Abstract
Recently we described a family of peptides, unrelated in sequence to IgE, that form stable β-hairpins in solution and inhibit IgE activity in the μM range [Nakamura, G. R., Starovasnik, M. A., Reynolds, M. E. & Lowman, H. B. (2001) Biochemistry 40, 9828–9835]. Using an expanded set of peptide–phage libraries, we found a simpler motif, X2CPX2CYX, for binding to the high-affinity IgE receptor. In solution, one of these peptides spontaneously formed a covalent antiparallel dimer. We subsequently linked these monomers in a single-chain construct on phage and optimized receptor binding. Ultimately, peptides with 30 nM affinity were produced. NMR studies showed that the peptide adopts a stable fold consisting of two “zeta” (ζ)-shaped moieties. Structure–activity analyses reveal a single binding site created by the zeta-dimer, with two tyrosine residues important for structural stability and two proline residues important for FcɛRI binding. The peptides inhibit histamine release from cultured cells and are extremely stable in biological fluids. The zeta peptides appear to act as competitive IgE inhibitors and suggest possibilities for design of novel IgE antagonists.
The binding of IgE to its high-affinity receptor, FcɛRI, is a key step in the manifestation of allergic disease; initiation of the allergic cascade depends on allergens, such as ragweed, binding to IgE⋅FcɛRI complexes that form on the surface of mast cells, basophils, and other leukocytes (1). The significance of this interaction is demonstrated by molecules that bind IgE and prevent receptor binding (2), thus preventing the release of inflammatory compounds that result in symptoms associated with allergic disease (3, 4). Molecules that target the high-affinity receptor, FcɛRI, and block IgE binding may be similarly efficacious in treating asthma, allergic rhinitis, and other forms of atopy. Phage-displayed libraries offer a means to obtain high-affinity peptide antagonists (5–8).
Previously, we described a class of β-hairpin-structured peptides that bind to FcɛRIα and inhibit IgE binding (9). These peptides were selected from naive peptide libraries displayed on phage, were active in inhibiting allergen-induced histamine release in cell-based assays, and remained active following exposure to serum and lung-associated matrix. In this report, we describe the identification of a different class of peptides with significantly higher potency. Peptides were selected from newly designed peptide–phage libraries that contained higher diversity and included a small putative loop, X2CX3CX2. Initial synthetic peptides based on clones from this library showed low activity for inhibiting IgE binding to cells. However, one of these peptides underwent conversion over time to a higher affinity, disulfide-dimer form. Subsequently, we used synthetic peptide chemistry, NMR structure determination, and further phage optimization in a concerted process of in vitro evolution to arrive at a nanomolar peptide inhibitor. We have designated these “zeta” (ζ) peptides on the basis of their three-dimensional structure.
Like the previously described β-hairpin peptides (9), the zeta peptides retain activity following exposure to biological fluids. However, unlike the β-hairpin peptide, these peptides contain two disulfide bonds and have an irregular but well defined structure. These results demonstrate that multiple peptide motifs can bind to FcɛRI and inhibit IgE binding. An understanding of the interaction between two structurally distinct families of peptides and FcɛRI may lead to the development of novel antagonists of IgE.
Methods
Phage-Displayed Peptide Libraries and Binding Selections.
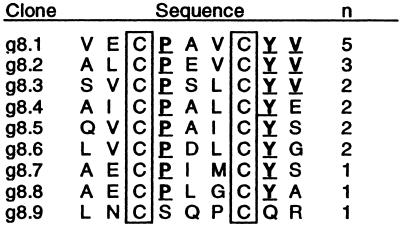
Twenty-four naive peptide libraries with high diversity (109 transformants each) were polyvalently displayed on the N terminus of gVIIIp by using an M13 phagemid vector with Ptac promoter as described (10). Briefly, peptide libraries were 9–20 residues in length and included a “linear” X8 motif, as well as motifs represented by X2–7CX3–10CX2–7. Peptide–phage libraries were propagated in XL-1 Blue Escherichia coli with VCSM13 helper phage (Stratagene). Binding selections against FcɛRIα-Ig, the alpha chain fused to the Fc region of human IgG, were as described (9). Before selection rounds 1–2, phage were propagated with 50 μM isopropyl-β-D-thiogalactopyranoside (IPTG) to induce high levels of peptide display; IPTG was omitted before round 3. DNA from selected clones (Fig. 1) was isolated and sequenced using standard Sequenase (Amersham Pharmacia) procedures.
Figure 1.
The sequences of peptides corresponding to the number, n, of phagemid clones sequenced after three rounds of binding selection to FcɛRIα-Ig. Cys residues (boxed) were fixed; all other positions were randomized in the library. Residues found at each position in more than half of the clones are underlined.
Peptide Synthesis.
