Abstract
Background
Postoperative acute pain is a common issue in children after surgery. Our study aimed to investigate whether preoperative use of a dexmedetomidine–esketamine combination could reduce postoperative pain in children undergoing tonsillectomy and adenoidectomy and reduce side effects, such as bradycardia, hypotension or emergence delirium.
Methods
In this double-blind, randomised controlled clinical trial, 180 children were randomly assigned to 3 groups. 30 min before surgery, the control group received 0.9% saline intranasally (Group C), the dexmedetomidine group received intranasal dexmedetomidine at 2.0 μg·kg−1 (Group D), and the combination group received intranasal dexmedetomidine at 1.0 μg·kg−1 and esketamine at 0.6 mg·kg−1 (Group DS). The primary outcome was the area under the curve (AUC) of the pain score within 24 h after surgery. Secondary outcomes included the incidence of emergence delirium (ED), pain scores after hospital discharge, and incidence of perioperative adverse events.
Results
A total of 173 children completed the study. The AUC of the pain score at rest within 24 h after surgery was 37.25 (20.25–51.75) in Group C, which was higher than those in Groups D (19.25 [12.50–39.13], P < 0.001) and DS (9.50 [9.00–16.25], P < 0.001). Compared with the control group, the DS group had a lower incidence of ED (12.3% vs. 44.8%, P = 0.001). Heart rates (HRs) in groups DS (P < 0.001) and C (P < 0.001) were higher than those in the dexmedetomidine group at all time points. No serious adverse events occurred.
Conclusions
Intranasal dexmedetomidine combined with esketamine for premedication was associated with reduced postoperative pain in children. It can also prevent ED and had fewer side effects.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12871-025-03203-x.
Keywords: Paediatric, Dexmedetomidine, Esketamine, Postoperative analgesia, Emergence delirium
Introduction
Tonsillectomy is one of the most common surgical procedures performed on children and is the preferred treatment for recurrent acute inflammation of the tonsils, as well as hypertrophic tonsils and adenoids. However, acute pain is a common issue that can persist for up to 2 weeks after surgery [1]. Postoperative pain is often overlooked due to the short hospital stay, which may affect patients’ quality of life and mental health, leading to postoperative behavioural changes [2].
Clinicians have explored various strategies to prevent and relieve postoperative pain in children. Low-dose, short-acting opioids and nonsteroidal anti-inflammatory drugs (NSAIDS), such as tramadol, fentanyl, acetaminophen, and ibuprofen, can be used for postoperative analgesia [3, 4]. Postoperative pain can also be relieved by using long-acting analgesics intraoperatively; compared with fentanyl, 0.15 mg·kg−1 intravenous methadone was opioid sparing after surgery [5], and 1 mg·kg−1 intravenous esketamine reduced pain scores at 1 and 24 h postoperatively [6].
Few studies have examined the effects of preoperative medication on postoperative pain in children. Anaesthesiologists often administer sedative medications in the preoperative period to reduce children’s anxiety and improve cooperation during induction [7]. If this medication provides adequate preoperative sedation and postoperative pain relief, it will be a convenient and efficient approach for children, and reduce postoperative analgesic usage, which may have unavoidable side effects.
Dexmedetomidine, when administered to children, achieves a good sedation level to improve comfort, relieves preoperative anxiety, and reduces postoperative pain [8–10]. Esketamine is the S-enantiomer of racemic ketamine and has a greater affinity for N-methyl-D-aspartate (NMDA) receptors, therefore having analgesic and anaesthetic effects approximately two to three times stronger than ketamine. Due to its minimal effects on respiration, esketamine is commonly used for preoperative sedation in children undergoing outpatient procedures or day surgery [11]. Both these two drugs can be administered intranasally, a non-invasive and well-accepted regimen widely used for paediatric preoperative sedation [7, 12]. However, esketamine is also known to induce hallucinogenic symptoms, so its use, especially at large doses, should be performed with caution.
When administered together, the sympathomimetic activity of esketamine, which increases blood pressure and causes tachycardia, may counteract the bradycardic and hypotensive effects of dexmedetomidine [13, 14]. Moreover, dexmedetomidine’s sedative properties may reduce the psychiatric side effects of esketamine in children [7, 15]. In a randomised study, the combination of dexmedetomidine and esketamine to supplement analgesia provided a better postoperative analgesic effect in patients undergoing scoliosis correction surgery [16]. However, whether intranasal dexmedetomidine, when used preoperatively, reduces the intensity of postoperative pain in children remains unclear. No high-quality studies have validated whether a preoperative intranasal combination of dexmedetomidine and esketamine offers superior analgesia and fewer side effects. To address these questions, we conducted a prospective, double-blind, randomised controlled trial to examine the effect of preoperative intranasal dexmedetomidine combined with esketamine on postoperative pain in children. We hypothesised that this premedication regimen would alleviate postoperative pain and reduce side effects such as bradycardia, hypotension, or emergence delirium.
Methods
Study design
This study was designed as a prospective, single-centre, double-blind, randomised controlled clinical trial conducted at The Children’s Hospital of Xuzhou (Xuzhou, Jiangsu, China) between July 2024 and December 2024. This trial was approved by the Medical Ethics Committee of the Children’s Hospital of Xuzhou (2024–05–13-H13; January 31, 2024) and was registered at the Chinese Clinical Trial Registration Centre (ChiCTR2400085642; June 14, 2024). The first participant was enrolled on July 1, 2024. The implementation of this trial followed CONSORT guidelines. Written informed consent was obtained from each participant's parent or guardian.
