Abstract
The transcriptional properties of unliganded thyroid hormone receptors are thought to cause the misdevelopment during hypothyroidism of several functions essential for adult life. To specifically determine the role of unliganded thyroid hormone receptor α1 (TRα1) in neuronal tissues, we introduced a mutation into the mouse TRα1 gene that lowers affinity to thyroid hormone (TH) 10-fold. The resulting heterozygous mice exhibit several distinct neurological abnormalities: extreme anxiety, reduced recognition memory, and locomotor dysfunction. The anxiety and memory deficiencies were relieved by treatment with high levels of TH in adulthood, an effect that correlated with a normalization of GABAergic inhibitory interneurons in the hippocampal CA1 region. In contrast, a post-natal TH treatment was necessary and sufficient for ameliorating the adult locomotor dysfunction. Here, the hormone treatment normalized the otherwise delayed cerebellar development. The data thus identify two novel and distinct functions of an unliganded TRα1 during development and adulthood, respectively.
Keywords: Thyroid hormones, nuclear receptors, cerebellum, hippocampus, GABA
Thyroid hormone (TH) is required for development of normal brain function. The effects of TH are mediated by the nuclear hormone receptors, encoded by the thyroid hormone receptor (TR) α and β genes in vertebrates. Like many nuclear hormone receptors, TRs exert potent transcription regulatory functions by acting not only as ligand-receptor complexes but also as apo-receptors. In the absence of ligand, TRs recruit transcriptional corepressors such as N-CoR or SMRT, thereby suppressing the innate basal level of target gene expression (Glass and Rosenfeld 2000). The binding of ligand causes release of corepressor and subsequent recruitment of factors required for gene expression above basal levels.
In endemic cretinism, severe iodine deficiency during pregnancy results in insufficient supply of thyroid hormone to the fetus during critical stages of neuronal development. This, in turn, leads to irreversible motor dysfunctions combined with mental retardation (Delange 1996). Untreated congenital hypothyroidism, which affects neuronal events occurring later than those causing endemic cretinism, leads to delayed post-natal development along with reduced mental capability (Delange 1996). Whether these deficiencies are caused by insufficient activation of target genes or by outright transcriptional repression by apo-receptors has been unclear. Recent evidence, however, suggested that mice devoid of all thyroid hormone receptor genes exhibit far fewer features of hypothyroidism as compared with mice made congenitally hypothyroid by treatment with thyrostatic drugs (Gauthier et al. 1999; Göthe et al. 1999), thus implicating TR apo-receptors as a cause of the detrimental effects of hypothyroidism. Indeed, subsequent genetic analyses identified the TRα1 apo-receptor as a general contributor to delayed post-natal maturation (Flamant et al. 2002) and cerebellar development in particular (Morte et al. 2002).
More than 300 patient families suffering from the syndrome of Resistance to Thyroid Hormone (RTH) have been shown to have mutant TRβ genes that encode a receptor with reduced affinity to ligand and dominant-negative properties. These patients display only weak neurological symptoms, in agreement with the concept that TRα1 is more relevant in central nervous system maturation and function than TRβ. No patient with a germline mutation in TRα1 has been found, raising the possibility that TRα1 mutations are lethal. However, mutations in human TRβ found in RTH have been transferred to the mouse TRα1 gene, causing retarded postnatal development, bradycardia, metabolic dysfunctions, and inappropriately elevated serum TSH (Kaneshige et al. 2001; Tinnikov et al. 2002; Liu et al. 2003). Since ∼70%-80% of TR in neuronal tissues is TRα1 (Ercan-Fang et al. 1996), we have here addressed the function of this receptor in governing neuronal development and adult brain function. By using mice expressing a dominant-negative TRα1 with a 10-fold lower affinity to T3 (Tinnikov et al. 2002), we have identified two distinct effects of TRα1. Firstly, the mice present an unusually prominent anxiety, which required an adult, ongoing treatment with T3 for amelioration. This treatment also normalized the pattern of GABAergic inhibitory interneurons in the CA1 region of the hippocampus, suggesting that TRα1 exerts a novel, adult neurophysiological role. Secondly, the mice exhibit an adult locomotor dysfunction that correlates with a post-natal delay in cerebellar development. Juvenile but not adult treatment with high levels of T3 normalized the locomotor activity in the adult mouse as well as accelerated their post-natal cerebellar development.
Thus, the analysis of the mutant mice identified two operationally distinct functions of TRα1 in behavior, and correlated each of them to specific and separate developmental events in the brain.
Results
Exploratory activity and anxiety-related behavior
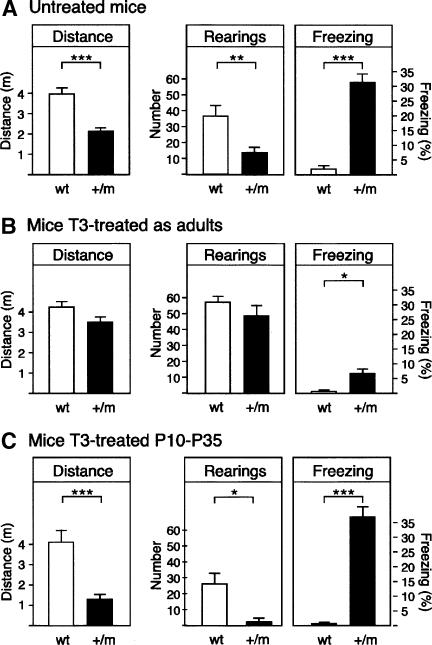
Since hypothyroidism in the adult is associated with mood disorders in man and elevated anxiety in the rat, we tested the hypothesis that a mutant TRα1 with a lower affinity to ligand would cause similar deficiencies in the mouse. For this we used knock-in mice harboring such an allele, the TRα1R384C mutant, that exerts a dominant-negative effect in heterozygotes (Tinnikov et al. 2002). Exploratory behavior of 12-14-wk-old male heterozygous mice (TRα1+/m) was examined in a novel environment, by subjecting them to the open field test. Figure 1A shows that 12-14-wk-old TRα1+/m mice exhibited a reduced exploratory activity in a brightly lit open field arena compared with wild-type mice, as indicated by a lower total distance traveled (horizontal activity; P < 0.001) and a reduced number of rearings (vertical activity; P < 0.01) during the testing session. Moreover, in contrast to wild-type mice, mutant mice exhibited a pronounced tendency for retropulsion (data not shown) and a strongly increased freezing behavior (31% vs. 1.8%) (Fig. 1A, right panel). Interestingly, the freezing behavior exhibited by mutant mice was accompanied by an enhanced sympathetic reactivity as indicated by the higher number of fecal boli compared with wild-type animals (6.2 vs. 2.1; P < 0.05). Given that the decrease in locomotion and the high freezing behavior that TRα1+/m mice displayed in the arena can be interpreted as an increase in anxiety-like behavior (Crawley and Paylor 1997), we decided to use a more specific test for evaluating anxiety-related behavior: the elevated plus maze.
Figure 1.
Exploration and anxiety as determined in the open field test. Total distance traveled, number of rearings, and percentage of freezing behavior were evaluated during 10 min in the open field. (A) Untreated TRα1+/m mice (n = 16) showed a lower exploratory activity and higher freezing behavior compared with wild-type control (n = 9) mice. (B) TRα1+/m (n = 7) mice treated with T3 as adults showed a normal exploratory behavior in the open field compared with wild-type mice (n = 9). (C) Injection of T3 during P10-P35 failed to normalize the exploratory behavior of TRα1+/m mice (n = 6 for both mutant and wild-type [wt] animals). All parameters were analyzed by means of a two-way (“genotype” × “T3 treatment”) ANOVA. Post hoc analysis showed that there was a statistically significant difference between mutant mice treated with T3 as adults and untreated mutant mice (all P < 0.05, Tukey's test). Data are presented as means ± SEMs; (★) P < 0.05; (★★) P < 0.01; (★★★) P < 0.001.
