Abstract
Cytochrome c is often released from mitochondria during the early stages of apoptosis, although the precise mechanisms regulating this event remain unclear. In this study, with isolated liver mitochondria, we demonstrate that cytochrome c release requires a two-step process. Because cytochrome c is present as loosely and tightly bound pools attached to the inner membrane by its association with cardiolipin, this interaction must first be disrupted to generate a soluble pool of this protein. Specifically, solubilization of cytochrome c involves a breaching of the electrostatic and/or hydrophobic affiliations that this protein usually maintains with cardiolipin. Once cytochrome c is solubilized, permeabilization of the outer mitochondrial membrane by Bax is sufficient to allow the extrusion of this protein into the extramitochondrial environment. Neither disrupting the interaction of cytochrome c with cardiolipin, nor permeabilizing the outer membrane with Bax, alone, is sufficient to trigger this protein's release. This mechanism also extends to conditions of mitochondrial permeability transition insofar as cytochrome c release is significantly depressed when the electrostatic interaction between cytochrome c and cardiolipin remains intact. Our results indicate that the release of cytochrome c involves a distinct two-step process that is undermined when either step is compromised.
Mitochondria play a central role in the initiation of apoptosis. Specifically, the release of different proteins that are usually present in the intermembrane space of these organelles has been observed during the early stages of apoptotic cell death. Among these proteins are apoptosis-inducing factor, adenylate kinase-2 (AK-2), Smac/DIABLO, and cytochrome c (1–4). Once in the cytosol, cytochrome c interacts with its adaptor molecule, Apaf-1, resulting in the processing and activation of pro-caspase-9 in the presence of dATP (5). Caspase-9, in turn, cleaves and activates pro-caspase-3 and -7; these effector caspases are responsible for the cleavage of various proteins leading to biochemical and morphological features characteristic of apoptosis (6).
Release of cytochrome c from mitochondria is therefore considered a key initial step in the apoptotic process, although the precise mechanisms regulating this event remain elusive. With isolated liver mitochondria, we demonstrated that cytochrome c release occurs by distinct mechanisms that are either Ca2+-dependent or Ca2+-independent (7). In the first case, mitochondrial Ca2+ overload promotes the opening of the permeability transition pore. This increased permeability of the inner mitochondrial membrane leads to matrix swelling, rupture of the outer mitochondrial membrane, and the release of cytochrome c. Ca2+-independent cytochrome c release seems to be governed by different members of the Bcl-2 family of proteins. In particular, the oligomeric form of the pro-apoptotic protein Bax stimulates cytochrome c release, although the precise way in which this protein permeabilizes the outer mitochondrial membrane remains unclear.
Cytochrome c is bound to the inner membrane by anionic phospholipids, primarily cardiolipin. This binding involves at least two conformations: (i) a loosely bound conformation provided by electrostatic—i.e., ion–ion interaction with positively charged lysine residues of cytochrome c and negatively charged phosphate groups of cardiolipin (8)—and (ii) a tightly bound conformation wherein hydrophobic interactions accompany a loosening of the tertiary structure, resulting in partial embedding of the protein into the membrane (9, 10). An alternate model for the tightly bound conformation has been proposed such that the hydrophobic interaction between an expanded acyl chain of cardiolipin and a hydrophobic inlet of cytochrome c anchors the protein to the membrane (11). In either case, because of its association with cardiolipin, it seems that permeabilization of the outer membrane, alone, would be insufficient to stimulate the release of cytochrome c. In other words, a disruption of the cytochrome c-cardiolipin interaction would seem to have to occur before, or concomitantly with, permeabilization of the outer membrane in order for cytochrome c to be released from mitochondria.
To that end, this study demonstrates that cytochrome c release from isolated rat liver mitochondria occurs by a two-step process, first involving the detachment of this protein from the inner membrane, followed by permeabilization of the outer membrane and the release of cytochrome c into the extramitochondrial milieu. In addition, our data suggest that, depending on the detachment stimulus, two distinct pools of cytochrome c can be mobilized. The first pool is sensitive to electrostatic alterations that can be elicited by changes in ionic strength, surface-charge density, or pH (11, 12) and thus most likely reflects cytochrome c present in the loosely bound conformation. The second pool can be mobilized by oxidative modification of mitochondrial lipids, specifically cardiolipin, and therefore likely represents tightly bound cytochrome c that is detached because of disturbances in membrane structure.
