Abstract
Movement therapy using Rhythmic Auditory Stimulation (RAS) has been proven beneficial in Parkinson’s disease (PD). However, research regarding RAS-therapy using wearable devices in all neurological disorders is needed. The aim of this study is to investigate the effectiveness of RAS-therapy using wearable devices on movement in individuals with neurological disorders. Systematic review and meta-analysis. Data sources June 27, 2024. PubMed, Web of Science, Medline, PEDro and ScienceDirect were searched. Following PRISMA-guidelines 2020. Inclusion criteria: all neurological disorders, Rhythmic auditory stimulation, wearable devices, movement parameters, studies written in Dutch or English. Exclusion criteria: non-neurological disorders, children, animals, healthy individuals, other interventions, EMG and EEG outcome parameters, patient reported outcome parameters, systematic reviews, meta-analyses, and other languages besides Dutch or English. Risk of bias was assessed using the QualSyst tool. 7993 articles after double-blind screening; thirty studies were included in the review and fifteen in the meta-analysis. Results showed improvements in stride length, step length, gait velocity, double support time, arm swing peak velocity and arm swing ROM. The meta-analysis confirmed significant improvements in gait velocity and stride length within a longitudinal design as well as when compared to a control group. Improvement in cadence was only significant in a longitudinal design but non-significant when compared to a control group (p = 0.247). RAS-therapy can be implemented for rehabilitation of PD, MS and stroke.
Keywords: Rhythmic auditory stimulation (RAS), Wearable devices, Neurological disorders, Movement, Gait, Parkinson’s disease
Subject terms: Motor control, Multiple sclerosis, Parkinson's disease, Stroke
Introduction
Due to the increasing life expectancy, the population of elderly people amongst the general population has begun (and will continue) to grow, leading to an increase in neurological disorders and thus a growing number of people with movement-related disorders. In a study of J.P. Bach et al. (2011)1, it was predicted that the prevalence of movement disorders would increase considerably between 2010 and 2050 with the greatest increase in Lewy Body Dementia. Interestingly, the authors suggested that the prevalence of Parkinson’s disease could double in some countries. In 2022, the World Health Organisation projected a two-fold increase of the sixty-plus population by 2050 (up to 2.1 billion). Additionally, an increase of the eighty-plus population of up to 436 million by 2050, was also estimated2. Consequently, the pressure on society to accommodate the increasing amount of those suffering with movement disorders will continue to rise3. Movement disorders can manifest themselves in several ways, of which tremor being the most common symptom worldwide4. Besides this, they may occur in various parts of the body. Upper extremity dysfunctions appear to be very common amongst stroke survivors5. Gait deviations also appear a common result of certain neurological disorders6. To clarify, gait disturbances are described as any deviation from the normal gait pattern, which may be outed in several ways due to the wide range of potential aetiologies at the root of these deviations7. Moon et al.6 concluded that among various neurological pathological groups, gait variability had increased compared to healthy individuals. These gait disorders have been directly correlated with poor quality of life and increased mortality8. A study of Varghese et al.9 showed that subjects with a neurological gait, had an increased risk of falls. Recurrent falls had also been associated with a neurological gait pattern in contrast to the population of subjects with non-neurologic disorders10. Depending on their nature, falls might lead to additional burdens for the diagnosed individual as well as the caregivers and by extension, the general healthcare system11. Thus, it is crucial to develop adequate treatment options to improve general movement among this population. Music therapy based on Rhythmic Auditory Stimulation (RAS) could potentially improve the gait and movement of those suffering with neurological disorders. RAS is a safe, inexpensive, free of adverse health effects and non-invasive neurological Music Therapy technique that synchronizes gait movements with predictable time cues to facilitate the rehabilitation of intrinsically rhythmic movements12–14. RAS can be applied in daily life using a musical stimulus to enhance the adherence to physical activity15. When applied to gait training, RAS could be provided in the form of regular isochronous auditory pulses like metronome clicks or metrical acoustical beat incorporated music, mostly matched to the preferred cadence of the subject14. RAS can be gradually increased or decreased to accommodate for the optimal cadence, velocity and stride length of the subjects in question14,16. Mostly, studies use a fixed-tempo RAS stimulus (e.g., metronome sound), to which the subjects have to synchronize their steps to, thus demanding a certain amount of attention from the subject12. However, RAS could also be implemented as an adaptive, interactive cueing system which adjusts (in real-time) to the subjects movements and gait pattern, potentially being more effective than the fixed-tempo RAS intervention12. Currently, reviews regarding the effects of RAS or musical therapy on neurological disorders already exists. Zhou et al.17 concluded that the music-based movement therapy is an effective treatment for improving several parameters including: motor function, balance, freezing of gait (FOG), gait velocity and mental health in subjects with Parkinson’s disease. Ye et al.18 supported these findings and concluded that RAS improved the stride length, gait speed, FOG and UPDRS-III19 in subjects with PD. Furthermore, López-Ortiz et al.20 concluded that dance and RAS provided beneficial effects in terms of balance, gait and walking for patients with Cerebral Paresis (CP). These reviews are often limited to one group of neurological disorders like PD and generally lack the use of wearable devices. There is still a gap in the literature regarding the use those wearable devices in combination with RAS-therapy targeted at the greater population of subjects with neurological disorders. Wearable systems such as inertial measurement units or wearable foot pressure insole, could overcome the limitations of non-wearable devices when it comes to data capturing during motion or gait. The use of such system makes it possible to continuously capture data outside the clinical setting, thus providing more accurate and complete data on the movements21. Wearable devices such as headphones could be used to provide the rhythmical beats to the subjects during RAS interventions. The usage of RAS on neurological patients using wearable devices has already been studied in a previous systematic review of Scataglini et al.22. Subjects included in this study suffered from either PD, MS, stroke or spinal cord injuries. The authors discovered that RAS in combination with these wearable devices was both effective and favourable as an intervention during the rehabilitation phase. However, few included articles covered neurological disorders other than PD22. There is still a need for further research in the field of wearable technology and the role it plays in RAS interventions for other neurological disorders. Therefore, the aim of this systematic review is to compile all available evidence regarding the effectiveness of RAS therapy using wearable devices for providing stimuli in persons diagnosed with a neurological disorder. Additionally, a meta-analysis will be conducted to provide a clearer and more comprehensive understanding of the effect of RAS-therapy. Furthermore, it is possible to reveal variations in outcomes between different gait parameters.
Materials and methods
This systematic review and meta-analysis were conducted according to the PRISMA guidelines set in 202023 and registered into PROSPERO International Prospective Register of Systematic Review (n = CRD42024527928).
Eligibility criteria
To be included, studies had to explore the effect of wearable RAS interventions on individuals with a neurological disorder (all neurological disorders were included). Following definition based on the research of Choi et al.24 was used to describe wearable devices: Wearables based on-body that can stimulate (RAS) and/or monitor physical characteristics (such as spatiotemporal gait parameters). Effects regarding the motoric system of subjects were included. All other outcome measures not pertaining to the motoric system were excluded. Comparisons made with other interventions or population groups were not considered. No limitations regarding date of publication. A visual summary of the eligibility criteria according to the PICOST method can be found in Table 1.
Table 1.
Eligibility criteria.
| PICOST-question | Inclusion criteria | Exclusion criteria | Medical Subjects Headings (MeSH) | Free Keywords | |
|---|---|---|---|---|---|
| P | Patient/ population |
Alzheimer disease Dementia (Lewy Body, frontotemporal) Mild cognitive impairment (MCI) Tourette syndrome (TS) Autism spectrum disorder (ASD) Amyotrophic lateral sclerosis (ALS) Charcot-Marie-Tooth (CMT) Traumatic brain injury Epilepsy Brain tumors (brain neoplasms) Ataxia Parkinson’s disease Multiple sclerosis Stroke Spinal cord injury Other neurological disorders |
Non-neurological disorders Children Animals Healthy people |
"Nervous System Diseases"[Mesh] Included Mesh terms: - Alzheimer Disease - Dementia - Frontotemporal Dementia - Lewy Body Disease - Tourette Syndrome - Autism Spectrum Disorder - Amyotrophic Lateral Sclerosis - Charcot-Marie-Tooth Disease - Brain Injuries, Traumatic - Epilepsy - Brain Neoplasms - Ataxia - Parkinson Disease - Multiple sclerosis - Stroke - Spinal Cord Injuries |
Alzheimer* Dementia Lewy Bod* Frontotemporal dementia Mild cognitive impairment* MCI Tourette syndrome TS Autism spectrum disorder ASD Amyotrophic lateral sclerosis ALS Charcot-Marie-Tooth CMT Traumatic brain injury Epilepsy Brain tumo* Ataxia Parkinson Parkinson disease PD Multiple sclerosis MS SCI Spinal cord injur* Stroke Neurologic* disorder* Neuro* Neurology* Neurologic* Nervous system Nervous system disorder Nervous system disease |
| I | Intervention |
Music Rhythmic auditory stimulation Wearable devices |
Other interventions RAS not delivered using wearable devices |
“Music”[Mesh] “Music Therapy”[Mesh] "Wearable Electronic Devices"[Mesh] |
Rhythmic auditory cue* Rhythmic auditory stim* Rhythmic auditory stimuli RAS Music rehabilitation Rhythm* Rhythmic Music therapy Music therap* Melody Beat Metronome Music Tone |
| C | Comparison | – | – | – | – |
| O | Outcome | Movement parameters |
EMG-parameters EEG-parameters Patient-reported |
“Movement”[Mesh] “Gait”[Mesh] “Gait Analysis”[Mesh] |
Capture wear* smart* intelligent Wearable Electronic Devices Movement Motion Motor* Gait gait analysis |
| S | Study design | Language: Dutch, English |
Systematic review Meta-analyses Language: other languages |
– | – |
| T | Timeframe | – | – | – | – |
Information sources
A systematic search of five electronic databases (PubMed, Web of Science, PEDro, Medline and ScienceDirect) was carried out on June 27, 2024. Subsequently the articles were transferred to Endnote 2025.
Search strategy
Each database was searched using a search strategy specifically designed for that respective database (Table 2). The search strategies used, consisted of keywords related to various neurological disorders, rhythmic auditory stimulation and motoric parameters. Some databases required multiple separate search strategies to find all relevant articles.
Table 2.
Search strategies.
| Pubmed 27/06/2024 | ((("Nervous System Diseases"[Mesh]) OR (Alzheimer*) OR (dementia) OR (“Lewy Body”) OR (“Frontotemporal dementia”) OR ("mild cognitive impairment*") OR (MCI) OR (“Tourette syndrome”) OR (TS) OR ("Autism spectrum disorder") OR (ASD) OR (autism) OR ("Amyotrophic lateral sclerosis") OR (ALS) OR ("Charcot-Marie-Tooth") OR (CMT) OR ("neurologic* disorder*") OR (neuro*) OR (neurology*) OR (neurologic*) OR (“nervous system”) OR ("nervous system disorder") OR ("nervous system disease") OR (“Lewy Bodies”) OR (“Traumatic brain injury”) OR (“Epilepsy”) OR (“Brain tumo*”) OR (“Ataxia”) OR (“Parkinson”) OR (“Parkinson disease”) OR (“PD”) OR (“multiple sclerosis”) OR (“MS”) OR (“SCI”) OR (“Spinal cord injur*”) OR (“Stroke”)) AND (("rhythmic auditory cue*") OR ("rhythmic auditory stim*") OR (RAS) OR (“music rehabilitation”) OR (rhythm*) OR (rhythmic) OR (“music therapy”) OR (melody) OR (beat) OR (metronome) OR ("rhythmic auditory stimuli") OR ("music therap*") OR (music) OR (tone) OR (“Music”[Mesh]) OR (“music therapy”[MeSH Terms])) AND ((capture*) OR (wear*) OR (smart*) OR (intelligent) OR ("Wearable Electronic Devices"[Mesh])) AND ((movement) OR (“Movement”[Mesh]) OR (motion) OR (motor*) OR (gait) OR (“Gait”[Mesh]) OR (“gait analysis”) OR (“Gait Analysis”[Mesh]))) |