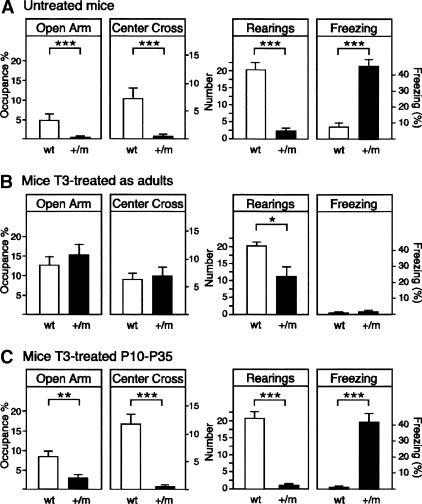
Figure 2A shows that TRα1+/m mice exhibited increased anxiety-like behavior in the elevated plus maze, as they spent less time in the open arms as compared with wild-type mice (P < 0.001). In addition, the mutant mice showed a lower number of center crossings (P < 0.0001) and rearings (P < 0.0001) than controls, indicating a reduced exploratory activity in this test. As previously observed in the open field, the mutant mice exhibited a high percentage of freezing behavior (43.9% vs. 5.5%; P < 0.0001) during the exposure to the elevated plus maze.
Figure 2.
Anxiety-like behavior in the elevated plus maze test. (A) Untreated TRα1 mutant mice (n = 16) showed an increased anxiety behavior compared with wild-type controls (n = 9) mice. (B) TRα1+/m (n = 7) mice treated with T3 as adults significantly ameliorated anxiety-like behavior in the elevated plus maze compared with wild-type mice (n = 9). (C) Injection of T3 during P10-P35 did not alleviate anxiety-like behavior of TRα1+/m (n = 6) compared with wild-type (n = 6) mice. Data were analyzed by means of a two-way (“genotype” × “T3 treatment”) ANOVA. Post hoc analysis showed that there was a statistically significant difference in the measured variables between mutant mice treated with T3 as adults and untreated mutant mice (all P < 0.01, Tukey's test). Data are presented as means ± SEMs; (★) P < 0.05; (★★) P < 0.01; (★★★) P < 0.001.
To investigate if high TH levels could restore the behavioral alterations observed in adult TRα1+/m mice, another group of animals was treated with hormone by addition of T3 to the drinking water of adult mice for 12 d, or by injecting juvenile mice during their post-natal development (post-natal days 10-35 [P10-P35]). The juvenile mice were then tested when adult. Figure 1B (left and center panels) shows that T3 treatment during adulthood abolished the differences between wild-type and mutant mice in exploratory activity of the open field test. Two-way ANOVA revealed a significant interaction between “genotype” and “treatment” for freezing behavior in the open field test [F(2,46) = 3.93; P < 0.05], but not for distance traveled [F(2,46) = 1.86; P = 0.13] or rearing behavior [F(2,46) = 0.01; P = 0.99]. A post hoc analysis indicated that freezing levels were still higher in T3-mutant mice treated as adults compared with corresponding T3-treated wild-type mice (Fig. 1B, right panel). However, this treatment significantly attenuated freezing levels in mutant mice as compared with untreated mutant mice (P < 0.0001, Tukey's test). In the elevated plus maze, T3 treatment during adulthood also normalized several behavioral parameters (percentage of time in the open arms of the maze, center crossing, and freezing) in mutant mice, although significant differences were still observed in the number of rearings (P < 0.05, Tukey's test) (Fig. 2B). In contrast, the T3 treatment during P10-P35 failed to ameliorate any of the behavioral alterations (exploratory activity and anxiety) described in untreated mutant mice (Figs. 1C, 2C). We conclude that the increased anxiety is readily reduced by T3 only in the adult animal.
Novel object recognition task
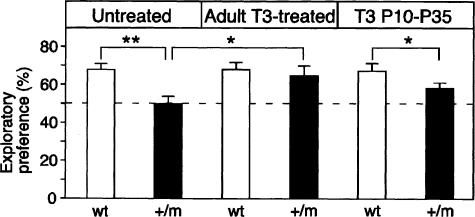
We used the novel object recognition task (Ennaceur and Delacour 1988) to measure visual recognition memory, a model of declarative memory that depends on hippocampal function (Myhrer 1989; Pittenger et al. 2002). This task takes advantage of a rodent's natural tendency to explore novel objects. Thus, no negative or positive reinforcers need to be applied that may interfere with the memory performance (e.g., swimming-induced stress in the Morris water maze). In addition, locomotor ability is not a critical factor in this task, in contrast to other hippocampal-dependent tasks like the Morris water maze. During training there was no significant difference between genotypes in the exploratory preference of the novel objects (data not shown). At the retention test (1 d later), one of the familiar objects used in the training session was replaced with a novel object, and the animals' behavior was videotaped during 5 min for later analysis. As indicated in Figure 3, marked differences were found in the response of mutant and wild-type animals to the presentation of a novel object. Two-way ANOVA indicated a significant “genotype” effect in the preference for the novel object, [F(1,42) = 11.09, P < 0.002], but not a significant “genotype” × “treatment” interaction [F(2,42) = 1.82, P = 0.17, n.s.]. A post hoc analysis using Dunnett's test revealed a significant difference in the object recognition test between untreated mutant and wild-type mice (P < 0.01). Moreover, mutant mice did not exhibit novel object preference during the test session (49.7%), since the discrimination index did not differ from the chance level [t(11) = 0.88; P = 0.98, n.s.]. Interestingly, mutant mice that were treated with T3 during adulthood showed a clear exploratory preference for the novel object (64.8%) similar to that seen with the wild-type controls (68.0%, P = 0.53), and which was significantly higher than that observed with untreated mutant mice (P < 0.05). In contrast, mutant mice treated with T3 as juveniles still showed a lower object recognition memory index (58.1%) compared with wild-type mice (67.3%; P < 0.05).
Figure 3.
Normalization of visual recognition memory by adult T3 treatment as measured by the novel object recognition task. TRα1 mutant mice (n = 12) showed a reduced exploratory preference in the object recognition task compared with wild-type control mice (n = 9). Mutant untreated mice displayed a memory impairment in this task since the discrimination ratio did not differ from the chance level [t(11) = 12.7, P < 0.0001]. TRα1+/m (n = 5) mice treated with T3 as adults showed a similar preference for the new object as wild-type treated mice (n = 8) and performed significantly better in this task than mutant untreated mice. Injection of T3 during P10-P35 failed to improve object recognition memory in TRα1+/m (n = 8) compared with wild-type (n = 6) mice. The dashed line represents equal exploration of the novel and familiar objects (50%). Data were analyzed by means of a two-way (“genotype” × “T3 treatment”) ANOVA. Data are presented as means ± SEMs; (★) P < 0.05; (★★) P < 0.01; (★★★) P < 0.001.
Alterations in hippocampal GABAergic interneurons
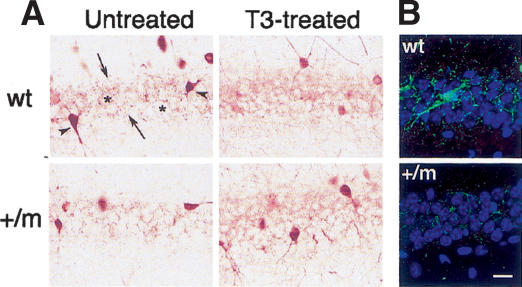
Previous analyses showed that mice lacking the TRα1 gene (TRα1-/- mice) showed anxiety in the open field and enhanced fear in the contextual fear conditioning test. These mice also presented a reduction in the density of GABAergic terminals from interneurons of the CA1 region in the hippocampus (Guadaño-Ferraz et al. 2003). We therefore examined the TRα1+/m mice for the presence of similar alterations in inhibitory cortical circuits by analyzing the immunostaining patterns for parvalbumin (PV). PV is a Ca2+-binding protein that labels the subpopulation of GABAergic interneurons (chandelier and basket cells) (Celio et al. 1986) representing the most powerful inhibitory neurons of the cerebral cortex. In the hippocampus they are the main cells implicated in perisomatic control of pyramidal cells, and their somata are mainly localized in the strata pyramidale and oriens (Freund and Buzsaki 1996; DeFelipe 1999). Figure 4A shows a comparison of immunohistochemical staining between normal and mutant animals, and the result of T3 treatment. In the untreated wild-type animals, PV-immunoreactive neuronal bodies were clearly seen (arrowheads). The axon terminals of these cells were also stained, resulting in a punctate appearance, ending on the cell bodies of pyramidal neurons, which delimit a clear, stain-free region (Fig. 4A, asterisks). Expression of the mutant receptor was associated with a lower number of PV-immunoreactive neurons as shown in Table 1. The mutant mice displayed a 40%-50% reduction in the number of PV-positive cell bodies. In addition, they showed a significantly lower immunohistochemical staining (Fig. 4A; Table 1), indicating a decreased density of terminals and therefore a reduced inhibitory synaptic innervation of pyramidal cells. A similar pattern was found using immunofluorescence, as illustrated in Figure 4B. Adult treatment of the mice for 12 d with high levels of T3 in the drinking water prior to analysis normalized the number of cells and the staining pattern (Fig. 4A; Table 1).
