Abstract
Background
The Russian invasion of Ukraine in 2022 has placed extraordinary pressure on hospitals there. One consequence of this has been the alarming increase in infections caused by multi-drug resistant organisms (MDROs), both within Ukraine and among the Ukrainian diaspora. The original source of these MDROs remains obscure although nosocomial origin is suspected. Here, we analyzed a collection of Acinetobacter baumannii and Pseudomonas aeruginosa collected from Ukraine before and after the invasion to glean a greater understanding of their relationship and origins.
Methods
Genomic analysis was conducted on 167 A. baumannii and 93 P. aeruginosa cultured from 223 Ukrainian patients hospitalized in Ukraine or other European countries. Fifty-three isolates were cultured between 2014 and 2021, prior to the invasion, and the remaining 207 after.
Results
Highly genetically related extensively-drug resistant (XDR) clones were identified that spanned the pre- and post-invasion periods. For A. baumannii, isolates encompassed three sequence types (STs), including carbapenemase-producing strains from ST-2 (blaOXA-23) and ST-78 (blaOXA-72), as well as ST-400 carrying the ESBL blaGES-11. For P. aeruginosa, isolates encompassed three STs: ST-773 carrying blaNDM-1, ST-1047 carrying blaIMP-1, and ST-244. For all, the mobile genetic elements associated with carbapenemase carriage were fully characterized. Notably, post-invasion ST-773 and ST-1047 P. aeruginosa had a signature of host adaptation with multiple loss-of-function mutations in the quorum-sensing regulator LasR, known to modulate immune responses and provide survival advantages in animal models of infection.
Conclusions
XDR epidemic clones circulating in Ukraine and across Europe since 2022 share a close genetic relationship to historical strains from Ukraine. In some cases, direct links to medical facilities within Ukraine can be inferred. These data suggest that surveillance efforts should focus on tracking nosocomial transmission within Ukrainian hospitals while infection control efforts are being disrupted by the ongoing Russian invasion.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13073-025-01500-1.
Keywords: A. baumannii, P. aeruginosa, Surveillance, Carbapenem resistance, Nosocomial outbreak, Microbial genomics, Antimicrobial resistance, Molecular epidemiology
Background
Human conflict plays an important role in the development and spread of antimicrobial resistance (AMR) [1], and the current war in Ukraine is no exception [2]. The Russian invasion has placed extraordinary pressure on Ukrainian healthcare services, with medical facilities struggling with large numbers of casualties while dealing with constant bombing, constrained supply chains, and greatly reduced ability to provide basic services [3]. One area where these challenges have been acutely felt is infection control, where large increases in multi-drug resistant (MDR) and extensively-drug resistant (XDR) bacteria have been reported among wounded service members and civilians receiving care in Ukrainian hospitals [4–6].
Prior to the invasion in February 2022, Ukraine was engaged in a conflict with separatists in the Donbas and Luhansk regions of Eastern Ukraine since 2014. Bacterial infections among injured service members during that Eastern conflict were characterized by initial contamination with Gram-positive microbes of low pathogenicity that gradually transitioned to more pathogenic Gram-negative rods during the wound healing process [7]. Notably, non-fermentative Gram-negative bacilli accounted for 68% of all positive wound swab cultures after the first week of healing, with Acinetobacter baumannii and Pseudomonas aeruginosa being the most prevalent [7].
A genetic analysis of these earlier (2014–2020) isolates from Ukraine revealed A. baumannii belonging to sequence type (ST)−1PAS and ST-78PAS and P. aeruginosa belonging to ST-235 and ST-773 carrying an array of AMR determinants, including the class D carbapenemases OXA-23 and OXA-72 in A. baumannii and the class B carbapenemase VIM-2 in P. aeruginosa [8]. These strains also carried 16S methyltransferases genes (16S RMTases; armA and rmtB4, respectively), and ST-78PAS A. baumannii also carried the extended spectrum β-lactamase (ESBL) genes blaCTX-M-115. Notably, since 2022, MDR and XDR strains from these lineages have been cultured from Ukrainian refugees and military personnel receiving medical care in other European countries [9–13], suggestive of a common reservoir before evacuation.
The origins of these MDR and XDR strains remains obscure and while historical isolates have been described at the lineage level [8], no comprehensive genomic comparison has been made between pre-invasion and post-invasion isolates. Here, we conduct such analysis on A. baumannii and P. aeruginosa and show that ongoing outbreaks are largely due to MDR and XDR clones already circulating in Ukraine before the invasion, and whose spread is now likely exacerbated because of the war.
Methods
Strain collection
Two hundred and sixty genomes, representing 260 isolates cultured from 223 patients, were available for analysis. Fifty-three were collected between 2014 and 2021 (pre-invasion) and 207 between March 2022 and September 2023 (post-invasion) (Table S1, [14]). Of these 260 isolates, 173 isolates from 136 patients were physically available for additional testing. These 173 isolates consisted of 161 cultured at eight Ukrainian medical facilities and 12 cultured at two German medical facilities (Table S1, [14]). All 161 Ukrainian isolates (45 pre-invasion and 116 post-invasion) were cultured from injured Ukrainian service members with clinical evidence of infection (edema, erythema, lymphangitis, wound discharge/purulence). The majority (63%) were recovered from wound cultures, followed by surveillance swabs (14.6%, rectal and groin), respiratory (8.5%), burn (4.2%), blood (3.8%), or various other (5.8%) cultures. Isolates were cultured from patients using two nutrient media: tryptone soy agar and chromogenic agar for Acinetobacter (Graso Biotech, Poland). For post-invasion isolates, the soldiers had been injured 6.2 ± 3.9 days prior to culture and had been transferred through 4–5 evacuation hospitals ranging from NATO level II (qualified medical care) to III (specialized medical care) [15] before arriving at the final facility. All 12 German isolates (1 pre-invasion and 11 post-invasion) were cultured from soldiers injured in Ukraine and receiving care at two military hospitals in Germany. Notably, the single pre-invasion German isolate (MRSN 483036) was cultured in 2017 from a peri-rectal surveillance swab of a US Army servicemember transferred to Landstuhl Regional Medical Center (LRMC) in Germany after prior hospitalization in Ukraine following a motor vehicle collision there.
These 173 isolates were supplemented with 87 genomes of isolates collected as part of surveillance programs in Denmark (n = 3), Germany (n = 75), and The Netherlands (n = 9). Sixty-six of the genomes from Germany were provided by the National Reference Centre for Multidrug-Resistant Gram-Negative Bacteria at Ruhr-University Bochum, Germany and were collected as part of a nationwide surveillance program. The remaining nine genomes were obtained from the National Center for Biotechnology Information (NCBI) and represent five and four isolates cultured from Ukrainian patients receiving care at German facility #3 and Germany facility #5, respectively (no associated publications). Similarly, the Danish and Dutch genomes were obtained from NCBI and represent bacteria collected from Ukrainian patients receiving care at a medical facility in The Netherlands and Denmark (no associated publication).
Antibiotic susceptibility testing (AST)
AST was performed on all 173 isolates using a Vitek 2 (Biomerieux) with cards N808 and XN-32, or broth microdilution, as previously described [16]. Strains were defined as MDR if they were resistant to ≥ 1 agent in three categories and XDR if they were non-susceptible to ≥ 1 agent in all but ≤ 2 categories of the tested antimicrobials.
