Abstract
Mitochondria, often referred to the powerhouse of the cell, are essential for cellular energy production, and their dysfunction can profoundly affect various organs. Transplantation of healthy mitochondria can restore the bioenergetics in diseased cells and address multiple conditions, but more potentials of this approach remain unclear. In this study, I demonstrated that the source of transplanted mitochondria is not limited by species, as exhibit no significant responses to mitochondria derived from different germlines. Moreover, I identified that metabolic compatibility between the recipient and exogenous mitochondria as a crucial factor in mitochondrial transplantation, which confers unique metabolic properties to recipient cells, enabling them to combat different diseases. Additionally, my findings indicated competitive interactions among mitochondria with varying functions, with more bioenergetic-active mitochondria yielded superior therapeutic benefits. Notably, no upper limit for the bioenhancement provided by exogenous mitochondria has been identified. Based on these insights, I proposes a novel therapeutic approach—adaptive bioenhancement through mitochondrial transplantation.
Subject terms: Biotechnology, Cell biology
Introduction
Mitochondrial transplantation involves isolating mitochondria from normal cells or tissues and transferring them to diseased areas, either directly or indirectly, to enhance the function of damaged cells, thereby facilitating therapeutic interventions [1–3]. Compared to traditional organ, tissue, or cell transplantation, mitochondrial transplantation offers a novel and less invasive alternative. In 2009, researchers at Boston Children ‘s Hospital first reported this technique by injecting mitochondria into ischemic regions of the rabbit heart, resulting in significant therapeutic effects [4]. Since then, mitochondrial transplantation has shown promising potential in treating various organ and tissue injuries, including those affecting the heart [5, 6], brain [7, 8], spinal cord [9], lungs, kidneys [10], liver [11], skeletal muscle [12], and skin [13]. It has also been explored as a treatment for conditions such as sepsis [14] and cancer [15].
Despite the proven safety and therapeutic efficacy of mitochondrial transplantation in numerous diseases [16], its full potential remains to be fully elucidated. The transplanting of functionally normal mitochondria to restore the function of dysfunctional mitochondria in diseased cells involves differences in mitochondrial function, which can enhance the function of diseased cells [17–19]. Furthermore, preemptively introducing exogenous mitochondria into healthy cells may increase their resistance to damage, thereby providing bioenergetic enhancement [6, 20]. These insights raise a topic that warrants further investigation: the potential to select mitochondria with specific characteristics tailored to different disease populations or to employ mitochondria with superior functions instead of general mitochondria as an adaptive bio-enhancement strategy.
In this study, I expanded the sources of mitochondria for transplantation and demonstrated the broad applicability of this approach. By leveraging the germline-specific characteristics of mitochondria, I further treated cells under various disease conditions, highlighting the importance of metabolic matching. Additionally, hybrid mitochondria were generated with enhanced therapeutic effects by inducing cell fusion to combine multiple germline characteristics. Furthermore, I found that mitochondria with greater functional potency exerted markedly superior therapeutic effect compared to their germline-specific counterparts, even enhancing the function of normal cells to some extent. Similar results were observed in animal experiments, suggesting that mitochondrial transplantation could serve as a potential strategy for biological enhancement. These findings significantly expand the therapeutic potential of mitochondrial transplantation and open a new avenue for research into biological augmentation.
Results
Universality of mitochondrial transplantation
Current research on mitochondrial transplantation predominantly utilizes mitochondria from three sources: various cell lines [17–19, 21] such as human umbilical cord mesenchymal stem cells [22], and tissues such as liver [23], skeletal muscle [24], brain [25], and platelet-derived extracellular vesicles [26], The experimental models commonly employed include humans [27, 28], rabbits [4], mice [29], pigs [30], and rats [31]. My study aimed to broaden the species diversity of mitochondria used for transplantation, thus enhance the therapeutic potential of this approach.
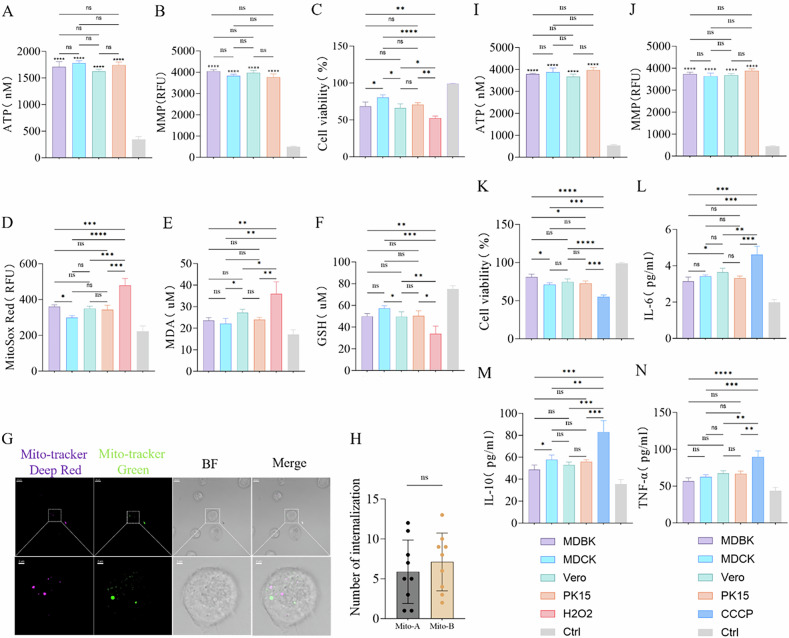
I obtained mitochondria from 13 different species including African green monkey kidney cell (Vero), bovine kidney cell (MDBK), canine kidney cell (MDCK), cat kidney cell (CRFK), pig kidney cell (PK15), chicken liver cancer cell (LMH), spodoptera frugiperda cell (Sf9), turtle liver tissue, bullfrog liver tissue, sparrow liver tissue, lizard liver tissue, salmon liver tissue, and eel liver tissue, and assessed their functionally through mitochondrial membrane potential (MMP) (Fig. 1A), adenosine triphosphate(ATP) production (Fig. 1B), transmission electron microscopy (Figs. 1C and S1B), and mitochondrial respiratory chain complex activity assays (Fig. 1D–G). My results confirmed that the isolated mitochondria were functionally intact and structurally well-preserved. Subsequently I co-cultured mitochondria from these species with human cardiomyocytes (AC16), human hepatoma cell (HepG2), and mouse fibroblast cell (L929), and efficient internalization as well as significant co-localization of the various mitochondria within recipient cells (Figs. 1H and S1A). To evaluate the safety of these transplantations, supernatants from the co-cultured cells were collected and their immune and inflammatory responses were assessed using enzyme-linked immunosorbent assay (ELISA). No significant changes were observed in the levels of IL-6, IL-10, and TNF-α in the mitochondria-transplanted groups compared to normal cells (Fig. 1I–N), indicating a relative high safety for multispecies mitochondrial transplantation.
Fig. 1. Universality of mitochondrial transplantation.
A Analysis of membrane potential in both cells and animals isolated mitochondria. Ctrl: Mitochondria were subjected to two freeze-thaw cycles at −80 °C and 37 °C to completely disrupt their structure and function, serving as a comparison, n = 3. B ATP production capacity in both cells and animals isolated mitochondria. Ctrl: Mitochondria were subjected to two freeze-thaw cycles at −80 °C and 37 °C, used for comparison, n = 3. C Mitochondria were randomly selected from cells (Vero) and animals (Bull Frog) for electron microscopy to observe their morphology. Scale bar: 500 nm. D–G Activity of respiratory chain complexes I and III in both cells and animals isolated mitochondria, n = 3. H Co-localization fluorescence imaging at 24 h of transplanted mitochondria labeled with Mito-Tracker Green (green fluorescence) from Eel, MDCK, CRFK, Sparrow, Vero, and Lizard, alongside AC16, L929, and HepG2 cells labeled with WGA594 (red fluorescence). Blue fluorescence (DAPI) represents the nucleus. Scale bar: 10 μm. I–N Immunoreactivity of IL-6, IL-10, and TNF-α in cell culture medium collected 24 h after the transplantation of exogenous mitochondria, n = 3. Ctrl: Untreated group. ns no statistical significance, ****p < 0.0001.
Additionally, a group of mitochondria from Vaucheria litorea was introduced to further investigate interspecies barriers. The mitochondria were labeled with Mito-Tracker Red and co-cultured with HepG2 cells pre-stained with WGA488 green fluorescence. Due to limitations in the extraction technique, most Vaucheria litorea mitochondria were damaged (Fig. S2D). However, some of them with intact structure and function was successfully internalized (Fig. S2A, B). During the 25-day transplantation, no significant immune or inflammatory responses were recorded in the first 15 days. From day 16 onward, the contamination led to a rapid increase in IL-6, IL-10, and TNF-α levels, ultimately causing cell death (Fig. S2E–H). These outcomes are acceptable because of the high contamination risk associated with plant mitochondrial isolation. The genomic differences between plant and animal mitochondria indicate substantial phylogenetic divergence [32, 33]. Nevertheless, the early-stage safety observed following Vaucheria litorea mitochondrial transplantation strengthens my hypothesis, further supporting the universality of mitochondrial transplantation. Moreover, the internalization of mitochondria from multiple species demonstrates the cellular “inclusiveness” to such foreign organelles.
Mitochondria with matching metabolic characteristics provide improved therapeutic outcomes
Metabolic compatibility plays a crucial role in achieving optimal therapeutic outcomes during organ and tissue transplantation, and this principle extends to mitochondrial transplantation [34–36]. This raises a question: When mitochondrial functions are similar, do metabolically matched mitochondria offer better therapeutic outcomes? I hypothesized that metabolically matched mitochondria could yield superior therapeutic effects without significant functional differences. To minimize variables, mitochondria were selected from four different kidney cell species that were functionally similar, as determined by mitochondria membrane potential (MMP) and ATP assessments (Fig. 2A, B, I, J). These mitochondria were then co-cultured with cells under various disease model conditions to assess varying therapeutic effects.
Fig. 2. Mitochondria with matching metabolic characteristics provide better therapeutic outcomes.