Figure 4.
Reduced number of PV-ir terminals in the CA1 region in the mutant mice. This figure shows sections of the dorsal hippocampus of wild-type and mutant mice immunostained for PV and developed by the peroxidase method (A) or by immunofluorescence (B). The density of PV-ir perisomatic terminals is lower in the untreated mutant mice (+/-) than in the wild-type mice (wt). T3 treatment did not modify the wild-type pattern, whereas it increased the mutant mice pattern as shown in A. In B, the same difference in the density of terminals, as revealed by immunofluorescence, is observed between the untreated wild-type and mutant mice.
Table 1.
Number of parvalbumin-positive cells and density of parvalbumin-positive terminals in t he hippocampus
| PV+ neuronsa
|
PV+ terminal density (arbitrary units)
|
||
|---|---|---|---|
| Mice | Experiment 1 | Experiment 2 | |
| Wild type | 37 ± 4 (n = 2) | 24 ± 0 (n = 2) | 1.94 ± 0.03 (n = 3) |
| Wild type | |||
| + T3 | 40 ± 1 (n = 3) | 26 ± 4 (n = 3) | 1.76 ± 0.16 (n = 3) |
| +/m | 22 ± 4 (n = 4)b | 13 ± 2 (n = 3)b | 1.25 ± 0.09 (n = 3)b |
| +/m + T3 | 39 ± 4 (n = 3) | 25 ± 2 (n = 3) | 1.69 ± 0.09 (n = 3) |
PV+ neurons: The number of parvalbumin-positive cell somata was counted in the CA1 region of the hippocampus, in two different experiments. PV+ terminal density in the pyramidal layer was measured as indicated in Materials and Methods. The number of animals used for each measurement is indicated.
ANOVA indicated that the differences were significant when comparing the data from the +/m mice to the untreated wild-type or T3-treated mice. There were no differences when wild-type, wild-type + T3, or +/m + T3 mice were compared with one another.
The lower density of PV terminals was probably due to the lower number of PV-immunoreactive neurons, although an effect of the mutant receptor on PV expression cannot be discarded. Quantification of the total number of GABAergic cells in the CA1 region, after in situ hybridization with digoxigenin-labeled probes for the two isoforms of glutamic acid decarboxylase (data not shown), indicated a reduction in the number of cells in the mutant mice (108 ± 5) as compared with the wild-type mice (118 ± 3, P < 0.05). The experiments thus indicate that the high anxiety and the reduced exploratory behavior correlate with an adult, neurophysiological effect imposed by the mutant receptor.
Impaired locomotor activity
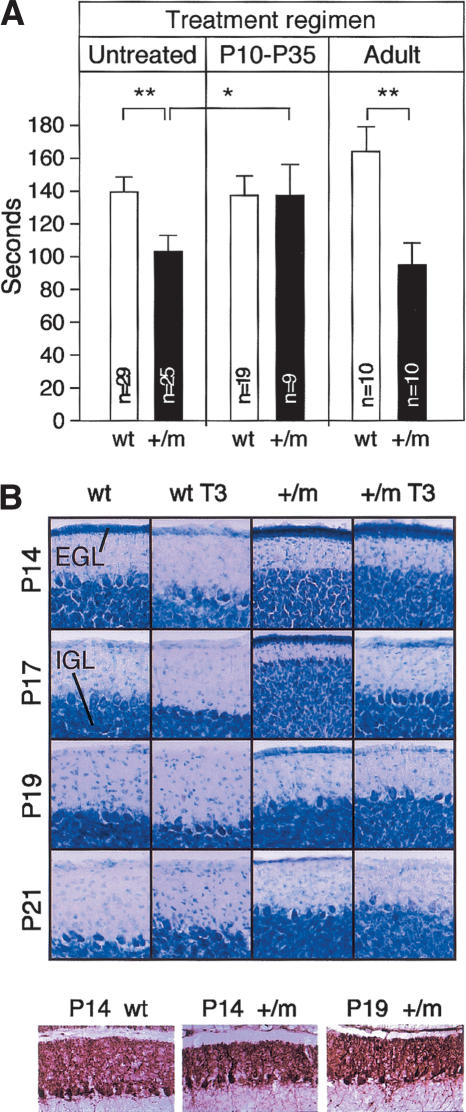
Since congenital hypothyroidism in rodents is well known to cause cerebellar misdevelopment and impaired locomotor activity, we tested the hypothesis that the mutant TRα1 would cause similar deficiencies. Male mice, 12-14 wk old, were trained on the Rotarod and then tested for their ability to stay on the accelerating rod. Figure 5A (left panel) shows that the mutant male animals (TRα1+/m) had a significantly reduced capacity. No difference was seen between wild-type and mutant female mice (data not shown).
Figure 5.
Locomotor dysfunction and its amelioration by T3. (A) Littermate wild-type (wt) and heterozygous mutant (+/m) mice, 12-14 wk old, were trained on the Rotarod, whereafter the time until they fall off the accelerating rod was determined. The left section shows that mutant mice were less capable than wild-type controls, the middle section demonstrates that injection of T3 during P10-P35 resulted in complete normalization of the adult mutant mice, and the right section illustrates the inefficacy of an adult, ongoing T3 treatment to improve performance. The number of animals tested is shown in each bar. Pairwise t-tests were used for determination of significance. (★) P < 0.05; (★★) P < 0.005. (B) The ability of post-natal injections to accelerate cerebellar development. Littermate wild-type or mutant mice were injected daily with T3, starting at P10. Three mice were analyzed for each panel. (Upper section) Lobes IIX of the cerebella were sectioned and stained with Nissl. Representative sections showing the external granular, molecular, and internal granular layers are shown. (Lower section) Cerebellar Purkinje cells of the TRα1+/m mice show a delayed but otherwise normal arborization.
The TH-sensitive period of cerebellar development occurs mainly post-natally in the mouse. Since the mutant receptor used had a reduced but not abolished affinity for T3, we tested if post-natal injections of TH would rescue normal locomotor ability. Accordingly, 10-d-old pups were injected i.p. daily with a 1:10 mixture of T3 and T4 (25 and 250 μg/kg body weight, respectively) for 25 d (P10-P35), whereafter the injections were stopped. Hormone injections rapidly but transiently increase thyroid hormones in serum to very high levels (Wikström et al. 1998) and result in transcriptional regulation of target genes also by the mutant TRα1 (Tinnikov et al. 2002). However, the injection regimen used here allows the free serum hormone levels to decrease to near normal after 24 h in young mice (data not shown). The proficiency on the Rotarod was determined when the mice were 12-14 wk old. The results show (Fig. 5A, middle panel) that such a treatment fully restored locomotor ability. In contrast, treatment only during adulthood by addition of T3 to the drinking water (0.5 μg/mL) for 12 d and during the test failed to enhance the locomotor ability of the mutant mice (Fig. 5A, right panel).