DNA extraction, whole genome sequencing (WGS), and de novo assemblies
-
Short-read sequencing:
DNA was extracted using the DNeasy UltraClean 96 Microbial Kit (Qiagen, Germantown, MD, USA) and libraries were constructed using the KAPA Hyperplus Library preparation kit (Roche Diagnostics, Indianapolis, IN, USA). Libraries were quantified using the KAPA Library Quantification Kit—Illumina/Bio-Rad iCycler™ (Roche Diagnostics) on a CFX96 real-time cycler (Biorad, Hercules, CA, USA). Libraries were normalized to 2 nM, pooled, denatured, and diluted to 1 nM. Whole genome sequencing was performed using a MiSeq, NextSeq 500, or NextSeq 2000 Benchtop Sequencer (Illumina Inc., CA, USA) with MiSeq Reagent Kit v3 (600 cycles; 2 × 300 bp), NextSeq Reagent Kit 500/550 v2 (300 cycles; 2 × 150 bp), or NextSeq 1000/2000 P2 Reagents (300 cycles) v3 kit (Illumina, San Diego, CA, USA). bbduk v38.96 [17] was used to remove barcode and adapter sequence as well as to perform quality trimming (ktrim = “r”, k = “23”, mink = “11”, hdist = “1”, qtrim = “r”, trimq = “15”, minlen = “100”). Kraken2 v2.1.2 [18] was used for initial taxonomic assignment (top hit = “1”, undetermined reads = “ < 10%”) and to screen for contamination (2 + genus level hits > 5% = “isContaminated”). De novo draft genome assemblies were produced using shovill v1.1.0 [19] with coverage estimates generated using bbmap v38.96 [20]. Minimum thresholds for contig size and coverage were set at 200 bp and 49.5 + , respectively. Quality controls for the assembly were standardized with a decision tree including the following parameters: total length of contigs > 1 Mb; total length of contigs < 1 Mb over expected genome size for taxon; average read depth for each contig ≥ 20; total length of contigs filtered for low coverage < 100 kb; total length of contigs filtered for length < 100 kb; and number of contigs filtered for length + numbers of contigs filtered for low coverage < 1000. In cases where the Kraken2-derived taxonomic assignment was ambiguous, GTDB [21] was used via the GTDB-Tk v2.4.0 [22] and a > 95% average nucleotide identity threshold for species level identification.
-
Long-read sequencing:
Long-read sequencing was performed on a Minion platform using a MinION Mk1B device (Oxford Nanopore Technologies, Oxford, England). DNA was extracted using the DNeasy UltraClean Microbial Kit (Qiagen, Germantown, MD, USA). Library preparation on genomic DNA was performed using SQK-RBK114-96 rapid barcoding kit and sequenced on a 10.4.1 flowcell. The resulting POD5 output were basecalled with Dorado V9.0.0 [23] using the super-accurate model dna_r10.4.1_e8.2_400bps_sup@v5.0.0. After demultiplexing fastq into samples, a hybrid long-read/short-read assembly approach was used to assemble genomes. First, de novo assembly from long reads only was performed using Autocycler v0.1.2 [24]. Briefly, Autocycler outputs a consensus assembly from multiple long-read dedicated assemblers. The dedicated assemblers used were Flye, Raven, Miniasm, Metamdbg, Necat, NextDenovo, and Redbean. Next, the consensus assembly was polished with Illumina short reads using Polypolish [25].
Molecular typing and annotations
Genomes were annotated using Bakta v.1.10.4 [26]. Antimicrobial resistance genes were annotated using a combination of ARIBA v2.14.6 [27] and AMRFinderPlus v3.12.8 [28] and the following parameters: ident_min < 0.9 > and coverage_min < 0.5 > for AMRFinderPlus; nucmer_min_id < 90 > nucmer_min_len < 20 > and nucmer_breaklen < 200 > for ARIBA. Finally, a 10 × minimum coverage was applied for ARIBA-only hits and deduplication was performed with priority given to the assembly-based hit. MLST assignment was performed using mlst v2.22.1 [29]. Mobile genetic elements were identified and annotated with tools and databases such as mobileOG-db (beatrix-1.6) [30], ISFinder [31], and TnCentral [32]. Plasmid replication initiation genes (Rep types) were determined using the Acinetobacter Plasmid Typing scheme [33] and plasmidFInder [34]. Comparisons of mobile genetic elements (MGE) were generated using the BLAST comparison tool version 1.4.1 integrated in Proksee.ca [35]. Parameters included an expect value cutoff of 0.0001, filtering of low complexity regions, and filtering of regions with < 95% nucleotide identity. When necessary, manual annotation and superficial edits (e.g., adding a sequence break on Fig. 6A) were made for the final illustration in CorelDRAW.
Fig. 6.
Genetic context of major resistance genes in P. aeruginosa epidemic clones. A Chromosomal co-localization of rmtB4 and blaNDM-1 in ST-773 isolates. Reference is a 117 kb chromosomal fragment from a complete assembly of MRSN740681. B Chromosomal blaIMP-1 in ST-1047 isolates. Reference is a 22.6 kb chromosomal fragment from a complete assembly of MRSN567204
Core genome MLST, SNP calling, and phylogenetic analysis
Detection of clusters of high genetic relatedness was performed in two stages. First, core genome MLST was performed in SeqSphere + Software v 7.7.2 (Ridom, Germany) using the cgMLST schemes developed for P. aeruginosa [36] and A. baumannii [37] with a cutoff of 90%. Isolates related by ≤ 20 alleles were considered part of a cluster of interest and additional whole genome single nucleotide polymorphism (SNP) analysis was performed. While more stringent thresholds (e.g., ≤ 5 allelic differences) have been applied to identify transmission clusters within a single hospital or healthcare network [38], we chose this conservative threshold in agreement with other studies of prolonged outbreaks [39, 40]. This was done to better suit our dataset which spanned 4 countries for up to 8 years and was not the result of a systematic and thorough sampling/surveillance strategy. As a result, we did not aim to reconstruct local patient-to-patient transmission but rather to track the spread of epidemic clones with origins from Ukraine. The cgMLST minimum-spanning tree was generated using the species-specific cgMLST schemes and allelic distance matrices. When necessary, superficial edits (e.g., changing node shape from circle to square) were made in CorelDRAW for the final illustration.
Second, to further investigate these putative clusters, an internal reference genome (first isolate temporally) was picked and whole genome SNP analysis was individually performed. SNP calling was performed with Snippy v.4.4.5 [41] using error corrected (Pilon v1.23) [42] and annotated draft assemblies with the earliest isolate (by date) within each cgMLST cluster chosen as reference. For each cluster, the core SNP alignment (with respective lengths of 3,972,412 bp for A. baumannii ST-2; 4,074,973 bp for A. baumannii ST-78 cluster 1; 4,123,489 bp for A. baumannii ST-78 cluster 2; 4,134,951 bp for A. baumannii ST-78 cluster 3; 4,022,063 bp for A. baumannii ST-400; 6,850,243 bp for P. aeruginosa ST-773; and 7,070,241 bp for P. aeruginosa ST-1047) was filtered for recombination using Gubbins v2.3.156 [43] and a SNP-based phylogeny was created by inferring a maximum-likelihood tree with RaxML-NG v0.9.0 [44] using the GTR + G model and 50–50 parsimony and random starting trees.
Results
Identification of clusters of high genetic relatedness grouping pre- and post-invasion isolates
Of the 260 available genomes, 167 were A. baumannii and 93 were P. aeruginosa (Table S1, [14]). The A. baumannii were distributed across 10 different ST, with 28 isolates collected pre-invasion and 139 collected post-invasion. Notable globally distributed and clinically important clones were identified, including ST-2 with the blaOXA-23 or blaOXA-72 carbapenemase genes, ST-78 with blaOXA-72, and ST-400 with the ESBL gene blaGES-11 (Fig. 1). P. aeruginosa were distributed across 17 STs, with 25 collected pre-invasion and 68 post-invasion. High-risk clones were also identified, including ST-773 and ST-1047 carrying the blaNDM-1 and blaIMP-1 carbapenemase genes, respectively (Fig. 2).
