Abstract
Objective
This study investigated the effects of sleep duration and sleep disorders on metabolic dysfunction-associated steatotic liver disease (MASLD) in older adults and provided a scientific basis for the development of targeted health management strategies.
Methods
The present study utilized data from the National Health and Nutrition Examination Survey database of older adults from 2005 to 2014 and included 4,731 participants. The assessment of sleep duration and sleep disorders was conducted using the “Sleep Disorders” questionnaire, while the diagnosis of MASLD was determined based on the fatty liver index and cardiometabolic criteria. The relationship between sleep duration, sleep disorders, and MASLD was analyzed using logistic regression modeling, and the dose-response relationship between sleep duration and MASLD was explored using the restricted cubic spline (RCS) model. Furthermore, subgroup analyses were conducted to investigate the association between sleep disorders and MASLD across diverse population characteristics.
Results
The study indicated that individuals who slept less than six hours per night exhibited a 21% higher prevalence of MASLD than those who slept six to eight hours. Notably, the prevalence increased substantially, by 38%, in individuals who slept more than eight hours per night. The presence of sleep disorders in individuals was found to be associated with a 2.38-fold elevated prevalence of MASLD compared to those without sleep disorders. Furthermore, the RCS analysis revealed a non-linear relationship between sleep duration and MASLD, indicating an overall “U”-shaped trend with a turning point at 7 h. Subgroup analyses demonstrated that the association remained significant in all subgroups except those with low education levels and stroke patients.
Conclusion
The present study found that short or long sleep duration and the presence of sleep disorders were significantly associated with MASLD in older adults. The result provides a rationale for the prevention of MASLD in the elderly population. The study suggests that clinical practice should focus on the potential value of sleep assessment in managing metabolic liver disease.
Clinical trial number
Not applicable.
Keywords: Metabolic dysfunction-associated steatotic liver disease, Sleep duration, Sleep disorders, Older adults, Nonlinear relationship
Introduction
The accelerated aging of the global population has led to a growing focus on the health concerns of older adults. Metabolic dysfunction-associated steatotic liver disease (MASLD), a chronic liver disease associated with metabolic disorders, has seen a steady rise in incidence among older adults, jeopardizing their health and quality of life [1, 2]. The repercussions of MASLD extend beyond the immediate well-being of the affected individuals, as it has the potential to culminate in advanced stages of liver disease, including cirrhosis and even hepatocellular carcinoma [3–5]. This underscores the grave challenges it poses to public health. Recent studies have indicated that both sleep duration abnormalities, such as protracted or diminished sleep, and sleep disorders, including insomnia and sleep apnea, are independently associated with an elevated risk of metabolic diseases, including obesity and type 2 diabetes [6–8]. However, the relationship between sleep duration and sleep disorders and MASLD among older adults remains to be fully elucidated, impeding the development of effective strategies for the prevention and management of MASLD.
Sleep is an integral component of the physiological processes of the human body, including the restoration of physical strength, the consolidation of memory, and the regulation of metabolism [9, 10]. It plays a critical role in maintaining the health of the body. Sleep is an essential safeguard in older adults’ physical and mental health. However, as individuals age, they are more prone to sleep disturbances, including decreased sleep duration and quality [11–13]. These sleep disorders not only affect the mental state and quality of daily life of older adults but also may adversely affect their health through complex physiological mechanisms. For instance, sleep deprivation has been demonstrated to interfere with the immune system of older adults, reducing their resistance and rendering them more susceptible to diseases [14]. Concurrently, sleep disorders have been shown to affect cognitive function in older adults, increasing their risk of developing Alzheimer’s disease [15, 16]. Additionally, sleep disorders may trigger or exacerbate chronic diseases such as cardiovascular disease and diabetes mellitus (DM) in older adults [17, 18], and they may also adversely affect the health of the liver [19], which may increase the risk of developing MASLD. Sleep deprivation has been demonstrated to inhibit the insulin signaling pathway by enhancing sympathetic nerve activity [20, 21], which triggers insulin resistance and promotes hepatic fat synthesis. Meanwhile, sleep disorders have been shown to directly injure hepatocytes through the activation of systemic inflammatory responses and oxidative stress [22, 23]. Furthermore, sleep disorders have been demonstrated to disrupt gut flora homeostasis, increase intestinal permeability, contribute to endotoxin entry into the bloodstream, and activate hepatic stellate cells, exacerbating hepatic inflammation [24]. Imbalances in neuroendocrine regulation (e.g., a decline in leptin levels and an increase in gastric starvation hormone) may result in a heightened inclination toward high-calorie foods, thereby indirectly fostering hepatic lipid accumulation [25–27].
A substantial body of research has demonstrated a significant association between sleep disturbances (e.g., insufficient or excessive sleep duration, sleep disorders) and an elevated risk of metabolic disorders (e.g., type 2 diabetes, obesity) in the general population [6–8]. However, there remains a notable gap in the sleep research targeting metabolic syndrome in the elderly population. A preliminary review of the extant literature reveals a predominant focus on the effects of a single sleep dimension, such as sleep duration or sleep disorders, on liver health [28, 29]. However, a paucity of literature specifically explores the impact on this metabolically vulnerable group within the elderly population. Furthermore, most studies employed linear models to hypothesize the association between sleep duration and MASLD, overlooking the possibility of nonlinear trends and threshold effects. In this study, the independent and joint effects of sleep duration and sleep disorders on MASLD in the elderly were assessed simultaneously for the first time using data from the National Health and Nutrition Examination Survey (NHANES) large-sample elderly population. The researchers employed restricted cubic spline (RCS) modeling to elucidate the nonlinear association between sleep duration and MASLD. We also explored population heterogeneity through subgroup analysis. This study addresses a critical gap in the research on the association between sleep and MASLD in the elderly population, providing a significant theoretical and clinical translational basis for preventing MASLD in this demographic.
Methods
Study population
This study was cross-sectional and observational, drawing upon the NHANES database. The database incorporates data from the older adult population from 2005 to 2014, with sleep variables (e.g., duration, disorder) and MASLD diagnoses based on data collected during the same survey cycle. The study design was formally approved by the National Center for Health Statistics (NCHS) Ethics Review Board, which rigorously ensured that all participants signed consent forms on a fully informed basis. Following relevant NIH policies, given that the NHANES data were not collected directly through contact with participants, they could be used directly for subsequent data analysis without further review by the institutional ethics committee.
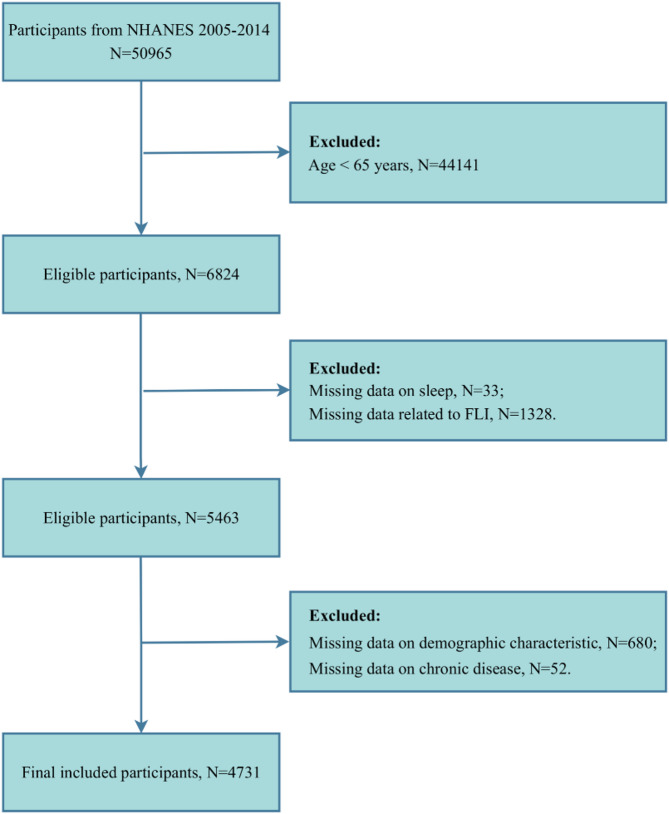
In the initial phase of the study, we included information on a total of 50,965 participants derived from five consecutive cycles of the NHANES survey. After this, a screening and exclusion process was implemented, with participants being removed based on the following criteria: being younger than 65 years of age, missing sleep-related data, missing the calculation of fatty liver index (FLI) metrics (including body mass index (BMI), waist circumference (WC), gamma-glutamyl transferase (GGT), and triglycerides (TG)), missing data on demographic characteristics, and missing data on chronic diseases. Following the application of these criteria, a total of 4,731 older participants were enrolled in this study (Fig. 1).
