Abstract
This research examined the impact of exogenous Glycine Betaine (GB) at various concentrations (0, 5, 10, and 15 mM) on cap browning and the overall quality of button mushrooms during a 15-day storage period at 4 °C. The study found that a concentration of 10 mM GB resulted in the least amount of cap browning. Additionally, mushrooms treated with 10 mM GABA showed lower levels of weight loss, electrolyte leakage, and malondialdehyde (MDA) content, while maintaining higher firmness. The use of 10 mM GB also led to an increase in the accumulation of total phenolic compounds, which was associated with higher expression and activity of phenylalanine ammonia-lyase (PAL) and lower expression and activity of polyphenol oxidase (PPO). Furthermore, GB treatment effectively reduced H2O2 content and increased proline and ascorbic acid (AsA) content. The application of exogenous GB resulted in an increase in the endogenous GB content and BADH activity. In conclusion, GB treatment can be utilized to slow down cap browning and preserve the nutritional quality and sensory of button mushrooms during chilling storage.
Keywords: Button mushroom, Cap browning, Glycine betaine, Phenolic compound, Storage period
Subject terms: Plant biotechnology, Plant physiology, Plant stress responses
Introduction
Button mushroom (Agaricus bisporus) is highly sensitive to cold temperatures and is particularly prone to experiencing chilling injury (CI) when stored in cold conditions below 8 °C for more than 20 days. The symptoms of CI that occur in button mushrooms after cold storage include the development of internal browning (IB), a fuzzy or leathery texture, and an inability to reach the desired stages of ripeness Yang, et al.1,2. The occurrence of CI poses a significant challenge in mushroom refrigeration as it diminishes the quality attributes of the fruiting body, including its appearance, texture mushrooms, and nutritional content3. Several approaches have been explored to mitigate CI in mushrooms, such as maintaining low-temperature conditions, employing hot air treatments, and utilizing substances like methyl jasmonate or oxalic acid4–6. These methods have shown promise in reducing the incidence of CI in mushrooms. At present, there remains a necessity for the continued advancement of more efficient techniques in order to control or diminish chilling injury (CI) in white button mushrooms stored under cold conditions7,8.
Glycine betaine (GB), derived from the amino acid glycine, is a quaternary ammonium compound with significant roles in plants. It acts as an osmotic regulator, protecting plant tissues from environmental stresses9–11. GB maintains cell membrane structure, stabilizes intracellular proteins, and regulates osmotic pressure12. It enhances tolerance to cold and drought stresses, bolstering a plant’s resilience11–13. GB serves as a protective agent, safeguarding plant tissues and supporting their ability to withstand external pressures10.
Studies have revealed that the application of exogenous glycine betaine (GB) has proven effective in mitigating the damage caused by cold stress in Arabidopsis and strawberry plants14,15. The utilization of GB resulted in improved resistance to cold temperatures in mushrooms by stimulating energy levels and increasing the presence of endogenous GB, γ-aminobutyric acid, and proline16. Nevertheless, the specific mechanism through which GB enhances the chilling tolerance of peach fruit remains to be investigated17,18.
The accumulation of phenolics and flavonoids is a notable outcome of cold stress in plants, and it plays a crucial role in enhancing stress tolerance19–21. Studies have shown that cold stress can lead to an increase in the levels of phenolic acids or anthocyanins in soybean roots, indicating their potential involvement in cold acclimation processes22,23. In terms of fruits post-harvesting, chilling injury (CI) was reduced in mushrooms through the use of 2,4-epibrassinolide or temperature conditioning, which led to increased levels of phenolic and flavonoid compounds23,24. However, the way that glycine betaine (GB) treatment regulates phenolic metabolism in mushrooms stored under cold conditions remains uncertain.
However, the precise mechanisms by which glycine betaine (GB) treatment influences the phenylpropanoid pathway and sugar metabolism in mushrooms during cold storage remain largely unexplored. Addressing this knowledge gap is crucial for developing effective strategies to enhance post-harvest quality. Therefore, the primary objectives of this study were to systematically evaluate the alterations in phenylpropanoid and sugar metabolism in button mushrooms following exogenous application of GB under chilling conditions, and to investigate the potential regulatory roles of phenolic compound profiles and soluble sugars in the mechanism of GB-induced tolerance to chilling stress in mushrooms. Based on previous research on plant stress responses, we hypothesize that GB application will modulate key enzymes and metabolites within these pathways, ultimately contributing to improved cold tolerance and extended shelf life of button mushrooms.
Materials and methods
Mushroom materials and treatments
Commercially mature button mushrooms (Agaricus bisporus) were obtained from a local mushroom farmer located in Parand, Iran in 2022 for the study. Only mushrooms displaying uniform ripeness and similar sizes were chosen. Any mushrooms exhibiting physical damage were excluded to ensure standardization.
Glycine betaine (CAS 58823-88-4) was purchased from Aikeda, Chengdu, China. To perform the Glycine betaine treatment, the method described by Yang, et al.1 was followed. A total of 540 mushrooms were utilized, divided into 3 groups of 180 mushroom each (3 replications; 60 mushrooms per replication). These mushrooms were subjected to immersion in Glycine betaine solutions of different concentrations including 0 mM (control), 10 mM, and 15 mM for a duration of 15 min at a temperature of 20 °C.
After the treatment, the mushrooms were thoroughly dried at a temperature of 25 °C for a period of 1 h. Subsequently, they were packaged in propylene boxes with a thickness of 0.15 mm, with 20 mushrooms placed in each bag. The packaged boxes were then stored in a dark environment at a temperature of 4 ± 0.5 °C and with a relative humidity of 80–90% for a duration of 15 days. To assess the changes in quality, samples of 20 mushrooms from each treatment were collected at intervals of 5, 10, and 15 days during the storage period. The evaluation included measurements of cap browning, weight loss, firmness, and electrolyte leakage, with 10 mushroom caps from each replication being analyzed. Additionally, a separate set of 10 caps per replication was processed, powdered using liquid nitrogen, and stored at -80 °C for subsequent gene expression and biochemical analysis.