A, I ATP production capacity in isolated mitochondria. Ctrl: Mitochondria subjected to two freeze-thaw cycles at −80 °C and 37 °C, used for comparison, n = 3. B, J Membrane potential in isolated mitochondria. Ctrl: Mitochondria subjected to two freeze-thaw cycles at −80 °C and 37 °C, used for comparison, n = 3. C Effects of mitochondrial transplantation from four different species on the viability of BMDM cells after H2O2 treatment. Ctrl: Untreated group, n = 3. D Mitochondrial ROS fluorescence intensity in BMDM cells 24 h after transplantation of mitochondria from four different species, followed by H2O2 treatment. Ctrl: Untreated group, n = 3. E, F Effects of mitochondrial transplantation from four different species on MDA and GSH levels in BMDM cells after H2O2 treatment. Ctrl: Untreated group, n = 3. G, H Fluorescence imaging and statistical analysis of the internalization of mitochondria A labeled with Mito-Tracker Red and mitochondria B labeled with Mito-Tracker Green in BMDM cells after H2O2 treatment. Original image scale bar: 10 µm; magnified region scale bar: 2 µm, n = 9. K Effects of mitochondrial transplantation from four different species on the viability of AC16 cells after CCCP treatment. Ctrl: Untreated group, n = 3. L–N Changes in IL-6, IL-10, and TNF-α levels in AC16 cells after CCCP treatment, following mitochondrial transplantation from four different species. Ctrl: Untreated group, n = 3. ns no statistical significance, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
With the H2O2-induced Bone Marrow-Derived Macrophage (BMDM) oxidative stress model [37], all mitochondrial transplant groups showed significant therapeutic effects, but the MDCK group notably exhibited enhanced cell viability compared to the MDBK and Vero groups (Fig. 2C). Fluorescence quantitative analysis revealed that mitochondrial ROS levels were significantly reduced in the MDCK group compared to those in the MDBK group (Fig. 2D). Additionally, lipid peroxidation was significantly decreased, and glutathione levels were notably increased in the MDCK group compared to the Vero group (Fig. 2E, F). Overall, these results suggest that MDCK mitochondrial transplantation is more effective in treating H2O2-induced oxidative stress in BMDMs. Then, I paired four types of mitochondria in pairs and labeled them with different fluorescent dyes, and co-culturing them with H2O2-treated BMDMs for 24 h, fluorescence immunoimaging revealed no significant competition between the different mitochondria, and their internalization amounts in the recipient cells were similar (Fig. 2G, H). According to these results, I inferred that the key distinguishing factor was their species of origin. Although the primary function of mitochondria is to provide energy, their auxiliary functions, which vary among species, appear to have made the MDCK mitochondria more metabolically compatible with the H2O2-treated BMDMs, resulting in improved therapeutic effects.
The results of mitochondrial transplantation from CCCP-treated AC16 cells further supported the hypothesis [38]. Despite comparable mitochondrial function assessed by ATP and MMP assessments (Fig. 2I, J), the MDBK group did not demonstrate a significant advantage. Nevertheless, its therapeutic effect was superior, markedly enhancing cell viability compared to the MDCK and PK15 groups (Fig. 2K). Additionally, these four mitochondrial transplantation groups significantly reduced the inflammatory response, the MDBK group exhibited lower IL-6 levels compared to the Vero group (Fig. 2L) and reduced IL-10 levels compared to the MDCK group (Fig. 2M), although no significant differences were observed in TNF-α levels among four transplanted groups (Fig. 2N). Overall, mitochondria derived from MDBK cells displayed enhanced anti-inflammatory effects in CCCP-treated AC16 cells. Despite lacking functional advantages, the improved therapeutic outcomes observed in the MDBK group suggest that its mitochondria were more metabolically compatible with the CCCP-treated AC16 cells. Similar results were also verified in the subsequent HepG2 cell model processed with LPS (Fig. S3A–F).
Mitochondrial fusion generates more potent pluripotent hybrid mitochondria
Mitochondrial transplantation can endow recipient cells with the characteristics of donor mitochondria. For instance, co-culturing mitochondria derived from human mammary epithelial cells with breast cancer cells has been demonstrated to inhibit glycolysis, reduce glucose uptake, suppress cancer cell proliferation, and enhance sensitivity to paclitaxel [39]. Similarly, co-culturing mitochondria that contain antibiotic resistance genes with antibiotic-sensitive cells can bolster resistance to antibiotics [40]. Given that cell fusion can lead to metabolic reprogramming, enabling fused cells to acquire traits from different cell types [41], a question emerges: Can the mitochondria within fused cells obtain multi-germline characteristics? Furthermore, could mitochondria possessing multi-germline traits offer enhanced therapeutic efficacy under equivalent functional conditions?
Specifically, HL1 and H9C2 cells were fused, and extracted for analysis using immunofluorescence and flow cytometry (Fig. 3A, B). Then, the resulting cells were cultured and analyzed by Western blot on the second day, revealing a significantly higher expression of Opa1 in the HL1 + H9C2 hybrid mitochondria compared to either HL1 or H9C2. Additionally, Drp1 and Mfn1 expression levels were notably elevated compared to those in HL1, with no significant difference observed compared to H9C2 (Fig. 3C, D). Overall, the functional evaluation suggested that HL1 + H9C2 hybrid mitochondria exhibited enhanced dynamic activity, indicating an active state of mitochondrial fusion within the cells. The three types of cells were further cultured until the fourth generation to ensure functional stability. Subsequently, oxidative stress was induced in AC16 cells with H₂O₂, and mitochondria were extracted from HL1, H9C2, and HL1 + H9C2 cells for mitochondrial function evaluation through measurement of MMP and ATP levels. The results demonstrated no significant functional differences among these three types of mitochondria, all of which maintained normal functionality (Fig. 3E, F). In addition, these mitochondria were co-cultured with AC16 cells. The AC16 cell viability was significantly enhanced within all groups, with the HL1 + H9C2 group showing the most pronounced effect (Fig. 3G). Quantitative fluorescence analysis revealed a significant reduction in mitochondrial ROS levels across all groups, with the HL1 + H9C2 group indicating superior oxidative stress mitigation compared to the HL-1 group (Fig. 3H). Notably, no significant differences in malondialdehyde (MDA) levels were observed among the three groups (Fig. 3I). However, glutathione (GSH) levels in the HL1 + H9C2 group were significantly higher than those in the H9C2 group (Fig. 3J). These findings indicate that hybrid mitochondria derived from HL1 + H9C2 could provide superior therapeutic effects on AC16 cells compared to the other two mitochondrial types.
Fig. 3. Generation of more potent pluripotent hybrid mitochondria through mitochondrial fusion.
A Co-localization fluorescence imaging 24 h after the fusion of HL1 cells labeled with WGA647 and H9C2 cells labeled with WGA594. Scale bar: 20 µm. B Flow cytometric sorting of hybrid cells 24 h after the fusion of HL1 cells labeled with WGA647 and H9C2 cells labeled with WGA488. C, D Western blot analysis of the mitochondrial fusion and fission proteins Mfn1, Opa1, and Drp1 in HL1, H9C2, and HL1 + H9C2 cells on the second day after cell fusion, n = 3. E ATP production capacity in isolated mitochondria, Ctrl: Mitochondria subjected to two freeze-thaw cycles at −80 °C and 37 °C, used for comparison, n = 3. F JC-1 membrane potential detection of isolated mitochondria. Ctrl: Mitochondria subjected to two freeze-thaw cycles at −80 °C and 37 °C, used for comparison, n = 3. G Effects of transplantation of three different types of mitochondria on the viability of AC16 cells after H2O2 treatment. Ctrl: Untreated group, n = 3. H Effects of transplantation of three different types of mitochondria on mitochondrial ROS levels in AC16 cells after H2O2 treatment. Ctrl: Untreated group, n = 3. I, J Effects of transplantation of mitochondria from three different species on MDA and GSH levels in AC16 cells after H2O2 treatment. Ctrl: Untreated group, n = 3. ns no statistical significance, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Competitive internalization of mitochondria: the fittest functions best
Natural selection operates on the principle that the fittest organisms are more likely to survive [42], a principle that also applies to mitochondrial transplantation. I think that the significantly differences in the bioenergetic function of transplanted mitochondria may allow more suitable mitochondria to provide better therapeutic outcomes.
Given the variability in bioenergetic function attributable to differences in the physiological state and the rearing environment of animals, I extracted mitochondria from the liver tissues of sparrows, lizards, salmon, and bullfrogs and co-cultured them with CCCP-treated HepG2 cells [43]. Subsequently, ATP and MMP assessments indicated that the mitochondria from bullfrogs exhibited the strongest bioenergetic function among the four groups (Fig. 4A, B). The cell viability of HepG2 was improved within all groups, with the Bull Frog group showing a more pronounced effect compared to the Sparrow, Salmon and Lizard groups (Fig. 4C). Furthermore, the mitochondria ROS production was markedly reduced within the Bull Frog group compared to both Sparrow and Salmon groups (Fig. 4D). The Bull Frog group also exhibited greater efficacy in repairing DNA oxidative damage than Sparrow and Lizard groups (Fig. 4E). Immunofluorescence further revealed a significant competition between mitochondria with different functions, HepG2 cells internalized a higher quantity of bullfrog derived mitochondria than those derived from lizards (Fig. 4F, G). These findings indicate that although metabolic compatibility is essential for similar bioenergetic function, significant improved bioenergetic performance offers greater therapeutic benefits in case of substantial disparity. That is to say, mitochondria with stronger overall function are more suitable for treatment.
Fig. 4. Competitive internalization of mitochondria: the fittest functions best.
A, H ATP production capacity in isolated mitochondria. Ctrl: Mitochondria subjected to two freeze-thaw cycles at −80 °C and 37 °C, used for comparison, n = 3. B, I Membrane potential in isolated mitochondria. Ctrl: Mitochondria subjected to two freeze-thaw cycles at −80 °C and 37 °C, used for comparison, n = 3. C Effects of mitochondria transplantation from four different species on the viability of HepG2 cells after CCCP treatment. Ctrl: Untreated group, n = 3. D Effects of mitochondria transplantation from four different species on mitochondria ROS in HepG2 cells after CCCP treatment. Ctrl: Untreated group, n = 3. E Detection of DNA damage repair in HepG2 cells after CCCP treatment following mitochondria transplantation from four different species. Ctrl: Untreated group, n = 3. F, G Fluorescence imaging and statistical analysis of the internalization of Lizard mitochondria labeled with Mito-Tracker Deep-Red and Bull Frog mitochondria labeled with Mito-Tracker Green in HepG2 cells after CCCP treatment. Original image scale bar: 10 µm, magnified region scale bar: 2 µm, n = 6. J Effects of mitochondria transplantation from four different species on the viability of BMDM cells after LPS treatment. Ctrl: Untreated group, n = 3. K–M Changes in IL-6, IL-10, and TNF-α levels in BMDM cells after LPS treatment following mitochondria transplantation from four different species. Ctrl: Untreated group, n = 3. N Detection of DNA damage repair in BMDM cells after LPS treatment following mitochondria transplantation from four different species, n = 3. O, P Fluorescence imaging and statistical analysis of the internalization of Lizard mitochondria labeled with Mito-Tracker Green, Sparrow mitochondria labeled with Mito-Tracker Deep-Red, and Salmon mitochondria labeled with Mito-Tracker Red in BMDM cells after LPS treatment. Original image scale bar: 10 µm, magnified region scale bar: 5 µm, n = 6. ns no statistical significance, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Next, I extracted mitochondria from the liver tissues of the four animals and co-culturing them with LPS-treated BMDM cells [44]. Assessments of ATP and MMP demonstrated that salmon-derived mitochondria exhibited superior bioenergetic function compared to the other three groups (Fig. 4H, I). Leveraging their robust biological effects, salmon mitochondria displayed enhanced therapeutic outcomes. Specifically, there were effective in increasing the viability of BMDM, reducing inflammation, and mitigating DNA oxidative damage. The Salmon group showed higher cell viability than the other three groups (Fig. 4J), lower IL-6 levels compared to the Sparrow and Lizard groups (Fig. 4K), lower TNF-α levels than the other three groups (Fig. 4L), lower IL-10 levels than the Sparrow and Bull Frog groups (Fig. 4M), and lower 8-OHDG levels than the other three groups (Fig. 4N). Immunofluorescence analysis revealed that the Salmon group exhibited a dominant advantage, with its internalization quantity far exceeding that of the Lizard and Sparrow groups (Fig. 4O, P). Similar results were observed in the D-gal-treated HepG2 cells model (Fig. S3G–L). These findings also indicate that, under equal conditions, the mitochondria compete to out-survive each other, and cells will preferentially select mitochondria that are more beneficial to them, demonstrating the classic law of “survival of the fittest.”