The data above indicated that the mutant TR caused deficiencies similar to those seen in congenital hypothyroidism. Histological analyses of the adult mutant cerebellum revealed no obvious abnormalities (data not shown). However, as hypothyroidism causes a transient delay in the post-natal migration of cerebellar external granular (EGL) cells to the internal granular layer (IGL), we compared the thickness of the developing EGL of wild-type and mutant mice between P14 and P21 as well as monitored the ability of injected TH to accelerate the process. Figure 5B, upper panels, shows that among the untreated animals, the mutant mice had a delay of 4-6d. Hormone treatment by daily injections of TH accelerated development in both animal groups, suggesting that the mutant TRα1 is activated by the higher hormone levels. Although a minor delay in Purkinje cell development was observed in the mutant mice (Fig. 5B, lower panel), no major perturbations were seen in the cellular arborization, as expected since these cells are known to express mainly TRβ (Strait et al. 1991; Bradley et al. 1992). Taken together, the data suggest that the dominant-negative TRα1 confers both a morphological and a functional impairment to the cerebellum, which, however, could be circumvented by an appropriately timed hormone treatment.
Effects of hormone treatment on tissues and target genes in adult mice
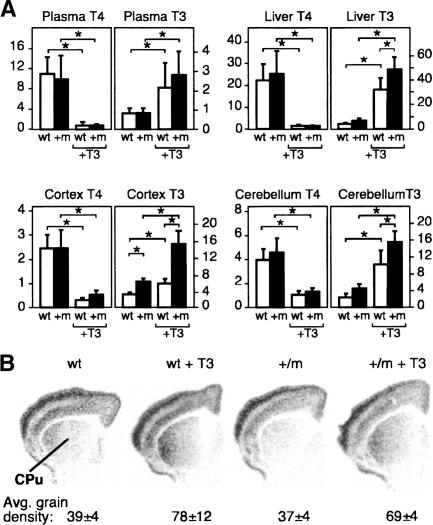
Since TH treatments dramatically improved the performance of the TRα1+/m mice, we aimed to confirm that the administration of T3 to adult animals appropriately increased serum and tissue levels of the hormone. Adult mice having received T3 for 12 d in the drinking water experienced sharply decreased plasma total T4 levels as expected (Fig. 6A). The plasma T3 levels were increased about threefold in both wild-type and the mutant mice (Fig. 6A). The tissue concentrations (nanograms per gram wet weight), however, showed greater increases in total T3, ranging from seven- to eightfold in the livers and about fourfold in the cerebella, to twofold in the cortices (Fig. 6A). The reductions seen in the tissue concentrations of T4 in all instances paralleled those seen for plasma. The data thus confirm that the TH treatment significantly increased plasma and tissue concentrations of T3.
Figure 6.
Effect of hormone administration to adult mice on tissue levels of T3 and on the target gene RC3. (A) Plasma and tissue concentrations of T4 and T3 in untreated and T3-treated wild-type and mutant mice. The data show concentrations of T4 and T3 in nanograms per deciliter, as measured by radioimmunoassay in whole plasma or tissue extracts as described in Materials and Methods. Differences between groups were analyzed by two-way ANOVA with the following results: (Plasma T4) F(3,38) = 26.877, P < 0.001; (Plasma T3) F(3,38) = 6.651, P < 0.001; (Liver T4) F(3,47) = 56.594, P < 0.001; (Liver T3) F(3,52) = 103.43, P < 0.001; (Cortex T4) F(3,25) = 54.666, P < 0.001; (Cortex T3) F(3,26) = 63.514, P < 0.001; (Cerebellum T4) F(3,27) = 50,68, P < 0.001; (Cerebellum T3) F(3,27) = 47.506, P < 0.001. (B) Expression of the thyroid hormone-regulated gene RC3 in adult wild-type and mutant mice, treated and untreated with T3. The data were quantified by measuring the optical density of autoradiograms in the caudate nucleus (CPu). Three wild-type and mutant mice, treated or not with T3, were used for quantification, and the mean values ± SD are shown as average grain density in arbitrary units. The data were analyzed by one-way ANOVA: F(3,6) = 4.94, P < 0.05. Post hoc analysis showed that there was a statistically significant difference (P < 0.05) in the treated wild-type or mutant mice compared with the untreated mice.
As an indication that the increased tissue concentrations of T3 were translated into a biological response at the genomic level, we analyzed the expression of a thyroid-hormone-regulated gene. RC3 is one of the few known thyroid-hormone-sensitive target genes in the adult brain and is regulated by T3, especially in the caudate nucleus. In addition, it is selectively induced by T3 through TRα1 (Manzano et al. 2003). RC3, therefore, is a good genomic target to verify that T3, by raised tissue concentrations, could act through the mutant TRα1. Tissues from adult mutant and wild-type mice that had been treated with TH in the drinking water for 12 d were analyzed for RC3 expression by in situ hybridization and compared with untreated controls. Figure 6B shows representative autoradiographs of brain slices at the level of the caudate nucleus (CPu). Expression in the caudate was increased by T3 treatment both in the wild-type and in the mutant animals, indicating that the treatment regimen was efficacious.
Discussion
Our findings provide the first data on the specific effect of a dominant-negative TRα1 on brain function and structure. In addition to the general developmental delay previously described, the mice harboring the mutant TRα1 exhibit a phenotype of CNS damage that resembles the behavioral and structural features of hypothyroidism. Most importantly, the serum and tissue concentrations of T4 and T3 were normal, and the deficiencies were reduced or abolished by appropriately timed treatments with high doses of thyroid hormone. This argues that the mutant receptor, having a reduced affinity to T3, caused the abnormalities by acting as an unliganded TR (apo-receptor) and that ligand, when present at sufficiently high levels, bound the mutant TR to allow normal signaling to occur. By correlating the properties specific to the mutant TRα1 with defects in behavior and locomotion that were ameliorated by hormone, the data in addition identify previously unclear roles of thyroid hormones in development and CNS function in the adult.
Our data suggest that the dominant-negative TRα1 causes two distinct major insults: a delayed cerebellar development, resulting in adult cerebellar locomotor dysfunction that is preventable by T3 treatment during the period of cerebellar development; and another, a reduced number of GABAergic interneurons and decreased density of GABAergic terminals in the CA1 region of the hippocampus, which presents as increased anxiety and reduced cognition. In contrast to cerebellar morphological changes, alterations in hippocampal GABAergic interneurons could be normalized by the adult but not the juvenile T3 treatment.
Previously, mice with a dominant-negative TRβ allele were described (Hashimoto et al. 2001). However, these data and their interpretations are different from those described above. TRα1 and TRβ are known to be expressed at very different levels and in distinct regions in the brain. The severe locomotor deficiencies reported by Hashimoto et al. (2001) correlated with a delayed development of cerebellar Purkinje cells, a tissue known to express TRβ and little if any TRα1. Furthermore, the mutant TRβ used binds no hormone, and as this isoform is critical for the pituitary-thyroid axis, the mice tested had severalfold elevated serum levels of TH. Identification of the developmental time for the insults and a study of the effects of hormone acting via TRα1 or TRβ were therefore not possible. The authors also mention that the memory impairments reported could not be un-ambiguously ascribed to hippocampal or cortical activities of the mutant TRβ as the Morris water maze test used is also sensitive to deficiencies in locomotor function.
