Abstract
Ceramides are bioactive lipids that play a crucial role in cellular signaling and structural integrity (Nat Rev Mol Cell Biol 19:175-191, 2018). As members of the sphingolipid family, ceramides consist of a sphingoid base attached to a fatty acid (Annu Rev Biophys 47:633-654, 2018). Their unique structure confers both hydrophobic and amphipathic properties, enabling them to organize into membrane microdomains that influence cellular dynamics (Annu Rev Biophys 47:633-654, 2018). In recent years, ceramides have garnered attention for their role in modulating a range of cellular and organismal functions. Unlike other lipids that primarily serve structural roles, ceramides act as bioactive lipids in key signaling pathways, mediating stress responses such as inflammation, oxidative stress, growth inhibition, metabolism, autophagy, and apoptosis (J Lipid Res 60:913-918, 2019). Their regulatory effects are particularly important in immune cells, where ceramides can influence cell fate, modulate cellular metabolism, affect cytokine production, and dictate responses to external stimuli (Nature 510:58-67, 2014). Since ceramides maintain a dynamic equilibrium with other sphingolipids within a cell, understanding their role in immune cells in isolation provides only a partial perspective. Nevertheless, as a bioactive lipid and the central precursor of other sphingolipids, ceramides play a pivotal role in immune cells, deserving focused attention.
Keywords: Ceramides, Sphingolipids, Dyslipidemia, Inflammation, Immune cells, Signaling
Highlights
Highlights of ceramides roles in immune cells:
• Immune cell activation: Ceramide modulate the activation of immune cells, influencing their cytotoxic activity and cytokine secretion (Cell Death Dis 6:e1828, 2015).
• Immune cell trafficking: Ceramide regulate the migration of immune cells to sites of inflammation by influencing chemokine receptors and cell adhesion molecules (Exp Mol Med 55:1110-1130, 2023).
• Inflammatory response:Ceramides influence inflammatory responses by modulating the production of inflammatory cytokines and chemokines, impacting the activity of immune cells (Sci Rep 11:8259, 2021).
• Cell death regulation: Ceramide is a key mediator of programmed cell death (apoptosis) in immune cells, triggering cell death pathways when its levels are elevated (Adv Med Sci 68:417-425, 2023).
• Interaction with other signaling pathways: Ceramide interact with other signaling pathways (e.g., Akt and MAPK), affecting cell survival, proliferation, and differentiation (J Biol Chem 275:13330-13335, 2000).
Ceramide biosynthesis
Endogenous ceramides are comprised of a sphingoid base with 18 carbons, a 4,5-trans double bond, and an acyl chain that ranges from 12 to greater than 26 carbons in length [10]. The acyl chain and the sphingoid base can have varying degrees of unsaturation. However, emerging evidence indicates that sphingolipids with sphingosine bases of varying carbon lengths—such as d16, d17, and d20—are also biosynthesized under specific physiological or pathological conditions [11–13].
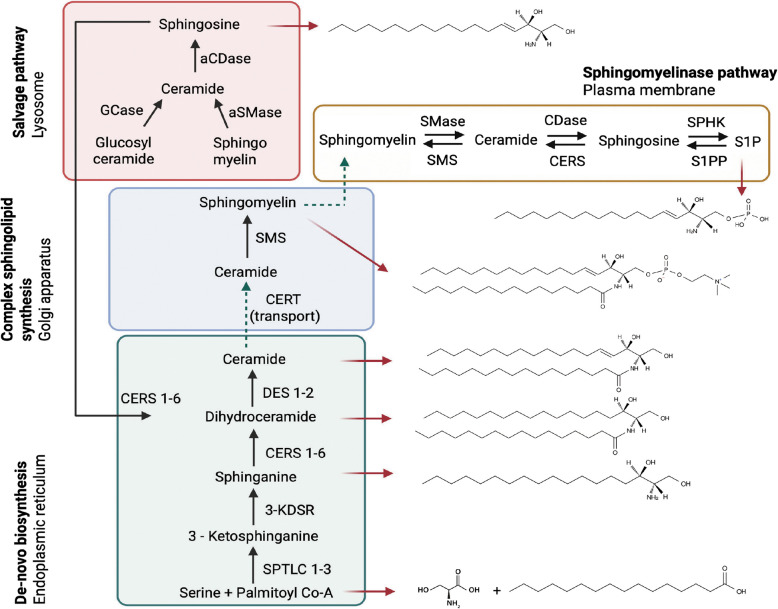
The sphingolipid family encompasses over 4,000 distinct lipid species that play key roles in cellular integrity and function. Ceramides lacking the 4,5-trans double bond are called dihydroceramides and are an important intermediate for de novo ceramide biosynthesis. Ceramides are central precursors for critical cellular sphingolipids, including sphingomyelins and gangliosides which are formed by the addition of chemical groups such as the phosphocholine and carbohydrates respectively, to carbon 1 (Fig. 1). Unlike other lipids, ceramides are not appreciably absorbed from the diet but are either synthesized de novo from saturated fats and proteins or regenerated from other sphingolipids—both processess that are finely regulated by specific enzymes that control ceramide production in response to physiological cues [14].
Fig. 1.
Sphingolipid metabolic pathways, organelles, and key sphingolipid structures. Schematic depicting the key pathways and enzymes involved in ceramide biosynthesis and degradation. Abbreviations: aSMase, acid sphingomyelinase; CDase, ceramidase; aCDase, acid ceramidase; CERS, ceramide synthase; CERT, ceramide transporter; Co-A, coenzyme A; DES, dihydroceramide desaturase; GCS, glucosylceramide synthase; GCase, glucosylceramidase; KDSR, 3-ketodihydrosphingosine reductase; SPTLC, serine palmitoyltransferase; SMS, sphingomyelin synthase; SPHK, sphingosine kinase; SMase, sphingomyelinase; S1PP, sphingosine 1-phosphate phosphatase
De novo ceramide biosynthesis takes place on the cytosolic surface of the endoplasmic reticulum (ER) in a multi-step cascade. The first and rate-limiting step is catalyzed by serine palmitoyltransferase (SPT), which typically condenses palmitoyl-CoA (C16:0) with L-serine to form 3-ketodihydrosphingosine, the precursor to sphinganine (d18:0) [1, 15]. However, SPT exhibits notable substrate flexibility, allowing for the incorporation of alternative acyl-CoAs such as myristoyl-CoA (C14:0) or stearoyl-CoA (C18:0), resulting in the synthesis of non-canonical sphingoid bases with 16 and 20 carbon atoms, respectively [16, 17]. These atypical sphingoid bases are less abundant than the canonical C18-sphinganine but are increasingly recognized for their physiological and pathological relevance [18, 19]. In addition to acyl-CoA variability of the sphingoid base, SPT can also utilize alternative amino acid substrates, such as alanine and glycine, although with reduced catalytic efficiency [20]. These substitutions yield atypical 1-deoxysphingolipids (deoxySLs), which lack the critical C1-hydroxyl group found in typical sphingoid bases. This structural difference renders deoxySLs unable to form complex sphingolipids or be degraded via canonical catabolic pathways, often resulting in their accumulation and are associated in the development of neurotoxicity and metabolic disorders [21–23].
SPT is an evolutionarily conserved enzyme and functions as a homodimer in prokaryotes and as a heterodimer in eukaryotes, primarily composed of SPTLC1 paired with either SPTLC2 or SPTLC3 [24]. Among these, SPTLC2 harbors the catalytic activity. The specificity and activity of the SPT complex are further modulated by small subunits ssSPTa and ssSPTb, which influence the enzyme’s acyl-CoA chain length preference for the synthesis of sphingoid base [17]. Additionally, ORMDL proteins (ORMDL1-3), integral ER membrane proteins, form a feedback loop to negatively regulate SPT activity in response to intracellular sphingolipid levels [25]. Together, these components—SPTLC1/2 or SPTLC1/3, ssSPTs, and ORMDLs—assemble into a higher-order regulatory complex that integrates cellular lipid status to finely tune sphingolipid biosynthesis, thereby maintaining membrane integrity and lipid homeostasis [26].
The reaction catalyzed by SPT yields 3-ketosphinganine, which is rapidly reduced to sphinganine and subsequently acylated by ceramide synthase enzymes (CERS1-6) to form dihydroceramides with variable acyl chain lengths [27]. Dihydroceramide desaturases then convert these to ceramides by introducing a 4,5-trans double bond, imparting ceramides its unique biophysical properties [14]. Ceramides are subsequently transported to the Golgi apparatus by ceramide transporter (CERT), where head groups such as phosphocholine or sugar moieties are added to form complex sphingolipids like sphingomyelins. CERT specifically mediates non-vesicular transport of ceramide from the endoplasmic reticulum (ER) to the Golgi, where sphingomyelin synthase catalyzes the transfer of a phosphocholine group from phosphatidylcholine (PC) to ceramide, producing sphingomyelin (SM) [28] (Fig. 1). CERT is capable of efficiently transferring various ceramide molecular species that naturally exist in mammalian cells, including those with C14–C20 saturated acyl chains, as well as C16-dihydroceramide and C16-phytoceramide [29]. Structurally, CERT is a cytosolic lipid transfer protein composed of three key functional domains: a pleckstrin homology (PH) domain, which targets the Golgi by binding phosphatidylinositol 4-phosphate (PI4P), an FFAT motif, which anchors CERT to the ER via interaction with VAMP-associated proteins (VAP), and a START domain, which binds and transfers ceramide [30, 31].This modular domain architecture enables CERT to bridge the ER and Golgi membranes and efficiently transport ceramide [14].
Ceramides can be regenerated through the breakdown of sphingomyelin by sphingomyelinases (sphingomyelin hydrolysis), which liberate ceramides while releasing the choline head group [32]. Sphingomyelinases (SMases) are classified into acidic (ASMase), neutral (NSMase), and alkaline (Alk-SMase) based on optimal pH and subcellular localization [1]. ASMase (encoded by Smpd1) primarily functions in lysosomes, while NSMases, including NSMase-1 and NSMase-2 (encoded by Smpd2 and Smpd3, respectively), are located in the plasma membrane [33]. NSMase-2, in particular, is regulated by inflammatory cytokines like TNF-α and IL-1β and is involved in cellular stress responses [34]. Alk-SMase (encoded by Enpp7), primarily acts in the gastrointestinal tract to hydrolyze dietary SM [35]. Depending on the SMase type activated, ceramide is rapidly generated at specific intracellular sites, influencing distinct signaling pathways [33]. Ceramides can be regenerated through a process known as the salvage pathway [1]. Constitutive degradation of sphingolipids and glycosphingolipids occurs in the acidic environments of late endosomes and lysosomes, where they are broken down into ceramides. Ceramides are then further hydrolyzed by acid ceramidase into sphingosine and free fatty acids, both of which can exit the lysosome. The released sphingosine can be recycled through the salvage pathway, where ceramide synthases converts it back into ceramide [36] (Fig. 1).
Ceramides can be degraded by ceramidases [37]. This occurs primarily in lysosomes [38]. In mammals, five ceramidases have been identified, each with a distinct optimal pH: acid ceramidase (Asah1/AC), neutral ceramidase (Asah2/NC), and three alkaline ceramidases (Acer1, Acer2, and Acer3). Among them, the lysosome-localized Asah1 is the physiologically most important [38]. In contrast, neutral ceramidase is primarily found at the plasma membrane, where it contributes to extracellular ceramide metabolism, while alkaline ceramidases are distributed across the endoplasmic reticulum and Golgi apparatus, involved in various cellular processes such as differentiation, apoptosis, and autophagy [39, 40]. Sphingosine produced by ceramides can then be converted to sphingosine-1-phosphate (S1P), a bioactive lipid with roles opposing those of ceramides, particularly in cell survival and proliferation pathways [41]. S1P may either be recycled back to ceramides via the salvage pathway, secreted out of the cell for autocrine or paracrine binding with its receptors (S1PR1-5), or irreversibly broken down by S1P lyase, diverting the molecules out of the sphingolipid pathway (Fig. 1). The activity of the ceramide biosynthetic pathways determines the sphingolipid levels in cells, and they are regulated in cell and context-specific manners.
Biophysical properties of ceramides that regulates its function
Ceramides, as a bioactive lipids significantly influence cellular processes, including membrane dynamics and signaling pathways, the effects that could be influenced by its biophysical properties, some of which are discussed here. The hydrophobic nature of ceramides and their ability to form strong hydrogen bonds lead to the formation of gel-like domains within lipid bilayers, reducing membrane fluidity [42]. Ceramide generation or enrichment within the membrane, alters membrane order and induces phase separation, forming gel domains in the membrane [43]. These domains cause significant changes in membrane structure and function, including vesicle aggregation, fusion, and the release of intravesicular contents [44–46]. Moreover, ceramide induces mitochondrial-mediated apoptosis by regulating membrane permeability and pore formation that allows the release of small proteins from the mitochondria [47, 48].
Biophysical studies on both model and cellular membranes have revealed that elevated ceramide content in lipid rafts increases membrane order and stability, making these regions more mechanically rigid [49, 50]. This change enhances the interaction between proteins and lipids, facilitating the formation of signaling complexes. Moreover, the acyl chain length of ceramide influences its biophysical effects, with long-chain ceramides leading to less tightly packed gel domains compared to shorter-chain variants, affecting membrane properties and cell signaling outcomes [51].
Ceramide’s biophysical effects are highly dependent on lipid composition. In membranes rich in cholesterol, ceramide's ability to form gel domains is inhibited, while in cholesterol-poor membranes, ceramide-enriched domains can form more readily [52, 53]. This ability to modulate membrane properties is crucial in regulating cell signaling and receptor activity. For example, ceramide’s effects on membrane organization can entrap receptors and signaling molecules, potentially activating or inhibiting specific pathways [54]. Studies show that ceramide can alter membrane protein dynamics, such as the lateral diffusion of GPI-anchored proteins, by stabilizing their interactions in ceramide-rich domains [2, 55].
Mechanisms by which ceramides modulate immune cell functions
Ceramides are known to modulate immune cell functions mainly through two mechanisms [3].
Through modulation of membrane dynamics
Ceramides have distinct physical properties, which play a crucial role in modulating membrane dynamics [2]. One of the key functions of ceramides is their ability to form ceramide-rich platforms (CRPs) within the lipid bilayer, influencing the organization of membrane proteins and facilitating various cellular processes, including immune response and cell death [56]. These CRPs promote receptor clustering and protein recruitment, which are essential for cellular signaling [57]. For example, ceramide-induced clustering of the Fas cell surface death receptor (Fas receptor; CD95) at the plasma membrane is critical for the formation of the death-inducing signaling complex and the subsequent activation of caspases, emphasizing ceramides’ involvement in regulating cell death pathways [51, 58–60].
