Abstract
Sertoli cells play a pivotal role in spermatogenesis through their interactions with germ cells. To set up a strategy for treating male infertility caused by Sertoli cell dysfunction, we developed a Sertoli cell gene transfer system by using an adenovirus vector, which maintained long-term transgene expression in the testes of infertile mice. Introduction of an adenovirus carrying the mouse Steel (Sl) gene into Sertoli cells restored partial spermatogenesis in infertile Steel/Steeldickie (Sl/Sld) mutant mouse testes. Although these males remained infertile, round spermatids and spermatozoa from the testes produced normal fertile offspring after intracytoplasmic injection into oocytes. None of the offspring showed evidence of germ line transmission of adenoviral DNA. Thus, we demonstrate a successful treatment for infertility by using a gene therapy vector. Therefore, adenovirus-mediated gene delivery into Sertoli cells not only provides an efficient and convenient means for studying germ cell–Sertoli cell interactions through manipulation of the germ cell microenvironment in vivo, but also is a useful method to treat male infertility resulting from a Sertoli cell defect.
Infertility affects ≈20% of couples, and severe spermatogenic defects are present in ≈5% of these cases, representing ≈1% of the male population (1). Spermatogenesis depends on an intimate interaction between germ cells and Sertoli cells (2). Sertoli cells are the only somatic cells in the seminiferous tubules that have direct contact with germ cells. A defect in Sertoli cells may result in abnormal spermatogenesis and male infertility. It is possible that the germ cells in some infertile testes might be functionally competent but deficiencies in Sertoli cells could possibly inhibit the normal differentiation of germ cells. There are currently no effective methods to correct such genetic defects in animals or humans (3).
The Sl mouse is used as a murine model of infertility (4). The Sl locus encodes soluble and membrane-bound forms of Sl factor that binds to the c-kit tyrosine kinase receptor produced by the W locus (5–7). In the testis, c-kit and Sl are expressed in spermatogonia and Sertoli cells, respectively, and the interaction between these two factors play an important role in the regulation of spermatogonial cell proliferation (8). A mutation in Sl deletes the entire Sl gene, whereas the Sld mutation deletes the transmembrane and intracellular domains (9). Thus, mice with the Sl/Sld mutation do not have the membrane-bound Sl factor and spermatogenesis does not occur in the male. In addition, the seminiferous tubules of these mice are virtually devoid of germ cells, a histological outcome that is similar to the clinical condition called Sertoli cell-only syndrome (10), although Sertoli cell-only is often associated with very small numbers of sperm (11, 12). Nonetheless, studies have shown the presence of healthy spermatogonia in the Sl/Sld testis. Aggregation chimeras between Sl and wild-type embryos produced progeny with the Sl phenotype, indicating the presence of functional Sl germ cells (13). Furthermore, transplantation of germ cells from Sl/Sld mice into infertile W mutant mice restored fertility to the latter, and the donor haplotype was transmitted to the offspring of W mutant mice (14). Thus, the Sl mutant mouse is an example of male infertility arising from defective Sertoli cells. The evidence suggests that primitive spermatogonia in the Sl testis may undergo spermatogenesis if provided with healthy Sertoli cells, despite long-term exposure to a defective environment.
Bearing in mind the close interaction between germ cells and Sertoli cells, a valuable approach to better understanding of spermatogenesis would be to introduce foreign genes into Sertoli cells and then monitor the outcome. Furthermore, genetic modification of Sertoli cells could be used to correct defective Sertoli cells, thus providing a method to treat those cases of male infertility that might be the results of defects in Sertoli cell function. However, although germ cells can be transfected by using several methods (15–17), there is currently no technique that allows long-term, widespread transgene expression in Sertoli cells (16, 18). Adenoviruses have potential as gene therapy vectors in human patients because of their relatively high cloning capacity and amenability to production in high titers (19). Sertoli cells represent an ideal target for adenoviral infection for two reasons. First, Sertoli cells may have the ability to express the DNA from infecting adenoviruses over long periods of time because they are mitotically quiescent after puberty (2). Although an adenovirus can transduce both dividing and nondividing cells (19), the division of infected cells results in viral DNA loss because an adenovirus cannot integrate into the host genome (19). Second, Sertoli cells have immunosuppressive activity. Because testis may be an immune-privileged organ and Sertoli cells can protect immunogenic tissues by Fas ligand expression (20, 21), an adenovirus infection may not trigger an immune reaction, a major drawback in this technology (19). The absence of an immune response could thus be beneficial.
The studies described here sought to determine whether an adenovirus vector could be used in mice to treat male infertility caused by a Sertoli cell defect. Earlier attempts to infect Sertoli cells in wild-type testes met with limited success, as transgene expression was transient and inflammation was apparent (18). Nonetheless, we hoped that adenovirus infection of infertile testes might provide a better outcome. Microinjection of adenovirus vectors into the seminiferous tubules of infertile mouse testes resulted in long-term transgene expression. By using this technique, we determined the potential of adenovirus infection system to rescue spermatogenesis in the Sl/Sld testis. This approach may be a powerful method to study germ cell–Sertoli cell interaction and might provide a novel treatment strategy for male infertility caused by defects in Sertoli cells.
