Abstract
A human Elongator complex was purified from HeLa cells and found to be composed of three polypeptides. Human Elongator contains histone acetyltransferase activity with specificity to histone H3 and, to a much lesser extent, to histone H4. Although many reports have suggested a role for the yeast Elongator in transcription elongation through chromatin templates, no direct evidence supporting this function exists. In the present study, we demonstrate that the human Elongator facilitates transcription by RNA polymerase II in a chromatin- and acetyl-CoA-dependent manner. The complex was found to directly interact with RNA polymerase II but failed to interact with other factors that facilitated RNA polymerase II to traverse through nucleosomes. From our results, we postulate that different mechanisms operate to ensure efficient transcription by RNA polymerase II on chromatin templates.
The formation of a transcription initiation complex at a particular promoter is a complicated and highly regulated process. However, the establishment of a transcription initiation complex seems much simpler than the process of transcription elongation with respect to the chromatin impediment, where the elongating polymerase has to traverse a nucleosome approximately every 200 base pairs (1).
The complexity of elongation by RNA polymerase II (RNAPII) was recognized early by Price, who postulated the existence of positive and negative elongation-specific regulatory factors (2). This hypothesis gained validity when factors such as P-TEFb (3), and the positive and negative regulatory elongation factors DSIF (4) and NELF (5), respectively, were isolated. However, whether these factors are chromatin specific or general elongation factors affecting the efficiency of elongation, like TFIIS (6), remains an open question. Thus far, only one functional biochemical assay has been devised to score for chromatin-specific elongation factors (7). This biochemical assay resulted in the identification of FACT, an abundant factor conserved through evolution and composed of two subunits, SSRP1 and Spt16, which in a reconstituted transcription system allows RNAPII to traverse nucleosomes (8). The finding that Spt16 interacts with SAS3, the catalytic subunit of the histone acetyltransferase (HAT) NuA3 complex (9), provides a possible mechanism explaining the extent of acetylation observed at transcriptionally active regions, as it is unlikely that promoter activity can account for such extended acetylation. An alternative possibility that could account for the extended acetylation observed within transcriptionally active regions is that the elongating RNAPII is associated with a HAT complex (1). Evidence supporting this possibility was provided by the identification of the Elongator in yeast (10).
Yeast Elongator was isolated as a complex that associates with the chromatin fraction and interacts with the elongating phosphorylated form of RNAPII (10). Elongator was initially found to be composed of three polypeptides (10–12). Its larger subunit is encoded by IKI3, a gene isolated earlier in a screen conferring resistance to the Kluyveromyces lactis killer toxin (13), and renamed ELP1 (10). The two other subunits are encoded by genes denoted ELP2 (11) and ELP3 (14). Importantly, ELP3 was found to encode a polypeptide possessing HAT activity with specificity to the four core histones (14). Deletion of ELP3 (or other ELP genes) confers a set of phenotypes, some of which are associated with elongation defects (10–12, 14).
The yeast Elongator complex was also isolated from the soluble cell fraction and found to be composed of the Elp1/2/3 core complex in association with three other polypeptides, termed Elp4, Elp5, and Elp6, to form the holo-Elongator (15, 16). It was suggested that the Elp4/5/6 complex interacts preferentially with the core complex (rather than in complex with RNAPII) and was therefore proposed to have a regulatory function (16). Interestingly, studies analyzing genes targeted by the K. lactis killer toxin identified genes encoding different subunits of Elongator (17). The function of the Elongator is not clear, and although many reports have suggested a role during elongation by RNAPII, likely through chromatin, no direct biochemical evidence has been demonstrated. In the present studies, we isolated a mammalian core-Elongator and provide direct evidence linking Elongator function with chromatin.
Materials and Methods
Purification of Human Elongator (hElongator) from HeLa Nuclear Extracts.