| Web of science 27/06/2024 | TS = (((Alzheimer*) OR (dementia) OR (“Lewy Body”) OR (“Frontotemporal dementia”) OR (“mild cognitive impairment*”) OR (MCI) OR (“Touret* syndrome”) OR (TS) OR (“Autism spectrum disorder”) OR (ASD) OR (autism) OR (“Amyotrophic lateral sclerosis”) OR (ALS) OR (“Charcot-Marie-Tooth”) OR (CMT) OR (“neurologic* disorder*”) OR (neuro*) OR (neurology) OR (neurologic*) OR (“nervous system”) OR (“nervous system disorder”) OR (“nervous system disease”) OR (“Lewy Bodies”) OR (Parkinson) OR (“Parkinson disease”) OR (PD) OR (“multiple sclerosis”) OR (MS) OR (SCI) OR (“Spinal cord injur*”) OR (Stroke) OR (“Traumatic brain injury”) OR (Epilepsy) OR (“Brain tumo*”) OR (Ataxia)) AND ((“rhythmic auditory cue*”) OR (“rhythmic auditory stim*”) OR (RAS) OR (“music rehabilitation”) OR (rhythm*) OR (rhythmic) OR (“music therapy”) OR (melody) OR (beat) OR (metronome) OR (“rhythmic auditory stimuli”) OR (“music therap*”) OR (music) OR (tone)) AND ((capture*) OR (wear*) OR (smart*) OR (intelligent) OR ("Wearable Electronic Devices")) AND ((movement) OR (motion) OR (motor*) OR (gait) OR (“gait analysis”))) |
| Pedro 27/06/2024 | Rhythmic auditory stimulation |
| Music based therapy | |
| Rhythmic auditory cueing | |
| Medline 27/06/2024 | (((Alzheimer*) OR (dementia) OR (traumatic brain injury) OR (epilepsy) OR (brain tumo*) OR (ataxia) OR (Lewy Body) OR (Frontotemporal dementia) OR (mild cognitive impairment*) OR (Touret* syndrome) OR (Autism spectrum disorder) OR (autism) OR (Amyotrophic lateral sclerosis) OR (Charcot-Marie-Tooth) OR (neurologic* disorder*) OR (neuro*) OR (neurology) OR (neurologic*) OR (nervous system) OR (nervous system disorder) OR (nervous system disease) OR (Lewy Bodies) OR (Parkinson* Disease) OR (Parkinson) OR (Spinal Cord Injur*) OR (Stroke) OR (multiple sclerosis)) AND ((rhythmic auditory cue*) OR (rhythmic auditory stim*) OR (RAS) OR (music rehabilitation) OR (rhythm*) OR (rhythmic) OR (music therapy) OR (melody) OR (beat) OR (metronome) OR (rhythmic auditory stimuli) OR (music therap*) OR (music) OR (tone)) AND ((capture*) OR (wear*) OR (smart*) OR (intelligent) OR (Wearable Electronic Devices)) AND ((movement) OR (motion) OR (motor*) OR (gait) OR (gait analysis))) |
| Science direct 27/06/2024 | ((dementia) OR (Alzheimer)) AND ((“rhythmic auditory cueing”) OR (“Rhythmic auditory stimulation”) OR (RAS) OR (“music rehabilitation”) OR (“music therapy”)) AND (wearables) |
| ((“mild cognitive impairment”) OR (MCI)) AND ((“rhythmic auditory cueing”) OR (“Rhythmic auditory stimulation”) OR (RAS) OR (“music rehabilitation”) OR (“music therapy”)) AND (wearables) | |
| ((“Tourette syndrome”) OR (TS)) AND ((“rhythmic auditory cueing”) OR (“Rhythmic auditory stimulation”) OR (RAS) OR (“music rehabilitation”) OR (“music therapy”)) AND (wearables) | |
| ((“autism spectrum disorder”) OR (ASD) OR (autism)) AND ((“rhythmic auditory cueing”) OR (“Rhythmic auditory stimulation”) OR (RAS) OR (“music rehabilitation”) OR (“music therapy”)) AND (wearables) | |
| ((“Amyotrophic lateral sclerosis”) OR (ALS)) AND ((“rhythmic auditory cueing”) OR (“Rhythmic auditory stimulation”) OR (RAS) OR (“music rehabilitation”) OR (“music therapy”)) AND (wearables) | |
| ((“Charcot-Marie-Tooth”) OR (CMT)) AND ((“rhythmic auditory cueing”) OR (“Rhythmic auditory stimulation”) OR (RAS) OR (“music rehabilitation”) OR (“music therapy”)) AND (wearables) | |
| ((“neurologic disorder”) OR (“neurologic disease”) OR (neurologic)) AND ((“rhythmic auditory cueing”) OR (“Rhythmic auditory stimulation”) OR (RAS) OR (“music rehabilitation”) OR (“music therapy”)) AND (wearables) | |
| ((“Traumatic brain injury”) OR (TBI)) AND ((“rhythmic auditory cueing”) OR (“Rhythmic auditory stimulation”) OR (RAS) OR (“music rehabilitation”) OR (“music therapy”)) AND (wearables) | |
| ((epilepsy)) AND ((“rhythmic auditory cueing”) OR (“Rhythmic auditory stimulation”) OR (RAS) OR (“music rehabilitation”) OR (“music therapy”)) AND (wearables) | |
| ((“brain tumor”) OR (“brain tumors”) OR (“brain neoplasms”)) AND ((“rhythmic auditory cueing”) OR (“Rhythmic auditory stimulation”) OR (RAS) OR (“music rehabilitation”) OR (“music therapy”)) AND (wearables) | |
| ((ataxia)) AND ((“rhythmic auditory cueing”) OR (“Rhythmic auditory stimulation”) OR (RAS) OR (“music rehabilitation”) OR (“music therapy”)) AND (wearables) | |
| ((Parkinson) OR (“Parkinson disease”) OR (PD)) AND ((“rhythmic auditory cueing”) OR (“Rhythmic auditory stimulation”) OR (RAS) OR (“music rehabilitation”) OR (“music therapy”)) AND (wearables) | |
| ((“Multiple sclerosis”)) AND ((“rhythmic auditory cueing”) OR (“Rhythmic auditory stimulation”) OR (RAS) OR (“music rehabilitation”) OR (“music therapy”)) AND (wearables) | |
| ((SCI) OR (“Spinal cord injury”)) AND ((“rhythmic auditory cueing”) OR (“Rhythmic auditory stimulation”) OR (RAS) OR (“music rehabilitation”) OR (“music therapy”)) AND (wearables) | |
| ((stroke)) AND ((“rhythmic auditory cueing”) OR (“Rhythmic auditory stimulation”) OR (RAS) OR (“music rehabilitation”) OR (“music therapy”)) AND (wearables) |
Selection process
The selection process was carried out in two stages using the online screening tool Rayyan26, which allowed double blinding during each stage of the process. The selection process was conducted by three independent reviewers (LJ, LVE and CVL), with each researcher reviewing two-thirds of the total number of articles, ensuring that each article was screened at least twice. Firstly, articles were screened based on their titles as well as their abstracts to quickly assess their relevance to the research question. Secondly, articles underwent a secondary screening based on their full texts. Following each stage, any conflicts were discussed, and a unanimous decision was made. Thirty articles were included in the review. The reasons leading to the exclusion of certain studies included; study design, language, topic, population (no neurological disorders), incorrect interventions (no rhythmic auditory stimulation and/or no wearable devices and/or no headphones) and lastly no motoric parameters as outcome. Articles were excluded if RAS was not provided using a wearable device, such as headphones (Table 3).
Table 3.
QualSyst criteria for evaluating quantitative studies.
| Nr | Question or condition |
|---|---|
| 1 | Question or objective sufficiently described? |
| 2 | Design evident and appropriate to answer study question? |
| 3 | Method of subject selection (and comparison group selection, if applicable) or source of information/input variables (e.g., for decision analysis) is described and appropriate |
| 4 | Subject (and comparison group, if applicable) characteristics or input variables/information (e.g., for decision analyses) sufficiently described? |
| 5 | If random allocation to treatment group was possible, is it described? |
| 6 | If interventional and blinding of investigators to intervention was possible, is it reported? |
| 7 | If interventional and blinding of subjects to intervention was possible, is it reported? |
| 8 | Outcome and (if applicable) exposure measure(s) well defined and robust to measurement / misclassification bias? Means of assessment reported? |
| 9 | Sample size appropriate? |
| 10 | Analysis described and appropriate? |
| 11 | Some estimate of variance (e.g., confidence intervals, standard errors) is reported for the main results/outcomes (i.e., those directly addressing the study question/objective upon which the conclusions are based)? |
| 12 | Controlled for confounding? |
| 13 | Results reported in sufficient detail? |
| 14 | Do the results support the conclusions? |
Data collection process
The task of extracting data from the included studies was evenly divided amongst the three authors. Each researcher screened their portion of the included articles, independently (Fig. 1). Disagreements were resolved by the decision of a third reviewer. The extracted data can be consulted in Table 4.
Fig. 1.
Prisma 2020 flowchart study selection.
Table 4.
Evidence table.
| Article Author (year) Study design |
POPULATION Neurological disorder - sample size (dropouts) - mean age in years - gender ratio (♂/♀) |
INTERVENTION RAS Procedure Medication |
WEARABLE DEVICE(S) |
RESULTS | |||
|---|---|---|---|---|---|---|---|
|
Baram et al (2007) non-RCT46 |
MS - N: 14 - 48.6 - 4/10 Healthy controls - N: 11 - 25.5 - 5/6 |
RAS - auditory feedback cue, closed-loop responding to P own steps Procedure - walking 4 × a straight track of 10 m - stage 0: baseline - stage 1: no device - stage 2: with device, make auditory cue as rhythmic as possible - stage 3: no device, after a 10’ break |
headphones Belt-mounted box with a motion sensor |
MS: stage 2 vs stage 1 - gait velocity: ↑ * - stride length: ↑ * MS: stage 3 vs stage 1 - gait velocity: ↑ * - stride length: ↑ * |
|||
|
Calvano et al. (2023) RCT28 |
PD (H&Y: 1–2) - N: 25 - 61.0 - 15/10 |
RAS - no stimulation - BiBS: Binaural beat stimulation audiofile of 30’, L/R: 320 Hz/355 Hz - CAS: Conventional acoustic stimulation audiofile of 30’, L/R: 340 Hz/340 Hz Procedure - on 2 separate consecutive days - part 1: OFF medication; no stimulation, 2 × acoustic stimulation - part 2: ON medication; no stimulation, 2 × acoustic stimulation Medication: dopaminergic medication, ON and OFF phase |
headphones MP3-player hand & foot sensors of the validated Kinesia 360™ device, attached to the side of the more-affected limb |
PD: part 1—OFF medication Motor symptoms both sides - BiBS < no stimulation: * - CAS < no stimulation: ** - BiBS vs CAS: ns Motor symptoms more affected side - BiBS < no stimulation: * - CAS vs no stimulation: ns - BiBS vs CAS: ns Walking (number of steps) - no effect for stimulation PD: part 2—ON medication Motor symptoms both sides - BiBS vs no stimulation: ns - CAS vs no stimulation: ns - BiBS vs CAS: ns Motor symptoms more affected side - BiBS vs no stimulation: ns - CAS vs no stimulation: ns - BiBS vs CAS: ns Walking (number of steps) - no effect for stimulation |
|||
|
Chomiak et al. (2017) non-RCT prospective pilot study |
PD (H&Y: 2.6) - N: 11 - 69.9 - 9/2 PD music (H&Y: 2.5) - n: 5 - 70.8 - 5/0 PD podcast (H&Y: 2.7) - n: 6 - 69.0 - 4/2 |
RAS - music - CBC podcast Procedure - pre: baseline - in-home Ambulosono SIP-training min. 3x/w, 10–20’, for 4w - post: SIP dual-task assessment • 1 × mono-task stepping trail • 4 × dual-task stepping trails Medication: consistent medication regimen |
headphones leg sensor iPod touch strapped to knee |
PD: pre vs post DT step automaticity (step automaticity = ratio step height DT/MT) - group 1—music: ↑ ** - group 2—podcast: ↓ ns PD music: pre vs post - FES: ↑ ns - FOG-Q: = ns PD podcast: pre vs post - FOG: ↑ ns - FOG-Q: ↑ ns |
|||
|
Cochen De Cock et al (2021) non-RCT |
PD (H&Y: 2.4) - N: 45 (6) - 65.0 - 25/20 |
RAS - individualized musical stimulation - tempo of music modified with gait P - P chooses ⩾2 genres/session (disco, soft pop, pop rock, instrumental or variety) - online stimulus adaptation Procedure - pre: before rehabilitation program - gait rehabilitation program with BeatWalk app at home 5x/w, 30’, for 4w - post: after rehabilitation program Medication: usual medication, ON phase |
BeatWalk: smartphone application and ankle worn sensors headphones 5 IMUs including 3D-accelerometers and gyroscopes - 2 × feet - 2 × anterior side tibia - 1 × sternum |
PD: pre vs post rehabilitation program 6MWT - distance: ↑ ** - cadence: ↑ ** - gait velocity: ↑ ** - stride length: ↑ * - asymmetry index: ↓ ns UPDRS-III: ↓ ns Falls self-efficacy score: ↓ * Mini Best test: ↑ ns |
|||
|
Collimore et al. (2023) non-RCT |
Chronic stroke - N: 10 - 60.2 - 7/3 |
RAS - closed-loop control of music with real-time gait analysis Procedure - pre: 3’ treadmill assessment before training - 1 session of 30’ overground gait training automated patient-tailored walking rehabilitation - post: 3’ treadmill assessment after training |
bone conduction headphones 2 inertial sensors with a 3D-gyroscope, attached to each shoe |
Stroke: pre vs post automated training More symmetric walking - step time asymmetry: ↓ * - stance time asymmetry: ↓ ** - swing time asymmetry: ↓ ** - step length asymmetry: ns - cadence: ns |
|||
|
Conklyn et al. (2010) RCT pilot study 29 |
MS - N: 10 - 48.6 - 3/7 MS—intervention group - n: 5 - 47.0 - 2/3 MS—control group - n: 5 - 50.2 - 1/4 |
RAS - songs with a tempo that is 10% above the spontaneous cadence - 8 instrumental songs in different genres (classical, folk and jazz) - beat embedded in music Procedure - home-based walking program 7x/w, 20’ for 2 or 4w + 2 × with RAS at spontaneous cadence + 2 × with RAS 10% above spontaneous cadence - week 1–2: HBWP intervention group - week 3–4: HBWP control & intervention group - week 4–6: no treatment - V1: baseline - V2: end of week 1 - V3: end of week 2 - V4: end of week 3 - V5: 2w after end of treatment • 2 walks on GAITRite • T25FW on regular floor • 2 additional walks on GAITRite |
MP3-player with headphones (music) electronic metronome with headphones (RAS) |
MS: V1 vs V3 after RAS (both groups) - double support time % L: ↓ * - double support time % R: ↓ * - cadence: ns - stride length L: ns - stride length R: ns - gait velocity: ns - step length L: ns - step length R: ns - normalized velocity: ns - T25FW: ns MS: after 1 week of RAS (both groups) - double support time % L: = ns - double support time % R: ↑ ns - cadence: ↑ * - stride length L: ↑ * - stride length R: ↑ * - gait velocity: ↑ * - step length L: ↑ * - step length R: ↑ * - normalized velocity: ↑ * - T25FW: ↓ ns |