Materials and Methods
Isolation of Rat Liver Mitochondria.
Male Harlan Sprague–Dawley rats (6–8 weeks old) were killed by CO2 inhalation in accordance with the European directive of protection of vertebrate animals for scientific research. The liver was minced on ice, resuspended in 50 ml of MSH buffer (210 mM mannitol/70 mM sucrose/5 mM Hepes, pH 7.5) supplemented with 1 mM EDTA, and homogenized with a glass Dounce homogenizer and Teflon pestle. Homogenates were centrifuged at 600 × g for 8 min at 4°C. The supernatant was decanted and recentrifuged at 5,500 × g for 15 min to form a mitochondrial pellet that was resuspended in MSH buffer without EDTA and centrifuged again at 5,500 × g for 15 min. The final mitochondrial pellet was resuspended in MSH buffer at a protein concentration of 80–100 mg/ml.
Measurement of Functional Activity of Isolated Mitochondria.
Mitochondria (1 mg/ml) were incubated in MSH buffer, mixed buffer (100 mM KCl/100 mM sucrose/5 mM Tris⋅HCl, pH 7.4) or KCl buffer (150 mM KCl/5 mM Tris⋅HCl, pH 7.4) with continuous stirring to avoid anoxia. Mitochondria were energized with 5 mM succinate, and rotenone (2 μM) was added to maintain pyridine nucleotides in a reduced form. For experiments involving antimycin A, mitochondria were energized with 1 mM ATP. EGTA (1 mM) and the absence of Pi assured non-mitochondrial permeability transition (MPT) conditions. For the MPT experiments, EGTA was omitted from the buffers, and 150 nmol of Ca2+ per mg of protein and 5 mM Pi were used to induce swelling. Estimation of Δψ was performed by using an electrode sensitive to the lipophilic cation tetraphenylphosphonium (TPP+). Energized mitochondria rapidly accumulate TPP+ from the incubation buffer and release this cation as Δψ decays. Ca2+ fluxes across the inner mitochondrial membrane were monitored by using a Ca2+-sensitive electrode (model 97-20, Orion Research, Beverly, MA). Mitochondrial swelling was monitored continuously as changes in OD540. At the end of the incubation period, mitochondrial suspensions were centrifuged at 10,000 × g for 5 min and the resulting supernatants and/or pellets were used for Western blot analysis. Fresh mitochondria were prepared for each experiment and used within 4 h.
Western Blot Analysis.
Samples were mixed with Laemmli's loading buffer, boiled for 5 min, and subjected to SDS/15% PAGE at 130 V followed by electroblotting to nitrocellulose membranes for 2 h at 100 V. Membranes were blocked for 1 h with 5% nonfat milk in PBS at room temperature and subsequently probed overnight with an anti-cytochrome c (1:2,500) or anti-AK-2 (1:2,000) antibody. The membranes were rinsed and incubated with a horseradish-peroxidase-conjugated secondary antibody (1:10,000). After the secondary antibody incubation, the membranes were rinsed, and bound antibodies were detected by using enhanced chemiluminescence according to the manufacturer's instructions. In all instances, gels were loaded with the supernatant or pellet obtained from 30 μg of mitochondrial protein, and individual band densities were integrated by using QUANTISCAN software (Biosoft, Cambridge, U.K.).
Expression and Purification of Bax.