Chilling injury, cap browning, weight loss, firmness and color analysis
Chilling injury was assessed using a 1–5 hedonic scale, as described by Kebbeh et al.25where 0 indicated no visible disorder, 1 represented visible disorder covering ≤ 1/4 of the fruit surface, 2 denoted disorders covering > 1/4 but ≤ 1/3, 3 indicated disorder covering > 1/3 but ≤ 1/2, and 4 signified disorder covering > 1/2. The scale evaluated the severity of symptoms such as pulp lignification, grainy texture, water-soaking, or pitting. The incidence of chilling injury (%) was calculated using the formula: Σ [(chilling injury level) × (number of fruit at the chilling injury level)]/(4 × total number of fruit) × 100.
The surface color of the mushroom caps was evaluated every 5 days during the storage period at 4 °C using a Minolta spectrophotometer on 10 mushrooms from each group. The percentage of cap browning, as an indicator of brown color purity, was assessed based on the method of Xu, et al.7. Weight loss was quantified following the methodology outlined by Nasiri, et al.26. The firmness of the mushrooms, which indicated softening, was assessed using the penetration test as described in the methodology by Nasiri, et al.26.
The color parameters of mushrooms were measured at 0, 5, 10, and 15 days of storage. Color measurements (CIE L*, a*, b* values) were determined using a WSC-S colorimeter (Shanghai Precision Instrument Co., Ltd., China). For each mushroom, a single measurement was taken on the upper surface of the cap. In each replication, three mushrooms were evaluated, resulting in a total of nine measurements per treatment per experimental trial.
Electrolyte leakage, H2O2, proline, and MDA accumulation
Electrolyte leakage was assessed following the method outlined by Malekzadeh, et al.27. To measure MDA accumulation, 1 gram of the mushroom powder was homogenized in 25 mL of 5% (w/v) trichloroacetic acid, as described in the protocol by Malekzadeh, et al.27. MDA accumulation was expressed as µmol kg− 1 fresh weight. For evaluation of H2O2 accumulation using the titanium (IV) method, 1 g of frozen powder was homogenized in 5 mL of acetone at 0 °C as outlined by Liu, et al.28. The endogenous proline accumulation was quantified using the acid ninhydrin method, as previously described by Malekzadeh et al.27. Briefly, one gram of frozen tissue powder was homogenized with 10 mL of 3% (v/v) sulfosalicylic acid. The resulting solution was then subjected to the acid ninhydrin assay to determine proline levels. The endogenous proline concentration was calculated and expressed in millimoles per kilogram (mmol kg⁻¹) on a fresh weight basis.
Determination of PAL enzyme activity
The method outlined by Sánchez-Rodríguez, et al.29was employed to determine PAL activity. To determine the activity of the PAL enzyme, one gram of frozen powder was homogenized with 20 milliliters of 50 millimolar borate buffer (pH 8.5) containing 5 millimolar β-mercaptoethanol and 0.5 g of polyvinylpolypyrrolidone (PVPP). The sample was then centrifuged at 18,000×g for 20 min at a temperature of 4 °C. The activity of the PAL enzyme was measured in the supernatant, where 0.3 milliliters of the supernatant was added to a reaction mixture containing 0.7 milliliters of 100 millimolar L-phenylalanine and 3 milliliters of 50 millimolar borate buffer (pH 8.5). The reaction mixture was incubated at 40 °C for one hour, after which the reaction was stopped by adding 0.1 milliliters of 5 millimolar HCl. The activity of the PAL enzyme was calculated based on the absorbance of the reaction mixture at a wavelength of 290 nanometers.
Determination of PPO enzyme activity
To measure the PPO activity, the method described by Liu et al. was used30. To prepare the sample, 1 gram of the frozen powder was homogenized in 15 mL of a phosphate buffer solution with a concentration of 0.1 M and a pH of 7.8. The buffer solution also contained 1 gram of polyvinyl pyrrolidone (PVP). After homogenization, the absorbance of the sample was measred at a wavelength of 480 nm using a spectrophotometer. The activity of polyphenol oxidase (PPO) was expressed as units (U) per milligram (mg) of protein.
Determination of total phenolic, flavonoids, GB content and BDAH activity
The method used to assess the accumulation of total phenolic compounds is called the Folin-Ciocalteu method, which was first described by Singleton and Rossi31. Total flavonoids content measured according the method described by Shahbazi et al.32. Endogenous content of GB measured according to the method of Malekzadeh13. Betaine aldehyde hydrogenase (BADH) activity was measured according to the method of Muñoz-Clares and Mújica-Jiménez33.
RNA extraction and qRT-PCR assay
The primer sequences utilized for qRT-PCR analysis in Agaricus bisporus samples were as follows. Total RNA was extracted from mushroom samples stored at − 80 °C using a plant RNA extraction kit (Azmaelixir® kit, Tehran, Iran) following the manufacturer’s instructions. High-quality RNA was employed for cDNA synthesis using the Takara PrimeScript RT Reagent Kit (TaKaRa, Dalian, China). Quantitative real-time PCR (qRT-PCR) was conducted on a LightCycler® 96 detection system (Roche, Switzerland) using the FastStart Essential DNA Green Master Mix (Roche, Germany). The primers for all genes were designed based on homologous sequences from Arabidopsis and tomato, and the specific details are presented in Table 1. Each reaction mixture consisted of 10 µL of 2× FastStart Essential DNA Green Master Mix (Roche, Germany), 0.5 µL of each primer, 2 µL of cDNA, and 7 µL of ddH2O. A uniform thermal profile was employed for all PCR runs, comprising an initial denaturation step at 94 °C for 10 min, followed by 45 cycles of denaturation at 94 °C for 30 s, annealing at 57–60 °C for 10 s, extension at 72 °C for 15 s, and a final extension step at 72 °C for 10 min. The relative expression levels were calculated using the 2−ΔΔCt method, with eggplant actin serving as an internal reference. Three replicates of PCR reactions and three biological replicates were performed for each target gene.
Table 1.