Fig. 1.
Participant screening flowchart. FLI, Fatty Liver Index
MASLD assessment
In the early stages of MASLD, abnormal fat accumulation in liver tissue is a distinguishing feature. Due to the paucity of data on ultrasound assessment of hepatic steatosis and transient elastography of the liver during the follow-up cycle of the present study, the evaluation of the status of MASLD relied principally on the FLI [30]. The following formula calculates the FLI:
According to the findings of previous studies, individuals with an FLI of less than 60 are at a lower risk of hepatic steatosis. In contrast, individuals with an FLI of 60 or more are at a higher risk and are diagnosed with hepatic steatosis [31]. Furthermore, a diagnosis of MASLD is substantiated by the presence of any of the following five cardiometabolic criteria: (1) a BMI ≥ 25 kg/m², or WC ≥ 94 cm in men and ≥ 80 cm in women; (2) a fasting plasma glucose (FPG) ≥ 100 mg/dL, or a 2-hour post-load glucose level ≥ 140 mg/dL, or a hemoglobin A1c (HbA1c) ≥ 5.7%, or a previous diagnosis of DM, or current treatment with glucose-lowering therapy for DM; (3) blood pressure ≥ 130/85 mmHg, or treatment with antihypertensive medications; (4) TG ≥ 150 mg/dL, or treatment with lipid-lowering therapy; and (5) high-density lipoprotein cholesterol (HDL-c) level < 40 mg/dL for men and < 50 mg/dL for women, or being treated with lipid-lowering therapy [32].
Assessment of sleep disorders
The assessment of sleep duration and disorders was conducted using the “Sleep Disorders” questionnaire. These inquiries were posed by trained interviewers in the subjects’ homes using a computer-assisted personal interview (CAPI) system. The assessment of sleep duration was conducted through the inquiry, “How many hours of sleep do you get on average per night?” and ranged from 2 to 12 h/day. The assessment of sleep disorders was conducted through the inquiry, “Have you ever informed your physician or other healthcare professional of a sleep disorder?” The presence or absence of a sleep disorder was determined based on the participant’s response (yes or no) [33].
Assessment of covariates
The analysis controlled for sociodemographic characteristics, lifestyle habits, and chronic disease history. Specifically, the following covariates were included: gender (male/female), age (in years), racial classification (Mexican American, non-Hispanic white, non-Hispanic black, and other race), educational attainment (categorized into three levels: less than 9th grade, 9th-12th grade, and more than 12th grade), marital status (simplified as cohabitation and solitude), and family income status (categorized into low (PIR ≤ 1.3), the medium category (1.3 < PIR ≤ 3.5), and the high category (PIR > 3.5)). The drinking habits inquired about included the number of alcoholic beverages consumed by the participant in one year. The smoking status was determined by the number of cigarettes smoked by the participant in their lifetime, as well as their current smoking status. The physical activity level was categorized as inactive, moderate, and vigorous. Regarding medical history variables, the diagnosis of DM was based on a physician’s diagnosis, FPG ≥ 126 mg/dl, HbA1c ≥ 6.5%, and diabetes medication or insulin use. Conversely, the determination of hypertension was contingent on physician notification and the utilization of prescription medications. The presence of coronary heart disease, stroke, and cancer was determined by physician notification of the diagnosis [34].
Statistical analysis
The Kolmogorov-Smirnov test was used to assess their normal distribution for continuous variables. The results indicated that all continuous variables in this study did not conform to a normal distribution. Consequently, these variables were described using the median (and the 25th and 75th percentiles) and analyzed for between-group differences using the Mann-Whitney test. Categorical variables, conversely, were presented as frequencies and percentages, and the chi-square test compared differences between groups.
We constructed logistic regression models to explore the relationship between sleep duration, sleep disorders, and MASLD and calculated the odds ratios (ORs) and their 95% confidence intervals (CIs). To accurately assess these relationships and to control for the potential problem of confounding variables, three models were constructed: model 1 was unadjusted; Model 2 adjusted for gender, age, and race based on Model 1; and Model 3 further adjusted for the inclusion of the variables of educational attainment, marital status, family PIR, smoking, alcohol consumption, physical activity, DM, hypertension, coronary heart disease, stroke, and cancer based on model 2.
The nonlinear relationship between sleep duration and MASLD was analyzed using a RCS model. The selection of degrees of freedom was guided by the Akaike Information Criterion (AIC) and the Bayesian Information Criterion (BIC), leading to the determination of a 3-node model as the optimal fit. The model was configured with three nodes (i.e., knots) based on the 10th, 50th, and 90th percentiles of sleep duration. The RCS model was selected based on the following rationale: first, previous studies suggested that sleep duration was nonlinearly associated with the risk of metabolic diseases [35, 36]; second, the 3-node model significantly improved the goodness of fit compared with the linear model, as validated by AIC; furthermore, the RCS has been demonstrated to be capable of capturing the nonlinear trend of continuous variables while avoiding the loss of information that is often associated with the division of categorical variables. This ability to avoid such a loss of information allows for a more precise revelation of the complex relationship between sleep duration and MASLD.
To further analyze the relationship between sleep disorders and MASLD in different subgroups, we performed stratified and interaction analyses based on variables such as race, education, marital status, family PIR, smoking, alcohol consumption, physical activity, DM, hypertension, coronary heart disease, stroke, and cancer. To verify the robustness of the findings, this study used multiple imputation to fill in the missing values of the covariates and reconstructed the logistic regression model to assess the potential bias of missing data on the results. The RCS model was augmented with the number of nodes (4 nodes, quantile positions: 5%, 35%, 65%, 95%) to assess the stability of the nonlinear relationship between sleep duration and MASLD under varying parameters.
A two-sided test was employed to conduct statistical analyses, with P < 0.05 designated as the criterion for statistical significance. All statistical analyses were performed using R 4.4.0 (courtesy of the R Foundation, http://www.R-project.org) and SPSS version 23.0 (IBM Corp., Armonk, NY, USA), and graphing was performed using GraphPad Prism version 9.0 (GraphPad Software, Inc., USA).
Results
Baseline characteristics of participants with and without MASLD
Four thousand seven hundred thirty-one participants were included in this study, of which 2,337 were diagnosed with MASLD, and 2,394 were diagnosed with non-MASLD. The results indicated that males were significantly more represented in the MASLD group than in the non-MASLD group (54.56% vs. 47.08%, P < 0.001). The median age was 72 years in the MASLD group, which was lower than 74 years in the non-MASLD group (P < 0.001). Regarding race, Mexican American participants constituted a higher percentage of the MASLD group (11.13% vs. 7.77%, P < 0.001). Furthermore, the MASLD group exhibited a higher prevalence of participants with lower levels of education (17.16% vs. 15.46%, P = 0.005). Individuals with a low family PIR exhibited a higher prevalence in the MASLD group (29.31% vs. 26.69%, P = 0.028). A significant disparity was observed in the prevalence of smoking, with a higher proportion of smokers identified in the MASLD group compared to the non-MASLD group (55.50% vs. 49.67%, P < 0.001). Regarding physical activity, the proportion of inactive persons was higher in the MASLD group (44.46% vs. 35.34%, P < 0.001). The prevalence of DM, hypertension, and coronary heart disease was significantly higher in the MASLD group than in the non-MASLD group (DM: 42.02% vs. 21.43%, P < 0.001; hypertension: 70.09% vs. 55.97%, P < 0.001; coronary heart disease: 12.54% vs. 10.36%, P = 0.019). Furthermore, a comprehensive analysis revealed that BMI, WC, FPG, HbA1c, TG, and alanine transaminase (ALT) levels were significantly higher in the MASLD group compared to the non-MASLD group (P < 0.001). In comparison, HDL-c levels were significantly lower in the non-MASLD group (P < 0.001). A notable finding was the significantly higher incidence of sleep disorders in MASLD patients compared to non-MASLD patients (12.97% vs. 5.10%, P < 0.001) (Table 1).
