Abstract
Extraordinary preservation in amber of the Miocene termite Mastotermes electrodominicus has led to the discovery of fossil symbiotic microbes. Spirochete bacteria and wood-digesting protists were identified in the intestinal tissue of the insect. Fossil wood (xylem: developing vessel-element cells, fibers, pit connections), protists (most likely xylophagic amitochondriates), an endospore (probably of the filamentous intestinal bacterium Arthromitus = Bacillus), and large spirochetes were seen in thin section by light and transmission electron microscopy. The intestinal microbiota of the living termite Mastotermes darwiniensis, a genus now restricted to northern Australia, markedly resembles that preserved in amber. This is a direct observation of a 20-million-year-old xylophagus termite fossil microbial community.
Mastotermes, a genus of large tropical wood-ingesting termites, is of evolutionary importance for two reasons: (i) they harbor Mixotricha paradoxa and other amitochondriate many-genomed protists considered key to the early history of nucleated cells, and (ii) they are phylogenetically proximal to cockroaches (1). Both morphological and molecular features consistently place the single extant species (Mastotermes darwiniensis, family Mastotermitidae) as an early taxon ancestral to other termites. For example, Mastotermes is the only termite that, like cockroaches, oviposits an ootheca (egg case), although it is rudimentary (2). Numerous mastotermitid fossil specimens, corresponding to four extinct genera and about 20 species, occur from the Eocene [40 million years ago (mya)] to the Miocene (20–5 mya) of Australia, Brazil, the Caribbean, Central America, and especially Europe (3, 4). The two finest preserved fossil species, extremely similar to M. darwiniensis, occur in Oligocene and Miocene amber from southern Mexico (Mastotermes electromexicus) (5) and the Dominican Republic (Mastotermes electrodominicus) (6). The notoriously polyphagous “living fossil” M. darwiniensis is limited to northern Australia.
Termites exhibit a complex and unique symbiosis with prokaryotic (eubacterial and archaebacterial) and eukaryotic microorganisms that live in their hindgut. The symbiotic microbiota digest cellulose to sugars and acetate and produce hydrogen, methane, and carbon dioxide (7). In addition to Mixotricha paradoxa, a giant trichomonad motile by means of a unique set of attached surface spirochetes (8, 9), M. darwiniensis harbors other Archaeprotista (class Parabasalia, those with distinctive parabasal bodies, a type of Golgi apparatus), including the two hypermastigote genera (Koruga and Deltotrichonympha), a devescovinid (Metadevescovina extranea), and a trichomonad (Pentatrichomonoides scroa) that harbors endosymbiotic methanogenic bacteria (10).
Where and when did this symbiosis evolve? Here we use the remarkable preservative properties of termites in amber to seek direct paleontological evidence for the evolution of termite intestinal symbionts. Cellular and subcellular structures of amber-preserved tissues, e.g., myofibrils and mitochondria, within amber are well documented (11, 12).
Materials and Methods
M. electrodominicus fossils are common in Dominican amber. We examined five specimens from the American Museum of Natural History (New York) and three from the Museum of Science (Barcelona), which all came from mines near the city of Santiago, Dominican Republic; these deposits are stratigraphically dated as Miocene, ≈15–20 mya (13). Most amber specimens are workers and alate termites (Fig. 1); soldiers, essential for identification to the species level, are exceedingly rare (4). Worker specimens were selected based on the lack of fine fractures between the inner amber surface and the termite inclusion, because such fractures allow deterioration of contents during burial and diagenesis.
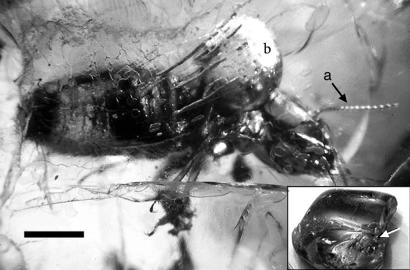
Figure 1.
M. electrodominicus in Miocene fossil resin of H. portera from the Dominican Republic in which the typical gas bubble (b) extrudes between the thorax and abdomen. (Bar = 3 mm). The right antenna is seen at a. (Inset) Hand sample of fractured amber, source of the photograph of the protists in Fig. 2; arrow at the head of the termite.