In addition to their role in receptor clustering, as mentioned above, ceramides also affect membrane fluidity and permeability. The length and saturation of ceramide acyl chains influence membrane fluidity, which in turn regulates processes such as cell migration. Moreover, ceramides can impact membrane permeabilization by forming channels in organellar membranes, including those of mitochondria and lysosomes, contributing to cellular homeostasis [61–64]. Together, these effects on membrane properties highlight ceramides as key modulator of cellular function and signaling.
Through ceramide-binding proteins
Ceramides influence cell functions by binding to ceramide-binding proteins (CBPs) located in both the membrane and intracellular compartments. Some of the most studied CBPs include protein phosphatases (PP1 and PP2A), referred to as ceramide-activated protein phosphatases (CAPPs), along with protein kinase C zeta (PKC-ζ) and cathepsin D [65]. CAPPs play essential roles in regulating processes like apoptosis, mitosis, glycogen metabolism, and insulin signaling, and are crucial for controlling Akt phosphorylation, which has implications in cancer and insulin resistance [66, 67].
In addition to these well-known CBPs, an expanding list of putative ceramide-binding proteins is being identified, which participate in a variety of cellular functions. Moreover, ceramides affect mitochondrial processes by interacting with proteins involved in mitophagy and electron transport. For instance, C18-ceramide in mitochondria has been shown to interact with LC3BII, anchoring autophagosomes to mitochondria to promote lethal mitophagy [8, 67], while C16-ceramide disrupts fatty acid oxidation and electron transport by inactivating complexes II and IV of the respiratory chain [68, 69]. C16-ceramide also binds with high affinity to the DNA-binding domain of p53 [70].
On the mitochondrial membrane, voltage-dependent anion channels (VDAC1 and VDAC2), especially VDAC2 – function as ceramide-binding proteins. The interaction of ceramide with VDAC2 increases mitochondrial membrane permeability, facilitating cytochrome c release and promoting intrinsic apoptosis, a mechanism particularly relevant in cancer cells [71, 72]. In addition, ceramide synthesized by CerS6, especially C16:0-ceramide, binds to mitochondrial fission factor (MFF), a key regulator of mitochondrial fragmentation. This interaction promotes mitochondrial fission, contributing to obesity-related insulin resistance by impairing mitochondrial function and disrupting energy metabolism. Notably, deficiency in either CerS6 or MFF protects against fatty acid-induced mitochondrial fragmentation in vitro and mitigates obesity-induced mitochondrial dysfunction in vivo [73].
In the lysosomal and endosomal system, lysosome associated protein transmembrane 4B (LAPTM4B) regulates ceramide levels in late endosomes via a sphingolipid interaction motif and an acidic transmembrane residue that mediate ceramide binding [74]. This interaction modulates mammalian target of rapamycin complex 1 (mTORC1) signaling, linking sphingolipid metabolism to cell growth and autophagy [75, 76]. PAQR4, a member of the progestin and adipoQ receptor (PAQR) family, regulates adipocyte function and systemic metabolic health by mediating ceramide levels. It stabilizes ceramide synthases (CERS2 and CERS5), enhancing their activity and leading to increased ceramide accumulation. This accumulation impairs adipogenesis and triggers adipocyte de-differentiation, contributing to metabolic disorders [77]. Similarly, ER-localized translocating chain-associated membrane proteins (TRAM1 and TRAM2), have been shown to bind ceramide and its analogs, influencing protein translocation across the ER [78].
Moreover, ceramide mediates the interaction of cytosolic proteins, such as S-palmitoylated, acetylated α-tubulin, with cellular membranes by forming ceramide-rich platforms. These ceramide-rich platforms play a crucial role in stabilizing microtubules and supporting ciliogenesis [79].
Notably, the above mentioned ceramide-protein interactions were found in numerous cells types (e.g., cancer cells, muscle cells, adipocytes etc.) and influence their functions. Future studies are warranted to determine whether these interactions and their functions are conserved in immune cells.
Role of ceramides in innate immune cells
Macrophages
Macrophages are essential components of the innate immune system, responsible for phagocytosis, antigen presentation, and the production of pro-inflammatory cytokines. Macrophage activation is a dynamic and essential process that enables these versatile cells to adapt to various stimuli and perform diverse functions. Initially classified into two categories—M1 (pro-inflammatory) and M2 (anti-inflammatory)—macrophages can be classically activated (M1) by pro-inflammatory cytokines like interferon-γ (IFN-γ) and tumor necrosis factor (TNF-α), leading to enhanced inflammatory responses and pathogen clearance [80]. Alternatively, they can undergo alternative activation (M2) in response to anti-inflammatory signals such as IL-4 and IL-13, promoting tissue repair and immune regulation [81, 82].
However, recent studies have shown that the M1/M2 dichotomy oversimplifies macrophage activation, as phenotypes are highly plastic and influenced by a wide range of intrinsic and extrinsic factors, including cytokines, chemokines, pathogen-associated molecular patterns (PAMPs), and pathogen recognition through toll-like receptors (TLRs) [83]. This results in a broad spectrum of macrophage phenotypes that vary in gene expression and cytokine production, complicating the identification of distinct M1 and M2 markers [84]. Macrophages can shift between intermediate or hybrid phenotypes—such as M4 and Mhem in atherosclerosis, and regulatory macrophages (MRs) in immune tolerance—further emphasizing the complexity of macrophage activation [85–87]. These phenotypes reflect the spatiotemporal nature of macrophage polarization, which is influenced by the microenvironment and can be reversible in response to changing conditions. The remarkable plasticity of macrophages allows them to fulfill critical roles in both homeostasis and disease, underscoring the need to understand macrophage activation as a continuum rather than a fixed classification [80, 88].
In macrophages, ceramides are generated in response to various stimuli, including PAMPs, pro-inflammatory cytokines, and nutrient cues [7, 89–92]. Activation of macrophages with Kdo2-lipid A (KLA), a specific (TLR4 ligand, significantly increases sphingolipid content, particularly ceramides, leading to larger cells with numerous autophagosomes crucial for autophagy [93]. Specifically, saturated fatty acids (SFA), which are components of Western diets, mimic pathogen-associated molecular patterns that regulate innate immune function, contributing to hyper-responsiveness to inflammatory stimuli. Exposure of macrophages to SFA, particularly palmitic acid (PA), significantly enhances the inflammatory responses of monocytes and macrophages to lipopolysaccharide (LPS) through a novel, TLR4-independent mechanism. This amplification occurs due to the activation of the ER stress-induced IRE1α pathway, increasing the expression of SPT [94]. Increased ceramide synthesis activates the inflammatory signaling pathways involving PKC-ζ and mitogen-activated protein kinase (MAPK) [95] leading to enhanced production of pro-inflammatory cytokines like IL-1β and TNF-α [90, 92]. This effect appears to be specific to saturated fatty acids, as unsaturated fatty acids either suppress inflammatory responses or have no significant effect [96]. Interestingly, IL-10, a crucial anti-inflammatory cytokine that suppresses macrophage activation, was recently found to reduce inflammation by potently inhibiting ceramide biosynthesis [97]. Moreover, macrophages lacking ASMase and reduced ceramide synthesis exhibit partial resistance to apoptosis during growth factor withdrawal, highlighting the importance of both ASMase activity and ceramides in apoptotic processes (Fig. 2) [89].
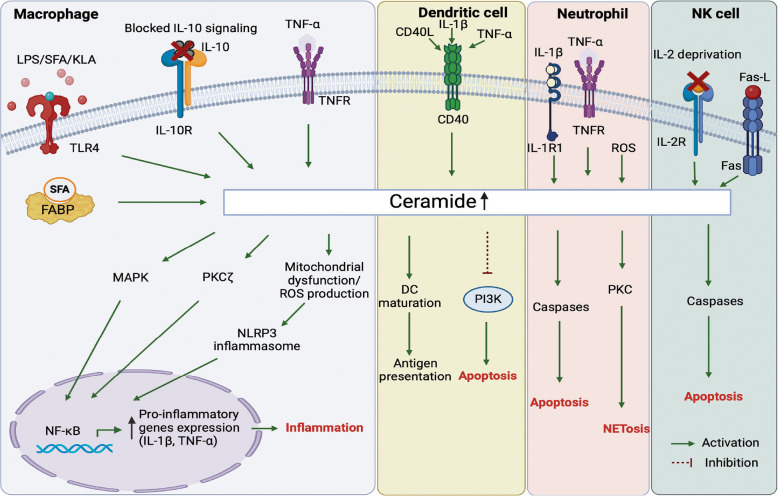
Fig. 2.
Ceramide signaling in innate immune cells. Summary of the pathways involved in promoting ceramide biosynthesis and its molecular and functional effects in innate immune cell subsets (macrophages, dendritic cells, neutrophils) and NK cells. Abbreviations: FABP, Fatty Acid- Binding Protein; IL-10, Interleukin 10; IL-10R, Interleukin receptor 10; KLA, Kdo2-lipid A; LPS, Lipopolysaccharide; MAPK, Mitogen-activated protein kinase; NETosis, Neutrophil Extracellular Trap formation; NF-kB, Nuclear Factor kappa-light-chain-enhancer of activated B cells; NLRP3, NOD-like receptor family pyrin domain-containing 3; PKC, Protein Kinase C; PI3K, Phosphoinositide 3-kinase; ROS, Reactive oxygen species; SFA, Saturated fatty acid; TLR4, Toll-like receptor 4; TNF, Tumor Necrosis Factor; TNFR, Tumor Necrosis Factor Receptor; TNF-α, Tumor necrosis factor-alpha
SFAs-induced ceramide biosynthesis also activates the NOD-like receptor family pyrin domain-containing 3 (NLRP3) inflammasome via activation of AMP-activated protein kinase (AMPK) and reactive oxygen species (ROS) production, leading to enhanced IL-1β secretion, which impairs insulin signaling in adipose tissue [98, 99]. Furthermore, adipose fatty acid-binding protein (A-FABP) in macrophages also induces ceramide production in macrophages in the presence of excess SFA and causes cell death [100]. This process contributes to chronic inflammation associated with obesity and metabolic disorders. Interestingly, while ceramide production through the enzyme SPT was hypothesized to be critical for this process, two independent studies revealed that myeloid cell-specific deletion of Sptlc2 does not significantly alter macrophage responses or insulin resistance [101, 102].
Ceramides also influence macrophage polarization, which refers to the ability of macrophages to adopt to different functional states based on environmental cues. Ceramide treatment has been shown to promote the differentiation of macrophages into a pro-inflammatory M1 phenotype by increasing the expression of M1 marker CD68 and suppressing the expression of M2 markers [103, 104]. Sun et al., identified elevated ceramide accumulation in M1 macrophages, leading to increased hepatocyte apoptosis and liver dysfunction, while M2 macrophages promote sphingosine-1-phosphate generation, aiding hepatocyte proliferation and recovery [105].
Moreover, elevated ceramide production by treatment with long-chain saturated fatty acids like palmitic acid, promotes pro-inflammatory M1 macrophage polarization by inhibiting peroxisome proliferator-activated receptor (PPARγ) [106]. In contrast, monounsaturated fats (MUFAs), such as oleate activate PPARγ and support an anti-inflammatory M2 phenotype [107]. Additionally, distinct macrophage subsets demonstrate varying lipid utilization patterns, suggesting that their polarization state influences responses to environmental fatty acids and inflammation [108].
Dendritic cells
Dendritic cells (DCs) are key antigen-presenting cells (APCs) that play a crucial role in linking innate and adaptive immune responses [109]. Upon exposure to pathogens or inflammatory signals, ceramide levels increase in DCs, leading to enhanced expression of co-stimulatory molecules like CD80 and CD86 [110]. This upregulation is vital for effective T cell activation, as DCs provide essential signals for T cell priming. However, it has been shown that CD40L, TNF-α, and IL-1β, all inducers of DC maturation, also trigger ceramide accumulation in DCs (Fig. 2). This accumulation impairs the cells'ability to capture and process antigens. For example, exogenous C2-ceramide treatment inhibits both macropinocytosis and receptor-mediated endocytosis in DCs, significantly reducing their capacity to present soluble antigens to T cells [111]. Furthermore, ceramide accumulation—following inhibition of acid ceramidase, disrupts major histocompatibility complex (MHC) class II-mediated antigen presentation, leading to reduced stimulation of CD4+ T cells and diminished cytokine production (e.g., IL-2), and impairing optimal immune responses [112].
While DCs are generally resistant to FasL-mediated apoptosis, a feature essential for their role in immune activation, ceramide accumulation disrupts this resistance, promoting cell death [113]. Tumor-derived ceramide species (such as C16, C24:1, and C24:0) have also been shown to induce apoptosis in DCs by inhibiting survival pathways like phosphoinositide 3-kinase/protein kinase B (PI3K/Akt) and extracellular signal-regulated kinase (ERK) (Fig. 2) [9, 114, 115]. Ceramides are also involved in regulating the migration of DCs to lymph nodes, where they present antigens to T cells [116]. The cellular outcomes of ceramide accumulation in DCs depend on factors such as its location, the extent of accumulation, and the timing of its production. The intricate balance between the enzymes of the sphingolipid synthesis and catabolism pathways plays a critical role in regulating DC lifespan, thereby influencing immune responses [117].
In contrast to the pro-apoptotic effects observed in tumor cells, a recent study identified a novel immune-stimulatory role for a synthetic ceramide analog, C8-ceramide, in viral infections. Specifically, C8-ceramide enhanced DC activation during lymphocytic choriomeningitis virus (LCMV) and influenza infections without inducing cell death. Instead, it promoted DC maturation, increasing the surface expression of MHC-I, MHC-II, and co-stimulatory markers, thereby enhancing T cell activation (Fig. 2). These findings suggest that C8-ceramide may offer a promising approach to modulate immune responses in viral infections without the adverse effects typically associated with ceramide-induced apoptosis [110].
Further investigation into the role of ceramides in DC maturation and immune responses reveals differences in lipid dynamics during tolerance-inducing versus inflammatory maturation of DCs. A key finding is the role of switch-associated protein 70 (SWAP-70), which regulates ceramide accumulation in DCs [118]. These findings underscore ceramide as a critical mediator of immune responses, particularly in DC activation and apoptosis, and highlight the importance of SWAP-70 in regulating lipid metabolism during immune activation. This research provides new insights into how lipid signaling influences immune responses and could inform therapeutic strategies targeting lipid pathways for immune modulation.
Neutrophils
Neutrophils, as key players in the immune response, have been shown to interact with ceramides in several ways that impact their function [119] (Fig. 2). Ceramides can be generated in neutrophils through various signaling pathways, including those activated by oxidative stress, infection, or inflammatory stimuli (Fig. 2). Once produced, ceramide can modulate multiple aspects of neutrophil biology, including migration, adhesion, and apoptosis [120]. Neutrophil functions such as extravasation, migration, cytokine production, superoxide generation, and neutrophil extracellular trap (NETosis) formation are essential for immune responses to extracellular pathogens (Fig. 2) [121]. Early research identified ceramides as critical regulators in these processes, linking them to TNF-α signaling [122]. However, subsequent research revealed that ceramides negatively regulate superoxide production. Specifically, C2-ceramide inhibited arachidonic acid-induced superoxide formation and respiratory bursts in N-formyl-methionyl-leucyl-phenylalanine (fMLP) – stimulated neutrophils [123]. Neutral sphingomyelinase-derived ceramides modulate Rac1/2 and RhoA GTPases, affecting neutrophil polarity and chemotactic responses [124]. These findings suggest that ceramides play a dual role, initially delaying superoxide production to facilitate migration and later regulating inflammatory responses.