Peptides were prepared manually or by machine, typically on a 0.25-mmol scale, using standard solid phase peptide chemistry with fluorenylmethoxycarbonyl (Fmoc)-protected amino acids, on a p-alkoxybenzyl alcohol resin, or on a Rink amide aminomethyl resin, as described (ref. 9 and references therein). e107 and e108 (Fig. 2) were synthesized as monomers, orthogonally protected on Cys as Trityl and Acm (S-acetamidomethyl aminothioacetal), and dimerized sequentially from the Trityl-protected Cys then from the Acm-protected Cys. The zeta peptides (e.g., e109, Fig. 2) were cyclized sequentially by means of oxidation of the “inner” cysteines (C7-C15, made Trityl-protected) then the “outer” cysteines (C3-C19, made Acm-protected). Oxidations were carried out at room temperature; the first was performed with an aqueous solution of K3Fe(CN)6 over 10 h, and the second with a saturated solution of I2 in acetic acid over 6–7 h for complete Acm deprotection and disulfide oxidation. Purification and characterization were performed as described (9).
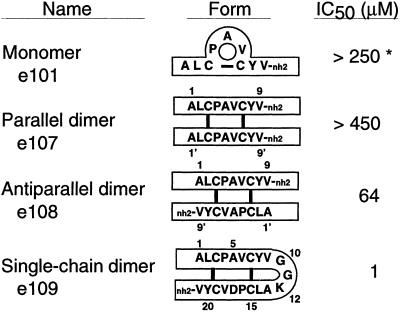
Figure 2.
Schematic diagram of peptide forms with [125I]IgE-binding inhibition data obtained from cell-based assays. *, The monomeric form of e101 showed improved activity with time (see text).
Peptides were also produced from fusion proteins consisting of the peptide sequence, followed by a Gly-Arg linker (for trypsin cleavage), and the Z-domain of protein A (11). K12H was introduced to prevent internal trypsin cleavage. After secretion from E. coli, the fusion protein was purified on an IgG-Sepharose column and cleaved with trypsin; thereafter, the Z-domain was removed using an IgG-Sepharose column, and the FcɛRI-binding peptide was purified by HPLC as described (11).
Binding and Activity Assays.
Surface plasmon resonance assays were carried out on a BIAcore-2000 instrument (BIAcore, Piscataway, NJ) to determine apparent dissociation constants (Kd) for the peptide–FcɛRIα (residues 1–172) interaction under steady-state conditions as described (9). Cell-based binding assays were used to determine the IC50 values of peptide inhibition of [125I]IgE binding to FcɛRI expressed on CHO cells (9, 12). These studies used IgE derived from a myeloma cell line (13). Rat basophil line RBL-48 (14), expressing the human FcɛRIα subunit, was used to determine peptide potency in inhibiting IgE-dependent, ragweed-induced histamine release as described (9).
Zeta Peptide–Phage Analysis and Optimization.
A single-chain dimer was designed (see Fig. 2) for optimization on a gIIIp display vector that displayed human growth hormone (hGH). The phagemid phGHam-g3 (15) was mutated by site-directed mutagenesis (16) to express an e101 monomer connected by a GGK linker to a second e101 monomer, followed by a (GGGS)2 linker, the mature hGH sequence, and the C-terminal domain of M13 gIIIp (249). Peptide libraries (see Tables 1–3, which are published as supporting information on the PNAS web site, www.pnas.org) and Ala mutations were constructed with synthetic oligonucleotides by using site-directed mutagenesis (6, 16). Degenerate oligonucleotides contained NNS codons for randomization to all possible residues, or a nucleotide mixture (70% wild-type and 10% of the remaining nucleotides) at each codon for bias toward wild type (5). Binding selections and competitive phage-ELISA assays (15) were performed on Maxisorp plates coated with FcɛRIα derived from baculovirus-infected insect cells (J. Stamos and G.R.N., unpublished results). In some cases, hGH-receptor (15) was used to select against hGH-deleted clones, which occasionally arose during selections (see Tables 1–3).
NMR Spectroscopy.
Two-dimensional (2D) NMR experiments were run on a Bruker DRX-500 or DRX-600 spectrometer typically at 288 K with samples containing 1–2 mM peptide in 92% H2O/8% D2O, 0.1 mM NaN3, with 0.1 mM 3-(trimethylsilyl)-1-propane-1,1,2,2,3,3-d6-sulfonic acid (DSS) as a chemical shift reference, and with pH adjusted for optimal solubility and line width (pH 5.4 for e109; pH 6.0 for e131). For e131, the peaks in the one-dimensional (1D) NMR spectrum at 288 K were rather broad, so 8% acetonitrile-d3 was added to the sample and the temperature was raised to 308 K. Under these conditions, the NMR peaks were significantly sharper and allowed for complete three-dimensional structure determination. Based on the lack of significant changes in chemical shifts, the cosolvent appears to have only minimized the extent of nonspecific aggregation without perturbing structure.