Expression and purification of full-length Bax protein was performed as described (13). The full-length human Bax cDNA sequence was amplified by standard PCR techniques. The PCR DNA fragment was isolated by using the QIAquick kit (Qiagen, Chatsworth, CA) and subcloned into the NcoI and HindIII sites of the plasmid pBAD. The plasmid was transformed into Escherichia coli and transformants were isolated by selection for ampicillin resistance. Cultures of the resistant colony were grown to an OD650 of ≈7. After induction, the culture was further incubated for several hours and cells were harvested by centrifugation. The cells were resuspended in lysis buffer (100 mM Hepes-NaOH, pH 8.0/100 mM NaCl/1 mM MgCl2/0.1% 2-mercaptoethanol/1% Triton X-100, a mixture of protease inhibitors, 30 μg/ml DNase I and 50 μg/ml lysozyme) and broken by sonication. After centrifugation, Bax was recovered in the supernatant. Because the protein was expressed with a His tag at the N terminus, the purification by affinity chromatography on Ni-NTA-agarose (Qiagen) followed by ion-exchange chromatography on Q-Sepharose (Amersham Pharmacia) was performed according to the manufacturer's instructions. The protein was at least 95.8% pure as determined by SDS/PAGE, and it could be concentrated up to 0.4 mg/ml in the presence of 1% octyl glucoside. The used concentrations of this detergent did not interfere with mitochondrial functional parameters.
Lipid Extraction and Standards.
Lipids were extracted by using a slightly modified version of a method described (14). Mitochondria (2 mg protein) were incubated in 150 mM KCl buffer for 13 min and centrifuged at 10,000 × g for 5 min; the pellet was resuspended in 2 ml of methanol and sonicated. Next, chloroform (4 ml) was added and the samples were stirred overnight at 4°C. The next morning, samples were filtered and dried under a stream of nitrogen. Lipids were redissolved in 100 μl chloroform/methanol (2:1), and 10-μl aliquots were subjected to high-performance liquid chromatography (HPLC) analysis. Another aliquot was used for Pi analysis. Lipid standards were purchased from Sigma, and commercial cardiolipin was oxidized overnight at 37°C (15). The resulting peaks revealed a distribution similar to the distribution reported by Parinandi et al. (16).
HPLC.
The HPLC system consisted of two Waters 501 pumps connected to a Waters automate gradient controller. The separation was performed on a Silice Uptisphere 120A 5-μm column (Interchim, Montluçon, France) maintained at 37°C. The absorption of the eluate was monitored either at 205 nm (double bonds, attenuation 128) or 234 nm (conjugated dienes, attenuation 32) with a Waters 484 multiwavelength detector. Data were analyzed with a Waters 740 data module by using a tailing peak command for the cardiolipin hydroperoxide (ClOOH) peak. All solvents were HPLC grade. Solvent A consisted of hexane/2-propanol (3:2), 3.5% H2O, and 125 μM ammonium sulfate. Solvent B consisted of hexane/2-propanol (3:2), 5.5% H2O, and 250 μM ammonium sulfate. Total flow rate was 1.5 ml/min and the gradient was as follows: 1–15 min, 100% A; 15–20min, 100–0% A; 20–35min, 0% A; 35–37min, 0–100% A. After each sample, the column was reequilibrated for 15 min with 100% A. With this gradient, all lipids were successfully eluted from the column. Significant baseline separation of the hydroperoxide peaks (234 nm) was achieved, and data were expressed as ClOOH peak area (234 nm) per injected Pi.
Statistical Analysis.
Data are expressed as means ± SD, and significance was determined by using a Student's t test. A value of P < 0.05 was considered to be significant.
Results and Discussion
By now, few investigators would challenge the notion of cytochrome c release being a key event during the early stages of apoptosis in response to various chemical triggers. Recent findings have implicated a role for cardiolipin in cytochrome c release (15, 17, 18). In particular, cardiolipin was reported to provide specificity for the targeting of a pro-apoptotic Bcl-2 protein, truncated Bid, to mitochondria, although the precise effect this event had on the cytochrome c–cardiolipin interaction was not addressed (17). A different study suggested that peroxidation of cardiolipin might induce the detachment of cytochrome c from the inner mitochondrial membrane, because overexpression of mitochondrial phospholipid hydroperoxide glutathione peroxidase suppressed this event (15). However, details regarding the mechanism by which “free” cytochrome c was released from the outer mitochondrial membrane were not described (15). Thus, the current study set out to clarify the steps required for cytochrome c release into the extramitochondrial environment.
Loosely Bound Cytochrome c Can Be Mobilized by Electrostatic Alterations.