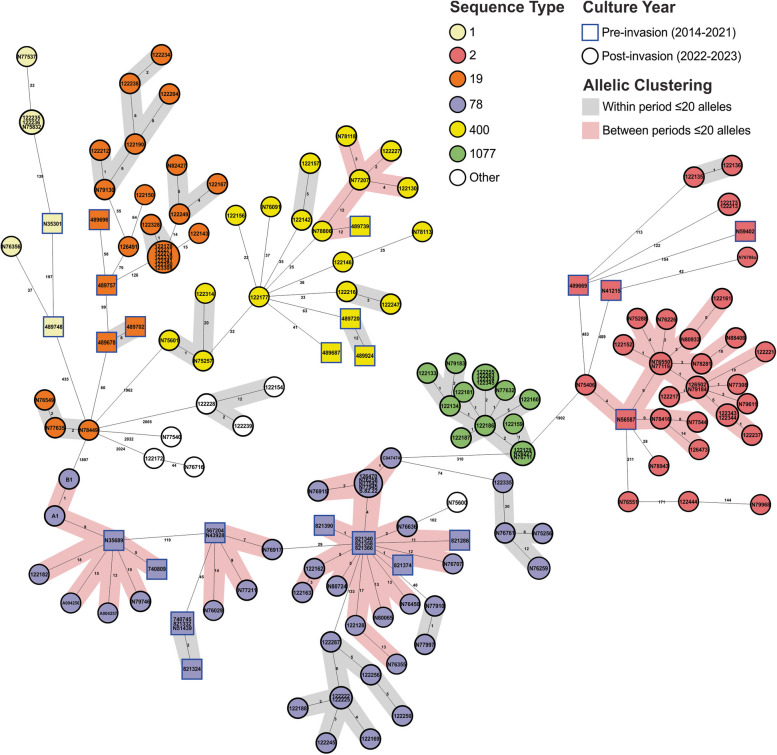
Fig. 1.
Genetic relationship of all A. baumannii in this study. Minimum spanning tree of all 167 A. baumannii analyzed in this study. Isolates are colored by sequence type and numbers between nodes are core genome MLST allelic differences and are highlighted if ≤ 20 alleles. Isolate nodes are shaped based on year of collection with square nodes indicating isolates collected pre-invasion and circular nodes indicating isolates collected post-invasion
Fig. 2.
Genetic relationship of all P. aeruginosa isolates in this study. Minimum spanning tree of all 93 P. aeruginosa analyzed in this study. Isolates are colored by sequence type and numbers between nodes are core genome MLST allelic differences and are highlighted if ≤ 20 alleles. Isolate nodes are shaped based on year of collection with square nodes indicating isolates collected pre-invasion and circular nodes indicating isolates collected post-invasion
Though distinct ST clusters were identified pre- and post-invasion, five A. baumannii and five P. aeruginosa STs were present during both periods (Figs. 1 and 2). Two of the A. baumannii STs (1 and 19) and two of the P. aeruginosa STs (235 and 2592) had pre-invasion isolates that were separated from the post-invasion isolates by > 20 allelic differences, and no further analysis was performed. The remaining three A. baumannii (ST-2, −78, and −400) and P. aeruginosa STs (ST-244, −773, and −1047) contained pre- and post-invasion isolates that were separated by < 20 allelic differences and these were selected for further SNP-based analysis (Table S2, [14]). This revealed that pre- and post-invasion strains differed by 3–87 SNPs across all isolates, though significant variation was observed between the six clusters.
An endemic clone of A. baumannii ST-2 in Ukraine and Germany
Although 35 isolates were assigned to ST-2, only 23 (16 from multiple hospitals in Germany and seven from 2 hospitals in Ukraine) formed a distinct cluster that contained both pre- and post-invasion isolates separated by ≤ 20 allelic differences (Figs. 1 and 3). All isolates carried armA and blaOXA-23. Only NRZ-56587, cultured from a Ukrainian patient being treated in a German hospital, was cultured before the invasion (2019), with the remaining isolates being collected in 2022 (n = 16) or 2023 (n = 6) (Fig. 3). Notably, NRZ-56587 was genetically nearly identical (5 SNPs) to NRZ-75406 collected during national surveillance 3 years later and shared a high level of genetic relatedness (16–54 SNPs) with the other 21 contemporary isolates from both Ukraine and Germany. A subset of 16 contemporary (2022–2023) isolates from German and Ukrainian hospitals were distinct by only 0–17 SNPs (Fig. 3). Interestingly, one isolate (NRZ-76226) acquired the ESBL gene blaGES-11, inserted as the first cassette within an Tn5086-like transposon, itself carried by a putative plasmid sharing homologies (99.99% nucleotide identity and 91.27% coverage) with a 77 kb rp-T1 type plasmid found in the six ST-400 A. baumannii collected from facilities in both Ukraine and Germany (Fig. 4A).
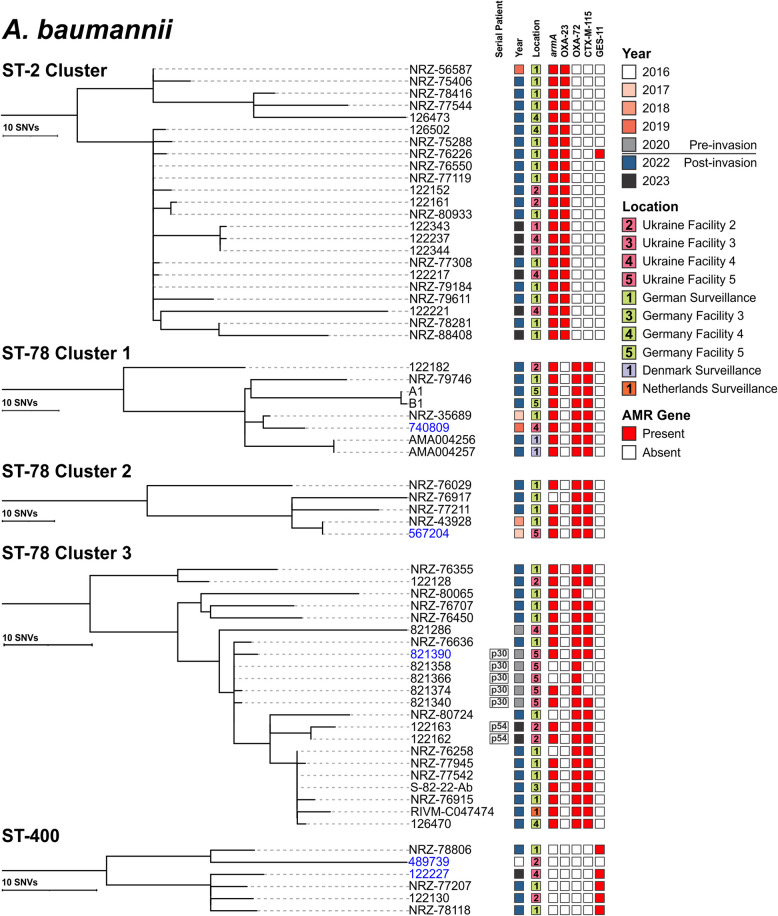
Fig. 3.
Single nucleotide variant phylogenies of select A. baumannii cgMLST clusters from three lineages. Isolate metadata include year and facility of culture collection, and AMR genes detected. Isolates sequenced with long-read platform(s) are shown in blue. All presented epidemic clones were detected in both European and Ukrainian facilities and included pre-/post-invasion isolates
Fig. 4.