Two-dimensional NMR spectra were acquired and analyzed as described (9). Complete 1H resonance assignments and 3JHN-Hα and 3JHα-Hβ for e109 and e131 are given in Tables 4 and 5, which are published as supporting information on the PNAS web site, www.pnas.org. Eighty initial structures were calculated using the hybrid distance geometry/simulated annealing program DGII (17); 50 of these were further refined by restrained molecular dynamics using the AMBER all-atom force-field implemented in DISCOVER as described (18). Twenty structures having the lowest restraint violation energy and good geometry represent the solution conformation of each peptide. The structure with the lowest rms deviation (rmsd) to the average coordinates of the ensemble was chosen as the representative structure (model 1 in the PDB file). The final ensemble of twenty models of each peptide satisfies the input data very well having no distance or dihedral angle restraint violations greater than 0.1 Å or 2°, respectively. The structures also have good covalent geometry as judged by the program PROCHECK with 77% ± 7% (e109) and 79% ± 7% (e131) of the residues in the most favored region of φψ space, with none in the disallowed or generously allowed regions (19).
Peptides were also evaluated to assess the effect of substitutions on their three-dimensional structures by using 2D NMR spectroscopy as described (9); backbone 1H resonances were assigned and 3JHN-Hα coupling constants were measured and compared with the reference peptide (e109). Peptides were defined to be of similar structure to e109 provided chemical shifts, coupling constants, and nuclear Overhauser effects (NOEs) were consistent with that structure. Peptides were defined to be less stable when 3JHN-Hα coupling constants were less extreme than the reference and/or when chemical shifts of backbone resonances were closer to random-coil values in the analog than in e109.
Results
Identification of FcɛRI Binding Peptides.
Twenty-four peptide–phage libraries of high diversity (109–1010), representing a more extensive array of motifs than those described (9), were separately subjected to receptor-binding selection to identify FcɛRI-binding peptides. After three rounds of selection, the putative disulfide-cyclic peptide library with the motif X2CX3CX2 was ≈100-fold enriched in binding FcɛRIα-Ig over controls (data not shown). Nine unique sequences were identified from 19 peptide–phage clones (Fig. 1). Most contained identical residues at two positions (P4 and Y8) and a conserved hydrophobic residue (M, L, I, or V) at position 6. Six peptides representing three sequences (each in nonacetylated and N-acetylated forms) were synthesized with C-terminal amides and C3-C7 disulfide bonds. e101 and e102 corresponded roughly to a consensus sequence, ALCPAVCYV-nh2; e103 and e104 corresponded to peptide–phage sequence g8.2; and e105 and e106 corresponded to g8.9 (Fig. 1). When assayed for inhibiting IgE binding to cell-surface FcɛRI, only peptide e101 (Fig. 2) had activity (IC50 = 250 μM). This activity, however, improved over time and stabilized at 37 μM when assayed under identical conditions 10 days after solubilization.
Peptide Dimerization.
Several analytical approaches were used to investigate the increase in activity of e101 with time. One-dimensional (1D) and 2D NMR spectra of freshly prepared e101 demonstrated that the nine-residue monomeric disulfide-bonded peptide did not adopt a well defined conformation in solution. At least four conformationally distinct forms of the peptide could be identified in the spectra, with the major form showing no evidence of stable structure. However, analysis of 1D NMR spectra over time showed that the relative populations of the different forms were changing. After 8 days at room temperature, ≈95% of the peptide had converted to a single new form that also showed significantly longer retention time than the original sample in an analytical HPLC run. This form presented a stable structure in solution as evidenced by significant chemical-shift dispersion from random coil and extreme values for backbone and side-chain coupling constants. Mass spectrometry showed multiple-charged ions differing in charge-to-mass ratios by units of two monomer masses, rather than one (as seen for reduced monomers), suggesting the presence of peptide dimers.
We postulated two potential covalent isomers, one with parallel arrangement of the monomers and the other antiparallel, that could form from e101. To test these possibilities, we synthesized peptides by using orthogonal thiol protection strategies to yield e107 and e108 (Fig. 2). The analytical HPLC retention time and NMR spectra for the antiparallel peptide were identical to that of active-form e101. When tested for inhibitory activity, e108 was equipotent to active-form e101, whereas e107 showed no detectable activity. These data indicate that monomeric e101 underwent a spontaneous rearrangement of disulfide bonds to form a covalent, antiparallel, homodimeric peptide corresponding to e108 (Fig. 2).
Engineering and Analysis of Zeta Peptides.
A single-chain variant was engineered to facilitate further phage-display efforts and structure determination. The first “monomer” was connected directly to the second by means of a GGK linker to generate a 21-residue “single-chain dimer.” The Gly residues were chosen for flexibility, and the Lys residue to maintain solubility and to substitute for the N-terminal amine of e101. When FcɛRIα-Ig binding clones were identified by phage-ELISA screening and sequenced, one clone contained a spontaneous A17D mutation (i.e., A5′ in the second “monomer”; Fig. 2). Fortuitously, this mutation improved receptor binding; the synthetic peptide e109 (Fig. 2) showed IgE inhibitory activity in cell-based assays with IC50 = 1 μM, a 60-fold improvement over e108.