To analyze qRT-PCR in Agaricus bisporus samples, the following primer sequences were used.
| Gene | Encoded proteins | Primer (5’ to 3’) |
|---|---|---|
| AbPPO | Polyphenol oxidase |
F: 5’-TCCTTCTGTCGCCGCCTTTG-3’ R: 5’-GGGTTCCATCCGCCAAGAG-3’ |
| AbPAL | Phenylalanine ammonia lyase |
F: 5’-CCCCATCCTGGTCAAATTGAAG-3’ R: 5’-TCCGTAGTCGAGTTGCATTCAG-3’ |
Data analysis
The data analysis was performed using SPSS version 25. Two-way analysis of variance (ANOVA) was performed on the data, and subsequent post-hoc multiple comparisons were carried out. The threshold for determining statistical significance was established at a significance level of P < 0.05. The results were presented as the mean ± standard error of triplicate experiments, indicating the average value and the variability among the three replicates. Data manipulation was performed using Microsoft Excel, while GraphPad Prism 8 software was used for graphing the results.
Results
Changes in the external and internal appearance, chilling injury, cap Browning and color
In Fig. 1A, B, the changes in the external and internal appearance of the button mushrooms are clearly shown. As observed, during days 5, 10, and 15, visible color changes occurred in all treated and control groups. However, the control and 5-GB groups showed an increasing trend in browning. Conversely, the 10-GB and 15-GB groups exhibited less external and internal color alteration. The least amount of browning and the external and internal appearance was observed on day 15 in the 10-GB treatment group.
Fig. 1.
depicts the impact of postharvest Glycine betaine (GB) treatment at various concentrations (0, 5, 10, and 15mM) on the external appearance (A), internal appearance (B), Chilling injury (C) and cap browning (D) of mushrooms during a 15-day storage period at 4 °C. Each data point represents the mean ± standard error (n = 3). Different letters are used to indicate significant differences (P < 0.05) between the treatments.
The results presented in Fig. 1C, illustrates the effect of different concentrations of GB on chilling injury incidence in mushroom over a 15-day storage period. The control group, which received no GB treatment, exhibited a steady increase in chilling injury incidence, starting at approximately 6.32% on day 5 and reaching around 24.6% by day 15. In contrast, the 10 mM GB-treated samples showed a lower incidence of chilling injury, beginning at 2.5% on day 5 and rising to about 11.5% by day 15. The 15 mM GB treatment demonstrated the significant reduction in chilling injury, with the incidence increasing from 2.8% on day 0 to only 13.6% by day 15.
The results presented in Fig. 1D, demonstrate that postharvest treatment of mushrooms with different concentrations of GB (0, 5, 0.5, 10, and 15 mM) resulted in increased mushroom cap browning percentages at the end of the cold storage period at 4 °C. Specifically, the cap browning was enhanced by 76.5%, 34.8%, 21.4%, and 28.1% for the respective GB concentrations mentioned above. Notably, the postharvest application of 10 mM GB significantly reduced mushroom cap browning to a much greater extent compared to other concentrations. Therefore, the optimal treatment to limit mushroom cap browning was determined to be the application of 10 mM GB. Consequently, for all qualitative and biochemical attribute assays, the concentration of 10 mM GB was utilized (as indicated in Fig. 1; statistically significant at p < 0.05).
Figure 2A–C illustrates the influence of exogenous glycine betaine (GB) application on the chromatic characteristics (L, a*, b*) of mushroom caps during refrigerated storage at 4 °C. A consistent temporal pattern emerged: lightness (L*) exhibited a progressive decline across all samples, whereas both redness (a*) and yellowness (b*) demonstrated a gradual augmentation throughout the storage period. Notably, GB-treated specimens displayed significantly attenuated L* values and elevated a*/b* indices relative to untreated controls immediately post-application (p < 0.05). Moreover, a dose-response relationship was evident, with incremental UV-C irradiation intensities correlating with enhanced reduction in lightness and more pronounced intensification of chromaticity parameters.
Fig. 2.
The figure illustrates the effect of postharvest Glycine betaine (GB) treatment at various concentrations (0, 5, 10, and 15mM) on L* (A), a* (B) and b* (C) values of mushrooms during a 15-day storage period at 4 °C. The data presented are expressed as mean ± standard error (n = 3). Different letters are used to indicate significant differences (P < 0.05) between the treatments.
Changes in the electrolyte leakage, weight loss, MDA content and firmness under exogenous GB
During storage, the weight loss of mushrooms was higher, but it was less pronounced in mushrooms treated with GB compared to the untreated ones (as shown in Fig. 3A; statistically significant at p < 0.05). The firmness of both GB-treated and untreated mushrooms decreased significantly throughout the storage period. However, the decline in firmness of the mushroom cap tissue was slower and less severe in mushrooms treated with 10 mM GB compared to the untreated mushrooms during cold storage (as depicted in Fig. 3B; statistically significant at p < 0.01).
Fig. 3.
The figure illustrates the effect of postharvest Glycine betaine (GB) treatment at a concentration of 10 mM on WL (A), firmness (B), EL (C), and MDA (D) of mushrooms during a 15-day storage period at 4 °C. The data presented are expressed as mean ± standard error (n = 3). Different letters are used to indicate significant differences (P < 0.05) between the treatments.
As shown in Fig. 3C, electrolyte leakage (EL) significantly increased in both control and GB-treated mushrooms throughout the 15-day cold storage period. However, the application of 10 mM GB consistently and significantly mitigated this increase compared to the control group. At day 0, both groups exhibited similar initial EL levels. By day 5, while EL in the control group had risen to approximately 31%, the GB-treated mushrooms showed a lower leakage of around 7.14%. This trend became more pronounced as storage progressed. By the end of the storage period (Day 15), the control mushrooms exhibited the highest EL, reaching approximately 25.1%, whereas the GB-treated mushrooms maintained significantly lower EL levels, around 14.2%.
Similarly, the malondialdehyde (MDA) content, an indicator of lipid peroxidation and oxidative stress, showed a time-dependent increase in both mushroom groups during cold storage (Fig. 3D). However, 10 mM GB treatment effectively suppressed the accumulation of MDA. At the initial storage time (Day 0), MDA levels were low and comparable between the two groups. As storage progressed, MDA content in control mushrooms escalated rapidly, reaching approximately 3.89 µmol g⁻¹ FW by Day 5, and peaking at around 14.3 µmol g⁻¹ FW by Day 15. In contrast, the GB-treated mushrooms consistently exhibited significantly lower MDA levels throughout the storage duration. For instance, at Day 15, MDA content in GB-treated mushrooms was approximately 7.20 µmol g⁻¹ FW, which was considerably lower than that of the control group. These results indicate that GB application significantly reduced oxidative damage to cell membranes in mushrooms during cold storage.