Genetic context of major resistance genes in A. baumannii epidemic clones. A Plasmid-encoded blaGES-11 in ST-2 and ST-400 isolates. Reference is circularized 77.6 kb plasmid from ST-400 MRSN122227. Short-read contig mapping to the reference revealed that isolates with blaGES-11 possessed the Tn5086-like transposon, including ST-2 isolate NRZ-76226. B Chromosomal co-localization of armA and blaCTX-M-115 in ST-78 isolates. Reference is a 77.7 kb chromosomal fragment from MRSN567204. Short-read contig mapping revealed that the region is highly recombinant. C Plasmidic blaOXA-72 in ST-78 isolates. Reference is circularized 17.8 kb plasmid from MRSN567204
Three distinct clones of A. baumannii ST-78 detected in Ukraine and other European countries
A high degree of genetic diversity was observed within ST-78, and cgMLST segregated the 53 isolates into 5 broad clusters that were separated by up to 133 allelic variants (Fig. 1). Of these, 35 isolates formed 3 clusters with ≤ 20 allelic differences that contained both pre- and post-invasion isolates (Fig. 1, Table S2). Ten isolates (from 6 patients) were collected pre-invasion, eight from 4 Ukrainian soldiers injured during the Eastern conflict in 2017, 2019, and 2020, and two from Ukrainian soldiers receiving treatment in Germany in 2017 and 2018 (Table S2, [14]). The remaining 25 isolates were cultured post-invasion from injured Ukrainian civilians and military receiving care in Denmark (n = 2), Germany (n = 18), The Netherlands (n = 1), and Ukraine (n = 4). Twenty-eight of the 35 isolates carried blaOXA-72, the ESBL gene blaCTX-M-115, and armA (Fig. 3). Long-read sequencing revealed that armA and blaCTX-M-115 were chromosomally integrated on a large 77.7 kb fragment (in reference genome MRSN567204), flanked by insertion sequence (IS) 26-like, part of the IS6 family (Fig. 4B). Similar islands, detected by Vuillemenot et al. in blaCTX-M-115-carrying A. baumannii from France, ranged from 20 to 79 kb in size and contained different combinations of determinants encoding multiple resistances and flanked by several ISs promoting possible sequence rearrangements [45]. Here, with partial loss of internal sections of the resistance island, five isolates lacked armA while blaCTX-M-115 was missing in four isolates (Fig. 4B). Finally, in every isolate, blaOXA-72 was encoded on a 15–17 kb plasmid (Fig. 4C) with a replication origin identical to that of pMMA2 [46], and high homologies (> 99.9% nucleotide identity) with pAbCTX5 from a ST-78 isolate from France [45].
SNP-based analysis of the three sub-clusters revealed that within each sub-cluster, isolates differed by 0–55 SNPs; with pre-invasion isolates differing from post-invasion isolates by 2–55 SNPs (Fig. 3).
Sub-cluster 1 encompassed 8 isolates from three countries (Ukraine (n = 2), Germany (n = 4), and Denmark (n = 2)), with two (NRZ-35689 and 740,809) being collected before the full-scale invasion (2017 and 2019 from Germany and Ukraine, respectively). Both pre-invasion isolates differed from one another by 7 SNPs and were separated from post-invasion isolates by 12–38 SNPs (Fig. 3). Two isolates cultured from Ukrainian patients receiving care in Denmark in 2022 were separated from the German isolate NRZ-35689 by just 12–14 SNPs, despite having been cultured over 5 years apart.
Sub-cluster 2 encompassed five isolates from two countries (Ukraine (n = 1) and Germany (n = 4)) (Fig. 3). Two isolates were collected pre-invasion: one from Ukraine in 2017 (567,204) and the other from a Ukrainian soldier receiving care in a German hospital in 2018 (NRZ-43928). Notably, both pre-invasion isolates were genetically identical (0 SNPs), suggesting that both patients were exposed to the same reservoir, possibly in Ukraine facility #5. Unfortunately, no further patient movement information is available to test this hypothesis further. Finally, sub-cluster 3 encompassed 22 isolates from three countries: Germany (n = 12), Ukraine (n = 9), and The Netherlands (n = 1) (Fig. 2). Six isolates were collected pre-invasion including five highly genetically related isolates cultured from the same patient (P30) in Ukraine in May 2020 (Table S1 and Fig. 3). Though all isolates were separated by up to 55 SNPs, the 5 pre-invasion isolates from patient P30 were separated from NRZ-76636 by just 2–5 SNPs. This latter isolate was obtained as part of the national German surveillance program from an injured Ukrainian soldier in May 2022, but no further patient information is available.
Acquisition of an ESBL in an epidemic clone of A. baumannii ST-400
Of 21 isolates assigned to ST-400, just six (three each from Germany and Ukraine) met the criteria for inclusion (Fig. 1). Only MRSN 489739 was cultured prior to the invasion, from an injured Ukrainian soldier in 2016 at Ukraine facility #2 (Fig. 3). Though at least 6 years separates 489,739 from the other isolates, they differ by just 15–30 SNPs, with the closest relative being NRZ-78806, cultured in August 2022 from a wound of an injured Ukrainian soldier being treated in a German hospital. Notably, though none of the isolates carries an acquired carbapenemase, all five post-invasion isolates acquired blaGES-11 (within a Tn5086-like transposon harbored by an ~ 70 kb rp-T1 type plasmid, as discussed above, Fig. 4A), a variant of the ESBL blaGES-1 that confers increased carbapenem resistance [47]. In accordance, the 5 contemporary isolates were non-susceptible to ampicillin-sulbactam (≥ 32/16 µg/ml), ceftazidime (≥ 32 µg/ml), meropenem (≥ 38 µg/ml), and trimethoprim-sulfamethoxazole (≥ 4/76 µg/ml) while 489,739 (historical) remained susceptible.
Endemic NDM-carrying P. aeruginosa ST-773 clone shows signature of host adaption as it spreads from Ukraine
Twenty-seven isolates from three countries (Ukraine (n = 16), Germany (n = 7), and The Netherlands (n = 4)) were assigned to ST-773 (Fig. 2). All formed a cluster of highly related isolates, with just MRSN 740681 being cultured before the invasion (2019) at Ukraine facility #3 (Fig. 5). SNP-based analysis indicated that the isolates were separated by 0–32 SNPs and the 2019 isolate was separated from all other isolates by just by 4–18 SNPs, with 8 recent Ukrainian isolates being separated by ≤ 5 SNPs.
Fig. 5.
Single nucleotide variant phylogenies of two P. aeruginosa cgMLST clusters from two lineages. Isolate metadata include year and facility of culture collection, and AMR genes detected. Isolates collected from the same patient are indicated by the serial patient, if applicable. Isolates sequenced with long-read platform(s) are shown in blue
Twenty-four of the 27 isolates carried rmtB4 and blaNDM-1. Long-read sequencing detected no plasmids and both genes were chromosomally located within a 117 kb (in reference MRSN740681) integrated conjugative element (ICE) similar to those detected in ST-773 from South Korea, the USA, and Spain [10]. Three isolates lacked both genes because of a 22.4 kb internal region (with homologies to Tn125) excising from the ICE (Fig. 6A). As expected, the antibiotic susceptibility profile correlates with the presence or absence of these resistance genes, with only those isolates carrying rmtB4 and blaNDM-1 displaying non-susceptibility to all aminoglycosides, 3rd and 4th generation cephalosporins, and the carbapenems. Notably, recurring mutations were observed for the quorum-sensing regulator LasR, for which 9 independent mutational events (including 3 with predicted loss of function) were detected. Strains with loss-of-function mutations in this regulator have previously been shown to modulate immune responses and display survival advantages in animal models of infection [48].
International spread of an IMP-carrying P. aeruginosa ST-1047 clone endemic in Ukraine
Twenty-eight isolates from four countries (Ukraine (n = 16), Germany (n = 7), The Netherlands (n = 4), and Denmark (n = 1)) were assigned to ST-1047 (Fig. 2). All formed a single cluster of highly related isolates that were separated by 0–16 alleles. Five isolates were cultured pre-invasion: four from Ukraine facilities # 3, 5, and 7 between 2017 and 2019 and one from a patient receiving care at German facility #2 in 2017 (Fig. 5). Pre- and post-invasion isolates were separated by 14–54 SNPs. Notably, both MRSN 567277 and 122,260 from Ukraine facility #7 were separated by just 14 SNPs, despite being cultured 7 years apart (2017 and 2023, respectively).
All ST-1047 isolates had no plasmids and carried a chromosomally integrated blaIMP-1 within In1595, itself harbored on a 22.6 kb chromosomal fragment (Fig. 6B), which was identical to genomic island PAGI-97B [49]. A representative isolate for this clone was non-susceptible to all antibiotics tested, except colistin and cefiderocol. Finally, LasR was also recurringly mutated in ST-1047, with three of the contemporary isolates (MRSN 110606, 122,251, and 126,503) carrying distinct non-synonymous mutations, including a LOF mutation in 122,251.