Mitochondrial transplantation enhances physical function and biological potency in mice
The in vitro experiments indicated that mitochondria with greater functional potency exhibited superior therapeutic effects on diseases compared to mitochondria with normal function. I want to know in vivo experiments are equally applicable. Previous studies have suggested that preemptive administration of normally functioning mitochondria can enhance cellular resistance to stimuli [45], but the extent of its benefits to general recipients remains unclear. Mitochondrial function improved by training mitochondrial biogenesis [46, 47]. Therefore, mice with trained mitochondria may exhibit better therapeutic outcomes than those with autologous or allogeneic mitochondria with normal function. The functional enhancement of mitochondria in trained mice is based on their overall physical fitness. Natural wild mice, being more robust with stronger physical fitness and metabolism, are hypothesized to possess even more potent mitochondrial function.
By isolating mitochondria from young C57BL/6J and wild mice, I conducted tail vein injections in mice with an acute inflammation model and normal ones. Additionally, I co-cultured these mitochondria with normal and LPS-treated BMDMs. ATP and MMP analyses revealed that the mitochondria derived from wild mice exhibited the highest functional potency (Fig. 5A, B), with no immune inflammatory response observed after a 24 h period (Fig. S4B). Small animal imaging detected the absorption of exogenous mitochondria by severely damaged liver and kidney tissues through the circulatory system (Fig. 5C). At 3 days post-transplantation, grip strength and endurance were significantly improved in all acute inflammation model groups, with the Wild Mice group showing the most notable endurance improvement (Fig. 5D, I). In addition, no significant differences in grip strength were observed among the normal model groups, while the Wild Mice group demonstrated a substantial increase in endurance compared to the Ctrl group (Fig. 5E, I). After 1 week, all acute inflammation model groups exhibited recovery in body weight, with the Wild Mice group demonstrating a significant increase compared to the LPS-Ctrl group (Fig. 5G, H). In contrast, no significant differences in body weight were observed in the normal model groups (Fig. 5F, H). MRI assessment revealed substantial increases in hind limb muscle volume for both the Wild Mice and Young C57 groups within the acute inflammation model, with the Wild Mice group showing slightly outperforming the Young C57 group. Additionally, the muscle tissue damage recovery was also more effective in the Wild Mice group (Figs. S4D and 4E). After treatment, serum levels of IL-6, IL-10, and TNF-α were reduced in both the Wild Mice and Young C57 groups of acute inflammation model, with the Wild Mice group showing lower levels of IL-10 and TNF-α compared to the Young C57 group, and no significant changes were observed in the normal model groups (Fig. 6A–C). Furthermore, liver and kidney function were significantly improved in the Wild Mice and Young C57 groups of acute inflammation model, with serum AST levels significantly lower in the Wild Mice group compared to the Young C57 group. No significant differences were observed in the normal model groups (Fig. 6D–F). Both the Wild Mice and Young C57 groups significantly reduced oxidative stress within the acute inflammation model. Characterized by decreased levels of MDA and increased superoxide dismutase (SOD) activity in the Wild Mice group compared to the Young C57 group. Moreover, the Wild Mice group demonstrated a notable increase in SOD activity among normal model groups. However, no difference in MDA levels were observed across the normal model groups (Fig. 6G, H). Mitochondrial transplantation significantly reduced the expression of inflammatory proteins in the liver and kidney in the acute inflammation model group, with the Wild Mice group showing lower P-JNK protein expression compared to the Young C57 group (Fig. 6I–K). Real-time quantitative PCR (Primer Sequence Table) revealed a downregulation of IL-6, IL-8, IL-18, TGF-β, MPO, CXCL1, CXCL2, and HMGB1 as well as upregulation of IL-13, SOD1, ARG1, and COX1 in the kidneys of both Wild Mice and Young C57 groups when compared to the LPS-Ctrl group. In the liver, IL-6, IL-8, IL-18, IL-1β, CXCL2, CCL5, CCL10, and TGF-β were downregulated, while IL-4, IL-13, ARG1, and SOD1 were upregulated (Fig. S4F, G). Notably, significant differences were identified between the Wild Mice and Young C57 groups, indicating that wild mice mitochondria could exert a superior therapeutic effect.
Fig. 5. Mitochondrial Transplantation Enhances Physical Function in Mice.
A, B Evaluation of isolated mitochondrial functions through ATP production and mitochondrial membrane potential (MMP) assays, with mitochondria subjected to two freeze-thaw cycles at −80 °C and 37 °C to completely disrupt their structure and function as a control, n = 3. C Biodistribution of exogenous mitochondria in the mouse liver, kidney, and heart detected by in vivo imaging 24 h after intravenous tail vein injection of mitochondria. The blank group consists of untreated LPS-Ctrl group mice. D, E Assessment of motor function in acute inflammation model mice and normal model mice 3 days after the transplantation of two different types of mitochondria, with untreated groups as control, n = 6. F–H Changes in body weight of acute inflammation model mice and normal model mice before and 1 week after the transplantation of two different types of mitochondria, with untreated groups as control, n = 6. I Grip strength measurements in acute inflammation model mice and normal model mice 3 days after the transplantation of two different types of mitochondria, with untreated groups as control, n = 6. ns no statistical significance, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Fig. 6. Mitochondrial Transplantation Enhances Biological Potency in Mice and BMDM cells.
A–C Changes in serum levels of IL-6, TNF-α, and IL-10 in acute inflammation model mice and normal model mice 1 week after transplantation of two different mitochondria, with untreated groups as control, n = 3. D–F Evaluation of kidney and liver function via serum Cr, ALT, and AST levels in acute inflammation model mice and normal model mice 1 week after transplantation of two different mitochondria, with untreated groups as control, n = 3. G, H Measurement of serum MDA levels and SOD activity in acute inflammation model mice and normal model mice 1 week after transplantation of two different mitochondria, with untreated groups as control, n = 3. I–K Analysis of JNK and P-JNK, p65, and P-p65 protein expression levels in liver and kidney tissues via Western blot 1 week after transplantation of two different mitochondria in acute inflammation model mice and normal model mice, with untreated groups as control, n = 3. L Effects of two different mitochondria on the viability of LPS-treated BMDM cells 24 h after transplantation, with untreated groups as control, n = 3. M Quantitative analysis of mitochondrial ROS levels in LPS-treated BMDM cells 24 h after transplantation of two different mitochondria, with untreated groups as control, n = 3. ns no statistical significance, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Both mitochondria transplantation exhibited improved cell viability in LPS-treated BMDM (Fig. 6L) and lower mitochondrial ROS production (Fig. 6M). Notably, the Wild Mice group demonstrated superior alleviation of oxidative stress as well as enhanced cell viability. By co-culturing the two mitochondria with normal BMDM, the enhancement of bioenergy was also observed, and the ATP level in the Wild Mice group increased more significantly (Fig. S4C). This animal experiment provided a more systematic investigation into the potential of mitochondrial transplantation than the first experiment (Extend Figs. 2 and 3). Although the Young C57 group showed good therapeutic effects, the robust results observed in the Wild Mice group indicated better therapeutic outcomes for both acute inflammation in mice and LPS-treated BMDM. Furthermore, this mitochondrial exhibited a greater capability to raise antioxidation ability and improve the exercise endurance of normal mice and enhance cellular bioenergy.
Discussion
Mitochondrial transplantation, as an emerging therapeutic strategy, has shown efficacy in various disease models, attracting increasing attention [30, 48–50]. Successful mitochondrial transplantation requires multiple factors, with the source of mitochondria being a critical determinant. Currently, mitochondria are primarily derived from autologous tissues or allogeneic cultured cells [1, 6, 7, 27, 51, 52], which significantly limits the clinical application of this approach. My study offers a preliminary solution to this limitation. I demonstrated the successful internalization of mitochondria from 13 heterologous sources by three different cell germlines, underscoring the safety of cross-germline mitochondrial transplantation. Given that the genomic differences between plant and animal mitochondria are even more significant than those between different animal species, I further confirmed the safety of Vaucheria litorea mitochondrial transplantation. These findings indicate that mitochondrial transplantation is not restricted by phylogenetic barriers. Based on this, I propose that mitochondrial transplantation is universally applicable. The unique immunological properties of mitochondria may stem from their endosymbiotic origin [53–56], while further research is needed to substantiate this hypothesis.
Mitochondria are primarily responsible for energy production, a function that is universally conserved across species [57, 58]. However, they also exhibit germline-specific functional differences, highlighting their role in adaptive evolution [59–61]. While current research mainly focuses on mitochondrial transplantation as an approach to provide damaged cells with energy [24, 45], emerging evidence suggests additional benefits [17, 18, 23]. My results are consistent with these findings, further demonstrating that mitochondria from different germlines can offer diverse advantages in various diseases. Specifically, mitochondria derived from MDCK cells were more effective in alleviating oxidative stress in BMDMs, MDBK-derived mitochondria exhibited superior attenuation of CCCP-induced apoptosis in AC16 cells, and Vero-derived mitochondria demonstrated greater efficiency in reducing inflammation in HepG2 cells. Although these mitochondria exhibited superior performance in specific assays, no statistically significant differences were observed across all metrics, which is both acceptable and explainable. I meticulously controlled for extraneous variables, and when overall mitochondrial function was maintained at a comparable level, the observed differential benefits are likely attributable to germline-specific mitochondrial characteristics. This observation reflects a selective and reciprocal compatibility between mitochondria and recipient cells, driven by metabolic matching to the disease status of cells. The competitive internalization assay has verified this hypothesis.
Furthermore, hybrid mitochondria were generated by fusing cells from different species to create multifunctional mitochondria with diverse germline characteristics [62]. By maintaining overall mitochondrial function at comparable levels, I observed results consistent with those in the previous section. Although hybrid mitochondria exhibited advantages in specific functions, they did not demonstrate absolute superiority because of the distinction between mitochondria’s primary and secondary roles. Specifically, germline-specific functions cannot surpass energy production, which remains the mitochondria’s primary role [56]. This also represents the contrast between quantity and quality. In this study, I created hybrid mitochondria from rat and mouse cardiac muscle cells, two species with relatively limited phylogenetic divergence [63, 64]. Rats have 21 chromosome pairs, while mice have 20, and their genomes are connected by ~280 homologous blocks of sequence similarity. Ninety percent of rat genes have orthologs in mice, and the two species share about 10% of their genes. However, there are still significant differences, including genes related to immune response and protein degradation [65–69]. Given this context, hybrid mitochondria derived from fused cells did not acquire sufficient germline-specific traits to surpass primary mitochondrial functions. Although the overall function of hybrid mitochondria was not markedly improved, they demonstrated superior therapeutic potential compared to single-germline mitochondria, likely due to functional optimization and selective compatibility with recipient cells.
