ABSTRACT
Aim
To perform an indirect treatment comparison of safety and anemia outcomes between the Janus kinase (JAK) inhibitors momelotinib and pacritinib in patients with myelofibrosis.
Methods
Treatment-emergent adverse events (AEs) and anemia outcomes were compared in a pooled population of JAK inhibitor – experienced and – naive patients treated with momelotinib (SIMPLIFY-1/SIMPLIFY-2/MOMENTUM) or pacritinib (PERSIST-2/PAC203).
Results
Momelotinib had statistically significantly lower odds and risk for all grades of diarrhea, nausea, peripheral edema, and vomiting as well as grade 3/4 and serious AEs vs pacritinib. Momelotinib-treated patients also had greater odds/possibility of hemoglobin improvement of ≥ 1 g/dL and clinical improvement in hemoglobin.
Conclusions
Momelotinib provides a more favorable safety profile and a higher chance for hemoglobin improvement vs pacritinib.
KEYWORDS: Myelofibrosis, JAK inhibitor, momelotinib, indirect treatment comparison, safety, pacritinib
Plain Language Summary
What is this article about?Myelofibrosis is a rare blood cancer that causes abnormal blood cell production. A class of medications known as Janus kinase (JAK) inhibitors can help treat symptoms, which include feeling tired and weak from too few red blood cells, as well as enlarged spleen. So far, four JAK inhibitors have been approved by the US FDA for treating myelofibrosis: ruxolitinib, fedratinib, pacritinib, and momelotinib. However, these JAK inhibitors have different side effects, and clinical trials that directly compare these medications are not always available. We used a method known as indirect comparison to further understand differences in the side effects between momelotinib and pacritinib.What were the results?The findings show that momelotinib was associated with a lower risk of some side effects, including anemia, diarrhea, nausea, swelling from fluid buildup, and vomiting. The study also showed that momelotinib is more likely to increase the amount of hemoglobin, which is a measure of anemia improvement, compared with pacritinib.What do the results of the study mean?These results suggest that momelotinib may be a treatment option with fewer unwanted side effects for people with myelofibrosis.
1. Introduction
Janus kinase (JAK) inhibitors are effective for improving symptoms and reducing spleen volume for most patients with myelofibrosis (MF) [1–4]. However, the safety profiles of JAK inhibitors can vary, and some have been associated with hematologic toxicity [5]. For example, anemia and thrombocytopenia were the most common adverse events (AEs) associated with ruxolitinib treatment in the pivotal phase 3 COMFORT-I trial [6]. In the phase 3 JAKARTA trial of fedratinib, diarrhea and nausea were reported in > 60% of patients, while hematologic AEs were more frequent in patients treated with fedratinib vs placebo [7].
The JAK1/JAK2/activin A receptor type 1 (ACVR1) inhibitor momelotinib was recently approved for treatment of adults with intermediate- or high-risk MF and anemia [8] and has been studied in three phase 3 trials: SIMPLIFY-1, SIMPLIFY-2, and MOMENTUM [9–11]. Across these trials, momelotinib showed consistent spleen, symptom, and anemia benefits for patients with MF. In particular, momelotinib treatment in JAK inhibitor – naive patients led to higher transfusion independence (TI) rates (defined as no transfusions and no hemoglobin [Hb] levels <8 g/dL in the 12 weeks immediately preceding week 24), reduced transfusion dependence rates, and lower transfusion burden vs ruxolitinib at week 24, all of which were prespecified secondary endpoints [9]. Similar anemia outcomes vs ruxolitinib were observed in patients on momelotinib compared with best available therapy (BAT; 88.5% on ruxolitinib) in JAK inhibitor – experienced patients in the SIMPLIFY-2 trial [10]. Momelotinib also has a well-characterized safety profile, with results from a long-term safety analysis of all 3 trials showing that the most common nonhematologic AEs (diarrhea, nausea, and fatigue) were typically low grade; no cumulative effects or long-term toxicity was observed [12]. Thus, momelotinib has demonstrated anemia benefit vs ruxolitinib as well as a manageable safety profile, but comparisons with other JAK inhibitors have not been performed.
Pacritinib is an oral kinase inhibitor targeting JAK2/fms-related receptor tyrosine kinase 3/interleukin 1 receptor associated kinase 1 that is indicated under US Food and Drug Administration accelerated approval for patients with MF and severe thrombocytopenia (platelet counts < 50 × 109/L) [2]. In addition to spleen and symptom improvement in patients with MF, results from retrospective analyses of subpopulations from the PERSIST-2 trial suggest that pacritinib may also provide anemia benefits via inhibition of ACVR1 [13,14]. One such analysis found that pacritinib led to higher rates of Hb improvement of ≥ 1 or ≥2 g/dL vs BAT among patients with baseline Hb levels of <10 g/dL [13]. Diarrhea, nausea, thrombocytopenia, and anemia were the most common AEs to be reported in PERSIST-2, with anemia and thrombocytopenia being the most common grade ≥ 3 AEs [4].
Thus far, SIMPLIFY-1 and SIMPLIFY-2 are the only trials with direct head-to-head comparison of momelotinib vs another JAK inhibitor (ruxolitinib). Differences in patient populations and endpoints, such as the definition of TI, limit the interpretation of cross-trial comparisons among other JAK inhibitors. However, understanding differences in safety, tolerability, and anemia outcomes between momelotinib and pacritinib could help to inform treatment choices in MF. Thus, in this analysis, we report the results from an indirect treatment comparison (ITC) of safety and anemia outcomes between momelotinib and pacritinib in JAK inhibitor – experienced and – naive patients with MF.
2. Materials and methods
2.1. Data sources
Outcomes were analyzed through week 24 from the momelotinib arms of MOMENTUM (NCT04173494), SIMPLIFY-1 (NCT01969838), and SIMPLIFY-2 (NCT02101268) and the 200-mg pacritinib arms of PERSIST-2 (NCT02055781) and PAC203 (NCT04884191). Detailed study designs and protocols for all five studies have been previously reported [4,9–11,15].