|||
|
De Bartolo et al. (2020) non-RCT |
PD - N: 20 - 72.5 - 14/6 Elderly adults - N: 20 - 72.1 - 8/12 Young adults - N: 20 - 32.3 - 8/12 |
RAS - 6 salient music tracks • classical andante 92 bpm • classical allegro 126 bpm • pop 118 bpm • motivational hard rock 120 bpm • rock arena 148 bpm • heavy metal 120 bpm Procedure - walk barefoot an unobstructed 18 m corridor at comfortable speed while listening to one of the tracks of music - trial 1: walking + no music - trial 2–7: walking + music track - trial 8–13: walking + music track (reverse order 2–7) - trail 14: walking + no music Medication: dopaminergic medication, ON phase |
headphones 1 IMU sensor with a 3D-accelerometer, gyroscope and magnetometer worn with a waist belt (S1-S2) |
Main effect of music - gait velocity: *** - stride length: *** - stride duration: *** - stance: * - swing: * - first double support phase: * Main effect of subjects’ group - gait velocity: *** - stride length: *** - stride duration: ns - stance: ns - swing: ns - first double support phase: ns |
|||
|
Elsner et al. (2020) RCT pilot study |
Chronic stroke - N: 12 - 67.0 - 3/9 Chronic stroke—RAS - n: 6 - 68.7 - 1/5 Chronic stroke—non RAS - n: 6 - 65.3 - 2/4 |
RAS - classical wandering songs with a clearly accentuated beat Procedure - overground gait training program, with or without RAS 3x/w, 30’, for 4w - T1: baseline - T2: end of intervention period, 4w - T3: follow-up, 12w |
MP3-player headphones |
Chronic stroke: T1 vs T2 (both groups) - gait velocity: ↑ ** - distance: ↑ *** - Berg balance scale: ↑ ** - stride length: ↑ *** Chronic stroke: T1 vs T3 (both groups) - gait velocity: ↑ *** - distance: ↑ *** - Berg balance scale: ↑ *** - stride length: ↑ *** Chronic stroke: RAS group vs non RAS group - ns differences between groups |
|||
|
Erra et al. (2019) non-RCT |
PD - N: 30 - 72.0 - 20/10 - Control group - N: 18 - age matched - sex matched |
RAS - no RAS - 90% of preferred walking cadence P - 100% of preferred walking cadence P - 110% of preferred walking cadence P Procedure - walk along a 20 m pathway in 4 conditions - part 1: OFF medication; no RAS, RAS 90, RAS 100 and RAS 110 - part 2: ON medication; no RAS, RAS 90, RAS 100 and RAS 110 Medication: dopaminergic medication, ON and OFF phase |
4 force resistive sensors placed under each foot 2 wireless modules, one for each foot 7 IMUs with a 3D-accelerometer, gyroscope and magnetometer - 2 × insteps of feet - 2 × lateral midshanks - 2 × lateral mid-thighs - 1 × pelvis headphones |
PD—OFF: preferred velocity vs RAS | |||
| RAS 90 | RAS 100 | RAS 110 | |||||
|
step length stride length cadence gait velocity stride time swing % single support % |
↑ ns ↑ ns ↓ * ↓ * ↑ * ↑ ns ↑ ns |
↑ ns ↑ ns ↓ ns ↓ ns ↑ * ↑ ns ↑ ns |
↑ * ↑ * ↓ ns ↓ ns ↓ ns ↑ ns ↑ ns |
||||
| PD—ON: preferred velocity vs RAS | |||||||
| RAS 90 | RAS 100 | RAS 110 | |||||
|
step length stride length cadence gait velocity stride time swing % single support % |
↑ ns ↑ ns ↓ * ↓ * ↑* ↓ ns ↓ ns |
↑ ns ↑ ns ↓ ns ↓ ns ↑* ↓ ns ↓ ns |
↑ * ↑ * ↓ ns ↑ ns ↓ ns ↓ ns ↑ ns |
||||
|
Ginis et al. (2017) non-RCT |
PD (H&Y: 1–3) - N: 28 - 62.0 - 23/5 PD—FOG + - n: 15 - 62.80 - 14/1 PD—FOG- - n: 13 - 61.2 - 9/4 |
RAS - ConCue: continuous cueing - IntCue: intelligent cueing - IntFB: intelligent feedback - NoInfo: no information Procedure - 1’ comfortable reference walk before the 30’ walk - 4 walks of 30’ in a period of 6w, min. 1w interval - 24 m × 9 m elliptical walking trajectory - 1 condition of RAS during the entire walk Medication: dopaminergic medication, ON phase |
headphones 2 foot-mounted IMUs with a 3D- accelerometer, gyroscope and magnetometer, attached on top of the shoes |
PD: gait deviations FOG + > FOG - ConCue: ns - IntCue: * - IntFB: * - NoInfo: ns PD: gait deviations within FOG + group - ConCue < NoInfo: * - ConCue < IntFB: ** - ConCue < IntCue: ns - IntCue < NoInfo: * - IntFB < NoInfo: * PD: gait deviations within FOG- group - ns differences between the conditions |
|||
|
Ginis et al. (2017) non-RCT42 |
PD (H&Y: 1–3) - N: 28 - 62.0 - 23/5 Healthy controls - N: 13 - 60.2 - 7/6 |
RAS - ConCue: continuous cueing - IntCue: intelligent cueing - IntFB: intelligent feedback - NoInfo: no information Procedure - 1’ comfortable reference walk before the 30’ walk - 4 walks of 30’ in a period of 6w, min. 1w interval - 24 m × 9 m elliptical walking trajectory - 1 condition of RAS during the entire walk Medication: dopaminergic medication, ON phase |
headphones 2 foot-mounted IMUs with a 3D- accelerometer, gyroscope and magnetometer, attached on top of the shoes 5 IMUs with a 3D-accelerometer, gyroscope and magnetometer - 2 × wrists - 2 × ankles - 1 × lower back |
PD: cadence 1–5 min - ns differences between the conditions PD: cadence 26–30 min - ConCue > NoInfo: * - IntFB > NoInfo: * PD: stride length - 1–5’ < 26–30’: * - 6–10’ < 16–20’: * - 6–10’ < 21–25’: * - 6–10’ < 26–30’: * |
|||
|
Guimarães et al. (2015) non-RCT51 |
PD (H&Y: 2.4) - N: 12 - 71.2 - 7/5 |
RAS - rhythmic auditory cues - several types of sounds: metronome sounds, musical beats, clapping, verbal cueing or combination of sounds Procedure - 2 × 20 m non-cued walking test for reference values - walking with RAS 10% below natural step rate - walking at will, with supervision + using the auditory cueing system |
smartphone 1 (step detection) connected to smartphone 2 (cueing rate) with headphones |
PD: non-cued vs cued walking - gait velocity: ns - step length: ns - cadence: ns PD: applied vs measured - rhythm (steps/minute): ** |
|||
|
Hove et al. (2012) non-RCT |
PD (H&Y: 2–3) - N: 20 - 69.2 - 8/12 Healthy controls - N: 18 - 24.7 - 16/2 |
RAS - 100 ms sine tones at 523 and 700 Hz - WalkMate: cueing with period and phase adjustment - RAS: fixed-tempo rhythmic auditory stimulation - silent control: unassisted silent control condition Procedure - walk at natural, comfortable velocity around a corridor of 200 m - 1 block consists of 3 trials - pretest trial: no auditory stimulation - test trial: 1 condition of auditory stimulation - post-test trial: no auditory stimulation Medication: dopaminergic medication, ON phase |
headphones pressure sensors attached to shoes |
PD: rhythmic treatment DFA Fractal Scaling Exponent - silent control < WalkMate: * - silent control > RAS: ns - WalkMate > RAS: * PD: post-treatment DFA Fractal Scaling Exponent - silent control < WalkMate: * - silent control < RAS: ns - WalkMate > RAS: * |
|||
|
Hutchinson et al. (2020) non-RCT |
Chronic stroke - N: 11 (4) - 57.7 - 9/2 |
RAS - closed-loop control of the rhythm of musical stimuli - music from familiar genres with salient beat strength - increase tempo + 5%: > 60% of steps within entrainment zone - decrease tempo -5%: < 60% of steps within entrainment zone Procedure - sessions of music-based rhythmic locomotor training; personalized and progressive rhythmic gait training by a music-based digital therapeutic platform, sensor-driven - visit 1–3: training, 30’ of continuous walking - visit 4: walking evaluation |
bone conduction headphones inertial sensor (3D gyroscope) smartphone application |
Chronic stroke: within-session speed changes (after 1 training, n = 11) - usual walking speed: ↑ * - fast walking speed: ↑ * Chonic stroke: across-session speed changes (after 3 trainings, n = 7) - usual walking speed: ↑ * - fast walking speed: ↑ ns - usual cadence: ↑ ** fast cadence: ↑ * |
|||
|
Kim et al. (2012) RCT |
Subacute stroke—RAS - N: 9 (1) - 58.3 - 6/4 Subacute stroke—non RAS - N: 9 (1) - 51.8 - 7/3 |
RAS - metronome beat Procedure - 3x/w, 30’, for 5w and NDT 10x/w, 30’ for 5w - gait training sessions in rectangular space 20 × 5 m - 5 stages of 5’ + 1’ break • forward walking, backward walking, and side walking • stand up from a chair with arm rests, walk 3 m, turn around, return to the chair, and sit down • cross obstacles placed in font • climb upstairs and downstairs • forward walking was performed by increasing the cadence of a comfortable speed by 5% |
smartphone metronome application + earphones |
Subacute: pre vs post RAS group - DGI: ↑ * - FSST: ↓* - TUG: ↓ * - Up stair (step/s): ↓ * - Down stair (step/s): ↓ * - gait velocity: ↑ *** - cadence: ↑ *** - stride length (affected side): ↑ *** - stride length (non-affected side): ↑ *** - cycle time (affected side): ↓ ns - cycle time (non-affected side): ↓ * |
|||
|
Li et al. (2022) RCT |
PD (H&Y: 2–3) music-based group - N: 23 - 64.1 - 12/11 PD (H&Y: 2–3) exercise group - N: 23 - 65.7 - 11/12 PD (H&Y: 2–3) control group - N: 24 - 61.6 - 11/13 |
RAS - music selected by therapists following considerations - repeated played music, in order of the playlist, with 2’ interval between music Procedure - 5x/w, 1 h, for 4w - exercise training on a trail 5 × 1 m with music - perform exercises to the beat of the music • flat start walking • turning • narrow space walking • step training Medication: dopaminergic medication |
music player + headphones |
PD: music-based vs exercise group after 4 weeks - stride length: ns - gait velocity: * - cadence: * - double support time (%GC): * - UPDRS-III: * - UPDRS-II: * - FOG-Q: * PD: music-based vs control group after 4 weeks - stride length: * - gait velocity: * - cadence: ** - double support time (%GC): ** - UPDRS-III: ** - UPDRS-II: ** - FOG-Q: ** |
|||
|
Lopez et al. (2014) non-RCT |
PD (H&Y: 2.5–3) - N: 10 (2) - 55.0 - 7/3 |
RAS - auditory rhythmic cues matching step frequency - auditory cueing rate (bpm): 25% faster than uncued cadence Procedure - single session, walking on a 7.62 m walkway - synchronize steps with auditory tones - walking at fastest speed, without Listenmee® - walking with Listenmee® without auditory cues - walking with Listenmee® delivering auditory cues Medication: dopaminergic medication, OFF phase |
Listenmee®: glasses system, auditory device with headphones and smartphone application smartwatch with accelerometer |
PD: non cued vs auditory cue of Listenmee® - cadence: ↑ * - stride length: ↑ * - gait velocity: ↑ * |
|||
|
Mainka et al. (2018) RCT |
Stroke—RAS treadmill - N: 15 (4) - 63.7 - 7/4 Stroke—treadmill - N: 15 (2) - 65.5 - 11/2 Stroke—NDT - N: 15 (4) - 61.1 - 8/3 |
RAS - functional training music, according to some criteria - beat rate of music: match cadence P on treadmill - musical tempo was a little slowed down to induce greater step lengths Procedure - 5x/w, w1: 15’, w2: 17’, w3 and w4: 20’, for 4w - training time increased during therapy - RAS treadmill training: walking on treadmill while listening to music + extra conventional physiotherapy 30’ or 60’/week |
earplugs + MP3-player |
Stroke: pre vs post RAS treadmill group - gait velocity: ↑ *** - cadence: ↑ *** - stride length: ↑** Stroke: pre vs post RAS treadmill group - distance 3 MWT (walking endurance): ↑ *** |
|||
|
Mainka et al. (2018) RCT |
Stroke—RAS treadmill - N: 15 (4) - 63.7 - 7/4 Stroke—treadmill - N: 15 (2) - 65.5 - 11/2 Stroke—NDT - N: 15 (4) - 61.1 - 8/3 |
RAS - functional training music, according to some criteria - beat rate of music: match cadence P on treadmill - musical tempo was a little slowed down to induce greater step lengths Procedure - 5x/w, w1: 15’, w2: 17’, w3 and w4: 20’, for 4w - training time increased during therapy - RAS treadmill training: walking on treadmill while listening to music + extra conventional physiotherapy 30’ or 60’/week |
earplugs + MP3-player |
Stroke: pre vs post RAS treadmill group - gait velocity: ↑ *** - cadence: ↑ *** - stride length: ↑** Stroke: pre vs post RAS treadmill group - distance 3 MWT (walking endurance): ↑ *** |
|||
|
Moumdjian et al. (2019) non-RCT case–control study (experimental session) |
MS - N: 31 (4) - 53.5 - 8/23 Healthy controls - N: 30 (2) - 51.8 - 8/22 |
RAS - music (beats), metronome (ticks) and silence - individualized optimal tempo in the auditory conditions Procedure - familiarisation: 3 × 1’ walking at comfort tempo in a square of 4.5 × 6 m - 3 × 12’ uninterrupted walking to the 3 conditions, 15’ break in-between - synchronize stepping to the auditory-stimuli |
D-Jogger: adaptive music player, headphones and 2 IMUs attached to the ankles (3D-accelerometers, gyroscopes and pressure sensors) 3 OPAL sensors with 3D-accelerometer, gyroscope and magnetometer - 2 × ankles - 1 × sternum |
Participants: cadence - metronomes > silence: *** - music > silence: *** MS: gait velocity - metronomes < music: ** - metronomes < silence: *** MS: stride length - music < metronomes: *** |
|||
|
Murgia et al. (2018) RCT |
PD (H&Y: 1.5–3) ecological RAS - N: 19 (3) - 66.5 - not mentioned PD (H&Y: 1.5–3) artificial RAS - N: 19 (3) - 69.9 - not mentioned |
RAS - ecological: stimuli consisted of footstep recordings - artificial: stimuli consisted of metronome sound Procedure - 2x/w, 45’, for 5w + train min. 3x/w at home - supervise rehabilitation (20’ specific gait training with RAS) + 12 weeks daily home-exercises unsupervised - T0: before treatment - T5: after treatment - T17: 17 weeks after first assessment Medication: dopaminergic medication, ON phase |
MP3-player + headphones | PD: T0 vs T5 and T17 ecological & artificial RAS group | |||
| T5 | T17 | ||||||
|
gait velocity cadence stride length step length step width stance phase (%GC) swing phase (%GC) double support (%GC) UPDRS-III Tinetti SPPB 4 m test FES FOG-Q |
↑ ** ↑ * ↑ ns ↑ ** = ns ↓ ns ↑ *** ↓ ** ↓ *** ↑ * ↓ * ↓ * ↓ ** |
↑ *** ↑ * ↑ ns ↑ *** ↑ *** ↓ ns ↑ * ↓ * ↓ *** ↑ * ↓ ** ↓ * ↓ * |