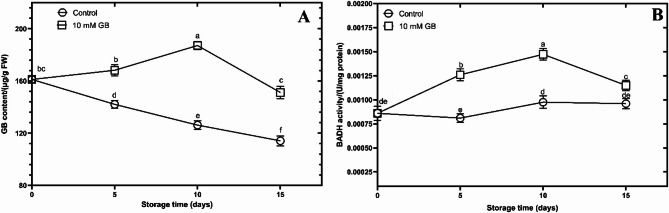
Changes in the endogenous GB content, and BADH activity under exogenous GB
During cold storage, accumulation of endogenous GB of the GB treated mushrooms increased until 10 d and continually declined in a similar trend at the end of the storage (Fig. 4A). endogenous GB content in the control group decreased with a constant trend until the end of the storage period.
Fig. 4.
The figure showcases the impact of postharvest Glycine betaine (GB) treatment at a concentration of 10 mM on the Endogenous GB content (A) and BADH (Betaine aldehyde dehydrogenase) activity (B) of mushrooms during a 15-day storage period at 4 °C.
As shown in Fig. 4B, BADH enzyme activity showed an increasing trend in both the control and GB-treated groups. But the BADH activity level of GB treated group was much higher than the control group. During the 5th and 10th days of storage, the BADH enzyme activity increased steeply, but on the 15th day, this activity showed a decreasing trend. However, in the control group, the BADH activity showed a slight increase during the storage period.
Changes in the endogenous GB content, and BADH activity under exogenous GB
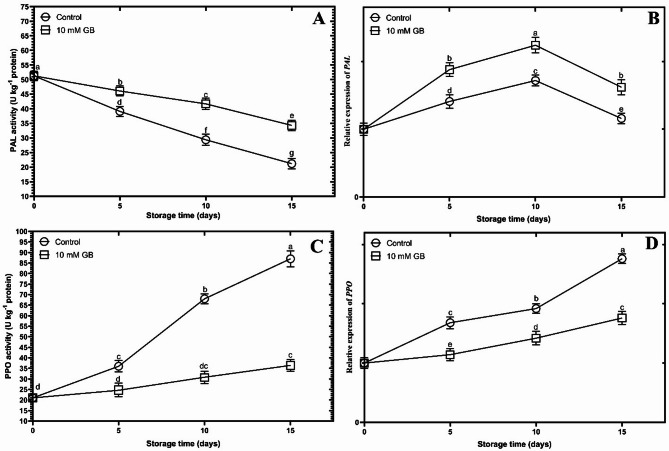
The GB-treated mushrooms exhibited a significantly higher activity and gene expression of PAL compared to the control group (p < 0.05) (Fig. 5A, B). The activity of PAL in the treated mushrooms showed a significant decrease (p < 0.05) until day 15 of the storage period. In contrast, the activity of PAL in the control mushrooms consistently decreased throughout the storage period.
Fig. 5.
The figure demonstrates the effect of postharvest Glycine betaine (GB) treatment at a concentration of 10 mM on PAL activity (A), PAL expression (B), PPO activity (C), and PPO expression (D) of mushrooms during a 15-day storage period at 4 °C.
During the storage period, both the GB-treated and control mushrooms exhibited an increase in the activity and gene expression of PPO (Fig. 5C, D) (p < 0.05). However, the rate of increase was slower in the treated mushrooms compared to the control group. In the control group, the activity of PPO enzyme increased with a very sharp slope during the 10th and 15th day (Fig. 5C, D). These results were consistent with the level of PPO gene expression in the control and treated groups.
Changes in the proline, H2O2, total phenol and flavonoid content
As shown in Fig. 6A, B, both the GB-treated and control mushrooms exhibited an increasing trend in the proline and H2O2 content. However, the exogenous application of GB significantly increased the proline content during the 15-day storage period at 4 °C (p < 0.05).
Fig. 6.
The figure demonstrates the effect of postharvest Glycine betaine (GB) treatment at a concentration of 10 mM on H2O2 (A), proline (B), total phenol (C), and total flavonoid (D) content of mushrooms during a 15-day storage period at 4 °C.
In the control group, the H2O2 content gradually increases over the storage period, indicating the accumulation of H2O2 due to oxidative stress. In contrast, in the group treated with 10 mM GB, the H2O2 content remains significantly lower than that of the control group throughout the storage period. This suggests that glycine betaine effectively reduces oxidative stress by minimizing the accumulation of H2O2.
Figure 6B illustrates a significant variation in proline content between the control and GB-treated samples. On day 5, the control group exhibited a proline content of approximately 7.21 µmol kg⁻¹ FW, whereas the GB-treated sample showed a content of around 9.34 µmol kg⁻¹ FW. Throughout the storage period, the proline content in the control group steadily increased, reaching approximately 35 µmol kg⁻¹ FW by day 15. Conversely, the GB-treated sample displayed a more substantial increase, peaking at nearly 36.21 µmol kg⁻¹ FW by day 15.
Figure 6C demonstrates the changes in the total phenol content of button mushrooms during storage at 4 °C. Both the control and GB-treated mushrooms exhibited a decreasing trend in total phenol content, but the rate of decline was lower in the treated mushrooms (p < 0.05). In the GB-treated group, the decrease in total phenol content was less pronounced, and the exogenous treatment was able to mitigate the reduction in phenol levels. Additionally, the GB treatment increased the amount of phenol reduction compared to the control group.
As shown in Fig. 6D, the total flavonoid content of both the control and treatment groups increased during the storage period. On the fifth day, there was no significant difference between the control and treatment groups in terms of total flavonoid content. However, on the 10th and 15th days of storage, the treated group showed a significant increase in total flavonoid content compared to the control group.