Sporadic detection of P. aeruginosa ST-244
Just two isolates were assigned to ST-244 and both were cultured from patients in Ukraine: one from facility #6 in 2017 and the other from facility #4 in 2023. Despite being cultured 6 years apart, they differed by just 28 SNPs. Neither carried a carbapenemase and the earlier isolate (MRSN 567329) only carried nine AMR genes (Table S2, [14]). Interestingly, MRSN 122207 from 2023 acquired an additional three genes, including the aminoglycoside-modifying genes aac(6′)-Ib4 and aph(3′)-IIb and the ciprofloxacin-modifying gene crpP.
Discussion
Since the full-scale invasion of Ukraine by the Russian Federation, multiple reports of XDR bacteria infecting or colonizing patients have been published, both within Ukraine [4–6] and among Ukrainian civilians in other European countries [10–13]. As a direct result of the war, the loss of infection control in Ukraine has contributed significantly to the spread of these organisms. However, the original source of these bacteria remains unknown. Here, we leveraged a unique international collection of XDR A. baumannii and P. aeruginosa cultured from Ukrainian patients both pre- and post-invasion to explore this relationship. We demonstrate that highly genetically related strains span both time periods and provide the first evidence that strains infecting Ukrainian patients, including Ukrainian refugees in Europe, have been circulating within Ukraine for at least the past 10 years.
The findings in this study reflect previous observations that Gram-negative organisms are the most common cause of war wound infectious complications after hospitalization [50]. This paradigm has been evident since World War II (1939–1945) and has supplanted the previous paradigm from earlier wars, like World War I (1914–1918) and the American Civil War (1861–1865), where infections were predominantly due to Gram-positive and anaerobic organisms [50]. This etiological shift can be attributed to the increased use of antiseptics (and subsequently antibiotics) alongside other surgical advancements throughout the twentieth century, where Gram-positive organisms that were ostensibly introduced to the wound during the initial trauma were eliminated by these methods. In contrast, Gram-negative organisms were often associated with infectious complications later in the healing process [51], suggesting a likely nosocomial origin. This observation has persisted throughout the modern era, with Gram-negative organisms, especially A. baumannii and P. aeruginosa, being signature pathogens of the Iraq and Afghanistan conflicts, with infections primarily developing following hospitalization [52]. In Ukraine, this same pattern was already observed in soldiers injured in Eastern Ukraine between 2014 and 2107, where initial wound cultures grew predominantly Gram-positive organisms, but these were supplanted by Gram-negative species after 8 weeks of hospitalization [7].
Modern molecular typing has provided greater insights to the global epidemiology of bacteria. For P. aeruginosa, a small number of “high-risk” clones are responsible for the majority of infections [53]. In this study, notable high-risk clones belonging to ST-235, 244, 357, and 654 were observed, but ST-773 and ST-1047 were the most prevalent. While ST-773 is increasingly recognized as an emerging global clone [10], reports of ST-1047 have been confined to Asia [54–56], and further surveillance would be required to determine the extent of their global distribution. Both lineages carried acquired carbapenemases, blaNDM-1 in ST-773 and blaIMP-1 in ST-1047, making them particularly challenging to treat. In the latter, the blaIMP-1 gene was chromosomally bound, within a class I integron (In1595) itself harbored on a genomic island named PAGI-97B when first identified from an extensively resistant ST-234 strain from Ghana [49]. In both the ST-234 from Ghana and the ST-1047 from Ukraine, PAGI-97B was inserted within the PrrF1/PrrF2 locus. This intergenic locus, which revealed to be a hot spot for chromosomal integration [49], encodes tandem small RNAs involved in the regulation of iron homeostasis and pathogenesis in P. aeruginosa [57, 58]. Whether the acquisition of PAGI-97B carrying blaIMP-1 impacts the virulence of ST-1047 from Ukraine remains to be determined.
A. baumannii is also a leading cause of HAIs worldwide and is a prominent cause of infections during conflicts. It was the signature pathogen of war wound infections during the Iraq and Afghanistan conflicts [59] and was frequently isolated from injured Ukrainian soldiers from 2014 to 2017 [8, 60]. A small number of dominant “high-risk” clones are also responsible for most infections worldwide, with two major global clones, known as global clones 1 and 2 (ST-1 and ST-2, respectively), being the most common [61]. However, additional lineages have also been reported in many countries, such as the recent detection of carbapenemase-producing ST-19 in Ukraine and Georgia [62]. In this study, A. baumannii was the 3rd most frequently identified organism infecting war wounds, after P. aeruginosa and Klebsiella pneumoniae, with ST-2 predominating. However, strains belonging to ST-1, ST-19, ST-78, ST-400, and ST-1077 were also represented, and all carried a variety of carbapenemases (Table S1, [14]). Notably, all these lineages have recently been reported in Ukraine and other European countries [4–6, 10–13] and likely represent endemic reservoirs in this region.
Besides the high prevalence of carbapenemase-producing isolates (79%), a remarkable 47% of A. baumannii from Ukraine carried an ESBL. These genes have rarely been found in A. baumannii globally and high prevalence of ESBL is usually associated with localized outbreaks, with numerous reports from hospitals in the Middle East [63–65]. Here, epidemic clones within lineages ST-400 (GES-11) and ST-78 (CTX-M-115) were responsible for the high ESBL prevalence. Interestingly, besides Ukraine, isolates of ST-78 also carrying the rare combination of a chromosomal blaCTX-M-115 and a plasmid-bound blaOXA-72 carbapenemase have previously been reported from around the globe, including from France, Germany, Russia, and the USA [45, 66, 67]. While their origin remains to be proven, it could be hypothesized that these isolates shared a common ancestor with the “Italian clone,” an ST-78 outbreak strain circulating in Italy in the mid-2000s [68], which emerged and spread globally after the acquisition of the blaCTX-M-115 ESBL gene.
The protracted nature of the outbreak clusters and the low level of genetic divergence accumulated though time (e.g., 14 SNPs between isolates from two patients 7 years apart at the same facility) are suggestive of persisting reservoirs in the hospital, a known capability of P. aeruginosa and A. baumannii [69]. Here, echoing previous reports [70, 71] and possibly contributing to their persistence [72], we found that both ST-400 and ST-78 A. baumannii strains from Ukraine carried a qacE disinfectant resistance gene colocalized with the acquired ESBL genes. Further, we found evidence of the similar + blaGES-11/qacE-carrying plasmid, previously described as self-transferable [47], in genetically distinct strains (ST-2 and ST-400) from the same hospital. This is most concerning as recent genomic studies are providing growing evidence of the role of horizontal gene transfer within hospital environments for the maintenance and spread of resistance genes [73, 74]. Altogether, our data suggests that to better understand and control the spread of MDRO, future surveillance efforts will likely need to extend beyond clinical samples in Ukraine and encompass hospital environmental and patient colonization screening surveillance also.
This report has several limitations. First, patient movement is largely unknown, and many patients moved through multiple hospitals over a short period. This, and the lack of environmental sampling discussed in the above paragraph, makes identifying chains of transmission difficult. However, some clear facilities of interest can be identified in the data; for example, nearly identical ST-2 A. baumannii are present in Ukrainian facilities #1 and #4 (Fig. 3) and highly related ST-773 P. aeruginosa can be identified in Ukrainian facilities #1 and #2 (Fig. 5). Second, albeit a unique resource, a relatively small number of pre-war isolates (54 isolates over 6 years) were available for analysis, constraining the ability to accurately identify potential reservoirs. Finally, recent data associated with Ukraine indicates that K. pneumoniae is a leading cause of infections among war wounded [4]. This contrasts with historical reports from Ukraine, where war wound infections due to K. pneumoniae were significantly lower than A. baumannii and P. aeruginosa [7]. For this study, just 3 non-clonal K. pneumoniae were available from the pre-invasion period and none was closely related to post-invasion strains.