Patients
Children aged 3 to 7 years with an American Society of Anesthesiologists physical status (ASA) I or II, and scheduled to undergo elective tonsillectomy and/or adenoidectomy were eligible for inclusion. The exclusion criteria were as follows: (a) children whose parents refused consent for participatation in the study; (b) children who were recent participants in other clinical trials; (c) children with long-term use of narcotic drugs or esketamine; (d) children with an allergy to dexmedetomidine or esketamine; (e) children whose body weight exceeded 95% of children of the same age and sex; (f) children with known cardiorespiratory diseases; (g) children who were neurologically delayed or had vision and hearing impairments that prevented their ability to communicate; (h) children with high intraocular and intracranial pressure; (i) children with electrocardiogram evidence of conduction block or sick sinus syndrome; and (j) children with a family history of psychiatric illnesses. Additional exclusion criteria included: (1) serious unforeseen intraoperative conditions (haemorrhage, cardiac arrest, etc.); (2) request to withdraw from the trial; (3) need for reoperation; and (4) inability to complete follow-up visits.
Randomisation and blinding
Children were divided into three groups in a 1:1:1 ratio according to computer-generated randomization (SPSS Inc., USA) by a researcher, and group allocations were concealed in sealed envelopes. Nasal drip medications were prepared in 1 ml syringes by an anaesthetist who was not involved in the follow-up study. The test drugs or placebo were administered by a qualified anaesthetist. The investigators responsible for data collection and the patients were blinded to the group allocation.
Anaesthesia, perioperative care, and intervention
One day before surgery, a researcher screened potential participants from the elective surgery list and collected baseline data. Thirty minutes before surgery, patients entered the anaesthesia preparation room and were assessed by a nurse to obtain their anxiety scores using the m-YPAS (Modified Yale Preoperative Anxiety Scale). The children were randomly divided into 3 groups. Children in Group D received 2 μg·kg−1 dexmedetomidine (Dexmedetomidine Hydrochloride Injection; Yichang Renfu Pharmaceutical Co., Ltd.; 2 mL: 200 μg) and those in Group DS received 1 μg·kg−1 dexmedetomidine combined with 0.6 mg·kg−1 esketamine (Esketamine Hydrochloride Injection; Jiangsu Hengrui Medicine Co., Ltd.; 2 mL: 50 mg), with 0.9% saline added to a final volume of 1 mL, while the control group received 1 ml of saline intranasally. The prepared drug solution was administered cautiously into both nostrils using a needleless 1-mL syringe, drop by drop. During administration, the children were positioned in a recumbent position on their parents’ laps. After administration, heart rate and pulse oximetry were routinely monitored. The sedation depth of each child was scored using the University of Michigan Sedation Scale (UMSS), and the time from intervention to a UMSS score of 2 was recorded for the children in groups D and DS. If sedation failed, the time was not recorded.
General anaesthesia was induced via inhalation. Once a sufficient depth of anaesthesia had been reached, the nurse established peripheral intravenous access. Fentanyl (2 μg·kg−1), cisatracurium (0.1–0.2 mg·kg−1), and propofol (2 mg·kg−1) were administered. After muscle relaxation, a tracheal tube was inserted, and volume-controlled ventilation was initiated. All children received 0.15 mg·kg−1 dexamethasone and 0.1 mg·kg−1 tropisetron at induction. Anaesthesia was maintained intraoperatively with 1–3% sevoflurane and a target-controlled infusion (TCI) of remifentanil. The remifentanil rate was adjusted according to intraoperative haemodynamics.
After surgery, children were sent to the PACU for observation. They received antagonists containing 0.02 mg·kg−1 neostigmine and 0.01 mg·kg−1 atropine. They were extubated when they resumed spontaneous breathing, their tidal volume reached 6–8 ml·kg−1 or more, and their respiratory rate reached 16 breathes· min−1 or more. Patients returned to the ward once the Steward score exceeded 4 points. If the postoperative pain score (FLACC scale) > 4 points, 0.5–1.0 μg·kg−1 fentanyl was administered. Upon return to the general ward or after discharge, oral acetaminophen (maximum daily dose: 50 mg·kg−1) and ibuprofen (maximum daily dose: 30 mg·kg−1) should be administered on time every day.
Primary and secondary outcomes
The primary outcome was the area under the curve (AUC) of pain scores (FLACC scale) at rest within 24 h (recorded at 15 min, 1 h, 3 h, 6 h, 9 h, 12 h and 24 h after extubation). The AUC was calculated using the trapezoidal rule:
 |
The secondary outcomes included: mean arterial pressure (MAP) and heart rate (HR) after induction (T1), 10 min (T2) and 20 min (T3) after induction; incidence of emergence delirium (Paediatric Anaesthesia Emergence Delirium (PAED) score ≥ 10); sedation score in the PACU (Ramsay score); time to extubation; pain scores after hospital discharge (Parents’ Postoperative Pain Measure, PPPM); quality of sleep on the 1 st postoperative day (NRS score, 0 = best sleep and 10 = worst sleep, a difference of ≥ 1 point was considered clinically meaningful [17]); and incidence of perioperative adverse events (nausea and vomiting, bradycardia, increased secretions, respiratory depression, hallucinations, nightmares, etc.).