An initial aim for this study was to understand the ability of electrostatic changes to influence cytochrome c release from mitochondria. Recombinant oligomeric Bax protein was used as a trigger because we had already demonstrated the ability of this protein to stimulate cytochrome c release in the absence of MPT. To ensure further that MPT was not contributing to cytochrome c release, experiments were performed under strict non-MPT conditions. Specifically, EGTA was used to chelate all available Ca2+, rotenone was used to keep pyridine nucleotides in a reduced state, and Pi was excluded from the incubation buffers. Under these conditions, large-amplitude swelling was not observed on the addition of recombinant oligomeric Bax to isolated liver mitochondria (Fig. 1a).
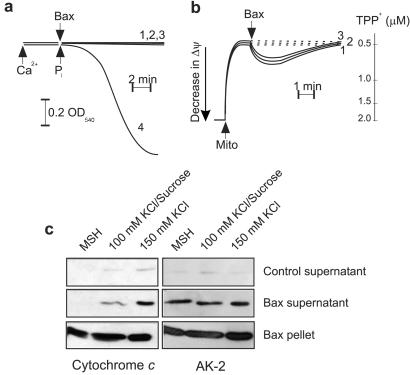
Figure 1.
Alterations in the electrostatic interaction between cytochrome c and cardiolipin influences Bax-induced cytochrome c release from isolated mitochondria. (a and b) Mitochondria (1 mg/ml protein) were incubated under strict non-MPT (described in text) conditions in either 150 mM KCl (trace 1), 100 mM KCl/sucrose (trace 2), or MSH (trace 3) buffer as described under Materials and Methods, and Bax (10 μg/ml) was added after a 5-min stabilization period. (a) Mitochondrial swelling was monitored continuously at 540 nm. A typical swelling curve induced by 150 nmol of Ca2+ per mg of protein and 5 mM Pi is shown as a positive control (trace 4). (b) Alternatively, with use of a TPP+-sensitive electrode, changes in Δψ of mitochondria incubated in the three different buffers were evaluated, after the addition of Bax. (c) Eight minutes after the addition of Bax, samples were centrifuged, and the resulting supernatants separated by SDS/PAGE and Western-blotted as described in Materials and Methods.
Because only the soluble form of cytochrome c can be released, both loosely and tightly bound proteins must be converted to a soluble form before being released by outer membrane permeabilization. As seen in Fig. 1c, disrupting the electrostatic interaction by increasing the ionic strength of the incubation buffer had a profound influence on Bax-induced cytochrome c release. In the low ionic strength MSH buffer, no release of cytochrome c was stimulated by the addition of recombinant Bax protein. Approximately 9% of cytochrome c was released when Bax-treated mitochondria were incubated in a buffer containing 100 mM KCl. However, Bax was most effective, triggering the release of ≈19% of cytochrome c, when mitochondria were incubated in a buffer containing 150 mM KCl (an ionic composition classically used to detach loosely bound cytochrome c from the inner membrane) (9). To exclude the possibility that the observed effect could be caused by an enhanced pore forming activity of Bax in the different buffers, we investigated the release of a non-membrane-bound intermembrane space protein, AK-2. As evidenced from Fig. 1c, the different incubation buffers had no effect on AK-2 release stimulated by Bax. When the mitochondrial membrane potential was estimated with a TPP+-sensitive electrode (Fig. 1b), it was determined that the addition of Bax caused a submaximal drop in mitochondrial membrane potential (Δψ) that was partially restored after 5 min, an effect that could be mimicked with 0.01% digitonin (data not shown). In addition, this effect was similar among mitochondria incubated in the different incubation buffers and thus did not correlate with the amount of cytochrome c released. Taken together, it was concluded that the amount of cytochrome c released under these conditions was largely, if not exclusively, determined by the degree to which the electrostatic interaction between cytochrome c and cardiolipin was disrupted.
Tightly Bound Cytochrome c Can Be Mobilized by Oxidative Stress.