Conclusions
The invasion of Ukraine by Russia has placed extraordinary strain on the Ukrainian healthcare system. While this resulted in the proliferation of MDROs, the source of these isolates remains obscure. Herein, we provide the first evidence indicating that many of the strains circulating since 2022 share a close genetic relationship to historical strains from Ukraine. In some cases, direct links to medical facilities within Ukraine can be inferred, but where these reservoirs ultimately exist remains unknown. These data suggest that surveillance efforts should focus on hospital environments with the ultimate aim of identifying and eliminating these sources of infection and enhancing transmission-based infection and prevention control practices.
Supplementary Information
Additional file 1: Table S1 All isolates in the study. Basic characteristics of all isolates used in this study. Table S2 All clustered isolates in the study. Basic characteristics of all isolates that formed related clusters in this study.
Acknowledgements
The authors are thankful to all the staff of the MRSN. The manuscript has been reviewed by the Walter Reed Army Institute of Research. There is no objection to its presentation and/or publication. The opinions or assertions contained herein are the private views of the authors, and are not to be construed as official, or as reflecting true views of the Defense Health Agency or the Department of Defense.
Abbreviations
- ESBL
Extended spectrum β-lactamase
- HAI
Hospital acquired infection
- ICE
Integrated conjugative element
- kb
Kilobase
- MDR
Multi-drug resistant
- MDRO
Multi-drug resistant organism
- MLST
Multi-locus sequence type
- MRSN
Multidrug-Resistant Organism Repository and Surveillance Network
- SNP
Single nucleotide polymorphism
- ST
Sequence type
- Tn
Transposon
- XDR
Extensively-drug resistant
Authors’ contributions
F.L., Vi.K., Va.K., N.P., S.G., J.W.B., and P.M.G designed research; A.O., J.E., F.F., N.F., performed research. F.L., Vi.K., N.P., T.L.L., B.T.J., F.F, J.B.H., and J.E. analyzed research; Vi.K., N.P., N.F., J.B.H., J.E. and S.G. contributed clinical metadata and isolates. F.L. and P.M.G. wrote the paper with input from all authors.
Funding
This study was funded by Defense Health Program (DHP) Operation & Maintenance (O&M) to PMG and JB, the German Ministry of Health (Grant no. 1369–402) to SG and NP, and the Ukrainian Ministry of Health (National Registration No. 0117U006903 and State Registration No. 0123U101070) to ViK and VaK.
Data availability
The genomes of all new isolates have been deposited in the National Center for Biotechnology Information under bioproject ID PRJNA1162747.
Declarations
Ethics approval and consent to participate
The isolates and clinical information were collected as part of the public health surveillance activities of the MRSN, as determined by the WRAIR Commander in accordance with Institutional Review Board (IRB) Policy Memorandum #10; Public Health activity Determination and Oversight Requirements issued June 10th 2019. For Ukrainian samples, hospital permission to conduct the surveillance study was obtained from Hospital Bioethics Committee of MMCC CR, Ministry of Defense, Vinnitsa, Ukraine, protocol reference number 18/2 from 05 May 2014. The patients were included after understanding the study and had signed an informed consent. The research conformed to the principles of the Helsinki Declaration.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Francois Lebreton and Viacheslav Kondratiuk contributed equally to this work.
References
- 1.Pallett SJC, Boyd SE, O’Shea MK, Martin J, Jenkins DR, Hutley EJ. The contribution of human conflict to the development of antimicrobial resistance. Commun Med (Lond). 2023;3(1):153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loban G, Faustova M, Dobrovolska O, Tkachenko P. War in Ukraine: incursion of antimicrobial resistance. Ir J Med Sci. 2023;192(6):2905–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haque U, Bukhari MH, Fiedler N, Wang S, Korzh O, Espinoza J, et al. A comparison of Ukrainian hospital services and functions before and during the Russia-Ukraine war. JAMA Health Forum. 2024;5(5):e240901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kovalchuk V, Kondratiuk V, McGann P, Jones BT, Fomina N, Nazarchuk O, et al. Temporal evolution of bacterial species and their antimicrobial resistance characteristics in wound infections of war-related injuries in Ukraine from 2014 to 2023. J Hosp Infect. 2024;152:99–104. [DOI] [PubMed] [Google Scholar]
- 5.Ljungquist O, Nazarchuk O, Kahlmeter G, Andrews V, Koithan T, Wasserstrom L, et al. Highly multidrug-resistant Gram-negative bacterial infections in war victims in Ukraine, 2022. Lancet Infect Dis. 2023;23(7):784–6. [DOI] [PubMed] [Google Scholar]
- 6.Stepanskyi D, Ishchenko O, Luo T, Lebreton F, Bennett JW, Kovalenko I, et al. Phenotypic and genomic analysis of bacteria from war wounds in Dnipro, Ukraine. JAC Antimicrob Resist. 2024;6(3):dlae090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valentine KP, Viacheslav KM. Bacterial flora of combat wounds from eastern Ukraine and time-specified changes of bacterial recovery during treatment in Ukrainian military hospital. BMC Res Notes. 2017;10(1):152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kondratiuk V, Jones BT, Kovalchuk V, Kovalenko I, Ganiuk V, Kondratiuk O, et al. Phenotypic and genotypic characterization of antibiotic resistance in military hospital-associated bacteria from war injuries in the Eastern Ukraine conflict between 2014 and 2020. J Hosp Infect. 2021;112:69–76. [DOI] [PubMed] [Google Scholar]
- 9.Fuchs F, Xanthopoulou KBT, Arazo Del Pino R, Wohlfarth E, Pavlu F, Hagen RM, Higgins PG. Coexistence of seven different carbapenemase producers in a single hospital admission screening confirmed by whole-genome sequencing. Journal of Global Antimicrobial Resistance. 2024;39:184–8. [DOI] [PubMed] [Google Scholar]
- 10.Hernandez-Garcia M, Cabello M, Ponce-Alonso M, Herrador-Gomez PM, Gioia F, Cobo J, et al. First detection in Spain of NDM-1-producing Pseudomonas aeruginosa in two patients transferred from Ukraine to a university hospital. J Glob Antimicrob Resist. 2024;36:105–11. [DOI] [PubMed] [Google Scholar]
- 11.Sandfort M, Hans JB, Fischer MA, Reichert F, Cremanns M, Eisfeld J, et al. Increase in NDM-1 and NDM-1/OXA-48-producing Klebsiella pneumoniae in Germany associated with the war in Ukraine, 2022. Euro Surveill. 2022;27(50):2200926. [DOI] [PMC free article] [PubMed]
- 12.Stolberg RS, Hansen F, Porsbo LJ, Karstensen KT, Roer L, Holzknecht BJ, et al. Genotypic characterisation of carbapenemase-producing organisms obtained in Denmark from patients associated with the war in Ukraine. J Glob Antimicrob Resist. 2023;34:15–7. [DOI] [PubMed] [Google Scholar]
- 13.Zwittink RD, Wielders CC, Notermans DW, Verkaik NJ, Schoffelen AF, Witteveen S, et al. Multidrug-resistant organisms in patients from Ukraine in the Netherlands, March to August 2022. Euro Surveill. 2022;27(50):2200896. [DOI] [PMC free article] [PubMed]
- 14.Lebreton F, Kondratiu V, Kovalchuk V, Pfennigwerth N, Luo TL, Jones BT, et al. High genetic relatedness between pre and post-invasion multidrug resistant bacteria associated with Ukraine. 2025. 10.6084/m9.figshare.29247410 and 10.6084/m9.figshare.29247455.