|||||
|
Nieuwboer et al. (2009) non-RCT |
PD (H&Y: 2–4) - N: 133 - 66.6 - 78/55 |
RAS - 3 different cue modalities: auditory, visual or somatosensory - synchronize steps with the rhythmical auditory tone Procedure - walk to a chair, placed 6 m away, pick up a tray with 2 cups, turn 180° and carry the tray back to the start position - baseline 1, no cue - 3 × 2x cue trials - baseline 2, no cue Medication: dopaminergic medication, ON phase |
cueing device, worn on a belt around the waist; auditory tone was delivered via an earphone portable data recorder worn on a belt around the waist 5 accelerometers attached to the body - 2 × legs - 3 × sternum |
PD: turn times compared with baseline - auditory cue trial: ↓ ** - baseline 2 trial: ↓ *** |
|||
|
Park et al. (2015) RCT pilot study |
Chronic stroke TRAS group - N: 9 - 51.8 - 4/5 Chronic stroke ORAS group - N: 10 - 55.0 6/4 |
RAS - metronome program for computers - increase tempo of RAS each week: w1 90%, w2 100% and w3 110% Procedure - 5x/w, 30’, for 3w + NDT - performed walking training (3 × 10’ RAS + 1’ no RAS + 2’ rest) - stepping in time with RAS • TRAS: treadmill walking with RAS • ORAS: overground walking with RAS (10 m walking path) |
headphones |
TRAS: pre vs post training - gait velocity: ↑* - step cycle: ↑ * - step length (AS): ↑* - step length (NAS): ↑ * - TUG: ↓ ns - 6MWD: ↑ * - FGA: ↑ * ORAS: pre vs post training - gait velocity: ↑ ns - step cycle: ↓ ns - step length (AS): ↑ ns - step length (NAS): ↑ ns - TUG: ↓ ns - 6MWD: ↑ * - FGA: ↑ * |
|||
|
Park et al. (2021) non-RCT |
PD (H&Y: 1–3) - N: 20 - 68.9 - 13/7 |
RAS - 1 familiar song, tempo 90–120 bpm - 1 unfamiliar song, tempo 107 or 120 bpm (acc. cadence P) - tempo of music cues: adjusted to cadence P - volume of cues: 89 dB Procedure - walk around boundaries of indoor gym court 29 × 15 m - baseline: 2’ walking without cues - session 1: 2’ walking to familiar & unfamiliar music - session 2: gait trails with familiar & unfamiliar music Medication: dopaminergic medication, ON phase |
Ambulatory Parkinson’s disease Monitoring system 6 sensors with a 3D- accelerometer, gyroscope and magnetometer - 2 × feet - 2 × wrist - 1 × waist - 1 × sternum headphones |
PD: baseline vs session 1 and session 2 | |||
|
Familiar music gait velocity stride length cadence stride time arm swing peak velocity arm swing ROM Unfamiliar music gait velocity stride length cadence stride time arm swing peak velocity arm swing ROM |
Session 1 ↑ *** ↑ *** ↑ ns ↓ ns ↑ *** ↑ *** Session 1 ↑ ** ↑ ** ↑ * ↓ * ↑ ** ↑ ** |
Session 2 ↑ ns ↑ ns ↑ ns ↓ ns ↑ ns ↑ ns Session 2 ↑ * ↑ * ↑ns ↓ ns ↑ns ↑ns |
|||||
|
Park et al. (2020) non-RCT |
PD (H&Y: 1–2.5) - N: 23 - 69.5 15/8 |
RAS: - neutral: isochronous drumbeat of 110 Hz - pleasant: one song out of favourite music P at 91–127 bpm - unpleasant: disharmonious counterparts of pleasant music - volume of rhythmic auditory cues: 89 dB - tempo of rhythmic auditory cues: matched to pace P Procedure - walk around boundaries of indoor gym court 29 × 15 m - 2’ walking by stepping into the rhythm of the auditory cue - 3 × 2’ walking in time with the auditory cues Medication: dopaminergic medication, ON phase |
Ambulatory Parkinson’s disease Monitoring system 6 sensors with a 3D-accelerometer, gyroscope and magnetometer - 2 × feet - 2 × wrist - 1 × waist - 1 × sternum headphones |
PD: gait velocity change (%) - neutral < pleasant: ** - neutral < unpleasant: ns - pleasant > unpleasant: ** PD: stride length change (%) - neutral < pleasant: ** - neutral < unpleasant: ns - pleasant > unpleasant: * PD: arm swing velocity change (%) - neutral < pleasant: * - neutral < unpleasant: ns - pleasant > unpleasant: ns PD: arm swing ROM change (%) - neutral < pleasant: * - neutral < unpleasant: ns - pleasant > unpleasant: * |
|||
| PD: change (%) from baseline for the different conditions | |||||||
| neutral | pleasant | unpleasant | |||||
|
gait velocity stride length arm swing peak velocity arm swing ROM |
↑ ns ↑ ns ↑* ↑ ns |
↑** ↑** ↑** ↑** |
↑ ns ↑* ↑** ↑** |
||||
|
Shahraki et al. (2017) RCT |
MS—RAS group - N: 9 - 40.3 - 2/7 MS—control group - N: 9 - 38.1 - 2/7 |
RAS - metronome beat, 10% higher than preferred cadence Procedure - match steps to metronome beat - walk 6 m, rotate 180° and return - 3x/w, 30’, for 3w |
headphones |
MS—RAS group: pre vs post after training program - stride length: ↑ * - stride time: ↓ * - double support time: ↓ * - cadence: ↑ * - gait velocity: ↑ * |
|||
|
Thaut et al. (1996) RCT |
PD (H&Y: 2.4) RAS group - N: 15 - 69.0 - 10/5 PD (H&Y: 2.6) no training group - N: 11 - 71.0 - 8/3 PD (H&Y: 2.5) self-paced group - N: 11 - 74.0 - 8/3 |
RAS - 4 instrumental music tapes of 30’ ( folk, classical, jazz, country) - 3 different tempos: normal, quick and fast - tempo increased each week with 5 to 10% Procedure - 7x/w, 30’, for 3w - walking on a flat surface, stair stepping, and stop-and-go exercises to rhythmically accentuated music, 10’ each tempo - pre: walking at normal speed, without rhythmic timekeeper - training period of 3 weeks, - post: walking at normal speed, without rhythmic timekeeper Medication: dopaminergic medication, ON phase |
portable tape players with headphones |
PD—RAS group: pre vs post after 3 weeks - gait velocity (flat): ↑ ** - gait velocity (inclined): ↑ ** - cadence (flat): ↑ ** - stride length (flat): ↑ ** PD—RAS vs no training & self-paced group after 3 weeks - gait velocity on flat surface: ↑ * - gait velocity on inclination: ↑ * - cadence: ↑ * stride length: ↑ * |
|||
|
Thaut et al. (2019) RCT |
PD (H&Y:3–4) RAS group - N: 30 (5) - 71.0 - 17/13 PD (H&Y: 3–4) discontinued RAS group - N: 30 (8) - 73.0 - 15/15 |
RAS - metronome click-embedded music - folk and classical instrumental music - metronome beats were inserted into the music - week 1–8: frequency 100%, 105% and 110% of cadence - week 8–16: frequency 105%, 110% and 115% of cadence - week 16–24: frequency 110%, 115% and 120% of cadence Procedure - 7x/w, 30’, for 24w - home-based gait training with RAS - group 1: RAS training week 1–24 - group 2: RAS training week 1–8 & week 16–24 - assessment at baseline, week 8, 16 and 24 Medication: dopaminergic medication, ON phase |
stride analyzer system: portable microprocessor worn on a gait belt, 4 sensors worn imbedded in the insoles of shoes MP3-player headphones |
PD: baseline vs week 8, 16 and 24 | |||
|
RAS cadence gait velocity stride length DF ankle L DF ankle R Fall index TUG BBS discontinued RAS cadence gait velocity stride length DF ankle L DF ankle R Fall index TUG BBS |
week 8 ↑ ns ↑ ns ↑ ns ↑ ns ↑ ns ↓ ns ↓ ns ↑ ns week 8 ↑ ns ↑ ns ↑ ns ↑ ns ↑ ns ↓ ns ↓ ns ↑ ns |
week 16 ↑ ns ↑ ** ↑ ** ↑ * ↑ * ↓ ** ↓ ns ↑ ns week 16 ↑ ns ↓ ns ↓ ns ↑ ns ↑ ns ↓ ns ↓ ns ↑ ns |
week 24 ↑ * ↑ ** ↑ ** ↑ * ↑ * ↓ ns ↑ ns ↑ ns week 24 ↑ ns ↑ ns = ns ↑ ns ↑ ns ↓ ns ↑ ns ↑ ns |
||||
|
Uchitomi et al. (2016) non-RCT |
PD (H&Y: 2.8) experimental group - N: 30 - 74.9 - 16/14 Healthy controls - N: 18 - 70.6 - 12/6 |
RAS - interactive rhythmic cues generated by WalkMate - interpersonally synchronized with gait rhythm of P Procedure - walking along 80 m corridor in a straight line - pre-interaction condition: walking alone without audible cues - interaction condition: walking and listening to interactive rhythmic cues - post-interaction condition: walking alone without audible cues Medication: dopaminergic medication |
Walk-Mate system headphones + foot pressure sensors |
PD: rates of change in stride interval - pre-interaction < interaction: *** - pre-interaction < post-interaction: *** - pre-interaction < control group: *** - interaction < post-interaction: ns - interaction < control: ns - post-interaction < control: ns PD: mean stride interval - pre-interaction vs control: ns - interaction vs control: ns - post-interaction vs control: ns |
|||
|
Uchitomi et al. (2013) RCT |
PD (H&Y: 2.4) - N: 32 - 70.4 - 18/14 |
RAS - interactive WalkMate, rhythmic cue - fixed tempo cue - 1/f fluctuating tempo cue - no cue Procedure - walking along a 200 m corridor - gait experiment program of 4 days, 3 walking trials per day (d4: only baseline trial) - 1 × baseline trial: walking alone without rhythmic cues - 2 × rhythmic cue trial: walking with a condition of rhythmic cues Medication: dopaminergic medication, ON phase |
Walk-Mate system headphones + foot pressure sensors |
PD: gait relearning effect in fractal scaling of stride intervals - interactive WalkMate > no cue: * - interactive WalkMate > fixed tempo: * - interactive WalkMate > 1/f fluctuating tempo: * PD: stride intervals synchronization with rhythmic cue - fixed tempo: ns - 1/f fluctuating tempo: ns - interactive WalkMate: ** |
|||
|
Hutin et al. (2024) RCT |
PD (H&Y: 2–3) - N: 15 - 70 8/7 |
RAS - RAC: rhythmic auditory cue • constant stimulation • 110% above patients cadence • using numeric metronome - ASAC: adaptive spatial auditory cue • verbal instruction if stride length is less than predetermined threshold • threshold is 110% of patient’s stride length • using GAIT Tutor ® Procedure - 20 min gait training with RAC - 20 min gait training with ASAC - 1 week apart - walking around a 21.6 m oval walkway - gait assessment: • T0: before intervention • T1: just after intervention • T2: 20 min after intervention Medication: dopaminergic medication, OFF phase |
Headphones + smartphone attached to patient’s waist using a belt RAC: Natural Metronome, version 1.6.2, APK, Single Minded Productions, LLC, Margate, FL, USA ASAC: GAIT Tutor® + 3 IMU’s (sternum & shoes) |
PD: RAC & ASAC T1 vs T0 - gait velocity: ↑* - step length: ↑* - cadence: ns PD: RAC & ASAC T2 vs T0 - gait velocity: ↑* - step length: ↑* - cadence: ↑* PD: RAC & ASAC T1 vs T2 - ns - PD: 20-min walking distance - ASAC > RAC: ** |
|||
RCT: randomized controlled trial; N: sample size group; n: sample size subgroup; MS: multiple sclerosis; PD: Parkinson disease; APD: atypical parkinsonian disorders; CS: chronic stroke; H&Y: Hoehn and Yahr Scale; FOG: freezing of gait; PSP: Progressive Supranuclear Palsy; CBS: Corticobasal Syndrome; MSA: Multiple System Atrophy; DLB: Dementia with Lewy Bodies; NDT: neurodevelopmental therapy; TRAS: treadmill walking with RAS; ORAS: overground walking with RAS; PT: physiotherapy; BATRAC: Bilateral Arm Training with Rhythmic Auditory Cueing; DMTE: Dose Matched Therapeutic Exercises; RAS: rhythmic auditory stimulation; P: patient; ‘: minute; “ : second; ms: milliseconds; h: hour; d: day; w: week; m: meter; min.: minimal, at least; Hz: Hertz; bpm: beats per minute; dB: decibel; BiBS: Binaural beat stimulation; CAS: Conventional acoustic stimulation; L/R: left/right; SIP: stepping-in-place; MT: mono task; DT: dual task; HBWP: home-based walking program; ConCue: continuous cueing; IntCue: intelligent cueing; IntFB: intelligent feedback; NoInfo: no information; RAC: rhythmic auditory cueing; MAC: melodic auditory cueing; NAC: no auditory cueing; IMUs: inertial measurements units; APDM system: Ambulatory Parkinson’s Disease Monitoring system; AS: affected side; NAS: non-affected side; GC: gait cycle; UE: upper extremity; FES: Falls Efficacy Scale; FOG-Q: Freezing of Gait Questionnaire; 6MWT: 6 min walking test; 3MWT: 3 min walking time test; UPDRS-III: Unified Parkinson Disease Rating Scale Part 3; UPDRS-II: Unified Parkinson Disease Rating Scale Part 2; T25FW: Timed 25-Foot Walk; DFA: Detrended Fluctuation Analysis; DGI: dynamic gait index; FSST: Four Square Step Test; SPPB: Short physical performance battery; 6MWD: 6 min walking distance; FGA: functional gait assessment; ROM: range of motion; DF: dorsiflexion; TUG: timed up and go; BBS: Berg balance scale; ↑: increase of value; ↓: decrease of value; = : value is the same; vs: versus, compared to; ns: not significant (p-value > 0.05); *: p-value ≤ 0.05; **: p-value ≤ 0.01; ***: p-value ≤ 0.001; EEG: electroencephalogram; EMG: electromyogram; ex.: example.
Data items
The extracted data consisted of several components; (1) general information about the article such as author, publication year and study design; (2) population information including the type of neurological disorder, sample size, number of dropouts, mean age and gender ratio; (3) data on the intervention and wearables used; (4) results regarding RAS intervention and motoric parameters.
Methodology study risk of bias assessment
The risk of bias of the included articles was assessed under double-blind conditions by all three investigators. Any disagreements between the researchers regarding the risk of bias, was resolved via consensus. A summary of this process is described on Table 5. The quality of the included articles was assessed using the QualSyst tool27. This tool can be used for a variety of primary research articles and is made up of two systems. The first of these two systems is used for qualitative research, whilst the other is used to assess quantitative studies. Due to the nature of this review, only the quantitative analysis system was used to assess all potential studies. A score indicating the internal validity of the article can be calculated based on fourteen questions listed in Table 3. The score corresponds to the percentage of confidence and can be calculated by a formula based on the number of times researchers provided certain answers during the assessment of each article. A higher percentage indicates a lower risk of bias.
Table 5.