Notably, the more powerful therapeutic outcomes could be driven by the superior functionality of mitochondria with robust overall performance. In the alleviation of HepG2 apoptosis, inflammation in BMDM cells, and HepG2 senescence, mitochondria from Bullfrog, Salmon, and PK15 cells consistently exhibited significantly greater therapeutic efficacy than other tested mitochondria. These results suggest that, at the current stage of research, mitochondria with superior overall performance are more suitable for therapeutic applications. The mitochondria of normal HepG2 cells internalized were significantly fewer than those of functionally impaired HepG2 cells (Fig. S2A–C), indicating the variable quality of extracted mitochondria and differential internalization patterns based on cellular needs. This selective process resembles a “refinement” mechanism, exemplifying the bidirectional selection between mitochondria and recipient cells. The overarching biological implication of this study is that mitochondria with greater bioenergetic capacity are more conducive to survival, echoing the classic “survival of the fittest” paradigm of natural selection.
Mitochondrial transplantation has shown promising results in clinical trials for ischemic heart disease and Single Large-Scale Mitochondrial DNA Deletion Syndromes (SLSMD) [1, 70]. While autologous [1, 20] and allogeneic [52, 71, 72] mitochondria are preferable sources, extensive research has already demonstrated the safety of xenogeneic mitochondrial transplantation [27, 71, 73]. My study further corroborates these findings and expands their applicability across a broader range of germlines. The outcomes of these clinical trials, particularly in SLSMD, could show good therapeutic effects. To investigate this, I explored the therapeutic potential of mitochondrial transplantation in animal models, based on prior in vitro experiments. Specifically, a significant therapeutic advantage was observed, with mitochondria possessing superior overall functionality, across both allogeneic and xenogeneic transplants. This suggests that mitochondria sourced from physically fit wild animals or well-conditioned individuals may serve as optimal candidates for this technique [74, 75]. This insight holds particular relevance for future human studies, where autologous mitochondria from patients may be suboptimal due to disease-related impairments. In such cases, mitochondria from healthier, younger allogeneic, or even xenogeneic donors may offer enhanced functionality and improved therapeutic effects [76–79]. My findings are consistent with those from mitochondrial competitive internalization assays, demonstrating that while exercise may increase oxidative stress damage [80, 81], the competition among donor mitochondria promotes the internalization of more robust mitochondria and rejects those with inferior function. This result was consistently observed in both rounds of in vivo experiments, where I recorded significant enhancements in biological function. Notably, mitochondria from wild mice exhibited superior therapeutic efficacy, suggesting that mitochondria from highly fit animals with enhanced mitochondrial functionality represent an optimal choice for transplantation. Additionally, wild mice mitochondria not only improved disease models but also enhanced the biological function of normal recipient cells, significantly expanding the potential applications and value of mitochondrial transplantation. These findings also provide a potential solution to tolerance issues that may arise with repeated mitochondrial transplantation in future therapeutic regimens.
Despite these promising findings, this study has several limitations that should be acknowledged. First, I did not evaluate mitochondrial heterogeneity, which is a crucial factor in safety assessments. Second, the evaluation indicators used in this study to assess mitochondrial function are relatively superficial, and more rigorous standards are needed for precise definition. Most importantly, my research may lack proteomic and metabolomic data, which are essential for understanding the compatibility between recipient cells and transplanted mitochondria, as well as for comparing pre- and post-transplant functional and metabolic changes. Therefore, future studies should integrate these data to establish a comprehensive database of multispecies mitochondria, enabling more precise matching to specific diseases and thus providing personalized and more effective clinical solutions. Furthermore, some experiments could not be completed due to funding and other reasons constraints, highlighting the need for further research to strengthen the evidence base for future advancements in the field.
In summary, my results demonstrate that mitochondrial transplantation is not constrained by phylogenetic barriers, with mitochondria from diverse species could provide adaptable and versatile therapeutic options for patients. Moreover, my study indicates that this transplantation could serve as a bio-enhancement strategy for healthy individuals. These findings offer significant insights and lay a solid foundation for the future clinical application of mitochondrial transplantation.
Material and methods
Reagents and antibodies
DMEM (C11965500BT), SF-900™ II SEM (10902088), FBS (10099141 C), Penicillin-Streptomycin (15140122), and phenol-red-free DMEM (12348017) were purchased from Gibco (USA). ProLong™ Antifade reagent (P36975) was obtained from Invitrogen (USA). Wheat Germ Agglutinin (WGA594) (W11262), Wheat Germ Agglutinin (WGA488) (W11261), Wheat Germ Agglutinin (WGA647) (W32466), MitoTracker™ Deep Red FM (M22426), MitoTracker™ Green FM (M7514), MitoTracker™ Red CMXRos (M7512), BCA Protein Assay Kit (23250), and SYBR™ Green PCR Master Mix (4309155) were purchased from ThermoFisher (USA). Lipopolysaccharide (LPS) (L2880), Hydrogen peroxide solution (H2O2) (323381), and PEG1450 solution (50% sterile-filtered) (P7181) were obtained from Sigma Aldrich (USA). D-Galactose (D-gal) (HY-N0210) and MitoSox Red (HY-D1055) were purchased from MCE (USA). Carbonylcyanide-m-chlorophenylhydrazone (CCCP) was obtained from Abcam (UK). Mercuric chloride (HgCl2) (10013616) was purchased from Sinoreagent (Shanghai, China). Mitochondria-Cytosol Protein Isolation Kit (C0010-50) was purchased from Applygen (Beijing, China), and Plant Mitochondrial Extraction Kit (SM0020) was also obtained from Beijing, China. PrimeScript™ RT Reagent Kit (RR037Q) was purchased from Takara (Japan). Mitochondrial Complex III Activity Assay Kit (E-BC-K149-M) and Mitochondrial Complex I Activity Assay Kit (E-BC-K151-M) were obtained from Elabscience (Wuhan, China). ATP Assay Kit (S0026), Mitochondrial Membrane Potential Assay Kit with JC-1 (C2006), Cell Counting Kit-8 (C0037), Mouse IL-6 ELISA Kit (PI326), Mouse IL-10 ELISA Kit (PI522), and Mouse TNF-α ELISA Kit (PT512) were purchased from Beyotime (Jiangsu, China). Superoxide Dismutase (SOD) Assay Kit (A001-2-2), Malondialdehyde (MDA) Assay Kit (A003-1-2), Glutathione Peroxidase (GSH-PX) Assay Kit (A005-1-2), Total Nitric Oxide Synthase Assay Kit (A014-2-2), Aspartate Aminotransferase Assay Kit (C010-2-1), Alanine Aminotransferase Assay Kit (C009-2-1), and Creatinine (Cr) Assay Kit (C011-2-1) were obtained from NJJCBIO (Nanjing, China).
The following antibodies were used for Western blotting: anti-p65 (1:1000, Abcam, ab32536), anti-phospho-p65 (1:1000, Cell Signaling, 3033), anti-JNK (1:1000, Cell Signaling, 9252), anti-phospho-JNK (1:1000, Cell Signaling, 9251), anti-Drp1 (1:1000, Cell Signaling, 8570), anti-Mfn1 (1:1000, Cell Signaling, 14739), anti-Opa1 (1:1000, Cell Signaling, 80471), β-actin (1:1000, Cell Signaling, 4970), and Goat Anti-Rabbit IgG (H+L) (1:3000, Abmart, M213811S).
Cell lines
Bovine kidney cells (MDBK) (GNO7), porcine kidney cells (PK15) (GNO31), African green monkey kidney cells (Vero) (SCSP-520), feline kidney cells (CRFK) (GNO16), canine kidney cells (MDCK) (GNO23), human cardiomyocytes (AC16) (SCSP-555), rat cardiomyocytes (H9C2) (GNR5), mouse cardiomyocytes (HL1) (GNM46), mouse myoblasts (C2C12) (SCSP-505) and human hepatocellular carcinoma cells (HepG2) (SCSP-510) were obtained from the Cell Resource Center, Chinese Academy of Sciences (Beijing, China). Chicken hepatocellular carcinoma cells (LMH) (SNL-494) were purchased from Sunncell (Wuhan, China), while Spodoptera frugiperda cells (Sf9) (CL-0205) and mouse fibroblasts (L929) (CL-0089) were obtained from Procell (Wuhan, China). MDCK, MDBK, Vero, PK15, CRFK, LMH, AC16, H9C2, HL1, C2C12, L929 and HepG2 were cultured in DMEM (Gibco) supplemented with 10% (v/v) FBS (Gibco) and 1% (v/v) penicillin-streptomycin (Gibco) at 37 °C in a humidified incubator with 5% CO₂. Sf9 were cultured in SF-900™ II SEM (Gibco) medium under suspension conditions in a shaker flask at 28 °C.
Animals
The animal experiments conducted in this study utilized C57BL/6J strains, which were all C57 B/L 6J male mice purchased from the Animal Protection Center of Southern Medical University. All mice were maintained in a specific pathogen-free SPF environment. All mice were used in accordance with the scheme approved by the Review Committee of Southern Medical University. The mouse experimental scheme has also been approved by the Animal Care and Use Committee of Southern Medical University, and the experimental animal facilities are licensed by the Guangdong Medical Experimental Animal Center (SCXK-2021-0041). Additionally, lizards, salmon, bullfrogs, sparrows, turtles, and eels were acquired from animal breeding markets. Wild mice were captured using traps in their natural habitat, disinfected, and subsequently housed in the laboratory under appropriate conditions.
Plant
Vaucheria litorea was collected and transported under expert supervision and used immediately after thorough disinfection.
Detailed methods
Isolation of mouse bone marrow-derived macrophages (BMDMs)
Bones were aseptically harvested from the hind legs of C57BL/6 mice, and muscle tissues were removed. Bone marrow was flushed out of the bones using a 25-gauge needle attached to a syringe containing BMDM growth medium, which consists of DMEM (Gibco), 20% L929 cell-conditioned medium to generate M-CSF, 10% FBS (Gibco), and 1% penicillin/streptomycin (Gibco). BMDMs were allowed to differentiate for 7 days at 37 °C with 5% CO2, with the growth medium changed every 2 days during ex vivo culture.
Establishment of cell disease models
Oxidative stress models were created by treating both BMDM and AC16 cells with 500 µM H2O2 (Sigma) for 24 h and HepG2 cells with 700 µM H2O2 (Sigma) for 24 h. Inflammation models were developed by treating BMDM cells with 100 nM LPS (Sigma) for 24 h and HepG2 cells with 1 µM LPS (Sigma) for 24 h. Mitophagy models were established by treating both AC16 and HepG2 cells with 30 µM CCCP (Abcam) for 24 h. The senescence model was created by treating HepG2 cells with 30 g/L D-gal (MCE) for 48 h.
Disinfection and sterilization of plant mitochondria
Weigh 1 g of tissue and rinse it with clean water for 3 h. Transfer the tissue to a laminar flow hood and soak it in 75% ethanol for 1 min. Then, wash the tissue twice with DPBS containing 1% streptomycin/penicillin (Gibco). After soaking in 2% NaClO for 8 min, wash the tissue three times with DPBS containing 1% streptomycin/penicillin (Gibco), rinsing for 1 min each time. Next, rinse the tissue repeatedly in a 1% mercuric chloride (Sinoreagent) solution for 10 min. Afterward, wash the tissue three more times with DPBS containing 1% streptomycin/penicillin (Gibco). Finally, place the sterilized tissue blocks into a 100 mm² culture dish (Corning, USA, 43107) and cut them into 0.1 cm² pieces for further use.