MOMENTUM was a multicenter, double-blind, phase 3 study that enrolled symptomatic (Total Symptom Score [TSS] ≥10) and anemic (Hb levels <10 g/dL) patients with MF who were previously treated with an approved JAK inhibitor (N = 195). Patients were randomized 2:1 to once-daily momelotinib (200 mg; n = 130) plus placebo or twice-daily danazol (300 mg; n = 65) plus placebo following a 21-day JAK inhibitor taper/washout and treated for 24 weeks [11].
SIMPLIFY-1 was a multicenter, double-blind, phase 3 study that enrolled patients with MF who were JAK inhibitor naive (N = 432). Patients were randomized 1:1 to once-daily momelotinib (200 mg; n = 215) plus placebo or twice-daily ruxolitinib (20 mg or dose adjustments per label; n = 217) plus placebo and treated for 24 weeks [9].
SIMPLIFY-2 was a multicenter, open-label, phase 3 study that enrolled patients with MF who were previously treated with ruxolitinib (N = 156). Patients were randomized 2:1 to once-daily momelotinib (200 mg; n = 104) or BAT (88.5% were receiving ruxolitinib alone or in combination; n = 52) and treated for 24 weeks; there was no washout period [10].
PERSIST-2 was a multicenter, open-label, phase 3 study that enrolled both JAK inhibitor – naive and – experienced, symptomatic (TSS ≥13) patients with MF and thrombocytopenia (platelet counts ≤ 100 × 109/L; N = 311). Patients were randomized 1:1:1 to once-daily pacritinib (400 mg; n = 104), twice-daily pacritinib (200 mg; n = 107), or BAT (n = 100) and treated for 24 weeks [4]. The present analysis considered only patients treated with pacritinib at the 200-mg twice-daily dose.
PAC203 was a multicenter, open-label, dose-finding, phase 2 study that enrolled symptomatic (TSS ≥10) patients with MF who were intolerant of or resistant to ruxolitinib (N = 161). Patients were randomized 1:1:1 to twice-daily pacritinib (200 mg; n = 54), once-daily pacritinib (100 mg; n = 52), or twice-daily pacritinib (100 mg; n = 55) and treated for 24 weeks [15]. The present analysis considered only patients treated with pacritinib at the 200-mg twice-daily dose.
For momelotinib, pooled individual patient data from the SIMPLIFY-1, SIMPLIFY-2, and MOMENTUM studies were used [9,10,12]. For pacritinib, publicly available aggregate data from the PERSIST-2 and PAC203 trials reported in available journal articles, conference abstracts, US Food and Drug Administration regulatory documents, and European Medicines Agency regulatory documents were used [4,14,15]. Data from PERSIST-1 were not included as that study did not use the approved dose of pacritinib. The analysis population was a combination of JAK inhibitor – experienced and – naive patients, as PERSIST-2 enrolled both populations and separate data were not reported. Because not all outcomes of interest were reported in PAC203, pooled MOMENTUM/SIMPLIFY-1/SIMPLIFY-2 data were compared vs PERSIST-2/PAC203 data or vs PERSIST-2 data alone. All authors had access to the final clinical study reports.
2.2. Assessments
2.2.1. Safety outcomes
Safety outcomes were assessed using the National Cancer Institute’s Common Terminology Criteria for Adverse Events version v.3.0, v.4.03, or v.5.0. This analysis included safety outcomes occurring in ≥ 15% of patients in the MOMENTUM, SIMPLIFY-1, SIMPLIFY-2, PERSIST-2, and PAC203 trials, to align with what was reported in the pacritinib trials. This cutoff was chosen to ensure a sufficient number of events for each safety outcome in the analysis. Primary safety outcomes were grade 3/4 anemia and thrombocytopenia; other safety outcomes occurring in ≥ 15% of patients were considered secondary outcomes. All safety outcomes were also assessed in a momelotinib subpopulation with baseline platelet counts ≤ 100 × 109/L.
2.2.2. Efficacy outcomes
For Hb-related efficacy outcomes, analyses were carried out in a subpopulation of patients with available Hb data such that they were evaluable through week 24, to match the population assessed in the PERSIST-2 analysis [13]. Outcomes, assessed at week 24, included Hb improvement of ≥ 1 and ≥2 g/dL (in patients with baseline Hb levels of <10 g/dL) and clinical improvement in Hb (per the International Working Group criteria [16], defined as Hb improvement of ≥2 g/dL any time through week 24 for ≥8 weeks on a rolling basis, not taking into account Hb levels ≤4 weeks from red blood cell [RBC] transfusion, or no RBC transfusion for ≥8 weeks on a rolling basis). For the main analysis, Hb levels ≤4 weeks from RBC transfusion were excluded; scenarios including post-transfusion Hb levels were also analyzed. All Hb efficacy outcomes were also assessed in a momelotinib subpopulation with baseline platelet counts of ≤ 100 × 109/L.
For transfusion-related efficacy outcomes, analyses were explored in a subpopulation of patients who were not transfusion independent at baseline, had baseline platelet counts of ≤ 100 × 109/L, had baseline TSS ≥ 13, and had ≥12 weeks of available data from randomization, to match the population assessed in the PERSIST-2 analysis [4,14]. These outcomes included the proportion of patients achieving TI according to Gale criteria [17] (as defined in PERSIST-2: no RBC transfusions over 12 weeks on a rolling basis with no minimum Hb level) and the SIMPLIFY-like criteria (as defined in PERSIST-2: no RBC transfusions and no Hb levels of <8 g/dL on a rolling 12-week basis) through week 24. The cumulative incidence of achieving TI according to Gale criteria from week 12 to week 24 and the proportion of patients achieving a ≥ 50% transfusion reduction according to Gale criteria were also considered.