Approximately 6.0 g of HeLa nuclear extracts was applied to a 500-ml phosphocellulose column equilibrated in BC100 [100 mM KCl containing buffer C, which was 20 mM Tris⋅HCl, pH 7.9/0.2 mM EDTA/10 mM 2-mercaptoethanol/10% (vol/vol) glycerol/0.2 mM PMSF]. The proteins were step-eluted with buffer C containing 0.3, 0.5, and 1.0 M KCl. hElp1 and hElp3 polypeptides eluted in the 0.3 M KCl wash. This fraction was dialyzed against BC150 and then loaded onto a 150-ml DEAE-Sepharose column equilibrated with BC150. Proteins were eluted with washes of buffer C containing 0.35, 0.55, and 1.0 M KCl. The hElp1 and hElp3 polypeptides eluted in the 0.35 M wash. This fraction was dialyzed against buffer C containing 1.0 M (NH4)2SO4 and loaded onto a phenyl-Sepharose HiLoad 26/10 FPLC column. Proteins were step-eluted with 1.0, 0.55, 0.35, and 0 M (NH4)2SO4 washes in buffer C. The 0 M (NH4)2SO4 wash fraction (30 mg) was loaded onto a 5-ml HiTrap Q-Sepharose column equilibrated in BC100. Proteins were eluted with a 20-column volume linear gradient from 0.1 M to 1 M KCl in buffer C. The hElongator complex eluted between 0.2 M and 0.3 M KCl. The hElp1- and hElp3-containing fractions were pooled (12 mg), dialyzed against BC100, and applied to a 3.1-ml DEAE-5PW HPLC column (TosoHaas); proteins eluted with a 15-column volume linear gradient from 0.1 M to 1.0 M KCl in buffer C. The hElp1 and hElp3 polypeptides eluted between 0.25 M and 0.3 M KCl. These fractions were pooled (0.5 mg) and loaded onto a Superose-6 hr 10/30 gel filtration column previously equilibrated in buffer C containing 0.5 M KCl and 0.1% Nonidet P-40.
Ion Trap Tandem Mass Spectrometry.
After in-gel tryptic digestion of the SDS/PAGE-isolated polypeptides, multiple peptide sequences were determined by microcapillary reverse-phase chromatography coupled to a Finnigan LCQ DECA quadrupole ion trap mass spectrometer equipped with a custom nanoelectrospray source. Flow rate was nominally 200 nl/min. The ion trap repetitively surveyed the range m/z 395 to 1400, acquiring data-dependent MS/MS spectra for peptide sequence information on the three most abundant ions in each survey scan. Relative collision energy was 30% at an isolation width of 2.5 daltons, with recurring ions dynamically excluded. Interpretation of the resulting MS/MS spectra of the peptides was facilitated by programs developed in the Harvard Microchemistry Facility and by database correlation with SEQUEST.
Protein–Protein Interaction Assays.
For the interaction between Elongator and RNAPII, RNAPII (300 ng) was mixed with Elongator (100 ng) in buffer C containing 0.15 M KCl/0.01% Nonidet P-40 in a final volume of 30 μl on ice. The mixture was incubated at 4°C for 1 h before loading onto a Superose 6 PC3.2/30 gel filtration column previously equilibrated in buffer C containing 0.15 M KCl and 0.1% Nonidet P-40.
Immunodepletion of the Elongator Subunits from HeLa Nuclear Extracts.
HeLa cell nuclear extracts (NE) were prepared as described previously (18). Purified polyclonal antibodies (30 mg) against Elp1 and Elp3 were coupled to 0.5 ml of protein G-agarose with 20 mM dimethylpimelimidate as described (19). The anti-Elp1-coupled resin was incubated with HeLa NE (1.0 ml, 0.8 mg/ml) for 1 h at 4°C. The mixture was decanted, and the extract was passed through the column 10 times. The unbound material was recovered and incubated with anti-Elp3-coupled resin, and the procedure was repeated as described above. The unbound material from this column was used as Elongator-depleted NE.
Chromatin Assembly and Transcription Assays.
Standard transcription assays were performed as described previously (7). Chromatin was assembled with Drosophila S-190 extract with 3 μg of plasmid DNA and 2 μg of HeLa-purified histones in a 400-μl volume. Chromatin was purified by CL-4B gel filtration chromatography as previously described (7).
Immunofluorescence.
FITC-labeled CTD, FITC-labeled Elp1, rhodamine-labeled Elp2, and rhodamine-labeled Elp3 antibodies were prepared by using FluoReporter protein labeling kit according to the supplier's instructions (Molecular Probes). HeLa, HepG2, 293T, and NIH 3T3 cells grown on coverslips were fixed with either cold 100% methanol or 4% paraformaldehyde, then blocked with 1% BSA. For competition experiments of immunostaining of HeLa cells with rhodamine-labeled Elp3 antibodies, cells were incubated for 2 h at 37°C with 0.5 mg of Elp3 polypeptide and 0.2% Tween-20 in PBS per coverslip before incubation with rhodamine-labeled Elp3 antibodies. Cells were stained by sequential incubation with fluorescence-labeled antibodies and visualized by using a Zeiss Axiovert 200M system.
Results
Purification of a hElongator.