NMR spectroscopy revealed that e109 adopts a well defined structure in solution. Backbone and side chain coupling constants and 1H resonance frequencies are significantly different from those of an unstructured peptide; 3JHN-Hα values are greater than 8.5 Hz for residues C3, Y8, C15, and Y20 and less than 6 Hz for residues A5, V6, C7, D17, V18, and C19, and 3JHα-Hβ values indicate that the side chains of residues C3, Y8, C15, C19, and Y20 have fixed χ1 angles. Thus, the structure of e109 was calculated from a total of 113 distance and 18 dihedral angle restraints (see Methods). The final ensemble of 20 models representing the solution structure of e109 is shown in Fig. 3.
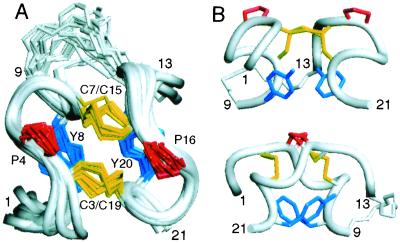
Figure 3.
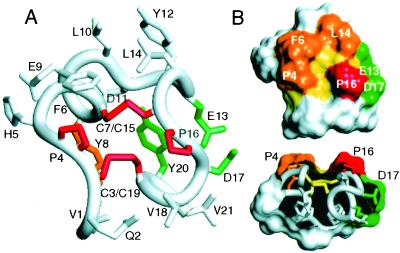
Three-dimensional structure of e109 determined by 2D NMR spectroscopy. (A) Ensemble of 20 models of e109. The backbone of residues 1–9 and 13–21 is represented in ribbon format. Only those side chains conserved during the phage peptide selections are shown. Backbone rmsd from the mean coordinates of the ensemble for residues 3–8 and 15–20 is 0.30 ± 0.06 Å (0.61 ± 0.06 Å for all heavy atoms). (B) Two orthogonal views of a representative structure of e109. The top view represents a 90° rotation of that in A. [Figs. 3 and 5 were generated with INSIGHT98.0 (Molecular Simulations, Waltham, MA).]
e109 has little regular secondary structure, but presents two small antiparallel 310 helices comprised of residues 4–8 and 16–20, connected by two disulfide bonds (Fig. 3). The backbone conformation is nearly symmetric with residues 1–9 adopting essentially the same structure, resembling the Greek letter zeta (ζ), as residues 13–21. We therefore refer to the peptide as a “zeta-dimer.” The helical backbone conformation appears to be stabilized by tight packing of the Tyr rings (Y8 and Y20) “below” the disulfide bonds with each Tyr hydroxyl proton donating a hydrogen bond across the “dimer interface” to the backbone carbonyl oxygen of a Cys, Y8HH/C19CO and Y20HH/C7CO, in eight and ten of the twenty models, respectively. The linker region is not defined by the NMR data and appears flexible in solution. The chemical shifts, coupling constants, and nuclear Overhauser effects (NOEs) observed for e108 are very similar to those of e109 (data not shown), indicating that both forms adopt the same fold, differing only by whether there is a flexible linker connecting the ends.
Peptide Optimization and Structure–Activity Analysis.
Additional improvements in binding affinity were obtained through a combination of secondary libraries based on e109 peptide–phage, point mutations, and additive effects. Several libraries were designed, each with a small number of randomized residues including the first “monomer,” the “linker” region, and the second “monomer.” Among these secondary libraries, only an N-terminal library with randomization at positions 1, 2, 5, and 6 gave rise to clones with improved affinity (see Table 1). Peptides based on the two most abundant clones, e111 and e113 (Fig. 4 A and B), were synthesized and found to bind receptor more tightly (IC50 = 290 nM and 170 nM, respectively) than e109, both in direct-binding (BIAcore) assays and in inhibition of IgE binding on cells. Another selectant, e110, sharing in common only a large hydrophobic residue at position 6, also showed improved affinity (Fig. 4A).
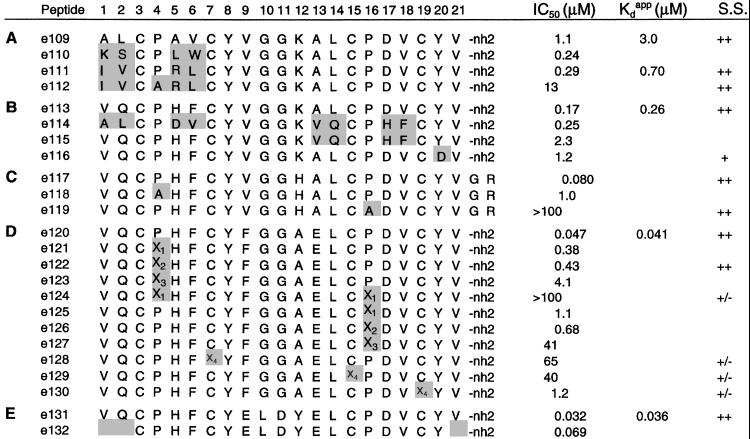
Figure 4.