Discussion
The current study investigated the visual characteristics, chemical compositions, and enzymatic activities of button mushrooms throughout the storage period. It revealed that treatment with GB effectively preserves the visual quality of the mushrooms, thus maintaining their aesthetic appeal and marketability (Fig. 1A, B). Analysis of gene expression and enzymatic activity indicated that a concentration of 10 mM GB significantly inhibits enzymatic browning and chilling-induced damage, leading to a reduction in the transcription levels of the PPO gene and its associated enzymatic activity in the treated samples compared to the control group. Browning in button mushrooms is a complex chemical reaction where phenolic compounds are transformed into quinones by oxidative enzymes, which then react with one another to form melanin7,30. Damage to the cell membrane’s integrity can expedite this process, as demonstrated in the control group by increased electrolyte leakage and MDA accumulation, as shown in Fig. 2C, D. The membrane permeability in the control group (untreated mushrooms) was recorded at 25.1 units, whereas in the GB-treated group, it measured 14.2 units. These findings align with previous research conducted by Huang et al.34. Moreover, it has been noted that the elevated expression of the PPO gene and its corresponding enzymatic activity likely enhance the oxidation of phenolic compounds, thereby contributing to the browning of mushrooms post-harvest during storage35,36. Additionally, Liu et al. demonstrated that high concentrations of melatonin can inhibit browning in mushrooms by preserving cell membrane integrity and suppressing the activities of PPO and PAL4.
As observed in Fig. 2, the L* value exhibited a decreasing trend throughout the experiment. Specifically, at the conclusion of the 15-day treatment period, the control mushrooms registered the lowest L* value, indicating the darkest appearance among the samples. Conversely, the mushroom samples treated with glycine betaine maintained the highest L* value at the end of this period, suggesting they retained the most luminosity compared to the other groups. Despite the overall decrease in L* values, the a* and b* values demonstrated an increasing trend in both the control and treated mushrooms. At the conclusion of the storage period, the highest a* and b* values were observed in the group treated with 10GB. Based on the reflectivity of the determined surface, the L* value was utilized to express the luminosity of the sample surface. A decrease in L* indicates a darkening of the mushroom, while an increase in a* signifies increasing redness, and an elevated b* value suggests increasing yellowness formed on the samples5,37.
Chilling injury (CI) is identified as a major challenge in the postharvest storage of button mushrooms at 4 °C. To mitigate CI, various strategies have been explored by researchers6,16,30,38–41. Glycine betaine (GB), an osmotic regulator, has emerged as a promising solution due to its ability to preserve cellular membrane integrity and stabilize intracellular proteins, thereby enhancing the mushroom’s tolerance. According to our findings, we observed that as the storage period for button mushrooms increased (15 days), there was a concurrent increase in weight loss, chilling injury incidence and cap browning, accompanied by reduced firmness. However, when we treated the mushrooms with 10 mM GB, we observed a decrease in weight loss and cap browning in mushroom. As a result, the application of 10 mM GB led to an improvement in the visual quality of the mushrooms during low-temperature storage compared to the untreated control group.
In recent research, a series of investigations have disclosed that the postharvest application of Glycine betaine (GB) can effectively prevent the loss of weight and alleviate the decline in pulp firmness in banana fruit9,42. Additionally, it has been found that GB treatment preserves the natural color of the strawberry fruit’s crust while simultaneously enhancing fruit firmness, which can be attributed to the reduction in the activities of pectin methyl esterase and polygalacturonates enzymes43. Furthermore, GB treatment has demonstrated the ability to suppress the loss of pulp firmness in kiwifruit44alleviate fruit peel yellowing, resulting in increased fruit peel firmness in bananas42and modulate the metabolism of phenolic compounds, thereby reducing browning in fresh-cut lotus slices and button mushrooms42,45. These recent findings offer compelling evidence for the efficacy of postharvest exogenous GB treatment in preserving fruit quality and addressing various quality-related concerns across a range of fruits.
Postharvest, the biochemical changes that occur in fresh button mushrooms during the onset of senescence involve several processes such as transpiration, browning, and softening. These changes are characterized by significant alterations in the cell wall structure and compromised integrity of the cell membranes. As a consequence, there is an escalation in water loss within the mushroom’s texture, leading to the deterioration of its qualitative characteristics. These destructive changes contribute to the overall senescence process observed in the fruit bodies of button mushrooms after harvest37,46,47.
As depicted in Fig. 5A, B, PAL enzyme activity exhibited a decrease over time, contrasting with a concurrent increase in its gene expression. This observed divergence can likely be attributed to factors such as post-translational modifications, including phosphorylation, which can rapidly alter PAL activity independent of mRNA levels, and feedback inhibition by accumulated phenolics, such as flavonoids, during storage, despite compensatory transcriptional upregulation6,7. By upregulating the PAL/PPO gene and increasing its enzyme activity, a higher accumulation of total phenolic compounds enhances the cellular ROS scavenging capacity, as evaluated by the proline and H2O2 content. Alongside the accumulation of total phenolic compounds, the higher ABTS, FRAP, and DPPH radical scavenging capacities may also be attributed to the increased content of proline34,48. In this study, the application of GB resulted in increased accumulation of total phenolic compounds, upregulation of the PAL gene, and increased enzyme activity. However, it reduced the expression of the PPO gene and its enzyme activity. A lower expression of the PPO gene indicates lower activity of this enzyme, leading to a decrease in enzymatic browning of mushroom caps during a 15-day storage at 4 °C. The increased expression of the PAL gene enhances its activity, resulting in the accumulation of total phenolic compounds as a defensive response to oxidative stress induced by ROS, which occurs during post-harvest senescence49. The elevated PAL activity leads to increased biosynthesis of phenolic compounds and reduces ROS accumulation in mushrooms20,49,50. This helps maintain quality and minimize enzymatic browning of mushroom caps6,7. Phenolic compounds have dual functions: (1) they can be oxidized by PPO, leading to browning, and (2) they can accumulate in horticultural products as antioxidant compounds in response to post-harvest stress, reducing browning17,49,51. The higher accumulation of total phenolic compounds in GB-treated plum fruits may be attributed to the higher activity of PAL, leading to higher FRAP and DPPH radical scavenging capacities, which may be responsible for delaying browning during cold storage52,53. Additionally, the delayed browning of spotted areas in GB-treated papaya fruits may be attributed to the higher accumulation of total phenolic compounds resulting from higher PAL activity and lower PPO activity during cold storage54. In addition to the accumulation of total phenolic compounds, the higher content of proline in GB-treated mushrooms may contribute to enhancing the activity of the intracellular ROS scavenging system, which could be beneficial for reducing oxidative stress, as indicated by the low H2O2 content. Thus, it delays the enzymatic browning of mushroom caps during a 15-day storage at 4 °C. Lower H2O2 content, along with higher proline content, has been reported in GB-treated papaya, mango, and banana fruits during a 15-day storage at 4 °C16,42,54,55.