Ceramides also mediate apoptosis in neutrophils through caspase activation, particularly involving C16- and C24-ceramide species (Fig. 2) [125]. Anti-apoptotic factors like granulocyte–macrophage colony-stimulating factor (GM-CSF) reduce ceramide levels to delay apoptosis. ASMase-generated ceramide is essential for early neutrophil apoptosis, with delayed apoptosis observed in ASMase-deficient mice [126]. Furthermore, ceramides contribute to antimicrobial responses, such as ROS-induced mitochondrial ceramide production during Pseudomonas aeruginosa infections, which triggers cytochrome c release and cell death [127].
Ceramides have been shown to play an anti-inflammatory role in neutrophils as well by interacting with the inhibitory receptor CD300f. Ceramide-CD300f interaction suppressed the release of neutrophil chemoattractants from Escherichia coli-stimulated mast cells and neutrophils. Disruption of ceramide-CD300f interactions leads to increased neutrophil infiltration and heightened inflammatory responses in sepsis, protecting CD300f knockout mice from sepsis-induced death [128]. Ceramide-CD300f binding inhibited lipopolysaccharide-induced skin inflammation as well [129]. These findings highlight ceramides' diverse regulatory effects in neutrophil biology.
Recent studies have highlighted the critical roles of IL-1β-induced ceramide synthesis and protein kinase C (PKC) isoforms in the regulation of NETosis. IL-1β triggers ceramide production in neutrophils, which in turn promotes NET formation, a process linked to the progression of diseases such as abdominal aortic aneurysms [130]. Concurrently, PKC isoforms, including PKCβ, PKCδ, and PKCζ, are essential for mediating oxidative burst, cell spreading, and NETosis [131] (Fig. 2). Ceramide synthesis may activate PKC [132], creating a feedback loop that enhances NETosis and contributes to chronic inflammation.
Natural Killer cells
Natural Killer (NK) cells play a pivotal role in immune surveillance, particularly in the elimination of infected and tumor-transformed cells [133]. Recent studies have highlighted the importance of ceramide in regulating NK cell functions, including activation, cytokine production, and apoptosis [134, 135]. Similar to its role in other immune cells, ceramide acts as a key mediator of apoptosis signaling in NK cells, particularly following receptor-mediated activation, such as Fas ligation [134].
In contrast to their role in activating NK cells, ceramides have been implicated in regulating NK cell apoptosis. A study published in 1998 showed that ceramide plays a critical role in Fas-induced apoptosis in NK cells and that redox regulation influences both ceramide generation and downstream signaling events, including protein tyrosine dephosphorylation, to control NK cell apoptosis [134] (Fig. 2). Additionally, C6-ceramide nanoliposomes and 1-phenyl-2-palmitoylamino-3-morpholino-1-propanol (PPMP), an inhibitor of GCS was shown to have synergistic effects, leading to increased ceramide levels in leukemic NK cells, inducing mitochondrial dysfunction, and triggering apoptosis. The mechanism of cell death involved the mitochondrial intrinsic apoptosis pathway, as evidenced by mitochondrial membrane depolarization and decreased levels of anti-apoptotic proteins like survivin and myeloid cells leukemia (Mcl)−1, which are key survival factors in NK leukemia [136]. ASM-generated ceramide in IL-2 deprivation induces apoptosis in NK/T lymphoma cells via a novel apoptotic pathway where ceramide activates cathepsin B in lysosomes, leading to the degradation of x-linked inhibitor of apoptosis (XIAP), which subsequently facilitates the nuclear translocation of cleaved caspase-3 [137] (Fig. 2).
IL-2 is known to promote NK cell expansion, and it has been shown that it does so by inhibiting ceramide generation through the regulation of ASMase, glucosylceramide synthase (GCS), and sphingomyelin synthase (SMS) [138]. This process is linked to the activation of PI-3 kinase, which not only supports cell growth but also inhibits ceramide generation through the regulation of ASMase, GCS, and SMS [138]. The balance between ceramide-induced survival and cell death signals is critical for maintaining proper NK cell function. Interestingly, ceramide regulation is not limited to apoptosis but also extends to NK cell activation. For example, α-galactosylceramide (αGalCer) strongly activates NK cells and triggers cytokine production (e.g., IL-4, IFN-γ) [135, 139]. This activation of NK cells increases the resistance to allogeneic bone marrow transplantation.
In parallel with ceramide signaling, a recent study highlights the critical role of glycosphingolipid (GSL) metabolism in NK cell biology. Specifically, super-enhancer loci in NK cells were found to contain genes Ugcg and B4galt5 that encodes for UDP-glucose ceramide glucosyltransferase or GCS and β−1,4-galactosyltransferase 5 respectively, the two enzymes essential for GSL biosynthesis [140]. The study further showed that glycosphingolipids are essential for NK cell survival and cytotoxic function. Moreover, authors found that inhibition of UGCG activity disrupts granzyme B localization and the formation of functional immunological synapses, leading to NK cell apoptosis [141].
Role of ceramides in adaptive immune cells
B cells
B cells are a crucial component of the adaptive immune system, primarily responsible for producing antibodies that help to fight infections. They originate in the bone marrow and undergo a series of developmental stages, including the rearrangement of their immunoglobulin (Ig) genes, which allow them to produce unique antibodies that recognize specific antigens [142]. Once matured, B cells circulate in the bloodstream and migrate to secondary lymphoid organs, such as the spleen and lymph nodes, where they encounter antigens [143]. B cells are activated when their B cell receptors (BCRs) bind to specific antigens, a process often assisted by helper T cells through CD40 ligation and cytokine release [144]. Upon activation, B cells differentiate into plasma cells, which secrete large quantities of antibodies, and memory B cells, which retain the ability to respond rapidly to future infections by the same pathogen [145]. In addition to antibody production, B cells also act as antigen-presenting cells (APCs), helping to initiate immune responses by presenting antigens to T cells [146]. The function of B cells is tightly regulated to prevent autoimmunity, with mechanisms in place to eliminate or inactivate self-reactive B cells during their development [147, 148]. However, dysregulation of B cell activity can lead to autoimmune diseases like systemic lupus erythematosus (SLE) and rheumatoid arthritis, or immunodeficiency disorders when B cell function is insufficient [149–151].
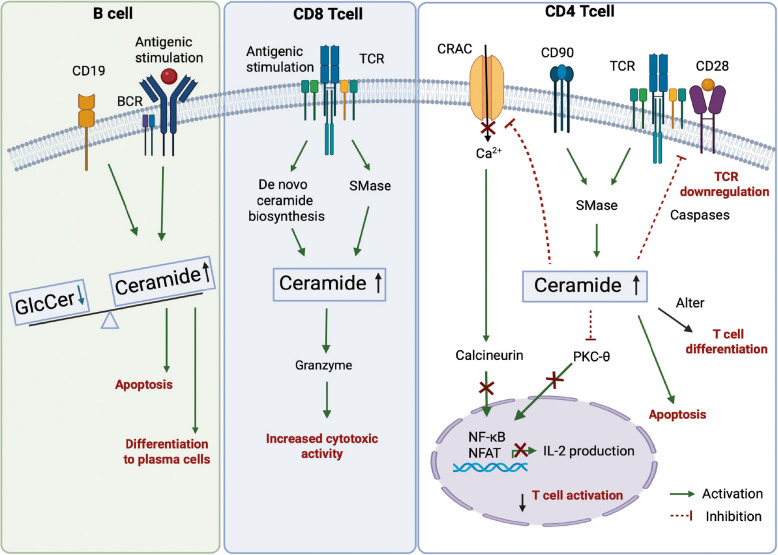
The studies highlights the multifaceted role of ceramide in regulating B cell fate, particularly in chronic lymphocytic leukemia (CLL) and other B cell types. Upon B-cell receptor (BCR) activation, CLL cells exhibit a metabolic shift—ceramide levels decrease while glucosylceramide increases, promoting cell survival and resistance to apoptosis (Fig. 3). This shift in the ceramide-to-glucosylceramide ratio is critical, as inhibiting glucosylceramide synthesis using UGCG inhibitors restores apoptosis, especially when combined with ceramide-inducing agents like ABT-737 [152]. In parallel, other studies show that BCR-triggered apoptosis involves a two-phase ceramide response: an early, caspase-independent rise in C16-ceramide leading to proteasomal activation and XIAP degradation, followed by caspase-dependent accumulation of C24-ceramide that amplifies apoptosis [153–155]. Additionally, long term exposure of ceramide levels suppress antigen-triggered calcium responses in B lymphocytes, while mature B cells like Burkitt lymphoma and marginal zone B cells show resistance [156]. In contrast to these detrimental effects, ceramide plays a crucial role in promoting B cell differentiation into plasma cells (PCs) during immune responses (Fig. 3). LPS activation induces metabolic changes, including increased ceramide biosynthesis, regulated by X-linked inhibitor of apopotosis protein (XBP-1) [157]. This process supports plasma cell differentiation, ER membrane expansion, and protein glycosylation for antibody production. Furthermore, ceramide promotes sphingolipid remodeling, particularly sphingomyelin synthesis, vital for membrane biogenesis and signaling. Together, these findings underscore the therapeutic potential of modulating ceramide metabolism in B cells, especially for targeting malignant B cell survival and promoting efficient plasma cell differentiation in immune responses.
Fig. 3.
Ceramide signaling in adaptive immune cells. Overview of the pathways involved in inducing ceramide biosynthesis and its molecular and functional impacts on adaptive immune cell subsets (B cells, CD8 and CD4-Tcells). Abbreviations: BCR, B cell receptor; CRAC: Calcium Release-Activated Calcium channels; GlcCer, Glucosylceramide; NF-kB, Nuclear Factor kappa-light-chain-enhancer of activated B cells; NFAT, Nuclear Factor of Activated T cells; SMase, sphingomyelinase; TCR, T cell receptor
CD4+ T cell lymphocytes
T cells are a central component of the adaptive immune system, responsible for recognizing and responding to various pathogens, tumors, and self-antigens [158]. They arise from hematopoietic stem cells in the bone marrow and mature in the thymus, where they undergo selection processes that ensure self-tolerance and functional competence [159]. Upon encountering antigens presented by major histocompatibility complex (MHC) molecules on APCs, T cells become activated and differentiate into distinct subtypes with specialized functions. The main subtypes of T cells include CD4+ helper T cells, CD8+ cytotoxic T cells, regulatory T cells (Treg), and memory T cells, each playing a pivotal role in immune defense and homeostasis [160]. T cells can initiate, sustain, or suppress the immune response. Initiation and enhancement of immune response are caused by the cytotoxic T cells and helper T cells, while the suppression of immune response is caused by the Treg cells [161].
Ceramides in T cell differentiation
Numerous studies have implicated the requirement of sphingolipids in the differentiation, and function of CD4+ T cells, including Th1, Th2, Th17, and Treg subsets [162, 163]. Employing genome-scale metabolic modeling (GSMM) in human CD4+ T cells, Sen and colleagues highlighted the essential and selective role of sphingolipids, particularly ceramide and GSL pathways, in Th17 cell differentiation [164]. These findings were further corroborated by Kanno and colleagues, who found that indeed numerous ceramide and GSL species were markedly induced in in vitro differentiated Th17 and were crucial for their differentiation and proinflammatory cytokine expression (IL-17A) [164–166]. While these two studies indicated that sphingolipids selectively modulate Th17 cell differentiation, other studies have found that ceramides are required for Th1 and Treg cell differentiation in vitro [167, 168]. In contrast to these in vitro findings indicating the requirement of ceramides for specific T cell subset differentiation, other studies have found that ceramides impair the differentiation of naïve CD4+ T cells into Treg cells but no other CD4+ T cell subtypes [169]. In vivo, while inhibition of Sptlc1 in CD4+ T cells impairs Th1 and Th17 differentiation [170], ablation of ASMase, which reduces ceramide levels, increases Treg cell differentiation [169]. Thus, while these studies provide support for the role of ceramides in modulating CD4+ T cell differentiation, there is a lack of clarity on how and which T cell subtypes are selectively modulated by ceramides and whether these effects are mediated by ceramides or other sphingolipids.
Regulation of T cell activation by ceramides
TCR modulation
T cell activation begins when the T cell receptors (TCRs), composed of TCR chains and CD3 molecules, recognize antigens presented by the MHC. T cells express either α-β or γ-δ TCR chains, with α-β T cells being the most common [171]. Co-receptors CD4 and CD8 further mediate antigen recognition by binding to MHC class II and class I molecules, respectively [172]. Full TCR activation requires co-stimulation via CD28, which binds CD80/86 on antigen-presenting cells, activating the PI3K pathway and phospholipase C-γ (PLC-γ) and downstream signaling [173–175]. This cascade involves the phosphorylation of zeta-chain-associated protein kinase 70 (ZAP-70) and the activation of linker for activation of T cells (LAT), which activates key transcription factors [e.g., nuclear factor-κB (NFκB), activator protein 1 (AP-1), signal transducer and activator of transcription 5 (STAT5), and nuclear factor of activated T cells (NFAT)], promoting T cell proliferation, differentiation, and survival [176]. TCR signaling occurs in lipid rafts, specialized membrane regions that concentrate signaling molecules into a supramolecular activation cluster (SMAC), ensuring efficient signal transduction [177, 178].
Ceramides play a pivotal role in regulating TCR signaling and modulating T cell fate, exhibiting both pro-apoptotic and regulatory effects [5, 179]. Ceramides are generated upon engagement of death receptors like Fas/CD95 [180] or exposure to stress/inflammatory stimuli through sphingomyelinase activation [181]. When TCR engages with APCs, it triggers a cascade of signaling events that rapidly induce ceramide production (Fig. 3) [182]. Ceramide has a dual role in regulating T cell fate, depending on the concentration and duration of the stimulus. High levels of ceramide, especially when generated during prolonged or strong Fas activation, trigger apoptosis in T cells [183]. This apoptosis is a key mechanism for eliminating exhausted effector T cells, thus maintaining immune homeostasis and preventing autoimmune diseases. Conversely, ceramide also exerts non-apoptotic effects, particularly at lower concentrations when treated for shorter durations. At lower concentrations, ceramide inhibits T cell activation without inducing cell death. This modulation occurs through ceramide's impact on membrane raft reorganization, ion channels, and phosphorylation events, which influences calcium signaling and TCR-mediated activation (Fig. 3) [184]. For example, short-term exposure to ceramide has been shown to protect cytotoxic T cells (CTLL-2; cytotoxic T lymphocyte cell line-2, clone of cytotoxic T cells derived from a C57BL/6 mouse) from apoptosis by inhibiting the degradation of Bcl-xL, a protein involved in cell survival [185].