Structure and activity of synthetic peptides. Shading indicates changes compared with the parental peptides (A) e109, (B) e113, (C) e117, made biosynthetically, (D) e120, and (E) e131. IC50 values are from cell-based binding assays of [125I]IgE inhibition. Apparent dissociation constants, Kdapp, are from FcɛRIα direct binding (BIAcore) experiments. Errors (SD) in IC50 and Kdapp were typically ±30–50%. Peptides with a mark in the structural score (S.S.) column were evaluated by NMR (see Methods): ++, structured similarly to e109; +, structured similarly to e109, but less stable; +/−, showing evidence of structure that differed from e109. x1, N-methyl Ala; x2, (L)-pipecolic acid; x3, (D)-pipecolic acid; x4, homo-Cys.
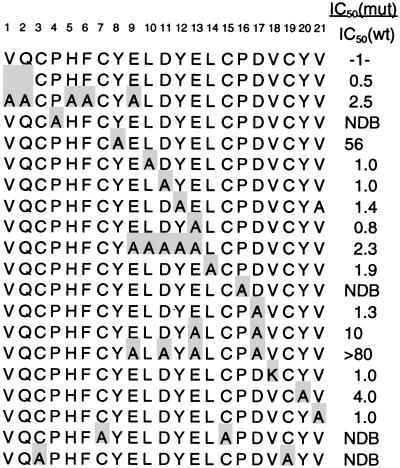
To analyze the structure–activity relationships of e111, Ala residues were substituted throughout the peptide–phage, and receptor binding was assayed by phage-ELISA (data not shown). Residues conserved in the initial binding selection appeared critical for receptor interaction because P4A, P16A, Y8A, and Y20A all resulted in undetectable phage binding. D17A caused a 25-fold reduction in binding. Receptor binding was not significantly affected by Ala replacements at other positions. A small improvement (about 2-fold) was found with the linker-region variant K12A.
Peptide variants were also made synthetically or biosynthetically to test certain positions. P4A caused a 45-fold reduction in binding in the context of e111 (or 13-fold in e117), and P16A caused a >1,000-fold reduction in binding in the context of e117 (Fig. 4 A and C). Importantly, NMR analysis of peptides having either P4 or P16 replaced by Ala (e112 and e119, respectively) indicates that neither P4 nor P16 is essential for maintaining peptide structure (Fig. 4). The asymmetry of the zeta-dimer's binding to FcɛRIα is apparent from comparison of peptides e113, e114, and e115. Whereas substitutions A1V, L2Q, A5H, and V6F (i.e., in the first “monomer”), or alternatively A13V, L14Q, A17H, and V18F (i.e., in the second “monomer”), improved affinity by about 5-fold compared with e109, the combination of mutations in both monomers actually decreased affinity by about 2-fold (Fig. 4B). These results led to the design of new libraries, based on an e111 or e113 template, in which the peptide was mutated at selected positions, avoiding the critical residues P4, P16, Y8, and Y20.
Among tertiary libraries randomized in the linker region (see Table 2), one library derived from e111 produced a predominant clone at round 4, in which the linker region was nonconservatively substituted (ELDYE replacing VGGKA13). Another library, derived from e113 (see Table 3) yielded a predominant clone with FGGKE13 replacing VGGKA13 in the linker region. Phage-ELISA assays indicated that each of these clones was indeed improved in affinity over e113. We postulated that small improvements in binding affinity would be additive (20). Therefore, we constructed two synthetic peptides, e120, corresponding to e113 with a FGGAE13 linker (based on the phage clone, but incorporating the K12A mutation described above), and e131, incorporating the ELDYE13 linker in the e113 background. These combinations proved effective; peptides e120 and e131 were improved in activity by ≈4–6 fold over e113 with IC50's of 50 and 30 nM, respectively (Fig. 4 D and E).
The structure of e131 was calculated using a total of 143 distance and 24 dihedral angle restraints derived from 2D NMR analysis. A representative structure of e131 is shown in Fig. 5. The backbone conformation of e131 is essentially indistinguishable from that of e109, with a backbone rms deviation (rmsd) between the average coordinates of each ensemble of 0.45 Å (N, Cα, C′ of residues 3–8, 15–20), within the uncertainty of the structure determinations. The conformations of V1, Q2, and residues 10–12 in the linker region are less well defined by the NMR data and appear to be more flexible than the core of the molecule. Y8 and Y20 pack inside the core of e131 as was seen for e109; a peptide that has Y20 replaced by Asp (e116) shows loss of stable structure for the C-terminal half of the peptide, demonstrating the structural role of the Tyr side chain.
Figure 5.
Structure of e131. (A) Representative structure of e131 with side chains colored based on their relative importance to receptor binding as measured by phage Ala-scanning (Fig. 6): red, no detectable binding; orange, >50-fold loss; green, 4–10-fold loss; white, <3-fold loss in binding. Note, however, that a 10-fold loss in binding is only seen for the E13A/D17A double mutant, whereas each single Ala mutant shows no effect. The backbone rmsd from the mean coordinates of the ensemble for residues 3–8 and 15–20 is 0.50 ± 0.16 Å (0.85 ± 0.10 Å for all heavy atoms). The side chain χ1 angles of F6, C7, Y8, E9, L10, C15, V18, C19, and Y20 are well defined by the NMR data. (B) Surface representation of e131 (same view as in A), highlighting key features of the proposed receptor-binding site. The groove in the surface is lined by the disulfides (yellow) and bordered by the essential P16 (red) as well as P4, F6, and L14 (orange); a negative charge provided by E13 and/or D17 (green) is adjacent to P16. (C) Cut-away view (90° rotation from that in B), highlighting the groove and proline ridges.