Previous studies in yeast described the Elongator, with one of its subunits, Elp3, possessing HAT activity and suggested that the complex facilitates elongation by RNAPII through nucleosomes in an acetyl-CoA-dependent manner (14). In light of these results, we attempted to isolate the hElongator by using a functional transcription assay. In this assay, we used our chromatin reconstituted transcription system composed of all human general transcription factors and RNAPII, in addition to factors that facilitate transcription on chromatin templates, i.e., activator, coactivators, RSF, and FACT (20). In this assay, however, FACT, a factor isolated based on its ability to allow RNAPII to traverse nucleosomes (7), was replaced by aliquots of HeLa-nuclear extracts. We assayed for the synthesis of a 390-nt RNA that depended on acetyl-CoA. Despite testing different assay conditions, we failed to establish an acetyl-CoA-dependent elongation assay (data not shown).
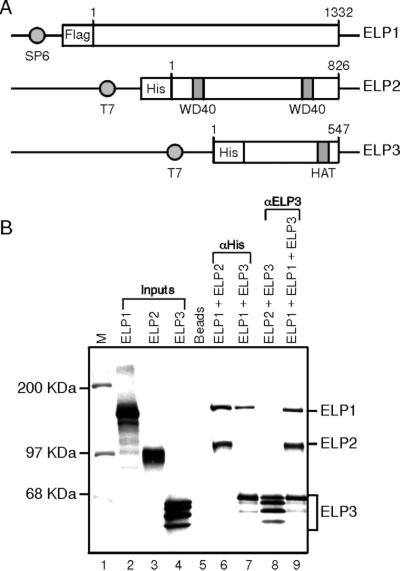
Because of our desire to study the mechanism of Elongator function and our inability to detect an acetyl-CoA-dependent elongation activity, we isolated cDNAs encoding the human homologues of the three subunits of the yeast Elongator (11). The proteins were expressed in bacteria and used to generate polyclonal antibodies, which were used to follow the hElongator through different steps of purification in Western blot analysis. Using the procedure outlined in Fig. 1A, an hElongator was purified to three polypeptides (Fig. 1B). Sequencing of the three polypeptides composing an hElongator by ion trap tandem mass spectrometry identified the 150-kDa, 92-kDa, and 58-kDa peaks as Elp1, Elp2, and Elp3, respectively (data not shown). These three polypeptides coeluted on a gel filtration column as demonstrated by silver staining of an SDS/PAGE gel as well as by Western blot analysis (Fig. 1C). In agreement with studies performed in yeast, human Elp3 contains signature domains of polypeptides with HAT activity. Indeed, the hElongator contains intrinsic HAT activity (Fig. 1D). However, in contrast to the studies in yeast claiming that the Elongator acetylates each of the histone polypeptides (14), the HAT activity associated with the human complex was highly specific for histone H3 and exhibited much reduced activity toward histone H4 (Fig. 1D). No activity toward the H2A and H2B polypeptides could be detected (Fig. 1D and data not shown). Surprisingly, the HAT activity associated with the hElongator complex was impaired when nucleosomal histones were used as substrate (data not shown).
Figure 1.
Purification of a hElongator. (A) Schematic representation of the steps used to purify a hElongator. (B) Fractions derived from each step of purification and containing approximately equal amounts of protein were resolved by electrophoresis on an SDS/4–15% polyacrylamide gel and stained with silver. (C Upper) Silver staining of an SDS/10% polyacrylamide gel containing aliquots of fractions derived from the Superose-6 gel filtration column. The subunits of core Elongator are indicated on the left, and the elution from the gel filtration column of protein markers is indicated on the top. (Lower) Western blot of the fractions derived from the gel filtration column analyzed by using antibodies specific for Elp1 and Elp3. (D) Human Elp3 is an HAT. (Upper) Schematic representation of human Elp3-conserved motifs D, A, and B of the GNAT superfamily (34) is indicated. The Q(R)XXGXG motif (central part of GNAT motif A) critical for acetyl-CoA recognition (35) is conserved in the human Elp3 subunit. Numbers on the top denote the amino acid sequence of hElp3. (Lower) HAT assay was performed by using the purified hElongator (20 and 100 ng of the gel filtration pool). P/CAF used as control is indicated. [3H]Acetylated core histones were analyzed by SDS/15% PAGE followed by fluorography.
Elongator Subunit Interaction and Cellular Localization of the Subunits.