2.3. Statistical analysis
Matching-adjusted indirect comparison (MAIC) was used to compare outcomes from momelotinib and pacritinib trials [18,19]. Pooled individual patient data from the momelotinib arms of MOMENTUM, SIMPLIFY-1, and SIMPLIFY-2 were reweighted to match the momelotinib population to the pacritinib populations on baseline characteristics prioritized by expert clinical input to adjust for baseline differences between populations. For safety and Hb-related efficacy outcomes, the base case model selected for the analysis of the momelotinib vs the PERSIST-2 pacritinib populations included adjustment for baseline characteristics of JAK inhibitor exposure, Dynamic International Prognostic Scoring System (DIPSS), TSS, platelet counts, spleen length, TI, Hb levels, Eastern Cooperative Oncology Group performance status, and age, as these factors are known prognostic factors or effect modifiers. Analyses of safety outcomes in the momelotinib vs the pooled PERSIST-2/PAC203 population included additional adjustments for sex and MF subtype for the momelotinib population. For transfusion-related efficacy outcomes, the base case model selected for the analysis of the momelotinib vs the PERSIST-2 pacritinib populations included adjustment for baseline characteristics of age, MF type, time since diagnosis, JAK inhibitor exposure, platelet counts, Hb levels, median RBC transfusions per month, and JAK2 V617F mutation status.
Because SIMPLIFY-1 used International Prognostic Scoring System (IPSS) to classify MF risk, high- and intermediate-risk MF in SIMPLIFY-1 was reclassified according to the DIPSS definition to facilitate comparability across studies. If the conversion could not be made precisely, the IPSS to DIPSS conversion was conducted using a conservative approach with the lower score. Median age was 67 and 68.5 years in PERSIST-2 and PAC203, respectively; thus, the median age in the pooled pacritinib cohort was assumed to be 68 years. Median palpable spleen length was 15 and 14 cm in PERSIST-2 and PAC203, respectively; thus, the median palpable spleen length in the pooled pacritinib cohort was assumed to be 14.5 cm. Several different scenarios for age and spleen length were explored, including setting the median age as 67, 67.5, or 68.5 years (base case model used 68 years) and median spleen length as 14 or 15 cm (base case model used 14.5 cm). Definitions of baseline TI were harmonized with the Gale criteria definitions [17] used in PERSIST-2 and PAC203 (no RBC transfusions over 12 weeks on a rolling basis with no minimum Hb level).
Differences between momelotinib and pacritinib for binary outcomes were reported using odds ratios (ORs) and risk differences (RDs), and weighted Wald’s z-tests were used. For safety outcomes, ORs of < 1 or RDs of < 0 favored momelotinib; for Hb outcomes, ORs of > 1 or RDs of > 0 favored momelotinib. Parallel “naive” comparisons (using unadjusted data) were performed in the trial populations before matching (using unweighted versions of the aforementioned tests, based on the aggregate results reported) and reviewed along with the adjusted results. Standard errors, 95% CIs, and p values for these indirect comparisons were based on a robust sandwich estimator.
3. Results
3.1. Patient demographics and disposition
Before adjustment, the momelotinib group consisted of 448 patients, while the PERSIST-2/PAC203 and PERSIST-2 groups had 160 and 106 patients, respectively. Multiple models adjusting for different combinations of baseline characteristics were considered (Supplemental Table S1), and one was selected as the base case due to acceptable effective sample size (ESS), distribution of matching weights, and number of factors adjusted. After adjustment, patient populations were well balanced between groups in the selected base case model, with an acceptable ESS (Table 1). For safety outcomes, post-adjustment momelotinib ESSs were 97.43 and 80.13 for comparisons vs pooled PERSIST-2/PAC203 and PERSIST-2 alone, respectively.
Table 1.
Demographics and baseline characteristics before and after adjustment.
| Pooled momelotinib population before adjustment (SIMPLIFY-1, SIMPLIFY-2, and MOMENTUM;N = 448) |
Pooled momelotinib vs pooled PERSIST-2/PAC203 |
Pooled momelotinib vs PERSIST-2 |
|||
|---|---|---|---|---|---|
| Momelotinib after adjustment(ESS = 97.43) |
Pacritinib (PERSIST-2/ PAC203; N = 128) |
Momelotinib after adjustment (ESS = 80.13) |
Pacritinib (PERSIST-2;N = 106†) |
||
| Age, median, years‡ | 68 | 68 | NA (assumed to be 68) | 67 | 67 |
| Male, % | 60.5 | 63.0 | 63.0 | 49.3 | 64.9 |
| Prior JAK inhibitor treatment, % | 52.2 | 63.2 | 63.2 | 44.6 | 44.6 |
| MF subtype, % | |||||
| PMF | 60.3 | 72.4 | 72.4 | 58.5 | 74.3 |
| PPV | 20.5 | 18.8 | 18.8 | 27.5 | 18.9 |
| PET | 19.2 | 8.8 | 8.8 | 14.0 | 6.8 |
| DIPSS/IPSS risk category, % | |||||
| Intermediate-1 | 16.7 | 20.0 | 20.0 | 18.9 | 18.9 |
| Intermediate-2 | 54.7 | 51.5 | 51.5 | 51.4 | 51.4 |
| High | 28.4 | 28.5 | 28.5 | 29.7 | 29.7 |
| NA | 0.2 | 0.0 | 0.0 | 0.0 | 0.0 |
| TSS, % | |||||
| ≥5 | 90.1 | 100 | 100 | 100 | NA |
| ≥13 | 69.1 | 76.9 | 100 | 100 | 100 |
| ECOG PS, % | |||||
| 0–1 | 87.7 | 86.9 | 86.9 | 87.8 | 87.8 |
| 2–3 | 12.3 | 13.1 | 12.2 | 12.2 | 10.8 |
| NA | 0.0 | 0.0 | 0.9 | 0.0 | 1.4 |
| RBC transfusion status, %¶ | |||||
| Dependent | 38.9 | 38.5 | 25.6 | 35.6 | 18.9 |
| Independent | 43.5 | 45.7 | 45.7 | 50.0 | 50.0 |
| Requiring | 17.6 | 15.8 | 27.8 | 14.4 | 29.7 |
| NA | 0.0 | 0.0 | 0.9 | 0.0 | 1.4 |
| Hemoglobin <10 g/dL, % | 62.2 | 65.0 | 65.0 | 59.5 | 59.5 |
| Platelet count < 50 × 109/L, % | 6.1 | 42.7 | 42.7 | 41.9 | 41.9 |
| Spleen length, cm# | 12 | 14.5 | NA (assumed to be 14.5) | 15 | 15 |
Bolded numbers indicate factors that have been matched in these analyses.