Materials and Methods
Recombinant Adenoviruses.
The replication-defective adenovirus vectors, AxCALacZ and Adex1CAmKL, constructed by inserting the bacterial LacZ gene into the Ax1w1 and full-length mouse Sl factor into Adex1cw, respectively, under the control of the chicken β-actin and cytomegalovirus enhancer (CAG) promoter (22), were obtained from The Institute of Physical and Chemical Research (Tsukuba, Japan). The recombinant adenovirus was isolated from 293 cells and purified by CsCl centrifugation (23). The titer of original virus was 2 × 108 plaque-forming units (pfu)/ml, which was diluted with PBS before injection into the testes.
Animals and Surgical Procedures.
Male C57BL/6JCr × DBA/2Cr F1 (B6D2F1) hybrids and Sl/Sld mice were purchased from Japan SLC (Hamamatsu, Japan). B6D2F1 recipients were treated with busulfan (44 mg/kg) to destroy the endogenous germ cells (24). In the first experiment, AxCALacZ was injected into the seminiferous tubules of untreated and busulfan-treated B6D2F1 mouse testes at 10 wk of age. In the second experiment, Adex1CAmKL was injected into the seminiferous tubules of Sl/Sld testes at 6 wk of age. Microinjection was performed through the efferent duct, and 75 to 85% of the tubules in each testis were filled (25). Approximately 10 μl was introduced into the seminiferous tubules of a B6D2F1 testis, whereas only ≈3 μl could be introduced into a Sl/Sld testis because of their smaller size.
Analysis of Testes.
In the first experiment, the X-gal staining was used to detect LacZ expression resulting from the injected AxCALacZ (17). In the second experiment, the testes were fixed in 10% neutral-buffered formalin and processed for paraffin sectioning. All sections were stained with hematoxylin and eosin. Four histological sections were made from the testes of each mouse, with a 12-μm interval between sections, and each slide was viewed at a magnification of ×400 to determine the extent of spermatogenesis in Sl/Sld mutant mice. The number of tubule cross-sections showing spermatogenesis (defined as the presence of multiple layers of germ cells in the seminiferous tubule) or not showing spermatogenesis was recorded for one section from each testis.
Microinsemination and Embryo Transfer to Foster Mothers.
The seminiferous tubules of Adex1CAmKL-injected Sl/Sld testes were carefully dissected, and germ cells were collected by using a mechanical method (26). Microinsemination was undertaken by intracytoplasmic injection into B6D2F1 oocyte (27, 28). Embryos that reached the 4-cell stage after 48 h in culture were transferred into the oviducts of day-1 pseudopregnant ICR females. Lactating foster mothers were used to raise the live pups.
DNA Analysis.
Genomic DNA (8 μg) isolated from tail tissue samples was digested overnight with EcoRI, separated by electrophoresis, and blotted onto a nylon membrane (Hybond-N+; Amersham Pharmacia). The Sl cDNA probe used for hybridization comprised the 434-bp NciI–BglI fragment of the full-length Sl cDNA (provided by Y. Matsui). The genomic DNA also was hybridized with a pAdex1cw cosmid that contained the entire Adex1cw genome. Controls contained 5, 50, or 500 pg of viral DNA. Assuming a diploid mammalian genome size of 9 × 109 bp, these amounts represent 0.1, 1, or 10 copies of viral DNA per diploid genome. Hybridization was carried out as described (14). A 309-bp region of Adex1cw was PCR-amplified by using the following primers: forward, 5′-ACCTGAGAAAAACACCTGG-3′ and reverse, 5′-ACGTAGTAGTGTGCCGGA-3′.
PCR conditions were 92°C for 3 min, followed by 35 cycles of 94°C for 30 s, 56°C for 30 s, and 72°C for 1 min.
Results
Adenoviral Infection of Busulfan-Treated Mouse Testes.
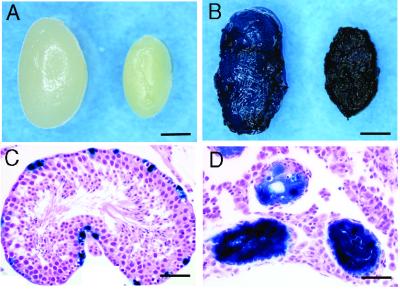
In the first experiment, we examined the efficiency of adenoviral infection in the infertile mouse testis by using busulfan treatment. One month after busulfan injection, the spermatogenic cells were completely destroyed (Fig. 1A), and only Sertoli cells remained in the seminiferous tubules (24). Four experiments were performed and 12 wild-type and 22 busulfan-treated testes were injected with the purified AxCALacZ adenovirus (1 × 106 pfu/ml). These testes were then examined for LacZ gene expression. All 22 busulfan-treated testes showed LacZ staining beginning 3 days after injection, and strong LacZ expression was still observed 3 mo after infection (Fig. 1B). Although LacZ staining in the wild-type testis appears striking (Fig. 1B), histological analysis revealed limited LacZ gene expression in a small number of Sertoli cells (Fig. 1C). In contrast, most Sertoli cells in the busulfan-treated testis showed extensive LacZ staining (Fig. 1D). Thus the results demonstrate that adenoviral gene expression is enhanced in Sertoli cells in germ cell-depleted testes.