In contrast, prolonged or higher ceramide concentrations, such as those induced by chronic Fas engagement, promote apoptosis in T cells through mechanisms like caspase activation [186]. Moreover, ceramide modulates various intracellular signaling pathways, such as the inhibition of protein kinases like PKC-θ (protein kinase C-theta). Ceramide’s inhibition of PKC-θ disrupts NF-κB activation, leading to reduced IL-2 production which negatively regulates T cell activation [187] (Fig. 3). In addition, increased accumulation of ceramides in the plasma membranes interferes with TCR nanoclustering in CD4+ T cells [188] (Fig. 3). Moreover, sphingomyelinase-mediated ceramide enrichment in the T cell plasma membrane disrupts C-X-C chemokine receptor type 4 (CXCR4) receptor dynamics, impairing the T cell's ability to respond to chemokine gradients and hindering T cell migration, the critical processes in T cell recruitment during autoimmune responses and cancer metastasis [189].
Calcium signaling
Calcium (Ca2+) signaling plays a critical and multifaceted role in T cell activation, differentiation, and immune responses [190]. Upon T cell receptor engagement, intracellular Ca2+ stores are mobilized through store-operated calcium enty mechanism, predominantly involving calcium release-activated calcium (CRAC) channels, composed of Orai1/2 proteins and Stim1 proteins [191, 192]. This Ca2+ influx is essential for various downstream signaling pathways, most notably the activation of calcineurin, a protein phosphatase that dephosphorylates the transcription factor NFAT [193]. This dephosphorylation allows NFAT to translocate into the nucleus and promote the transcription of genes like IL-2, a key cytokine for T cell proliferation and survival (Fig. 3). Membrane composition critically modulates calcium signaling efficiency. For example, the unsaturated fatty acid oleic acid enhances calcium flux upon CD3/CD28 stimulation in CD4⁺ T cells by incorporating into membrane lipids, suggesting that lipid membrane fluidity and structure play a role in optimizing Ca2+2⁺ channel function and signal transduction [194]. In contrast, ceramide exerts an inhibitory influence on Ca2+ signaling in T cells [2]. By altering membrane biophysical properties, ceramide can disrupt the function of CRAC channels, thereby blocking Ca2⁺ influx (Fig. 3). This suppresses the activation of calcineurin and NFAT, ultimately downregulating IL-2 production and impairing T cell activation (Fig. 3). Thus, ceramide dampens immune responses not only through the induction of apoptosis but also by directly antagonizing calcium-dependent signaling pathways [180, 195, 196].
Role of ceramide species in T cells
TCR stimulation in activated T helper cells show a significant increase in ceramide production, particularly through the upregulation of ceramide synthases, CerS2 and CerS5 enzymes, which produce long-chain (C22–C24) and short-chain (C14–C16) ceramide species respectively [165]. This shift enhances glycosphingolipid production and supports TCR signaling, highlighting a potential regulatory role for ceramide species in immune activation [165]. However, in mice lacking CerS2, Th2 differentiation from naïve CD4+ T cells is reduced, while Th17 differentiation is enhanced. This shift is partly due to changes in TCR signaling and membrane properties, as CerS2 influences lipid rafts and TCR nanoclustering [197].
CerS4 is an essential enzyme in the synthesis of long-chain ceramides (C18-C20), and its role extends beyond lipid metabolism to regulate critical immune responses [198]. CerS4 plays a critical role in T cell function and immune responses in colitis and colitis-associated cancer. In T cells, CerS4 regulates several key pathways, including TGFβ signaling, NF-κB activation, and cytokine production [199]. CerS4 deficiency in T cells impairs T cell proliferation, and immune resolution, and enhances tumor progression in models of colitis and cancer, respectively [200, 201].
CerS5 and CerS6 both contribute to the generation of short-chain ceramides, but their physiological effects differ. For example, CerS5 and CerS6 knockout mice show increased susceptibility to DSS-induced colitis and colitis-associated colon cancer [60, 202]. However, CerS6 deficiency is linked to increased neutrophil infiltration, while CerS5 deficiency leads to a reduction in CD3+ T cells, including CD4+, CD8+, and Treg subsets, in the colon, blood, and spleen. This reduction suggests that CerS5 affects T cell migration and activation. Moreover, CerS5 depletion in T cells impairs TCR signaling, reduces NF-κB activation, and impairs cytokine production (e.g., IFN-γ and IL-4) [202].
In inflammatory models, such as colitis and graft-vs-host disease (GVHD), CerS6-deficient T cells exhibit reduced inflammation, suggesting a protective effect [203]. CerS6 contributes to T cell-driven inflammation by enhancing T cell proliferation and IFN-γ production. In contrast, pharmacological inhibition or genetic ablation of CerS6 impairs TCR signaling in response to alloantigens, leading to reduced T cell responses in GVHD and colitis [204]. This effect is mediated through the regulation of TCR signaling in CD4+ T cells via the N-Ras/ERK pathway, which is critical for T cell activation, migration, and inflammatory responses [204]. In addition, CerS6-induced ceramide accumulation during aging has been shown to disrupt T cell antitumor activity [205]. Aging-related ceramide stress contributes to T cell dysfunction via mitophagy, limiting their antitumor and immune functions. Mechanistically, this is mediated by CerS6-induced ceramide accumulation, which inhibits protein kinase A (PKA) activation, and mitochondrial dysfunction, and induces mitophagy [205].
CD8+ T cell lymphocytes
Ceramides regulate the cytotoxic functions of CD8+ T cells, which are essential for clearing infected or tumorigenic cells [206]. CD8+ T cells execute their cytotoxic response primarily through the secretion of perforin and granzymes, which induce apoptosis in target cells [207, 208]. The formation and function of the immunological synapse — the interface between the CD8+ T cell and its target — is critical for this process, and ceramide plays a significant role in this dynamic. Ceramide levels influence TCR signaling in CD8+ T cells and are required to protect against viral infections (Fig. 3) [209]. Specifically, in AC-deficient mice, ceramide-enriched platforms in the immunological synapse were found to enhance TCR signaling, suggesting that ceramide plays a crucial role in modulating TCR signaling strength and improving CD8+ T cell function [206]. Additionally, ASMase deficiency in CD8+ T cells led to reduced granzyme B production, impaired cytotoxicity, and diminished tumor size/burden [206]. Moreover, ablation of Sptlc2 impairs the metabolic fitness of CD8+ T cells to elicit protective T cell responses [209]. Conversely, elevated ceramide levels promoted increased T cell activation and improved granzyme B production in CD8+ T cells, enhancing their cytotoxic function and anti-tumoral immune response, which ultimately resulted in reduced tumor growth [104, 206, 210].
Diseases associated with ceramide mediated immune cell dysfunction
Ceramide-mediated immune dysfunction in chronic inflammatory diseases (obesity, diabetes mellitus, atherosclerosis, inflammatory bowel disease (IBD), etc.) and autoimmune diseases (systemic lupus erythematosus (SLE), multiple sclerosis (MS), Alzheimer’s disease (AD), etc.) are well established [4, 14, 211–215]. A common underlying factor for obesity-associated diseases like type 2 diabetes mellitus and atherosclerosis is chronic low-grade inflammation [216]. This inflammation is characterized by ceramide accumulation in adipose tissue and contributes to insulin resistance and activation of pro-inflammatory immune cells, which further exacerbates metabolic dysfunction. Moreover, ceramides activate NLRP3 inflammasomes in macrophages to induce cytokine secretion [99, 217]. Macrophage secretion of inflammatory cytokines such as TNF-α, IL-6, and IL-1β further induces ceramide production leading to a vicious cycle of inflammation in adipose tissue and subsequent insulin resistance [218]. In atherosclerosis, ceramide promotes the activation of endothelial cells and macrophages, leading to the formation of atherosclerotic plaques and vascular inflammation [219–221].
Recent studies suggest that ceramide metabolism is closely linked to the pathogenesis of inflammatory bowel disease, with both ceramide synthesis and catabolism influencing disease outcomes. Altered ceramide levels, due to changes in ceramide synthase isoforms, contribute to intestinal inflammation by disrupting the epithelial barrier and enhancing immune responses [60, 213, 222]. Increased ceramide levels in intestinal tissues can drive the differentiation of pro-inflammatory Th17 cells, leading to the secretion of cytokines such as IL-17 and IL-22, which intensify gut inflammation [223]. Furthermore, ceramides hinder the functions of regulatory T cells, impairing their ability to suppress effector T cell activity [224]. Ceramides also promote T cell migration to inflamed tissues and enhance cytokine secretion, further fueling the inflammatory response [225]. This dysregulation of T cell activity contributes to the chronic inflammation and immune dysfunction observed in IBD [226, 227]. For instance, myeloid-specific ceramidase knockouts protects against colitis by reducing neutrophil recruitment [203]. These findings suggest that ceramide regulation plays a vital role in immune cell activation, and inflammatory responses, presenting ceramide-related pathways as potential targets for therapeutic intervention in IBD.
Ceramide signaling has been implicated in other chronic inflammatory conditions including autoimmune diseases like SLE and MS where it triggers the activation of autoreactive immune cells, induces cytokine production, and enhances the inflammatory processes that drive tissue damage [228, 229]. In the central nervous system (CNS), elevated ceramide levels have been shown to activate the NF-κB signal transduction in microglia, shifting the glial response towards inflammation and prompting the release of TNF-α, IL-1β, and IL-6 from astrocytes, thereby inducing neuroinflammation in conditions like AD [230, 231]. In AD, ceramide is also implicated in neuroinflammation, neuronal apoptosis, and the formation of amyloid plaques, which are characteristic of the disease’s pathology [211, 232, 233]. Through these mechanisms, ceramide mediates immune dysfunction by modulating immune cell activation, promoting chronic inflammation, and contributing to tissue damage in these diseases. Although most of the studies on the role of ceramides in disease conditions have focused on the role of ceramides in instigating inflammation and immune cell activation, the evidence of ceramide dysregulation within the immune cells in these disease contexts is limited and warrants future attention.
Therapeutic targeting of ceramide pathways in immune cells
Targeting ceramides in immune cells offers a compelling avenue for therapeutic intervention in a variety of diseases where immune dysfunction plays a pivotal role. Dysregulated immune cell ceramide signaling has been implicated in a spectrum of pathological conditions, including chronic inflammatory diseases like rheumatoid arthritis, autoimmune disorders such as lupus, metabolic diseases like obesity and diabetes, and cancer progression [99, 200, 214, 227, 232, 234]. By modulating immune cell ceramide levels, it may be possible to restore immune homeostasis and counteract disease progression.
Ceramide signaling in immune cells can impact their activation and function, offering the potential for immune modulation in cancer immunotherapy. Ceramide influences the immune response in the tumor microenvironment, and modulating ceramide levels enhances the effectiveness of immunotherapies [235]. Ceramide acts as a pro-inflammatory mediator by activating inflammatory signaling pathways, such as NF-κB signaling and cytokine production in macrophages. Thus, elevated ceramide levels can contribute to the amplification of the anti-cancer immune response. The frequencies and activities of different T cell subtypes in the tumor microenvironment regulate tumor progression, and ceramides can modulate this process. For example, exogenous C2-ceramide induces a strong anti-tumor response by increasing frequencies of cytotoxic CD8+ and IFN-γ-producing CD4+ T cells [104]. Using acid sphingomyelinase and acid ceramidase knockouts in CD4+ cells, which exhibit decreased or increased ceramide levels in T cells, respectively, it was revealed that increased ceramide concentrations improve the anti-tumoral T cell response during melanoma progression [206]. These findings highlight ceramides as important modulators of T cell function in the tumor microenvironment (TME). Treg cells also play a key role in modulating anti-cancer immune response. The infiltration of Treg cells into the tumor is considered a critical step during tumorigenesis, enhancing tumor progression [236, 237] and these cells are known to adapt to the tumor microenvironment by shifting their lipid metabolism – particularly through increased fatty acid uptake and oxidation, to sustain their suppressive function [238]. Although unclear, growing evidence suggests that ceramides play a role in the metabolic reprogramming essential for Treg survival and function within the TME [201, 239]. As ceramides emerge as key regulators of Treg biology, targeting their metabolic pathways presents a promising strategy to modulate Treg-mediated immunosuppression and enhance anti-tumor immune responses. Targeting immune cell ceramides is a promising strategy for treating autoimmune diseases as elevated ceramide levels within immune cells contribute to excessive inflammation by activating immune cell signaling pathways [6, 119, 234], making them a potential therapeutic target to modulate immune responses in autoimmune conditions such as rheumatoid arthritis, systemic lupus erythematosus, and multiple sclerosis.
Ceramide is required for maintaining intestinal homeostasis [213, 240]. Modulation of ceramides in neutrophils alters their migration to inflamed sites within the intestine. Depletion of acid ceramidase in myeloid cells, which increases ceramide levels, leads to decreased migration of neutrophils to the intestine and protects from experimental models of colitis and colitis-associated cancer development [241]. Interestingly, decreasing C16-ceramide species in T-lymphocytes was found to protect against experimental colitis development partly due to the decreased migratory capacity of the T cells [203]. Thus further opening the therapeutic opportunity to target specific ceramide species in inflammatory diseases.
Future directions and open questions
While the role of ceramides in immune dysregulation across various disease contexts is well established, there remain significant gaps in understanding their precise role within specific immune cell subsets. Ceramides are known to modulate several critical immune processes, including immune cell activation, trafficking, inflammation, and programmed cell death [117, 234]. However, the cell-type-specific mechanisms by which ceramides influence these processes in T cells, macrophages, dendritic cells, neutrophils, and other immune populations are not fully elucidated.
The effects of different ceramide species—shaped by their acyl chain length, saturation, and subcellular localization—likely vary across immune cell types. To address this, future research employing single-cell lipidomics and metabolomics will aid in resolving the spatial and metabolic heterogeneity of ceramide signaling at the single-cell level. These approaches will help decipher how specific ceramide species regulate immune activation, cytokine production, migration to inflammatory sites, and cell death pathways. Complementary flux studies using stable isotope-labeled precursors can further illuminate the real-time dynamics of ceramide biosynthesis and turnover under different immunological conditions. To understand the molecular underpinnings of ceramide function, future studies could utilize genetic models, such as conditional knockout mice targeting ceramide synthases or sphingomyelinases in specific immune cell types. Moreover, CRISPR-Cas9-based gene editing in primary immune cells will provide a powerful strategy to dissect the roles of individual enzymes or regulatory nodes in the ceramide biosynthetic and signaling pathways.