To test whether an oxidative stress could be involved in cytochrome c release in our system, experiments were performed with well characterized inducers of reactive oxygen species (ROS) production. Moreover, to determine whether an oxidative stress would target a pool of cytochrome c distinct from the pool that was mobilized by electrostatic changes, we used 150 mM KCl buffer to first detach loosely bound cytochrome c from the membrane. Fig. 2 a–c shows that, after adding Bax to isolated mitochondria, a portion of cytochrome c was released. This release was increased by ≈85% by either the ROS-generating system Fe(II)SO4/ascorbate (Fe2+/Asc) or the addition of cumene hydroperoxide, an effect that was mitigated by the inclusion of the antioxidant butylated hydroxytoluene (Fig. 2 a and b, and data not shown). Furthermore, when ROS were produced endogenously by the addition of antimycin A, an inhibitor of complex III of the electron transport chain, a similar enhanced release of cytochrome c was observed (Fig. 2c). Cytochrome c release was not observed without the addition of Bax, indicating that oxidation of cardiolipin (15) alone is not sufficient to stimulate the release of cytochrome c (Fig. 2 a–c). In agreement with this finding, an increased oxidative stress alone did not trigger MPT, as evidenced by the absence of any changes in organelle volume during the incubation period (Fig. 2d). When the release of AK-2 under the same conditions was investigated, no differences from those results obtained in Fig. 1c were detected (Fig. 2e).
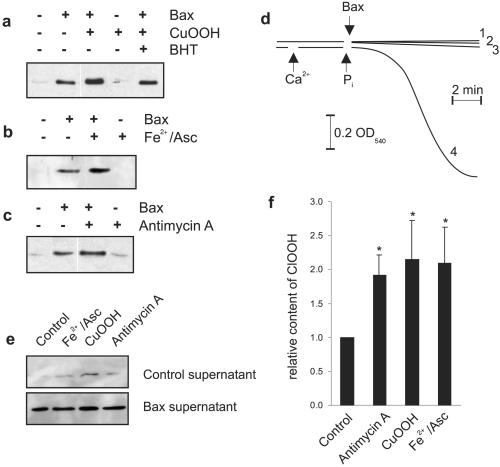
Figure 2.
Oxidative stress mobilizes a unique pool of cytochrome c that involves peroxidation of cardiolipin. (a–c) Mitochondria (1 mg/ml) were incubated as described in Materials and Methods and treated with different ROS-generating systems. After a 5-min stabilization period, Bax and inducers of ROS were added. After 8 min, samples were centrifuged, and the supernatants were separated by SDS/PAGE and Western-blotted for the presence of cytochrome c as described in Materials and Methods. The concentrations of the different reagents are as follows: cumene hydroperoxide (250 μM), butylated hydroxytoluene (5 μM), Fe(II)SO4 (60 μM)/ascorbate (500 μM), antimycin A (5 μg/ml) + ATP (1 mM). (d) Changes in mitochondrial (0.5 mg/ml) volume were evaluated in the presence of the different ROS-generating systems as follows: trace 1, cumene hydroperoxide; trace 2, Fe2+/Asc; trace 3, antimycin A; trace 4, shows a positive control induced by 150 nmol of Ca2+ per mg of protein and 5 mM Pi. (e) Duplicate samples (a–c) were separated by SDS/PAGE and Western-blotted for the presence of AK-2. (f) Mitochondria were incubated as described for a–c, and lipids were extracted after 13 min as presented in Materials and Methods. An aliquot was subjected to HPLC analysis, and quantitative analysis of the cardiolipin hydroperoxide content obtained from three independent experiments is presented. Data are expressed as means ± SD. *, significantly different from control mitochondria. BHT, butylated hydroxytoluene; CuOOH, cumene hydroperoxide.
Because it was suggested that lipid peroxidation, and in particular cardiolipin peroxidation (15, 18), could disrupt binding of cytochrome c to the inner membrane, we developed a method for measuring ClOOH formation under these conditions. As seen in Fig. 2f, a significant and similar increase in ClOOH content was stimulated by the different ROS-generating systems used. The difference in the level of ClOOH among treated and untreated samples led us to conclude that the additional pool of cytochrome c released in response to an oxidative stress reflects the tightly bound pool that is detached from the membrane because of the oxidation of cardiolipin. Taken together, it was concluded that cytochrome c release requires a two-step process, consisting of the detachment of this protein from its membrane-anchoring lipid, cardiolipin, followed by permeabilization of the outer mitochondrial membrane, in this case by Bax insertion, enabling the release of cytochrome c into the extramitochondrial environment.