Amber was trimmed and polished close to the insect, and a fine groove was cut into the surface with a Dremel cutting wheel. A sharp blade inserted into the groove was then slowly twisted, which caused the amber to split. The split tends to occur along a plane parallel to the groove through the termite abdominal cavity. Dark consolidated remains of the gut tissue were removed with flame-sterilized forceps and placed in Eppendorf tubes for analysis. Amber-embedded termite fragments were placed directly in resin (Epon-Araldite for at least 24 h), which was changed once after 48 h (without fixation or dehydration). Resin was polymerized at 60°C overnight and samples were sectioned with a DDK diamond knife (Wilmington, DE). The sections were stained with lead citrate and uranyl acetate on carbon-coated copper grids; they were examined at 60 kV with a JEOL electron microscope. Light microscopic examination of translucent intact amber pieces was done by phase-contrast, Nomarski differential interference contrast light microscopy (Nikon Optiphot), and videomicrography (Optronix camera connected to a Sony U-matic video deck).
Results and Discussion
Protist symbionts were found on the surface of a bubble extruded from the thorax of an intact amber specimen, whereas ultrastructural details were seen in hindgut contents of other specimens. Although identification to genus (i.e., Mixotricha, Deltotrichonympha, Pentatrichomonoides, etc.) was not possible in the fossils, the intestinal microbiota of M. electrodominicus greatly resembles that of the extant M. darwiniensis. Overall shapes of the protists (Fig. 2) and details of structure, including protist membrane remnants studded with epibiotic bacteria (Fig. 3), and two specimens of large termite (Hollandina-like pillotinaceous) spirochetes (Fig. 4) indicate that little morphological change has occurred in 20 million years of mastotermitid evolution. The fossil membranes and outer wall (“sheath”) of a Hollandina-like spirochete are particularly clear in transverse section.
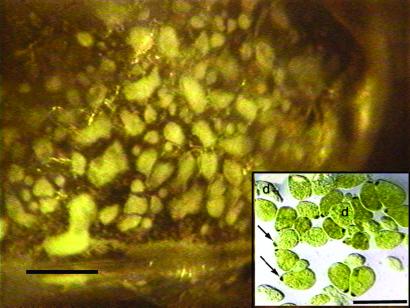
Figure 2.
Optical microscope observation of three size classes interpreted to be fossil protists; the large one at least is comparable in size and shape to modern Mixotricha paradoxa (with the dark-spotted anteriors at arrows in Inset) and Deltotrichonympha operculata (d). (Inset) Live protists, light micrographs. (Bars = 500 μm.)
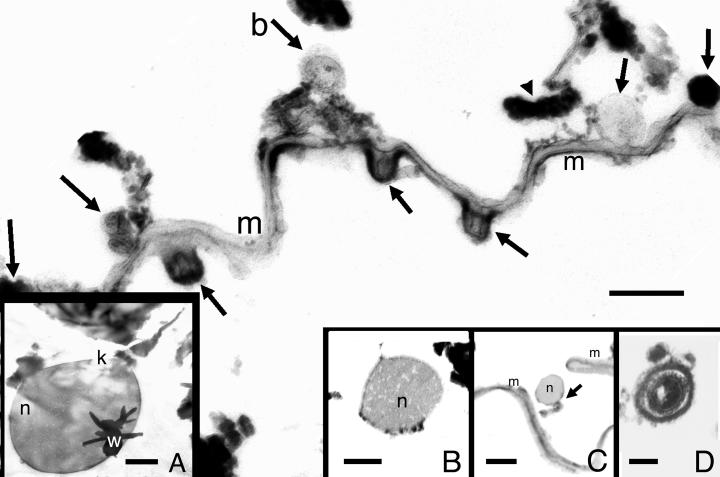
Figure 3.
Membranous material (m) with adhering Gram-negative bacteria (b) comparable to those on the surface of the Mixotricha and other protist genera (such as those in Fig. 2 Inset). Cytoplasmic tubule is visible in cross section in bacterium labeled b. The sections of all epibionts are transverse except that at the arrowhead at the upper right, which is longitudinal. (Bar = 1.5 μm.) (Inset A) Karyomastigont (k) nucleus (n) in amber similar to that of live Mixotricha; w, wood. (Bar = 0.5 μm.) (Inset B) Protist nucleus (n) with intact membranes not associated with a mastigont. (Bar = 1.0 μm.) (Inset C) Karyomastigont nucleus (n) at arrow, with membrane (m). (Bar = 2 μm.) (Inset D) Bacterial endospore similar to those produced by the extant termite bacillus Arthromitus. (Bar = 0.4 μm.) Images were produced by transmission electron microscopy.