Another major gap is the limited understanding of the temporal dynamics and subcellular compartmentalization of ceramide production during immune activation. This could be addressed through live-cell imaging techniques using ceramide-sensitive fluorescent probes, coupled with metabolic flux assays to track the synthesis and turnover of ceramides in different intracellular compartments. In addition, the composition and organization of ceramide-enriched membrane microdomains, which serve as signaling hubs for receptor clustering and downstream signaling—remain largely uncharacterized. Advanced proteomic tools, such as proximity labeling proteomics can be employed to identify the protein complexes assembled within these microdomains and define how ceramide scaffolds immune signaling at the membrane interface.
The role of ceramides in maintaining immune tolerance and preventing autoimmunity also remains poorly understood. Key questions include whether ceramide profiles are distinct in regulatory T cells and tolerogenic dendritic cells, and how alterations in ceramide metabolism may contribute to the breakdown of peripheral tolerance. Experimental autoimmune models, combined with selective ceramide modulation, can be used to investigate whether altering ceramide signaling restores immune homeostasis and prevents autoimmune responses.
Ceramides are emerging as key regulators of immune cell metabolism [115, 123, 136, 205], yet several fundamental questions remain unanswered. One major question is how ceramide impact metabolic pathways such as glycolysis, oxidative phosphorylation, and fatty acid oxidation across various immune cells. It is unclear whether these effects are cell-type specific or influenced by environmental cues. Another open question is how ceramides impair mitochondrial function and alter redox balance in immune cells. Do they directly affect mitochondrial integrity, or act via upstream regulators like PI3K/Akt or AMPK? The timing of ceramide accumulation is also not well understood—whether it serves as an early metabolic switch, or a marker of chronic stress remains to be determined. Further questions include how ceramides interact with other metabolic pathways, such as amino acid metabolism and mTOR signaling, and whether these interactions influence immune cell fate—activation, exhaustion, or memory formation. To explore these questions, future studies could combine lipidomics and metabolomics with genetic manipulation (e.g., ceramide synthase knockouts or pathway inhibitors), real-time metabolic assays (e.g., Seahorse), and isotope tracing. Single-cell multi-omics and in vivo models will be essential to define the context-specific metabolic roles of ceramides.
Finally, to translate these mechanistic insights into viable clinical applications, there is a need to develop precision-targeted therapeutic strategies. This includes the use of nanoparticle-based or antibody-conjugated liposomal delivery systems to modulate ceramide pathways in specific immune cell populations. Additionally, enzyme-specific modulators will ensure targeted manipulation of ceramide signaling with minimal off-target effects and systemic immune suppression.
Over the past decades, research has collectively shown that ceramides are tightly regulated and play a key role in modulating immune cell differentiation and function, thereby influencing the development of various inflammatory and potentially metabolic diseases. With the advent of novel tools and technologies, this field is now well-positioned to uncover how ceramides are regulated, trafficked between organelle and modulate metabolism within immune cells during their activation or suppression. Moreover, defining their cell-autonomous effects and underlying mechanisms could lay the foundation for developing new strategies to target ceramides within immune cells for the treatment of numerous diseases such as the inflammatory diseases, cancer and metabolic disorders.
Acknowledgements
The authors received research support from the National Institutes of Health (DK124326 to B.C.), American Diabetes Association (7-21-JDF-033 to B.C.), Diabetes Action Research and Education Foundation (522 to B.C.), Roy J. Carver Charitable Trust (25-5906 to B.C.), American Heart Foundation postdoctoral fellowship (25POST1374595 to H.T) and (25POST1372103 to VV).
Authors' contributions
H.T and V.V wrote the initial draft of the manuscript with input from B.C. B.C. and H.T edited the manuscript.
Funding
The authors received research support from the National Institutes of Health (DK124326 to B.C.), American Diabetes Association (7-21-JDF-033 to B.C.), Diabetes Action Research and Education Foundation (522 to B.C.), Roy J. Carver Charitable Trust (25-5906 to B.C.), American Heart Foundation postdoctoral fellowship (25POST1374595 to H.T) and (25POST1372103 to V.V).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Himani Thakkar, Email: himani-thakkar@uiowa.edu.
Bhagirath Chaurasia, Email: bhagirath-chaurasia@uiowa.edu.
References
- 1.Hannun YA, Obeid LM. Sphingolipids and their metabolism in physiology and disease. Nat Rev Mol Cell Biol. 2018;19:175–91. 10.1038/nrm.2017.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alonso A, Goñi FM. The physical properties of ceramides in membranes. Annu Rev Biophys. 2018;47:633–54. 10.1146/annurev-biophys-070317-033309. [DOI] [PubMed] [Google Scholar]
- 3.Stith JL, Velazquez FN, Obeid LM. Advances in determining signaling mechanisms of ceramide and role in disease. J Lipid Res. 2019;60:913–8. 10.1194/jlr.S092874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maceyka M, Spiegel S. Sphingolipid metabolites in inflammatory disease. Nature. 2014;510:58–67. 10.1038/nature13475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bai A, Kokkotou E, Zheng Y, Robson SC. Role of acid sphingomyelinase bioactivity in human CD4+ T-cell activation and immune responses. Cell Death Dis. 2015;6:e1828. 10.1038/cddis.2015.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee M, Lee SY, Bae YS. Functional roles of sphingolipids in immunity and their implication in disease. Exp Mol Med. 2023;55:1110–30. 10.1038/s12276-023-01018-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Rashed F, et al. Ceramide kinase regulates TNF-α-induced immune responses in human monocytic cells. Sci Rep. 2021;11:8259. 10.1038/s41598-021-87795-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pilátová MB, Solárová Z, Mezencev R, Solár P. Ceramides and their roles in programmed cell death. Adv Med Sci. 2023;68:417–25. 10.1016/j.advms.2023.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Schubert KM, Scheid MP, Duronio V. Ceramide inhibits protein kinase B/Akt by promoting dephosphorylation of serine 473. J Biol Chem. 2000;275:13330–5. 10.1074/jbc.275.18.13330. [DOI] [PubMed] [Google Scholar]
- 10.Carreira AC et al. Mammalian sphingoid bases: biophysical, physiological and pathological properties. Prog Lipid Res. 2019:100995. 10.1016/j.plipres.2019.100995. [DOI] [PubMed]
- 11.Zhao L, et al. Elevation of 20-carbon long chain bases due to a mutation in serine palmitoyltransferase small subunit b results in neurodegeneration. Proc Natl Acad Sci U S A. 2015;112:12962–7. 10.1073/pnas.1516733112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Russo SB, Tidhar R, Futerman AH, Cowart LA. Myristate-derived d16:0 sphingolipids constitute a cardiac sphingolipid pool with distinct synthetic routes and functional properties. J Biol Chem. 2013;288:13397–409. 10.1074/jbc.M112.428185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hannun, Y. A., Merrill, A. H., Jr. & Luberto, C. The bioactive sphingolipid playbook. A primer for the uninitiated as well as sphingolipidologists. J Lipid Res. 2025:100813. 10.1016/j.jlr.2025.100813. [DOI] [PubMed]
- 14.Chaurasia B, Summers SA. Ceramides - lipotoxic inducers of metabolic disorders. Trends Endocrinol Metab. 2015;26:538–50. 10.1016/j.tem.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 15.Merrill AH Jr. De novo sphingolipid biosynthesis: a necessary, but dangerous, pathway. J Biol Chem. 2002;277:25843–6. 10.1074/jbc.R200009200. [DOI] [PubMed] [Google Scholar]
- 16.Hornemann T, et al. The SPTLC3 subunit of serine palmitoyltransferase generates short chain sphingoid bases. J Biol Chem. 2009;284:26322–30. 10.1074/jbc.M109.023192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han G, et al. Identification of small subunits of mammalian serine palmitoyltransferase that confer distinct acyl-CoA substrate specificities. Proc Natl Acad Sci U S A. 2009;106:8186–91. 10.1073/pnas.0811269106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quehenberger O, et al. Lipidomics reveals a remarkable diversity of lipids in human plasma. J Lipid Res. 2010;51:3299–305. 10.1194/jlr.M009449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weishaupt N, Caughlin S, Yeung KK, Whitehead SN. Differential anatomical expression of ganglioside GM1 species containing d18:1 or d20:1 sphingosine detected by MALDI Imaging mass spectrometry in mature rat brain. Front Neuroanat. 2015;9:155. 10.3389/fnana.2015.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ikushiro H, et al. Structural insights into the substrate recognition of serine palmitoyltransferase from Sphingobacterium multivorum. J Biol Chem. 2023;299:104684. 10.1016/j.jbc.2023.104684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bejaoui K, et al. SPTLC1 is mutated in hereditary sensory neuropathy, type 1. Nat Genet. 2001;27:261–2. 10.1038/85817. [DOI] [PubMed] [Google Scholar]
- 22.Penno A, et al. Hereditary sensory neuropathy type 1 is caused by the accumulation of two neurotoxic sphingolipids. J Biol Chem. 2010;285:11178–87. 10.1074/jbc.M109.092973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Handzlik MK, et al. Insulin-regulated serine and lipid metabolism drive peripheral neuropathy. Nature. 2023;614:118–24. 10.1038/s41586-022-05637-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y, et al. Structural insights into the regulation of human serine palmitoyltransferase complexes. Nat Struct Mol Biol. 2021;28:240–8. 10.1038/s41594-020-00551-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li S, Xie T, Liu P, Wang L, Gong X. Structural insights into the assembly and substrate selectivity of human SPT-ORMDL3 complex. Nat Struct Mol Biol. 2021;28:249–57. 10.1038/s41594-020-00553-7. [DOI] [PubMed] [Google Scholar]
- 26.Ikushiro H, et al. Racemization of the substrate and product by serine palmitoyltransferase from Sphingobacterium multivorum yields two enantiomers of the product from d-serine. J Biol Chem. 2024;300:105728. 10.1016/j.jbc.2024.105728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zelnik ID, Rozman B, Rosenfeld-Gur E, Ben-Dor S, Futerman AH. A stroll down the CerS lane. Adv Exp Med Biol. 2019;1159:49–63. 10.1007/978-3-030-21162-2_4. [DOI] [PubMed] [Google Scholar]
- 28.Crivelli SM, et al. Function of ceramide transfer protein for biogenesis and sphingolipid composition of extracellular vesicles. J Extracell Vesicles. 2022;11:e12233. 10.1002/jev2.12233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumagai K, et al. CERT mediates intermembrane transfer of various molecular species of ceramides. J Biol Chem. 2005;280:6488–95. 10.1074/jbc.M409290200. [DOI] [PubMed] [Google Scholar]
- 30.Kumagai K, Hanada K. Structure, functions and regulation of CERT, a lipid-transfer protein for the delivery of ceramide at the ER-Golgi membrane contact sites. FEBS Lett. 2019;593:2366–77. 10.1002/1873-3468.13511. [DOI] [PubMed] [Google Scholar]
- 31.Sugiki T, et al. Phosphoinositide binding by the PH domain in ceramide transfer protein (CERT) is inhibited by hyperphosphorylation of an adjacent serine-repeat motif. J Biol Chem. 2018;293:11206–17. 10.1074/jbc.RA118.002465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Claus RA, Dorer MJ, Bunck AC, Deigner HP. Inhibition of sphingomyelin hydrolysis: targeting the lipid mediator ceramide as a key regulator of cellular fate. Curr Med Chem. 2009;16:1978–2000. 10.2174/092986709788682182. [DOI] [PubMed] [Google Scholar]
- 33.Insausti-Urkia N, Solsona-Vilarrasa E, Garcia-Ruiz C, Fernandez-Checa JC. Sphingomyelinases and liver diseases. Biomolecules. 2020;10. 10.3390/biom10111497. [DOI] [PMC free article] [PubMed]
- 34.Marchesini N, et al. Role for mammalian neutral sphingomyelinase 2 in confluence-induced growth arrest of MCF7 cells. J Biol Chem. 2004;279:25101–11. 10.1074/jbc.M313662200. [DOI] [PubMed] [Google Scholar]
- 35.Gorelik A, Liu F, Illes K, Nagar B. Crystal structure of the human alkaline sphingomyelinase provides insights into substrate recognition. J Biol Chem. 2017;292:7087–94. 10.1074/jbc.M116.769273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kitatani K, Idkowiak-Baldys J, Hannun YA. The sphingolipid salvage pathway in ceramide metabolism and signaling. Cell Signal. 2008;20:1010–8. 10.1016/j.cellsig.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coant N, Sakamoto W, Mao C, Hannun YA. Ceramidases, roles in sphingolipid metabolism and in health and disease. Adv Biol Regul. 2017;63:122–31. 10.1016/j.jbior.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parveen F. et al. Role of ceramidases in sphingolipid metabolism and human diseases. Cells. 2019;8. 10.3390/cells8121573. [DOI] [PMC free article] [PubMed]
- 39.Xu R. et al. Alkaline ceramidase 2 and its bioactive product sphingosine are novel regulators of the DNA damage response. Oncotarget. 2016;7:18440–18457. 10.18632/oncotarget.7825. [DOI] [PMC free article] [PubMed]
- 40.Wang Y, et al. Alkaline ceramidase 2 is a novel direct target of p53 and induces autophagy and apoptosis through ROS generation. Sci Rep. 2017;7:44573. 10.1038/srep44573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cartier A, Hla T. Sphingosine 1-phosphate: lipid signaling in pathology and therapy. Science. 2019;366. 10.1126/science.aar5551. [DOI] [PMC free article] [PubMed]
- 42.Ventura AE, Mestre B, Silva LC. Ceramide domains in health and disease: a biophysical perspective. Adv Exp Med Biol. 2019;1159:79–108. 10.1007/978-3-030-21162-2_6. [DOI] [PubMed] [Google Scholar]