Mitochondrial isolation
Mitochondria were isolated from cultured cells, animal tissues, and plant tissues using the Mitochondria-Cytosol Protein Isolation Kit (Applygen) and the Plant Mitochondrial Extraction Kit (Solarbio). For tissue homogenization, 100–200 mg of fresh animal tissue or 1 g of plant tissue was cut into 0.5 cm² pieces and placed into a Teflon-glass homogenizer containing 2.5 mL of ice-cold mitochondrial extraction buffer. The tissue was homogenized 20 times using a tight-fitting pestle. For cell homogenization, 2−5 × 107 cells were collected, digested, centrifuged, washed, and resuspended in 1.5 mL of ice-cold mitochondrial extraction buffer. The cell suspension was transferred to a small glass homogenizer and homogenized 30 times with a tight-fitting pestle. The homogenate was then transferred to a centrifuge tube and centrifuged at 800 × g for 5 min at 4 °C. The supernatant was collected and transferred to a new centrifuge tube, followed by centrifugation at 10,000 × g for 10 min at 4 °C. The mitochondrial pellet was resuspended in 0.2 mL of mitochondrial extraction buffer and subjected to centrifugation at 12,000 × g for 10 min at 4 °C. The supernatant was discarded, and the mitochondrial pellet was resuspended in mitochondrial respiration buffer for subsequent use.
Mitochondrial quality control
The extracted mitochondria were quantified using a BCA Protein Assay Kit (ThermoFisher). The integrity of the isolated mitochondria was assessed with the Mitochondrial Complex I and III Activity Assay Kit (Elabscience). The mitochondrial membrane potential was measured using the Mitochondrial Membrane Potential Assay Kit with JC-1 (Beyotime), and the capacity for energy synthesis was evaluated using an ATP Assay Kit (Beyotime).
Electron microscopy
For transmission electron microscopy (TEM), the isolated mitochondria were fixed overnight in 0.2 M sodium cacodylate buffer containing 2.5% formaldehyde, 5% glutaraldehyde, and 0.06% picric acid at pH 7.4. The samples were washed in cacodylate buffer, stained with 1% osmium tetroxide and 1.5% potassium ferricyanide, and then dehydrated using ethanol and propylene oxide. After infiltration and embedding in Epon-Araldite (Electron Microscopy Sciences, Hatfield, PA), sections (60–80 nm thick) were cut using an Ultracut-S ultramicrotome (Reichert Technologies, Depew, NY) and mounted on copper grids (200 mesh) for observation with a transmission electron microscope (Hitachi H-7500, Japan).
Mitochondrial transplantation in cells
After isolating the mitochondria, their concentration was quantified using a BCA Protein Assay Kit (ThermoFisher). For the co-culture, 10 µg of mitochondria were added per 10⁵ cells [17]. For competitive mitochondrial internalization, 1 µg of mitochondria was added per 10⁵ cells, using 0.5 µg of each type for dual mitochondrial competition or 0.33 µg of each type for triple mitochondrial competition.
Mitochondrial internalization and observation
Recipient cell membranes were labeled with WGA594 (ThermoFisher) or WGA488 (ThermoFisher), while isolated exogenous mitochondria were labeled with MitoTracker™ Green FM (ThermoFisher) or MitoTracker™ Red CMXRos (ThermoFisher). After 30 min, both the cells and mitochondria were washed twice with DPBS. The labeled mitochondria and recipient cells were co-cultured in a 35-mm confocal culture dish (Beyotime, Jiangsu, China, FCFC020-150pcs) for 24 h. Following the removal of the culture medium, the cells were washed twice with DPBS and fixed with glutaraldehyde. After an additional 30 min, the cells were washed twice with DPBS, and an antifade reagent (Prosperich, Israel, PL739) was added. Mitochondrial and cellular co-localization was observed using an inverted laser confocal microscope (LSM900 with Airyscan2, USA).
ATP content measurement
ATP content was measured using an ATP Assay Kit (Beyotime) according to the manufacturer’s instructions. One hundred micrograms of isolated mitochondria or cells seeded in a 6-well plate (at a density of 5 × 10⁵ cells per well) were treated with 200 μL of ATP lysis buffer and then centrifuged at 12,000 × g for 5 min at 4 °C. Twenty microliters of the supernatant were mixed with 100 μL of ATP detection solution in a 96-well white solid flat-bottom polystyrene microplate (Corning, USA, 3599), and luminescence (RLU) was measured using a multifunctional microplate reader (Tecan, Switzerland).
Mitochondrial membrane potential fluorescence quantification
The isolated mitochondria were subjected to protein quantification, followed by an assessment of mitochondrial membrane potential using the Mitochondrial Membrane Potential Assay Kit with JC-1 (Beyotime). In brief, 50 µg of mitochondria were mixed with 90 µL of JC-1 staining solution (2 µM) and transferred to a 96-well plate (Corning, USA, 3522). The mixture was thoroughly mixed, and the membrane potential was immediately measured using the BioTek Cytation5 Multifunctional Cell Imaging Microplate Reader (Agilent, USA), with an excitation wavelength of 485 nm and an emission wavelength of 590 nm.
Measurement of mitochondrial reactive oxygen species (ROS) fluorescence intensity
Cells were seeded at a density of 1 × 10⁴ cells per well in a 96-well solid white flat-bottom polystyrene tissue culture-treated microplate (Corning, USA, 3599). After applying the respective treatment conditions, exogenous mitochondria were co-cultured with the cells for 24 h. The medium was then removed, and the cells were washed twice with DPBS. A 5 µM working solution of MitoSOX Red (MCE) dye (100 µL) was added to each well, and the plate was gently agitated to ensure complete coverage of the cells. Following a 20-min incubation, the dye was removed, and the cells were washed twice with the medium. The mitochondrial ROS fluorescence intensity was measured using a multifunctional microplate reader (Tecan, Switzerland) with an excitation wavelength of 510 nm and an emission wavelength of 580 nm.
Measurement of intracellular reactive oxygen species
Cells were seeded at a density of 1 × 10⁵ cells per well in a 24-well plate (Corning, USA, 3524). After treating the cells under the specified conditions, exogenous mitochondria were co-cultured with the cells for 24 h. The medium was then removed, and the cells were washed twice with DPBS. Subsequently, the cells were incubated with 10 µM DCFH-DA (Beyotime) or 5 µM MitoSOX Red (MCE) at 37 °C for 30 min. The stained cells were washed with PBS and observed using the LSM900 with Airyscan2 (Zeiss, Germany) or the EVOS M5000 (ThermoFisher, USA).
Measurement of mitochondrial membrane potential
Cells were seeded at a density of 1 × 10⁵ cells per well in a 24-well plate (Corning, USA, 3524). After treating the cells under the specified conditions, exogenous mitochondria were co-cultured with the cells for 24 h. The medium was subsequently removed, and the cells were washed twice with DPBS. Following the protocol for the Mitochondrial Membrane Potential Assay Kit using JC-1, 500 µL of JC-1 staining solution (2 µM) was added to each well and incubated for 20 min. The cells were then washed and observed using the LSM900 with Airyscan2 (Zeiss, Germany) or the EVOS M5000 (ThermoFisher, USA) in phenol-red-free DMEM (Gibco).
CCK8 assay
According to the Cell Counting Kit-8 (Beyotime) protocol, 100 µL of cell suspension (density of 1 × 10⁴ cells/mL) was seeded in a 96-well plate (Corning, USA, 3522). After treating the cells under the specified conditions, 10 µL of CCK8 reagent was added, and the cells were incubated at 37 °C for 2 h. The absorbance at 450 nm was measured using a multifunctional microplate reader (Tecan, Switzerland). The percentage of cell viability was calculated based on the average optical density (OD) of each group.
DNA oxidative damage detection
The supernatant from the cultured cells was collected 24 h after mitochondrial transplantation. The concentration of 8-OHdG was measured using the 8-OHdG ELISA Kit (Elabscience), and absorbance at 450 nm was recorded to quantify DNA oxidative damage.
Immunoinflammatory cytokines detection
The supernatant from the cultured cells was collected 24 h after mitochondrial transplantation. The concentrations of IL-6, IL-10, and TNF-α were measured using the Mouse IL-6 ELISA Kit (Beyotime), Mouse IL-10 ELISA Kit (Beyotime), and Mouse TNF-α ELISA Kit (Beyotime), respectively, following the manufacturer’s instructions, with absorbance readings taken at 450 nm.
Measurement of MDA, GSH, SOD, ALT, AST, Cr
The supernatants of cultured cells following mitochondrial transplantation, as well as serum from model mice, were collected. According to the manufacturer’s instructions (NJJCBIO), the optical density (OD) values for MDA, GSH, SOD, ALT, AST, and Cr were measured at wavelengths of 532 nm, 412 nm, 550 nm, 505 nm, 510 nm, and 546 nm, respectively.
Cell fusion
When the cells reached high proliferation rates and optimal conditions, 3 × 10⁶ HL-1 and H9C2 cells were harvested separately. HL1/MDCK/Vero cells were stained with WGA647, while H9C2/MDBK/PK15 cells were stained with WGA594. The staining was conducted at 37 °C for 25 min, followed by two washes with DPBS. The cells were then combined and centrifuged at 200 g for 5 min. The supernatant was discarded, and pre-warmed 50% PEG1450 (Sigma) was gradually added while stirring the pellet. A total of 700 µL of 50% PEG1450 (Sigma) was added within 1 min. The mixture was incubated at 37 °C for 1 min, after which 10 mL of DMEM was added to dilute the PEG1450. The centrifuge tube was placed in a 37 °C incubator for 10 min before being centrifuged again at 200 × g for 5 min. The cells were washed twice with DPBS, resuspended in complete culture medium, and incubated at 37 °C with 5% CO₂ for 24–48 h. Cell fusion was observed using the LSM900 with Airyscan2 (Zeiss, Germany), and fused HL1 + H9C2 cells were sorted using a MoFlow XDP flow sorter (Beckman, USA) for further culture.
Competitive internalization of mitochondria
For the competitive internalization of two types of mitochondria, the mitochondria were stained with MitoTracker™ Deep Red FM (ThermoFisher) and MitoTracker™ Green FM (ThermoFisher) for 30 min. Following two washes with DPBS, the mitochondria were co-cultured with recipient cells for 24 h. The medium was then replaced with phenol-red-free medium containing ProLong™ antifade reagent (Invitrogen), and live-cell imaging was conducted using the LSM900 with Airyscan2 (Zeiss, Germany). For the competitive internalization of three types of mitochondria, the mitochondria were stained with MitoTracker™ Deep Red FM (ThermoFisher), MitoTracker™ Green FM (ThermoFisher), and MitoTracker™ Red CMXRos (ThermoFisher) for 30 min. After two washes with DPBS, the mitochondria were co-cultured with recipient cells for 24 h. The medium was then replaced with phenol-red-free DMEM (Gibco) containing ProLong™ antifade reagent (Invitrogen), and live-cell imaging was performed using the LSM900 with Airyscan2 (Zeiss, Germany).