Locomotor dysfunctions
The male TRα1+/m mice exhibited a significant reduction in motor coordination as assessed by the Rotarod test, whereas females were not affected. The reason for the sex difference is unclear, but could be related to differences in the effects of sex hormones on neuronal locomotor functions or be due to differences in body weight, reflecting muscle strength. For example, estradiol reduces neuronal damage during ethanol withdrawal syndrome (Rewal et al. 2003). Although TRα1 has been ascribed important roles in muscle function (Johansson et al. 2000), we consider this possibility unlikely since determination of soleus and edl muscle function in explanted tissues failed to reveal major functional dysfunctions (H. Westerblad and B. Vennström, unpubl.). The impaired locomotor activity on the Rotarod was ameliorated by a juvenile TH but not an adult treatment with TH. This suggests that a major part of the insult imposed by the mutant receptor resides in the cerebellum, since the time for the post-natal treatment coincided with the known TH-dependent period of cerebellar development. This is further supported by the observation that the maturation of the external granular layer was delayed in the mutant mice but accelerated by TH, which is in congruence with our previous demonstration that unliganded TRα1 selectively impairs the development of this cell type (Morte et al. 2002). In contrast to these alterations, only minor effects were seen on the cerebellar Purkinje cells: The developing TRα1+/- mice exhibited marginally shorter dendrites but normal arborization. Although hypothyroidism normally leads to shortening of dendritic fibers and markedly less arborization, our data are fully compatible with the delayed overall maturation of 4-6 d seen with these animals, especially as Purkinje cells are known to express TRβ and not TRα1 (Strait et al. 1991). Taken together, our data implicate the cerebellum as the target for an insult by the mutant TRα1 that caused the locomotor deficiency revealed by the Rotarod. This conclusion is further supported by the observations that defects in locomotion and balance are associated with cerebellar or basal ganglia damage, as described for many mouse strains with specific genetic alterations (Lalonde et al. 1995).
We have also detected many additional parameters of locomotor deficiencies in the mutant mice (M. Sjögren and B. Vennström, unpubl.). Here, the developmental insult(s) occur invariably during late embryonal/early fetal development and, as with the Rotarod parameter, affect male mice significantly more than female. The underlying tissue defect(s) are as yet unidentified.
Altered exploration and increased anxiety
The open field task was used to test for differences in the exploratory activity of the animals in a novel environment, another parameter known to be affected by thyroid hormone disorders in rodents (Eayrs 1966). Mutant TRα1 mice displayed a low activity exploration compared with wild-type mice. This could be influenced by the altered locomotor coordination that these mice present, as previously evidenced in the Rotarod test (Fig. 5A), and/or by a high anxiety-like behavior in a novel environment (Crawley and Paylor 1997). The former possibility is unlikely since adult mutant mice treated with TH did not show significant differences in the total distance traveled or number of rearings in the open field when compared with controls (Fig. 1B), in contrast to the deficit in locomotor coordination on the Rotarod (Fig. 5A). Therefore, the reduced exploratory activity displayed by the mutant mice on the open field and the elevated plus maze is likely to be a consequence of a high anxiety behavior. This possibility is supported by the fact that, during open field (Fig. 1A) and elevated plus maze sessions (Fig. 2A), TRα1+/m mice displayed high levels of freezing behavior accompanied by an increased defecation, a feature that is associated with high anxiety behavior (Gray 1971). Importantly, most of these altered behavioral responses displayed by mutant mice were normalized or ameliorated by a TH supplement to adult but not juvenile animals (cf. Figs. 1B, 5B,C). In conclusion, our results provide novel evidence linking genetic variation in TRα1 with cognitive and emotional processes.
Hippocampal abnormalities
The observation that only the adult treatment with TH ameliorated the anxiety identifies the dysfunction as one caused by the action of the mutant receptor in the adult brain, in contrast to the developmental effect on locomotor activity. Furthermore, the results of the open field and elevated plus maze tests indicated that the defect could be located at the level of the hippocampus, similar to that previously seen with mice lacking TRα1 (Guadaño-Ferraz et al. 2003). Other brain areas, particularly the amygdala, play important roles in anxiety- and fear-related behaviors (LeDoux 2000). Although at present we cannot discard a defect at the level of the amygdala, recent neuroanatomical studies suggest that the hippocampus may have a preferential role in brain processes associated with anxiety-related behaviors, while the amygdala would be associated specifically with fear (for review, see Bannerman et al. 2004). Indeed, the TRα1+/m mice showed a prominent and selective reduction of perisomatic terminals expressing the Ca2+-binding protein PV in the CA1 field of the hippocampus, suggesting that TH affects the specification of inhibitory hippocampal circuitry via TRα1. This is supported by our previous demonstration (Guadaño-Ferraz et al. 2003) that PV-immunoreactive interneurons in the hippocampus express predominantly TRα1 over TRβ. PV immunohistochemistry in the hippocampus selectively labels the cell bodies and axons of basket and chandelier cells (Sik et al. 1995; Freund and Buzsaki 1996), the most powerful inhibitory cells involved in regulation of efferent activity (Miles et al. 1996). Our results indicate that these inhibitory interneurons are affected in the mutant mice. It is thus possible that a decreased GABAergic inhibition could lead to hippocampal hyperactivity, resulting in the increased emotionality and anxiety observed in the mutant mice. In support of this interpretation, it is noteworthy that mice with a disrupted gene for urokinase plasminogen activator receptor show increased anxiety probably as a consequence of diminished interneuronal activity (Powell et al. 2003): These features are accompanied by a near complete loss of parvalbumin-positive subtype neurons with little or no effects on other interneuronal subtypes. Our data do not allow discriminating between a decrease in the number of cells or a decrease in PV expression leading to fewer PV-immunoreactive neurons, although the whole population of glutamic acid decarboxylase-expressing cells is reduced in the CA1 field of the mutant mice.
The high anxiety/low exploration represents novel effects of a dominant-negative TRα1, especially as it correlated with abnormalities in the hippocampal GABAergic neurons involved in cognitive functions. The hippocampus is well known to play a role in emotion and fear- or anxiety-related behavior (LeDoux 2000; McHugh et al. 2004). A hippocampal hyperfunction has been suggested to lead to anxiety because of a greatly increased perception of threat in situations involving conflicting stimuli or response alternatives (McNaughton 1997). This raises the possibility that the reduced GABAergic input observed in TRα1+/m mice may be caused by reduced tonic inhibition in the hippocampus. This could contribute not only to the development of core behavioral symptoms of mood disorders, but also include deficits in novel object recognition memory that depend on the hippocampus.
Object recognition impairment
Object recognition memory is a model of declarative memory in which the hippocampus and parahippocampal regions of the temporal lobe have been implicated (Clark et al. 2000). In our study, mutant mice showed a deficit in visual recognition memory accompanied by an altered inhibitory input in the CA1 area of the hippocampus. Interestingly, this area has been indicated to play a crucial role in long-term object recognition memory since inactivation of this region impairs recognition memory in this task after a 24-h retention interval (Hammond et al. 2004). Moreover, a similar deficit was found in mice with a region-specific knockout of NMDA receptor 1 subunits in the CA1 (Rampon et al. 2000), while transgenic mice with forebrain NMDA receptor 2B subunit overexpression exhibit enhanced object recognition memory (Tang et al. 1999). As cognitive and morphological alterations in hippocampus observed in TRα1+/m mice were normalized when animals were treated with T3 during adulthood, it is reasonable to suggest that, by regulating the GABAergic input in the CA1 area, TRα1 plays a critical role for hippocampal-dependent memory.
Tissue hormone levels
The mutant TRα1 has previously been shown to be activated by high levels of T3 to increase expression of target genes, and to restore normal growth (Tinnikov et al. 2002). The present results of thyroid hormone determinations in the different tissues of mutant animals show that the behavioral alterations are due to the dominant-negative action of the mutant receptor and not decreased hormone concentrations leading to hypothyroidism. Administration of T3 led to a significant decrease of plasma and tissue T4, as a result of pituitary TSH inhibition, in both wild-type and mutant mice, and to an increase of tissue T3 compatible with activation of the mutant receptor. T3 increased similarly in plasma and in tissues in both types of animals, but the increase in tissues of the mutant mice was significantly larger than in the wild-type mice. The reason for this is unknown, but may be caused by a lack of comparable induction, in wild-type and mutant animals, of type 3 deiodinase, an enzyme that degrades T4 and T3 in tissues and that is under thyroid hormone control.
Treatment of the mutant mice with T3 increased the tissue concentrations to levels compatible with activation of the receptor and accelerated cerebellar development (Fig. 5). Locomotor dysfunction is known to result from TH deficiency during the known period of TH dependency for cerebellar development (Legrand 1984). Mice lacking TRα1 expression exhibit no deficiency in the Rotarod locomotor test (B. Vennström, unpubl.), and our results therefore indicate (1) that the apparent hypothyroid feature is not caused by TH deficiency, and (2) that the dysfunction results from a failure of the dominant-negative TRα1 to act upon genes involved in developing locomotor function, that is, that the mutation causes a receptor-mediated, hypothyroid effect.