Sample size calculation
Preliminary data showed that the AUC of the FLACC scores in children who underwent tonsillectomy and adenoidectomy was approximately 38.31, with an SD of 23.75 within 24 h after surgery. We assumed that the difference in the AUC of the 24 h was one point per hour. Using the multiple comparison correction Tukey‒Kramer method in PASS version 15.0, with a 2-sided significance level of 5% and a power of 90%, 162 participants were required, with a 1:1:1 group allocation. Accounting for a potential loss to follow-up rate of approximately 10%, a total of 180 participants (60 per group) were enrolled.
Statistical methods
Data were analysed using SPSS 27.0 statistical software. The Kolmogorov‒Smirnov test was performed to determine normality. Normally distributed data are presented as means (SD), and non-normally distributed data are expressed as medians (interquartile ranges), M (IQR). Normally distributed continuous data among the three groups were analysed with one-way analysis of variance (ANOVA), and comparisons between two groups were made using the least significant difference (LSD) test. The Kruskal‒Wallis H rank-sum test was used for non-normally distributed continuous data among the three groups, and the Mann‒Whitney U test was used for two-way comparisons. Differences between the two medians and 95% CIs were estimated using the Hodges‒Lehmann method. Categorical data were analysed using the chi-square or Fisher’s exact tests. The generalised estimating equation (GEE) was used for comparisons of repeated-measures data. P values were adjusted using the Bonferroni method and fixed at 0.017 for two-way comparisons. A P value < 0.05 indicated statistical significance.
Results
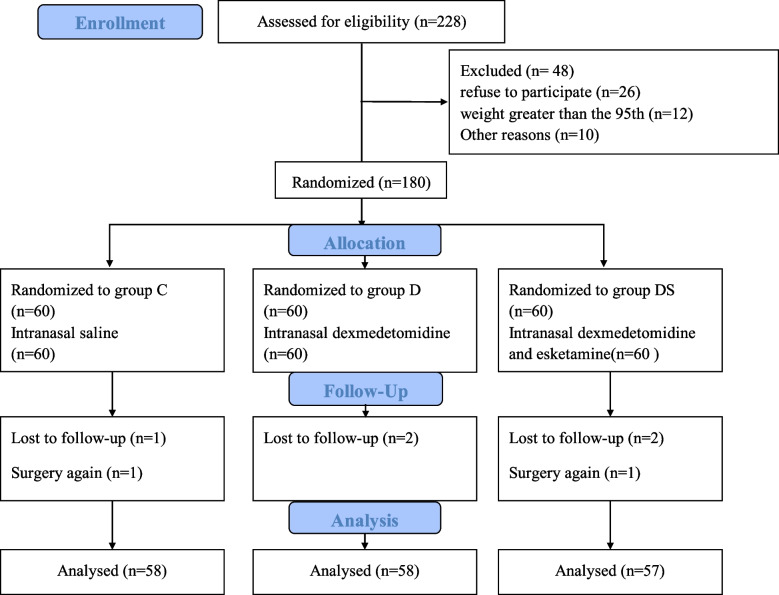
Between 1 July 2024 and 30 September 2024, 180 patients were recruited, 11 of whom were excluded from the analysis (2 patients underwent a second surgery, and 5 were lost to follow-up). Complete data sets were available for 173 participants (58 in the control group, 58 in the D group, and 57 in the DS group) (Fig. 1). Baseline characteristics of each group are presented in Table 1.
Fig. 1.
CONSORT flow diagram. CONSORT indicates Consolidated Standards of Reporting Trials. Group C: the control group; Group D: the dexmedetomidine group; Group DS: the combination group
Table 1.
Characteristics of patients
| Group C (N = 58) |
Group D (N = 58) |
Group DS (N = 57) |
SMD Group C vs Group D |
SMD Group C vs Group DS |
SMD Group D vs Group DS |
|
|---|---|---|---|---|---|---|
| Male | 42(72.4%) | 39(67.2%) | 38(66.7%) | 0.11 | 0.12 | 0.01 |
| Age (yr) | 5(4–7) | 6(4–6) | 6(4–6) | 0.03 | 0.07 | 0.12 |
| Weight (kg) | 21.00(19–25) | 22.75(18–25) | 20.58(20–25) | 0.11 | 0.10 | 0.00 |
| ASA physical status I, (n, %) | 56(96.6%) | 57(98.3%) | 57(100%) | 0.10 | 0.26 | 0.18 |
| Prioperative anxiety score | 28.3 (26.7–38.3) | 27.5(23.3–33.3) | 26.7(23.3–38.3) | 0.11 | 0.00 | 0.12 |
| Heart rate (times/min) | 96.72(12.13) | 94.91(12.08) | 95.65(10.37) | 0.14 | 0.09 | 0.07 |
| MAP (mmHg) | 80.97(8.21) | 82.68(8.03) | 81.83(8.69) | 0.20 | 0.11 | 0.11 |
| Duration of surgery (min) | 20(18–28) | 20(18–29) | 23(17–30) | 0.00 | 0.05 | 0.04 |
| Duration of anaesthesia (min) | 30(25–36) | 30(25–37) | 30(25–38) | 0.10 | 0.13 | 0.02 |
| Type of surgery (n, %) | ||||||
| Tonsillectomy and adenoidectomy | 50(86.2%) | 53(91.4%) | 51(89.5%) | 0.16 | 0.10 | 0.06 |
| Adenoidectomy | 6(10.3%) | 5(8.6%) | 5(8.8%) | 0.05 | 0.05 | 0.00 |
| Tonsillectomy | 2(3.5%) | 0 | 1(1.7%) | 0.26 | 0.10 | 0.18 |
Data are presented as the mean (SD), number (proportion), or median (inter-quartile range). Group C: the control group; Group D: the dexmedetomidine group; Group DS: the combination group
ASA physical status: American Society of Anesthesiologists physical status
MAP: mean arterial pressure; HR heart rates
SMD: Standardized mean difference
a: P < 0.017 vs Group C b: P < 0.017 vs Group D
Primary outcome