- 15.Kazmirchuk A, Yarmoliuk Y, Lurin I, Gybalo R, Burianov O, Derkach S, et al. Ukraine’s experience with management of combat casualties using NATO’s four-tier “changing as needed” healthcare system. World J Surg. 2022;46(12):2858–62. [DOI] [PubMed] [Google Scholar]
- 16.Russo TA, Alvarado CL, Davies CJ, Drayer ZJ, Carlino-MacDonald U, Hutson A, et al. Differentiation of hypervirulent and classical Klebsiella pneumoniae with acquired drug resistance. mBio. 2024;15(2):e0286723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bushnell, B. BBDuk: high-performance tool that can perform various operations on DNA sequences, such as trimming, filtering, masking, and kmer matching. 2015. https://github.com/BioInfoTools/BBMap/blob/master/sh/bbduk2.sh. Source code.
- 18.Wood DE, Lu J, Langmead B. Improved metagenomic analysis with Kraken 2. Genome Biol. 2019;20(1):257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seemann, T. Shovill: assemble bacterial isolate genomes from Illumina paired-end reads. Github. 2020. https://github.com/tseemann/shovill.
- 20.Bushnell, B. BBMap: short read aligner for DNA and RNA-seq data. 2014. https://github.com/BioInfoTools/BBMap. Source code.
- 21.Parks DH, Chuvochina M, Rinke C, Mussig AJ, Chaumeil PA, Hugenholtz P. GTDB: an ongoing census of bacterial and archaeal diversity through a phylogenetically consistent, rank normalized and complete genome-based taxonomy. Nucleic Acids Res. 2022;50(D1):D785–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chaumeil PA, Mussig AJ, Hugenholtz P, Parks DH. GTDB-Tk v2: memory friendly classification with the genome taxonomy database. Bioinformatics. 2022;38(23):5315–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nanoporetech. Dorado is a high-performance, easy-to-use, open source analysis engine for Oxford Nanopore reads. Github. 2025. https://github.com/nanoporetech/dorado.
- 24.Wick RR, Howden BP, Stinear TP. Autocycler: long-read consensus assembly for bacterial genomes. bioRxiv. 2025;05(12):653612. 10.1101/2025.05.12.653612.
- 25.Wick RR, Holt KE. Polypolish: short-read polishing of long-read bacterial genome assemblies. PLoS Comput Biol. 2022;18(1):e1009802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwengers O, Jelonek L, Dieckmann MA, Beyvers S, Blom J, Goesmann A. Bakta: rapid and standardized annotation of bacterial genomes via alignment-free sequence identification. Microb Genom. 2021;7(11):000685. [DOI] [PMC free article] [PubMed]
- 27.Hunt M, Mather AE, Sanchez-Buso L, Page AJ, Parkhill J, Keane JA, et al. ARIBA: rapid antimicrobial resistance genotyping directly from sequencing reads. Microb Genom. 2017;3(10):e000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feldgarden M, Brover V, Gonzalez-Escalona N, Frye JG, Haendiges J, Haft DH, et al. AMRFinderPlus and the Reference Gene Catalog facilitate examination of the genomic links among antimicrobial resistance, stress response, and virulence. Sci Rep. 2021;11(1):12728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jolley KA, Maiden MC. BIGSdb: scalable analysis of bacterial genome variation at the population level. BMC Bioinformatics. 2010;11:595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown CL, Mullet J, Hindi F, Stoll JE, Gupta S, Choi M, et al. mobileOG-db: a manually curated database of protein families mediating the life cycle of bacterial mobile genetic elements. Appl Environ Microbiol. 2022;88(18):e0099122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siguier P, Perochon J, Lestrade L, Mahillon J, Chandler M. ISfinder: the reference centre for bacterial insertion sequences. Nucleic Acids Res. 2006;34(Database issue):D32-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ross K, Varani AM, Snesrud E, Huang H, Alvarenga DO, Zhang J, et al. TnCentral: a prokaryotic transposable element database and web portal for transposon analysis. mBio. 2021;12(5):e0206021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lam MMC, Koong J, Holt KE, Hall RM, Hamidian M. Detection and typing of plasmids in Acinetobacter baumannii using rep genes encoding replication initiation proteins. Microbiol Spectr. 2023;11(1):e0247822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carattoli A, Zankari E, Garcia-Fernandez A, Voldby Larsen M, Lund O, Villa L, et al. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob Agents Chemother. 2014;58(7):3895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grant JR, Enns E, Marinier E, Mandal A, Herman EK, Chen CY, et al. Proksee: in-depth characterization and visualization of bacterial genomes. Nucleic Acids Res. 2023;51(W1):W484–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stanton RA, McAllister G, Daniels JB, Breaker E, Vlachos N, Gable P, et al. Development and application of a core genome multilocus sequence typing scheme for the health care-associated pathogen Pseudomonas aeruginosa. J Clin Microbiol. 2020;58(9):e00214–20. [DOI] [PMC free article] [PubMed]
- 37.Higgins PG, Prior K, Harmsen D, Seifert H. Development and evaluation of a core genome multilocus typing scheme for whole-genome sequence-based typing of Acinetobacter baumannii. PLoS ONE. 2017;12(6):e0179228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cunningham SA, Eberly AR, Beisken S, Posch AE, Schuetz AN, Patel R. Core genome multilocus sequence typing and antibiotic susceptibility prediction from whole-genome sequence data of multidrug-resistant Pseudomonas aeruginosa isolates. Microbiol Spectr. 2022;10(6):e0392022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cabal A, Hortenhuber A, Salaheddin Y, Stoger A, Springer B, Bletz S, et al. Three prolonged outbreaks of metallo-beta-lactamase-producing Pseudomonas aeruginosa in an Upper Austrian hospital, 2017–2023. Microbiol Spectr. 2024;12(10):e0074024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stribling William HLR, Powell Aubrey, Harless Casey, Martin Melissa J, Corey Brendan W., Snesrud Erik, Ong Ana, Maybank Rosslyn, Stam Jason, Bartlett Katie, Jones Brendan T, Preston Lan N, Lane Katherine F, Thompson Bernadette, Young Lynn M, Kwak Yoon I, Barsoumian Alice E, Markelz Ana-Elizabeth, Kiley John L, Cybulski Robert J, Bennett Jason W, Mc Gann Patrick T, Lebreton Francois. Detecting, mapping, and suppressing the spread of a decade-long Pseudomonas aeruginosa nosocomial outbreak with genomics. eLife. 2024;13:RP93181.
- 41.Seemann, T. Snippy: rapid haploid variant calling and core genome alignment. Github. 2014. https://github.com/tseemann/snippy.