Table 1.
Baseline characteristics of participants with and without MASLD
| Variables | Total (n = 4731) |
NON-MASLD (n = 2394) |
MASLD (n = 2337) |
P |
|---|---|---|---|---|
| Gender, n (%) | < 0.001 | |||
| Male | 2402 (50.77) | 1127 (47.08) | 1275 (54.56) | |
| Female | 2329 (49.23) | 1267 (52.92) | 1062 (45.44) | |
| Age (years) | 73.00 (68.00, 79.00) | 74.00 (69.00, 80.00) | 72.00 (68.00, 77.00) | < 0.001 |
| Race, n (%) | < 0.001 | |||
| Mexican American | 446 (9.43) | 186 (7.77) | 260 (11.13) | |
| Non-Hispanic White | 2896 (61.21) | 1465 (61.19) | 1431 (61.23) | |
| Non-Hispanic Black | 823 (17.40) | 412 (17.21) | 411 (17.59) | |
| Other Race | 566 (11.96) | 331 (13.83) | 235 (10.06) | |
| Education Level, n (%) | 0.005 | |||
| Less than 9th grade | 771 (16.30) | 370 (15.46) | 401 (17.16) | |
| 9–12th grade | 1943 (41.07) | 948 (39.60) | 995 (42.58) | |
| More than 12th grade | 2017 (42.63) | 1076 (44.95) | 941 (40.27) | |
| Marital Status, n (%) | 0.180 | |||
| Cohabitation | 2707 (57.22) | 1347 (56.27) | 1360 (58.19) | |
| Solitude | 2024 (42.78) | 1047 (43.73) | 977 (41.81) | |
| Family PIR, n (%) | 0.028 | |||
| Low (≤ 1.3) | 1324 (27.99) | 639 (26.69) | 685 (29.31) | |
| Medium (1.3–3.5) | 2141 (45.25) | 1078 (45.03) | 1063 (45.49) | |
| High (> 3.5) | 1266 (26.76) | 677 (28.28) | 589 (25.20) | |
| Smoking, n (%) | < 0.001 | |||
| Yes | 2486 (52.55) | 1189 (49.67) | 1297 (55.50) | |
| No | 2245 (47.45) | 1205 (50.33) | 1040 (44.50) | |
| Alcohol, n (%) | 0.650 | |||
| Yes | 3082 (65.14) | 1567 (65.46) | 1515 (64.83) | |
| No | 1649 (34.86) | 827 (34.54) | 822 (35.17) | |
| Physical Activity, n (%) | < 0.001 | |||
| Inactive | 1885 (39.84) | 846 (35.34) | 1039 (44.46) | |
| Moderate | 2155 (45.55) | 1177 (49.16) | 978 (41.85) | |
| Vigorous | 691 (14.61) | 371 (15.50) | 320 (13.69) | |
| Diabetes mellitus, n (%) | < 0.001 | |||
| Yes | 1495 (31.60) | 513 (21.43) | 982 (42.02) | |
| No | 3236 (68.40) | 1881 (78.57) | 1355 (57.98) | |
| Hypertension, n (%) | < 0.001 | |||
| Yes | 2978 (62.95) | 1340 (55.97) | 1638 (70.09) | |
| No | 1753 (37.05) | 1054 (44.03) | 699 (29.91) | |
| Coronary heart disease, n (%) | 0.019 | |||
| Yes | 541 (11.44) | 248 (10.36) | 293 (12.54) | |
| No | 4190 (88.56) | 2146 (89.64) | 2044 (87.46) | |
| Stroke, n (%) | 0.102 | |||
| Yes | 423 (8.94) | 198 (8.27) | 225 (9.63) | |
| No | 4308 (91.06) | 2196 (91.73) | 2112 (90.37) | |
| Cancer, n (%) | 0.841 | |||
| Yes | 1095 (23.15) | 557 (23.27) | 538 (23.02) | |
| No | 3636 (76.85) | 1837 (76.73) | 1799 (76.98) | |
| BMI (kg/m2) | 27.81 (24.62, 31.60) | 24.76 (22.62, 26.80) | 31.54 (29.00, 35.09) | < 0.001 |
| WC (cm) | 100.90 (92.15, 110.20) | 92.50 (86.00, 98.10) | 110.10 (104.30, 118.00) | < 0.001 |
| FPG (mg/dL) | 100.00 (90.00, 117.00) | 96.00 (89.00, 108.00) | 105.00 (93.00, 126.00) | < 0.001 |
| HbA1c (%) | 5.80 (5.50, 6.20) | 5.70 (5.40, 6.00) | 5.90 (5.60, 6.50) | < 0.001 |
| TC (mg/dL) | 188.00 (160.00, 218.00) | 189.50 (162.00, 219.00) | 186.00 (159.00, 218.00) | 0.045 |
| TG (mg/dL) | 129.00 (88.00, 190.00) | 102.00 (75.00, 143.00) | 166.00 (116.00, 236.00) | < 0.001 |
| HDL-c (mg/dL) | 52.00 (43.00, 64.00) | 58.00 (48.00, 70.00) | 47.00 (39.00, 56.00) | < 0.001 |
| AST (U/L) | 24.00 (21.00, 28.00) | 24.00 (21.00, 27.94) | 24.00 (20.00, 28.00) | 0.727 |
| ALT (U/L) | 19.00 (16.00, 24.00) | 19.00 (15.00, 23.00) | 21.00 (17.00, 26.00) | < 0.001 |
| GGT (IU/L) | 19.00 (15.00, 28.00) | 17.00 (13.00, 22.75) | 23.00 (17.00, 35.00) | < 0.001 |
| Sleep disorder, n (%) | < 0.001 | |||
| Yes | 425 (8.98) | 122 (5.10) | 303 (12.97) | |
| No | 4306 (91.02) | 2272 (94.90) | 2034 (87.03) |
Data are shown as median (25th, 75th percentiles) or percentages, p < 0.05 considered statistically significant. MASLD, Metabolic dysfunction-associated steatotic liver disease; PIR, Poverty-to-income ratio; BMI, Body mass index; WC, Waist circumference; FPG, Fasting plasma-glucose; HbA1c, Hemoglobin A1c; TC, Total cholesterol; TG, Triglyceride; HDL-c, High density lipoprotein cholesterol; AST, Aspartate aminotransferase; ALT, Alanine transaminase; GGT, Gamma-glutamyl transferase
Baseline characteristics of participants with and without sleep disorders
Among the 4,731 participants, 425 were diagnosed with sleep disorders, while 4,306 did not exhibit any sleep disorders. The results indicated that males were significantly more represented in the sleep disorder group than in the no sleep disorder group (61.18% vs. 49.74%, P < 0.001). The median age in the sleep disorder group was 71 years, which was lower than 73 years in the no sleep disorder group (P < 0.001). A higher percentage of participants with higher levels of education were found in the sleep disorder group (48.94% vs. 42.01%, P = 0.022). A significant disparity was observed in the prevalence of smoking, with a higher proportion of smokers identified in the sleep disorder group compared to the no sleep disorder group (61.18% vs. 51.70%, P < 0.001). The prevalence of DM, hypertension, coronary heart disease, and stroke was all significantly higher in the sleep disorder group than in the no sleep disorder group (DM: 43.29% vs. 30.45%, P < 0.001; hypertension: 68.94% vs. 62.35%, P = 0.007; coronary heart disease: 17.41% vs. 10.85%, P < 0.001; stroke: 11.76% vs. 8.66%, P = 0.032). Furthermore, patients with sleep disorders exhibited higher BMI, WC, FPG, HbA1c, TG, and GGT, while HDL-c levels were lower. Of particular interest is the observation that the prevalence of MASLD was significantly higher in patients with sleep disorders compared to those without sleep disorders (71.29% vs. 47.24%, P < 0.001) (Table 2).