HIIT functional training
Three 3-month-old mice underwent a 1-week pre-adaptation exercise program on a small animal treadmill (Shxinruan) before commencing an 8-week high-intensity interval training (HIIT) regimen. The maximum exercise capacity was assessed weekly to adjust the running speed accordingly. Each session began with a 10-min warm-up at a speed of 10 m/min. During the first 2 weeks, the regimen included high-intensity exercise at 24 m/min (80% VO2max) for 3 min, followed by 3 min of low-intensity recovery at 15 m/min with a 0° incline. In weeks 3 and 4, the regimen increased to 26 m/min (80% VO2max) for 3 min, followed by 3 min at 15 m/min with an 8° incline. In weeks 5 and 6, the speed was further increased to 28 m/min (80% VO2max) for 3 min, followed by 3 min at 17 m/min with a 16° incline. Finally, in weeks 7 and 8, the speed reached 30 m/min (80% VO2max) for 3 min, followed by 3 min at 17 m/min with a 25° incline. Each session concluded with 8 min at 15 m/min. The regimen consisted of 7 sets (3 + 3) per session, with each session lasting 1 h, conducted 5 days per week, and included 2 days of rest.
Aging model
The aging model was categorized into two categories. Disease Model: A total of 60 3-month-old C57 B/L mice were randomly assigned to five groups: D-gal group, C2C12 group, Young C57 group, Trained C57 group, and Wild Mice group, with 12 mice in each group. Aging was induced through subcutaneous injection of D-gal (MCE) at a dosage of 500 mg/kg once daily for 8 consecutive weeks. Normal Model: A total of 36 5-month-old C57 B/L mice (subhealth) were divided into three groups: Ctrl group, Young C57 group, and Wild Mice group, with 12 mice in each group. These mice received no drug treatment and were maintained under standard conditions.
Acute inflammation model
The purchased 2-month-old mice were not treated and normally cultured to 4-month-old (subhealth). The acute inflammation model was categorized into two categories. A total of 30 4-month-old C57BL/6 mice were divided into three groups: the LPS group, the Young C57 group, and the Wild Mice group, with 10 mice in each group. Acute inflammation was induced through intraperitoneal injection of LPS (Sigma) at a dosage of 2.5 mg/kg/day for 5 consecutive days. Normal Model: Additionally, another 30 4-month-old C57BL/6 mice were divided into three groups: the Ctrl group, the Young C57 group, and the Wild Mice group, with 10 mice in each group. These mice received no drug treatment and were maintained under standard conditions.
Animal mitochondrial transplantation
For the aging model, mitochondria were isolated from the gastrocnemius muscle tissues of 5-month-old Young C57, Trained C57, and Wild Mice, as well as C2C12 cells, using the Mitochondria-Cytosol Protein Isolation Kit (Applygen). Mitochondrial protein concentration was quantified using the BCA Protein Assay Kit (ThermoFisher). The isolated mitochondria were resuspended in DPBS and injected via the tail vein at a dosage of 100 μL/100 μg [82] into mice from the C2C12 group, Young C57 group, Trained C57 group, and Wild Mice group, while the D-gal group received no treatment. For the normal model, mitochondria from Trained C57 and Wild Mice were injected into their respective groups at the same dosage, with the Ctrl group receiving 100 μL of mitochondrial carrier solution. In the acute inflammation model, mitochondria were isolated from the gastrocnemius muscle of 2-month-old Young C57 and Wild Mice, quantified, and injected via the tail vein at a dosage of 100 μL/100 μg into the Young C57 and Wild Mice groups. The LPS group received no treatment. For normal model, Young C57 group and Wild Mice group were injected 100 μL/100 μg with the corresponding mitochondria through the tail vein, and the Ctrl group received 100 μL of mitochondrial carrier solution.
Mitochondrial biodistribution
The isolated mitochondria were stained with MitoTracker™ Deep-Red FM (ThermoFisher) at room temperature for 20 min, followed by two washes with DPBS. The mitochondria were then resuspended in DPBS and injected into mice via the tail vein. After 24 h, the hearts, livers, and kidneys were harvested, and the in vivo biodistribution of the mitochondria was analyzed using the PerkinElmer IVIS Spectrum (PerkinElmer, USA) multimodal small animal imaging system.
Motor function detection (rotarod test)
Adaptive training was performed before the experiment. During the formal experiment, the mice were placed on a rotating rod apparatus (Shxinruan, China) set to 5 rpm/min for 5 min, after which the speed was gradually increased to 40 rpm/min. The duration for which each mouse remained on the rotating rod was recorded using a stopwatch until they fell off. The maximum duration allowed for aging or acutely inflamed mice was 180 s, while normal mice were permitted a maximum of 360 s. Each mouse was tested three times, with a 3-min interval between trials, ensuring a quiet environment throughout the experiment.
Body weight measurement
All experimental groups of mice were weighed 3 days before and 1 week after mitochondrial transplantation, and their weights were recorded.
Mouse forelimb grip strength test
Adaptive training was performed before the experiment. During the formal experiment, each mouse was positioned on a mouse grip tester (Shxinruan, China), and gentle traction was applied to the tail, encouraging the mouse to grip the probe firmly with its forelimbs. The grip strength reading was recorded when the experimenter applied maximum force, and the experiment was conducted in a quiet environment.
Lower limb muscle volume and injury assessment
One week after mitochondrial injection, mice were briefly anesthetized with a mixture of isoflurane (3.0%) and O2 (2.0 L/min) via a nose cone and placed in a custom-made restraint tube, with the right lower limb fixed with a coil. During MRI imaging, mice were anesthetized with a mixture of isoflurane (1.5%) and O2 (0.6 L/min). The rectal temperature was maintained at 37 ± 1 °C using a heated water blanket. A small animal magnetic resonance imaging system, PharmaScan70/16S (Bruker, USA), was used to scan the horizontal plane of lower limb muscles, employing spin echo (SE) T1WI (TR4000ms, TE 28.5 ms), fast spin echo (FSE) T2WI (TR4000ms, TE 47.5 ms), with a slice thickness of 4 mm and an interval of 1 mm. Process the images using Paravision6.0.1 (Bruker Software, USA).
Western blot
For Cells: On the second day after cell fusion, HL1, H9C2, and HL1 + H9C2 cells were lysed, and proteins were extracted from each cell type. For Tissues: Mice were randomly selected from each of the two experimental groups in the second animal experiment. Three mice were chosen from each group of the acute inflammation model and three from each group of the normal model. Proteins were then extracted from the liver and kidney tissues of these mice. Protein concentrations were measured using a BCA protein assay kit. Proteins were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and subsequently transferred onto polyvinylidene fluoride (PVDF) (Millipore, USA) membranes. The membranes were blocked with 5% bovine serum albumin (BSA) or 5% milk for detecting phosphorylated proteins (P-p65, P-JNK) and total proteins (p65, JNK, β-actin, Drp1, Mfn1, Opa1). The PVDF membranes were incubated with primary antibodies at 4 °C overnight, followed by incubation with horseradish peroxidase-conjugated secondary antibodies at room temperature for 2 h. Gel images were captured using the Automatic Luminous System Tanon-5200Multi (Tanon, China), and data were analyzed using ImageJ (ImageJ Software, National Institutes of Health, USA).
Quantitative PCR
Twenty-four hours after mitochondrial transplantation, total RNA was extracted using Trizol reagent. Purified RNA was reverse-transcribed into cDNA using the PrimeScript™ RT reagent Kit (Takara). RT-qPCR was conducted with SYBR Green Master Mix (ThermoFisher) using the ABI QuantStudio 5 Real-Time PCR system (ThermoFisher, USA). Expression levels were normalized to the internal control 18 s rRNA. The primer sequences are listed in Primer Sequence Table.
| Oligonucleotides | ||
|---|---|---|
|
Mouse 18S rRNA RT-qPCR Forward: CGATCCGAGGGCCTCACTA Reverse: AGTCCCTGCCCTTTGTACACA |
This paper | N/A |
|
Mouse IL-4 RT-qPCR Forward: ATGGGTCTCACCTCCCAACTG Reverse: TCAGCTCGAACACTTTGAATATTTCTCTCTCAT |
This paper | N/A |
|
Mouse IL-6 RT-qPCR Forward: CCAGAGTCCTTCAGAGAGATACA Reverse: CCTTCTGTGACTCCAGCTTATC |
This paper | N/A |
|
Mouse IL-8 RT-qPCR Forward: ATGACTTCCAAGCTGGCCGTG Reverse: TTATGAATTCTCAGCCCTCTTCAAAAACTTCTC |
This paper | N/A |
|
Mouse IL-13 RT-qPCR Forward: TGAGCAACATCACACAAGACC Reverse: GGCCTTGCGGTTACAGAGG |
This paper | N/A |
|
Mouse IL-18 RT-qPCR Forward: GAAGGACACTTTCTTGCTTGC Reverse: GTGAGAGTGAACATTACAGATTTATCC |
This paper | N/A |
|
Mouse IL-1β RT-qPCR Forward: GGTGTGTGACGTTCCCATTA Reverse: ATTGAGGTGGAGAGCTTTCAG |
This paper | N/A |
|
Mouse TGF-β RT-qPCR Forward: ATGACACCACCTGAACGTCTCTTC Reverse: CTACAGAGCGAAGGCTCCAAAGAAGACAGTACT |
This paper | N/A |
|
Mouse SOD1 RT-qPCR Forward: AACCAGTTGTGTTGTCAGGAC Reverse: CCACCATGTTTCTTAGAGTGAGG |
This paper | N/A |
|
Mouse HMGB1 RT-qPCR Forward: GCTGACAAGGCTCGTTATGAA Reverse: CCTTTGATTTTGGGGCGGTA |
This paper | N/A |
|
Mouse COX1 RT-qPCR Forward: GACCGCAATGAACTTCGGGA Reverse: TCCATTAGGTCTCTAAAGCCGAG |
This paper | N/A |
|
Mouse ARG1 RT-qPCR Forward: CTCCAAGCCAAAGTCCTTAGAG Reverse: GGAGCTGTCATTAGGGACATCA |
This paper | N/A |
|
Mouse MPO RT-qPCR Forward: CGACATTCTGCCTGGATAAGG Reverse: TCTCGAACACTCTCCATTTGC |
This paper | N/A |
|
Mouse CCL5 RT-qPCR Forward: TTTGCCTACCTCTCCCTCG Reverse: CGACTGCAAGATTGGAGCACT |
This paper | N/A |
|
Mouse CCL1 RT-qPCR Forward: TGCCGTGTGGATACAGGATG Reverse: GTTGAGGCGCAGCTTTCTCTA |
This paper | N/A |
|
Mouse CXCL1 RT-qPCR Forward: CCGAAGTCATAGCCACACTCA Reverse: CTCCGTTACTTGGGGACACC |
This paper | N/A |
|
Mouse CXCL2 RT-qPCR Forward: CCAACCACCAGGCTACAGG Reverse: GCGTCACACTCAAGCTCTG |
This paper | N/A |
Quantification and statistical analysis
Cell experiments were repeated at least three times except for mitochondrial competitive internalization and animal experiments except for body weight measurement, grip strength, and motor function tests. GraphPad Prism 9.01 (GraphPad Software Inc., USA) software was used for statistical analysis. The data were expressed as mean ± standard error (SEM). For normally distributed data and comparisons between two groups, the significance was calculated using unpaired two-tailed Student’s t-tests. Comparisons between three or more groups were performed using ordinary One-way ANOVA, Using Two-way ANOVA, and Bonferroni post hoc test were used to compare multi-factor variance for multiple data. The significance threshold was determined by p value < 0.05, and the annotation was ns no statistical significance, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Supplementary information
Acknowledgements
I am very grateful to Professor Yong Jiang of Southern Medical University for the experimental site and many equipment support provided for this experiment. I sincerely thank the People’s Hospital of Dongguan City, affiliated with Southern Medical University, for providing free access to experimental instruments. I also extend our gratitude to Dr. Guiming Chen from the Department of Pathology and Pathophysiology at Southern Medical University and Dr. Rong Wu from the School of Traditional Chinese Medicine for their technical support with mitochondrial tail vein injections. Additionally, I appreciate the assistance of Master Xin Heng in the experiments involving fluorescence microscopy for detecting reactive oxygen species and mitochondrial membrane potential. Moreover, I express strong condemnation towards those who deliberately sabotage others’ experiments. I call for all laboratories actively weed out the bad apples.