- 43.Ostermeyer-Fay AG, et al. The steady-state level of plasma membrane ceramide is regulated by neutral sphingomyelinase 2. J Lipid Res. 2025;66:e1001290. 10.1016/j.jlr.2024.100719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Silva LC, Futerman AH, Prieto M. Lipid raft composition modulates sphingomyelinase activity and ceramide-induced membrane physical alterations. Biophys J. 2009;96:3210–22. 10.1016/j.bpj.2008.12.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Quadri Z, et al. Ceramide-mediated orchestration of oxidative stress response through filopodia-derived small extracellular vesicles. J Extracell Vesicles. 2024;13:e12477. 10.1002/jev2.12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arya SB, Chen S, Jordan-Javed F, Parent CA. Ceramide-rich microdomains facilitate nuclear envelope budding for non-conventional exosome formation. Nat Cell Biol. 2022;24:1019–28. 10.1038/s41556-022-00934-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ramírez-Camacho I, et al. Role of sphingomyelinase in mitochondrial ceramide accumulation during reperfusion. Biochim Biophys Acta. 2016;1862:1955–63. 10.1016/j.bbadis.2016.07.021. [DOI] [PubMed] [Google Scholar]
- 48.Chang KT, et al. Ceramide channels: destabilization by Bcl-xL and role in apoptosis. Biochim Biophys Acta. 2015;1848:2374–84. 10.1016/j.bbamem.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huo Y. et al. Ceramide mediates cell-to-cell ER stress transmission by modulating membrane fluidity. J Cell Biol. 2025;224. 10.1083/jcb.202405060. [DOI] [PMC free article] [PubMed]
- 50.Boulgaropoulos B, Rappolt M, Sartori B, Amenitsch H, Pabst G. Lipid sorting by ceramide and the consequences for membrane proteins. Biophys J. 2012;102:2031–8. 10.1016/j.bpj.2012.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pinto SN, Silva LC, Futerman AH, Prieto M. Effect of ceramide structure on membrane biophysical properties: the role of acyl chain length and unsaturation. Biochim Biophys Acta. 2011;1808:2753–60. 10.1016/j.bbamem.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 52.Bennett WFD, Tieleman DP. Molecular simulation of rapid translocation of cholesterol, diacylglycerol, and ceramide in model raft and nonraft membranes. J Lipid Res. 2012;53:421–9. 10.1194/jlr.M022491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chiantia S, et al. Role of ceramide in membrane protein organization investigated by combined AFM and FCS. Biochim Biophys Acta. 2008;1778:1356–64. 10.1016/j.bbamem.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 54.Kinoshita M, Matsumori N. Inimitable impacts of ceramides on lipid rafts formed in artificial and natural cell membranes. Membranes (Basel). 2022;12. 10.3390/membranes12080727. [DOI] [PMC free article] [PubMed]
- 55.Rodriguez-Gallardo S, et al. Quality-controlled ceramide-based GPI-anchored protein sorting into selective ER exit sites. Cell Rep. 2022;39:110768. 10.1016/j.celrep.2022.110768. [DOI] [PubMed] [Google Scholar]
- 56.Stancevic B, Kolesnick R. Ceramide-rich platforms in transmembrane signaling. FEBS Lett. 2010;584:1728–40. 10.1016/j.febslet.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grassmé H, Cremesti A, Kolesnick R, Gulbins E. Ceramide-mediated clustering is required for CD95-DISC formation. Oncogene. 2003;22:5457–70. 10.1038/sj.onc.1206540. [DOI] [PubMed] [Google Scholar]
- 58.Eberle M, et al. Exacerbation of experimental autoimmune encephalomyelitis in ceramide synthase 6 knockout mice is associated with enhanced activation/migration of neutrophils. Immunol Cell Biol. 2015;93:825–36. 10.1038/icb.2015.47. [DOI] [PubMed] [Google Scholar]
- 59.Pinto SN, et al. Changes in membrane biophysical properties induced by sphingomyelinase depend on the sphingolipid N-acyl chain. J Lipid Res. 2014;55:53–61. 10.1194/jlr.M042002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Helke K, et al. Ceramide synthase 6 deficiency enhances inflammation in the DSS model of colitis. Sci Rep. 2018;8:1627. 10.1038/s41598-018-20102-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Samanta S, Stiban J, Maugel TK, Colombini M. Visualization of ceramide channels by transmission electron microscopy. Biochim Biophys Acta. 2011;1808:1196–201. 10.1016/j.bbamem.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yamane M, Moriya S, Kokuba H. Visualization of ceramide channels in lysosomes following endogenous palmitoyl-ceramide accumulation as an initial step in the induction of necrosis. Biochem Biophys Rep. 2017;11:174–81. 10.1016/j.bbrep.2017.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Artetxe I, et al. Does ceramide form channels? The ceramide-induced membrane permeabilization mechanism. Biophys J. 2017;113:860–8. 10.1016/j.bpj.2017.06.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Siskind LJ, Kolesnick RN, Colombini M. Ceramide channels increase the permeability of the mitochondrial outer membrane to small proteins. J Biol Chem. 2002;277:26796–803. 10.1074/jbc.M200754200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim SW, Kim HJ, Chun YJ, Kim MY. Ceramide produces apoptosis through induction of p27(kip1) by protein phosphatase 2A-dependent Akt dephosphorylation in PC-3 prostate cancer cells. J Toxicol Environ Health A. 2010;73:1465–76. 10.1080/15287394.2010.511553. [DOI] [PubMed] [Google Scholar]
- 66.Ghosh N, et al. Ceramide-activated protein phosphatase involvement in insulin resistance via Akt, serine/arginine-rich protein 40, and ribonucleic acid splicing in L6 skeletal muscle cells. Endocrinology. 2007;148:1359–66. 10.1210/en.2006-0750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sentelle RD, et al. Ceramide targets autophagosomes to mitochondria and induces lethal mitophagy. Nat Chem Biol. 2012;8:831–8. 10.1038/nchembio.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Raichur S, et al. CerS2 haploinsufficiency inhibits β-oxidation and confers susceptibility to diet-induced steatohepatitis and insulin resistance. Cell Metab. 2014;20:919. 10.1016/j.cmet.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 69.Park JW, Park WJ, Futerman AH. Ceramide synthases as potential targets for therapeutic intervention in human diseases. Biochim Biophys Acta. 2014;1841:671–81. 10.1016/j.bbalip.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 70.Fekry B, et al. C(16)-ceramide is a natural regulatory ligand of p53 in cellular stress response. Nat Commun. 2018;9:4149. 10.1038/s41467-018-06650-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dadsena S, et al. Ceramides bind VDAC2 to trigger mitochondrial apoptosis. Nat Commun. 2019;10:1832. 10.1038/s41467-019-09654-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yuan Z, Dewson G, Czabotar PE, Birkinshaw RW. VDAC2 and the BCL-2 family of proteins. Biochem Soc Trans. 2021;49:2787–95. 10.1042/bst20210753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hammerschmidt P, et al. CerS6-derived sphingolipids interact with Mff and promote mitochondrial fragmentation in obesity. Cell. 2019;177:1536-1552.e1523. 10.1016/j.cell.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 74.Blom T, et al. LAPTM4B facilitates late endosomal ceramide export to control cell death pathways. Nat Chem Biol. 2015;11:799–806. 10.1038/nchembio.1889. [DOI] [PubMed] [Google Scholar]
- 75.Dichlberger A, et al. LAPTM4B controls the sphingolipid and ether lipid signature of small extracellular vesicles. Biochim Biophys Acta Mol Cell Biol Lipids. 2021;1866:158855. 10.1016/j.bbalip.2020.158855. [DOI] [PubMed] [Google Scholar]
- 76.Zhou K, et al. a ceramide-regulated element in the late endosomal protein LAPTM4B controls amino acid transporter interaction. ACS Cent Sci. 2018;4:548–58. 10.1021/acscentsci.7b00582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhu Q, et al. PAQR4 regulates adipocyte function and systemic metabolic health by mediating ceramide levels. Nat Metab. 2024;6:1347–66. 10.1038/s42255-024-01078-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Deng Y, et al. Identification of TRAMs as sphingolipid-binding proteins using a photoactivatable and clickable short-chain ceramide analog. J Biol Chem. 2021;297:101415. 10.1016/j.jbc.2021.101415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tripathi P, et al. Palmitoylation of acetylated tubulin and association with ceramide-rich platforms is critical for ciliogenesis. J Lipid Res. 2021;62:100021. 10.1194/jlr.RA120001190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shapouri-Moghaddam A, et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018;233:6425–40. 10.1002/jcp.26429. [DOI] [PubMed] [Google Scholar]
- 81.Locati M, Curtale G, Mantovani A. Diversity, mechanisms, and significance of macrophage plasticity. Annu Rev Pathol. 2020;15:123–47. 10.1146/annurev-pathmechdis-012418-012718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kiseleva V, Vishnyakova P, Elchaninov A, Fatkhudinov T, Sukhikh G. Biochemical and molecular inducers and modulators of M2 macrophage polarization in clinical perspective. Int Immunopharmacol. 2023;122:110583. 10.1016/j.intimp.2023.110583. [DOI] [PubMed] [Google Scholar]
- 83.Chen S, et al. Macrophages in immunoregulation and therapeutics. Signal Transduct Target Ther. 2023;8:207. 10.1038/s41392-023-01452-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang LX, Zhang SX, Wu HJ, Rong XL, Guo J. M2b macrophage polarization and its roles in diseases. J Leukoc Biol. 2019;106:345–58. 10.1002/jlb.3ru1018-378rr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Luo M, Zhao F, Cheng H, Su M, Wang Y. Macrophage polarization: an important role in inflammatory diseases. Front Immunol. 2024;15:1352946. 10.3389/fimmu.2024.1352946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pérez S, Rius-Pérez S. Macrophage polarization and reprogramming in acute inflammation: a redox perspective. Antioxidants (Basel). 2022;11. 10.3390/antiox11071394. [DOI] [PMC free article] [PubMed]
- 87.Di Benedetto P, Ruscitti P, Vadasz Z, Toubi E, Giacomelli R. Macrophages with regulatory functions, a possible new therapeutic perspective in autoimmune diseases. Autoimmun Rev. 2019;18:102369. 10.1016/j.autrev.2019.102369. [DOI] [PubMed] [Google Scholar]
- 88.Stout RD, Suttles J. Functional plasticity of macrophages: reversible adaptation to changing microenvironments. J Leukoc Biol. 2004;76:509–13. 10.1189/jlb.0504272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang SW, et al. Regulation of ceramide generation during macrophage apoptosis by ASMase and de novo synthesis. Biochim Biophys Acta. 2015;1851:1482–9. 10.1016/j.bbalip.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Korbecki J, Bajdak-Rusinek K. The effect of palmitic acid on inflammatory response in macrophages: an overview of molecular mechanisms. Inflamm Res. 2019;68:915–32. 10.1007/s00011-019-01273-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Olona A, et al. Sphingolipid metabolism during Toll-like receptor 4 (TLR4)-mediated macrophage activation. Br J Pharmacol. 2021;178:4575–87. 10.1111/bph.15642. [DOI] [PubMed] [Google Scholar]
- 92.Schilling JD, et al. Palmitate and lipopolysaccharide trigger synergistic ceramide production in primary macrophages. J Biol Chem. 2013;288:2923–32. 10.1074/jbc.M112.419978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sims K. et al. Kdo2-lipid A, a TLR4-specific agonist, induces de novo sphingolipid biosynthesis in RAW264.7 macrophages, which is essential for induction of autophagy. J Biol Chem. 2010;285:38568–38579. 10.1074/jbc.M110.170621. [DOI] [PMC free article] [PubMed]
- 94.Lone MA, et al. Subunit composition of the mammalian serine-palmitoyltransferase defines the spectrum of straight and methyl-branched long-chain bases. Proc Natl Acad Sci U S A. 2020;117:15591–8. 10.1073/pnas.2002391117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schwartz EA, et al. Nutrient modification of the innate immune response: a novel mechanism by which saturated fatty acids greatly amplify monocyte inflammation. Arterioscler Thromb Vasc Biol. 2010;30:802–8. 10.1161/atvbaha.109.201681. [DOI] [PubMed] [Google Scholar]
- 96.Seufert AL. et al. Enriched dietary saturated fatty acids induce trained immunity via ceramide production that enhances severity of endotoxemia and clearance of infection. Elife. 2022;11. 10.7554/eLife.76744. [DOI] [PMC free article] [PubMed]
- 97.York AG, et al. IL-10 constrains sphingolipid metabolism to limit inflammation. Nature. 2024;627:628–35. 10.1038/s41586-024-07098-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wen H, et al. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol. 2011;12:408–15. 10.1038/ni.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vandanmagsar B, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17:179–88. 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang Y, et al. Adipose fatty acid binding protein promotes saturated fatty acid–induced macrophage cell death through enhancing ceramide production. J Immunol. 2017;198:798–807. 10.4049/jimmunol.1601403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Camell CD et al. Macrophage-specific de Novo synthesis of ceramide is dispensable for inflammasome-driven inflammation and insulin resistance in obesity*. J Biol Chem. 2015;290:29402–29413. 10.1074/jbc.M115.680199. [DOI] [PMC free article] [PubMed]
- 102.Chaurasia B, et al. Adipocyte ceramides regulate subcutaneous adipose browning, inflammation, and metabolism. Cell Metab. 2016;24:820–34. 10.1016/j.cmet.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 103.de Araujo Junior RF et al. Ceramide and palmitic acid inhibit macrophage-mediated epithelial-mesenchymal transition in colorectal cancer. Mol Cell Biochem. 2020;468:153–168. 10.1007/s11010-020-03719-5. [DOI] [PMC free article] [PubMed]
- 104.Ghosh S, et al. PKCζ mediated anti-proliferative effect of C2 ceramide on neutralization of the tumor microenvironment and melanoma regression. Cancer Immunol Immunother. 2020;69:611–27. 10.1007/s00262-020-02492-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sun H, et al. Ceramides and sphingosine-1-phosphate mediate the distinct effects of M1/M2-macrophage infusion on liver recovery after hepatectomy. Cell Death Dis. 2021;12:324. 10.1038/s41419-021-03616-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Odegaard JI, et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–20. 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pararasa C, et al. Age-associated changes in long-chain fatty acid profile during healthy aging promote pro-inflammatory monocyte polarization via PPARγ. Aging Cell. 2016;15:128–39. 10.1111/acel.12416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Morgan PK, et al. Macrophage polarization state affects lipid composition and the channeling of exogenous fatty acids into endogenous lipid pools. J Biol Chem. 2021;297:101341. 10.1016/j.jbc.2021.101341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Palucka K, Banchereau J. Dendritic cells: a link between innate and adaptive immunity. J Clin Immunol. 1999;19:12–25. 10.1023/a:1020558317162. [DOI] [PubMed] [Google Scholar]