Table 2.
Baseline characteristics of participants with and without sleep disorder
| Variables | Total (n = 4731) |
Non-Sleep disorder (n = 4306) |
Sleep disorder (n = 425) |
P |
|---|---|---|---|---|
| Gender, n (%) | < 0.001 | |||
| Male | 2402 (50.77) | 2142 (49.74) | 260 (61.18) | |
| Female | 2329 (49.23) | 2164 (50.26) | 165 (38.82) | |
| Age (years) | 73.00 (68.00, 79.00) | 73.00 (68.00, 80.00) | 71.00 (68.00, 77.00) | < 0.001 |
| Race, n (%) | 0.118 | |||
| Mexican American | 446 (9.43) | 411 (9.54) | 35 (8.24) | |
| Non-Hispanic White | 2896 (61.21) | 2615 (60.73) | 281 (66.12) | |
| Non-Hispanic Black | 823 (17.40) | 764 (17.74) | 59 (13.88) | |
| Other Race | 566 (11.96) | 516 (11.98) | 50 (11.76) | |
| Education Level, n (%) | 0.022 | |||
| Less than 9th grade | 771 (16.30) | 711 (16.51) | 60 (14.12) | |
| 9–12th grade | 1943 (41.07) | 1786 (41.48) | 157 (36.94) | |
| More than 12th grade | 2017 (42.63) | 1809 (42.01) | 208 (48.94) | |
| Marital Status, n (%) | 0.003 | |||
| Cohabitation | 2707 (57.22) | 2435 (56.55) | 272 (64.00) | |
| Solitude | 2024 (42.78) | 1871 (43.45) | 153 (36.00) | |
| Family PIR, n (%) | 0.369 | |||
| Low (≤ 1.3) | 1324 (27.99) | 1209 (28.08) | 115 (27.06) | |
| Medium (1.3–3.5) | 2141 (45.25) | 1957 (45.45) | 184 (43.29) | |
| High (> 3.5) | 1266 (26.76) | 1140 (26.47) | 126 (29.65) | |
| Smoking, n (%) | < 0.001 | |||
| Yes | 2486 (52.55) | 2226 (51.70) | 260 (61.18) | |
| No | 2245 (47.45) | 2080 (48.30) | 165 (38.82) | |
| Alcohol, n (%) | 0.085 | |||
| Yes | 3082 (65.14) | 2789 (64.77) | 293 (68.94) | |
| No | 1649 (34.86) | 1517 (35.23) | 132 (31.06) | |
| Physical Activity, n (%) | 0.909 | |||
| Inactive | 1885 (39.84) | 1716 (39.85) | 169 (39.76) | |
| Moderate | 2155 (45.55) | 1964 (45.61) | 191 (44.94) | |
| Vigorous | 691 (14.61) | 626 (14.54) | 65 (15.29) | |
| Diabetes mellitus, n (%) | < 0.001 | |||
| Yes | 1495 (31.60) | 1311 (30.45) | 184 (43.29) | |
| No | 3236 (68.40) | 2995 (69.55) | 241 (56.71) | |
| Hypertension, n (%) | 0.007 | |||
| Yes | 2978 (62.95) | 2685 (62.35) | 293 (68.94) | |
| No | 1753 (37.05) | 1621 (37.65) | 132 (31.06) | |
| Coronary heart disease, n (%) | < 0.001 | |||
| Yes | 541 (11.44) | 467 (10.85) | 74 (17.41) | |
| No | 4190 (88.56) | 3839 (89.15) | 351 (82.59) | |
| Stroke, n (%) | 0.032 | |||
| Yes | 423 (8.94) | 373 (8.66) | 50 (11.76) | |
| No | 4308 (91.06) | 3933 (91.34) | 375 (88.24) | |
| Cancer, n (%) | 0.576 | |||
| Yes | 1095 (23.15) | 992 (23.04) | 103 (24.24) | |
| No | 3636 (76.85) | 3314 (76.96) | 322 (75.76) | |
| BMI (kg/m2) | 27.81 (24.62, 31.60) | 27.57 (24.50, 31.12) | 31.32 (27.40, 35.71) | < 0.001 |
| WC (cm) | 100.90 (92.15, 110.20) | 100.10 (91.60, 109.20) | 110.00 (101.70, 120.90) | < 0.001 |
| FPG (mg/dL) | 100.00 (90.00, 117.00) | 99.00 (90.00, 116.00) | 104.00 (93.00, 128.00) | < 0.001 |
| HbA1c (%) | 5.80 (5.50, 6.20) | 5.80 (5.50, 6.20) | 5.90 (5.60, 6.50) | < 0.001 |
| TC (mg/dL) | 188.00 (160.00, 218.00) | 189.00 (162.00, 220.00) | 176.00 (149.00, 209.00) | < 0.001 |
| TG (mg/dL) | 129.00 (88.00, 190.00) | 128.00 (87.00, 189.00) | 143.00 (99.00, 198.00) | < 0.001 |
| HDL-c (mg/dL) | 52.00 (43.00, 64.00) | 53.00 (43.00, 65.00) | 47.00 (40.00, 57.00) | < 0.001 |
| AST (U/L) | 24.00 (21.00, 28.00) | 24.00 (21.00, 28.00) | 24.00 (21.00, 28.00) | 0.878 |
| ALT (U/L) | 19.00 (16.00, 24.00) | 19.00 (16.00, 24.00) | 21.00 (16.00, 26.00) | < 0.001 |
| GGT (IU/L) | 19.00 (15.00, 28.00) | 19.00 (14.00, 28.00) | 21.00 (15.00, 31.00) | 0.003 |
| MASLD, n (%) | < 0.001 | |||
| Yes | 2337 (49.40) | 2034 (47.24) | 303 (71.29) | |
| No | 2394 (50.60) | 2272 (52.76) | 122 (28.71) |
Data are shown as median (25th, 75th percentiles) or percentages, p < 0.05 considered statistically significant. PIR, Poverty-to-income ratio; BMI, Body mass index; WC, Waist circumference; FPG, Fasting plasma-glucose; HbA1c, Hemoglobin A1c; TC, Total cholesterol; TG, Triglyceride; HDL-c, High density lipoprotein cholesterol; AST, Aspartate aminotransferase; ALT, Alanine transaminase; GGT, Gamma-glutamyl transferase; MASLD, Metabolic dysfunction-associated steatotic liver disease
Relationship between sleep duration, sleep disorders, and MASLD
This study employed multi-model logistic regression analysis to investigate the association between sleep duration and sleep disorders with MASLD. In unadjusted model 1, sleep duration shorter than 6 h/night or longer than 8 h/night and sleep disorders were found to be significantly associated with an increased prevalence of MASLD (P < 0.05). These associations remained significant in Models 2 and 3, adjusted for potential confounders. Specifically, compared with those who slept 6–8 h per night, those who slept less than 6 h per night had an increased prevalence of MASLD onset of approximately 33% (model 2) to 21% (model 3). In contrast, those who slept more than 8 h per night had a more significant increase in prevalence of 45% (model 2) and 38% (model 3), respectively. Furthermore, the prevalence of MASLD was found to be 2.61 times (model 2) to 2.38 times (model 3) higher in individuals with sleep disorders compared to those without sleep disorders (Table 3).
Table 3.