DIPSS, Dynamic International Prognostic Scoring System; ECOG PS, Eastern Cooperative Oncology Group performance status; ESS, effective sample size; IPSS, International Prognostic Scoring System; ITT, intent to treat; JAK, Janus kinase; MF, myelofibrosis; NA, not available; PET, post – essential thrombocythemia; PMF, primary myelofibrosis; PPV, post – polycythemia vera; RBC, red blood cell; TSS, Total Symptom Score.
†Baseline characteristics were available for the subset of 74 patients from the ITT population in PERSIST-2; population matching was performed assuming that baseline characteristics in both sets (n = 106 and n = 74) were similar.
‡The median age in PERSIST-2 and PAC203 was 67 and 68.5 years, respectively; thus, the median age in the pooled pacritinib cohort was assumed to be 68 years (scenarios using 67, 67.5, and 68.5 years provided similar results).
§High- and intermediate-risk myelofibrosis in SIMPLIFY-1 was reclassified according to the DIPSS definition to facilitate comparability across studies.
¶Definitions of baseline transfusion independence and transfusion dependence were harmonized with the definitions used in PERSIST-2 and PAC203.
#The median palpable spleen length in PERSIST-2 and PAC203 was 15 and 14 cm, respectively; thus, the median palpable spleen length in the pooled pacritinib cohort was assumed to be 14.5 cm (scenarios using 14 and 15 cm provided similar results).
For Hb-related efficacy outcomes in a subpopulation of patients with Hb levels of ≤10 g/dL, the sample size was 278 patients in the momelotinib group; in the pacritinib group, sample sizes were 33 for the analysis of Hb improvement and 44 for the analysis of clinical Hb improvement. Following adjustment and selection of the base case model, the momelotinib ESS was 47.64 for comparisons vs PERSIST-2. For TI-related efficacy outcomes in a subpopulation of patients who were not transfusion independent at baseline, the sample sizes were 64 and 41 patients in the momelotinib and pacritinib groups, respectively. A majority of patients in the momelotinib (89.1%) and pacritinib (56%) groups were JAK inhibitor experienced, with 42.2% and 50.0% receiving < 1.5 units of RBC transfusions per month, respectively. The ESS was small following matching, ranging from 4.8 to 62.44, depending on which baseline factors were matched (Supplemental Table S2).
3.2. Safety outcomes
In total, 14 AEs occurred at an incidence of ≥ 15% and met the criteria for inclusion in this analysis. Momelotinib had numerically favorable results for most safety outcomes, with statistically significantly lower odds and risk for all grades of diarrhea (OR, 0.51; 95% CI, 0.28–0.91; RD, −0.15; 95% CI, −0.28 to − 0.03), nausea (OR, 0.41; 95% CI, 0.23 to − 0.73; RD, −0.15; 95% CI, −0.25 to − 0.06), peripheral edema (OR, 0.34; 95% CI, 0.13–0.90; RD, −0.11; 95% CI, −0.20 to − 0.03), and vomiting (OR, 0.32; 95% CI, 0.14–0.70; RD, −0.12; 95% CI, −0.20 to − 0.03) as well as grade 3/4 AEs (OR, 0.26; 95% CI, 0.14–0.49; RD, −0.32; 95% CI, −0.46 to − 0.18) and serious AEs (OR, 0.44; 95% CI, 0.23–0.83; RD, −0.19; 95% CI, −0.33 to − 0.05) (Figures 1 and 2). Odds and risk of grade 3/4 or any-grade anemia and thrombocytopenia favored momelotinib vs pacritinib; grade 3/4 events of anemia (OR, 0.41; 95% CI, 0.20–0.87; RD, −0.11; 95% CI, −0.20 to − 0.03) and thrombocytopenia (OR, 0.48; 95% CI, 0.25–0.93; RD, −0.14, 95% CI, −0.25 to − 0.02) were significantly lower with momelotinib. Of note, no identified safety outcomes were significantly less likely with pacritinib.
Figure 1.

ORs for safety outcomes.
Safety outcomes with ORs of < 1 indicate outcomes favoring momelotinib.
AE, adverse event; ESS, effective sample size; OR, odds ratio.
Figure 2.
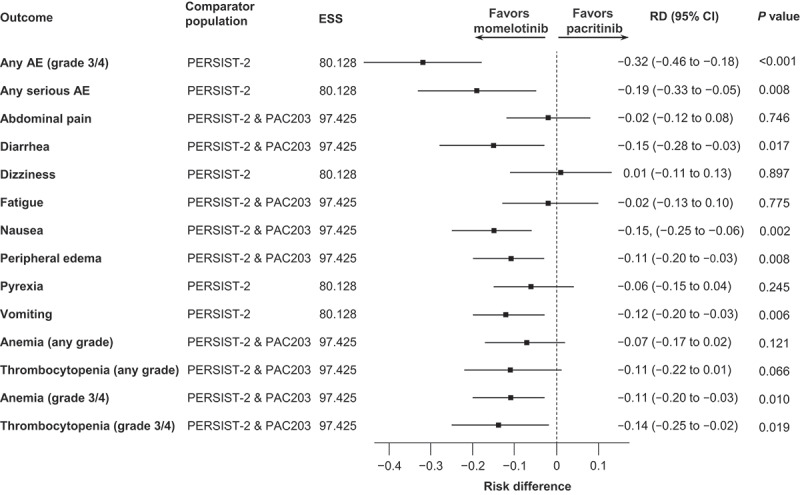
RDs for safety outcomes.
Safety outcomes with RDs of < 0 indicate outcomes favoring momelotinib.
AE, adverse event; ESS, effective sample size; RD, risk difference.