Possible relation to human disease
In patients, dominant-negative mutations in TRβ cause the RTH syndrome, which is associated with pituitary resistance leading to elevated serum levels of thyroid hormones and goiter. Human syndromes caused by dominant-negative TRα1 have not yet been detected. Some reports have found no mutations in TRα1 despite direct scanning for mutations in the TRα1 gene that could be associated with different psychiatric syndromes (Feng et al. 2001). Despite this, we believe that our results raise the possibility that dominant-negative mutations in TRα1 in humans may have a correlate in behavior, as in the mutant mice. RTH mutations involving TRβ occur at a rate of 1/50,000 births, and despite the often considerable consequences for TH homeostasis, heart rate, and metabolism, most of these patients appear at the clinic while presenting a goiter (Refetoff et al. 1993). Mice with the dominant-negative TRα1 have normal TH levels, and association of any neurological disorder caused by such a receptor may not be easily recognized in a clinical setting. Interestingly, a subset of patients with rapidly cycling polar disorder is refractory to conventional treatment, unless treated with supraphysiological doses of thyroid hormone (Bauer et al. 2003). These patients show normal heart rate and no signs of osteoporosis despite months of hormone treatment, which has led to the notion that they exhibit a resistance to thyroid hormone (Bauer et al. 2004). The action of thyroid hormone on these two tissues is mediated through TRα1, raising the intriguing possibility that the resistance of the psychiatric disease to conventional treatment may involve TRα1. Treatment of the mutant mice with thyroid hormone improves not only growth, but also the locomotor dysfunction, the elevated anxiety, and the recognition memory impairment. Therefore our studies suggest that if the consequences of a mutant receptor are carefully identified, the corresponding patients can be properly diagnosed and hormone therapies developed.
Materials and methods
Animals
The knock-in mouse strain carrying the dominant-negative R384C mutation in TRα1 was previously described (Tinnikov et al. 2002). In short, the mutation was introduced in embryonic stem (ES) cells (derived from 128/Ola), and the founder mice were crossed to C57Bl/CR for at least three generations. Animals were kept on a 12-h dark-light cycle under controlled humidity and temperature conditions, and had free access to water and food. For experiments, wild-type female mice were mated with heterozygous males of the same genetic background, yielding ∼50% of wild-type and mutant offspring. Wild-type mice were weaned at 3 wk of age, whereas mutants, due to their delayed post-natal development (Tinnikov et al. 2002), were weaned at 5 wk. Littermate wild-type and mutant male mice, 3-4 mo old, were used for all experiments unless stated otherwise. All mice used were genotyped by PCR. Animal care procedures were conducted in accordance with the guidelines set by the European Community Council Directives (86/609/EEC). Necessary animal permissions were obtained from the appropriate local ethical committees.
Treatment of adult mice with thyroid hormone was done by adding T3 to the drinking water, containing 0.01% albumin to stabilize the hormone, at a final concentration of 0.5 μg/mL. This solution was administered to the mice 12 d before onset of behavioral studies and was continued during the test periods. Juvenile T3 treatment was done by injecting a mixture of T3 and T4 daily (T3: 25 μg/kg body weight; T4: 250 μg/kg) starting at P10 and ending at P35.
Behavioral tests
Mice used in open field or elevated plus maze tests (Current Protocols in Neuroscience 2003) were individually housed for 3 wk before analysis. They were weighed once a week and handled daily for 4 d before the experiment to allow habituation to experimental manipulations. The experiments were done between 08.00 and 14.30.
A circular open field of 140 cm diameter and 32 cm height was used. Each mouse was transferred to the center of the field, and its behavior and locomotor activity were monitored for 5 min using a video camera and computerized system (Ethovision 1.90, Noldus IT).
The elevated plus maze apparatus consisted of two opposing open arms (30 × 5 cm) and two opposing enclosed arms (30 × 5 × 15 cm) with an open roof, connected by a central square (5 × 5 cm). The maze was elevated 38 cm above floor level. The mouse was placed on the center square facing an open arm and allowed to freely explore the apparatus for 5 min. The apparatus was cleaned with 0.1% acetic acid solution between subjects. Behavior was scored using a video camera and computerized system (Ethovision 1.90, Noldus IT). Behaviors scored were open and closed arm entries (an arm entry was defined as all four paws into an arm) and the time spent in the open arms.
Novel object recognition task
For the novel object recognition task, mice were individually familiarized to an open-field box (35 × 35 × 15 cm) with sample objects, in order to reduce the anxiety-related behavior that mutant mice exhibit in a novel environment. The mice received one sample-exposure session per day for three consecutive days. In these sessions, the mouse was placed into the area with two copies of the sample and left to explore for 5 min before it was returned to its home cage. The light intensity was equal in the different parts of the apparatus (∼50 lx). During training sessions, two novel objects (small plastic toys (10 × 15 cm) were placed in a symmetrical position ∼5 cm away from walls, and the animal was allowed to explore for 5 min. The objects were always thoroughly cleaned between sessions using a 0.1% acetic acid solution. A preference index, a ratio of the amount of time spent exploring any of the two objects (training session) or the novel object (retention session) over the total time spent exploring both objects, was used to measure recognition memory. Exploration was defined as follows: directing the nose to the object at a distance of no more than 2 cm and/or touching the object with the nose. Novel and familiar object location was counterbalanced across animals.
Rotarod tests
Mice were housed three to five together from weaning. Twelve- to 14-mo-old mice were trained for three consecutive days by allowing them to run three times daily on the accelerating rod. Testing was done on the fourth and fifth days (three runs each day), and the time an individual animal stayed on the rod was averaged. The animals had been habituated to the experimental environment and personnel prior to testing. The Rotarod was purchased from Panlab SL.
Hormone assays
Thyroid hormone concentrations in plasma, liver, cerebral cortex, and cerebellum were determined by radioimmunoassay using whole plasma or tissue extracts as described (Escobar-Morreale et al. 1996).
Tissue analyses
Mice to be used for histological analyses were anesthetized by intraperitoneal injection of a mixture of ketamine (4 mg/100 g body weight) and metedomidine (15 mg/100 g body weight) or with Avertin. The mice were perfused transcardially with 4% paraformaldehyde in 0.1 M phosphate buffer (PB) at pH 7.4. Analyses of cerebellar structures were done as previously described (Morte et al. 2002). For analyses of hippocampal structures, the brains were removed, post-fixed overnight in the same solution, and dissected across the midline for analysis of hippocampal structures. The left hemibrain was processed for in situ hybridization and the right for immunohistochemistry. A total of four wild-type and TRα1+/m and three wild-type and T3-treated TRα1+/m were used. The hemibrains were cut serially at 50 μm on a vibratome in the coronal plane. Sections were pre-treated with a solution of 10% methanol and 3% hydrogen peroxide (Merk, 107210) in PB to remove endogenous peroxidase activity, then preincubated in a blocking solution (3% normal horse serum [Vector Laboratories, S-2000], 0.1% Triton X-100 [Merk, 86031000], and 4% BSA in PB) for 2 h at room temperature. Sections were then incubated overnight at 4°C in the above solution containing primary monoclonal mouse anti-PV (1/4000; Sigma, C-8666). The sections were subsequently washed in PB, incubated in a species-specific biotinylated antibody (Vector Laboratories, BA-2020), diluted 1:200 in PB for 1 h at room temperature, and processed by the avidin-biotin-peroxidase method. Sections were mounted on glass slides, dehydrated, cleared in xylene, and coverslipped. For microscopy we used a Nikon Elite optical microscope equipped with a Nikon dn 100 digital camera. Immunofluorescence was carried out using streptavidin Alexa Fluor 488 after incubation with biotinylated secondary antibody, and the sections were counterstained with DAPI. For microscopy we used a Leica TCS SP2 confocal microscope (Leica Microsystems). Comparisons between animals were made using sections incubated simultaneously in the same well.