Relationships between sleep duration, sleep disorder and MASLD
| Variables | Model 1 | Model 2 | Model 3 | |||||
|---|---|---|---|---|---|---|---|---|
| OR (95%CI) | P | OR (95%CI) | P | OR (95%CI) | P | |||
| Sleep duration | ||||||||
| 6–8 h/night | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | |||||
| < 6 h/night | 1.27 (1.07 ~ 1.51) | 0.007 | 1.33 (1.11 ~ 1.58) | 0.002 | 1.21 (1.01 ~ 1.46) | 0.041 | ||
| > 8 h/night | 1.33 (1.11 ~ 1.59) | 0.002 | 1.45 (1.20 ~ 1.74) | < 0.001 | 1.38 (1.14 ~ 1.66) | < 0.001 | ||
| Sleep disorder | ||||||||
| No | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | |||||
| Yes | 2.77 (2.23 ~ 3.45) | < 0.001 | 2.61 (2.09 ~ 3.25) | < 0.001 | 2.38 (1.89 ~ 3.00) | < 0.001 | ||
Model 1: crude; Model 2: adjusted for Gender, Age, Race; Model 3: adjusted for Gender, Age, Race, Education Level, Marital Status, Family PIR, Smoking, Alcohol, Physical Activity, Diabetes mellitus, Hypertension, Coronary heart disease, Stroke, Cancer. MASLD, Metabolic dysfunction-associated steatotic liver disease; OR, Odds ratio; CI, Confidence interval
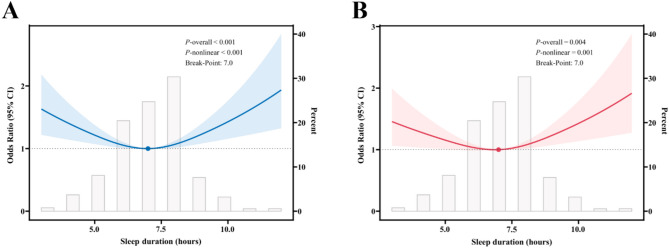
RCS analysis of sleep duration and MASLD
Figure 2A illustrates the association between unadjusted sleep duration and MASLD, and the results indicate that both the P-value and the nonlinear P-value are less than 0.001, suggesting a significant nonlinear relationship between sleep duration and MASLD. The spline fitting identified a turning point of 7.0 (95% CI: 6.4–7.6) hours, suggesting a substantial difference in the effect of sleep duration on MASLD before and after this turning point. Furthermore, Fig. 2B illustrates the relationship between sleep duration and MASLD, adjusted for a variety of potential confounders, including gender, age, race, educational attainment, marital status, family PIR, smoking, alcohol consumption, physical activity, DM, hypertension, coronary heart disease, stroke, and cancer. The adjusted results yielded a P-value and nonlinear P-value of 0.004 and 0.001, respectively, thereby sustaining support for a nonlinear relationship between sleep duration and MASLD. The turning point remained at 7 h, suggesting that 7 h of sleep per night may be critical in influencing MASLD. Specifically, before 7 h, a 1-hour increase in sleep duration was associated with a significant decrease in the prevalence of MASLD. In contrast, after 7 h, an increase in sleep duration increased the prevalence of MASLD.
Fig. 2.
Restricted cubic spline fitting for the association between sleep duration and MASLD. A: Unadjusted; B: Adjusted for gender, age, race, education level, marital status, family PIR, smoking, alcohol, physical activity, diabetes mellitus, hypertension, coronary heart disease, stroke, and cancer. The solid line displays the odds ratio, with the 95% CI represented by shading. MASLD, Metabolic dysfunction-associated steatotic liver disease; CI, Confidence interval
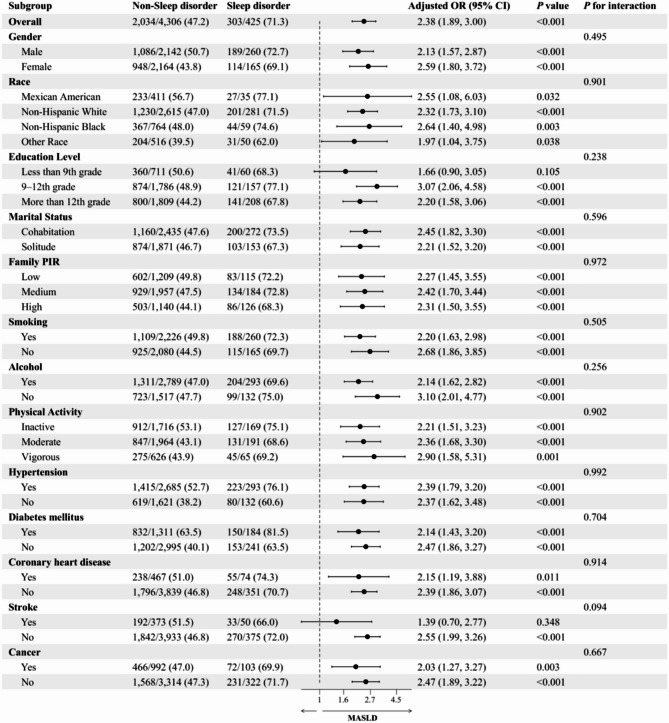
Subgroup analysis of the relationship between sleep disorders and MASLD
This study conducted a subgroup analysis to examine the association between sleep disorders and MASLD. Potential confounders, including gender, age, race, education, marital status, family PIR, smoking, alcohol consumption, physical activity, DM, hypertension, coronary heart disease, stroke, and cancer, were considered in the analysis. The results of this analysis are presented in Fig. 3. The analysis revealed that individuals with sleep disorders exhibited 2.38 times (95% CI: 1.89-3.00) elevated prevalence of MASLD compared to those without sleep disorders, with a P value of less than 0.001. Across all subgroups, the association between sleep disorders and MASLD remained statistically significant, except for the subgroups comprising individuals with low education and those who had suffered a stroke (P < 0.05). Notably, no significant interactions were observed among all subgroups (P-value > 0.05 for interaction). These findings further substantiate the robust association between sleep disorders and MASLD, thereby underscoring the persistency of this relationship across diverse population characteristics.
Fig. 3.
Subgroup analysis of the relationship between sleep disorder and MASLD. Adjusted variables: gender, age, race, education level, marital status, family PIR, smoking, alcohol, physical activity, diabetes mellitus, hypertension, coronary heart disease, stroke, and cancer. The model was not adjusted for the stratification variables themselves in the corresponding stratification analysis. MASLD, Metabolic dysfunction-associated steatotic liver disease; OR: odds ratio; CI: confidence interval
Sensitivity analysis
The multiple imputation model demonstrated a strong correlation between sleep disorder and MASLD (OR = 2.35, 95% CI: 1.86–2.98), which is consistent with the findings of the primary analysis (OR = 2.38). This suggests that including missing data did not substantially influence the conclusions drawn. Furthermore, the Bootstrap resampling method confirmed the turning point estimate’s reliability, which involved 1,000 replications. This analysis yielded a 95% confidence interval of 6.4–7.6 h, aligning with the initial analysis. The turning point of sleep duration and MASLD in the 4-node RCS model remained 7.0 (95% CI: 6.8–7.2), consistent with the main analysis of 7.0 (95% CI: 6.4–7.6), thereby confirming the stability of the nonlinear trend. In summary, the primary conclusion of this study (the association between sleep disturbances and MASLD) remained consistent when different data processing strategies and model parameters were employed. This finding serves to bolster the credibility of the study’s results.