Figure 1.
Adenovirus-mediated gene transfer into wild-type and busulfan-treated mouse testes. (A) Macroscopic appearance of wild-type (left) and busulfan-treated (right) testes. The busulfan-treated testis was significantly smaller (≈25 mg) than the wild-type testis because of the absence of spermatogenesis. (B) Macroscopic appearance of the wild-type (left) and busulfan-treated (right) testes injected with AxCALacZ. Blue staining represented LacZ expression from AxCALacZ. Whole mount of testes were stained 1 wk (wild type) or 3 mo (busulfan-treated) after virus injection. (C) Histological section of a wild-type testis, analyzed 1 wk after AxCALacZ injection. Expression of transgene indicated by blue staining was found on the basement membrane. (D) Histological section of a busulfan-treated testis, analyzed 3 mo after AxCALacZ injection. Note the absence of germ cells in the seminiferous tubules and the strongly stained Sertoli cells. Hematoxylin and eosin stain. Scale bars represent 2 mm (A and B) and 50 μm (C and D).
Spermatogenesis from Sl/Sld Germ Cells After Adenoviral Transfer of the Sl Gene.
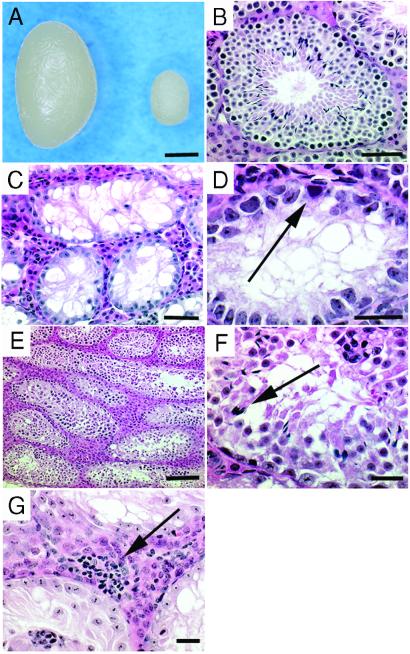
In the second experiment, Sl mutant mice were used as a model of male infertility to examine whether spermatogenic cells from adult males with Sertoli cell-based infertility could regain functional spermatogenesis after adenoviral gene transfer (4). The testes of Sl mutant mice were considerably smaller (weighing ≈11 mg) than those of fertile wild-type mice (weighing ≈110 mg) (Fig. 2 A and B). Spermatogenesis was absent (Fig. 2C) because of the impaired microenvironment in the Sl/Sld testis (14); although undifferentiated spermatogonia were present in a few tubules (Fig. 2D). Therefore, the detection of spermatogenesis in Sl/Sld testes after the injection of a Sl factor-expressing adenovirus vector would indicate successful Sl factor expression from infected Sertoli cells.
Figure 2.
Adenovirus-mediated gene transfer into Sl/Sld testes. (A) Macroscopic appearance of wild-type (left) and Sl/Sld (right) testes. The Sl/Sldtestis was significantly smaller (≈11 mg) than the wild-type testis (≈110 mg) because of the absence of spermatogenesis. (B) Histological section of a wild-type testis with normal spermatogenesis. (C and D) Histological section of a Sl/Sld testis. Spermatogenesis was absent, but a few spermatogonia were present on the basement membrane in some tubules (arrow, D). (E–G) Histological section of a Sl/Sld testis 10 wk after AdexCA1mKL injection. Spermatogenesis occurred in many seminiferous tubules and some contain spermatozoa (arrow, F). Lymphocytic infiltration was occasionally found after infection (arrow, G). Hematoxylin and eosin stain. Scale bars represent 2 mm (A), 50 μm (B and C), 20 μm (D, F, and G), and 100 μm (E).
Five experiments were performed and 54 Sl/Sld testes were microinjected with different concentrations (ranging from 2 to 20 × 105 pfu/ml) of Adex1CAmKL. The testes of injected animals were examined histologically for the presence of spermatogenesis at 6 wk and 10 wk after injection. This time period represented 1.5–3 spermatogenic cycles in mice (2), which would allow sufficient time for sperm development from undifferentiated spermatogonia. The results showed the restoration of spermatogenesis in 30% (16/54 testes when the 6- and 10-wk observations were combined; Table 1) of the injected testes, indicating that the infected Sertoli cells expressed the full-length Sl factor. In contrast, spermatogenesis was not seen in control testes that did not receive adenovirus injection.
Table 1.