Study risk of bias assessment.
| Author (year) | Criteria | Score (%) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | ||
| Baram et al. (2007)46 | 2 | 2 | 1 | 2 | – | – | – | 2 | 1 | 1 | 0 | 1 | 2 | 2 | 73 |
| Calvano et al. (2023)28 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 96 |
| Chomiak et al. (2017)40 | 2 | 2 | 2 | 2 | – | – | –– | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 100 |
| Cochen De Cock et al. (2021)47 | 2 | 2 | 1 | 2 | – | – | – | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 95 |
| Collimore et al. (2023)44 | 2 | 2 | 1 | 2 | – | – | – | 2 | 1 | 2 | 2 | 1 | 2 | 2 | 86 |
| Conklyn et al. (2010)29 | 2 | 2 | 2 | 2 | 2 | 0 | – | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 92 |
| De Bartolo et al. (2019)48 | 2 | 2 | 1 | 2 | – | – | – | 2 | 2 | 2 | 0 | 1 | 2 | 2 | 91 |
| Elsner et al. (2019)30 | 2 | 2 | 2 | 2 | 2 | 2 | – | 2 | 1 | 2 | 0 | 2 | 2 | 2 | 96 |
| Erra et al. (2019)49 | 2 | 2 | 2 | 2 | – | – | –– | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 95 |
| Ginis et al. (2017)41 | 2 | 2 | 2 | 2 | – | – | – | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 100 |
| Ginis et al. (2017)42 | 2 | 2 | 2 | 2 | – | – | – | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 100 |
| Guimarães et al. (2015)51 | 2 | 2 | 0 | 2 | – | – | – | 1 | 1 | 2 | 2 | 1 | 2 | 2 | 73 |
| Hove et al. (2012)52 | 2 | 2 | 1 | 2 | – | – | – | 1 | 2 | 2 | 0 | 0 | 2 | 2 | 77 |
| Hutchinson et al. (2020)45 | 2 | 2 | 2 | 2 | – | – | – | 2 | 1 | 2 | 2 | 0 | 2 | 2 | 86 |
| Kim et al. (2012)31 | 2 | 2 | 2 | 2 | 2 | 0 | 0 | 2 | 1 | 1 | 0 | 1 | 2 | 2 | 64 |
| Li et al. (2022)32 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 86 |
| Lopez et al. (2014)54 | 2 | 2 | 2 | 2 | – | 2 | – | 2 | 1 | 2 | 0 | 0 | 2 | 2 | 79 |
| Mainka et al. (2018)33 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 89 |
| Moumdjian et al. (2019)43 | 2 | 2 | 2 | 2 | – | – | – | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 100 |
| Murgia et al. (2018)34 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 93 |
| Nieuwboer et al. (2009)50 | 2 | 2 | 2 | 2 | – | 2 | – | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 96 |
| Park et al. (2015)35 | 2 | 2 | 2 | 2 | 2 | 0 | 0 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 75 |
| Park et al. (2021)55 | 2 | 2 | 2 | 2 | – | – | – | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 95 |
| Park et al. (2020)56 | 2 | 2 | 2 | 2 | – | – | – | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 95 |
| Shakraki et al. (2017)36 | 2 | 2 | 2 | 2 | 2 | 0 | 0 | 2 | 1 | 2 | 0 | 1 | 2 | 2 | 71 |
| Thaut et al. (1996)37 | 2 | 2 | 1 | 2 | 2 | 0 | 0 | 2 | 2 | 2 | 0 | 1 | 2 | 2 | 71 |
| Thaut et al. (2019)13 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 93 |
| Uchitomi et al. (2016)53 | 2 | 2 | 2 | 2 | – | – | – | 1 | 2 | 2 | 0 | 1 | 2 | 2 | 82 |
| Uchitomi et al. (2013)38 | 2 | 2 | 1 | 2 | 2 | 0 | 0 | 2 | 2 | 2 | 0 | 1 | 2 | 2 | 71 |
| Hutin et al. (2024)39 | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 93 |
Legenda: 0 = no; 1 = partial; 2 = yes; – = not applicable
Score calculation: ((# yes × 2) + (# partials × 1)) – (28 – (# not applicable * 2)) × 100.
Methodology meta-analysis
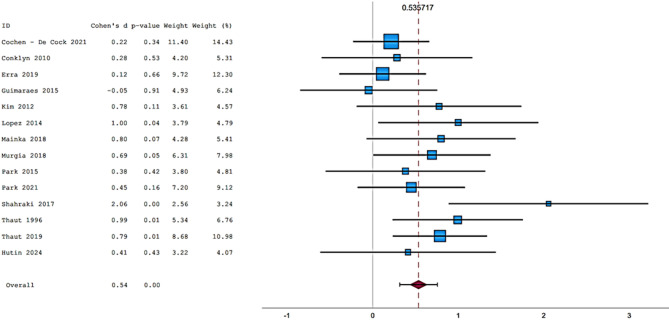
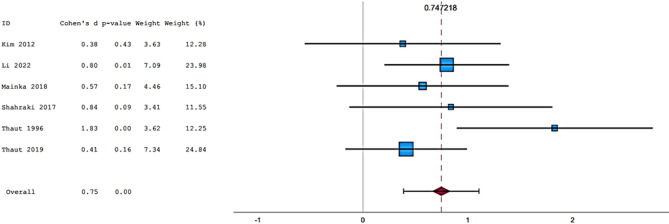
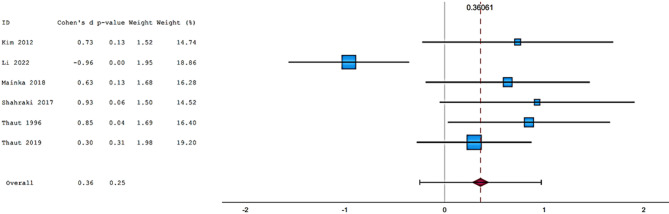
Fifteen articles were included in the meta-analysis. However, only articles reporting data on gait were included in the meta-analysis due to the insufficient number of studies providing outcome data related to the upper limb. The parameters assessed in the meta-analysis concerning gait were gait velocity, stride length, and cadence. The included articles provided data on individuals who received RAS-therapy (experimental group) compared to control group and/or data on pre-intervention versus post-intervention outcomes. The data was firstly entered manually into Microsoft Excel and then exported to IBM SPSS Statistics (version 29.0.2.0) for the analysis of the data. The aforementioned parameters were statistically examined in a longitudinal design using a random effects model. Additionally, heterogeneity was assessed using the I2 statistic and Cochran’s Q test.
Results
Study selection
In total, 7993 records were found in multiple databases. After removing duplicates, the remaining 4157 articles were screened based on their title and abstract. Following the initial screening process, a secondary full-text screening of the remaining eighty-three articles left thirty studies to be included in the final systematic review. The references pertaining to the included studies can be found within the bibliography. In addition, the study selection process can be referred to in Fig. 1.
Risk of bias within studies
The thirty articles included in this review were assessed using the QualSyst risk of bias tool. Table 5 provides a detailed description of the assessment. Risk of bias is determined based on several factors including; blinding, outcome measures, analysing process, estimation of variance and lastly identification of confounding factors. Thirteen papers are randomized controlled trails (RCT)13,28–39 of which six13,28–30,34,39 have a percentage of confidence higher than ninety percent, indicating a very low risk of bias. Li et al.32 and Mainka et al.33 have a percentage between eighty and ninety percent. Kim et al.31 achieves an internal validity of sixty-four percent, making it the article with the lowest score of all RCT studies. The remaining four RCTs35–38 have a score ranging between seventy and eighty percent. The other seventeen articles have scores ranging from seventy to a hundred percent. Chomiak et al.40, Ginis et al.41, Ginis et al.42 and Moumdjian et al.43 have achieved a hundred percent internal validity.
Study characteristics
In Table 6, a summary of characteristics (incl. study design, sample size, mean age, gender ratio, duration of intervention, type of RAS, used wearable devices and overall outcome) of the included studies is shown. Refer to Table 4 for detailed information regarding intervention procedures and results.
Table 6.
Study characteristics.
| Author (year) | Study design | Sample size (dropouts) |
Mean age | ♂/♀ | Intervention duration |
RAS | Wearable device(s) |
Outcome |
|---|---|---|---|---|---|---|---|---|
| Baram et al. (2007)46 | non-RCT | 14 MS + 11 healthy | 48.6 25.5 |
4/10 5/6 |
1 session | adaptive |
headphones sensor |
gait parameters |
| Calvano et al (2023)28 | RCT | 25 PD | 61.0 | 15/10 | 2 sessions in 2 days | fixed-tempo |
headphones sensors MP3-player |
motor function |
| Chomiak et al (2017)40 | non-RCT (pilot) | 11 PD | 69.9 | 9/2 | 3x/w, 10–20’, 4w | fixed-tempo |
headphones sensors |
step automaticity clinical test(s)/ questionnaire(s) |
| Cochen De Cock et al. (2021)47 | non-RCT | 45 (6) PD | 65.0 | 15/20 | 5x/w, 30’, 4w | adaptive |
BeatWalk smartphone application headphones sensors |
gait parameters clinical test(s)/ questionnaire(s) |
| Collimore et al. (2023)44 | non-RCT | 10 chronic stroke | 60.2 | 7/3 | 1 session | adaptive |
headphones sensors |
gait asymmetry |
| Conklyn et al (2010)29 | RCT (pilot) | 10 MS | 48.6 | 3/7 | 7x/w, 20’, 2 or 4w | fixed-tempo |
headphones MP3-player |
gait parameters |
| De Bartolo et al (2020)48 | non-RCT | 20 PD | 72.5 | 14/6 | 1 session | fixed-tempo |
headphones sensor |
gait parameters |
| Elsner et al (2020)30 | RCT (pilot) | 12 chronic stroke | 67.0 | 3/9 | 3x/w, 30’, 4w | fixed-tempo |
headphones MP3-player |
gait parameters clinical test(s)/ questionnaire(s) |
| Erra et al (2019)49 | non-RCT | 30 PD | 72.0 | 20/10 | 1 session | fixed-tempo |
headphones sensors |
gait parameters |
| Ginis et al (2017)41 | non-RCT | 28 PD | 62.0 | 23/5 | 1 session |
2 conditions adaptive 1 condition fixed-tempo |
headphones sensors |
gait deviations |
| Ginis et al (2017)42 | non-RCT | 28 PD + 13 healthy |
62.0 60.2 |
23/5 7/6 |
1 session |
2 conditions adaptive 1 condition fixed-tempo |
headphones sensors |
gait parameters |
| Guimarães et al (2015)51 | non-RCT | 12 PD | 71.2 | 7/5 | 1 session | fixed-tempo | headphones | gait parameters |
| Hove et al (2012)52 | non-RCT | 20 PD + 18 healthy |
69.2 24.7 |
8/12 16/2 |
1 session |
1 condition adaptive 1 condition fixed-tempo |
WalkMate headphones sensors |
fractal scaling |
| Hutchinson et al (2020)45 | non-RCT | 11 chronic stroke | 57.7 | 9/2 |
1 or 3 session(s) in 1 or 3 days |
adaptive |
headphones sensors |
gait parameters |
| Kim et al (2012)31 | RCT | 18 (2) subacute stroke | 55.1 | 13/7 | 3x/w, 30’, 5w | fixed-tempo | earphones |
gait parameters clinical test(s)/ questionnaire(s) |
| Li et al (2022)32 | RCT | 40 PD | 63.8 | 34/36 | 5x/w, 1h, 4w | fixed-tempo |
headphones music player |
gait parameters clinical test(s)/ questionnaire(s) |
| Lopez et al (2014)54 | non-RCT | 10 PD | 55.0 | 7/3 | 1 session | fixed-tempo |
Listenmee® smartphone application headphones glasses smartwatch |
gait parameters |
| Mainka et al (2018)33 | RCT | 45 (10) stroke | 63.4 | 26/9 | 5x/w, 15’-20’, 4w | fixed-tempo |
earplugs MP3-player |
gait parameters clinical test(s)/ questionnaire(s) |
| Moumdjian et al (2019)43 | non-RCT | 31 (4) MS + 30 (2) healthy |
53.5 51.8 |
8/23 8/22 |
1 session | adaptive |
D-Jogger headphones music player sensors |
gait parameters |
| Murgia et al (2018)34 | RCT | 38 (6) PD | 68.2 | / | 2x/w, 45’, 5w + train 3x/w at home | fixed-tempo |
headphones MP3-player |
gait parameters clinical test(s)/ questionnaire(s) |
| Nieuwboer et al (2009)50 | non-RCT | 133 PD | 66.6 | 78/55 | 1 session | fixed-tempo |
earphone sensors |
functional turning performance |
| Park et al (2015)35 | RCT (pilot) | 19 chronic stroke | 53.4 | 10/9 | 5x/w, 30’, 3w | fixed-tempo | headphones |
gait parameters clinical test(s)/ questionnaire(s) |
| Park et al (2021)55 | non-RCT | 20 PD | 68.9 | 13/7 | 2 sessions in 1 day | fixed-tempo |
APDM system headphones sensors |
gait parameters arm swing |
| Park et al (2020)56 | non-RCT | 23 PD | 69.5 | 15/8 | 1 session | fixed-tempo |
APDM system headphones sensors |
gait parameters arm swing |
| Shahraki et al. (2017)36 | RCT | 18 MS | 39.2 | 4/14 | 3x/w, 30’, 3w | fixed-tempo | headphones | gait parameters |
| Thaut et al (1996)37 | RCT | 37 PD | 71.3 | 26/11 | 7x/w, 30’, 3w | fixed-tempo |
headphones music players |
gait parameters clinical test(s)/ questionnaire(s) |
| Thaut et al (2019)13 | RCT | 60 (13) PD | 72.0 | 32/28 | 7x/w, 30’, 24w | fixed-tempo |
headphones MP3-player sensors |
gait parameters clinical test(s)/ questionnaire(s) |
| Uchitomi et al (2016)53 | non-RCT | 30 PD + 18 healthy |
74.9 70.6 |
16/14 12/6 |
1 session | adaptive |
Walk-Mate headphones sensors |
stride interval |
| Uchitomi et al (2013)38 | RCT | 32 PD | 70.4 | 18/14 |
4 sessions in 4 days |
2 conditions fixed-tempo 1 condition adaptive |
Walk-Mate headphones sensors |
stride interval |
| Hutin et al (2024)39 | RCT | 15 PD | 70 | 8/7 | 2 sessions | adaptive |
headphones smartphone applications sensors GAIT Tutor ® |
gait parameters clinical test(s)/ questionnaire(s) |
Study demographics
In the six studies on stroke30,31,33,35,44,45, 68 participants are men, while 39 participants are women. The youngest reported mean age of participants is 53 years35 while the oldest reported mean age is 67 years30. The studies have been conducted in the United States of America (Massachusetts)44,45, Germany30,33 and Republic of Korea31,35. The disease duration, defined as the time since the stroke occurred, ranges from 1.4 months to 99.5 months (8.3 years).
The distribution of sex in the multiple sclerosis studies by Baram et al.46, Conklyn et al.29, Moumdjian et al.43, and Shahraki et al.36 shows that eighty-two participants are women, while only thirty-two are men. The reported mean ages of participants were 49 years29,46, 54 years43, and 39 years36. The studies are conducted in Israel46, Ohio29, Belgium43 and Iran36, covering multiple continents of the world. The mean disease duration lays between 9 and 17 years amongst the studies. The expanded disability status scale (EDSS) reported by Baram et al.46 ranges from a score of three and a half to six, while Shahraki et al.36 reported a range from three to six.
