 10.1007/s00401-021-02275-6 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mattsson-Carlgren, N. et al. Cerebrospinal fluid biomarkers in autopsy-confirmed Alzheimer disease and frontotemporal lobar degeneration. Neurology98, e1137–e1150. 10.1212/WNL.0000000000200040 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Illan-Gala, I. et al. Plasma Tau and neurofilament light in frontotemporal lobar degeneration and Alzheimer disease. Neurology96, e671–e683. 10.1212/WNL.0000000000011226 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leitao, M. J. et al. Clinical validation of the Lumipulse G cerebrospinal fluid assays for routine diagnosis of Alzheimer’s disease. Alzheimers Res. Ther.11, 91. 10.1186/s13195-019-0550-8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dubois, B. et al. Clinical diagnosis of Alzheimer’s disease: Recommendations of the International Working Group. Lancet Neurol.20, 484–496. 10.1016/S1474-4422(21)00066-1 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lane-Donovan, C. & Boxer, A. L. Disentangling tau: One protein, many therapeutic approaches. Neurotherapeutics21, e00321. 10.1016/j.neurot.2024.e00321 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan, V. T. T. et al. Spectral domain-optical coherence tomography measurements in Alzheimer’s disease: A systematic review and meta-analysis. Ophthalmology10.1016/j.ophtha.2018.08.009 (2018).31047198 [Google Scholar]
- 14.Coppola, G. et al. Optical coherence tomography in Alzheimer’s disease: A meta-analysis. PLoS ONE10, e0134750. 10.1371/journal.pone.0134750 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim, B. J. et al. Optical coherence tomography identifies outer retina thinning in frontotemporal degeneration. Neurology89, 1604–1611. 10.1212/WNL.0000000000004500 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim, B. J. et al. Persistent and progressive outer retina thinning in frontotemporal degeneration. Front. Neurosci.13, 298. 10.3389/fnins.2019.00298 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun, J. Q. et al. Comparison of the Iowa reference algorithm to the Heidelberg spectralis optical coherence tomography segmentation algorithm. J. Biophotonics13, e201960187. 10.1002/jbio.201960187 (2020). [DOI] [PubMed] [Google Scholar]
- 18.Kim, B. J. et al. Retina tissue validation of optical coherence tomography determined outer nuclear layer loss in FTLD-tau. Acta Neuropathol. Commun.9, 184. 10.1186/s40478-021-01290-8 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim, B. J. et al. Retinal photoreceptor layer thickness has disease specificity and distinguishes predicted FTLD-Tau from biomarker-determined Alzheimer’s disease. Neurobiol. Aging125, 74–82. 10.1016/j.neurobiolaging.2023.01.015 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oertel, F. C. et al. Scientific commentary on: “Phosphorylated tau in the retina correlates with tau pathology in the brain in Alzheimer’s disease and primary tauopathies”. Acta Neuropathol.147, 30. 10.1007/s00401-023-02656-z (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li, M. et al. Relationship between Alzheimer’s disease and retinal choroidal thickness: A cross-sectional study. J. Alzheimers Dis.80, 407–419. 10.3233/JAD-201142 (2021). [DOI] [PubMed] [Google Scholar]
- 22.Salobrar-Garcia, E. et al. Ocular vascular changes in mild Alzheimer’s disease patients: Foveal avascular zone, choroidal thickness, and ONH hemoglobin analysis. J. Pers. Med.10, 231. 10.3390/jpm10040231 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lopez-de-Eguileta, A. et al. Evaluation of choroidal thickness in prodromal Alzheimer’s disease defined by amyloid PET. PLoS ONE15, e0239484. 10.1371/journal.pone.0239484 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cunha, J. P. et al. Choroidal thinning: Alzheimer’s disease and aging. Alzheimers Dement. (Amst.)8, 11–17. 10.1016/j.dadm.2017.03.004 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gharbiya, M. et al. Choroidal thinning as a new finding in Alzheimer’s disease: Evidence from enhanced depth imaging spectral domain optical coherence tomography. J. Alzheimers Dis.40, 907–917. 10.3233/JAD-132039. (2014). [DOI] [PubMed] [Google Scholar]
- 26.den Haan, J. et al. Is retinal vasculature a biomarker in amyloid proven Alzheimer’s disease?. Alzheimers Dement. (Amst.)11, 383–391. 10.1016/j.dadm.2019.03.006 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rascovsky, K. et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain134, 2456–2477. 10.1093/brain/awr179 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoglinger, G. U. et al. Clinical diagnosis of progressive supranuclear palsy: The movement disorder society criteria. Mov. Disord.32, 853–864. 10.1002/mds.26987 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Litvan, I. et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): Report of the NINDS-SPSP international workshop. Neurology47, 1–9 (1996). [DOI] [PubMed] [Google Scholar]
- 30.Litvan, I. et al. Accuracy of clinical criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome). Neurology46, 922–930 (1996). [DOI] [PubMed] [Google Scholar]
- 31.Josephs, K. A. et al. Clinicopathologic analysis of frontotemporal and corticobasal degenerations and PSP. Neurology66, 41–48. 10.1212/01.wnl.0000191307.69661.c3 (2006). [DOI] [PubMed] [Google Scholar]
- 32.Spinelli, E. G. et al. Typical and atypical pathology in primary progressive aphasia variants. Ann. Neurol.81, 430–443. 10.1002/ana.24885 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Montine, T. J. et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: A practical approach. Acta Neuropathol.123, 1–11. 10.1007/s00401-011-0910-3 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gobom, J. et al. Validation of the LUMIPULSE automated immunoassay for the measurement of core AD biomarkers in cerebrospinal fluid. Clin. Chem. Lab. Med.60, 207–219. 10.1515/cclm-2021-0651 (2022). [DOI] [PubMed] [Google Scholar]