Spermatogenesis in the Sl/Sld testis after AdexCA1mKL injection
| Virus titer, ×105 pfu/ml | 6 wk after injection
|
10 wk after injection
|
||||||
|---|---|---|---|---|---|---|---|---|
| No. of testes injected | Testis weight,* mg | No. of testes with spermatogenesis, % | Tubules with spermatogenesis, %† | No. of testes injected | Testis weight,* mg | No. of testes with spermatogenesis, % | Tubules with spermatogenesis, %† | |
| 2.0 | 6 | 12.0 ± 2.1 | 0 (0) | 0 (0/783) | 4 | 12.8 ± 1.3 | 0 (0) | 0 (0/527) |
| 4.0 | 12 | 12.1 ± 3.4 | 4 (33) | 0.3 (5/1822) | 8 | 9.8 ± 2.8 | 0 (0) | 0 (0/1059) |
| 8.0 | 8 | 11.0 ± 3.8 | 5 (63) | 1.4 (18/1273) | 8 | 11.3 ± 1.4 | 0 (0) | 0 (0/991) |
| 20.0 | 4 | 13.4 ± 1.4 | 4 (100) | 6.4 (40/624) | 4 | 10.2 ± 1.8 | 3 (75) | 24.0 (126/524) |
Data are presented as means ± SD.
In parenthesis, total number of tubule cross-sections containing spermatogenesis in all recipient testis/total number of cross-sections examined in all recipient testes.
The efficiency of spermatogenesis depended on the virus titer. Spermatogenesis occurred when the injected virus titer was higher than 4 × 105 pfu/ml (Table 1, line 2) and most efficiently at 20 × 105 pfu/ml (Table 1, line 4; Fig. 2 E and F). Although a focal inflammatory reaction was occasionally noticed with an infection of 20 × 105 pfu/ml (Fig. 2G), spermatogenesis still occurred in all (4/4 testes) infected testes, with an average 6.4% of tubules undergoing spermatogenesis. However, the level of spermatogenesis varied significantly among the samples; spermatogenesis was observed in 57% of the tubules in one case. Spermatogonia, spermatocytes, and round spermatids appeared morphologically normal, which indicated that germ cells underwent meiotic divisions (Fig. 2F). Although adenovirus-infected Sl/Sld testes showed efficient development of round spermatids, the numbers of elongated spermatids and spermatozoa were markedly reduced, indicating that adenovirus-mediated transduction of the Sl gene failed to restore the differentiation of round spermatids to spermatozoa (Fig. 2F). The number of mature germ cells did not increase after 10 wk after injection and no spermatozoa were observed in the epididymis. In total, 7.4% (4/54 testes when the 6- and 10-wk observations were combined) of the injected testes contained mature spermatozoa, and only 0.11% of tubules (8/7603 tubules in the 54 testes) underwent complete spermatogenesis. Consistent with these histological observations, the injected males remained infertile up to the time of analysis because wild-type females that were housed with adenovirus-infected males did not sire offspring.
Microinsemination and Analysis of Progeny.
To test whether offspring could be generated from differentiated germ cells, such as round spermatids or spermatozoa, in the Adex1CAmKL-infected Sl/Sld testis, in vitro microinsemination (a technique commonly used to derive offspring from infertile animals and humans) was used (26–30). Twenty-two Sl/Sld testes were injected with Adex1CAmKL (ranging from 1.0 to 20 × 105 pfu/ml), and used for microinsemination 6–12 wk after virus injection. Germ cells were recovered by repeated pipettings of tubule fragments. Of the testes examined, those injected with 8 × 105 pfu/ml contained a significant number of differentiated germ cells 8 wk after the virus injection. Round spermatids, characterized by their small size and centrally located chromatin mass, and spermatozoa were identified in the testis cell suspension. All 93 diploid zygotes constructed with 87 round spermatids or with six spermatozoa progressed to the four-cell stage by 48 h in culture. After embryo transfer into the oviducts, 20 (22%) normal offspring were obtained at term. Nineteen of them were derived from round spermatid injection, and one was from spermatozoa injection. All of the normal offspring (7 males and 13 females) grew into normal adults (Table 2).
Table 2.
Development of oocytes fertilized with round spermatids or spermatozoa recovered from AdexCA1mKL-injected Sl/Sld mice
| Type of cells | No. of embryos cultured | No. of embryos transferred,* % | No. of embryos implanted, % | No. of pups, % |
|---|---|---|---|---|
| Spermatid | 87 | 87 (100) | 68 (78) | 19 (22) |
| Spermatozoon | 6 | 5 (83) | 4 (80) | 1 (20) |
| Total | 93 | 92 (99) | 72 (78) | 20 (22) |
Embryos were cultured for 48 hr and transferred at the four-cell stage.