Glycine betaine acts as a crucial compatible osmolyte, accumulating in the cytoplasm to regulate osmotic potential, thereby maintaining cellular turgor and volume under chilling stress. Its protective mechanism extends to safeguarding macromolecules like proteins and enzymes from denaturation, and critically, preserving cell membrane integrity. Our findings corroborate this: reduced electrolyte leakage (EL) directly indicates superior membrane preservation, while lower malondialdehyde (MDA) content signifies mitigated lipid peroxidation and oxidative damage, collectively reflecting GB’s osmoprotectant and macromolecule-stabilizing roles. Beyond direct protection, GB’s beneficial effects also influence key metabolic pathways. While not a direct antioxidant, GB’s role in maintaining cellular structure indirectly alleviates oxidative stress by minimizing ROS overproduction, further supported by decreased MDA levels. Moreover, our investigation into phenylpropanoid metabolism revealed (enhanced phenolic compound accumulation or modulated PAL activity), suggesting GB regulates these protective compounds, bolstering the mushroom’s antioxidant defense. Thus, mushroom preservation under GB treatment is likely mediated by a complex interplay of direct protective mechanisms and indirect modulation of essential metabolic pathways, enhancing chilling stress resilience.
Conclusion
In conclusion, this study demonstrated the beneficial effects of postharvest 10 mM glycine betaine (GB) treatment on maintaining the quality of button mushrooms during 15 days of cold storage at 4 °C. GB treatment effectively mitigated several key deteriorative processes associated with postharvest senescence. Specifically, it significantly reduced cap browning, weight loss, electrolyte leakage, and MDA content, indicating a positive impact on membrane integrity and oxidative stress. Furthermore, GB application influenced key enzymatic activities related to browning, increasing PAL activity and gene expression while decreasing PPO activity and gene expression. This modulation of enzyme activity contributed to a higher accumulation of total phenolic compounds, which in turn enhanced the antioxidant capacity of the mushrooms, as evidenced by increased proline content and reduced H2O2 levels. These findings suggest that GB treatment can effectively prolong the shelf life and preserve the visual and nutritional quality of button mushrooms by delaying senescence and maintaining cellular integrity. This approach offers a promising strategy for the postharvest management of button mushrooms, contributing to reduced losses and improved marketability.
Building upon the foundational insights of this study, future research should aim to further elucidate the intricate mechanisms underlying GB’s protective effects by employing advanced molecular techniques such as transcriptomics, proteomics, and metabolomics to precisely identify the affected genes, proteins, and metabolites in mushrooms under chilling stress17,56,57. Expanding the applicability of GB, subsequent investigations could explore its efficacy on other economically important mushroom species to assess similar improvements in quality and shelf life. For practical implementation, optimization studies focusing on GB concentration, application methods, and integration with other post-harvest technologies (e.g., modified atmosphere packaging) are warranted to enhance its commercial viability. Finally, comprehensive sensory evaluations and detailed nutritional analyses of GB-treated mushrooms throughout storage are essential to fully understand consumer acceptance and the overall nutritional benefits.
Author contributions
Parviz Malekzadeh: Supervision, Investigation, Writing – review & editing. Zeinab Abbasi: Investigation, Methodology, Data curation, Formal analysis. Mehdi Sadeghi: Investigation, Methodology. Asghar Kmrani: Supervision, Investigation, Methodology.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request. There are no restrictions on data availability.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Yang, X. M. et al. Postharvest treatment of arginine maintains the storage quality of fresh-cut button mushrooms (Agaricus bisporus). J. Food Process. Preserv.46, e16488 (2022). [Google Scholar]
- 2.Park, D. H., Park, J. J., Olawuyi, I. F. & Lee, W. Y. Quality of white mushroom (Agaricus bisporus) under argon-and nitrogen-based controlled atmosphere storage. Sci. Hort.265, 109229 (2020). [Google Scholar]
- 3.Nagulwar, M., More, D. & Mandhare, L. Nutritional properties and value addition of mushroom: A review. Pharma Innov. J.9, 395–398 (2020). [Google Scholar]
- 4.Li, L. et al. Melatonin retards senescence via regulation of the electron leakage of postharvest white mushroom (Agaricus bisporus). Food Chem.340, 127833 (2021). [DOI] [PubMed] [Google Scholar]
- 5.Jebelli Javan, A. et al. Effect of citric acid dipping treatment on bioactive components and antioxidant properties of sliced button mushroom (Agaricus bisporus). J. Food Qual. Hazards Control. 2, 20–25 (2015). [Google Scholar]
- 6.Shekari, A., Naghsiband Hassani, R., Soleimani Aghdam, M., Rezaii, M. & Janatizadeh, A. The effect of postharvest treatments of melatonin and γ-aminobutyric acid on improving antioxidant activity and reducing Browning of fresh cut button mushroom (Agaricus bisporus) during cold storage. J. Plant. Prod. Res.29, 45–61 (2023). [Google Scholar]
- 7.Xu, L., Gao, L., Meng, J. & Chang, M. Browning Inhibition and postharvest quality of button mushrooms (Agaricus bisporus) treated with alginate and ascorbic acid edible coating. Int. Food Res. J.29 (2022).
- 8.Hou, F. et al. Bacterial community dynamics and metabolic functions prediction in white button mushroom (Agaricus bisporus) during storage. Food Res. Int. 113077 (2023). [DOI] [PubMed]
- 9.Denaxa, N. K., Tsafouros, A., Ntanos, E. & Roussos, P. A. In Plant Stress Mitigators127–158 (Elsevier, 2023).