For all safety outcomes, MAIC results were similar across different scenarios assessed to account for uncertainty around the exact median age and spleen length of the pooled PERSIST-2/PAC203 population. Consistency across scenarios also suggests that uncertainties around the conversion of IPSS to DIPSS in SIMPLIFY-1 did not affect MAIC outcomes. Safety outcomes in a momelotinib population with baseline platelet counts of ≤ 100 × 109/L were similar to the overall population (Supplemental Table S3).
3.3. Efficacy outcomes
Momelotinib was associated with statistically greater odds or possibility of Hb improvement of ≥1 g/dL (OR, 4.89; 95% CI, 1.52–15.71; RD, 0.31; 95% CI, 0.11–0.52) as well as clinical Hb improvement (OR, 3.00; 95% CI, 1.14–7.88; RD, 0.25; 95% CI, 0.04–0.46; defined as Hb improvement of ≥2 g/dL any time through week 24 for ≥8 weeks on a rolling basis, not taking into account Hb levels ≤4 weeks from RBC transfusion, or no RBC transfusion for ≥8 weeks on a rolling basis) (Figures 3 and 4). Odds and risk of Hb improvement of ≥2 g/dL numerically favored momelotinib but did not reach significance. Outcomes were similar regardless of whether post-transfusion Hb levels were excluded (Figures 3 and 4) or included (OR, 30.59; 95% CI, 8.74–107.01 and OR, 8.14; 95% CI, 2.08–31.92; RD, 0.69; 95% CI, 0.53–0.86 and RD, 0.36; 95%, CI 0.16–0.55 for Hb improvement of ≥ 1 and ≥2 g/dL, respectively; both significant). Hb outcomes in a momelotinib population with baseline platelet counts of ≤ 100 × 109/L were similar to the overall population (Supplemental Table S3).
Figure 3.

ORs for anemia outcomes.
Anemia outcomes with ORs of > 1 indicate outcomes favoring momelotinib.
BL, baseline; ESS, effective sample size; Hb, hemoglobin; OR, odds ratio; RBC, red blood cell. †Hb improvement of ≥2 g/dL at any time through week 24 (for ≥8 weeks, rolling) and not taking into account Hb levels of ≤4 weeks from RBC transfusion, or no RBC transfusion for ≥8 weeks (rolling).
Figure 4.

RDs for anemia outcomes.
Anemia outcomes with RDs of > 0 indicate outcomes favoring momelotinib.
BL, baseline; ESS, effective sample size; Hb, hemoglobin; RBC, red blood cell; RD, risk difference. †Hb improvement of ≥2 g/dL at any time through week 24 (for ≥8 weeks, rolling) and not taking into account Hb levels ≤4 weeks from RBC transfusion, or no RBC transfusion for ≥8 weeks (rolling).
There were substantial imbalances in the unmatched baseline characteristics of the populations considered in the analysis of TI efficacy outcomes (Supplemental Table S4). Patients in the momelotinib group tended to be older and were more likely to have prior JAK inhibitor therapy, lower Hb levels, and higher platelet counts. All MAIC models considered were associated with an unreliably small ESS and persistent imbalances, thus only a limited comparison of TI based on the unmatched naive data could be performed. At week 24, 40.6% and 24.4% of the momelotinib and pacritinib groups achieved TI according to SIMPLIFY-like criteria (as defined in PERSIST-2; using a rolling definition).
4. Discussion
The results from this ITC suggest that momelotinib is a tolerable treatment and may provide improved anemia outcomes in patients with MF. Overall, 14 safety outcomes met the criteria for inclusion in this study, with eight outcomes showing significantly lower odds and risk with momelotinib vs pacritinib: any-grade 3/4 AEs, any serious AEs, diarrhea, nausea, vomiting, peripheral edema, grade 3/4 thrombocytopenia, and grade 3/4 anemia. The odds of any-grade thrombocytopenia and any-grade anemia were also numerically lower with momelotinib. Furthermore, momelotinib treatment showed significantly higher odds of Hb improvement vs pacritinib.
JAK inhibitors improve splenomegaly and symptoms in many patients with MF but are associated with AEs that can lead to dose disruptions and reductions that might compromise efficacy [1–3,20]. In particular, gastrointestinal AEs are common with fedratinib and pacritinib [1,2]. As reported in PERSIST-2, common AEs in the 200-mg pacritinib arm were gastrointestinal in nature: diarrhea (48%), nausea (32%), and vomiting (19%) [4]. With momelotinib, as reported in MOMENTUM, SIMPLIFY-1, and SIMPLIFY-2, common gastrointestinal AEs were diarrhea (range, 18%-33%) and nausea (range, 16%-19%) [9–11]. Of note, patients in PERSIST-2 and PAC203 were prophylactically prescribed antidiarrheal medication at baseline, with instructions to initiate treatment at the first sign of gastrointestinal AEs [4,15]; prophylactic medication was not mandated in the momelotinib trials. Hematologic AEs are also common with JAK inhibitors [1,3,21]. In particular, anemia and thrombocytopenia were reported in 24% and 34% of patients, respectively, in the 200-mg pacritinib arm of PERSIST-2 and were cited as the most frequent reasons for discontinuation of treatment [4]. In patients receiving momelotinib, anemia and thrombocytopenia occurred in approximately 14% and 19% in SIMPLIFY-1, respectively, and in 17% and 13% of SIMPLIFY-2, respectively; anemia was an inclusion criterion for MOMENTUM [9–11]. Results from a long-term analysis of SIMPLIFY-1, SIMPLIFY-2, and MOMENTUM showed similar rates of hematologic AEs (anemia, 23%; thrombocytopenia, 25%) to those reported in the individual trials [12]. The results here corroborate observations from these studies and suggest that momelotinib-treated patients have lower odds of gastrointestinal and hematologic AEs vs pacritinib-treated patients.