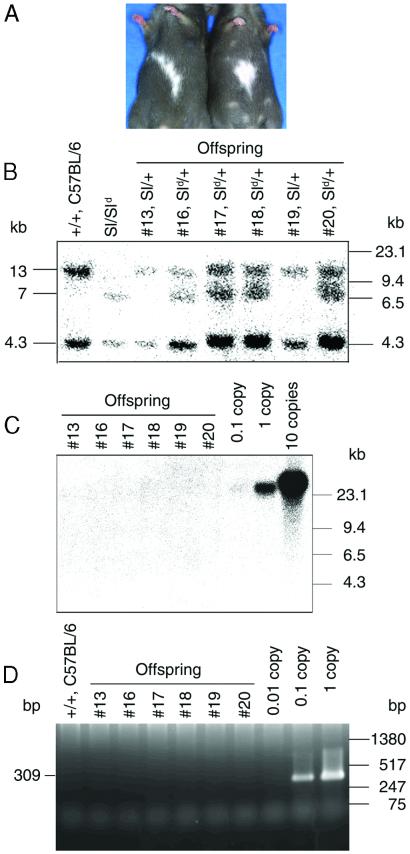
The progeny obtained by microinsemination (potentially Sl/+ or Sld/+ genotypes) had a dark coat color with a white belly patch (Fig. 3A), a coat pattern that is characteristic of heterozygous Sl mutants (4). To confirm that the Steel phenotype was transmitted, some F1 offspring were mated. A completely white phenotype is observed when both parental alleles have the Steel mutation. Offspring from the F1 cross consisted of pups with a dark coat color (Sl/+, Sld/+, or +/+) and pups with the completely white phenotype (Sl/Sld or Sld/Sld) that is typical of the homozygous Steel mutation. To further confirm the progeny genotypes, DNA from the F1 offspring was analyzed by Southern blot analysis using a Sl cDNA probe. The hybridization patterns showed that the offspring had either the Sl/+ or Sld/+ genotype (Fig. 3B). Four Sl/+ and 16 Sld/+ offspring were born after microinsemination. Adenoviral DNA was not detected by either Southern blot or PCR analysis in any of the progeny (Fig. 3 C and D), indicating the absence of germ line integration of the adenovirus vector.
Figure 3.
Offspring from the formerly infertile Sl/Sld male. (A) Offspring were born after microinsemination with round spermatids/spermatozoa recovered from AdexCA1mKL-injected Sl/Sld testes. Note the white patches on the belly, which are indicative of the Sl/+ or Sld/+ haplotype. (B) Southern blot analysis of DNA samples from six representative F1 offspring hybridized with Sl cDNA probe (see Materials and Methods). Both the Sl and Sld haplotypes of the donor germ cells were transmitted to the progeny, which indicated that both alleles of the Sl/Sld testes were segregated during meiosis. Left and right margins, molecular size markers. The wild-type locus produced bands of 4.3 and 13 kb, the Sl locus produced no bands, and the Sld locus produced bands at 4.3 and 7 kb (12). (C) Southern blot analysis of the same F1 DNA samples hybridized with a probe specific for adenoviral sequences (see Materials and Methods). Controls represent viral DNA in amounts equivalent to 0.1, 1, and 10 copies of viral DNA per diploid genome. (D) PCR analysis of the F1 DNA samples. The adenovirus-specific 309-bp fragment was amplified from the F1 DNA samples (see Materials and Methods). Controls containing 0.3 μg of normal mouse DNA were spiked with viral DNA representing 0.01, 0.1, and 1 copies of the viral genome.
Discussion
We extended the findings reported in other studies (18) by demonstrating that adenoviruses could be used to infect Sertoli cells in infertile mouse testes. Furthermore, we found that the introduction of full-length Sl factor cDNA into the Sertoli cells partially restored spermatogenesis up to round spermatid stage in the Sl/Sld testes, and offspring were produced after microinsemination. These findings represent the first application of gene therapy in the treatment of male infertility. The method also provides a novel system to study germ cell–Sertoli cell interactions in vivo.
A remarkable finding from the first experiment was the attainment of long-term adenoviral transgene expression. Earlier attempts at using adenoviruses to infect Sertoli cells in wild-type testes had limited success; transgene expression lasted for a few weeks and was accompanied by a severe inflammatory reaction (18). In contrast, our results show that infected Sertoli cells in germ cell-depleted testes continued to express the LacZ gene for up to 3 mo. This suggests that adenoviruses efficiently infected Sertoli cells in the absence of germ cells. The discrepancies between the various studies, in terms of successful adenovirus infection of Sertoli cells, may have arisen from differences in the promoters and vectors used. In several tested cell lines, the activity of CAG promoter was more than 10-fold when compared to Rous sarcoma virus promoter used in the previous study (18, 22). However, we also believe that Sertoli cells in the infertile testis offered at least two additional advantages for adenovirus infection. First, the concentration of Sertoli cells are increased >30-fold in the testis after germ cell-depletion (31). Sertoli cells constitute only 3% of the wild-type testis cell population (31) and are more difficult to target in the wild-type testis than in the infertile testis. Because the number of Sertoli cells remains constant in the adult testis and is not affected by busulfan treatment (2, 32), the elimination of germ cells increased the relative concentration of Sertoli cells in the testis cell population. Second, Sertoli cells are the only cells that would be directly exposed to injected adenovirus, because other types of cells are separated by the blood–testis barrier between Sertoli cells (2). These two biological factors must have increased accessibility of injected virus particles to Sertoli cells in the seminiferous tubules and may have allowed us to use low virus titers (1 to 20 × 105 pfu/ml) in inducing Sertoli cell infection without triggering inflammation, as observed in the previous study using significantly higher virus titer (3 × 1012 pfu/ml) (18). Although much remains to be investigated about the level of immune privilege in the testis, studies have shown that even dissociated Sertoli cells have ability to suppress rejection after transplantation (33, 34), and this local immunosuppresion by Sertoli cells must have contributed to the absence of inflammatory reaction. Other factors also may be involved, but the unique microenvironment in the infertile testis may account for the long-term transgene expression in Sertoli cells seen in this study.