Ala-scanning analysis was performed on e131-phage to identify individual residues critical for binding receptor (Figs. 5 and 6). Ala replacement of either Cys pair resulted in complete loss of binding. Similar results were obtained with P4A and P16A, which showed no detectable binding, as for e111-phage. Lesser impacts were observed with Y8A (56-fold weaker) and Y20A (4-fold weaker). Individual substitutions of the remaining residues had little impact on binding. In contrast to results with e111, D17A in the e131 context had no significant impact on binding (Fig. 6). Surprisingly, individual Ala replacements of linker residues 10–13, including E13A, also did not affect binding. We speculated that the side chain carboxylate of D17 could be substituted by one or more of the selected acidic groups in the linker region (i.e., residues 9–13) of e131. This indeed appears to be the case as the combination of mutation E13A with D17A yielded a 10-fold reduction in affinity, and the combination E9A/E13A/D17A yielded a >80-fold reduction (Fig. 6). Nevertheless, the linker variant AAAAA13 is 2.5-fold improved over the e113 linker region, VGGKA13 (data not shown); thus, binding affinity improved with increasingly acidic linkers.
Figure 6.
e131 peptide–phage Ala scan. Relative affinities, IC50 (mutant)/IC50 (e131), were obtained from competitive assays compared with e131-hGH-g3p (IC50 = 40 ± 9 nM). Residues differing from e131 are shaded. NDB, no detectable binding.
Synthetic variants of peptides e120 and e131 confirmed the importance of residues P4 and P16 and the C3-C19 and C7-C15 disulfides. In e120, even subtle substitutions of P4 or P16 with N-methyl Ala or pipecolic acid reduced affinity (Fig. 4D), and replacement of the Cys residues with homo-Cys were poorly tolerated (Fig. 4D). On the other hand, in e131, deletion of V1, Q2, and V21 (e132), resulted in only a 2-fold loss in affinity (Fig. 4E). Further truncations are possible, because peptides with shortened linkers retained activity (data not shown).
Biological Activity.
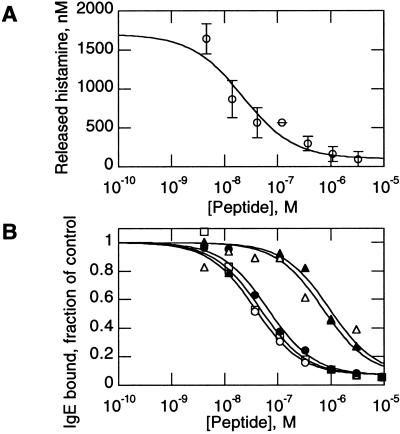
We tested whether e131 could inhibit histamine release in a relevant allergen setting (9) by adding peptides to cells expressing human FcɛRI, in the presence of ragweed-specific IgE. Peptides e109, e111, e113, e120, and e131 all inhibited ragweed-induced histamine release from these cells. e131 inhibited histamine release with a typical IC50 of 20–50 nM (Fig. 7A), corresponding closely to its activity in receptor-binding and IgE-binding inhibition assays (Fig. 4E).
Figure 7.
Biological assays. (A) Peptide e131 inhibition of IgE-induced histamine release from ragweed-treated cells. IC50 = 21 ± 7 nM for the experiment shown. (B) Peptide stability in biological matrices. e131 was incubated for 24 h at 37°C with lung lavage (□) or lung homogenate (○), or for 1 h at 37°C with murine gastric homogenate (▵) or intestinal lavage (▴), and compared with untreated e131 standard (●) in an IgE binding inhibition assay. Control extracts showed no inhibition of IgE binding.
A major safety concern for therapeutic use of molecules binding to FcɛRI is whether binding causes activation of the receptor, leading to cellular histamine release (9). To test this, we treated cells with peptide e131 alone. Control cells that received ragweed and ragweed-specific IgE released significant levels of histamine (data not shown). In contrast, cells that received e131 (over a concentration range up to 500 μM), buffer, or a nonbinding peptide control (e133; QCPHFCPFELDYELCPDVCY-nh2) showed only background histamine release, demonstrating that e131 does not activate cells, even at concentrations 104-fold above the peptide's apparent Kd.
Peptides commonly have a short half-life in biological matrices; linear and unstructured peptides are often quickly degraded, leading to rapid loss of activity (21). As e131 is structured in solution, it seemed likely to be stable in biological matrices. Indeed, after 24-h incubation at 37°C in murine lung lavage or lung homogenates, we observed no decline in e131 inhibitory activity (Fig. 7B). Even after 1-h incubations at 37°C in murine gastrointestinal lavage or homogenates, e131 still retained 10% activity (Fig. 7B).