Effects of Electrostatic Changes and Oxidative Stress on the Release of Cytochrome c During MPT.
To extend our knowledge of the determinants of cytochrome c release from mitochondria, we tested the notion of a two-step process under conditions of MPT. In these experiments, MPT was induced by the addition of 150 nmol Ca2+/mg protein in the presence of 5 mM Pi. Fig. 3a reflects the swelling pattern of these mitochondria in the presence (trace 4) or absence (trace 3) of the prooxidant-generating system Fe2+/Asc. Although the onset of swelling occurred earlier under conditions of oxidative stress, the extent and rate of swelling were similar in all three buffers (data not shown). This trend was also true for the kinetics of Ca2+ accumulation and its release (Fig. 3b). In addition, the release of the matrix protein adenylate kinase-3 (AK-3), a further indication of MPT (7), was similar under all conditions (data not shown). We concluded that the efficiency of mitochondrial swelling and hence MPT was similar in all three buffers.
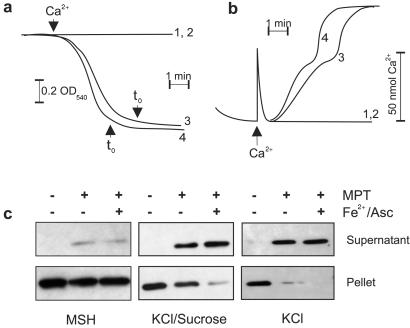
Figure 3.
Fe2+/Asc accentuates cytochrome c release stimulated by MPT in intermediate and high ionic strength buffers. (a) Mitochondria (0.5 mg/ml) were incubated as described in Materials and Methods, and swelling was monitored continuously at 540 nm. MPT was induced by 150 nmol of Ca2+ per mg of protein and 5 mM Pi in the presence or absence of Fe(II)SO4 (60 μM)/ascorbate (500 μM). Trace 1, control; trace 2, control + Fe2+/Asc; trace 3, MPT; trace 4, MPT in the presence of Fe2+/Asc. (b) Mitochondria (1 mg/ml) were incubated as described in Materials and Methods. After stabilization, MPT was induced, and Ca2+ release into the incubation medium was monitored. Trace 1, control; trace 2, control + Fe2+/Asc, trace 3, MPT; trace 4, MPT in the presence of Fe2+/Asc. (c) Mitochondria (1 mg/ml) were incubated as described in Materials and Methods and, in some cases, stimulated to undergo MPT ± Fe2+/Asc. Samples were taken for Western blot analysis exactly 8 min after t0 (a) to compensate for the shorter onset of MPT in the presence of Fe2+/Asc.
Fig. 3c reveals the relative amounts of cytochrome c that were released in the three different buffers. Consistent with our previous report, cytochrome c release during MPT is depressed in MSH buffer as compared with a buffer containing higher ionic strength (7). Moreover, this release was not enhanced by the production of lipid hydroperoxides with Fe2+/Asc. In contrast, the addition of prooxidants to mitochondria incubated in the mixed KCl/sucrose buffer stimulated the release of ≈87% of cytochrome c as compared with ≈51% release induced by MPT alone, suggesting that peroxidation of cardiolipin may, in addition to its ability to target the tightly bound form, also disturb the electrostatic interaction of the loosely bound form. Finally, nearly all cytochrome c was released in the high ionic strength buffer (150 mM KCl) during MPT, and the ≈7% of cytochrome c present in the pellet was effectively mobilized by oxidative stress.
Concluding Remarks.