Author contributions
Xiaomeng Lu leads all the experimental design, experimental operation, experimental analysis, article writing, and proofreading.
Funding
With no funding, this work was carried out with the XL’s full commitment, with all funding provided personally by the author (XL).
Data availability
All data generated in the present study may be requested from the corresponding author. Cell fusion and hybrid mitochondria in Fig. 3 are drawn by Biorender.
Competing interests
The author declares no competing interests.
Ethics approval and consent to participate
The experimental animal facilities have been licensed by the Guangdong Medical Experimental Animal Center (SCXK-2021-0041). All methods were performed in accordance with the relevant guidelines and regulations.
Footnotes
Edited by Stephen Tait
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41419-025-07643-8.
References
- 1.Emani SM, Piekarski BL, Harrild D, Del Nido PJ, McCully JD. Autologous mitochondrial transplantation for dysfunction after ischemia-reperfusion injury. J Thorac Cardiovasc Surg. 2017;154:286–9. [DOI] [PubMed] [Google Scholar]
- 2.Gollihue JL, Rabchevsky AG. Prospects for therapeutic mitochondrial transplantation. Mitochondrion. 2017;35:70–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCully JD, Cowan DB, Emani SM, Del Nido PJ. Mitochondrial transplantation: from animal models to clinical use in humans. Mitochondrion. 2017;34:127–34. [DOI] [PubMed] [Google Scholar]
- 4.McCully JD, Cowan DB, Pacak CA, Toumpoulis IK, Dayalan H, Levitsky S. Injection of isolated mitochondria during early reperfusion for cardioprotection. Am J Physiol Heart Circ Physiol. 2009;296:H94–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gorick C, Debski A. Mitochondrial transplantation for ischemic heart disease. Nat Nanotechnol. 2024;19:1247–1248. [DOI] [PubMed] [Google Scholar]
- 6.Moskowitzova K, Shin B, Liu K, Ramirez-Barbieri G, Guariento A, Blitzer D, et al. Mitochondrial transplantation prolongs cold ischemia time in murine heart transplantation. J Heart Lung Transpl. 2019;38):92–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun L, Zhao Z, Guo J, Qin Y, Yu Q, Shi X, et al. Mitochondrial transplantation confers protection against the effects of ischemic stroke by repressing microglial pyroptosis and promoting neurogenesis. Neural Regen Res. 2024;19:1325–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eo H, Yu SH, Choi Y, Kim Y, Kang YC, Lee H, et al. Mitochondrial transplantation exhibits neuroprotective effects and improves behavioral deficits in an animal model of Parkinson’s disease. Neurotherapeutics. 2024;21:e00355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu Z, Li X, Wang X, Zuo X, Ma Y, Gao X, et al. Photobiomodulation augments the effects of mitochondrial transplantation in the treatment of spinal cord injury in rats by facilitating mitochondrial transfer to neurons via Connexin 36. Bioeng Transl Med. 2023;8:e10473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Su Y, Zhu L, Yu X, Cai L, Lu Y, Zhang J, et al. Mitochondrial transplantation attenuates airway hyperresponsiveness by inhibition of cholinergic hyperactivity. Theranostics. 2016;6:1244–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ulger O, Kubat GB, Cicek Z, Celik E, Atalay O, Suvay S, et al. The effects of mitochondrial transplantation in acetaminophen-induced liver toxicity in rats. Life Sci. 2021;279:119669. [DOI] [PubMed] [Google Scholar]
- 12.Lee JM, Hwang JW, Kim MJ, Jung SY, Kim KS, Ahn EH, et al. Mitochondrial transplantation modulates inflammation and apoptosis, alleviating tendinopathy both in vivo and in vitro. Antioxidants. 2021;10:696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Z, Cao X, Liu Z, Wu F, Lin C, Wang CM. Therapeutic effect of mitochondrial transplantation on burn injury. Free Radic Biol Med. 2024;215:2–13. [DOI] [PubMed] [Google Scholar]
- 14.Kim S, Noh JH, Lee MJ, Park YJ, Kim BM, Kim YS, et al. Effects of Mitochondrial transplantation on transcriptomics in a polymicrobial sepsis model. Int J Mol Sci. 2023;24:15326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cruz-Gregorio A, Aranda-Rivera AK, Amador-Martinez I, Maycotte P. Mitochondrial transplantation strategies in multifaceted induction of cancer cell death. Life Sci. 2023;332:122098. [DOI] [PubMed] [Google Scholar]
- 16.Hosseinian S, Ali Pour P, Kheradvar A. Prospects of mitochondrial transplantation in clinical medicine: aspirations and challenges. Mitochondrion. 2022;65:33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang JC, Chang HS, Wu YC, Cheng WL, Lin TT, Chang HJ, et al. Mitochondrial transplantation regulates antitumour activity, chemoresistance and mitochondrial dynamics in breast cancer. J Exp Clin Cancer Res. 2019;38:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun C, Liu X, Wang B, Wang Z, Liu Y, Di C, et al. Endocytosis-mediated mitochondrial transplantation: transferring normal human astrocytic mitochondria into glioma cells rescues aerobic respiration and enhances radiosensitivity. Theranostics. 2019;9:3595–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun X, Gao R, Li W, Zhao Y, Yang H, Chen H, et al. Alda-1 treatment promotes the therapeutic effect of mitochondrial transplantation for myocardial ischemia-reperfusion injury. Bioact Mater. 2021;6:2058–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boutonnet L, Mallard J, Charles AL, Hucteau E, Gény B, Lejay A, et al. Autologous mitochondrial transplantation in male mice as a strategy to prevent deleterious effects of peripheral ischemia-reperfusion. Am J Physiol Cell Physiol. 2024;326:C449–56. [DOI] [PubMed] [Google Scholar]
- 21.Chen T, Majerníková N, Marmolejo-Garza A, Trombetta-Lima M, Sabogal-Guáqueta AM, Zhang Y, et al. Mitochondrial transplantation rescues neuronal cells from ferroptosis. Free Radic Biol Med. 2023;208:62–72. [DOI] [PubMed] [Google Scholar]
- 22.Yao X, Ma Y, Zhou W, Liao Y, Jiang Z, Lin J, et al. In-cytoplasm mitochondrial transplantation for mesenchymal stem cells engineering and tissue regeneration. Bioeng Transl Med. 2022;7:e10250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu Z, Hou Y, Zhou W, Zhao Z, Liu Z, Fu A. The effect of mitochondrial transplantation therapy from different gender on inhibiting cell proliferation of malignant melanoma. Int J Biol Sci. 2021;17:2021–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhattacharya D, Slavin MB, Hood DA. Muscle mitochondrial transplantation can rescue and maintain cellular homeostasis. Am J Physiol Cell Physiol. 2023;325:C862–84. [DOI] [PubMed] [Google Scholar]
- 25.Picone P, Porcelli G, Bavisotto CC, Nuzzo D, Galizzi G, Biagio PLS, et al. Synaptosomes: new vesicles for neuronal mitochondrial transplantation. J Nanobiotechnol. 2021;19:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baharvand F, Habibi Roudkenar M, Pourmohammadi-Bejarpasi Z, Najafi-Ghalehlou N, Feizkhah A, Bashiri Aliabadi S, et al. Safety and efficacy of platelet-derived mitochondrial transplantation in ischaemic heart disease. Int J Cardiol. 2024;410:132227. [DOI] [PubMed] [Google Scholar]
- 27.Bamshad C, Habibi Roudkenar M, Abedinzade M, Yousefzadeh Chabok S, Pourmohammadi-Bejarpasi Z, Najafi-Ghalehlou N, et al. Human umbilical cord-derived mesenchymal stem cells-harvested mitochondrial transplantation improved motor function in TBI models through rescuing neuronal cells from apoptosis and alleviating astrogliosis and microglia activation. Int Immunopharmacol. 2023;118:110106. [DOI] [PubMed] [Google Scholar]
- 28.Guariento A, Piekarski BL, Doulamis IP, Blitzer D, Ferraro AM, Harrild DM, et al. Autologous mitochondrial transplantation for cardiogenic shock in pediatric patients following ischemia-reperfusion injury. J Thorac Cardiovasc Surg. 2021;162:992–1001. [DOI] [PubMed] [Google Scholar]
- 29.Dubinin MV, Mikheeva IB, Stepanova AE, Igoshkina AD, Cherepanova AA, Semenova AA, et al. Mitochondrial transplantation therapy ameliorates muscular dystrophy in mdx mouse model. Biomolecules. 2024;14:316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaza AK, Wamala I, Friehs I, Kuebler JD, Rathod RH, Berra I, et al. Myocardial rescue with autologous mitochondrial transplantation in a porcine model of ischemia/reperfusion. J Thorac Cardiovasc Surg. 2017;153:934–43. [DOI] [PubMed] [Google Scholar]
- 31.Javani G, Babri S, Farajdokht F, Ghaffari-Nasab A, Mohaddes G. Mitochondrial transplantation improves anxiety- and depression-like behaviors in aged stress-exposed rats. Mech Ageing Dev. 2022;202:111632. [DOI] [PubMed] [Google Scholar]
- 32.Otten AB, Smeets HJ. Evolutionary defined role of the mitochondrial DNA in fertility, disease and ageing. Hum Reprod Update. 2015;21:671–89. [DOI] [PubMed] [Google Scholar]
- 33.Gray MW. Origin and evolution of mitochondrial DNA. Annu Rev Cell Biol. 1989;5:25–50. [DOI] [PubMed] [Google Scholar]
- 34.Broughton RE, Reneau PC. Spatial covariation of mutation and nonsynonymous substitution rates in vertebrate mitochondrial genomes. Mol Biol Evol. 2006;23:1516–24. [DOI] [PubMed] [Google Scholar]
- 35.Levoux J, Prola A, Lafuste P, Gervais M, Chevallier N, Koumaiha Z, et al. Platelets facilitate the wound-healing capability of mesenchymal stem cells by mitochondrial transfer and metabolic reprogramming. Cell Metab. 2021;33:283–99.e9. [DOI] [PubMed] [Google Scholar]
- 36.Zhang A, Liu Y, Pan J, Pontanari F, Chia-Hao Chang A, Wang H, et al. Delivery of mitochondria confers cardioprotection through mitochondria replenishment and metabolic compliance. Mol Ther. 2023;31:1468–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jin M, Wang Y, Huang M, Lu Z, Wang Y. Sulphation can enhance the antioxidant activity of polysaccharides produced by Enterobacter cloacae Z0206. Carbohydr Polym. 2014;99:624–9. [DOI] [PubMed] [Google Scholar]