The most striking result from this study was the generation of offspring from infertile Sl/Sld male mice. Considering that the number of spermatogonial stem cells in the Sl/Sld testis is only 5% of the wild-type control (35), the results suggest that some cases of idiopathic azoospermia in humans, even those that show severe spermatogenic defects at the spermatogonial level, may be treated by adenovirus-mediated gene transfer to somatic Sertoli cells. In addition, the absence of adenoviral DNA in the progeny of Sl/Sld male mice confirms previous observations that adenovirus vectors pose a minimal risk for male germline integration (36, 37), probably reflecting the absence of specific integrin molecules considered to be required for internalization of the adenovirus particle (36). Although several therapeutic options are available for the infertile male with differentiating germ cells, such as in vitro fertilization, and intracytoplasmic sperm injection (28, 30), there are no effective methods for treating the infertile male with primitive germ cells. Thus, induction of spermatogenesis from primitive spermatogonia by adenoviral gene transfer extends the application of current therapeutic techniques, and creates new possibilities to obtain offspring from males with more severe spermatogenic arrest if the problem is Sertoli cell gene defect. Our results show that if a few spermatogonial stem cells differentiate into the haploid stages, these cells may be used in assisted reproductive techniques for offspring production. Although spermatogonial transplantation has been shown to restore fertility in mice (14) and the technique is now being applied in different species (including humans) (38, 39), it requires histocompatible recipients and the treatment of the recipients with carcinogenic and/or immunomodulatory agents (24, 25). These requirements will be difficult to satisfy in a genetically outbred human population, and such heterologous transplantation is likely anyway to encounter legal and ethical problems because the offspring will be genetically unrelated to the host. Thus, our successful production of offspring from infertile mice has important implications for the development of gene therapy protocols to correct human male infertility.
Increasing the efficiency of spermatogenesis after gene transfer is a prerequisite for successful infertility treatment. Although spermatogenesis lasted long enough to support at least two to three spermatogenic cycles, the numbers of elongated spermatids and spermatozoa were markedly reduced in the Sl/Sld testes, which suggested spermatogenic arrest at the round spermatid level. The result was unexpected because Sl germ cells can complete normal spermatogenesis after transplantation into a healthy microenvironment (14, 40). A possible explanation for this outcome is that forced, widespread expression of the Sl factor in seminiferous tubules may affect the normal testicular microenvironment. In fact, several genes, including that for the Sl factor, are expressed on Sertoli cells at specific spermatogenic stages (41, 42), and ectopic Sl factor expression by adenovirus may have caused inefficient spermatogenesis. Alternatively, the absence of spermatogenesis during testicular development may have hampered Sertoli cell maturation, and exogenous Sl expression in the adult may not have completely restored normal function. Because the cytoplasmic portion of the Sl factor may have signaling function (43), the interaction between germ cells and Sertoli cells also could affect Sertoli cells. Thus, the discrepancies between our results and the transplantation studies raise interesting questions about the germ cell–Sertoli cell interactions. The method used in this study provides a novel tool to analyze these problems.
At present, no human equivalent of the Sl mutation has been reported, and the genes responsible for human male infertility have been specifically expressed on germ cells so far (44–46). In addition, it should be noted that the high success rate with round spermatid injection in mice may not be simply extrapolated to humans (47, 48). Therefore, it remains to be determined how many of human cases might be treated by the present approach. Nonetheless, our successful production of offspring from an infertile male mouse suggests a promising possibility for the gene therapy approach in the treatment of male infertility. A number of infertile mutants in mice are caused by genetic defects in Sertoli cell function, which strongly suggest that some cases of human male infertility may be associated with Sertoli cell dysfunction (49), and improvements in the gene transfer technology will certainly increase mature spermatozoa production. Extensive studies on sequencing spermatogenesis genes and their site of expression as well as how to safely correct genes are clearly required for the possibility of gene therapy for male infertility at this stage of research. We are currently conducting a more rigorous, large-scale assessment of the risk of vector transmission to progeny. Such basic studies will help us accurately evaluate the risks and potential benefits of this approach in the future treatment of human male infertility.
Acknowledgments
We thank Ms. Y. Doi for technical assistance, Dr. I. Saito for AxCALacZ, and Drs. K. Ikuta and T. Tsubata for critically reading the manuscript. We also thank Dr. R. L. Brinster for continuous encouragement. Financial support was derived from the Kanae Foundation for Life and Socio-Medical Science, and from the Ministry of Education, Science, and Culture of Japan.