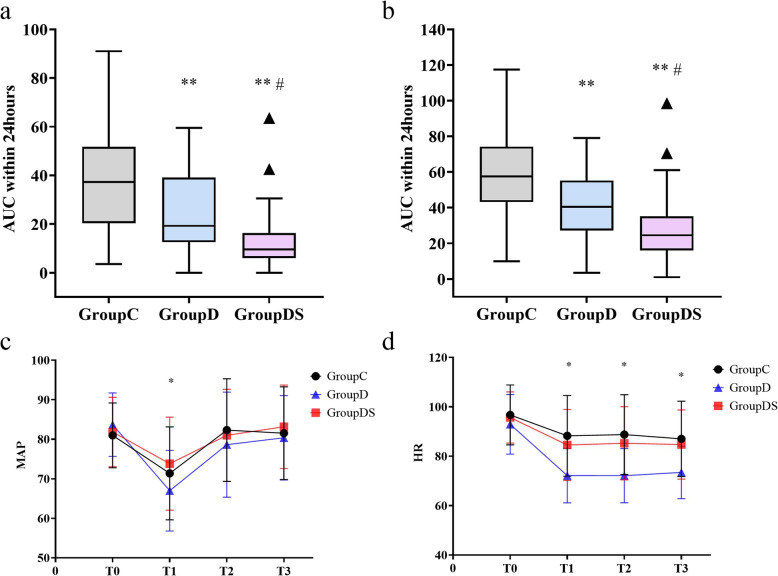
The primary outcome was the AUC of the 24-h postoperative pain score at rest. Compared with the control group, children in the dexmedetomidine group had lower 24-h pain scores [19.25 (12.50–39.13) vs. 37.25 (20.25–51.75), median difference (MD): −15; 95% CI: −22– −7; P < 0.001]. In contrast, children in the DS group had significantly lower 24-h postoperative pain scores than the children in the dexmedetomidine group [9.50 (9.00–16.25) vs. 19.25 (12.50–39.13), MD: −10; 95% CI: −17– −5; P < 0.001] (Table 2; Fig. 2.a). The 24-h pain while swallowing scores were also significantly different among groups (Table 2; Fig. 2.b). Generalised estimating equations revealed no interaction effect between time and group for the 24-h postoperative FLACC score (P = 0.082). However, the main effects analysis showed significant differences in FLACC scores across time points (P < 0.001). Similar results were observed for FLACC scores while swallowing (Supplementary Table S2).
Table 2.
The area under the curve (AUC) of FLACC scores of patients
| AUC of FLACC | Median difference (95% CI) | ||||||
|---|---|---|---|---|---|---|---|
| Group C (N = 58) |
Group D (N = 58) |
Group DS (N = 57) |
Group C vs Group D |
Group C vs Group DS |
Group D vs Group DS |
P-value | |
| At rest |
37.25 (20.25–51.75) |
19.25 (12.50–39.13) a |
9.50 (9.00–16.25)a,b |
15(7–22) | 26(20–32) | 10(5–17) | < 0.001 |
| During swallo-wing |
57.5 (43.13–74.25) |
40.5 (27.25–55.25) a |
24.5 (16.00–35.25)a,b |
18(10–27) | 32(25–40) | 15(8–21) | < 0.001 |
Data are presented as the median (inter-quartile range)
Group C the control group, Group D the dexmedetomidine group, Group DS the combination group
FLACC: the face, legs, activity, crying, consolability scale
a: P < 0.017 vs Group C
b: P < 0.017 vs Group D
Fig. 2.
Comparison of the AUC of FLACC scores (a) at rest and (b) during swallowing; Heart rate (c) and mean arterial pressure (MAP) (d) in three groups during operation; The box and whisker plots show medians, inter-quartile ranges, and outer ranges; The line chart shows the mean and standard deviation. MAP: mean arterial pressure; HR heart rates; Group C: the control group; Group D: the dexmedetomidine group; Group DS: the combination group. AUC: the area under the curve. **P < 0.017 vs Group C; #P < 0.017 vs Group D; *P < 0.05
Secondary outcomes
The GEE was used to compare the MAP or HR between groups (Supplemental Table S4; Supplementary Table S5; Fig. 2.c; Fig. 2.d). HRs in the DS (P < 0.001) and C (P < 0.001) groups were higher than those in the dexmedetomidine group.
The incidence of emergence delirium in the DS group (12.3%) was lower than in the control group (44.8%) (RR: 0.27; 95% CI: 0.13–0.58; P = 0.001), with no significant difference between the DS and the dexmedetomidine groups (25.9%) (P = 0.075). No significant difference was observed in the incidence of emergence delirium between the dexmedetomidine and control groups (P = 0.039). Time to extubation in the dexmedetomidine group was 21 (16–29) min, which was longer than in groups C (18 [12–23], P = 0.013) and DS (15 [13–21], P = 0.002). The PACU sedation score in the dexmedetomidine group was 2 (2–3), which was higher than in the DS group (2 [2-2], P < 0.001) and C group (2 [1, 2], P < 0.001), with no significant difference between the sedation scores in groups C and DS (P = 0.386). The incidence of remedial analgesia in the PACU was not significantly different among the three groups (P = 0.071). On postoperative day 1, the sleep disorder score (NRS) in the control group was higher than in the dexmedetomidine group (MD: 2; 95% CI: 1–2; P < 0.001) and DS groups (MD: 2; 95% CI: 1–3; P < 0.001), which were clinically significant (MD > 1). No cases of respiratory depression or laryngospasm were reported among the postoperative adverse events, and there were no differences in the incidence of the remaining adverse events among the groups (Table 3).