- 35.Lleo, A. et al. A 2-step cerebrospinal algorithm for the selection of frontotemporal lobar degeneration subtypes. JAMA Neurol.75, 738–745. 10.1001/jamaneurol.2018.0118 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Toledo, J. B. et al. A platform for discovery: The university of pennsylvania integrated neurodegenerative disease biobank. Alzheimers Dement.10, 477–484. 10.1016/j.jalz.2013.06.003 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suh, E. et al. Semi-automated quantification of C9orf72 expansion size reveals inverse correlation between hexanucleotide repeat number and disease duration in frontotemporal degeneration. Acta Neuropathol.130, 363–372. 10.1007/s00401-015-1445-9 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wood, E. M. et al. Development and validation of pedigree classification criteria for frontotemporal lobar degeneration. JAMA Neurol.70, 1411–1417. 10.1001/jamaneurol.2013.3956 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mrejen, S. & Spaide, R. F. Optical coherence tomography: Imaging of the choroid and beyond. Surv. Ophthalmol.58, 387–429. 10.1016/j.survophthal.2012.12.001 (2013). [DOI] [PubMed] [Google Scholar]
- 40.Yiu, G. et al. Characterization of the choroid-scleral junction and suprachoroidal layer in healthy individuals on enhanced-depth imaging optical coherence tomography. JAMA Ophthalmol.132, 174–181. 10.1001/jamaophthalmol.2013.7288 (2014). [DOI] [PubMed] [Google Scholar]
- 41.Agrawal, R. et al. Choroidal vascularity index as a measure of vascular status of the choroid: Measurements in healthy eyes from a population-based study. Sci. Rep.6, 21090. 10.1038/srep21090 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Robbins, C. B. et al. Characterization of retinal microvascular and choroidal structural changes in Parkinson disease. JAMA Ophthalmol.139, 182–188. 10.1001/jamaophthalmol.2020.5730 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li, X. Q., Larsen, M. & Munch, I. C. Subfoveal choroidal thickness in relation to sex and axial length in 93 Danish university students. Invest. Ophthalmol. Vis. Sci.52, 8438–8441. 10.1167/iovs.11-8108 (2011). [DOI] [PubMed] [Google Scholar]
- 44.Rhodes, L. A. et al. Peripapillary choroidal thickness variation with age and race in normal eyes. Invest. Ophthalmol. Vis. Sci.56, 1872–1879. 10.1167/iovs.14-16179 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liang, K. & Zeger, S. Longitudinal data analysis using generalized linear models. Biometrika73, 13–22 (1986). [Google Scholar]
- 46.Ying, G. S., Maguire, M. G., Glynn, R. & Rosner, B. Tutorial on biostatistics: Linear regression analysis of continuous correlated eye data. Ophthalmic. Epidemiol.24, 130–140. 10.1080/09286586.2016.1259636 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li, Z. B. et al. Evaluation of retinal and choroidal changes in patients with Alzheimer’s type dementia using optical coherence tomography angiography. Int. J. Ophthalmol.14, 860–868. 10.18240/ijo.2021.06.11 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.De Bernardo, M. et al. The correlation between retinal and choroidal thickness with age-related white matter hyperintensities in progressive supranuclear palsy. J. Clin. Med.12, 6671. 10.3390/jcm12206671 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsai, Y. et al. Ocular changes in TgF344-AD rat model of Alzheimer’s disease. Invest. Ophthalmol. Vis. Sci.55, 523–534. 10.1167/iovs.13-12888 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ning, A., Cui, J., To, E., Ashe, K. H. & Matsubara, J. Amyloid-beta deposits lead to retinal degeneration in a mouse model of Alzheimer disease. Invest. Ophthalmol. Vis. Sci.49, 5136–5143. 10.1167/iovs.08-1849 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mirzania, D. et al. Retinal and choroidal changes in men compared with women with Alzheimer’s disease: A case-control study. Ophthalmol. Sci.2, 100098. 10.1016/j.xops.2021.100098 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Robbins, C. B. et al. Choroidal structural analysis in Alzheimer disease, mild cognitive impairment, and cognitively healthy controls. Am. J. Ophthalmol.223, 359–367. 10.1016/j.ajo.2020.09.049 (2021). [DOI] [PubMed] [Google Scholar]
- 53.Asanad, S. et al. The retinal choroid as an oculovascular biomarker for Alzheimer’s dementia: A histopathological study in severe disease. Alzheimers Dement. (Amst.)11, 775–783. 10.1016/j.dadm.2019.08.005 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Canepa, E. & Fossati, S. Impact of Tau on neurovascular pathology in Alzheimer’s disease. Front. Neurol.11, 573324. 10.3389/fneur.2020.573324 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bennett, R. E. et al. Tau induces blood vessel abnormalities and angiogenesis-related gene expression in P301L transgenic mice and human Alzheimer’s disease. Proc. Natl. Acad. Sci. USA115, E1289–E1298. 10.1073/pnas.1710329115 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cennamo, G. et al. Correlation between retinal vascularization and disease aggressiveness in amyotrophic lateral sclerosis. Biomedicines10, 2390. 10.3390/biomedicines10102390 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Singer, M. B. et al. Abnormal retinal capillary blood flow in autosomal dominant Alzheimer’s disease. Alzheimers Dement. (Amst)13, e12162. 10.1002/dad2.12162 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ostergaard, L. et al. The capillary dysfunction hypothesis of Alzheimer’s disease. Neurobiol. Aging34, 1018–1031. 10.1016/j.neurobiolaging.2012.09.011 (2013). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. Raw data can be made available on request.