- 10.Ayub, M. A. et al. In Emerging Plant Growth Regulators in Agriculture335–356 (Elsevier, 2022).
- 11.Habibi, F., Sarkhosh, A., Guillén, F., Serrano, M. & Valero, D. Postharvest treatments with Methyl salicylate and glycine betaine synergistically enhanced chilling tolerance and maintained bioactive compounds of blood orange fruit subjected to cold quarantine storage. LWT, 115141 (2023).
- 12.Sharma, A., Pathania, A., Sharma, P., Bhardwaj, R. & Sharma, I. Role of glycine betaine in regulating physiological and molecular aspects of plants under abiotic stress. The Role Growth Regulators Phytohormones Overcoming Environ. Stress, 327–353 (2023).
- 13.Malekzadeh, P. Influence of exogenous application of Glycinebetaine on antioxidative system and growth of salt-stressed soybean seedlings (Glycine max L). Physiol. Mol. Biology Plants. 21, 225–232 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alaei, S. & Mahna, N. Influence of exogenous application of Glycine betaine on growth and ion accumulation in strawberry plants under saline condition. Int. J. Hortic. Sci. Technol.10, 41–52 (2023). [Google Scholar]
- 15.Xing, W. & Rajashekar, C. Glycine betaine involvement in freezing tolerance and water stress in Arabidopsis thaliana. Environ. Exp. Bot.46, 21–28 (2001). [DOI] [PubMed] [Google Scholar]
- 16.Wang, Z., Chen, L., Yang, H. & Wang, A. Effect of exogenous glycine betaine on qualities of button mushrooms (Agaricus bisporus) during postharvest storage. Eur. Food Res. Technol.240, 41–48 (2015). [Google Scholar]
- 17.Wang, L. et al. Glycine betaine reduces chilling injury in Peach fruit by enhancing phenolic and sugar metabolisms. Food Chem.272, 530–538 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Jia, Z., Wang, Y., Wang, L., Zheng, Y. & Jin, P. Amino acid metabolomic analysis involved in flavor quality and cold tolerance in Peach fruit treated with exogenous glycine betaine. Food Res. Int.157, 111204 (2022). [DOI] [PubMed] [Google Scholar]
- 19.Wang, H. et al. Effects of UV-B on vitamin C, phenolics, flavonoids and their related enzyme activities in mung bean sprouts (Vigna radiata). Int. J. Food Sci. Technol.52, 827–833 (2017). [Google Scholar]
- 20.Sharma, K., Kumar, M. & Chandran, D. In Plant Phenolics in Abiotic Stress Management, 369–390 (Springer, 2023).
- 21.Ramaroson, M. L. et al. Role of phenylpropanoids and flavonoids in plant resistance to pests and diseases. Molecules27, 8371 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang, Y. J. et al. Exogenous genistein enhances soybean resistance to Xanthomonas axonopodis pv. glycines. Pest Manag. Sci.78, 3664–3675 (2022). [DOI] [PubMed] [Google Scholar]
- 23.Wu, J. et al. Cold stress induces malformed tomato fruits by breaking the feedback loops of stem cell regulation in floral meristem. New Phytol.237, 2268–2283 (2023). [DOI] [PubMed] [Google Scholar]
- 24.Amini, S. et al. Effect of cold stress on polyamine metabolism and antioxidant responses in Chickpea. J. Plant Physiol.258–25910.1016/j.jplph.2021.153387 (2021). [DOI] [PubMed]
- 25.Kebbeh, M., Dong, J., Chen, H. & Yan, L. ZHENG, X.-l. Melatonin treatment alleviates chilling injury in Mango fruit ‘keitt’by modulating proline metabolism under chilling stress. J. Integr. Agric.22, 935–944 (2023). [Google Scholar]
- 26.Nasiri, M., Barzegar, M., Sahari, M. A. & Niakousari, M. Tragacanth gum containing Zataria multiflora boiss. Essential oil as a natural preservative for storage of button mushrooms (Agaricus bisporus). Food Hydrocoll.72, 202–209 (2017). [Google Scholar]
- 27.Malekzadeh, P., Hatamnia, A. A. & Tiznado-Hernández, M. E. Arginine catabolism induced by exogenous arginine treatment reduces the loss of green color rate in broccoli florets. Physiol. Mol. Plant Pathol.124, 101973 (2023). [Google Scholar]
- 28.Liu, Q. et al. γ-Aminobutyric acid treatment induced chilling tolerance in postharvest Kiwifruit (Actinidia chinensis cv. Hongyang) via regulating ascorbic acid metabolism. Food Chem.404, 134661 (2023). [DOI] [PubMed] [Google Scholar]
- 29.Sánchez-Rodríguez, E., Moreno, D. A., Ferreres, F. & del Differential responses of five Cherry tomato varieties to water stress: changes on phenolic metabolites and related enzymes. Phytochemistry72, 723–729 (2011). Rubio-WilhelmiM.Ruiz, J. M. [DOI] [PubMed] [Google Scholar]
- 30.Liu, Z. & Wang, X. Changes in color, antioxidant, and free radical scavenging enzyme activity of mushrooms under high oxygen modified atmospheres. Postharvest Biol. Technol.69, 1–6 (2012). [Google Scholar]
- 31.Singleton, V. L. & Rossi, J. A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Viticult.16, 144–158 (1965). [Google Scholar]
- 32.Shahbazi, S., Hatamnia, A. A. & Malekzadeh, P. Effect of melatonin treatment on Post-Harvest life of broccoli during storage. J. Veg. Sci.6 (2023).