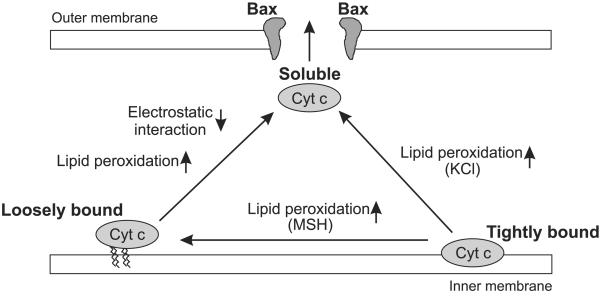
In this study, we provide evidence that the release of cytochrome c from mitochondria requires a two-step process, consisting of the detachment of this protein from its membrane-anchoring lipid, cardiolipin, followed by permeabilization of the outer mitochondrial membrane, permitting the release of cytochrome c into the extramitochondrial environment (Fig. 4).
Figure 4.
Model for the two-step release of cytochrome c from mitochondria.
Tightly bound cytochrome c is released by alterations in membrane structure by peroxidation of cardiolipin. Subsequently, the fate of cytochrome c—i.e., whether it adopts a loosely bound or soluble form—depends largely on the ability or inability of this protein to establish an electrostatic interaction with cardiolipin (Fig. 4). In our model, if the ionic strength is low (MSH buffer), cytochrome c remains bound to cardiolipin by its electrostatic interaction; however, if the ionic strength is high (KCl buffer), cytochrome c largely assumes a soluble form that renders this protein fit for release after permeabilization of the outer membrane. In the intermediate buffer (100 mM KCl), however, it seems that peroxidation of cardiolipin may also impair electrostatic binding of cytochrome c, at least in part, because a prominent increase in the release of this protein is observed during subsequent MPT.
Mitochondria are the primary sources of intracellular ATP and successful completion of the apoptotic process requires energy (19). Although part of this energy requirement is clearly needed for apoptosome formation, a recent study demonstrated the ability of ATP to bind and trigger a conformational change in cytochrome c that disrupted this protein's electrostatic interaction with cardiolipin (20). Thus, any disruption in Δψ and/or oxidative phosphorylation, resulting in decreased ATP production, could compromise the progression of this form of cell death. Treatment of mitochondria with oligomeric Bax, under strict non-MPT conditions, stimulates a recoverable submaximal shift in Δψ and the release of a portion of cytochrome c; the vast majority of this protein remains inside these organelles enabling them to maintain their ATP-producing capacity. In contrast, MPT, generally associated with late-stage apoptosis (21) or necrotic cell death (22), is associated with a sustained drop in Δψ, ATP depletion, and the release of cytochrome c. In this case, cytochrome c release is merely a consequence of the loss of mitochondrial integrity and does not bear significantly on the determination of the mode of cell death.
Acknowledgments
We thank Dr. Bruno Antonsson (Serono Pharmacetical Research Institute, Geneva, Switzerland) for kindly providing his pBadHisBax plasmid, Dr. Takafumi Noma (Yamaguchi University School of Medicine, Yamaguchi, Japan) for providing the anti-AK-2 antibody, Margareta Sandström (Karolinska Institutet) for preparation of the recombinant Bax proteins, Elisabeth Peterson (Karolinska Institutet) for HPLC technical assistance, and Professor Dieter Brdiczka (University of Konstanz, Konstanz, Germany) for helpful discussion. This work was supported by grants from the Swedish Medical Research Council (03X-2471) and the Swedish (3829-B98-03XAC) and Stockholm (00:148) Cancer Societies. J.D.R. is supported by Grant 1 F32 CA83273 from the National Cancer Institute, National Institutes of Health, and V.G. by a Visiting Scientist grant from The Swedish Foundation for International Cooperation in Research and Higher Education (STINT).