Table 3.
Other secondary outcomes of patients
| Group C (N = 58) | Group D (N = 58) | Group DS (N = 57) | P-value | |
|---|---|---|---|---|
| Intranasal sedation success patients (n, %) | / | 52(89.7%) | 52(91.2%) | |
| Time to achieve satisfactory sedation (min) | / | 22.41(2.47) | 18.65(2.15) | < 0.001 |
| ICC score | 3(2–5) | 2(1–2) a,b | 1(0–2) a | 0.001 |
| Consumption of remifentanil (ug·kg−1·min−1) | 0.35(0.30–0.45) | 0.35(0.26–0.45) | 0.31(0.27–0.40) | 0.081 |
| Incidence of emergence delirium (n, %) | 26(44.8%) | 15(25.9%) | 7(12.3%) | < 0.001 |
| Ramsay score | 2(1–2[1–5]) | 2(2–3 [1–5]) a | 2(2–2[1–4]) b | 0.001 |
| Extubation time (min) | 18(12–23) | 21(16–29) a | 15(13–21) b | 0.001 |
| Time out of PACU (min) | 16(15–20) | 20(16–22) a | 16(16–20) b | 0.001 |
| Duration of postoperative hospital stay (day) | 1.07(0.26) | 1.03(0.18) | 1.04(0.19) | 0.604 |
| Incidence of using fentanyl in PACU (n, %) | 12(20.7%) | 6(10.3%) | 4(7.0%) | 0.071 |
| Sleep quality on the first postoperative day | 5(4.00–6.00) | 3 (2.75–5.00) a | 3(2.00–4.00) a | < 0.001 |
| Adverse events (n, %) | ||||
| nausea and vomiting | 5(8.6%) | 3(5.2%) | 4(7%) | 0.708 |
| fever | 3(5.2%) | 4(6.9%) | 4(7%) | 0.902 |
| increased secretions | 4(6.9%) | 3(5.2%) | 6(10.5%) | 0.545 |
| nightmares | 2(3.4%) | 2(3.4%) | 5(8.8%) | 0.333 |
Data are presented as the mean (SD), number (proportion), or median (inter-quartile range) or median (inter-quartile range[range])
Group C the control group, Group D the dexmedetomidine group, Group DS the combination group
ICC: Induction Compliance Checklist
a: P < 0.017 vs Group C
b: P < 0.017 vs Group D
The pain score in the control group was higher than in the DS group on postoperative days 3 (MD: 2; 95% CI: 1–2; P < 0.001) and 7 (MD: 2; 95% CI: 1–3; P < 0.001) (Table 4).
Table 4.
PPPM scores of patients after hospital discharge
| PPPM scores | Median difference (95% CI) | ||||||
|---|---|---|---|---|---|---|---|
| Group C (N = 58) |
Group D (N = 58) |
Group DS (N = 57) |
Group C vs Group D |
Group C vs Group DS |
Group D vs Group DS |
P-value | |
| Day3 | 5(3–5) | 3(3–5) a | 3(2–3) a | 0(0,2) | 2(1,2) | 0(0,1) | < 0.001 |
| Day7 | 3(2–3) | 2(0–3[0–5) a | 0(0–2) a, b | 1(0,1) | 2(1,3) | 0(0,1) | < 0.001 |
| Month 1 | 0(0–1) | 0(0–0[0–2]) | 0(0–0) | 0(0,0) | 0(0,0) | 0(0,0) | 0.098 |
| Month 3 | 0(0–0) | 0(0–0) | 0(0–0) | 0(0,0) | 0(0,0) | 0(0,0) | 0.369 |
Data are presented as median (inter-quartile range)
Group C the control group, Group D the dexmedetomidine group, Group DS the combination group
PPPM: the parents postoperative pain measure scale
a: P < 0.017 vs Group C
b: P < 0.017 vs Group D
Discussion
In this study, we found that in children who underwent tonsillectomy and/or adenoidectomy, preoperative sedation with a combination of esketamine and dexmedetomidine resulted in lower pain scores within 24 h postoperatively. It also reduced the incidence of ED, improved postoperative sleep quality and was associated with fewer side effects.
Tonsillectomy is one of the most common surgical procedures performed in children. It is a day surgery requiring only a short postoperative hospital stay, with most children discharged on the second day after the procedure. However, pain can last for up to two weeks after surgery. Inadequately management often intensifies pain and therefore increases the risk of short- and long-term complications, such as emergence delirium, agitation, prolonged adverse behavioural changes and chronic pain. These outcomes can affect children’s ability to perform routine daily activities, such as eating, drinking, and sleeping, after surgery [18–20].