We next analyzed the interaction among the different subunits of the hElongator. Subunit interaction studies were performed by using in vitro translated polypeptides coupled with immunoprecipitation by using antibodies against a tag present in the Elongator subunits (Fig. 2A) or by using antibodies against Elp3. This analysis demonstrates that Elp1 interacts with Elp2 and Elp3 (Fig. 2B, lanes 6 and 7). This interaction seems specific because Elp1 failed to interact either with subunits of FACT (SSRP1 and Spt16) or DSIF (Spt4 and Spt5) (data not shown, see below). Moreover, antibodies against Elp3 failed to immunoprecipitate Elp2 (lane 8); however, in the presence of Elp1, anti-Elp3 antibodies effectively immunoprecipitate Elp2 (lane 9). The most logical interpretation to these results is that Elp1 interacts independently with Elp2 and Elp3.
Figure 2.
Subunit interaction of the core human Elongator. (A) Schematic representation of N-terminally tagged human Elp1, Elp2, and Elp3. Numbers on the top indicate the N- and C-terminal amino acids of each construct. The promoters used to transcribe the DNA in vitro are indicated. (B) Elp1 interacts with Elp2 and with Elp3. In vitro translated N-terminally His-tagged Elp1 was incubated with in vitro translated N-terminally flag-tagged Elp2 and/or Elp3. The complex was immunoprecipitated with antibodies against the His-tag or Elp3 as described in Materials and Methods. Immunoprecipitates were separated by SDS/10% PAGE and analyzed by autoradiography.
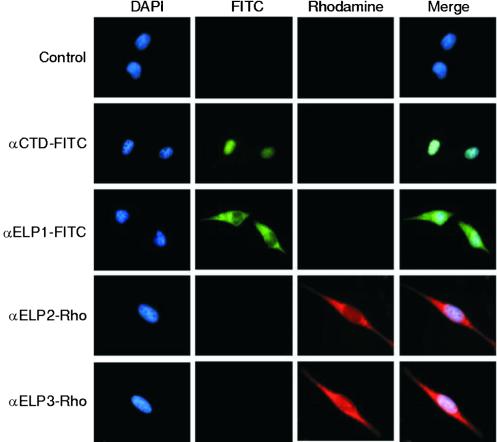
Next, we analyzed the cellular localization of the Elongator by immunofluorescence by using affinity-purified highly specific antibodies raised against each of the subunits of the human complex, which were conjugated to specific dyes. Surprisingly, this analysis revealed that, although some levels of these proteins exist in the nucleus, the majority of each subunit is localized to the cytoplasm (Fig. 3). This localization seems specific to Elongator subunits because RNAPII, as detected by using antibodies against the CTD (Fig. 3) or antibodies against Rpb7 (data not shown), was exclusively localized to the nucleus. More importantly, the immunofluorescence observed with antibodies against Elongator subunits was completely blocked by the addition of excess specific antigen but not by an irrelevant polypeptide (data not shown). This finding was not restricted to the cell line used, as similar results were observed with HeLa, HepG2, NIH 3T3, and 293T cells (data not shown). Also, similar results were observed when different fixation methods were used. Thus, we concluded that the majority of the Elongator subunits are localized within the cytoplasm of the cell.
Figure 3.
Subcellular localization of human core-Elongator subunits. HeLa cells were fixed and stained with various fluorescent-conjugated antibodies or 0.01 mg/ml 4′,6-diamidino-2-phenylindole (DAPI) as indicated.
Analyses of the Interaction between Elongator and Other Transcription Factors.
The results described above (Fig. 3) prompted us to analyze whether the hElongator interacts with RNAPII and other transcription factors. Interactions between Elongator and RNAPII, FACT, and DSIF were analyzed by glycerol gradient sedimentation, gel filtration, and immunoprecipitation. These analyses revealed a direct interaction between Elongator and RNAPII (Fig. 4) as previously reported for the yeast complex (10). However, in contrast to the finding with the yeast factor of a specific interaction between Elongator and the phosphorylated form of RNAPII, our analysis demonstrated interaction with both forms of RNAPII (Fig. 4 and data not shown). Similar results were observed when the interaction was analyzed by immunoprecipitation (data not shown). Although we were able to demonstrate a direct interaction between Elongator and RNAPII as well as between DSIF and RNAPII (data not shown), we failed to detect interaction between Elongator and factors that allow RNAPII to transcribe through chromatin templates, including FACT and DSIF (data not shown).
Figure 4.
Human core Elongator interacts with RNAPII (Pol II) in vitro. Elongator (100 ng) and RNAPII (300 ng) were incubated and then fractionated through a Superose-6 column as indicated in Materials and Methods. In parallel experiments, individual factors (top of each gel) were also fractionated on the column. The bottom of each gel denotes the fractions derived from the Elongator–RNAPII fractionation. The indicated fractions were blotted and probed with anti-Elp1, anti-Elp3, and anti-CTD as indicated. Elution positions of size markers are indicated on the top.