Discussion
The present study’s findings demonstrated that both abnormal sleep duration (i.e., insufficient or excessive sleep) and sleep disorders are significantly associated with a higher prevalence of MASLD in older adults. This finding aligns with existing literature on the relationship between sleep and liver health, further substantiating the pivotal role of sleep in maintaining liver health. Specifically, the prevalence of MASLD was significantly elevated in older individuals who slept less than 6 h or more than 8 h per night compared to those who slept 6–8 h per night. This finding indicates that maintaining optimal sleep duration is crucial for preserving liver health. The potential mechanisms by which sleep influences liver health may involve effects on insulin resistance, hormone secretion (e.g., cortisol and leptin), and other pathways. Research has demonstrated that sleep deprivation leads to increased sympathetic nervous system activity, which affects insulin signaling and improves insulin resistance, thereby promoting fat accumulation in the liver [20, 21]. Furthermore, the impact of sleep duration on appetite regulation, as evidenced by changes in leptin levels, suggests a potential mechanism through which sleep deprivation may exacerbate metabolic disorders [25–27]. Conversely, individuals with prolonged sleep duration and an increased prevalence of MASLD may be associated with chronic fatigue, reduced physical activity, and other factors. The prolonged sleep duration may indicate an individual’s state of chronic fatigue, leading to a subsequent reduction in physical activity, which in turn exacerbates metabolic disorders [37]. Also, prolonged sleep duration has been linked to poor sleep quality and various metabolic abnormalities [38].
The present study is the first to validate the nonlinear relationship between sleep duration and MASLD in an elderly population. This nonlinear relationship is consistent with previous studies on the general adult population, demonstrating a U-shaped association [39, 40]. These findings support the hypothesis that a sleep duration of 7–9 h per day is associated with a lower prevalence of MASLD. Furthermore, the strength of the association for sleep disorders (OR = 2.38) was significantly higher than in general population studies (OR = 1.40–1.78) [41, 42]. This finding supports the hypothesis that the metabolic system is more sensitive to sleep disorders in the elderly population.
The underlying mechanisms by which sleep disorders associated with MASLD in older adults may involve several potential pathways. Firstly, sleep disorders may induce a persistent state of stress within the body, which subsequently prompts inflammatory responses and oxidative stress [22–24, 43]. Research has demonstrated that sleep disorders are associated with elevated levels of inflammatory factors, such as C-reactive protein, interleukin-6, and tumor necrosis factor-α [22, 43]. These inflammatory factors can directly damage liver cells, triggering liver inflammation and promoting the development of liver fibrosis [44]. Furthermore, sleep disorders have been shown to lead to dysbiosis of the intestinal flora and increased intestinal permeability, which facilitates the entry of endotoxins (e.g., lipopolysaccharides) into the bloodstream, thereby triggering a systemic inflammatory response [24]. Upon entering the liver, endotoxins activate hepatic stellate and Kupffer cells, resulting in liver inflammation and fibrosis [45]. Furthermore, the presence of sleep disorders has resulted in an escalation in the production of free radicals, including an augmentation in the generation of reactive oxygen species (ROS) [23]. These free radicals, being highly reactive, can attack and damage biomolecules, such as lipid membranes, proteins, and DNA in hepatocytes, resulting in oxidative damage and dysfunction [46]. Free radicals also promote fat oxidation, forming oxidized fat, further exacerbating the development of MASLD [47].
Secondly, sleep disorders can potentially disrupt the neuroendocrine system’s regulation, particularly impacting insulin sensitivity and lipid metabolism [48–51]. Sleep disorders have increased sympathetic nervous system activity and activation of the hypothalamic-pituitary-adrenal axis [48]. This, in turn, has been demonstrated to affect insulin signaling. Specifically, sleep deprivation or subpar sleep quality has been shown to decrease insulin receptor sensitivity, rendering insulin ineffective in facilitating glucose entry into the cell and consequently inducing insulin resistance [49]. Insulin resistance is a significant pathophysiological basis of MASLD, leading to a reduction in glucose uptake by the liver while concurrently promoting fat synthesis and accumulation in the liver [52]. Furthermore, sleep disorders have been demonstrated to influence fat synthesis and catabolism [50, 51]. Specifically, sleep deprivation has been shown to enhance the liver’s capacity for fat synthesis, thereby promoting fat accumulation in the liver. Concurrently, sleep disorders impede lipolysis in adipose tissue, facilitating liver fat storage [53]. Furthermore, sleep deprivation has been shown to diminish fatty acid oxidation, thereby perpetuating fat accumulation in the liver [54].
Furthermore, sleep disorders have the potential to influence the lifestyle habits and dietary choices of older adults. For instance, sleep disturbances may predispose older adults to increased consumption of high-calorie, high-fat foods while reducing physical activity [55, 56], which places additional strain on the liver. Furthermore, the presence of sleep disorders has been associated with the development of mood disorders, including depression and anxiety [57, 58]. Research has demonstrated a strong correlation between mood disorders and the emergence and progression of MASLD [59, 60]. Mood disorders, including depression and anxiety, have been found to disrupt the endocrine system and induce metabolic disorders, thereby amplifying the risk of developing MASLD [59, 60]. In conclusion, sleep disorders are closely associated with the development of MASLD in older adults through multiple mechanisms, including inflammatory responses, oxidative stress, endocrine dysregulation, metabolic disorders, lifestyle habits, and psychological factors. These mechanisms interact, collectively contributing to the occurrence and development of MASLD.
Utilizing RCS analysis, we subsequently identified a nonlinear relationship between sleep duration and the prevalence of MASLD in older adults, determining that 7 h was the critical turning point. Before this threshold, a positive correlation was observed between sleep duration and the prevalence of MASLD, with each additional hour of sleep significantly reducing risk. However, after reaching 7 h, an increase in sleep duration was associated with an elevated prevalence of MASLD. This finding underscores the significance of optimal sleep duration for maintaining liver health in older adults. The results of the subgroup analyses further support the strong association between sleep disorders and MASLD. However, this relationship did not reach statistical significance in the subgroups of low education and stroke patients.
These results demonstrates a positive correlation between sleep disorders and the prevalence of MASLD in older adults. Moreover, they imply that the impact of sleep disorders on diverse population characteristics should be duly considered in formulating preventive and therapeutic strategies to more effectively mitigate the risk of developing MASLD in this demographic. At the individual level, it is recommended that clinicians incorporate sleep quality assessment into the standard care of elderly patients, particularly those at high risk of metabolic syndrome, including individuals with obesity and diabetes. Sleep interventions can serve as a complementary approach to prevent MASLD. At the public health level, it is necessary to promote health education on sleep in the elderly population, advocate for approximately seven hours of sleep per day, and enhance screening for sleep disorders using simple questionnaires such as the STOP-Bang. Future studies should prioritize validating sleep interventions, such as cognitive-behavioral therapy and continuous positive airway pressure ventilation for sleep apnea, on the incidence of MASLD and the reversal of early hepatic steatosis. Furthermore, for patients with limited education or a history of stroke (wherein the association did not reach statistical significance in subgroup analyses), exploring personalized sleep management programs in conjunction with community support and multidisciplinary collaboration (e.g., social workers, dietitians) is imperative. This approach aims to address the potential impact of population heterogeneity on the effectiveness of interventions.
Notwithstanding the significant strides made in elucidating the impact of sleep duration and sleep disorders on MASLD in older adults, this study has its limitations. First, it is imperative to acknowledge the limitations of this study’s cross-sectional design, which precludes the ability to make causal inferences. The observed association between sleep disturbances and MASLD may be influenced by unmeasured confounders, thereby compromising the study’s internal validity. Consequently, future studies must employ a longitudinal design to substantiate these findings and elucidate the causal relationship between sleep duration and sleep disorders in the context of the risk of developing MASLD. Secondly, this study’s assessment of sleep disorders relied primarily on self-report, which is subject to recall bias. To enhance the accuracy of the evaluation and mitigate the impact of recall bias on study outcomes, future research should employ more objective sleep monitoring methods, such as polysomnography. Furthermore, the present study addressed only the effects of sleep duration and sleep disorders on the risk of developing MASLD, neglecting other potential sleep-related factors (e.g., sleep apnea, circadian rhythm disorders, etc.). Subsequent studies may benefit from exploring the role of these factors in the development of MASLD in older adults to achieve a more comprehensive understanding of the relationship between sleep and liver health. Consequently, subsequent intervention studies should be conducted to investigate the effects of improving sleep duration and sleep quality on the prevention and treatment of MASLD, thereby providing a scientific basis for developing targeted interventions.