In this randomised controlled trial, preoperative dexmedetomidine administration relieved pain during the first 24 h after surgery, while dexmedetomidine combined with esketamine resulted in even lower pain scores. These findings are consistent with previous studies [12, 16]. Those studies also reported that dexmedetomidine can relieve postoperative pain, and the pain scores were lower when dexmedetomidine was combined with esketamine. However, we are the first to evaluate the effects of these two drugs as preoperative medications for managing postoperative pain in children, which may reduce the need for additional analgesics. Dexmedetomidine reduces pain by stimulating α−2 receptors in the dorsal horn of the spinal cord, thereby preventing injury to neurons and reducing the release of substance P [21]. Esketamine inhibits the activation of downstream signalling pathways by inhibiting NMDA receptors and blocking incoming pain signals from the reticular pathway of the spinal cord [22]. We believe these two drugs reduce pain together by lowering the body's response to pain via different pathways.
The sedative–hypnotic effects of dexmedetomidine are mediated by binding to α−2A receptors, which inhibit norepinephrine release and decrease activity in the upstream noradrenergic pathway. This negative feedback loop suppresses sympathetic responses, reducing heart rate and blood pressure [23]. Children have higher vagal tone and are thus more prone to bradycardia. Therefore, for children receiving dexmedetomidine, measures should be taken to relieve circulatory depression without reducing its sedative effect. In contrast, esketamine inhibits NMDA receptors and suppresses cardiac parasympathetic activity [24]. As a result, esketamine increases heart rate and blood pressure. This explains why heart rates were consistently higher in the DS group than in the dexmedetomidine group in this trial.
We also found that, in terms of sedative effect, the combined use of esketamine and dexmedetomidine resulted in a faster onset of sedation and better induction compliance compared to dexmedetomidine alone. This finding is inconsistent with a previous randomised controlled study [7], in which no significant difference in onset time was found between groups. This discrepancy may be due to our use of a higher dose of esketamine (0.6 mg·kg−1 esketamine vs. 0.5 mg·kg−1), thus accelerating the sedation onset and improving induction compliance. In addition, we chose inhalation induction anaesthesia once a good sedative effect was achieved. This enabled the children to transition from a sedated state to an anaesthetised state, avoiding invasive procedures such as opening venous access, which may stimulate the children. As a result, there was better induction compliance and improved anaesthesia experience for the children.
We also observed that esketamine mitigated the delayed sedation associated with dexmedetomidine. In a basic study, researchers reported that esketamine may promote awakening from isoflurane general anaesthesia in mice by increasing calcium signalling in PVT glutamatergic (PVT Glu) neurons during the awakening period and shortening the time to peak calcium signalling [25]. The terminal elimination half-life of dexmedetomidine is approximately 2 h, which can prolong sedation and delay extubation times for patients undergoing short daytime procedures. In this trial, the time to extubation was longer in the dexmedetomidine group than in the DS group, and the time to exit the recovery room was also longer in the dexmedetomidine group. Similarly, sedation scores during the PACU stay were higher in the dexmedetomidine group than in the DS and C groups, indicating a deeper level of sedation and therefore resulting in a longer time to discharge from the PACU. In contrast, dexmedetomidine combined with esketamine achieved a faster onset of sedation and ensured better cooperation during induction than dexmedetomidine alone. Therefore, we believe that esketamine, when combined with dexmedetomidine, reduces the side effects of dexmedetomidine without affecting the sedative effect.
Dexmedetomidine can reduce the incidence of emergency delirium (ED) after sevoflurane anaesthesia. Our findings are consistent with previous studies [26]. Notably, the incidence of ED was lower in the DS group than in the dexmedetomidine group in our study, suggesting that low-dose esketamine may reduce the incidence of postoperative ED in children. A prospective study reported that the incidence of postoperative delirium in preschool children was approximately 23.8% in the control group, while it was 20% for those infused with 0–0.3 mg·kg−1 esketamine and 41% for those infused with a medium dose (> 0.3, ≤ 0.5 mg·kg−1), suggesting that we should be aware of the dose-related psychiatric side effects of esketamine in paediatric patients [27]. Therefore, we selected a dose that was unlikely to cause ED. The esketamine dose we chose was 0.6 mg·kg−1, as the bioavailability of esketamine intranasal drops is approximately 50% [28]. In our study, although the incidence of ED was lower in the DS group than in Group D, it was not statistically significant. This may be due to our limited sample size, as we calculated that at least 128 patients per group would be needed to find a significant difference between the two groups. More large-sample, multicentre studies are required to determine whether esketamine can reduce the incidence of emergence delirium in children and to establish the optimal dosage and safety profile.
Dexmedetomidine can improve postoperative sleep quality in patients with chronic insomnia as well as those in the ICU. Additionally, esketamine has also been found to improve sleep quality after gynaecological surgery [29, 30]. The combination of dexmedetomidine–esketamine for postoperative analgesia improved sleep quality in patients following scoliosis correction surgery [16]. This combination regimen for sleep quality in paediatric patients was consistent with our hypothesis. Unfortunately, we did not use a scale specifically validated for assessing postoperative sleep quality in children aged 3–7 years, and NRS scores were determined through a combination of children’s self-reports and parents’ assessments. Therefore, this outcome requires further validation using a more authoritative scale.
Unfortunately, we did not observe a significant difference in pain among the three groups at the 3-month postoperative follow-up because this time point may mark the beginning of chronic pain. This may relate to the low incidence of chronic pain in children who underwent tonsillectomy and/or adenoidectomy, as only approximately 7.5% of children continued to experience significant pain during the second postoperative week [1]. Our sample size was insufficient to test for significance.