- 38.Ganote CE. Armstrong SC. Effects of CCCP-induced mitochondrial uncoupling and cyclosporin A on cell volume, cell injury and preconditioning protection of isolated rabbit cardiomyocytes. J Mol Cell Cardiol. 2003;35:749–59. [DOI] [PubMed] [Google Scholar]
- 39.Jiang XP, Elliott RL, Head JF. Exogenous normal mammary epithelial mitochondria suppress glycolytic metabolism and glucose uptake of human breast cancer cells. Breast cancer Res Treat. 2015;153:519–29. [DOI] [PubMed] [Google Scholar]
- 40.Clark MA, Shay JW. Mitochondrial transformation of mammalian cells. Nature. 1982;295:605–7. [DOI] [PubMed] [Google Scholar]
- 41.Song JH, Choi J, Hong YJ, La H, Hong TK, Hong K, et al. Developmental potency and metabolic traits of extended pluripotency are faithfully transferred to somatic cells via cell fusion-induced reprogramming. Cells. 2022;11:3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reznick DN, Ricklefs RE. Darwin’s bridge between microevolution and macroevolution. Nature. 2009;457:837–42. [DOI] [PubMed] [Google Scholar]
- 43.Hsu CC, Wang CH, Wu LC, Hsia CY, Chi CW, Yin PH, et al. Mitochondrial dysfunction represses HIF-1α protein synthesis through AMPK activation in human hepatoma HepG2 cells. Biochim Biophys Acta. 2013;1830:4743–51. [DOI] [PubMed] [Google Scholar]
- 44.Volchuk A, Ye A, Chi L, Steinberg BE, Goldenberg NM. Indirect regulation of HMGB1 release by gasdermin D. Nat Commun. 2020;11:4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ali Pour P, Kenney MC, Kheradvar A. Bioenergetics consequences of mitochondrial transplantation in cardiomyocytes. J Am Heart Assoc. 2020;9:e014501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shang Q, Bian X, Zhu L, Liu J, Wu M, Lou S. Lactate mediates high-intensity interval training-induced promotion of hippocampal mitochondrial function through the GPR81-ERK1/2 Pathway. Antioxidants. 2023;12:2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Souza-Tavares H, Santana-Oliveira DA, Vasques-Monteiro IML, Silva-Veiga FM, Mandarim-de-Lacerda CA, Souza-Mello V. Exercise enhances hepatic mitochondrial structure and function while preventing endoplasmic reticulum stress and metabolic dysfunction-associated steatotic liver disease in mice fed a high-fat diet. Nutr Res. 2024;126:180–92. [DOI] [PubMed] [Google Scholar]
- 48.Yan C, Ma Z, Ma H, Li Q, Zhai Q, Jiang T, et al. Mitochondrial transplantation attenuates brain dysfunction in sepsis by driving microglial M2 polarization. Mol Neurobiol. 2020;57:3875–90. [DOI] [PubMed] [Google Scholar]
- 49.Hwang JW, Lee MJ, Chung TN, Lee HAR, Lee JH, Choi SY, et al. The immune modulatory effects of mitochondrial transplantation on cecal slurry model in rat. Crit Care. 2021;25:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhu J, Wang Z, Xu M, Ma X, Shen M, Yan J, et al. Mitochondrial transplantation following cardiopulmonary resuscitation improves neurological function in rats by inducing M2-type MG/MΦ polarization. J Transl Med. 2024;22:1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao Z, Hou Y, Zhou W, Keerthiga R, Fu A. Mitochondrial transplantation therapy inhibit carbon tetrachloride-induced liver injury through scavenging free radicals and protecting hepatocytes. Bioeng Transl Med. 2021;6:e10209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maleki F, Rabbani S, Shirkoohi R, Rezaei M. Allogeneic mitochondrial transplantation ameliorates cardiac dysfunction due to doxorubicin: an in vivo study. Biomed Pharmacother. 2023;168:115651. [DOI] [PubMed] [Google Scholar]
- 53.Katz LA. Origin and diversification of eukaryotes. Annu Rev Microbiol. 2012;66:411–27. [DOI] [PubMed] [Google Scholar]
- 54.Lynch M, Marinov GK. Membranes, energetics, and evolution across the prokaryote-eukaryote divide. eLife. 2017;6:e20437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roger AJ, Muñoz-Gómez SA, Kamikawa R. The origin and diversification of mitochondria. Curr Biol. 2017;27:R1177–92. [DOI] [PubMed] [Google Scholar]
- 56.Schavemaker PE, Muñoz-Gómez SA. The role of mitochondrial energetics in the origin and diversification of eukaryotes. Nat Ecol Evol. 2022;6:1307–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mailloux RJ, Treberg J, Grayson C, Agellon LB, Sies H. Mitochondrial function and phenotype are defined by bioenergetics. Nat Metab. 2023;5:1641. [DOI] [PubMed] [Google Scholar]
- 58.Brand MD, Orr AL, Perevoshchikova IV, Quinlan CL. The role of mitochondrial function and cellular bioenergetics in ageing and disease. Br J Dermatol. 2013;169:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.James JE, Piganeau G, Eyre-Walker A. The rate of adaptive evolution in animal mitochondria. Mol Ecol. 2016;25:67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hill GE. Mitonuclear ecology. Mol Biol Evol. 2015;32:1917–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weaver RJ, Rabinowitz S, Thueson K, Havird JC. Genomic signatures of mitonuclear coevolution in mammals. Mol Biol Evol. 2022;39:msac233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yoon YG, Haug CL, Koob MD. Interspecies mitochondrial fusion between mouse and human mitochondria is rapid and efficient. Mitochondrion. 2007;7:223–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jensen-Seaman MI, Furey TS, Payseur BA, Lu Y, Roskin KM, Chen CF, et al. Comparative recombination rates in the rat, mouse, and human genomes. Genome Res. 2004;14:528–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhao S, Shetty J, Hou L, Delcher A, Zhu B, Osoegawa K, et al. Human, mouse, and rat genome large-scale rearrangements: stability versus speciation. Genome Res. 2004;14:1851–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brüggemann M, Free J, Diamond A, Howard J, Cobbold S, Waldmann H. Immunoglobulin heavy chain locus of the rat: striking homology to mouse antibody genes. Proc Natl Acad Sci USA. 1986;83:6075–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Djian P, Phillips M, Easley K, Huang E, Simon M, Rice RH, et al. The involucrin genes of the mouse and the rat: study of their shared repeats. Mol Biol Evol. 1993;10:1136–49. [DOI] [PubMed] [Google Scholar]
- 67.Brudno M, Poliakov A, Salamov A, Cooper GM, Sidow A, Rubin EM, et al. Automated whole-genome multiple alignment of rat, mouse, and human. Genome Res. 2004;14:685–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huang H, Winter EE, Wang H, Weinstock KG, Xing H, Goodstadt L, et al. Evolutionary conservation and selection of human disease gene orthologs in the rat and mouse genomes. Genome Biol. 2004;5:R47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shoja V, Zhang L. A roadmap of tandemly arrayed genes in the genomes of human, mouse, and rat. Mol Biol Evol. 2006;23:2134–41. [DOI] [PubMed] [Google Scholar]
- 70.Jacoby E, Bar-Yosef O, Gruber N, Lahav E, Varda-Bloom N, Bolkier Y, et al. Mitochondrial augmentation of hematopoietic stem cells in children with single large-scale mitochondrial DNA deletion syndromes. Sci Transl Med. 2022;14:eabo3724. [DOI] [PubMed] [Google Scholar]
- 71.Patel SP, Michael FM, Arif Khan M, Duggan B, Wyse S, Darby DR, et al. Erodible thermogelling hydrogels for localized mitochondrial transplantation to the spinal cord. Mitochondrion. 2022;64:145–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jain R, Begum N, Rajan S, Tryphena KP, Khatri DK. Role of F-actin-mediated endocytosis and exercise in mitochondrial transplantation in an experimental Parkinson’s disease mouse model. Mitochondrion. 2024;74:101824. [DOI] [PubMed] [Google Scholar]
- 73.Ali Pour P, Hosseinian S, Kheradvar A. Mitochondrial transplantation in cardiomyocytes: foundation, methods, and outcomes. Am J Physiol Cell Physiol. 2021;321:C489–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Befroy DE, Petersen KF, Dufour S, Mason GF, Rothman DL, Shulman GI. Increased substrate oxidation and mitochondrial uncoupling in skeletal muscle of endurance-trained individuals. Proc Natl Acad Sci USA. 2008;105:16701–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Campos JC, Marchesi Bozi LH, Krum B, Grassmann Bechara LR, Ferreira ND, Arini GS, et al. Exercise preserves physical fitness during aging through AMPK and mitochondrial dynamics. Proc Natl Acad Sci USA. 2023;120:e2204750120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jakovlić I, Zou H, Ye T, Zhang H, Liu X, Xiang CY, et al. Mitogenomic evolutionary rates in bilateria are influenced by parasitic lifestyle and locomotory capacity. Nat Commun. 2023;14:6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Phielix E, Meex R, Moonen-Kornips E, Hesselink MK, Schrauwen P. Exercise training increases mitochondrial content and ex vivo mitochondrial function similarly in patients with type 2 diabetes and in control individuals. Diabetologia. 2010;53:1714–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Granata C, Jamnick NA, Bishop DJ. Training-induced changes in mitochondrial content and respiratory function in human skeletal muscle. Sports Med. 2018;48:1809–28. [DOI] [PubMed] [Google Scholar]
- 79.Pileggi CA, Blondin DP, Hooks BG, Parmar G, Alecu I, Patten DA, et al. Exercise training enhances muscle mitochondrial metabolism in diet-resistant obesity. EBioMedicine. 2022;83:104192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Powers SK, Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev. 2008;88:1243–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kaliman P, Párrizas M, Lalanza JF, Camins A, Escorihuela RM, Pallàs M. Neurophysiological and epigenetic effects of physical exercise on the aging process. Ageing Res Rev. 2011;10:475–86. [DOI] [PubMed] [Google Scholar]
- 82.Nakamura Y, Lo EH, Hayakawa K. Placental mitochondria therapy for cerebral ischemia-reperfusion injury in mice. Stroke. 2020;51:3142–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated in the present study may be requested from the corresponding author. Cell fusion and hybrid mitochondria in Fig. 3 are drawn by Biorender.