While anemia is a common AE in MF clinical trials, there is an increasing appreciation for anemia-related outcomes as efficacy endpoints given that anemia is also a common manifestation of the disease itself. While anemia-related benefits have been reported with both pacritinib and momelotinib, comparisons are complicated by the fact that anemia-related efficacy endpoints and the populations they were assessed in have not been consistently defined. Although pacritinib does not have a known myelosuppressive profile, no substantial anemia benefits were observed in the primary analysis of PERSIST-2 [4]. Among 36 patients who were not transfusion independent at baseline (Gale criteria) in the 200-mg pacritinib arm, only one achieved TI (Gale criteria) at week 24 [4]. In the PERSIST-2 primary analysis and subsequent retrospective analyses, anemia response was defined in several different ways, using the Gale criteria [17] (no RBC transfusions for ≥12 weeks without a required minimum Hb level), TI response on a rolling basis (transfusion independent for ≥12 weeks at any time during randomized treatment), and TI response using the SIMPLIFY-like criteria (no RBC transfusions and no Hb levels of <8 g/dL for ≥12 weeks at any time during randomized treatment) [4,13,14]. While the SIMPLIFY-1/SIMPLIFY-2/MOMENTUM trials also defined TI response as no RBC transfusions and no Hb levels of <8 g/dL, the response assessment period was restricted to the last 12 weeks of randomized treatment and was reported in the intent-to-treat population [9–11]. With this conservative definition, TI response was observed in 66.5% of patients treated with momelotinib in SIMPLIFY-1, 43% in SIMPLIFY-2, and 30% in MOMENTUM. With the less stringent SIMPLIFY-like criteria, TI response was achieved by 24% of a subgroup of patients treated with pacritinib who were not transfusion independent at baseline in PERSIST-2 [14]. Hb improvement has also been reported in secondary analyses of MOMENTUM and PERSIST-2, although populations differed between studies; Hb improvement of ≥ 1 and ≥2 g/dL was noted in 53% and 29% of patients in the intent-to-treat population in MOMENTUM, respectively, and in 15% and 9% of patients with baseline Hb levels of <10 g/dL in PERSIST-2, respectively [13,22]. This present analysis highlights the anemia benefits of momelotinib vs pacritinib when the same definitions and matched populations are applied; with a 4.89 times higher odds of Hb improvement of >1 g/dL and a 3 times higher odds of clinical improvement in Hb with momelotinib vs pacritinib.
For TI, attempts were made to match momelotinib and pacritinib data, but imbalances persisted and the resulting ESS was small. A small ESS could be the result of a large discrepancy in values between trials or when there is a low prevalence in the index or comparator population [23]. Because a small ESS might indicate an irregular distribution with some patients receiving extreme weights, it may result in an outcome that is substantially influenced by only a small fraction of patients as well as low statistical power to detect differences between treatments [23]. Thus, MAIC could not be performed and limited comparisons were made based on naive unmatched data. Although there were imbalances in the naive data, in particular the number of patients who were JAK inhibitor naive, momelotinib had higher odds of achieving a TI response according to the SIMPLIFY-like criteria that was used in PERSIST-2 [14]. The naive momelotinib population was restricted to a subpopulation that matched the study design of the pacritinib TI analysis, which included patients who were not transfusion independent at baseline and had platelet counts of ≤ 100 × 109/L, TSS of ≥ 13, and ≥12 weeks of available data from randomization [14]. Thus, momelotinib had higher odds of TI specifically when aligning study design and endpoint definitions, although this interpretation is limited because the data were not matched.
Previously, SIMPLIFY-1 was the only trial to directly compare JAK inhibitors (momelotinib vs ruxolitinib); SIMPLIFY-2 compared momelotinib vs BAT; the BAT population was composed largely of patients treated with ruxolitinib [9,10]. In both trials, the incidence of all-grade and grade 3/4 anemia was lower with momelotinib vs the control arm [9,10]. Here, an ITC was used in the absence of head-to-head randomized trials to facilitate comparisons between momelotinib and pacritinib. The use of MAICs can reduce cross-trial differences and provide comparative evidence when comparing individual patient data vs published aggregate data [18]. However, indirect comparisons are limited by possible bias due to sensitivity to modeling assumptions (eg, consolidating the DIPSS/IPSS risk categories) and cross-trial differences in patient populations and outcome measures (eg, inclusion criteria, reported baseline characteristics, and potential differences in Hb improvement due to insufficient clarity in the comparator trial publications). MAIC analyses may also be subject to unmeasured or residual confounding, despite adjusting for prognostic factors/effect modifiers, and imbalances in some unmatched characteristics remained after adjustment. In particular, there were substantial imbalances in the unmatched baseline characteristics of the populations considered in the analysis of TI efficacy outcomes, including prior JAK inhibitor exposure. However, the lack of an established definition of anemia related to JAK inhibitor treatment vs anemia due to other disease mechanisms did not allow for the attribution of anemia-related outcomes to the effects of prior JAK inhibition. Other limitations of this analysis include the use of aggregate data for the pacritinib-treated population, which may have affected the comparability of the outcomes, reduced the robustness of the comparison, and restricted evaluable outcomes. Sample sizes also differed between the two treatment populations, largely due to differences in enrollment between the momelotinib and pacritinib trials that met this study’s inclusion criteria. Outcomes included only those reported during the 24-week trial period; long-term and potential late-emerging events were not assessed. Adjustment resulted in lower ESSes compared with the original momelotinib trial population; in particular, TI analyses were conducted in a subpopulation that met certain baseline criteria, and the resulting low ESS prevented any further statistical analysis.
5. Conclusions
This study highlights the favorable safety and anemia profile of momelotinib vs pacritinib in a population of pooled JAK inhibitor – experienced and – naive patients with MF. These results show that momelotinib had lower odds of key gastrointestinal and hematologic AEs, including grade 3/4 anemia and thrombocytopenia. If possible, these results should be validated through direct head-to-head clinical trials for more definitive conclusions. These findings are particularly notable given that pacritinib is indicated only for patients with baseline platelet counts of < 50 × 109/L [2], while momelotinib may be used regardless of baseline platelet counts, with safety findings favoring momelotinib in this broader patient population. Anemia-related efficacy outcomes that significantly favored momelotinib included Hb improvement of ≥1 g/dL, further supporting the use of momelotinib in patients with anemia. Overall, the results from this analysis provide evidence that momelotinib may be associated with a more tolerable safety profile with respect to the AEs examined and provide improved anemia outcomes vs pacritinib in patients with MF.