For Parkinson’s disease, a total of 596 participants of which 358 male and 200 female were examined within RAS conditions amongst 20 studies. Only one study by Murgia et al.33 didn’t mention the gender ratio. Within the studies, 61 years27 was the lowest mean age, whilst 75 years48 was the oldest. Like within the subgroups of MS and stroke, the studies were conducted around the world in different continents except for Africa and Oceania. The studies were conducted in Germany28, Canada13,40, France47, Italy34,42,48,49, Belgium41,50, Portugal and Spain51, Japan38,52,53, China32, Brazil54 and the United States of America37,55,56 All studies were conducted on participants with a Hoehn and Yahr scale between one and four.
Results of the individual studies – intervention
Looking at all the included studies, different modalities and types of RAS are used. On one hand, fixed-tempo RAS are used (e.g., metronomic beats matching the subjects´ cadence). On the other hand, an adaptive RAS (real-time adaptive stimulus interacting with the subjects´ gait pattern) could be implemented. Wearable systems such as BeatWalk47 and WalkMate38 have recently been invented, making it possible to investigate this type of RAS. BeatWalk includes an application that adapts the tempo of music in order encourage the synchronisation of the subjects´ gait with the auditory stimulus47. The WalkMate system (used in the studies of Hove et al.52 and Uchitomi et al. (2016 and 2013)38,53), generates rhythmic cues, interacting interpersonally with the individual gait rhythm of the participants. However, WalkMate uses pressure sensors in the shoes of the subjects and a real-time computer to either speed up or slow down the provided stimulus based on the speed of the subjects´ footsteps. This, in turn, has an impact on the gait timing52. The point of interest is to report the results of adaptive RAS versus fixed-tempo RAS to create an overview of any notable differences in the results. As mentioned in the introduction, the adaptive RAS might be more effective.
Adaptive RAS
Up until now, literature investigating adaptive RAS has been focussed solely on its impact on gait patterns. Baram et al.46 provided subjects with an auditory stimulus in the form of a ‘click’ every time the subject takes a step. The goal of this approach is to encourage subjects to create an even, rhythmic pattern, leading to an improved gait, significant for gait velocity and stride length. Cochen De Cock et al.47 used BeatWalk to improve the subjects´ cadence. The results of the 6MWT showed statistically significant improvements related to distance, cadence, gait velocity and stride length. Collimore et al.44 also studied the effect of adaptive RAS on the subjects´ gait, showing a significant statistical reduction in gait asymmetry, stance time asymmetry, swing time asymmetry and step time asymmetry (no significant reduction in neither step length asymmetry nor cadence was mentioned). Ginis et al.41 investigated both types of RAS (adaptive vs. fixed). Participants are subjected to either intelligent cueing (ten beats corresponding to the reference cadence) or intelligent feedback (verbal commands to either increase or decrease their tempo). Both feedback and cueing are provided when the mean of five consecutive left and right strides deviate more than five percent compared to the reference cadence. Participants receiving these stimuli show fewer gait deviations than those receiving neither cueing nor feedback. Hove et al.52 found that the Detrended Fluctuation Analysis of the fractal scaling exponent was significantly greater during and post-treatment (using the WalkMate system) when compared to silent control and fixed-tempo RAS trials. Uchitomi et al. (2016)53 determined that the rates of change in stride interval are significantly greater during and after the interactive WalkMate condition when compared to the pre-interaction condition where subjects walked without RAS. In another study, Uchitomi et al. (2013)38 demonstrated how the gait relearning effect in fractal scaling of stride intervals increased significantly following the interactive WalkMate trials, whereas no significant effects were observed in those receiving no cue, fixed cues and/or 1/f fluctuation cues. Furthermore, significant stride interval synchronisation was observed only in the interactive WalkMate intervention group. Hutchinson et al.45 used a sensor to measure the cadence of the subjects and subsequently applied specific algorithms, modifying the auditory stimulus in such a way to set a new target cadence. As a result, the subject is encouraged to adapt to the newly set target cadence by adjusting their gait pattern. Following one session, subjects demonstrated a statistically significant increase in both standard as well as fast gait velocity. Those participants who completed all three sessions had significantly increased both their standard as well as their fast cadence and walking velocity. Moumdjian et al.43 adopted the use of D-Jogger, an adaptive media player in which the tempo of the musical beats and metronomic ‘ticks’ are altered to match the tempo of the individual using this system. The use of this program lead to significant improvements regarding participant cadence when compared to receiving no stimulus. Interestingly, gait velocity was measured to be slower when walking to metronomic beats than when walking to music. In addition, stride length was shorter when walking to music than when walking to a metronomic stimulus. Hutin et al.41 used both types of RAS, the participants walked with a constant metronome stimulation and with an adaptive spatial auditory cue. In the adaptive cueing, they receive a verbal instruction to lengthen their steps if the threshold of 110 percent of the patient’s stride length is not achieved. For both types of interventions, the gait velocity, step length and cadence increased significantly comparing before the intervention and 20 min after the end of the intervention. But remarkable is that the 20-min walking distance is 15% higher while using adaptive spatial auditory cue comparing to a simple rhythmic auditory cue.
Fixed-tempo RAS
In general, most studies used a fixed tempo RAS, which could be a music track, or a metronomic beat. In some studies, the beat of the song is accentuated to make it clearer. Chomiak et al.40 demonstrated a significantly higher dual task step automaticity in subjects who trained using musical stimuli compared to those using stimuli such as podcasts. A multitude of recent studies have shown that training with RAS results in a significant increase in gait velocity, cadence, and stride length. Having said this, Mainka et al.33 and Shahraki et al.36 have all similarly demonstrated this effect. Furthermore, Li et al.32 and Lopez et al.54 have also shown the positive impact of being exposed to a RAS intervention when comparing intervention groups (RAS groups) to non-intervention groups. Although research into these RAS interventions appears promising, not all gait parameters are equally influenced, leading to some parameters enjoying statistically significant improvements, whilst others do not. Looking at parameters such as gait velocity, cadence and stride length, Murgia et al.34 demonstrated statistically significant improvements in regard to both gait velocity and cadence but not stride length. Park et al. (2015)35 found there to be an increase in gait velocity and bilateral stride length. This was especially significant when subjects were tested on a treadmill instead of normal pavement. Park et al. (2021)55 went on to show that gait velocity, stride length and cadence all improved when being exposed to familiar musical stimuli. In addition, Park et al. (2020)56 suggested that, based on their research, neutral musical stimuli lead to non-significant improvements in terms of gait velocity and stride length, pleasant musical stimuli lead to significant improvements in gait velocity and stride length and unpleasant musical stimuli lead to non-significant improvements to gait velocity, but significant improvements to stride length. Calvano et al.28 showed no effect of binaural beats and/or conventional acoustic stimulation on walking. Elsner et al.30 also showed no significant differences between the RAS intervention group and the non-intervention group.
Results of the individual studies—population
The research question included all neurological disorders. However, following the selection process, neurological disorders were limited to the following; PD, MS and stroke. Twenty articles specifically investigated PD, six articles investigated stroke and four articles investigated MS.
Effect of RAS in multiple sclerosis
When reviewing the results of Baram et al.46, Conklyn et al.29, Moumdjian et al.43 and Shahraki et al.36, different outcome measurements were identified and subsequently analysed. Certain outcome measures are consistent across several studies such as gait velocity, cadence, and stride length. Baram et al.46 aimed their study at investigating the impact of auditory feedback cues within a closed-loop system (in response to the steps of the patient) on gait management and rehabilitation. Both gait velocity and stride length were improved. Conklyn et al.29 focused on the evolution of gait parameters when RAS interventions are applied. A significant improvement of the double support time was observed when comparing baseline measurements with those taken after three weeks of RAS training. When effects were analysed after one week, significant improvements were identified in relation to cadence, stride length, gait velocity, step length and normalized gait velocity. The double support time was not significantly decreased after one week of the intervention. Furthermore, Moumdjian et al.43 found there to be a significant improvement in cadence when using music or a metronome in comparison to the absence of auditory stimuli. In addition, the authors demonstrated a significantly greater increase in gait velocity when musical beats were applied as well as in the absence of auditory stimuli (this in comparison to metronomic stimulation). However, stride length improved more when metronomic stimulation was applied in comparison to musical stimulation. Shahraki et al.36 demonstrated a significant increase in stride length, cadence and gait speed when subjects were exposed to metronomic stimuli. Stride time and double support time also significantly decreased when exposed to the same stimulus.
Effect of RAS in Parkinson’s disease
Cross-study results exhibit discrepancies concerning gait velocity. Both positive and negative effects have been documented. Erra et al.49 provided various different RAS tempos and found differing results. The authors concluded that individualized RAS treatment is needed to achieve optimal results. In most studies, stride length was increased32,34,37,42,48,49,54–56. However, this increase was not always statistically significant. Step length was also improved both significantly (RAS 110) and non-significantly (RAS 90 and RAS 100) in the study of Erra et al.49 for in two studies. Murgia et al.34 also found significant results for step length. Furthermore, the cadence parameter was increased but these increases were often not of any statistical significance13,32,34,37,42,47,54,55. Interestingly, Erra et al.49 discovered (in their study) that the cadence of both the ON- and OFF-group decreased when participants were sorted into RAS 90 groups (significant) and RASS 100–110 groups (non-significant). De Bartolo et al.48, Erra et al.49 and Park et al. (2021)55 additionally investigated stride length. Erra et al.49 reported non-significant increases in stepping time for some subgroups, whilst remaining subgroups demonstrated significant decreases in terms of the same parameter. Arm swing peak velocity and arm swing ROM were also both investigated by Park et al. in both 202056 and 202155. Although the results of their study appeared to be positive, various inconsistencies were identified regarding statistical significance. Thaut et al. (2019)13 examined the ROM of dorsiflexion and demonstrated improvements of both the left and right ankles. In addition, fall index, BBS57 and TUG58 were also investigated. BBS improved non-significantly, whilst both significant and non-significant results were shown in relation to the TUG parameter due to the applied RAS intervention. The fall index was determined at week 16 and had significantly improved. Remaining results related to the fall index were determined to be of no statistical significance. Chomiak et al.40, Li et al. and31urgia et al. 34 examined the impact of RAS interventions on freezing of gait (FOG). Majority of these studies reported decreases in in FOG-related incidents, with the exception of the study carried out by Chomiak et al.40 (no significant effect). Chomiak et al.40, Cochen De Cock et al.47 and Murgia et al.34 focussed their efforts on examining the effects of RAS interventions on the Falls Efficacy Scale (FES). Both the studies carried out by Cochen De Cock et al.47 and Murgia et al.34 identified significant improvements, while Chomiak et al.40 were unable to demonstrate any improvements to the FES following the application of their RAS intervention. The Movement Disorders Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS)59 examined by Li et al.32 and Murgia et al.34 showed significant improvement. Cochen De Cock et al.47 were unable to demonstrate any significant improvements to the MDS-UPDRS score. In relation to the double support time parameter, statistically significant reductions were identified by both Li et al.32 and Murgia et al.34 Furthermore, De Bartolo et al.48 also showed significant reductions in terms of double support time when exposed to musical stimuli, whereas the reductions observed in the remaining subgroups were of no statistical significance. Ginis et al. (2017)41 compared the effect of different types of cueing and feedback on gait deviations. Continuous cueing resulted in decreased gait deviations in comparison intelligent feedback and omission of feedback/cueing. In addition, continuous cueing was shown to be more effective than intelligent cueing at achieving decreased gait deviation. Ginis et al. (2017)41 also showed how both intelligent cueing and intelligent feedback lead to fewer gait deviations than when no stimulus was provided. Uchitomi et al. (2016)53 provided interactive rhythmic cues generated by the WalkMate system and examined their effect on different study groups. The authors concluded that those subjects with a festinating gait possess the ability to relearn a stable gait pattern. The authors also demonstrated that the WalkMate system can aid subjects in this process. These conclusions were made based on changes in subjects´ stride intervals. Uchitomi et al. (2013)38 conducted an RCT investigating the effect of interactive rhythmic cues on gait relearning. Interactive WalkMate appeared to be more effective in improving gait relearning effects in fractal scaling of stride interval and stride interval synchronization than no cues, fixed cues and 1/f fluctuation tempo cues, alike. In the pilot-RCT of Huntin et al. (61) similar effects on walking at free speed between the interventions are found. A significant increase in gait velocity and step length is found for both types of cueing before the intervention compared to just after the intervention and compared to 20 min after the intervention. The increase in cadence is only significant when comparing before and 20 min after the intervention. The total walking distance after 20 min of walking is significant higher for adaptive spatial auditory cueing compared to rhythmic auditory cueing.
Effect of RAS in stroke
The majority of studies30,31,33,35,45 suggest (based on their results) that gait velocity significantly increases following a RAS the intervention in subjects who have suffered a stroke. Three studies30,31,33, demonstrated a statistically significant improvement in relation to stride length. Additionally, a significant increase in cadence was found in a series of studies investigating the effect of RAS on this parameter31,33,45. Collimore et al.44 observed no significant changes to cadence. Walking distance of subjects who had suffered a stroke increased (p < 0.05) following the aforementioned intervention in studies conducted by Elsner et al.30, Mainka et al.33 and Park et al. (2015)35. Park et al. (2015)35 interestingly noted a difference between those walking on treadmills and those walking on pavement. RAS group subjects walking on treadmills showed significant improvements, whilst RAS group subjects walking on pavements only experienced non-significant improvements. Additional studies30,31,35,44 included in this review demonstrated significant improvements in regard to the asymmetry index, step time asymmetry, stance time asymmetry, swing time asymmetry, Berg Balance Scale (BBS), Dynamic Gait Index (DGI), Four Square Step Test (FSST), cycle time, Functional Gait Assessment (FGA), peak acceleration and the Fugl-Meyer Assessment (FMA). Both significant as unsignificant improvements were found for the timed up and go (TUG) test, symmetry in swing ratios, movement time and movement units. For the TUG,31 was significant, but35 was not.
Results meta-analysis
Three gait parameters are examined in the meta-analysis: gait velocity, stride length and cadence, all investigated using a random-effects model. Furthermore, the studies could be divided into two main groups: studies within a longitudinal design, comparing performance before and after the application of RAS stimuli and studies that used a control group. Therefore, when discussing data, a division was made between these two. In the forest plots, Cohen’s d, p-value, weight and weight (%) can be found for each individual study as well as Cohen’s d and p-value for the overall effect.
Gait velocity
For gait velocity in a longitudinal design, 14 trials including results of 231 participants pre and 222 participants post participants were investigated. A significant mean difference in gait velocity was found, favouring rhythmic auditory stimulation compared to pre-RAS values of the same participants suffering from neurological disorders (95% CI = [0.32; 0.76], p < 0.001; Fig. 2), with a low heterogeneity (I2 = 21.1%, 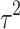 = 0.04, H2 = 1.27, Q = 17.57, df = 13, p = 0.175).