Counts of PV- or glutamic acid decarboxylase (GAD)-labeled somata were performed in the CA1 field of the dorsal hippocampus. All strata from stratum oriens to stratum lacunosum-moleculare were included in the analysis. The total number of cells in three areas of 0.275 mm2 of each section was counted directly from light microscope slides using the same counting frame. Two to five sections from each animal were analyzed in this way.
Quantitative measurements of the density of PV immunoreactive terminals in the hippocampal CA1 region were perfomed by using the Neurolucida software (MicroBrightField, Inc.). Three animals per group were analyzed. From each individual animal, B/W images were captured from five randomly selected microscope fields from the stratum pyramidale at high magnification (100×). Similar measurements were made in the white matter for background subtraction. The cell bodies from PV-stained cells were excluded from the measurements.
In situ hybridization
In situ hybridization was performed on floating sections following procedures previously described in detail (Bernal and Guadaño-Ferraz 2002). The RC3 sense and antisense riboprobes were synthesized from the cDNA template of RC3 spanning nucleotides 253-486 in the presence of 35S[UTP]. For quantitation, the autoradiographs were scanned and densitometry was measured with the AnalySIS software (Soft Imaging System GmbH). Sections from three animals of each group, that is, wild-type and TRα1+/m, treated, and untreated with T3, were used. In situ hybridization for GAD was performed to label the whole GABAergic cell population. A mixed, GAD65 and GAD67, digoxigenin-labeled riboprobe was used.
Statistical analyses
The data on the Rotarod experiments were subjected to the Student's t-test to check for differences between groups. Behavioral data were analyzed by two-way ANOVA with between-subjects factors for genotype and TH treatment. Post hoc analyses were performed using Tukey's test. All other data were subjected to one-way ANOVA and the protected least-significant differences test (LSD) for multiple comparisons, after validation of the homogeneity of variances by the Bartlett-Box F test. The Student's t-test was used to verify that novel object preference was above chance (50%). Differences were considered significant when P < 0.05. Calculations were performed using the SPSS statistical package (SPSS Inc.).
Acknowledgments
We thank Dr. Douglas Forrest for constructive advice and criticism. This project was supported by funds from the Swedish Cancer Society (to B.V.); the Wallenberg Foundation (to B.V.); Karobio AB (to B.V.); BFI2002-00489 (to J.B.), BFI2003-07524 (to C.V.), and BFU2004-5944 (to A.G.-F.) from the Spanish Ministery of Science and Technology (MCYT); 08.5/0042/2003 1 from the Community of Madrid (to J.B.); and FIS Red de Centros RCMN (C03/08) (to J.B.). A.I.H. was the recipient of a MCYT predoctoral fellowship. C.V. and A.G.-F. are the recipients of contracts from the Ramón y Cajal Program of the Spanish Ministry of Education and Science. We acknowledge Marina Sanz for expert technical help. The two isoforms of rat glutamic acid decarboxylase, GAD65 and GAD67, were provided by Dr. Eduardo Soriano, University of Barcelona, Spain.
Article published online ahead of print. Article and publication date are at http://www.genesdev.org/cgi/doi/10.1101/gad.346105.
Corresponding authors.
References
- Bannerman D.M., Rawlins, J.N., McHugh, S.B., Deacon, R.M., Yee, B.K., Bast, T., Zhang, W.N., Pothuizen, H.H., and Feldon, J. 2004. Regional dissociations within the hippocampus—Memory and anxiety. Neurosci. Biobehav. Rev. 28: 273-283. [DOI] [PubMed] [Google Scholar]
- Bauer M., London, E.D., Silverman, D.H., Rasgon, N., Kirchheiner, J., and Whybrow, P.C. 2003. Thyroid, brain and mood modulation in affective disorder: Insights from molecular research and functional brain imaging. Pharmacopsychiatry 36 Suppl 3: S215-S221. [DOI] [PubMed] [Google Scholar]
- Bauer M., Fairbanks, L., Berghofer, A., Hierholzer, J., Bschor, T., Baethge, C., Rasgon, N., Sasse, J., and Whybrow, P.C. 2004. Bone mineral density during maintenance treatment with supraphysiological doses of levothyroxine in affective disorders: A longitudinal study. J. Affect. Disord. 83: 183-190. [DOI] [PubMed] [Google Scholar]
- Bernal J. and Guadaño-Ferraz, A. 2002. Analysis of thyroid hormone-dependent genes in the brain by in situ hybridization. Methods Mol. Biol. 202: 71-90. [DOI] [PubMed] [Google Scholar]
- Bradley D.J., Towle, H.C., and Young III, W.S. 1992. Spatial and temporal expression of α- and β-thyroid hormone receptor mRNAs, including the β 2-subtype, in the developing mammalian nervous system. J. Neurosci. 12: 2288-2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celio M.R. 1986. Parvalbumin in most γ-aminobutyric acid-containing neurons of the rat cerebral cortex. Science 231: 995-997. [DOI] [PubMed] [Google Scholar]
- Clark R.E., Zola, S.M., and Squire, L.R. 2000. Impaired recognition memory in rats after damage to the hippocampus. J. Neurosci. 20: 8853-8860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley J.N. and Paylor, R. 1997. A proposed test battery and constellations of specific behavioral paradigms to investigate the behavioral phenotypes of transgenic and knockout mice. Horm. Behav. 31: 197-211. [DOI] [PubMed] [Google Scholar]
- Current protocols in neuroscience. 2003. (series ed. G.P. Taylor). John Wiley, New York.
- Delange F. 1996. Endemic cretinism. In Werner and Ingbar's `The thyroid' (eds. L.E. Braverman and R.D. Utiger), pp. 756-767, 7th ed. Lippincott-Raven Publishers, New York.
- DeFelipe J. 1999. Chandelier cells and epilepsy. Brain 122 (Pt 10): 1807-1822. [DOI] [PubMed] [Google Scholar]
- Eayrs J.T. 1966. Thyroid and central nervous development. Sci. Basis Med. Annu. Rev. 316: 338-339. [PubMed] [Google Scholar]
- Ennaceur A. and Delacour, J. 1988. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav. Brain Res. 31: 47-59. [DOI] [PubMed] [Google Scholar]
- Ercan-Fang S., Schwartz, H.L., and Oppenheimer, J.H. 1996. Isoform specific 3,5,3′-triiodothyronine receptor binding capacity and messenger ribonucleic acid content in rat adeno-hypophysis: Effect of thyroidal state and comparison with extrapituitary tissues. Endocrinology 137: 3228-3233. [DOI] [PubMed] [Google Scholar]
- Escobar-Morreale H.F., Escobar del Rey, F.E., Obregon, M.J., and Morreale de Escobar, G. 1996. Only the combined treatment with thyroxine and triiodothyronine ensures euthyroidism in all tissues of the thyroidectomized rat. Endocrinology 137: 2490-2502. [DOI] [PubMed] [Google Scholar]
- Feng J., Yan, J., Michaud, S., Craddock, N., Jones, I.R., Cook Jr., E.H., Goldman, D., Heston, L.L., Peltonen, L., Delisi, L.E., et al. 2001. Scanning of estrogen receptor α (ERα) and thyroid hormone receptor α (TRα) genes in patients with psychiatric diseases: Four missense mutations identified in ERα gene. Am. J. Med. Genet. 105: 369-374. [DOI] [PubMed] [Google Scholar]
- Flamant F., Poguet, A.L., Plateroti, M., Chassande, O., Gauthier, K., Streichenberger, N., Mansouri, A., and Samarut, J. 2002. Congenital hypothyroid Pax8-/- mutant mice can be rescued by inactivating the TRα gene. Mol. Endocrinol. 16: 24-32. [DOI] [PubMed] [Google Scholar]
- Freund T.F. and Buzsaki, G. 1996. Interneurons of the hippocampus. Hippocampus 6: 347-470. [DOI] [PubMed] [Google Scholar]
- Gauthier K., Chassande, O., Plateroti, M., Roux, J.P., Legrand, C., Pain, B., Rousset, B., Weiss, R., Trouillas, J., and Samarut, J. 1999. Different functions for the thyroid hormone receptors TRα and TRβ in the control of thyroid hormone production and post-natal development. EMBO J. 18: 623-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass C.K. and Rosenfeld, M.G. 2000. The coregulator exchange in transcriptional functions of nuclear receptors. Genes & Dev. 14: 121-141. [PubMed] [Google Scholar]
- Göthe S., Wang, Z., Ng, L., Kindblom, J.M., Barros, A.C., Ohlsson, C., Vennström, B., and Forrest, D. 1999. Mice devoid of all known thyroid hormone receptors are viable but exhibit disorders of the pituitary-thyroid axis, growth, and bone maturation. Genes & Dev. 13: 1329-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray J.A. 1971. The psychology of fear and stress. Weidenfeld and Nicholson, London.