Supplementary Material
Acknowledgments
The sponsor thanks all participating patients and their families as well as participating study sites. The analyses reported in this manuscript were previously presented in part at the EHA2023 hybrid Congress (poster P1057).
Funding Statement
This manuscript was funded GSK. GSK employees were involved in the study design, data collection and analysis, decision to publish, and preparation of the manuscript. GlaxoSmithKline.
Article highlights
Because Janus kinase (JAK) inhibitors have varying safety profiles, an indirect treatment comparison was performed to compare safety and anemia outcomes using data from phase 2/3 momelotinib and pacritinib trials
In a pooled population consisting of JAK inhibitor – experienced and – naive patients, momelotinib was associated with significantly lower odds and risk for all grades of diarrhea, nausea, peripheral edema, and vomiting as well as grade 3/4 and serious adverse events (AEs), anemia, and thrombocytopenia vs pacritinib; no identified safety outcomes were significantly less likely with pacritinib
Momelotinib was also associated with greater odds/possibility of hemoglobin (Hb) improvement of ≥1 g/dL and clinical improvement in Hb
These results underscore the safety profile of momelotinib as a tolerable treatment option for patients with myelofibrosis
Author contributions
Study conception or design: Lucia Masarova, Srdan Verstovsek, Francesca Palandri, Ruben Mesa, Claire Harrison, Balázs Dobi, Boris Gorsh, Zhaohui Wang, Catherine Ellis, Dwaipayan Patnaik, Tom Liu, Venediktos Kapetanakis.
Data collection/analysis: Balázs Dobi, Boris Gorsh, Zhaohui Wang, Catherine Ellis, Tom Liu.
Manuscript preparation: Lucia Masarova, Srdan Verstovsek, Francesca Palandri, Ruben Mesa, Claire Harrison, Balázs Dobi, Boris Gorsh, Zhaohui Wang, Catherine Ellis, Dwaipayan Patnaik, Tom Liu, Venediktos Kapetanakis.
Disclosure statement
Lucia Masarova reports advisory board participation with MorphoSys. Srdan Verstovsek reports consultancy fees from GSK. Francesca Palandri reports consultancy fees from AOP, Celgene, and Novartis and honoraria from AOP, Celgene, CTI, Novartis, and Sierra Oncology. Ruben Mesa reports honoraria/consultancy fees from AbbVie, BMS, Blueprint, CTI, Genentech, Geron, GSK, Incyte, MorphoSys, Novartis, Sierra Oncology, Sierra, and Telios. Claire Harrison reports consultancy fees from DISC, Galecto, and Keros; honoraria from BMS, CTI, GSK, Novartis, and Sierra; advisory/safety board participation for AOP, Keros, Sumitomo, and Telios; and leadership/fiduciary roles for EHA, MPN Voice, and HemaSphere. Balázs Dobi and Venediktos Kapetanakis are employees of Evidera, which was hired by the sponsor, GSK, to conduct this research. Boris Gorsh is a former employee and shareholder of GSK. Zhaohui Wang, Catherine Ellis, Dwaipayan Patnaik, and Tom Liu are current employees of and may hold stock/stock options with GSK. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Medical writing support for this manuscript was provided by Keng Jin Lee, PhD (Nucleus Global, an Inizio Company), based on the authors’ input and in accordance with ICMJE and GPP3 guidelines, and was supported by GSK.
Ethical declaration
As these analyses are based on previously published clinical trials, IRB approval for the present analyses was not required.
Data sharing statement
Data are available upon reasonable request. Information on GSK’s data sharing commitments and requesting access to anonymized individual participant data and associated study documents can be found at https://www.gsk-studyregister.com/en/.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14796694.2025.2511562
References
Papers of special note have been highlighted as of interest (•) to readers.
- 1.Bristol Myers Squibb . Inrebic [Prescribing information]. 2022. Available from: https://packageinserts.bms.com/pi/pi_inrebic.pdf
- 2.CTI Biopharma Corp . Vonjo (pacritinib). Prescribing information. 2022.
- 3.Incyte Corporation . Jakafi (ruxolitinib). Prescribing information. 2021. Available from: https://www.jakafi.com/pdf/prescribing-information.pdf
- 4.Mascarenhas J, Hoffman R, Talpaz M, et al. Pacritinib vs best available therapy, including ruxolitinib, in patients with myelofibrosis: a randomized clinical trial. JAMA Oncol. 2018. May 1;4(5):652–659. doi: 10.1001/jamaoncol.2017.5818 [DOI] [PMC free article] [PubMed] [Google Scholar]; • This phase 3 study found that pacritinib was more effective than BAT for reducing splenomegaly and symptoms in patients with myelofibrosis and thrombocytopenia, including patients who were JAK inhibitor experienced.
- 5.Arslan Davulcu E, Oğuz MB, Kılıç E, et al. Treatment of anemia in myelofibrosis: focusing on novel therapeutic options. Expert Opin Investig Drugs. 2024. Jan;33(1):27–37. doi: 10.1080/13543784.2023.2294324 [DOI] [PubMed] [Google Scholar]
- 6.Verstovsek S, Mesa RA, Gotlib J, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012. Mar 1;366(9):799–807. doi: 10.1056/NEJMoa1110557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pardanani A, Tefferi A, Masszi T, et al. Updated results of the placebo-controlled, phase III JAKARTA trial of fedratinib in patients with intermediate-2 or high-risk myelofibrosis. Br J Haematol. 2021. Oct;195(2):244–248. doi: 10.1111/bjh.17727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.GSK . Ojjaara [Prescribing information]. 2023. Available from: https://gskpro.com/content/dam/global/hcpportal/en_US/Prescribing_Information/Ojjaara/pdf/OJJAARA-PI-PIL.PDF
- 9.Mesa RA, Kiladjian JJ, Catalano JV, et al. SIMPLIFY-1: a phase III randomized trial of momelotinib versus ruxolitinib in Janus kinase inhibitor–naïve patients with myelofibrosis. J Clin Oncol. 2017. Dec 1;35(34):3844–3850. doi: 10.1200/JCO.2017.73.4418 [DOI] [PMC free article] [PubMed] [Google Scholar]; • This phase 3 study of momelotinib vs ruxolitinib in JAK inhibitor–naive patients is one of three phase 3 trials prospectively demonstrating the anemia benefits of momelotinib.