= 0.04, H2 = 1.27, Q = 17.57, df = 13, p = 0.175).
Fig. 2.
Forest plot gait velocity pre vs post.
For gait velocity using RAS, compared to a control group, 6 trials including results of 92 experimental participants and 88 control participants were investigated. A significant mean difference in gait velocity was found, favouring rhythmic auditory stimulation within neurological patients, compared to a control group (95% CI = [0.39; 1.11], p < 0.001; Fig. 3), with a low heterogeneity (I2 = 24.2%, 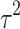 = 0.05, H2 = 1.32, Q = 7.27, df = 5, p = 0.202).
= 0.05, H2 = 1.32, Q = 7.27, df = 5, p = 0.202).
Fig. 3.
Forest plot gait velocity control vs experimental.
Stride length
For stride length in a longitudinal design, 11 trials including results of 202 participants pre and 194 participants post participants were investigated. A significant mean difference in stride length was found, favouring RAS compared to pre-RAS values of the same participants suffering from neurological disorders (95% CI = [0.26; 0.94], p < 0.001; Fig. 4), with a moderate to moderate heterogeneity (I2 = 60.8%, 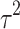 = 0.19, H2 = 2.55, Q = 26.30, df = 10, p = 0.003).
= 0.19, H2 = 2.55, Q = 26.30, df = 10, p = 0.003).
Fig. 4.
Forest plot stride length pre vs post.
For stride length using RAS, compared to a control group, 6 trials including results of 92 experimental participants and 88 control participants were investigated. A significant mean difference in stride length was found, favouring rhythmic auditory stimulation within neurological patients, compared to a control group (95% CI = [0.18; 0.78], p = 0.002; Fig. 5), with a low heterogeneity (I2 = 0.00%, 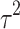 = 0.00, H2 = 1.00, Q = 2.82, df = 5, p = 0.728).
= 0.00, H2 = 1.00, Q = 2.82, df = 5, p = 0.728).
Fig. 5.
Forest plot stride length control vs experimental.
Cadence
For cadence in a longitudinal design, 12 trials including results of 202 participants pre and 193 participants post participants were investigated. A significant mean difference in cadence was found, favouring RAS compared to pre-RAS values of the same participants suffering from neurological disorders (95% CI = [0.34; 1.14], p < 0.001; Fig. 6), with a moderate heterogeneity (I2 = 70.2%, 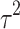 = 0.33, H2 = 3.35, Q = 33.28, df = 11, p < 0.001).
= 0.33, H2 = 3.35, Q = 33.28, df = 11, p < 0.001).
Fig. 6.
Forest plot cadence pre vs post.
For cadence using RAS, compared to a control group, 6 trials including results of 92 experimental participants and 88 control participants were investigated. A non-significant mean difference in cadence was found, favouring rhythmic auditory stimulation within neurological patients, compared to a control group (95% CI = [-0.25; 0.97], p = 0.247; Fig. 7), with a moderate to high heterogeneity (I2 = 73.7%, 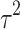 = 0.42, H2 = 3.80, Q = 21.07, df = 5, p < 0.001).
= 0.42, H2 = 3.80, Q = 21.07, df = 5, p < 0.001).
Fig. 7.
Forest plot cadence control vs experimental.
Discussion
The purpose of this systematic review and meta-analysis was to sum up the evidence concerning the topic of wearable rhythmic auditory stimulation (RAS) to enhance the movement of a broad neurological population. The search strategy was not limited to certain neurological disorders. Remarkably, only results related to stroke, multiple sclerosis and Parkinson’s disease were systematically obtained. Currently, only studies that examined the effect of RAS combined with wearable devices in the population of participants with stroke, multiple sclerosis and Parkinson’s disease are published.
All included studies examining the impact of RAS interventions on subjects suffering from MS29,36,43,46 demonstrated significant improvements regarding both gait velocity as well as stride length. Additionally, significant improvements were made to the cadence of said subjects29,36,43. Both metronomic as well as musical stimulation have been shown to be effective and it can be inferred that RAS is a good intervention for those suffering with MS.
A total of twenty studies investigating the effect of RAS interventions on movement (using wearable devices) of subjects suffering with Parkinson’s disease (PD) were included in this review. Analysis revealed conflicting evidence regarding parameters such as gait velocity, double support time and the MDS-UPDRS59. All evidence relating to stride length and step length leaned towards a general positive trend, with some of the included evidence being considered statistically significant for stride length32,37,42,48,49,54–56 and for step length34,39,49. Cadence improved in majority of the included studies where RAS interventions were implemented. However, Erra et al.49 showed the opposite, producing results leaning towards decreased cadence. Arm swing peak velocity was studied by Park et al. in both 202056 and 202155. Their results suggested that arm swing peak velocity increased significantly. In 202155, they also succeeded in demonstrating that a musical stimulus familiar to the subject did not lead to significant increases in either peak arm swing velocity or arm swing ROM (in session two of the experiment). Similarly, exposure to unfamiliar musical stimuli also failed to produce significant increases in these parameters. All in all, literature specifically related to both peak arm swing velocity and arm swing ROM is limited. Therefore, data should be interpreted with caution and further research carried out. Furthermore, studies concerning the effects of RAS interventions on the falls efficacy scale60, Murgia et al.34 and Chomiak et al.40 showed improvements. However, only Murgia et al.34 were able to produce statistically significant results. Lastly, the greater proportion of included studies investigating the effects of RAS interventions suggested that RAS therapy lead to improvements in regards to FOG32,34,40.
A total of six studies30,31,33,35,44,45 investigating the effect of RAS using wearable devices on subjects who had suffered a stroke were included in this review. Four of these30,35,44,45 specifically focussed on chronic stroke subjects, whereas one study31 investigated RAS interventions on subacute stroke subjects. Overall, subjects who had suffered a stroke experienced significant improvements in terms of gait velocity, step length, cadence and walking distance. One of the four studies investigating cadence failed to produce any significant differences in cadence following the implementation of a RAS intervention44.
The lack of information regarding RAS interventions on neurological disorders other than PD, MS, and stroke (using wearable devices) is too great to make any conclusions regarding the effect of this type of intervention on other neurological conditions, not mentioned in this review and meta-analysis. Future research could be useful to highlight the benefits of RAS interventions in other central and peripheral neurological disorders, thus potentially allowing professionals to help a greater population of those suffering from a neurological disorder.
As shown Table 6, a total of seven studies39,43–47,53 researched the effects of an adaptive-RAS intervention on subjects diagnosed with a neurological disorder using wearables. Four studies38,41,42,52 included both types of RAS interventions. The remaining nineteen studies13,28–37,40,48–51,54–56 used a fixed-RAS intervention. All thirty studies included in this review were effective at improving the target parameters. Both forms of RAS appear to be effective at improving various outcome measures, including gait and movement. All studies corroborate to this effect, except for two fixed-RAS studies, which failed to demonstrate any improvement to their respective outcome measures28,30. In conclusion, neither fixed nor adaptive RAS interventions can be favoured when attempting to improve movement-related parameters as both types of RAS are shown to improve various outcome measures. Further research is needed to compare the effect of adaptive RAS and fixed-tempo RAS.
All included studies included the use of wearable devices. Wearable headphones were used in every study to provide auditory stimuli to the test subjects. Other wearable devices used by the included studies, were smartphones, MP3-players, glasses or specific smartphone applications31,38,39,45,47,52–54. Besides those, motion sensing wearable systems were used. For example, hand and foot sensors or any sensor attached to an extremity to capture the movement of a patient measuring the angular rate or body’s specific force used to monitor movements such as gait61. These sensors were commonly used to retrieve the information of the patient’s movements to see whether the RAS-intervention improved those movements Cho et al.62 found that an inertial measurement unit-based system could potentially be a reliable alternative to a camera-based system in the assessment of clinical body motion as well as gait. Some studies used the sensor input (real-time gait pattern analysis) to adapt the given auditory cues.
A previous systematic review by Scataglini et al.22 showed that wearable devices have potential to contribute to RAS-therapy. In their study, they found that they can quantify the effect of a music-based therapy in external, non-clinical environments. This current systematic review has produced similar conclusions based on the overwhelming positive effects of RAS interventions using wearable devices. These findings could potentially pave the way towards providing both training and rehabilitation in environments that are more native (familiar) to the subject. Previous systematic reviews demonstrated the effectiveness of RAS interventions in subjects suffering from various neurological disorders. For example, in the systematic review and meta-analysis of Wang et al.63, it was concluded that RAS interventions improved the gait parameters, gait function and balance of subjects who had suffered a stroke. Additionally, in another study, Wang et al.64 found RAS interventions to be an effective option to improve motor performance. These results are in line with the findings of the current systematic review – an overall improvement in gait parameters and movement (whilst integrating a wearable device). Thus, by integrating a wearable device, the therapy remains effective. This current review is the first to include all neurological disorders in the search strategy, gathering all existing evidence on the topic. Compared to other studies concerning the topic of RAS, this review and meta-analysis investigates the effect of RAS using wearable devices like in previous research of Scataglini et al.22. However, this current study is the first meta-analysis investigating the effect of RAS using wearable devices on movement parameters, including all neurological disorders, thus giving a broad population.
When examining the clinical characteristics of the studies, two-thirds of the participants suffering from a stroke were male, in contrast to the predominantly female participants in the MS studies. An analysis of the mean ages suggests that the participants range from middle-aged to older adults, which is expected given that stroke most commonly occurs at older ages. The geographical locations of the conducted studies are limited, which suggests that a broader range of regions is needed to improve the generalizability of the results around the world. The wide range in disease duration indicates that the effect of RAS has been studied at different stages of post-stroke recovery, ranging from the acute phase to the more chronic phase.
Upon closer examination of the sex distribution of participants in the studies on MS conducted by Baram et al.46, Conklyn et al.29, Moumdjian et al.43, and Shahraki et al.36, it is evident that a disproportionate number of participants are female. Generalizability of the findings is limited due to the underrepresentation of male participants across the studies. On the other hand, the high number of female participants can provide valuable insights into the female population with MS. Additionally, age can be an important moderating factor when assessing the effects of RAS on MS symptoms. The mean ages suggest that most participants were middle-aged people. Age-related variability in disease progression and symptom severity may influence the differential responses observed in the studies. Therefore, further research is needed to assess the effects of RAS in studies with older and younger participants to improve the generalizability of the findings. Taking a closer look at the geographical locations where the studies were conducted, it is evident that a wide range of regions around the world are represented, enhancing the generalizability of the findings. However, Africa and Australia are not represented, which could limit the applicability of the results in these regions. When looking at the mean disease duration is only reported by three articles29,36,46. It can be concluded that the effects of RAS only have been investigated in people who have had MS for a relatively long period of time. Additionally, the range of the expanded disability status scale (EDSS) reported by the studies is logical, as a lower score is associated with no walking disabilities, while a higher score indicates an inability to walk. Therefore, RAS could not have been applied to participants with higher EDSS scores. However, the effect of RAS could have been studies in the lower score range of the EDSS, though this would be likely less relevant.
Lastly, for the population of participants with PD, there were more male subjects than female, with a ratio of 1.8/1 (male/female), comparable to an actual ratio of 2/1 within the general, healthy population65. For age, there was a narrow range of 61 to 75 years of age, which makes that the interpretation and comparison of these studies should lead to more similar results. However, there is a difference in disease severity measured by the Hoehn and Yahr scale amongst the studies, making them less comparable to one another. As of demographics, studies were conducted in Europe, North America, South America and Asia. No studies were found for the continents of Africa, Oceania, or Antarctica.
For the meta-analysis, three gait parameters could be researched: gait velocity, stride length and cadence. A division is made between de studies researching the effect of RAS within one group of participants with neurological disorders and the studies researching the effect of RAS within a group of participants compared to a healthy control group. This division between the studies makes it difficult to statistically compare the effect of RAS on these gait parameters between all studies.
Looking at the funnel plots of each separate meta-analysis concerning the effect of RAS on gait velocity and cadence within a longitudinal design, each plot seems to be symmetrical suggesting that there is no evidence of any publication bias. On the other hand, within the other three funnel plots looking at the effect of RAS on these gait parameters within a group of participants compared to a control group, the number of included articles was too low (< 10) to draw any conclusions out of the funnel plots. An overview of all these funnel plots can be found in Table 7.
Table 7.
Overview funnel plots.
| Pre vs post | Control vs experimental |
|---|---|
| Gait velocity | |
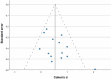 |
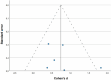 |
| Stride length | |
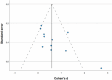 |
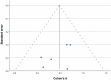 |
| Cadence | |
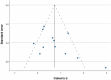 |
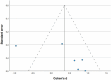 |
Four test situations demonstrated highly significant results (p < 0.001). This for gait velocity when compared to a control group and gait velocity, stride length and cadence in a longitudinal design. For stride length compared to a control group, a significant (p < 0.002) difference was found favouring RAS. Lastly, for cadence, when compared to a control group, no significant difference (p = 0.247) could be observed between a RAS and a control group. Within this comparison, it’s noticeable that only the study of Li et al.32 showed that the cadence was slower post-intervention when compared to a control group, which can be explained by a significant improvement in step length within a RAS experimental group. Overall, these findings align with expectations from the conducted systematic review and the earlier review of Scataglini et al.22.
This review and meta-analysis provide clinically relevant results for exploring the effects of RAS-therapy using wearable devices, focusing on three central neurological disorders; PD, MS, and stroke. Although the current evidence is limited to these conditions, it establishes a foundation for future research that includes other central and peripheral neurological disorders to explore potential therapeutic benefits. Establishing a standardized protocol regarding the intervention and use of wearable devices for studies investigating RAS-therapy would be beneficial for enhancing the results related to movement outcomes. Wearable devices facilitate the implementation of home-based therapy, offering an alternative to rehabilitation in clinical centers or private practices66. Several of the included studies have investigated home-based programs13,29,34,40,47. However, future research is required to assess the implementation of these programs.
Conclusion
This review and meta-analysis provide clinically relevant results for exploring the effects of RAS-therapy using wearable devices, focusing on three central neurological disorders; PD, MS, and stroke. The systematic review reveals clinically relevant improvements in stride length, step length, gait velocity, and double support time. The meta-analysis confirmed significant improvements in gait velocity and stride length within a longitudinal design as well as when compared to a control group. Improvement in cadence was only significant in a longitudinal design but non-significant when compared to a control group (p = 0.247). Future perspectives should be addressed to consider RAS-therapy using wearable technology for peripheral neurological disorders in clinical and home-based therapy.
Acknowledgements
Supported by FWO medium-scale research infrastructure: 4D scanner for Accelerating Advanced motion Analysis and Application (I002020N).
Author contributions
All authors contributed to the study conception and design. Idea for the article: SS, ST; Literature search and data analysis: LJ, LVE, CVL; Draft and/or critical revision: SS, ST, LJ, LVE, CVL and CVB. All authors read and approved the final manuscript. The authors have no relevant financial or non-financial interests to disclose.