Conclusion
In summary, the present study reveals the critical effects of sleep duration and sleep disorders on MASLD in older adults, providing new perspectives and strategies for preventing and treating MASLD in this population. Future studies should further explore these effects’ causality and potential mechanisms and consider the role of other possible sleep-related factors in developing MASLD in older adults. The development of rational sleep health promotion strategies for older adults is a crucial direction for future research. The results of this study underscore the significance of sleep in preserving liver health in older adults, underscoring the necessity of prioritizing not only the sleep health of older adults but also their liver health.
Acknowledgements
We thank the NHANES participants and staff for their contributions.
Author contributions
Conceptualization and methodology, F.Z. and W.L.; project administration, data curation, and investigation, F.Z. and Y.X.; formal analysis, F.Z. and W.L.; visualization and supervision, W.L.; Writing - original draft, F.Z.; Writing - review and editing, W.L.; funding acquisition, F.Z. and Y.X.; All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Science and Technology Project of Changzhou (CJ20243023), the Key Discipline Project of Changzhou Health Commission (CZXK202218), and the Key Talents Project of Changzhou Third People’s Hospital.
Data availability
The National Health and Nutrition Examination Survey dataset is publicly available at the National Center for Health Statistics of the Centers for Disease Control and Prevention (https://www.cdc.gov/nchs/nhanes/).
Declarations
Ethics approval and consent to participate
The studies involving humans were approved by the National Center for Health Statistics Ethics Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. As NHANES is a publicly accessible database, the Changzhou Third People’s Hospital Ethics Committee granted approval to waive ethical review and approved the study protocol (02A-A2024018).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.DiLeo DA, Gidener T, Aytaman A. Chronic liver disease in the older Patient-Evaluation and management. Curr Gastroenterol Rep. 2023;25(12):390–400. [DOI] [PubMed] [Google Scholar]
- 2.Qiu S, Cai J, Yang Z, He X, Xing Z, Zu J, et al. Trends in hepatocellular carcinoma mortality rates in the US and projections through 2040. JAMA Netw Open. 2024;7(11):e2445525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Owrangi S, Paik JM, Golabi P, de Avila L, Hashida R, Nader A et al. Meta-Analysis: global prevalence and mortality of cirrhosis in metabolic Dysfunction-Associated steatotic liver disease. Aliment Pharmacol Ther. 2025;61(3):433–43. [DOI] [PubMed]
- 4.Hwang SY, Danpanichkul P, Agopian V, Mehta N, Parikh ND, Abou-Alfa GK et al. Hepatocellular carcinoma: updates on epidemiology, surveillance, diagnosis and treatment. Clin Mol Hepatol. 2025;31(Suppl):S228–S254. [DOI] [PMC free article] [PubMed]
- 5.Stefan N, Yki-Järvinen H, Neuschwander-Tetri BA. Metabolic dysfunction-associated steatotic liver disease: heterogeneous pathomechanisms and effectiveness of metabolism-based treatment. Lancet Diabetes Endocrinol. 2025;13(2):134–48. [DOI] [PubMed]
- 6.Lee JH, Cho J. Sleep and obesity. Sleep Med Clin. 2022;17(1):111–6. [DOI] [PubMed] [Google Scholar]
- 7.Chasens ER, Imes CC, Kariuki JK, Luyster FS, Morris JL, DiNardo MM, et al. Sleep and metabolic syndrome. Nurs Clin North Am. 2021;56(2):203–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jaspan VN, Greenberg GS, Parihar S, Park CM, Somers VK, Shapiro MD, et al. The role of sleep in cardiovascular disease. Curr Atheroscler Rep. 2024;26(7):249–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iranzo A. An overview on sleep medicine. Adv Exp Med Biol. 2022;1384:3–15. [DOI] [PubMed] [Google Scholar]
- 10.Baranwal N, Yu PK, Siegel NS. Sleep physiology, pathophysiology, and sleep hygiene. Prog Cardiovasc Dis. 2023;77:59–69. [DOI] [PubMed] [Google Scholar]
- 11.Cohen ZL, Eigenberger PM, Sharkey KM, Conroy ML, Wilkins KM. Insomnia and other sleep disorders in older adults. Psychiatr Clin North Am. 2022;45(4):717–34. [DOI] [PubMed] [Google Scholar]
- 12.Jaqua EE, Hanna M, Labib W, Moore C, Matossian V. Common sleep disorders affecting older adults. Perm J. 2023;27(1):122–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brewster GS, Riegel B, Gehrman PR. Insomnia in the older adult. Sleep Med Clin. 2022;17(2):233–9. [DOI] [PubMed] [Google Scholar]
- 14.Garbarino S, Lanteri P, Bragazzi NL, Magnavita N, Scoditti E. Role of sleep deprivation in immune-related disease risk and outcomes. Commun Biol. 2021;4(1):1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pappas JA, Miner B. Sleep deficiency in the elderly. Sleep Med Clin. 2024;19(4):593–606. [DOI] [PubMed] [Google Scholar]
- 16.Gottesman RF, Lutsey PL, Benveniste H, Brown DL, Full KM, Lee JM, et al. Impact of sleep disorders and disturbed sleep on brain health: A scientific statement from the American heart association. Stroke. 2024;55(3):e61–76. [DOI] [PubMed] [Google Scholar]
- 17.Yeghiazarians Y, Jneid H, Tietjens JR, Redline S, Brown DL, El-Sherif N, et al. Obstructive sleep apnea and cardiovascular disease: A scientific statement from the American heart association. Circulation. 2021;144(3):e56–67. [DOI] [PubMed] [Google Scholar]
- 18.Schipper SBJ, Van Veen MM, Elders PJM, van Straten A, Van Der Werf YD, Knutson KL, et al. Sleep disorders in people with type 2 diabetes and associated health outcomes: a review of the literature. Diabetologia. 2021;64(11):2367–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marjot T, Ray DW, Williams FR, Tomlinson JW, Armstrong MJ. Sleep and liver disease: a bidirectional relationship. Lancet Gastroenterol Hepatol. 2021;6(10):850–63. [DOI] [PubMed] [Google Scholar]
- 20.Antza C, Kostopoulos G, Mostafa S, Nirantharakumar K, Tahrani A. The links between sleep duration, obesity and type 2 diabetes mellitus. J Endocrinol. 2021;252(2):125–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.St-Onge MP, Cherta-Murillo A, Darimont C, Mantantzis K, Martin FP, Owen L. The interrelationship between sleep, diet, and glucose metabolism. Sleep Med Rev. 2023;69:101788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhai S, Tao S, Wu X, Zou L, Yang Y, Xie Y, et al. Associations of sleep insufficiency and chronotype with inflammatory cytokines in college students. Nat Sci Sleep. 2021;13:1675–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davinelli S, Medoro A, Savino R, Scapagnini G. Sleep and oxidative stress: current perspectives on the role of NRF2. Cell Mol Neurobiol. 2024;44(1):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Z, Wang Z, Lu T, Chen W, Yan W, Yuan K, et al. The microbiota-gut-brain axis in sleep disorders. Sleep Med Rev. 2022;65:101691. [DOI] [PubMed] [Google Scholar]
- 25.Lin J, Jiang Y, Wang G, Meng M, Zhu Q, Mei H, et al. Associations of short sleep duration with appetite-regulating hormones and adipokines: A systematic review and meta-analysis. Obes Rev. 2020;21(11):e13051. [DOI] [PubMed] [Google Scholar]