- Guadaño-Ferraz A., Benavides-Piccione, R., Venero, C., Lancha, C., Vennström, B., Sandi, C., DeFelipe, J., and Bernal, J. 2003. Lack of thyroid hormone receptor α1 is associated with selective alterations in behavior and hippocampal circuits. Mol. Psychiatry 8: 30-38. [DOI] [PubMed] [Google Scholar]
- Hammond R.S., Tull, L.E., and Stackman, R.W. 2004. On the delay-dependent involvement of the hippocampus in object recognition memory. Neurobiol. Learn. Mem. 82: 26-34. [DOI] [PubMed] [Google Scholar]
- Hashimoto K., Curty, F.H., Borges, P.P., Lee, C.E., Abel, E.D., Elmquist, J.K., Cohen, R.N., and Wondisford, F.E. 2001. An unliganded thyroid hormone receptor causes severe neurological dysfunction. Proc. Natl. Acad. Sci. 98: 3998-4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson C., Lännergren, J., Lunde, P.K., Vennström, B., Thorén, P., and Westerblad, H. 2000. Isometric force and endurance in soleus muscle of thyroid hormone receptor-α(1)- or -β-deficient mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 278: R598-R603. [DOI] [PubMed] [Google Scholar]
- Kaneshige M., Suzuki, H., Kaneshige, K., Cheng, J., Wimbrow, H., Barlow, C., Willingham, M.C., and Cheng, S. 2001. A targeted dominant negative mutation of the thyroid hormone α 1 receptor causes increased mortality, infertility, and dwarfism in mice. Proc. Natl. Acad. Sci. 98: 15095-15100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalonde R., Bensoula, A.N., and Filali, M. 1995. Rotorod sensorimotor learning in cerebellar mutant mice. Neurosci. Res. 22: 423-426. [DOI] [PubMed] [Google Scholar]
- LeDoux J.E. 2000. Emotion circuits in the brain. Annu. Rev. Neurosci. 23: 155-184. [DOI] [PubMed] [Google Scholar]
- Legrand J. 1984. Effects of thyroid hormones on central nervous system. In Neurobehavioral teratology (ed. J. Yanai), pp. 331-363. Elsevier Science Publishers, Amsterdam.
- Liu Y.Y., Schultz, J.J., and Brent, G.A. 2003. A thyroid hormone receptor α gene mutation (P398H) is associated with visceral adiposity and impaired catecholamine-stimulated lipolysis in mice. J. Biol. Chem. 278: 38913-38920. [DOI] [PubMed] [Google Scholar]
- Manzano J., Morte, B., Scanlan, T.S., and Bernal, J. 2003. Differential effects of triiodothyronine and the thyroid hormone receptor β-specific agonist GC-1 on thyroid hormone target genes in the brain. Endocrinology 144: 5480-5487. [DOI] [PubMed] [Google Scholar]
- McHugh S.B., Deacon, R.M., Rawlins, J.N., and Bannerman, D.M. 2004. Amygdala and ventral hippocampus contribute differentially to mechanisms of fear and anxiety. Behav. Neurosci. 118: 63-78. [DOI] [PubMed] [Google Scholar]
- McNaughton N. 1997. Cognitive dysfunction resulting from hippocampal hyperactivity—A possible cause of anxiety disorder? Pharmacol. Biochem. Behav. 56: 603-611. [DOI] [PubMed] [Google Scholar]
- Miles R., Toth, K., Gulyas, A.I., Hajos, N., and Freund, T.F. 1996. Differences between somatic and dendritic inhibition in the hippocampus. Neuron 16: 815-823. [DOI] [PubMed] [Google Scholar]
- Morte B., Manzano, J., Scanlan, T., Vennström, B., and Bernal, J. 2002. Deletion of the thyroid hormone receptor α 1 prevents the structural alterations of the cerebellum induced by hypothyroidism. Proc. Natl. Acad. Sci. 99: 3985-3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myhrer T. 1989. Exploratory behavior and reaction to novelty in rats: Effects of medial and lateral septal lesions. Behav. Neurosci. 103: 1226-1233. [DOI] [PubMed] [Google Scholar]
- Pittenger C., Huang, Y.Y., Paletzki, R.F., Bourtchouladze, R., Scanlin, H., Vronskaya, S., and Kandel, E.R. 2002. Reversible inhibition of CREB/ATF transcription factors in region CA1 of the dorsal hippocampus disrupts hippocampus-dependent spatial memory. Neuron 34: 447-462. [DOI] [PubMed] [Google Scholar]
- Powell E.M., Campbell, D.B., Stanwood, G.D., Davis, C., Noebels, J.L., and Levitt, P. 2003. Genetic disruption of cortical interneuron development causes region- and GABA cell type-specific deficits, epilepsy, and behavioral dysfunction. J. Neurosci. 23: 622-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampon C., Tang, Y.P., Goodhouse, J., Shimizu, E., Kyin, M., and Tsien, J.Z. 2000. Enrichment induces structural changes and recovery from nonspatial memory deficits in CA1 NMDAR1-knockout mice. Nat. Neurosci. 3: 238-244. [DOI] [PubMed] [Google Scholar]
- Refetoff S., Weiss, R.E., and Usala, S.J. 1993. The syndromes of resistance to thyroid hormones. Endocr. Rev. 14: 348-399. [DOI] [PubMed] [Google Scholar]
- Rewal M., Jung, M.E., Wen, Y., Brun-Zinkernagel, A.M., and Simpkins, J.W. 2003. Role of the GABAA system in behavioral, motoric, and cerebellar protection by estrogen during ethanol withdrawal. Alcohol 31: 49-61. [DOI] [PubMed] [Google Scholar]
- Sik A., Penttonen, M., Ylinen, A., and Buzsaki, G. 1995. Hippocampal CA1 interneurons: An in vivo intracellular labeling study. J. Neurosci. 15: 6651-6665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strait K.A., Schwartz, H.L., Seybold, V.S., Ling, N.C., and Oppenheimer, J.H. 1991. Immunofluorescence localization of thyroid hormone receptor protein β 1 and variant α 2 in selected tissues: Cerebellar Purkinje cells as a model for β 1 receptor-mediated developmental effects of thyroid hormone in brain. Proc. Natl. Acad. Sci. 88: 3887-3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y.P., Shimizu, E., Dube, G.R., Rampon, C., Kerchner, G.A., Zhuo, M., Liu, G., and Tsien, J.Z. 1999. Genetic enhancement of learning and memory in mice. Nature 401: 63-69. [DOI] [PubMed] [Google Scholar]
- Tinnikov A., Nordström, K., Thorén, P., Kindblom, J.M., Malin, S., Rozell, B., Adams, M., Rajanayagam, O., Pettersson, S., Ohlsson, C., et al. 2002. Retardation of post-natal development caused by a negatively acting thyroid hormone receptor α1. EMBO J. 21: 5079-5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikström L., Johansson, C., Salto, C., Barlow, C., Campos Barros, A., Baas, F., Forrest, D., Thoren, P., and Vennstrom, B. 1998. Abnormal heart rate and body temperature in mice lacking thyroid hormone receptor α1. EMBO J. 17: 455-461. [DOI] [PMC free article] [PubMed] [Google Scholar]