- 33.Muñoz-Clares, R. A. & Mújica-Jiménez, C. Complexes of NADH with betaine aldehyde dehydrogenase from leaves of the plant Amaranthus hypochondriacus L. Chemico-Biol. Interact.130, 71–80 (2001). [DOI] [PubMed] [Google Scholar]
- 34.Huang, S., Lim, S. Y., Lau, H., Ni, W. & Li, S. F. Y. Effect of Glycinebetaine on metabolite profiles of cold-stored strawberry revealed by 1H NMR-based metabolomics. Food Chem.393, 133452 (2022). [DOI] [PubMed] [Google Scholar]
- 35.Guo, Y. et al. Effect of Shiitake mushrooms polysaccharide and Chitosan coating on softening and Browning of Shiitake mushrooms (Lentinus edodes) during postharvest storage. Int. J. Biol. Macromol.218, 816–827 (2022). [DOI] [PubMed] [Google Scholar]
- 36.Pu, H., Yu, J., Liu, Z., Paliwal, J. & Sun, D. W. Evaluation of the effects of vacuum cooling on moisture contents, colour and texture of mushroom (Agaricus Bisporus) using hyperspectral imaging method. Microchem. J.190, 108653 (2023). [Google Scholar]
- 37.Lian, L. et al. Calcium chloride treatment delays the quality deterioration of King oyster mushroom (Pleurotus eryngii) through positive regulation of antioxidants by PeCaMK1. Food Biosci., 104778 (2024).
- 38.Ojeda, G. A., Sgroppo, S. C., Martín-Belloso, O. & Soliva-Fortuny, R. Chitosan/tripolyphosphate nanoaggregates enhance the antibrowning effect of ascorbic acid on mushroom slices. Postharvest Biol. Technol.156, 110934 (2019). [Google Scholar]
- 39.Ko, J., Lee, B., Lee, J. & Park, H. J. Effect of UV-B exposure on the concentration of vitamin D2 in sliced Shiitake mushroom (Lentinus edodes) and white button mushroom (Agaricus bisporus). J. Agric. Food Chem.56, 3671–3674 (2008). [DOI] [PubMed] [Google Scholar]
- 40.Gholami, R., Aghili Nategh, N. & Rabbani, H. Evaluation the effects of temperature and packaging conditions on the quality of button mushroom during storage using e-nose system. J. Food Sci. Technol.60, 1355–1366 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lagnika, C., Zhang, M., Nsor-Atindana, J. & Tounkara, F. Extension of mushroom shelf-life by ultrasound treatment combined with high pressure argon. Int. Agrophys.28 (2014).
- 42.Chen, L. L. et al. Postharvest application of glycine betaine ameliorates chilling injury in cold-stored banana fruit by enhancing antioxidant system. Sci. Hort.287, 110264 (2021). [Google Scholar]
- 43.Rajashekar, C., Zhou, H., Marcum, K. & Prakash, O. Glycine betaine accumulation and induction of cold tolerance in strawberry (Fragaria X Ananassa Duch.) plants. Plant Sci.148, 175–183 (1999). [Google Scholar]
- 44.Dong, H. et al. Exogenous γ-Aminobutyric acid (GABA) treatment suppresses ethylene biosynthesis in hardy Kiwifruit (Actinidia aruguta) during postharvest storage. (2022).
- 45.Zhang, Y. et al. Exogenous melatonin maintains postharvest quality in Kiwiberry fruit by regulating sugar metabolism during cold storage. LWT174, 114385 (2023). [Google Scholar]
- 46.Gukasyan, N., Griffiths, R. R., Yaden, D. B., Antoine, D. G. & Nayak, S. M. Attenuation of psilocybin mushroom effects during and after SSRI/SNRI antidepressant use. J. Psychopharmacol.37, 707–716 (2023). [DOI] [PubMed] [Google Scholar]
- 47.Mehta, H., Chhipa, N. & Patani, P. Edible mushroom for medicinal use. J. Adv. Zool.45 (2024).
- 48.Meng, Z., Wang, T., Malik, A. U. & Wang, Q. Exogenous isoleucine can confer Browning resistance on fresh-cut potato by suppressing polyphenol oxidase activity and improving the antioxidant capacity. Postharvest Biol. Technol.184, 111772 (2022). [Google Scholar]
- 49.Jiang, Y. & Joyce, D. C. ABA effects on ethylene production, PAL activity, anthocyanin and phenolic contents of strawberry fruit. Plant. Growth Regul.39, 171–174 (2003). [Google Scholar]
- 50.Peng, W., Wang, N., Wang, S., Wang, J. & Bian, Z. Effects of microwave and exogenous l-phenylalanine treatment on phenolic constituents, antioxidant capacity and enzyme inhibitory activity of Tartary buckwheat sprouts. Food Sci. Biotechnol., 1–9 (2022). [DOI] [PMC free article] [PubMed]
- 51.Machado, M., Felizardo, C., Fernandes-Silva, A. A., Nunes, F. M. & Barros, A. Polyphenolic compounds, antioxidant activity and l-phenylalanine ammonia-lyase activity during ripening of Olive cv.cobrançosa under different irrigation regimes. Food Res. Int.51, 412–421 (2013). [Google Scholar]
- 52.Mahmoudi, R., Razavi, F., Rabiei, V., Gohari, G. & Palou, L. Application of Glycine betaine coated Chitosan nanoparticles alleviate chilling injury and maintain quality of Plum (Prunus domestica L.) fruit. Int. J. Biol. Macromol.207, 965–977 (2022). [DOI] [PubMed] [Google Scholar]
- 53.Mahmoudi, R., Razavi, F., Rabiei, V., Palou, L. & Gohari, G. Postharvest chitosan-arginine nanoparticles application ameliorates chilling injury in Plum fruit during cold storage by enhancing ROS scavenging system activity. BMC Plant Biol.22, 555 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pan, Y. et al. Effect of glycine betaine on chilling injury in relation to energy metabolism in Papaya fruit during cold storage. Food Sci. Nutr.7, 1123–1130 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Varlı Yunusoğlu, S. & Ekinci, N. The effect of Post-Harvest Salicylic acid and modified atmosphere packaging treatments on the storage of ‘roxana’apricots. Erwerbs-Obstbau, 1–9 (2023).
- 56.Molaei, S., Rabiei, V., Soleimani, A. & Razavi, F. Exogenous application of glycine betaine increases the chilling tolerance of pomegranate fruits cv. Malase Saveh during cold storage. J. Food Process. Preserv.45, e15315 (2021). [Google Scholar]
- 57.Badawy, A. A. et al. Glycine betaine mitigates heavy metal toxicity in beta vulgaris (L.): an antioxidant-driven approach. Agronomy14, 797 (2024). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. There are no restrictions on data availability.