- 42.Walker BJ, Abeel T, Shea T, Priest M, Abouelliel A, Sakthikumar S, et al. Pilon: an integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS ONE. 2014;9(11):e112963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Croucher NJ, Page AJ, Connor TR, Delaney AJ, Keane JA, Bentley SD, et al. Rapid phylogenetic analysis of large samples of recombinant bacterial whole genome sequences using Gubbins. Nucleic Acids Res. 2015;43(3):e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kozlov AM, Darriba D, Flouri T, Morel B, Stamatakis A. RAxML-NG: a fast, scalable and user-friendly tool for maximum likelihood phylogenetic inference. Bioinformatics. 2019;35(21):4453–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vuillemenot JB, Bour M, Beyrouthy R, Bonnet R, Laaberki MH, Charpentier X, et al. Genomic analysis of CTX-M-115 and OXA-23/-72 co-producing Acinetobacter baumannii, and their potential to spread resistance genes by natural transformation. J Antimicrob Chemother. 2022;77(6):1542–52. [DOI] [PubMed] [Google Scholar]
- 46.Merino M, Acosta J, Poza M, Sanz F, Beceiro A, Chaves F, et al. OXA-24 carbapenemase gene flanked by XerC/XerD-like recombination sites in different plasmids from different Acinetobacter species isolated during a nosocomial outbreak. Antimicrob Agents Chemother. 2010;54(6):2724–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moubareck C, Bremont S, Conroy MC, Courvalin P, Lambert T. GES-11, a novel integron-associated GES variant in Acinetobacter baumannii. Antimicrob Agents Chemother. 2009;53(8):3579–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao K, Yang X, Zeng Q, Zhang Y, Li H, Yan C, et al. Evolution of lasR mutants in polymorphic Pseudomonas aeruginosa populations facilitates chronic infection of the lung. Nat Commun. 2023;14(1):5976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Janice J, Agyepong N, Owusu-Ofori A, Govinden U, Essack SY, Samuelsen O, et al. Carbapenem resistance determinants acquired through novel chromosomal integrations in extensively drug-resistant Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2021;65(7): e0028921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Murray CK, Hinkle MK, Yun HC. History of infections associated with combat-related injuries. J Trauma. 2008;64(3 Suppl):S221–31. [DOI] [PubMed] [Google Scholar]
- 51.Tong MJ. Septic complications of war wounds. JAMA. 1972;219(8):1044–7. [PubMed] [Google Scholar]
- 52.Kaspar RL, Griffith ME, Mann PB, Lehman DJ, Conger NG, Hospenthal DR, et al. Association of bacterial colonization at the time of presentation to a combat support hospital in a combat zone with subsequent 30-day colonization or infection. Mil Med. 2009;174(9):899–903. [DOI] [PubMed] [Google Scholar]
- 53.Del Barrio-Tofino E, Lopez-Causape C, Oliver A. Pseudomonas aeruginosa epidemic high-risk clones and their association with horizontally-acquired beta-lactamases: 2020 update. Int J Antimicrob Agents. 2020;56(6):106196. [DOI] [PubMed] [Google Scholar]
- 54.Hyun JE, Chung TH, Hwang CY. Identification of VIM-2 metallo-beta-lactamase-producing Pseudomonas aeruginosa isolated from dogs with pyoderma and otitis in Korea. Vet Dermatol. 2018;29(3):186-e68. [DOI] [PubMed] [Google Scholar]
- 55.Tada T, Hishinuma T, Watanabe S, Uchida H, Tohya M, Kuwahara-Arai K, et al. Molecular characterization of multidrug-resistant Pseudomonas aeruginosa isolates in hospitals in Myanmar. Antimicrob Agents Chemother. 2019;63(5):e02397-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takahashi T, Tada T, Shrestha S, Hishinuma T, Sherchan JB, Tohya M, et al. Molecular characterisation of carbapenem-resistant Pseudomonas aeruginosa clinical isolates in Nepal. J Glob Antimicrob Resist. 2021;26:279–84. [DOI] [PubMed] [Google Scholar]
- 57.Reinhart AA, Powell DA, Nguyen AT, O’Neill M, Djapgne L, Wilks A, et al. The prrF-encoded small regulatory RNAs are required for iron homeostasis and virulence of Pseudomonas aeruginosa. Infect Immun. 2015;83(3):863–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wilderman PJ, Sowa NA, FitzGerald DJ, FitzGerald PC, Gottesman S, Ochsner UA, et al. Identification of tandem duplicate regulatory small RNAs in Pseudomonas aeruginosa involved in iron homeostasis. Proc Natl Acad Sci U S A. 2004;101(26):9792–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Calhoun JH, Murray CK, Manring MM. Multidrug-resistant organisms in military wounds from Iraq and Afghanistan. Clin Orthop Relat Res. 2008;466(6):1356–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Higgins PG, Hagen RM, Podbielski A, Frickmann H, Warnke P. Molecular epidemiology of carbapenem-resistant Acinetobacter baumannii Isolated from war-injured patients from the Eastern Ukraine. Antibiotics (Basel). 2020;9(9):579. [DOI] [PMC free article] [PubMed]
- 61.Hamidian M, Nigro SJ. Emergence, molecular mechanisms and global spread of carbapenem-resistant Acinetobacter baumannii. Microb Genom. 2019;5(10):e000306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Luo TL, Martin MJ, Kovalchuk V, Kondratiuk V, Trapaidze N, Metreveli M, et al. Detection of carbapenemase producing Acinetobacter baumannii ST19 from Georgia and Ukraine carrying bla(OXA-23), bla(OXA-72), and/or bla(NDM-5), December 2019 to June 2023. Euro Surveill. 2024;29(24):2400259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Al-Agamy MH, Khalaf NG, Tawfick MM, Shibl AM, El Kholy A. Molecular characterization of carbapenem-insensitive Acinetobacter baumannii in Egypt. Int J Infect Dis. 2014;22:49–54. [DOI] [PubMed] [Google Scholar]
- 64.Alyamani EJ, Khiyami MA, Booq RY, Alnafjan BM, Altammami MA, Bahwerth FS. Molecular characterization of extended-spectrum beta-lactamases (ESBLs) produced by clinical isolates of Acinetobacter baumannii in Saudi Arabia. Ann Clin Microbiol Antimicrob. 2015;14:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ranjbar R, Farahani A. Study of genetic diversity, biofilm formation, and detection of carbapenemase, MBL, ESBL, and tetracycline resistance genes in multidrug-resistant Acinetobacter baumannii isolated from burn wound infections in Iran. Antimicrob Resist Infect Control. 2019;8:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Munoz-Price LS, Arheart K, Nordmann P, Boulanger AE, Cleary T, Alvarez R, et al. Eighteen years of experience with Acinetobacter baumannii in a tertiary care hospital. Crit Care Med. 2013;41(12):2733–42. [DOI] [PubMed] [Google Scholar]
- 67.Pfeifer Y, Hunfeld KP, Borgmann S, Maneg D, Blobner W, Werner G, et al. Carbapenem-resistant Acinetobacter baumannii ST78 with OXA-72 carbapenemase and ESBL gene blaCTX-M-115. J Antimicrob Chemother. 2016;71(5):1426–8. [DOI] [PubMed] [Google Scholar]
- 68.Giannouli M, Cuccurullo S, Crivaro V, Di Popolo A, Bernardo M, Tomasone F, et al. Molecular epidemiology of multidrug-resistant Acinetobacter baumannii in a tertiary care hospital in Naples, Italy, shows the emergence of a novel epidemic clone. J Clin Microbiol. 2010;48(4):1223–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kizny Gordon AE, Mathers AJ, Cheong EYL, Gottlieb T, Kotay S, Walker AS, et al. The hospital water environment as a reservoir for carbapenem-resistant organisms causing hospital-acquired infections-a systematic review of the literature. Clin Infect Dis. 2017;64(10):1435–44. [DOI] [PubMed] [Google Scholar]
- 70.Elkhatib WF, Khalil MAF, Ashour HM. Integrons and antiseptic resistance genes mediate resistance of Acinetobacter baumannii and Pseudomonas aeruginosa isolates from intensive care unit patients with wound infections. Curr Mol Med. 2019;19(4):286–93. [DOI] [PubMed] [Google Scholar]
- 71.Gomaa FAM, Helal ZH, Khan MI. High prevalence of bla(NDM-1), bla(VIM), qacE, and qacEDelta1 genes and their association with decreased susceptibility to antibiotics and common hospital biocides in clinical isolates of Acinetobacter baumannii. Microorganisms. 2017;5(2):18. [DOI] [PMC free article] [PubMed]
- 72.Liu WJ, Fu L, Huang M, Zhang JP, Wu Y, Zhou YS, et al. Frequency of antiseptic resistance genes and reduced susceptibility to biocides in carbapenem-resistant Acinetobacter baumannii. J Med Microbiol. 2017;66(1):13–7. [DOI] [PubMed] [Google Scholar]
- 73.Diorio-Toth L, Wallace MA, Farnsworth CW, Wang B, Gul D, Kwon JH, et al. Intensive care unit sinks are persistently colonized with multidrug resistant bacteria and mobilizable, resistance-conferring plasmids. mSystems. 2023;8(4):e0020623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lerminiaux NA, Cameron ADS. Horizontal transfer of antibiotic resistance genes in clinical environments. Can J Microbiol. 2019;65(1):34–44. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1 All isolates in the study. Basic characteristics of all isolates used in this study. Table S2 All clustered isolates in the study. Basic characteristics of all isolates that formed related clusters in this study.
Data Availability Statement
The genomes of all new isolates have been deposited in the National Center for Biotechnology Information under bioproject ID PRJNA1162747.