- 110.Pritzl CJ, et al. A ceramide analogue stimulates dendritic cells to promote T cell responses upon virus infections. J Immunol. 2015;194:4339–49. 10.4049/jimmunol.1402672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sallusto F, Nicolò C, De Maria R, Corinti S, Testi R. Ceramide inhibits antigen uptake and presentation by dendritic cells. J Exp Med. 1996;184:2411–6. 10.1084/jem.184.6.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhao D, et al. Inhibition of acid ceramidase regulates MHC class II antigen presentation and suppression of autoimmune arthritis. Cytokine. 2020;135:155219. 10.1016/j.cyto.2020.155219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ashany D, Savir A, Bhardwaj N, Elkon KB. Dendritic cells are resistant to apoptosis through the Fas (CD95/APO-1) pathway. J Immunol. 1999;163:5303–11. [PubMed] [Google Scholar]
- 114.Herber DL, et al. Lipid accumulation and dendritic cell dysfunction in cancer. Nat Med. 2010;16:880–6. 10.1038/nm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kanto T, Kalinski P, Hunter OC, Lotze MT, Amoscato AA. Ceramide mediates tumor-induced dendritic cell apoptosis. J Immunol. 2001;167:3773–84. 10.4049/jimmunol.167.7.3773. [DOI] [PubMed] [Google Scholar]
- 116.Avota E, Gulbins E, Schneider-Schaulies S. DC-SIGN mediated sphingomyelinase-activation and ceramide generation is essential for enhancement of viral uptake in dendritic cells. PLoS Pathog. 2011;7:e1001290. 10.1371/journal.ppat.1001290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Franchi L, Malisan F, Tomassini B, Testi R. Ceramide catabolism critically controls survival of human dendritic cells. J Leukoc Biol. 2006;79:166–72. 10.1189/jlb.1004601. [DOI] [PubMed] [Google Scholar]
- 118.Ocaña-Morgner C, Sales S, Rothe M, Shevchenko A, Jessberger R. Tolerogenic versus immunogenic lipidomic profiles of CD11c(+) immune cells and control of immunogenic dendritic cell ceramide dynamics. J Immunol. 2017;198:4360–72. 10.4049/jimmunol.1601928. [DOI] [PubMed] [Google Scholar]
- 119.Espaillat MP, Kew RR, Obeid LM. Sphingolipids in neutrophil function and inflammatory responses: mechanisms and implications for intestinal immunity and inflammation in ulcerative colitis. Adv Biol Regul. 2017;63:140–55. 10.1016/j.jbior.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ohta H, Yatomi Y, Sweeney EA, Hakomori S, Igarashi Y. A possible role of sphingosine in induction of apoptosis by tumor necrosis factor-alpha in human neutrophils. FEBS Lett. 1994;355:267–70. 10.1016/0014-5793(94)01218-0. [DOI] [PubMed] [Google Scholar]
- 121.Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. 2011;11:519–31. 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
- 122.Kim MY, Linardic C, Obeid L, Hannun Y. Identification of sphingomyelin turnover as an effector mechanism for the action of tumor necrosis factor alpha and gamma-interferon. Specific role in cell differentiation. J Biol Chem. 1991;266:484–9. [PubMed] [Google Scholar]
- 123.Fuortes M, Jin W, Nathan C. Ceramide selectively inhibits early events in the response of human neutrophils to tumor necrosis factor. J Leukoc Biol. 1996;59:451–60. 10.1002/jlb.59.3.451. [DOI] [PubMed] [Google Scholar]
- 124.Sitrin RG, Sassanella TM, Petty HR. An obligate role for membrane-associated neutral sphingomyelinase activity in orienting chemotactic migration of human neutrophils. Am J Respir Cell Mol Biol. 2011;44:205–12. 10.1165/rcmb.2010-0019OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Seumois G, et al. De novo C16- and C24-ceramide generation contributes to spontaneous neutrophil apoptosis. J Leukoc Biol. 2007;81:1477–86. 10.1189/jlb.0806529. [DOI] [PubMed] [Google Scholar]
- 126.Scheel-Toellner D, et al. Clustering of death receptors in lipid rafts initiates neutrophil spontaneous apoptosis. Biochem Soc Trans. 2004;32:679–81. 10.1042/bst0320679. [DOI] [PubMed] [Google Scholar]
- 127.Managò A, et al. Pseudomonas aeruginosa pyocyanin induces neutrophil death via mitochondrial reactive oxygen species and mitochondrial acid sphingomyelinase. Antioxid Redox Signal. 2015;22:1097–110. 10.1089/ars.2014.5979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Izawa K, et al. Disrupting ceramide-CD300f interaction prevents septic peritonitis by stimulating neutrophil recruitment. Sci Rep. 2017;7:4298. 10.1038/s41598-017-04647-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shiba E, et al. Ceramide-CD300f binding inhibits lipopolysaccharide-induced skin inflammation. J Biol Chem. 2017;292:2924–32. 10.1074/jbc.M116.768366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Meher AK, et al. Novel role of IL (Interleukin)-1β in neutrophil extracellular trap formation and abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2018;38:843–53. 10.1161/atvbaha.117.309897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Vorobjeva N, Dagil Y, Pashenkov M, Pinegin B, Chernyak B. Protein kinase C isoforms mediate the formation of neutrophil extracellular traps. Int Immunopharmacol. 2023;114:109448. 10.1016/j.intimp.2022.109448. [DOI] [PubMed] [Google Scholar]
- 132.Bourbon NA, Yun J, Kester M. Ceramide directly activates protein kinase C zeta to regulate a stress-activated protein kinase signaling complex. J Biol Chem. 2000;275:35617–23. 10.1074/jbc.M007346200. [DOI] [PubMed] [Google Scholar]
- 133.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9:503–10. 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 134.Furuke K, Bloom ET. Redox-sensitive events in Fas-induced apoptosis in human NK cells include ceramide generation and protein tyrosine dephosphorylation. Int Immunol. 1998;10:1261–72. 10.1093/intimm/10.9.1261. [DOI] [PubMed] [Google Scholar]
- 135.Ortaldo JR, et al. Dissociation of NKT stimulation, cytokine induction, and NK activation in vivo by the use of distinct TCR-binding ceramides. J Immunol. 2004;172:943–53. 10.4049/jimmunol.172.2.943. [DOI] [PubMed] [Google Scholar]
- 136.Watters RJ, et al. Targeting glucosylceramide synthase synergizes with C6-ceramide nanoliposomes to induce apoptosis in natural killer cell leukemia. Leuk Lymphoma. 2013;54:1288–96. 10.3109/10428194.2012.752485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Taniguchi M, et al. Lysosomal ceramide generated by acid sphingomyelinase triggers cytosolic cathepsin B-mediated degradation of X-linked inhibitor of apoptosis protein in natural killer/T lymphoma cell apoptosis. Cell Death Dis. 2015;6:e1717. 10.1038/cddis.2015.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Taguchi Y, et al. Interleukin-2-induced survival of natural killer (NK) cells involving phosphatidylinositol-3 kinase-dependent reduction of ceramide through acid sphingomyelinase, sphingomyelin synthase, and glucosylceramide synthase. Blood. 2004;104:3285–93. 10.1182/blood-2004-03-0900. [DOI] [PubMed] [Google Scholar]
- 139.Kobayashi E, et al. Enhancing effects of alpha-, beta-monoglycosylceramides on natural killer cell activity. Bioorg Med Chem. 1996;4:615–9. 10.1016/0968-0896(96)00049-1. [DOI] [PubMed] [Google Scholar]
- 140.Sciumè G, et al. Rapid enhancer remodeling and transcription factor repurposing enable high magnitude gene induction upon acute activation of NK cells. Immunity. 2020;53:745-758.e744. 10.1016/j.immuni.2020.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Morrison TA et al. Selective requirement of glycosphingolipid synthesis for natural killer and cytotoxic T cells. Cell. 2025. 10.1016/j.cell.2025.04.007. [DOI] [PMC free article] [PubMed]
- 142.Pieper K, Grimbacher B, Eibel H. B-cell biology and development. J Allergy Clin Immunol. 2013;131:959–71. 10.1016/j.jaci.2013.01.046. [DOI] [PubMed] [Google Scholar]
- 143.LeBien TW, Tedder TF. B lymphocytes: how they develop and function. Blood. 2008;112:1570–80. 10.1182/blood-2008-02-078071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Elgueta R, et al. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol Rev. 2009;229:152–72. 10.1111/j.1600-065X.2009.00782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wang Y, Liu J, Burrows PD, Wang JY. B cell development and maturation. Adv Exp Med Biol. 2020;1254:1–22. 10.1007/978-981-15-3532-1_1. [DOI] [PubMed] [Google Scholar]
- 146.Rastogi I, et al. Role of B cells as antigen presenting cells. Front Immunol. 2022;13:954936. 10.3389/fimmu.2022.954936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lee DSW, Rojas OL, Gommerman JL. B cell depletion therapies in autoimmune disease: advances and mechanistic insights. Nat Rev Drug Discov. 2021;20:179–99. 10.1038/s41573-020-00092-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Schett G, Nagy G, Krönke G, Mielenz D. B-cell depletion in autoimmune diseases. Ann Rheum Dis. 2024;83:1409–20. 10.1136/ard-2024-225727. [DOI] [PubMed] [Google Scholar]
- 149.Choi SC, Morel L. B cell contribution of the CD4(+) T cell inflammatory phenotypes in systemic lupus erythematosus. Autoimmunity. 2017;50:37–41. 10.1080/08916934.2017.1280028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wu F, et al. B cells in rheumatoid arthritis: pathogenic mechanisms and treatment prospects. Front Immunol. 2021;12:750753. 10.3389/fimmu.2021.750753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wang Y, et al. Rheumatoid arthritis patients display B-cell dysregulation already in the naïve repertoire consistent with defects in B-cell tolerance. Sci Rep. 2019;9:19995. 10.1038/s41598-019-56279-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Schwamb J, et al. B-cell receptor triggers drug sensitivity of primary CLL cells by controlling glucosylation of ceramides. Blood. 2012;120:3978–85. 10.1182/blood-2012-05-431783. [DOI] [PubMed] [Google Scholar]
- 153.Kroesen BJ, et al. Induction of apoptosis through B-cell receptor cross-linking occurs via de novo generated C16-ceramide and involves mitochondria. J Biol Chem. 2001;276:13606–14. 10.1074/jbc.M009517200. [DOI] [PubMed] [Google Scholar]
- 154.Hannun YA, Obeid LM. Many ceramides. J Biol Chem. 2011;286:27855–62. 10.1074/jbc.R111.254359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kroesen BJ, et al. BcR-induced apoptosis involves differential regulation of C16 and C24-ceramide formation and sphingolipid-dependent activation of the proteasome. J Biol Chem. 2003;278:14723–31. 10.1074/jbc.M210756200. [DOI] [PubMed] [Google Scholar]
- 156.Kiss E, Sármay G, Matkó J. Ceramide modulation of antigen- triggered Ca2+ signals and cell fate: diversity in the responses of various immunocytes. Ann N Y Acad Sci. 2006;1090:161–7. 10.1196/annals.1378.017. [DOI] [PubMed] [Google Scholar]
- 157.Goldfinger M, et al. De novo ceramide synthesis is required for N-linked glycosylation in plasma cells. J Immunol. 2009;182:7038–47. 10.4049/jimmunol.0802990. [DOI] [PubMed] [Google Scholar]
- 158.Sun L, Su Y, Jiao A, Wang X, Zhang B. T cells in health and disease. Signal Transduct Target Ther. 2023;8:235. 10.1038/s41392-023-01471-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Takahama Y. Journey through the thymus: stromal guides for T-cell development and selection. Nat Rev Immunol. 2006;6:127–35. 10.1038/nri1781. [DOI] [PubMed] [Google Scholar]
- 160.Golubovskaya V, Wu L. Different subsets of T cells, memory, effector functions, and CAR-T immunotherapy. Cancers (Basel). 2016;8. 10.3390/cancers8030036. [DOI] [PMC free article] [PubMed]
- 161.Zhu J, Paul WE. CD4 T cells: fates, functions, and faults. Blood. 2008;112:1557–69. 10.1182/blood-2008-05-078154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Luckheeram RV, Zhou R, Verma AD, Xia B. CD4⁺T cells: differentiation and functions. Clin Dev Immunol. 2012;2012:925135. 10.1155/2012/925135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30:646–55. 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 164.Sen P, et al. Quantitative genome-scale metabolic modeling of human CD4(+) T cell differentiation reveals subset-specific regulation of glycosphingolipid pathways. Cell Rep. 2021;37:109973. 10.1016/j.celrep.2021.109973. [DOI] [PubMed] [Google Scholar]
- 165.Kanno T, et al. The integration of metabolic and proteomic data uncovers an augmentation of the sphingolipid biosynthesis pathway during T-cell differentiation. Commun Biol. 2024;7:622. 10.1038/s42003-024-06339-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhu Y, et al. Lowering glycosphingolipid levels in CD4+ T cells attenuates T cell receptor signaling, cytokine production, and differentiation to the Th17 lineage. J Biol Chem. 2011;286:14787–94. 10.1074/jbc.M111.218610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sofi MH. et al. Ceramide synthesis regulates T cell activity and GVHD development. JCI Insight. 2017;2. 10.1172/jci.insight.91701. [DOI] [PMC free article] [PubMed]
- 168.Kue CS, et al. C6-ceramide in combination with transforming growth factor-beta enhances Treg cell differentiation and stable FoxP3 expression in vitro and in vivo. Immunobiology. 2013;218:952–9. 10.1016/j.imbio.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 169.Zhou Y, et al. Acid Sphingomyelinase (ASM) is a negative regulator of regulatory T cell (Treg) development. Cell Physiol Biochem. 2016;39:985–95. 10.1159/000447806. [DOI] [PubMed] [Google Scholar]
- 170.Abimannan T, et al. Sphingolipid biosynthesis is essential for metabolic rewiring during T(H)17 cell differentiation. Sci Adv. 2024;10:eadk1045. 10.1126/sciadv.adk1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Gaulard P. et al. Expression of the alpha/beta and gamma/delta T-cell receptors in 57 cases of peripheral T-cell lymphomas. Identification of a subset of gamma/delta T-cell lymphomas. Am J Pathol. 1990;137:617–628. [PMC free article] [PubMed]
- 172.Lustgarten J, Waks T, Eshhar Z. CD4 and CD8 accessory molecules function through interactions with major histocompatibility complex molecules which are not directly associated with the T cell receptor-antigen complex. Eur J Immunol. 1991;21:2507–15. 10.1002/eji.1830211030. [DOI] [PubMed] [Google Scholar]
- 173.Courtney AH, Lo WL, Weiss A. TCR signaling: mechanisms of initiation and propagation. Trends Biochem Sci. 2018;43:108–23. 10.1016/j.tibs.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Carreno BM, Collins M. The B7 family of ligands and its receptors: new pathways for costimulation and inhibition of immune responses. Annu Rev Immunol. 2002;20:29–53. 10.1146/annurev.immunol.20.091101.091806. [DOI] [PubMed] [Google Scholar]