- 10.Harrison CN, Vannucchi AM, Platzbecker U, et al. Momelotinib versus best available therapy in patients with myelofibrosis previously treated with ruxolitinib (SIMPLIFY 2): a randomised, open-label, phase 3 trial. Lancet Haematol. 2018. Feb;5(2):e73–e81. doi: 10.1016/S2352-3026(17)30237-5 [DOI] [PubMed] [Google Scholar]; • Although this phase 3 study of momelotinib vs BAT in JAK inhibitor–experienced patients did not meet the primary endpoint of spleen volume reduction, the data suggested that momelotinib provided anemia benefits, fewer transfusion requirements, and symptom improvement.
- 11.Verstovsek S, Gerds AT, Vannucchi AM, et al. Momelotinib versus danazol in symptomatic patients with anaemia and myelofibrosis (MOMENTUM): results from an international, double-blind, randomised, controlled, phase 3 study. Lancet. 2023. Jan 28;401(10373):269–280. doi: 10.1016/S0140-6736(22)02036-0 [DOI] [PubMed] [Google Scholar]; • This phase 3 study showed that momelotinib treatment resulted in clinically significant improvements in myelofibrosis-associated symptoms, anemia measures, and spleen response compared with danazol in JAK inhibitor–experienced, symptomatic, and anemic patients.
- 12.Verstovsek S, Mesa RA, Gupta V, et al. Momelotinib long-term safety and survival in myelofibrosis: integrated analysis of phase 3 randomized-controlled trials. Blood Adv. 2023. Apr 12;7(14):3582–3591. doi: 10.1182/bloodadvances.2022009311 [DOI] [PMC free article] [PubMed] [Google Scholar]; • This analysis demonstrated the consistent safety profile of momelotinib without long-term or cumulative toxicity.
- 13.Oh ST, Mesa R, Harrison C, et al. Retrospective analysis of anemia benefit of pacritinib from the PERSIST-2 trial. SOHO. 2022;22:S327. doi: 10.1016/S2152-2650(22)01439-2 [DOI] [Google Scholar]; • In this retrospective analysis, pacritinib therapy was associated with increased transfusion independence, hemoglobin improvement, and reduced myelofibrosis symptom burden vs BAT in evaluable non–transfusion independent patients.
- 14.Oh ST, Mesa RA, Harrison CN, et al. Pacritinib is a potent ACVR1 inhibitor with significant anemia benefit in patients with myelofibrosis. Blood Adv. 2023;7(19):5835–5842. doi: 10.1182/bloodadvances.2023010151 [DOI] [PMC free article] [PubMed] [Google Scholar]; • Several definitions of RBC transfusion independence were applied in this retrospective analysis showing that pacritinib provides anemia benefit vs BAT in a subset of patients from PERSIST-2.
- 15.Gerds AT, Savona MR, Scott BL, et al. Determining the recommended dose of pacritinib: results from the PAC203 dose-finding trial in advanced myelofibrosis. Blood Adv. 2020. Nov 24;4(22):5825–5835. doi: 10.1182/bloodadvances.2020003314 [DOI] [PMC free article] [PubMed] [Google Scholar]; • This phase 2 study showed clinical activity with the pacritinib 200 mg twice daily dose, along with an acceptable safety profile; the dose was selected as the recommended dose for a pivotal phase 3 study in patients with myelofibrosis and severe thrombocytopenia.
- 16.Tefferi A, Cervantes F, Mesa R, et al. Revised response criteria for myelofibrosis: international working group-myeloproliferative neoplasms research and treatment (IWG-MRT) and European LeukemiaNet (ELN) consensus report. Blood. 2013. Aug 22;122(8):1395–1398. doi: 10.1182/blood-2013-03-488098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gale RP, Barosi G, Barbui T, et al. What are RBC-transfusion-dependence and -independence? Leuk Res. 2011. Jan;35(1):8–11. doi: 10.1016/j.leukres.2010.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Signorovitch JE, Sikirica V, Erder MH, et al. Matching-adjusted indirect comparisons: a new tool for timely comparative effectiveness research. Value Health. 2012. Sep;15(6):940–947. doi: 10.1016/j.jval.2012.05.004 [DOI] [PubMed] [Google Scholar]
- 19.Signorovitch JE, Wu EQ, Yu AP, et al. Comparative effectiveness without head-to-head trials: a method for matching-adjusted indirect comparisons applied to psoriasis treatment with adalimumab or etanercept. Pharmacoeconomics. 2010;28(10):935–945. doi: 10.2165/11538370-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 20.Talpaz M, Prchal J, Afrin L, et al. Safety and efficacy of ruxolitinib in patients with myelofibrosis and low platelet counts (50–100 × 109/L): final analysis of an open-label phase 2 study. Clin Lymphoma Myeloma Leuk. 2022. May;22(5):336–346. doi: 10.1016/j.clml.2021.10.016 [DOI] [PubMed] [Google Scholar]
- 21.Bristol Myers Squibb . Inrebic (fedratinib). Summary of product characteristics. 2022. Available from: https://www.ema.europa.eu/en/documents/product-information/inrebic-epar-product-information_en.pdf
- 22.Verstovsek S, Oh ST, Kiladjian JJ, et al., editors. Transfusion independence response (TI-R) as a potential surrogate for overall survival (OS) in Janus kinase inhibitor (JAKi)-experienced patients with myelofibrosis from MOMENTUM trial. New Orleans (LA): ASH; 2022. [Google Scholar]
- 23.Phillippo DM, Ades AE, Dias S, et al. Methods for population-adjusted indirect comparisons in health technology appraisal. Med Decis Making. 2018. Feb;38(2):200–211. doi: 10.1177/0272989X17725740 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