- 26.Chaput JP, McHill AW, Cox RC, Broussard JL, Dutil C, da Costa BGG, et al. The role of insufficient sleep and circadian misalignment in obesity. Nat Rev Endocrinol. 2023;19(2):82–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kurnool S, McCowen KC, Bernstein NA, Malhotra A. Sleep apnea, obesity, and Diabetes - an intertwined trio. Curr Diab Rep. 2023;23(7):165–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bu LF, Xiong CY, Zhong JY, Xiong Y, Li DM, Hong FF, et al. Non-alcoholic fatty liver disease and sleep disorders. World J Hepatol. 2024;16(3):304–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang J, Zhang Q, Zhao W, Ye B, Li S, Zhang Z, et al. Associations of traditional healthy lifestyle and sleep quality with metabolic dysfunction-associated fatty liver disease: two population-based studies. Nutr Diabetes. 2024;14(1):79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, et al. The fatty liver index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park J, Kim G, Kim BS, Han KD, Kwon SY, Park SH, et al. The associations of hepatic steatosis and fibrosis using fatty liver index and BARD score with cardiovascular outcomes and mortality in patients with new-onset type 2 diabetes: a nationwide cohort study. Cardiovasc Diabetol. 2022;21(1):53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J Hepatol. 2023;79(6):1542–56. [DOI] [PubMed] [Google Scholar]
- 33.Center for Disease Control and Prevention Sample Person Questionnaire: Sleep Disorders. Accessed 18. May 2025; 2005. https://wwwn.cdc.gov/nchs/data/nhanes/public/2005/questionnaires/sp_slq_d.pdf
- 34.Zhang F, Li W. Correlation between metabolic Dysfunction-Associated steatotic liver disease and the risk of urinary incontinence in adult women. Int J Womens Health. 2024;16:1607–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li J, Wu Q, Fan L, Yan Z, Shen D, Zhang M. Nonlinear associations between sleep duration and the risks of all-cause and cardiovascular mortality among the general adult population: a long-term cohort study. Front Cardiovasc Med. 2023;10:1109225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu Q, Lin Z, Chen Y, Huang M. Association between sleep duration and patterns and obesity: a cross-sectional study of the 2007–2018 National health and nutrition examination survey. BMC Public Health. 2025;25(1):1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakagawa T, Yasumoto S, Kabayama M, Matsuda K, Gondo Y, Kamide K, et al. Association between prior-night sleep and next-day fatigue in older adults: a daily diary study. BMC Geriatr. 2023;23(1):817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Addo PNO, Mundagowa PT, Zhao L, Kanyangarara M, Brown MJ, Liu J. Associations between sleep duration, sleep disturbance and cardiovascular disease biomarkers among adults in the united States. BMC Public Health. 2024;24(1):947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Q, Chen H, Deng H, Zhang M, Hu H, Ouyang H, et al. Association of daily sleep duration with risk of metabolic dysfunction-associated steatotic liver disease and adverse liver outcomes. Diabetes Metab. 2025;51(3):101628. [DOI] [PubMed] [Google Scholar]
- 40.Wang D, Zhang X, Cai Y, Dong H, Zhang Y. Multidimensional sleep impairment predicts steatotic liver disease spectrum risk. Sci Rep. 2025;15(1):10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mir HM, Stepanova M, Afendy H, Cable R, Younossi ZM. Association of sleep disorders with nonalcoholic fatty liver disease (NAFLD): A Population-based study. J Clin Exp Hepatol. 2013;3(3):181–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wei YT, Lee PY, Lin CY, Chen HJ, Lin CC, Wu JS, et al. Non-alcoholic fatty liver disease among patients with sleep disorders: a nationwide study of Taiwan. BMC Gastroenterol. 2020;20(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Veler H. Sleep and inflammation: bidirectional relationship. Sleep Med Clin. 2023;18(2):213–8. [DOI] [PubMed] [Google Scholar]
- 44.Xu HL, Wan SR, An Y, Wu Q, Xing YH, Deng CH, et al. Targeting cell death in NAFLD: mechanisms and targeted therapies. Cell Death Discov. 2024;10(1):399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gandhi CR. Pro- and Anti-fibrogenic functions of Gram-Negative bacterial lipopolysaccharide in the liver. Front Med (Lausanne). 2020;7:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Almalki WH, Almujri SS. Aging, ROS, and cellular senescence: a trilogy in the progression of liver fibrosis. Biogerontology. 2024;26(1):10. [DOI] [PubMed] [Google Scholar]
- 47.Raut SK, Khullar M. Oxidative stress in metabolic diseases: current scenario and therapeutic relevance. Mol Cell Biochem. 2023;478(1):185–96. [DOI] [PubMed] [Google Scholar]
- 48.Azuara-Alvarez LE, Díaz-Muñoz M, Báez Ruiz A, Saderi N, Ramírez-Plascencia OD, Cárdenas-Romero S, et al. Visceral fat sympathectomy ameliorates systemic and local stress response related to chronic sleep restriction. Exp Biol Med (Maywood). 2023;248(23):2381–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sondrup N, Termannsen AD, Eriksen JN, Hjorth MF, Færch K, Klingenberg L, et al. Effects of sleep manipulation on markers of insulin sensitivity: A systematic review and meta-analysis of randomized controlled trials. Sleep Med Rev. 2022;62:101594. [DOI] [PubMed] [Google Scholar]
- 50.Wang F, Zou J, Xu H, Huang W, Zhang X, Wei Z, et al. Effects of chronic intermittent hypoxia and chronic sleep fragmentation on gut microbiome, serum metabolome, liver and adipose tissue morphology. Front Endocrinol (Lausanne). 2022;13:820939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou C, Hu Z, Liu X, Wang Y, Wei S, Liu Z. Disruption of the peripheral biological clock May play a role in sleep deprivation-induced dysregulation of lipid metabolism in both the daytime and nighttime phases. Biochim Biophys Acta Mol Cell Biol Lipids. 2024;1869(7):159530. [DOI] [PubMed] [Google Scholar]
- 52.Ziamanesh F, Mohammadi M, Ebrahimpour S, Tabatabaei-Malazy O, Mosallanejad A, Larijani B. Unraveling the link between insulin resistance and Non-alcoholic fatty liver disease (or metabolic dysfunction-associated steatotic liver disease): A narrative review. J Diabetes Metab Disord. 2023;22(2):1083–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stefanovski D, Boston RC, Punjabi NM. Sleep-Disordered breathing and free fatty acid metabolism. Chest. 2020;158(5):2155–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gao T, Feng M, Wang Z, Cao J, Chen Y. Microbiota-gut-adipose axis: butyrate-mediated the improvement effect on inflammatory response and fatty acid oxidation dysregulation attenuates obesity in sleep-restricted mice. Microbes Infect. 2023;25(6):105125. [DOI] [PubMed] [Google Scholar]
- 55.Zuraikat FM, Wood RA, Barragán R, St-Onge MP. Sleep and diet: mounting evidence of a cyclical relationship. Annu Rev Nutr. 2021;41:309–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McCoy T, Sochan AJ, Spaeth AM. The relationship between sleep and physical activity by age, race, and gender. Rev Cardiovasc Med. 2024;25(10):378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Palagini L, Hertenstein E, Riemann D, Nissen C. Sleep, insomnia and mental health. J Sleep Res. 2022;31(4):e13628. [DOI] [PubMed] [Google Scholar]
- 58.Nielson SA, Kay DB, Dzierzewski JM. Sleep and depression in older adults: A narrative review. Curr Psychiatry Rep. 2023;25(11):643–58. [DOI] [PubMed] [Google Scholar]
- 59.Shea S, Lionis C, Kite C, Atkinson L, Chaggar SS, Randeva HS et al. Non-Alcoholic fatty liver disease (NAFLD) and potential links to depression, anxiety, and chronic stress. Biomedicines. 2021;9(11):1697. [DOI] [PMC free article] [PubMed]
- 60.Gu Y, Zhang W, Hu Y, Chen Y, Shi J. Association between nonalcoholic fatty liver disease and depression: A systematic review and meta-analysis of observational studies. J Affect Disord. 2022;301:8–13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The National Health and Nutrition Examination Survey dataset is publicly available at the National Center for Health Statistics of the Centers for Disease Control and Prevention (https://www.cdc.gov/nchs/nhanes/).