There are several limitations. First, this was a single-centre study involving children undergoing elective tonsillectomy and/or adenoidectomy, which limits the generalisability of the findings. Second, we did not investigate additional dosage combinations to ascertain the optimal combination. Third, 2 μg·kg−1 intranasal dexmedetomidine has been shown to reduce the incidence of perioperative respiratory adverse events (PRAEs) in children12. Few previous studies have examined the effects of esketamine on PRAEs. Esketamine antagonises muscarinic receptors, thus increasing upper airway secretions. Compared with 2 μg·kg−1 dexmedetomidine, we reduced the dexmedetomidine dose and added a low dose of esketamine. Despite adequate suctioning and comprehensive assessment before extubation, there is still a risk of PRAEs. Therefore, future studies are needed to determine whether this combination increases the incidence of PRAEs.
Conclusion
In this study, we found that in preschool children who underwent tonsillectomy and/or adenoidectomy, the combination of esketamine and dexmedetomidine for preoperative sedation resulted in lower pain scores within 24 h postoperatively, reduced the incidence of ED, and was associated with fewer side effects.
Supplementary Information
Acknowledgements
JWQ and CL contributed equally to this work. Junwei Qi: This author helped to design the study, analyse statistical, write, draft, and revise of the manuscript; Chuang Li: This author helped to collect data and revise of the manuscript; Xin-Yuan Qiu, Hua Yin, Xin-Ge Wen, Qing-Ling Meng: These author helped to collect data and analyse statistical; Qian Zhang, Li Li:These author helped to collect data; Yue-ying Zhang: This author helped to design the study and writing, drafting, and revision of the manuscript. The study was approved by the Medical Ethics Committee and conducted in the Children's Hospital of Xuzhou. This research did not receive any specific grant from funding agencies in the public, commercial, or not- for- profit sectors. No other competing interests declared.
Authors’ contributions
JWQ and CL contributed equally to this work. Junwei Qi: This author helped to design the study, analyse statistical, write, draft, and revise of the manuscript; Chuang Li: This author helped to collect data and revise of the manuscript; Xin-Yuan Qiu, Hua Yin, Xin-Ge Wen, Qing-Ling Meng: These author helped to collect data and analyse statistical; Qian Zhang, Li Li:These author helped to collect data; Yue-ying Zhang: This author helped to design the study and writing, drafting, and revision of the manuscript.
Funding
The authors have no sources of funding to declare for this manuscript.
Data availability
Article-related datasets are stored in the body manuscript and in supplementary date files in the submission system. Readers are encouraged to consult the article-related datasets themselves after publication.
Declarations
Ethics approval and consent to participate
This controlled trial was approved by the Medical Ethics Committee of the Children’s Hospital of Xuzhou (2024–05-13-H13; January 31, 2024) and registered at the Chinese Clinical Trial Registration Centre (ChiCTR2400085642; June 14, 2024). The implementation of this trial followed CONSORT guidelines. The study complied with the ethical standards set out in the 1964 Declaration of Helsinki. We obtained written informed consent from each participant's parent or guardian.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jun-wei Qi and Chuang Li contributed equally to this work.
References
- 1.Fortier MA, MacLaren JE, Martin SR, Perret-Karimi D, Kain ZN. Pediatric Pain After Ambulatory Surgery: Where’s the Medication? Pediatrics. 2009;124:e588–95. [DOI] [PubMed] [Google Scholar]
- 2.Moura LAD, Pereira LV, Minamisava R, Borges NDC, Castral TC, Souza LAF. Severe acute postoperative pain self-reported by children after ambulatory surgeries: a cohort study. Rev Bras Enferm. 2021;74:e20200151. [DOI] [PubMed] [Google Scholar]
- 3.Zieliński J, Morawska-Kochman M, Zatoński T. Pain assessment and management in children in the postoperative period: A review of the most commonly used postoperative pain assessment tools, new diagnostic methods and the latest guidelines for postoperative pain therapy in children. Adv Clin Exp Med. 2020;29:365–74. [DOI] [PubMed] [Google Scholar]
- 4.Lee CS, Merchant S, Chidambaran V. Postoperative Pain Management in Pediatric Spinal Fusion Surgery for Idiopathic Scoliosis. Pediatr Drugs. 2020;22:575–601. [DOI] [PubMed] [Google Scholar]
- 5.Einhorn LM, Hoang J, La JO, Kharasch ED. Single-dose Intraoperative Methadone for Pain Management in Pediatric Tonsillectomy: A Randomized Double-blind Clinical Trial. Anesthesiology. 2024;141:463–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu F, Kong F, Zhong L, Wang Y, Xia Z, Wu J. Preoperative Esketamine Alleviates Postoperative Pain after Endoscopic Plasma Adenotonsillectomy in Children. Clin Med Res. 2023;21:79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu X, Tang L, Lan H, Li C, Lin H. A Comparison of Intranasal Dexmedetomidine, Esketamine or a Dexmedetomidine-Esketamine Combination for Induction of Anaesthesia in Children: A Randomized Controlled Double-Blind Trial. Front Pharmacol. 2022;12:808930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yao J, Gong H, Zhao X, Peng Q, Zhao H, Yu S. Parental presence and intranasal dexmedetomidine for the prevention of anxiety during anesthesia induction in children undergoing tonsillectomy and/or adenoidectomy surgery: A randomized controlled trial. Front Pharmacol. 2022;13:1015357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee-Archer PF, Von Ungern-Sternberg BS, Reade M, et al. The effect of dexmedetomidine on postoperative behaviour change in children: a randomised controlled trial. Anaesthesia. 2020;75:1461–8. [DOI] [PubMed] [Google Scholar]