Discussion
We report here a family of highly structured “zeta” peptides that bind to FcɛRI with high affinity. Unlike the previously described β-hairpin family (9), which was identified from a peptide–phage library of the XnCX8CXn motif, these peptides initially appeared to have a smaller disulfide cycle with the motif X2CX3CX2. However, HPLC, mass spectrometry, and NMR of the initial peptides, along with synthesis of parallel and antiparallel dimeric forms, demonstrated that the active species was, in fact, an antiparallel dimer with two intermolecular disulfides. Analysis and optimization was facilitated by the design of a short linker connecting two monomers. From screening of the initial single-chain clones, a fortuitous mutation, A17D was found to improve affinity dramatically; successive randomization and addition of mutations yielded peptides e120 and e131, with 30–50 nM affinity for FcɛRI.
The relationship between the form of peptide expressed on the surface of phage particles and the active form in solution is not certain. However, several lines of evidence argue that dimeric forms resembling e108 occurred on phage. A synthetic monomer containing an intramolecular disulfide bond spontaneously converted to a covalent dimer, with the antiparallel form predominating. Tyr residues, conserved in the initial phage clones, were also conserved in the zeta-dimers and were shown to be critical for formation of a structural core. Finally, the conservation of Pro residues in the initial phage clones and throughout the optimization of the zeta peptides argues that the modes of receptor recognition by the initial phage peptides and the synthetic zeta peptides were similar.
We propose that the primary receptor-recognition surface on the zeta peptides consists of a hydrophobic surface that crosses the “dimer interface” (Fig. 5), with predominant contribution by the P16 side chain (cf. Fig. 4C) and contributions from P4, F6, and L14. Structurally, these residues form protrusions separated by a groove lined by the two disulfide bonds (Fig. 5). Multiple-site mutants (see Fig. 6) suggest that the acidic linker region may have long-range electrostatic interactions with receptor. Unlike P4 and P16, the Cys residues are critical for peptide structure; thus, distinguishing the structural role of these residues from a potential role in direct receptor interaction is difficult. For example, substitutions with the longer side chain homo-Cys (namely, e128-e130) perturb both structure and binding affinity (Fig. 4D). In contrast, the roles of Y8 and Y20 appear to be purely structural; these side chains are buried in the core of the zeta structure and are solvent-exposed only on the opposite side of the molecule from the prolines.
Interestingly, the presence of a Pro-binding pocket on FcɛRIα is evident in crystal structures of the receptor both alone and in complex with IgE (22, 23). Furthermore, a Pro residue in the turn region of the hairpin peptides (9) also had a predominant role in receptor binding. Structural analyses of both classes of peptide/receptor complexes are underway to determine the specific modes of receptor binding and how they compare with IgE/receptor interactions. Regardless of the details of the zeta peptide/receptor complex, the receptor-binding determinants of e131 appear to define a spatially compact epitope. In contrast to the suggestion that two distinct regions of FcɛRIα that normally interact with IgE may need to be simultaneously blocked (23), our observations suggest that a small inhibitor binding within or between these two regions may be sufficient to act as an effective antagonist.
Peptide dimers were previously identified from peptide–phage libraries selected for binding IL-5-receptor; however, these dimers were determined to be parallel, and their three-dimensional structure has not been reported (7). These peptides appear to have two binding sites per dimer, leading to IL-5 receptor dimerization. Importantly, the zeta-dimers described here present a single binding site and do not appear to dimerize IgE receptor at concentrations greatly exceeding those expected to achieve saturation of FcɛRI.
Like our previously described hairpin peptides, the zeta peptides are stable in solution as well as in biological fluids and are capable of inhibiting histamine release from cultured cells, making them attractive candidates for therapeutic development. In addition, the fact that two very distinct types of structure, each having no discernible sequence similarity to IgE or to each other, are able to bind and block the IgE receptor, raises the possibility that common elements from these two families might be combined in even more potent and pharmacologically useful molecules.
Supplementary Material
Acknowledgments
We thank B. Cunningham for preparation of peptide–phage libraries; C. Quan and J. Tom for peptide synthesis; M. Struble and J. Dotson for peptide purifications; J. Bourell for mass spectrometry; P. Lester for FcɛRIα-Ig and IgE; J. Stamos for baculovirus-derived receptor preparations; T. Sweeney and M. Aldrich for murine tissues; and D. Artis, N. Skelton, and W. Fairbrother for many helpful discussions.
Abbreviations
- FcɛRIα
extracellular domain of the alpha chain of the high-affinity IgE receptor
- FcɛRIα-Ig
FcɛRIα fused to the Fc region of human IgG
- 2D
two-dimensional
Footnotes
Data deposition: The structure factors of e109 and e131 have been deposited in the Protein Data Bank, www.rcsb.org (PDB ID codes 1KCN and 1KCO, respectively).