Abbreviations
- AK-2
adenylate kinase-2
- ClOOH
cardiolipin hydroperoxide
- Fe2+/Asc
Fe(II)SO4/ascorbate
- HPLC
high-performance liquid chromatography
- MPT
mitochondrial permeability transition
- MSH
mannitol/sucrose/Hepes
- ROS
reactive oxygen species
- TPP+
tetraphenylphosphonium
- Δψ
mitochondrial membrane potential
References
- 1.Loeffler M, Kroemer G. Exp Cell Res. 2000;255:19–26. doi: 10.1006/excr.2000.4833. [DOI] [PubMed] [Google Scholar]
- 2.Köhler C, Gahm A, Noma T, Nakazawa A, Orrenius S, Zhivotovsky B. FEBS Lett. 1999;447:10–12. doi: 10.1016/s0014-5793(99)00251-3. [DOI] [PubMed] [Google Scholar]
- 3.Du C, Fang M, Li Y, Li L, Wang X. Cell. 2000;102:33–42. doi: 10.1016/s0092-8674(00)00008-8. [DOI] [PubMed] [Google Scholar]
- 4.Verhagen A M, Ekert P G, Pakusch M, Silke J, Connolly L M, Reid G E, Moritz R L, Simpson R J, Vaux DL. Cell. 2000;102:43–53. doi: 10.1016/s0092-8674(00)00009-x. [DOI] [PubMed] [Google Scholar]
- 5.Li P, Nijhawan D, Budijardjo I, Srinivasula S M, Ahmad M, Alnemri E S, Wang X. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 6.Robertson J D, Orrenius S, Zhivotovsky B. J Struct Biol. 2000;129:346–358. doi: 10.1006/jsbi.2000.4254. [DOI] [PubMed] [Google Scholar]
- 7.Gogvadze V, Robertson J D, Zhivotovsky B, Orrenius S. J Biol Chem. 2001;276:19066–19701. doi: 10.1074/jbc.M100614200. [DOI] [PubMed] [Google Scholar]
- 8.Nichols P. Biochim Biophys Acta. 1974;346:261–310. doi: 10.1016/0304-4173(74)90003-2. [DOI] [PubMed] [Google Scholar]
- 9.Cortese J D, Voglino A L, Hackenbrock C R. Biochemistry. 1998;37:6402–6409. doi: 10.1021/bi9730543. [DOI] [PubMed] [Google Scholar]
- 10.Gorbenko G P. Biochim Biophys Acta. 1999;1420:1–13. doi: 10.1016/s0005-2736(99)00082-6. [DOI] [PubMed] [Google Scholar]
- 11.Rytömaa M, Kinnunen P K J. J Biol Chem. 1995;270:3197–3202. doi: 10.1074/jbc.270.7.3197. [DOI] [PubMed] [Google Scholar]
- 12.Rytömaa M, Mustonen P, Kinnunen P K J. J Biol Chem. 1992;267:22243–22248. [PubMed] [Google Scholar]
- 13.Montessuit S, Mazzei G, Magnenat E, Antonsson B. Protein Expression Purif. 1999;15:202–206. doi: 10.1006/prep.1998.1010. [DOI] [PubMed] [Google Scholar]
- 14.Bligh E G, Dyer W J. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 15.Nomura K, Imai H, Koumura T, Kobayashi T, Nakagawa Y. Biochem J. 2000;351:183–193. doi: 10.1042/0264-6021:3510183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parinandi N L, Weis B K, Schmid H H O. Chem Phys Lipids. 1988;49:215–220. doi: 10.1016/0009-3084(88)90009-6. [DOI] [PubMed] [Google Scholar]
- 17.Lutter M, Fang M, Luo X, Nishijima M, Xie X, Wang X. Nat Cell Biol. 2000;2:754–756. doi: 10.1038/35036395. [DOI] [PubMed] [Google Scholar]
- 18.Shidoji Y, Hayashi K, Komura S, Ohishi N, Yagi K. Biochem Biophys Res Commun. 1999;264:343–347. doi: 10.1006/bbrc.1999.1410. [DOI] [PubMed] [Google Scholar]
- 19.Leist M, Single B, Castoldi A F, Kühnle S, Nicotera P. J Exp Med. 1997;185:1481–1486. doi: 10.1084/jem.185.8.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tuominen E K, Zhu K, Wallace C J, Clark-Lewis I, Craig D B, Rytömaa M, Kinnunen P K J. J Biol Chem. 2001;276:19356–19362. doi: 10.1074/jbc.M100853200. [DOI] [PubMed] [Google Scholar]
- 21.Chen Q, Gong B, Almasan A. Cell Death Differ. 2000;7:227–233. doi: 10.1038/sj.cdd.4400629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crompton M. Biochem J. 1999;341:233–249. [PMC free article] [PubMed] [Google Scholar]