Abbreviations
- Sl
Steel
- Sl/Sld
Steel/Steeldickie
- pfu
plaque-forming unit
References
- 1.Hull M G, Glazener C M, Kelly N J, Conway D I, Foster P A, Hinton R A, Coulson C, Lambert P A, Watt E M, Desai K M. Br Med J. 1985;291:1693–1697. doi: 10.1136/bmj.291.6510.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Russell L D, Ettlin R A, Sinha Hikim A P, Clegg E D. Histological and Histopathological Evaluation of the Testis. Clearwater, FL: Cache River Press; 1990. pp. 1–40. [Google Scholar]
- 3.Silber S J. Clin Obstet Gynecol. 2000;43:854–888. doi: 10.1097/00003081-200012000-00017. [DOI] [PubMed] [Google Scholar]
- 4.Silvers W K. The Coat Colors of Mice. New York: Springer; 1979. pp. 242–267. [Google Scholar]
- 5.Zsebo K M, Williams D A, Geissler E N, Broudy V C, Martin F H, Atkins H L, Hsu R Y, Birkett N C, Okine K H, Murdock D C, et al. Cell. 1990;63:213–224. doi: 10.1016/0092-8674(90)90302-u. [DOI] [PubMed] [Google Scholar]
- 6.Chabot B, Stephenson D A, Chapman V M, Besmer P, Bernstein A. Nature (London) 1988;335:88–89. doi: 10.1038/335088a0. [DOI] [PubMed] [Google Scholar]
- 7.Geissler E N, Ryan M A, Housman D E. Cell. 1988;55:185–192. doi: 10.1016/0092-8674(88)90020-7. [DOI] [PubMed] [Google Scholar]
- 8.Yoshinaga K, Nishikawa S, Ogawa M, Hayashi S-I, Kunisada T, Fujimoto T, Nishikawa S-I. Development (Cambridge, UK) 1991;113:689–699. doi: 10.1242/dev.113.2.689. [DOI] [PubMed] [Google Scholar]
- 9.Flanagan J G, Chan D C, Leder P. Cell. 1991;64:1025–1035. doi: 10.1016/0092-8674(91)90326-t. [DOI] [PubMed] [Google Scholar]
- 10.Del-Castillo E B, Trabucco A, de la Balze F A. J Clin Endocrinol Metab. 1947;7:493–502. doi: 10.1210/jcem-7-7-493. [DOI] [PubMed] [Google Scholar]
- 11.Silber S J, Van Steirteghem A C, Devroey P. Hum Reprod. 1995;10:1031–1032. doi: 10.1093/oxfordjournals.humrep.a136085. [DOI] [PubMed] [Google Scholar]
- 12.Silber S J, Nagy Z, Devroey P, Tournaye H, Van Steirteghem A C. Hum Reprod. 1997;12:2422–2428. doi: 10.1093/humrep/12.11.2422. [DOI] [PubMed] [Google Scholar]
- 13.Nakayama H, Kuroda H, Onoue H, Fujita J, Nishimune Y, Matsumoto K, Nagano T, Suzuki F, Kitamura Y. Development (Cambridge, UK) 1988;102:117–126. doi: 10.1242/dev.102.1.117. [DOI] [PubMed] [Google Scholar]
- 14.Ogawa T, Dobrinski I, Avarbock M R, Brinster R L. Nat Med. 2000;6:29–34. doi: 10.1038/71496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim J-H, Jung H-S, Lee H-T, Chung K-S. Mol Reprod Dev. 1997;46:515–526. doi: 10.1002/(SICI)1098-2795(199704)46:4<515::AID-MRD10>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 16.Yamazaki Y, Fujimoto H, Ando H, Ohyama T, Hirota Y, Noce T. Biol Reprod. 1998;59:1439–1444. doi: 10.1095/biolreprod59.6.1439. [DOI] [PubMed] [Google Scholar]
- 17.Nagano M, Shinohara T, Avarbock M R, Brinster R L. FEBS Lett. 2000;475:7–10. doi: 10.1016/s0014-5793(00)01606-9. [DOI] [PubMed] [Google Scholar]
- 18.Blanchard K T, Boekelheide K. Biol Reprod. 1997;56:495–500. doi: 10.1095/biolreprod56.2.495. [DOI] [PubMed] [Google Scholar]
- 19.Wivel N A, Gao G-P, Wilson J M. In: The Development of Human Gene Therapy. Friedmann T, editor. Plainview, NY: Cold Spring Harbor Lab. Press; 1999. pp. 87–110. [Google Scholar]
- 20.Barker C F, Billingham R E. Adv Immunol. 1977;25:1–54. [PubMed] [Google Scholar]
- 21.Bellgrau D, Gold D, Selawry H, Moore J, Franzusoff A, Duke R C. Nature (London) 1995;377:630–632. doi: 10.1038/377630a0. [DOI] [PubMed] [Google Scholar]
- 22.Niwa H, Yamamura K-I, Miyazaki J. Gene. 1991;108:193–200. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- 23.Kanegae Y, Lee G, Sato Y, Tanaka M, Nakai M, Sakaki T, Sugano S, Saito I. Nucleic Acids Res. 1995;23:3816–3821. doi: 10.1093/nar/23.19.3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brinster R L, Zimmerman J W. Proc Natl Acad Sci USA. 1994;91:11298–11302. doi: 10.1073/pnas.91.24.11298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ogawa T, Aréchaga J M, Avarbock M R, Brinster R L. Int J Dev Biol. 1997;41:111–122. [PubMed] [Google Scholar]