References
- 1.Ishizaka T, Conrad D H, Schulman E S, Sterk A R, Ishizaka K. J Immunol. 1983;130:2357–2362. [PubMed] [Google Scholar]
- 2.Jardieu P. Curr Opin Immunol. 1995;7:779–782. doi: 10.1016/0952-7915(95)80047-6. [DOI] [PubMed] [Google Scholar]
- 3.Ishizaka K, Ishizaka T, Lee E H. Immunochemistry. 1970;7:687–702. doi: 10.1016/0019-2791(70)90175-8. [DOI] [PubMed] [Google Scholar]
- 4.Kinet J P. Annu Rev Immunol. 1999;17:931–972. doi: 10.1146/annurev.immunol.17.1.931. [DOI] [PubMed] [Google Scholar]
- 5.Wrighton N C, Farrell F X, Chang R, Kashyap A K, Barbone F P, Mulcahy L S, Johnson D L, Barrett R W, Jolliffe L K, Dower W J. Science. 1996;273:458–464. doi: 10.1126/science.273.5274.458. [DOI] [PubMed] [Google Scholar]
- 6.Dennis M S, Eigenbrot C, Skelton N J, Ultsch M H, Santell L, Dwyer M A, O'Connell M P, Lazarus R A. Nature (London) 2000;404:465–470. doi: 10.1038/35006574. [DOI] [PubMed] [Google Scholar]
- 7.England B P, Balasubramanian P, Uings I, Bethell S, Chen M-J, Schatz P J, Yin Q, Chen Y-F, Whitehorn E A, Tsavaler A, et al. Proc Natl Acad Sci USA. 2000;97:6862–6867. doi: 10.1073/pnas.110053997. . (First Published May 23, 2000; 10.1073/pnas.110053997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scherf T, Kasher R, Balass M, Fridkin M, Fuchs S, Katchalski-Katzir E. Proc Natl Acad Sci USA. 2001;98:6629–6634. doi: 10.1073/pnas.111164298. . (First Published May 29, 2001; 10.1073/pnas.111164298) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakamura G R, Starovasnik M A, Reynolds M E, Lowman H B. Biochemistry. 2001;40:9828–9835. doi: 10.1021/bi0109360. [DOI] [PubMed] [Google Scholar]
- 10.Sidhu S S, Lowman H B, Cunningham B C, Wells J A. Methods Enzymol. 2000;328:333–363. doi: 10.1016/s0076-6879(00)28406-1. [DOI] [PubMed] [Google Scholar]
- 11.Dennis M S, Roberge M, Quan C, Lazarus R A. Biochemistry. 2001;40:9513–9521. doi: 10.1021/bi010591l. [DOI] [PubMed] [Google Scholar]
- 12.Hakimi J, Seals C, Kondas J A, Pettine L, Danho W, Kochan J. J Biol Chem. 1990;265:22079–22081. [PubMed] [Google Scholar]
- 13.Nilsson K, Bennich H, Johansson S G O, Ponten J. Clin Exp Immunol. 1970;7:477–489. [PMC free article] [PubMed] [Google Scholar]
- 14.Gilfillan A M, Kado-Fong H, Wiggan G A, Hakimi J, Kent U, Kochan J P. J Immunol. 1992;149:2445–2451. [PubMed] [Google Scholar]
- 15.Lowman H B. In: Methods in Molecular Biology. Cabilly S, editor. Vol. 87. Totowa, NJ: Humana Press; 1998. pp. 249–264. [DOI] [PubMed] [Google Scholar]
- 16.Kunkel T A, Bebenek K, McClary J. Methods Enzymol. 1991;204:125–139. doi: 10.1016/0076-6879(91)04008-c. [DOI] [PubMed] [Google Scholar]
- 17.Havel T F. Prog Biophys Mol Biol. 1991;56:43–78. doi: 10.1016/0079-6107(91)90007-f. [DOI] [PubMed] [Google Scholar]
- 18.Starovasnik M A, Skelton N J, O'Connell M P, Kelley R F, Reilly D, Fairbrother W J. Biochemistry. 1996;35:15558–15569. doi: 10.1021/bi961409x. [DOI] [PubMed] [Google Scholar]
- 19.Laskowski R A, MacArthur M W, Moss D S, Thornton J M. J Appl Crystallogr. 1993;26:283–291. [Google Scholar]
- 20.Wells J A. Biochemistry. 1990;29:8509–8517. doi: 10.1021/bi00489a001. [DOI] [PubMed] [Google Scholar]
- 21.Latham P W. Nat Biotechnol. 1999;17:755–757. doi: 10.1038/11686. [DOI] [PubMed] [Google Scholar]
- 22.Garman S C, Wurzburg B A, Tarchevskaya S S, Kinet J P, Jardetzky T S. Nature (London) 2000;406:259–266. doi: 10.1038/35018500. [DOI] [PubMed] [Google Scholar]
- 23.Garman S C, Sechi S, Kinet J P, Jardetzky T S. J Mol Biol. 2001;311:1049–1062. doi: 10.1006/jmbi.2001.4929. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.