Funding
No funds, grants, or other support was received. The authors have no relevant financial or non-financial interests to disclose.
Data availability
The datasets generated and/or analysed during the current study are not publicly available due to privacy (Rayyan, SPSS) but are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bach, J. P., Ziegler, U., Deuschl, G., Dodel, R. & Doblhammer-Reiter, G. Projected numbers of people with movement disorders in the years 2030 and 2050. Mov. Disord.26(12), 2286–2290 (2011). [DOI] [PubMed] [Google Scholar]
- 2.WHO. Ageing and health 2022 [Available from: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health.
- 3.Deuschl, G. et al. The burden of neurological diseases in Europe: An analysis for the Global Burden of Disease Study 2017. Lancet Public Health.5(10), e551–e567 (2020). [DOI] [PubMed] [Google Scholar]
- 4.van de Jaron, W. et al. Systematic clinical approach for diagnosing upper limb tremor. J. Neurol. Neurosurg. Psych.91(8), 822 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi, L., Wang, R., Zhao, J., Zhang, J. & Kuang, Z. Detection of Rehabilitation Training Effect of Upper Limb Movement Disorder Based on MPL-CNN. Sensors.24(4), 1105 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moon, Y., Sung, J., An, R., Hernandez, M. E. & Sosnoff, J. J. Gait variability in people with neurological disorders: A systematic review and meta-analysis. Hum. Mov. Sci.47, 197–208 (2016). [DOI] [PubMed] [Google Scholar]
- 7.Ataullah AHM, De Jesus O. Gait Disturbances. StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2024, StatPearls Publishing LLC.; 2024.
- 8.Fasano, A. & Bloem, B. R. Gait disorders. Continuum Minneap Minn.19, 1344–1382 (2013). [DOI] [PubMed] [Google Scholar]
- 9.Verghese, J., Ambrose, A. F., Lipton, R. B. & Wang, C. Neurological gait abnormalities and risk of falls in older adults. J Neurol.257(3), 392–398 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahlknecht, P. et al. Prevalence and burden of gait disorders in elderly men and women aged 60–97 years: A population-based study. PLoS ONE8(7), e69627 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stolze, H. et al. Falls in frequent neurologicaldiseases. J. Neurol.251(1), 79–84 (2004). [DOI] [PubMed] [Google Scholar]
- 12.Ashoori, A., Eagleman, D. M. & Jankovic, J. Effects of Auditory Rhythm and Music on Gait Disturbances in Parkinson’s Disease. Front Neurol.6, 234 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thaut, M. H., Rice, R. R., Braun Janzen, T., Hurt-Thaut, C. P. & McIntosh, G. C. Rhythmic auditory stimulation for reduction of falls in Parkinson’s disease: A randomized controlled study. Clin Rehabil.33(1), 34–43 (2019). [DOI] [PubMed] [Google Scholar]
- 14.Braun Janzen, T., Koshimori, Y., Richard, N. M. & Thaut, M. H. Rhythm and music-based interventions in motor rehabilitation: Current evidence and future perspectives. Front. Hum. Neurosci.15, 789467 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alter, D. A. et al. Synchronized personalized music audio-playlists to improve adherence to physical activity among patients participating in a structured exercise program: A proof-of-principle feasibility study. Sports Medicine - Open.1(1), 23 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koshimori, Y. & Thaut, M. H. Rhythmic auditory stimulation as a potential neuromodulator for Parkinson’s disease. Parkinsonism Relat. Disord.113, 105459 (2023). [DOI] [PubMed] [Google Scholar]
- 17.Zhou, Z., Zhou, R., Wei, W., Luan, R. & Li, K. Effects of music-based movement therapy on motor function, balance, gait, mental health, and quality of life for patients with Parkinson’s disease: A systematic review and meta-analysis. Clin. Rehabil.35(7), 937–951 (2021). [DOI] [PubMed] [Google Scholar]
- 18.Ye, X., Li, L., He, R., Jia, Y. & Poon, W. Rhythmic auditory stimulation promotes gait recovery in Parkinson’s patients: A systematic review and meta-analysis. Front. Neurol.13, 940419 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Movement Disorder Society Task Force on Rating Scales for Parkinson’s D. The Unified Parkinson’s Disease Rating Scale (UPDRS): Status and recommendations. Movement Disorders. 2003; 18(7):738–50. [DOI] [PubMed]
- 20.López-Ortiz, C., Gaebler-Spira, D. J., McKeeman, S. N., McNish, R. N. & Green, D. Dance and rehabilitation in cerebral palsy: a systematic search and review. Dev. Med. Child Neurol.61(4), 393–398 (2019). [DOI] [PubMed] [Google Scholar]
- 21.Celik, Y., Stuart, S., Woo, W. L. & Godfrey, A. Gait analysis in neurological populations: Progression in the use of wearables. Med. Eng. Phys.87, 9–29 (2021). [DOI] [PubMed] [Google Scholar]
- 22.Scataglini, S. et al. Effect of music based therapy rhythmic auditory stimulation (RAS) using wearable device in rehabilitation of neurological patients: A systematic review. Sensors (Basel).23(13), 5933 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Page, M. J. et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ372, n160 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi, J.-Y. et al. Health-Related Indicators Measured Using Earable Devices: Systematic Review. JMIR Mhealth Uhealth.10(11), e36696 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gotschall, T. EndNote 20 desktop version. J. Med. Libr. Assoc.109(3), 520–522 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ouzzani, M., Hammady, H., Fedorowicz, Z. & Elmagarmid, A. Rayyan—a web and mobile app for systematic reviews. Syst. Rev.5(1), 210 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kmet L, Lee R. Standard Quality Assessment Criteria for Evaluating Primary Research Papers from a Variety of FieldsAHFMRHTA Initiative20040213. HTA Initiative. 2004; 2.
- 28.Calvano, A., Timmermann, L., Loehrer, P. A., Oehrn, C. R. & Weber, I. Binaural acoustic stimulation in patients with Parkinson’s disease. Front Neurol.14, 1167006 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Conklyn, D. et al. A home-based walking program using rhythmic auditory stimulation improves gait performance in patients with multiple sclerosis: a pilot study. Neurorehabil. Neural. Repair.24(9), 835–842 (2010). [DOI] [PubMed] [Google Scholar]
- 30.Elsner, B., Schöler, A., Kon, T. & Mehrholz, J. Walking with rhythmic auditory stimulation in chronic patients after stroke: A pilot randomized controlled trial. Physiother. Res. Int.25(1), e1800 (2020). [DOI] [PubMed] [Google Scholar]
- 31.Kim, J. H. et al. Effects of the combination of rhythmic auditory stimulation and task-oriented training on functional recovery of subacute stroke patients. J. Phys. Ther. Sci.24(12), 1307–1313 (2012). [Google Scholar]
- 32.Li, K. P. et al. Effect of music-based movement therapy on the freezing of gait in patients with Parkinson’s disease: A randomized controlled trial. Front Aging Neurosci.14, 924784 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mainka, S., Wissel, J., Völler, H. & Evers, S. The use of rhythmic auditory stimulation to optimize treadmill training for stroke patients: A randomized controlled trial. Front Neurol.9, 755 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murgia, M. et al. The use of footstep sounds as rhythmic auditory stimulation for gait rehabilitation in Parkinson’s disease: A randomized controlled trial. Front Neurol.9, 348 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park, J., Park, S. Y., Kim, Y. W. & Woo, Y. Comparison between treadmill training with rhythmic auditory stimulation and ground walking with rhythmic auditory stimulation on gait ability in chronic stroke patients: A pilot study. NeuroRehabilitation37(2), 193–202 (2015). [DOI] [PubMed] [Google Scholar]
- 36.Shahraki, M., Sohrabi, M., Taheri Torbati, H. R., Nikkhah, K. & NaeimiKia, M. Effect of rhythmic auditory stimulation on gait kinematic parameters of patients with multiple sclerosis. J. Med. Life.10(1), 33–37 (2017). [PMC free article] [PubMed] [Google Scholar]
- 37.Thaut, M. H. et al. Rhythmic auditory stimulation in gait training for Parkinson’s disease patients. Mov. Disord.11(2), 193–200 (1996). [DOI] [PubMed] [Google Scholar]
- 38.Uchitomi, H., Ota, L., Ogawa, K., Orimo, S. & Miyake, Y. Interactive rhythmic cue facilitates gait relearning in patients with Parkinson’s disease. PLoS ONE8(9), e72176 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hutin, E., Poirier, T., Meimoun, M., Mardale, V. & Ghédira, M. Model-based cueing-as-needed for walking in Parkinson’s disease: A randomized cross-over study. Rev. Neurol. (Paris).180(8), 798–906 (2024). [DOI] [PubMed] [Google Scholar]
- 40.Chomiak, T., Watts, A., Meyer, N., Pereira, F. V. & Hu, B. A training approach to improve stepping automaticity while dual-tasking in Parkinson’s disease: A prospective pilot study. Medicine (Baltimore)96(5), e5934 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ginis, P. et al. External input for gait in people with Parkinson’s disease with and without freezing of gait: One size does not fit all. J Neurol.264(7), 1488–1496 (2017). [DOI] [PubMed] [Google Scholar]
- 42.Ginis, P. et al. Prolonged walking with a wearable system providing intelligent auditory input in people with Parkinson’s disease. Front Neurol.8, 128 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moumdjian, L. et al. Continuous 12 min walking to music, metronomes and in silence: Auditory-motor coupling and its effects on perceived fatigue, motivation and gait in persons with multiple sclerosis. Mult. Scler. Relat. Disord.35, 92–99 (2019). [DOI] [PubMed] [Google Scholar]
- 44.Collimore, A. N. et al. Autonomous control of music to retrain walking after stroke. Neurorehabil. Neural. Repair.37(5), 255–265 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hutchinson, K. et al. A music-based digital therapeutic: proof-of-concept automation of a progressive and individualized rhythm-based walking training program after stroke. Neurorehabil. Neural. Repair.34(11), 986–996 (2020). [DOI] [PubMed] [Google Scholar]
- 46.Baram, Y. & Miller, A. Auditory feedback control for improvement of gait in patients with multiple sclerosis. J. Neurol. Sci.254(1–2), 90–94 (2007). [DOI] [PubMed] [Google Scholar]
- 47.Cochen De Cock, V. et al. BeatWalk: Personalized Music-Based Gait Rehabilitation in Parkinson’s Disease. Front Psychol.12, 655121 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.De Bartolo, D. et al. Effect of different music genres on gait patterns in Parkinson’s disease. Neurol. Sci.41(3), 575–582 (2020). [DOI] [PubMed] [Google Scholar]
- 49.Erra, C. et al. Immediate effects of rhythmic auditory stimulation on gait kinematics in Parkinson’s disease ON/OFF medication. Clin Neurophysiol.130(10), 1789–1797 (2019). [DOI] [PubMed] [Google Scholar]
- 50.Nieuwboer, A. et al. The short-term effects of different cueing modalities on turn speed in people with Parkinson’s disease. Neurorehabil. Neural. Repair.23(8), 831–836 (2009). [DOI] [PubMed] [Google Scholar]
- 51.Guimarães V, Castro R, Barros A, Cevada J, Bayés À, Garcá S, et al., editors. Development of an Auditory Cueing System to Assist Gait in Patients with Parkinson’s Disease. Bioinformatics and Biomedical Engineering; 2015 2015//; Cham: Springer International Publishing.
- 52.Hove, M. J., Suzuki, K., Uchitomi, H., Orimo, S. & Miyake, Y. Interactive rhythmic auditory stimulation reinstates natural 1/f timing in gait of Parkinson’s patients. PLoS ONE7(3), e32600 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Uchitomi, H., Ogawa, K., Orimo, S., Wada, Y. & Miyake, Y. Effect of interpersonal interaction on festinating gait rehabilitation in patients with Parkinson’s Disease. PLoS ONE11(6), e0155540 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lopez, W. O. et al. Listenmee and Listenmee smartphone application: synchronizing walking to rhythmic auditory cues to improve gait in Parkinson’s disease. Hum Mov Sci.37, 147–156 (2014). [DOI] [PubMed] [Google Scholar]
- 55.Park, K. S., Hass, C. J. & Janelle, C. M. Familiarity with music influences stride amplitude and variability during rhythmically-cued walking in individuals with Parkinson’s disease. Gait Posture.87, 101–109 (2021). [DOI] [PubMed] [Google Scholar]
- 56.Park, K. S., Hass, C. J., Patel, B. & Janelle, C. M. Musical pleasure beneficially alters stride and arm swing amplitude during rhythmically-cued walking in people with Parkinson’s disease. Hum Mov Sci.74, 102718 (2020). [DOI] [PubMed] [Google Scholar]
- 57.Berg, K., Wood-Dauphine, S., Williams, J. I. & Gayton, D. Measuring balance in the elderly: preliminary development of an instrument. Physiother. Can.41(6), 304–311 (1989). [Google Scholar]
- 58.Podsiadlo, D. & Richardson, S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc.39(2), 142–148 (1991). [DOI] [PubMed] [Google Scholar]
- 59.Goetz, C. G. et al. Movement disorder society-sponsored revision of the unified parkinson’s disease rating scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov. Disord.23(15), 2129–2170 (2008). [DOI] [PubMed] [Google Scholar]
- 60.Yardley, L. et al. Development and initial validation of the falls efficacy scale-International (FES-I). Age Ageing34(6), 614–619 (2005). [DOI] [PubMed] [Google Scholar]
- 61.Ribeiro NF, Santos CP, editors. Inertial measurement units: A brief state of the art on gait analysis. 2017 IEEE 5th Portuguese Meeting on Bioengineering (ENBENG); 2017 16–18 Feb. 2017.
- 62.Cho, Y. S. et al. Evaluation of Validity and Reliability of Inertial Measurement Unit-Based Gait Analysis Systems. Ann Rehabil Med.42(6), 872–883 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang, L., Peng, J. L., Xiang, W., Huang, Y. J. & Chen, A. L. Effects of rhythmic auditory stimulation on motor function and balance ability in stroke: A systematic review and meta-analysis of clinical randomized controlled studies. Front Neurosci.16, 1043575 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang, L. et al. Effects of rhythmic auditory stimulation on gait and motor function in Parkinson’s Disease: A systematic review and meta-analysis of clinical randomized controlled studies. Front Neurol.13, 818559 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Miller, I. N. & Cronin-Golomb, A. Gender differences in Parkinson’s disease: clinical characteristics and cognition. Mov Disord.25(16), 2695–2703 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Junior P, Souza P, Reis K, Filoni E. Home-based physiotherapy programmes for individuals with neurological diseases: systematic review. Fisioterapia em Movimento. 2019;32.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analysed during the current study are not publicly available due to privacy (Rayyan, SPSS) but are available from the corresponding author on reasonable request.