- 26.Ogura A, Yamamoto Y, Suzuki O, Takano K, Wakayama T, Mochida K, Kimura H. Theriogenology. 1996;45:1141–1149. doi: 10.1016/0093-691x(96)00070-2. [DOI] [PubMed] [Google Scholar]
- 27.Ogura A, Inoue K, Matsuda J. Hum Reprod. 1999;14:1294–1298. doi: 10.1093/humrep/14.5.1294. [DOI] [PubMed] [Google Scholar]
- 28.Kimura Y, Yanagimachi R. Biol Reprod. 1995;52:709–720. doi: 10.1095/biolreprod52.4.709. [DOI] [PubMed] [Google Scholar]
- 29.Meng, X., Akutsu, H., Schoene, K., Reifsteck, C., Fox, E. P., Olson, S., Sariola, H., Yanagimachi, R. & Baetscher, M. (2002) Biol. Reprod., in press. [DOI] [PubMed]
- 30.Palermo G, Joris H, Debroey P, Van Steirteghem A C. Lancet. 1992;340:17–18. doi: 10.1016/0140-6736(92)92425-f. [DOI] [PubMed] [Google Scholar]
- 31.Bellvé A R. Methods Enzymol. 1993;225:84–113. doi: 10.1016/0076-6879(93)25009-q. [DOI] [PubMed] [Google Scholar]
- 32.Bucci L R, Meistrich M L. Mutat Res. 1987;176:259–268. doi: 10.1016/0027-5107(87)90057-1. [DOI] [PubMed] [Google Scholar]
- 33.Selawry H P, Cameron D F. Cell Transplant. 1993;2:123–129. doi: 10.1177/096368979300200206. [DOI] [PubMed] [Google Scholar]
- 34.Sanberg P R, Borlongan C V, Saporta S, Cameron D F. Tissue Cell. 1999;31:461–472. [Google Scholar]
- 35.Shinohara T, Avarbock M R, Brinster R L. Dev Biol. 2000;220:401–411. doi: 10.1006/dbio.2000.9655. [DOI] [PubMed] [Google Scholar]
- 36.Hall S J, Bar-Chama N, Ta S, Gordon J W. Hum Gene Ther. 2000;11:1705–1712. doi: 10.1089/10430340050111359. [DOI] [PubMed] [Google Scholar]
- 37.Ye X, Gao G P, Pabin C, Raper S E, Wilson J M. Hum Gene Ther. 1998;9:2135–2142. doi: 10.1089/hum.1998.9.14-2135. [DOI] [PubMed] [Google Scholar]
- 38.Schlatt S, Rosiepen G, Weinbauer G F, Rolf C, Brook P F, Nieschlag E. Hum Reprod. 1999;14:144–150. doi: 10.1093/humrep/14.1.144. [DOI] [PubMed] [Google Scholar]
- 39.Brook P F, Radford J A, Shalet S M, Joyce A D, Gosden R G. Fertil Steril. 2001;75:269–274. doi: 10.1016/s0015-0282(00)01721-0. [DOI] [PubMed] [Google Scholar]
- 40.Ohta H, Yomogida K, Dohmae K, Nishimune Y. Development (Cambridge, UK) 2000;127:2125–2131. doi: 10.1242/dev.127.10.2125. [DOI] [PubMed] [Google Scholar]
- 41.Yomogida K, Ohtani H, Harigae H, Ito E, Nishimune Y, Engel J D, Yamamoto M. Development (Cambridge, UK) 1994;120:1759–1766. doi: 10.1242/dev.120.7.1759. [DOI] [PubMed] [Google Scholar]
- 42.Vincent S, Segretain D, Nishikawa S, Nishikawa S-I, Sage J, Cuzin F, Rassoulzadegan M. Development (Cambridge, UK) 1998;125:4585–4593. doi: 10.1242/dev.125.22.4585. [DOI] [PubMed] [Google Scholar]
- 43.Brannan C I, Bedell M A, Resnick J L, Eppig J J, Handel M A, Williams D E, Lyman S D, Donovan P J, Jenkins N A, Copeland N G. Genes Dev. 1992;6:1832–1842. doi: 10.1101/gad.6.10.1832. [DOI] [PubMed] [Google Scholar]
- 44.Wang P J, McCarrey J R, Yang F, Page D C. Nat Genet. 2001;27:422–426. doi: 10.1038/86927. [DOI] [PubMed] [Google Scholar]
- 45.Menke D B, Mutter G L, Page D C. Am J Hum Genet. 1997;60:237–241. [PMC free article] [PubMed] [Google Scholar]
- 46.Kuroda-Kawaguchi T, Skaletsky H, Brown L G, Minx P J, Cordum H S, Waterston R H, Wilson R K, Silber S, Oates R, Rozen S, Page D C. Nat Genet. 2001;29:279–286. doi: 10.1038/ng757. [DOI] [PubMed] [Google Scholar]
- 47.Silber S J, Johnson L. Hum Reprod. 1998;13:509–515. doi: 10.1093/humrep/13.3.509. [DOI] [PubMed] [Google Scholar]
- 48.Silber S J, Johnson L, Verheyen G, Van Steirteghem A. Fertil Steril. 2000;73:897–900. doi: 10.1016/s0015-0282(00)00488-x. [DOI] [PubMed] [Google Scholar]
- 49.Escalier D. Hum Reprod Update. 2001;7:191–210. doi: 10.1093/humupd/7.2.191. [DOI] [PubMed] [Google Scholar]