- 175.Fuse S, et al. CD80 and CD86 control antiviral CD8+ T-cell function and immune surveillance of murine gammaherpesvirus 68. J Virol. 2006;80:9159–70. 10.1128/jvi.00422-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wilcox RA. A three-signal model of T-cell lymphoma pathogenesis. Am J Hematol. 2016;91:113–22. 10.1002/ajh.24203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Dustin ML. The immunological synapse. Cancer. Immunol Res. 2014;2:1023–33. 10.1158/2326-6066.Cir-14-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zumerle S, Molon B, Viola A. Membrane rafts in T cell activation: a spotlight on CD28 costimulation. Front Immunol. 2017;8:1467. 10.3389/fimmu.2017.01467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Saravia J, Raynor JL, Chapman NM, Lim SA, Chi H. Signaling networks in immunometabolism. Cell Res. 2020;30:328–42. 10.1038/s41422-020-0301-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lepple-Wienhues A, et al. Stimulation of CD95 (Fas) blocks T lymphocyte calcium channels through sphingomyelinase and sphingolipids. Proc Natl Acad Sci U S A. 1999;96:13795–800. 10.1073/pnas.96.24.13795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Tonnetti L, Veri MC, Bonvini E, D’Adamio L. A role for neutral sphingomyelinase-mediated ceramide production in T cell receptor-induced apoptosis and mitogen-activated protein kinase-mediated signal transduction. J Exp Med. 1999;189:1581–9. 10.1084/jem.189.10.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Chan G, Ochi A. Sphingomyelin-ceramide turnover in CD28 costimulatory signaling. Eur J Immunol. 1995;25:1999–2004. 10.1002/eji.1830250730. [DOI] [PubMed] [Google Scholar]
- 183.Lin T, et al. Role of acidic sphingomyelinase in Fas/CD95-mediated cell death. J Biol Chem. 2000;275:8657–63. 10.1074/jbc.275.12.8657. [DOI] [PubMed] [Google Scholar]
- 184.Detre C, et al. Death or survival: membrane ceramide controls the fate and activation of antigen-specific T-cells depending on signal strength and duration. Cell Signal. 2006;18:294–306. 10.1016/j.cellsig.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 185.Flores I, Martinez AC, Hannun YA, Mérida I. Dual role of ceramide in the control of apoptosis following IL-2 withdrawal. J Immunol. 1998;160:3528–33. [PubMed] [Google Scholar]
- 186.Menné C, et al. T-cell receptor downregulation by ceramide-induced caspase activation and cleavage of the zeta chain. Scand J Immunol. 2001;53:176–83. 10.1046/j.1365-3083.2001.00852.x. [DOI] [PubMed] [Google Scholar]
- 187.Abboushi N, et al. Ceramide inhibits IL-2 production by preventing protein kinase C-dependent NF-kappaB activation: possible role in protein kinase Ctheta regulation. J Immunol. 2004;173:3193–200. 10.4049/jimmunol.173.5.3193. [DOI] [PubMed] [Google Scholar]
- 188.Martín-Leal A et al. CCR5 deficiency impairs CD4(+) T-cell memory responses and antigenic sensitivity through increased ceramide synthesis. Embo J. 2020;39:e104749. 10.15252/embj.2020104749. [DOI] [PMC free article] [PubMed]
- 189.Gardeta SR, et al. Sphingomyelin depletion inhibits CXCR4 dynamics and CXCL12-mediated directed cell migration in human T cells. Front Immunol. 2022;13:925559. 10.3389/fimmu.2022.925559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Fracchia KM, Pai CY, Walsh CM. Modulation of T cell metabolism and function through calcium signaling. Front Immunol. 2013;4:324. 10.3389/fimmu.2013.00324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Vaeth M, Kahlfuss S, Feske S. CRAC channels and calcium signaling in T cell-mediated immunity. Trends Immunol. 2020;41:878–901. 10.1016/j.it.2020.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Vaeth M, et al. ORAI2 modulates store-operated calcium entry and T cell-mediated immunity. Nat Commun. 2017;8:14714. 10.1038/ncomms14714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Panyi G, Vámosi G, Bodnár A, Gáspár R, Damjanovich S. Looking through ion channels: recharged concepts in T-cell signaling. Trends Immunol. 2004;25:565–9. 10.1016/j.it.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 194.von Hegedus JH, et al. Oleic acid enhances proliferation and calcium mobilization of CD3/CD28 activated CD4(+) T cells through incorporation into membrane lipids. Eur J Immunol. 2024;54:e2350685. 10.1002/eji.202350685. [DOI] [PubMed] [Google Scholar]
- 195.Dangel GR, Lang F, Lepple-Wienhues A. Effect of sphingosine on Ca2+ entry and mitochondrial potential of Jurkat T cells–interaction with Bcl2. Cell Physiol Biochem. 2005;16:9–14. 10.1159/000087726. [DOI] [PubMed] [Google Scholar]
- 196.Strauss G, et al. CD95 co-stimulation blocks activation of naive T cells by inhibiting T cell receptor signaling. J Exp Med. 2009;206:1379–93. 10.1084/jem.20082363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Shin SH et al. Ceramide synthase 2 null mice are protected from ovalbumin-induced asthma with higher T cell receptor signal strength in CD4+ T cells. Int J Mol Sci. 2021;22. 10.3390/ijms22052713. [DOI] [PMC free article] [PubMed]
- 198.Mullen TD, Hannun YA, Obeid LM. Ceramide synthases at the centre of sphingolipid metabolism and biology. Biochem J. 2012;441:789–802. 10.1042/bj20111626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Gencer S et al. TGF-β receptor I/II trafficking and signaling at primary cilia are inhibited by ceramide to attenuate cell migration and tumor metastasis. Sci Signal. 2017;10. 10.1126/scisignal.aam7464. [DOI] [PMC free article] [PubMed]
- 200.El-Hindi K et al. T-Cell-Specific CerS4 depletion prolonged inflammation and enhanced tumor burden in the AOM/DSS-induced CAC model. Int J Mol Sci. 2022;23. 10.3390/ijms23031866. [DOI] [PMC free article] [PubMed]
- 201.Wofford W, et al. Alterations of ceramide synthesis induce PD-L1 internalization and signaling to regulate tumor metastasis and immunotherapy response. Cell Rep. 2024;43:114532. 10.1016/j.celrep.2024.114532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.El-Hindi K et al. Ceramide synthase 5 deficiency aggravates dextran sodium sulfate-induced colitis and colon carcinogenesis and impairs T-cell activation. Cancers (Basel). 2020;12. 10.3390/cancers12071753. [DOI] [PMC free article] [PubMed]
- 203.Scheffel MJ, et al. Adoptive transfer of ceramide synthase 6 deficient splenocytes reduces the development of colitis. Sci Rep. 2017;7:15552. 10.1038/s41598-017-15791-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Sofi MH, et al. Ceramide synthase 6 impacts T-cell allogeneic response and graft-versus-host disease through regulating N-RAS/ERK pathway. Leukemia. 2022;36:1907–15. 10.1038/s41375-022-01581-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Vaena S, et al. Aging-dependent mitochondrial dysfunction mediated by ceramide signaling inhibits antitumor T cell response. Cell Rep. 2021;35:109076. 10.1016/j.celrep.2021.109076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Hose M et al. Cell-intrinsic ceramides determine T cell function during melanoma progression. Elife. 2022;11. 10.7554/eLife.83073. [DOI] [PMC free article] [PubMed]
- 207.Hay ZLZ, Slansky JE. Granzymes: the molecular executors of immune-mediated cytotoxicity. Int J Mol Sci. 2022;23. 10.3390/ijms23031833. [DOI] [PMC free article] [PubMed]
- 208.Zöphel D, et al. Faster cytotoxicity with age: increased perforin and granzyme levels in cytotoxic CD8(+) T cells boost cancer cell elimination. Aging Cell. 2022;21:e13668. 10.1111/acel.13668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Wu J et al. Loss of neurological disease HSAN-I-Associated gene SPTLC2 impairs CD8(+) T cell responses to infection by inhibiting T cell metabolic fitness. Immunity. 2019;50:1218–1231 e1215. 10.1016/j.immuni.2019.03.005. [DOI] [PMC free article] [PubMed]
- 210.Herz J, et al. Acid sphingomyelinase is a key regulator of cytotoxic granule secretion by primary T lymphocytes. Nat Immunol. 2009;10:761–8. 10.1038/ni.1757. [DOI] [PubMed] [Google Scholar]
- 211.Choudhary P, Kumari S, Bagri K, Deshmukh R. Ceramide: a central regulator in Alzheimer’s disease pathogenesis. Inflammopharmacology. 2025;33:1775–83. 10.1007/s10787-025-01719-9. [DOI] [PubMed] [Google Scholar]
- 212.Delcheva G, Stefanova K, Stankova T. Ceramides-emerging biomarkers of lipotoxicity in obesity, diabetes, cardiovascular diseases, and inflammation. Diseases. 2024;12. 10.3390/diseases12090195. [DOI] [PMC free article] [PubMed]
- 213.Li Y, Nicholson RJ, Summers SA. Ceramide signaling in the gut. Mol Cell Endocrinol. 2022;544:111554. 10.1016/j.mce.2022.111554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Varre JV, Holland WL, Summers SA. You aren't IMMUNE to the ceramides that accumulate in cardiometabolic disease. Biochim Biophys Acta (BBA) - Mol Cell Biol Lipids. 2022;1867:159125. 10.1016/j.bbalip.2022.159125. [DOI] [PMC free article] [PubMed]
- 215.Alexandropoulou I. et al. Ceramides in autoimmune rheumatic diseases: existing evidence and therapeutic considerations for diet as an anticeramide treatment. Nutrients. 2023;15. 10.3390/nu15010229. [DOI] [PMC free article] [PubMed]
- 216.Chaurasia B, Talbot CL, Summers SA. Adipocyte ceramides-the Nexus of inflammation and metabolic disease. Front Immunol. 2020;11:576347. 10.3389/fimmu.2020.576347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Barra NG, Henriksbo BD, Anhê FF, Schertzer JD. The NLRP3 inflammasome regulates adipose tissue metabolism. Biochem J. 2020;477:1089–107. 10.1042/bcj20190472. [DOI] [PubMed] [Google Scholar]
- 218.Xu J, et al. Involvement of de novo ceramide biosynthesis in tumor necrosis factor-alpha/cycloheximide-induced cerebral endothelial cell death. J Biol Chem. 1998;273:16521–6. 10.1074/jbc.273.26.16521. [DOI] [PubMed] [Google Scholar]
- 219.Zhang S. et al. Sensing ceramides by CYSLTR2 and P2RY6 to aggravate atherosclerosis. Nature. 2025. 10.1038/s41586-025-08792-8. [DOI] [PubMed]
- 220.Wang P, et al. Disruption of adipocyte HIF-1α improves atherosclerosis through the inhibition of ceramide generation. Acta Pharm Sin B. 2022;12:1899–912. 10.1016/j.apsb.2021.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Choi RH, Tatum SM, Symons JD, Summers SA, Holland WL. Ceramides and other sphingolipids as drivers of cardiovascular disease. Nat Rev Cardiol. 2021;18:701–11. 10.1038/s41569-021-00536-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Oertel S, et al. Ceramide synthase 2 deficiency aggravates AOM-DSS-induced colitis in mice: role of colon barrier integrity. Cell Mol Life Sci. 2017;74:3039–55. 10.1007/s00018-017-2518-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Feng T, et al. Th17 cells induce colitis and promote Th1 cell responses through IL-17 induction of innate IL-12 and IL-23 production. J Immunol. 2011;186:6313–8. 10.4049/jimmunol.1001454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Komuro M, et al. Glucosylceramide in T cells regulates the pathology of inflammatory bowel disease. Biochem Biophys Res Commun. 2022;599:24–30. 10.1016/j.bbrc.2022.02.004. [DOI] [PubMed] [Google Scholar]
- 225.Zhang S, et al. The alterations in and the role of the Th17/Treg balance in metabolic diseases. Front Immunol. 2021;12:678355. 10.3389/fimmu.2021.678355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Doll CL, Snider AJ. The diverse roles of sphingolipids in inflammatory bowel disease. Faseb j. 2024;38:e23777. 10.1096/fj.202400830R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Gomez-Bris R. et al. CD4 T-cell subsets and the pathophysiology of inflammatory bowel disease. Int J Mol Sci. 2023;24. 10.3390/ijms24032696. [DOI] [PMC free article] [PubMed]
- 228.Ladakis DC. et al. Metabolomics of multiple sclerosis lesions demonstrates lipid changes linked to alterations in transcriptomics-based cellular profiles. Neurol Neuroimmunol Neuroinflamm. 2024;11;e200219. 10.1212/nxi.0000000000200219. [DOI] [PMC free article] [PubMed]
- 229.Amatruda M, et al. Neuroprotective effect of neuron-specific deletion of the C16 ceramide synthetic enzymes in an animal model of multiple sclerosis. Glia. 2025;73:271–90. 10.1002/glia.24631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.de Wit NM, et al. Astrocytic ceramide as possible indicator of neuroinflammation. J Neuroinflammation. 2019;16:48. 10.1186/s12974-019-1436-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Katsel P, Li C, Haroutunian V. Gene expression alterations in the sphingolipid metabolism pathways during progression of dementia and Alzheimer’s disease: a shift toward ceramide accumulation at the earliest recognizable stages of Alzheimer’s disease? Neurochem Res. 2007;32:845–56. 10.1007/s11064-007-9297-x. [DOI] [PubMed] [Google Scholar]
- 232.Choi BJ, Park MH, Jin HK, Bae JS. Acid sphingomyelinase as a pathological and therapeutic target in neurological disorders: focus on Alzheimer’s disease. Exp Mol Med. 2024;56:301–10. 10.1038/s12276-024-01176-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Tallon C, et al. Inhibiting tau-induced elevated nSMase2 activity and ceramides is therapeutic in an Alzheimer’s disease mouse model. Transl Neurodegener. 2023;12:56. 10.1186/s40035-023-00383-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Albeituni S, Stiban J. Roles of ceramides and other sphingolipids in immune cell function and inflammation. Adv Exp Med Biol. 2019;1161:169–91. 10.1007/978-3-030-21735-8_15. [DOI] [PubMed] [Google Scholar]
- 235.Zou Y, et al. Ceramide metabolism-related prognostic signature and immunosuppressive function of ST3GAL1 in osteosarcoma. Transl Oncol. 2024;40:101840. 10.1016/j.tranon.2023.101840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Hansen W, et al. Neuropilin 1 deficiency on CD4+Foxp3+ regulatory T cells impairs mouse melanoma growth. J Exp Med. 2012;209:2001–16. 10.1084/jem.20111497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Pastille E, et al. Transient ablation of regulatory T cells improves antitumor immunity in colitis-associated colon cancer. Cancer Res. 2014;74:4258–69. 10.1158/0008-5472.Can-13-3065. [DOI] [PubMed] [Google Scholar]
- 238.Shan Y, et al. Lipid metabolism in tumor-infiltrating regulatory T cells: perspective to precision immunotherapy. Biomark Res. 2024;12:41. 10.1186/s40364-024-00588-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Qi D, et al. AKT1/FOXP3 axis-mediated expression of CerS6 promotes p53 mutant pancreatic tumorigenesis. Cancer Lett. 2021;522:105–18. 10.1016/j.canlet.2021.06.024. [DOI] [PubMed] [Google Scholar]
- 240.Li Y, et al. Ceramides increase fatty acid utilization in intestinal progenitors to enhance stemness and increase tumor risk. Gastroenterology. 2023;165:1136–50. 10.1053/j.gastro.2023.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Espaillat MP, et al. Loss of acid ceramidase in myeloid cells suppresses intestinal neutrophil recruitment. Faseb J. 2018;32:2339–53. 10.1096/fj.201700585R. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.