- 10.Li L-Q, Wang C, Xu H-Y, Lu H-L, Zhang H-Z. Effects of different doses of intranasal dexmedetomidine on preoperative sedation and postoperative agitation in pediatric with total intravenous anesthesia undergoing adenoidectomy with or without tonsillectomy. Medicine. 2018;97:e12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang J, Zeng J, Zhao N, et al.: Intranasal esketamine combined with oral midazolam provides adequate sedation for outpatient pediatric dental procedures: a prospective cohort study. International Journal of Surgery 2023; Publish Ahead of Print [DOI] [PMC free article] [PubMed]
- 12.Shen F, Zhang Q, Xu Y, et al. Effect of Intranasal Dexmedetomidine or Midazolam for Premedication on the Occurrence of Respiratory Adverse Events in Children Undergoing Tonsillectomy and Adenoidectomy: A Randomized Clinical Trial. JAMA Netw Open. 2022;5:e2225473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olofsen E, Sigtermans M, Noppers I, et al. The Dose-Dependent Effect of S(+)-Ketamine on Cardiac Output in Healthy Volunteers and Complex Regional Pain Syndrome Type 1 Chronic Pain Patients. Anesth Analg. 2012;115:536–46. [DOI] [PubMed] [Google Scholar]
- 14.Sigtermans M, Dahan A, Mooren R, et al. S(+)-ketamine Effect on Experimental Pain and Cardiac Output. Anesthesiology. 2009;111:892–903. [DOI] [PubMed] [Google Scholar]
- 15.Li H, Liu K, Yao L. Dexmedetomidine in combination with ketamine for pediatric procedural sedation or premedication: A meta-analysis. Am J Emerg Med. 2021;50:442–8. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Cui F, Ma J-H, Wang D-X. Mini-dose esketamine–dexmedetomidine combination to supplement analgesia for patients after scoliosis correction surgery: a double-blind randomised trial. Br J Anaesth. 2023;131:385–96. [DOI] [PubMed] [Google Scholar]
- 17.Myles PS, Myles DB, Galagher W, et al. Measuring acute postoperative pain using the visual analog scale: the minimal clinically important difference and patient acceptable symptom state. Br J Anaesth. 2017;118:424–9. [DOI] [PubMed] [Google Scholar]
- 18.Lee S-J, Sung T-Y. Emergence agitation: current knowledge and unresolved questions. Korean J Anesthesiol. 2020;73:471–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stargatt R, Davidson AJ, Huang GH, et al. A cohort study of the incidence and risk factors for negative behavior changes in children after general anesthesia. Pediatr Anesth. 2006;16:846–59. [DOI] [PubMed] [Google Scholar]
- 20.Vierola A, Suominen AL, Eloranta A-M, et al. Determinants for craniofacial pains in children 6–8 years of age: the PANIC study. Acta Odontol Scand. 2017;75:453–60. [DOI] [PubMed] [Google Scholar]
- 21.Virtanen R, Savola J-M, Saano V, Nyman L: Characterization of the selectivity, specificity and potency of medetomidine as an a2-adrenoceptor agonist.Eur J Pharmacol. 1988;150(1-2):9–14. [DOI] [PubMed]
- 22.Mion G, Villevieille T. Ketamine Pharmacology: An Update ( Pharmacodynamics and Molecular Aspects, Recent Findings ). CNS Neurosci Ther. 2013;19:370–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giovannitti JA, Thoms SM, Crawford JJ. Alpha-2 Adrenergic Receptor Agonists: A Review of Current Clinical Applications. Anesth Prog. 2015;62:31–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frank JG, Mendelowitz D. Synaptic and Intrinsic Activation of GABAergic Neurons in the Cardiorespiratory Brainstem Network. PLoS ONE. 2012;7:e36459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duan W-Y, Peng K, Qin H-M, et al. Esketamine accelerates emergence from isoflurane general anaesthesia by activating the paraventricular thalamus glutamatergic neurones in mice. Br J Anaesth. 2024;132:334–42. [DOI] [PubMed] [Google Scholar]
- 26.Shi M, Miao S, Gu T, Wang D, Zhang H, Liu J. Dexmedetomidine for the prevention of emergence delirium and postoperative behavioral changes in pediatric patients with sevoflurane anesthesia: a double-blind, randomized trial. DDDT. 2019;13:897–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen S, Yang J-J, Zhang Y, et al. Risk of esketamine anesthesia on the emergence delirium in preschool children after minor surgery: a prospective observational clinical study. Eur Arch Psychiatry Clin Neurosci. 2024;274:767–75. [DOI] [PubMed] [Google Scholar]
- 28.Jonkman K, Duma A, Olofsen E, et al. Pharmacokinetics and Bioavailability of Inhaled Esketamine in Healthy Volunteers. Anesthesiology. 2017;127:675–83. [DOI] [PubMed] [Google Scholar]
- 29.Wu J, Liu X, Ye C, Hu J, Ma D, Wang E. Intranasal dexmedetomidine improves postoperative sleep quality in older patients with chronic insomnia: a randomized double-blind controlled trial. Front Pharmacol. 2023;14:1223746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qiu D, Wang X-M, Yang J-J, et al. Effect of Intraoperative Esketamine Infusion on Postoperative Sleep Disturbance After Gynecological Laparoscopy: A Randomized Clinical Trial. JAMA Netw Open. 2022;5: e2244514. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Article-related datasets are stored in the body manuscript and in supplementary date files in the submission system. Readers are encouraged to consult the article-related datasets themselves after publication.