Functional Analysis of Elongator.
Having established a physical association between hElongator and RNAPII, we next asked whether this interaction is functional. We began the studies by asking whether the purified mammalian Elongator affected transcription of chromatin templates in an acetyl-CoA-dependent manner, by using our chromatin reconstituted transcription system (20, 21). Chromatin was deposited on DNA containing five Gal4 sites upstream of the adenovirus major late promoter that directs transcription of a 390-nt G-less cassette by using the S190 Drosophila extract (22) and by using the RSF-mediated nucleosome deposition system (21). Regardless of the assay conditions, the reconstituted system failed to demonstrate any effect by Elongator under conditions where DSIF (T. Wada, G. Orphanides, A. Loyola, J. Hasegawa, D.-K. Kim, D. Shima, Y. Yamaguchi, H. Handa, and D.R., unpublished data) and FACT allowed transcription from the chromatin template (data not shown). In light of these negative results, we attempted to measure Elongator activity in extracts that were depleted of Elongator subunits. Depletion was accomplished by successive passages of a HeLa-cell-derived NE onto columns containing antibodies to each of the Elongator subunits, as described in Materials and Methods. This procedure was successful in removing Elongator subunits as detected by Western blots probing for Elp1 and Elp3 (Fig. 5A). Immunodepletion was specific for the Elongator subunits, as other transcription factors and RNAPII were apparently not affected by the procedure (Fig. 5A).
Figure 5.
Human Elongator allows transcription from chromatin templates. (A) Immunoblot analysis of untreated HeLa NE and Elongator-depleted HeLa NE. Equal amounts (30 μg) of NE and Elongator-depleted NE were subjected to SDS/4–15% polyacrylamide gradient gel electrophoresis, transferred to nitrocellulose, and analyzed with antibodies to the proteins indicated. (B) Transcription from naked DNA and chromatin templates. Transcription was performed by using either normal NE or Elongator-depleted NE (NE[ΔELP]). Transcription reaction mixtures (40 μl) contained 80 μg of NE proteins and freshly assembled chromatin or histone-free DNA (100 ng) with (20, 40, and 80 ng) or without Elongator and acetyl-CoA (1.5 μM), as indicated. In addition, reactions were supplemented with Gal4-VP16. Reactions were incubated at 30°C for 30 min or as indicated. Products of the transcription reactions were separated by electrophoresis on a 6% sequencing gel.
Transcription analysis by using naked DNA shows that the Elongator-depleted NE was not affected, as compared with the untreated NE (Fig. 5B, lanes 1 and 2). Importantly, when the depleted NE was used to direct transcription from chromatin templates, the formation of the full-length transcript was severely affected (Fig. 5B, compare lanes 6–8 with lanes 3–5), and stimulation of transcription was observed upon addition of acetyl-CoA (Fig. 5B, compare lane 8 with lane 11). We interpret these findings to suggest that the depletion of the Elongator affected the properties of the extract to direct transcription. The low level of transcription stimulation observed upon addition of acetyl-CoA might be the result of limiting amounts of Elongator remaining in the extract that was activated upon cofactor addition. If this was the case, we expected that addition of purified hElongator to the depleted extract should stimulate transcription in an acetyl-CoA-dependent manner. This was found to be the case (Fig. 5B, lanes 12–14); moreover, stimulation of transcription depended on the amount of Elongator added (Fig. 5B, lanes 14–16), and, most importantly, the effect observed upon addition of Elongator depended on acetyl-CoA (Fig. 5B, compare lanes 17 and 18). We conclude that the Elongator complex stimulates transcription of chromatin templates in an acetyl-CoA-dependent manner.
Discussion
In the present study, we have isolated an hElongator complex and demonstrated the long-awaited function of this complex, its ability to stimulate transcription on chromatin templates.
In agreement with the findings that one of the subunits of the complex, Elp3, contains domains characteristic of proteins with acetyltransferase activity, the complex was found to acetylate histones, with specificity to H3 and to a much lesser extent H4. More importantly, the chromatin-specific transcription stimulatory activity associated with hElongator was found to be dependent on acetyl-CoA. The purified three-subunit complex (core complex) was not capable of acetylating nucleosomal histones. This finding correlates with the inability of the complex to allow RNAPII to traverse nucleosomes in a reconstituted chromatin transcription system. The ability of the hElongator to stimulate transcription on chromatin templates could be demonstrated only on NE depleted of Elongator subunits. The inability of the three-subunit human (and yeast) Elongator to acetylate nucleosomal histones and to function in a reconstituted chromatin transcription system is likely related to findings demonstrating that the yeast trimeric core-Elongator interacts with three other subunits suggested to have regulatory functions (15, 16). The identification of a core hElongator, active in extracts depleted of core-Elongator subunits, provides the foundation for future studies elucidating the mechanism by which Elongator, in combination with other factors, allows RNAPII to traverse nucleosomes.
FACT and DSIF are two factors that function in elongation through chromatin templates as demonstrated by genetic (8, 23) and biochemical (ref. 7; T. Wada, G. Orphanides, A. Loyola, J. Hasegawa, D.-K. Kim, D. Shima, Y. Yamaguchi, H. Handa, and D.R., unpublished results) studies. We therefore analyzed whether these factors interact with hElongator. We analyzed whether DSIF, which directly interacts with RNAPII (24), can also interact with Elongator and/or whether an RNAPII–DSIF–Elongator complex could be isolated. We failed to demonstrate an interaction between DSIF and Elongator, and although we successfully demonstrated independent interactions between RNAPII and DSIF or RNAPII and Elongator, we also failed to demonstrate a complex with all three factors. This failure suggests that the association of DSIF or Elongator with RNAPII is mutually exclusive. This is a likely possibility, as studies performed in yeast, although limited, demonstrate that not all genes are regulated by Elongator (12) and DSIF (4). Moreover, Elongator subunits, as well as Spt4, a subunit of DSIF, were found to be dispensable for viability in yeast, suggesting that more than one mechanism operates to allow efficient transcription through nucleosomes. Unfortunately, genetic interaction studies between ELPs and the genes encoding the subunits of DSIF (SPT4/5) have not been reported.
In support of the hypothesis that more than one mechanism operates to regulate elongation through nucleosomes are the studies with FACT, which is composed of two subunits encoded by essential genes (8). In agreement with the observation that FACT functions by directly interacting with nucleosomes (8) and Elongator functions by interacting with RNAPII (Fig. 4 and ref. 10), we failed to demonstrate a physical interaction between these two factors. Whether these factors interact functionally, like DSIF and FACT (T. Wada, G. Orphanides, A. Loyola, J. Hasegawa, D.-K. Kim, D. Shima, Y. Yamaguchi, H. Handa, and D.R., unpublished results), awaits the development of an in vitro reconstituted transcription system capable of recapitulating a requirement for Elongator. Relevant to the studies of Elongator function are findings demonstrating an interaction between Spt16, a subunit of FACT, and Sas3, the catalytic subunit of the NuA3 complex (9). In this regard, genetic interaction studies were performed analyzing whether GCN5, the catalytic subunit of the SAGA and the ADA complexes (25, 26), interacts with ELP3 (12). Although the most relevant experiment testing for a genetic interaction between SAS3 and Elp3 was not performed, the genetic studies suggest an interaction between SAGA and Elongator (12). SAGA is a factor recruited to promoters by interactions with activators (27) and functions to establish transcription initiation complexes (28). Whether SAGA is also required during transcription elongation is unknown. Previous studies performed with Elongator suggested that the complex is recruited to transcriptionally engaged genes through recognition of the phosphorylated CTD of RNAPII (10, 14). Because phosphorylation of the CTD is a postinitiation event (29), Elongator should then function exclusively during elongation. However, the genetic interaction between SAGA and Elongator suggests a role for Elongator during initiation of transcription. This observation might be in agreement with our finding that Elongator interacts with RNAPII independently of the phosphorylation state of the CTD, and thus it may function during initiation of transcription at some genes.
A surprising result was the finding that the subunits of core Elongator are localized primarily to the cytoplasm. Although unexpected, this finding reflects a possible mode of regulating Elongator function by cellular localization and transport to the nucleus. In support of our findings are results that uncovered interaction between the Elp2 subunit of Elongator and Stat3 and other polypeptides involved in the IFN signal transduction pathway (31). Importantly, Elp2 was found localized to the cytoplasm, but upon IL6 treatment, Elp2 translocated to the nucleus (30). It is therefore tempting to postulate that, similar to other transcription factors (31), Elongator might be regulated by cellular compartmentalization. However, the fact that during purification of hElongator we did not observe all Elp1 and Elp3 cofractionating, raises the possibility that Elongator subunits participate in cellular processes other than transcription. This, however, is in contrast to results in yeast demonstrating that all subunits of Elongator, including holo-Elongator, participate in the same cellular process (15). Elucidating whether in higher eukaryotes Elongator subunits participate independently in processes other than transcription is important. A mutation in the gene encoding Elp1 was shown to cause familial dysautonomia (32), a fatal disorder affecting the nervous system. Also, recent studies have indicated that single amino acid changes in Elp1 correlate with increased bronchial asthma in children (33). Thus, whether these abnormalities are because of defects on Elongator function and, therefore, transcription defects, is unknown, but with the identification of the hElongator, these studies can now be addressed.
Acknowledgments
We thank Alejandra Loyola for helpful discussions and Dr. J. Svesjstrup for communicating results before publication. We also thank Dr. S. Oh, from our laboratory, for providing recombinant FACT, Drs. T. Wada and H. Handa for providing recombinant DSIF, and members of the Reinberg laboratory for helpful suggestions. We thank Drs. M. Hampsey and L. Vales for comments on the manuscript. This work was supported by National Institutes of Health Grant GM-37120 and the Howard Hughes Medical Institute (to D.R.).
Abbreviations
- RNAPII
RNA polymerase II
- HAT
histone acetyltransferase
- NE
nuclear extract(s)
- hElongator
human Elongator
References
- 1.Orphanides G, Reinberg D. Nature (London) 2000;407:471–475. doi: 10.1038/35035000. [DOI] [PubMed] [Google Scholar]
- 2.Marshall N F, Price D H. Mol Cell Biol. 1992;12:2078–2090. doi: 10.1128/mcb.12.5.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Renner D B, Yamaguchi Y, Wada T, Handa H, Price D H. J Biol Chem. 2001;276:42601–42609. doi: 10.1074/jbc.M104967200. [DOI] [PubMed] [Google Scholar]
- 4.Wada T, Takagi T, Yamaguchi Y, Ferdous A, Imai T, Hirose S, Sugimoto S, Yano K, Hartzog G A, Winston F, et al. Genes Dev. 1998;12:343–356. doi: 10.1101/gad.12.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamaguchi Y, Takagi T, Wada T, Yano K, Furuya A, Sugimoto S, Hasegawa J, Handa H. Cell. 1999;97:41–51. doi: 10.1016/s0092-8674(00)80713-8. [DOI] [PubMed] [Google Scholar]
- 6.Reinberg D, Roeder R G. J Biol Chem. 1987;262:3331–3337. [PubMed] [Google Scholar]
- 7.Orphanides G, LeRoy G, Chang C H, Luse D S, Reinberg D. Cell. 1998;92:105–116. doi: 10.1016/s0092-8674(00)80903-4. [DOI] [PubMed] [Google Scholar]
- 8.Orphanides G, Wu W H, Lane W S, Hampsey M, Reinberg D. Nature (London) 1999;400:284–288. doi: 10.1038/22350. [DOI] [PubMed] [Google Scholar]
- 9.John S, Howe L, Tafrov S T, Grant P A, Sternglanz R, Workman J L. Genes Dev. 2000;14:1196–1208. [PMC free article] [PubMed] [Google Scholar]
- 10.Otero G, Fellows J, Li Y, de Bizemont T, Dirac A M, Gustafsson C M, Erdjument-Bromage H, Tempst P, Svejstrup J Q. Mol Cell. 1999;3:109–118. doi: 10.1016/s1097-2765(00)80179-3. [DOI] [PubMed] [Google Scholar]
- 11.Fellows J, Erdjument-Bromage H, Tempst P, Svejstrup J Q. J Biol Chem. 2000;275:12896–12899. doi: 10.1074/jbc.275.17.12896. [DOI] [PubMed] [Google Scholar]
- 12.Wittschieben B O, Fellows J, Du W, Stillman D J, Svejstrup J Q. EMBO J. 2000;19:3060–3068. doi: 10.1093/emboj/19.12.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yajima H, Tokunaga M, Nakayama-Murayama A, Hishinuma F. Biosci Biotechnol Biochem. 1997;61:704–709. doi: 10.1271/bbb.61.704. [DOI] [PubMed] [Google Scholar]
- 14.Wittschieben B O, Otero G, de Bizemont T, Fellows J, Erdjument-Bromage H, Ohba R, Li Y, Allis C D, Tempst P, Svejstrup J Q. Mol Cell. 1999;4:123–128. doi: 10.1016/s1097-2765(00)80194-x. [DOI] [PubMed] [Google Scholar]
- 15.Winkler G S, Petrakis T G, Ethelberg S, Tokunaga M, Erdjument-Bromage H, Tempst P, Svejstrup J Q. J Biol Chem. 2001;276:32743–32749. doi: 10.1074/jbc.M105303200. [DOI] [PubMed] [Google Scholar]
- 16.Li Y, Takagi Y, Jiang Y, Tokunaga M, Erdjument-Bromage H, Tempst P, Kornberg R D. J Biol Chem. 2001;276:29628–29631. doi: 10.1074/jbc.C100274200. [DOI] [PubMed] [Google Scholar]
- 17.Frohloff F, Fichtner L, Jablonowski D, Breunig K D, Schaffrath R. EMBO J. 2001;20:1993–2003. doi: 10.1093/emboj/20.8.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dignam J D, Lebovitz R M, Roeder R G. Nucleic Acids Res. 1983;11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harlow E, Lane D. Antibodies: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1988. [Google Scholar]
- 20.LeRoy G, Orphanides G, Lane W S, Reinberg D. Science. 1998;282:1900–1904. doi: 10.1126/science.282.5395.1900. [DOI] [PubMed] [Google Scholar]
- 21.Loyola A, LeRoy G, Wang Y H, Reinberg D. Genes Dev. 2001;15:2837–2851. doi: 10.1101/gad.937401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamakaka R T, Bulger M, Kadonaga J T. Genes Dev. 1993;7:1779–1795. doi: 10.1101/gad.7.9.1779. [DOI] [PubMed] [Google Scholar]
- 23.Hartzog G A, Wada T, Handa H, Winston F. Genes Dev. 1998;12:357–369. doi: 10.1101/gad.12.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamaguchi Y, Wada T, Watanabe D, Takagi T, Hasegawa J, Handa H. J Biol Chem. 1999;274:8085–8092. doi: 10.1074/jbc.274.12.8085. [DOI] [PubMed] [Google Scholar]
- 25.Grant P A, Duggan L, Cote J, Roberts S M, Brownell J E, Candau R, Ohba R, Owen-Hughes T, Allis C D, Winston F, et al. Genes Dev. 1997;11:1640–1650. doi: 10.1101/gad.11.13.1640. [DOI] [PubMed] [Google Scholar]
- 26.Eberharter A, Sterner D E, Schieltz D, Hassan A, Yates J R, III, Berger S L, Workman J L. Mol Cell Biol. 1999;19:6621–6631. doi: 10.1128/mcb.19.10.6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Utley R T, Ikeda K, Grant P A, Cote J, Steger D J, Eberharter A, John S, Workman J L. Nature (London) 1998;394:498–502. doi: 10.1038/28886. [DOI] [PubMed] [Google Scholar]
- 28.Cosma M P, Tanaka T, Nasmyth K. Cell. 1999;97:299–311. doi: 10.1016/s0092-8674(00)80740-0. [DOI] [PubMed] [Google Scholar]
- 29.Akoulitchev S, Makela T P, Weinberg R A, Reinberg D. Nature (London) 1995;377:557–560. doi: 10.1038/377557a0. [DOI] [PubMed] [Google Scholar]
- 30.Collum R G, Brutsaert S, Lee G, Schindler C. Proc Natl Acad Sci USA. 2000;97:10120–10125. doi: 10.1073/pnas.170192197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hendzel M J, Kruhlak M J, MacLean N A, Boisvert F, Lever M A, Bazett-Jones D P. J Steroid Biochem Mol Biol. 2001;76:9–21. doi: 10.1016/s0960-0760(00)00153-9. [DOI] [PubMed] [Google Scholar]
- 32.Anderson S L, Coli R, Daly I W, Kichula E A, Rork M J, Volpi S A, Ekstein J, Rubin B Y. Am J Hum Genet. 2001;68:753–758. doi: 10.1086/318808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takeoka S, Unoki M, Onouchi Y, Doi S, Fujiwara H, Miyatake A, Fujita K, Inoue I, Nakamura Y, Tamari M. J Hum Genet. 2001;46:57–63. doi: 10.1007/s100380170109. [DOI] [PubMed] [Google Scholar]
- 34.Neuwald A F, Landsman D. Trends Biochem Sci. 1997;22:154–155. doi: 10.1016/s0968-0004(97)01034-7. [DOI] [PubMed] [Google Scholar]
- 35.Wolf E, Vassilev A, Makino Y, Sali A, Nakatani Y, Burley S K. Cell. 1998;94:439–449. doi: 10.1016/s0092-8674(00)81585-8. [DOI] [PubMed] [Google Scholar]