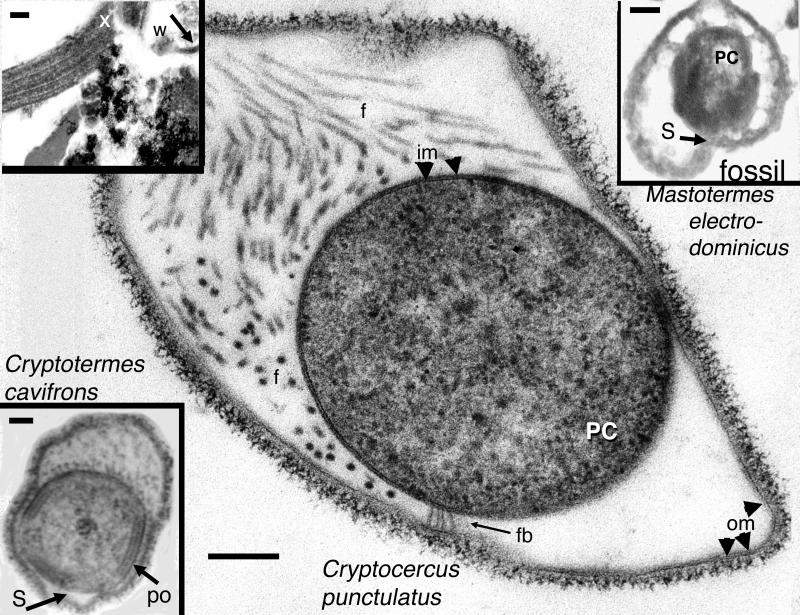
Figure 4.
Modern spirochetes. Hollandina pterotermitidis from the wood-eating cockroach (Cryptocercus punctulatus; bar = 0.25 μm) and a termite spirochete Hollandina (Hollandina cryptotermitidis; Lower Left Inset; bar = 0.20 μm) transverse section, compared with the putative fossil termite spirochete (Upper Right Inset, bar = 0.15 μm). Longitudinal section of spirochete formerly attached to wood fiber (Upper Left Inset; x = xylem, bar = 7.5 μm). PC, protoplasmic cylinder; om, outer membrane; w, wood; fb, fibers; f, flagella; po, polar organelle; s, sillon. These spirochetes belong to the family Pillotinaceae. Cryptocercus is considered an ancestral group to the termites (1, 14, 15). Images were produced by transmission electron microscopy.
Fragments of plant material that possess tracheid vessels indicate that the extinct termite fed on wood, the main diet of the Australian congeneric (Fig. 5). The extinct species inhabited tropical forests (16) and may have even ingested pieces of dead or dying roots of the Dominican amber tree species Hymenea portera (Leguminosae). M. electrodominicus specimens are invariably preserved with bubbles that emanate from thoracic or abdominal spiracles (b in Fig. 1). The bubble volume may occupy 10 times that of the body of the trapped termite. Such bubbles have not been reported in other amber fossil insects, including other species of termites. Gas excretion by M. darwiniensis includes copious quantities of ethylene (17), methane (1–26%), and CO2 (1.3–11.6%) (D.A.G., D. Bookwalter, and G. Landis, unpublished data). We propose that these gases were generated in large quantity by the unique hindgut microbial community, and exuded as the insect was immersed in the viscous resin. This interpretation is consistent with the report of intracellular methanogens of Pentatrichomonoides (10).
Figure 5.
Plant tissue. Immature xylem vessel element in production. The central decaying materials are remnants of the parent xylem cell with thickened cellulose walls, most likely from the resin-producing leguminous tree Hymenea sp. They shortly would have disappeared if they had not been trapped in amber and preserved for 20 million years. Pit connections between the xylem elements of the plant cell wall (w) are visible at the white arrows. (Bar = 25 μm.) Image was produced by transmission electron microscopy.
Preservation, especially of lipidic and polymeric carbohydrate materials, is notable at a resolution of less than 1 μm. Plant cells with layered lignified cellulosic walls (fragments of ingested wood in Figs. 3A, 4 Upper Left Inset, and 5), the decorated calcium dipicolinate spore coat of a Gram-positive Arthromitus-like bacterium (Fig. 3D), and the two-lipid membranous walls of Gram-negative spirochete bacteria (Fig. 4 Upper Right Inset) are recognizable. Most significant observation was the fine preservation of more than a dozen protist nuclei. Four or five (as in Fig. 3 A and C) were still attached to their nuclear connectors (interpreted to be karyomastigonts of parabasalids), some were free (Fig. 3 Inset B). Our results suggest the likelihood that symbiotic microbes may be found in other amber-fossilized anthropods: Plasmodium-like apicomplexans in Culicidae and Borrelia spirochetes in ticks; both of these groups have a Cretaceous record. Extinct termites are preserved in their entirety in Cretaceous amber from New Jersey (Carinotermes nascimbeni) (16), Burma (Kalotermes swinhoei), and especially the undescribed hodotermitid (damp wood-eating termites) in the Lower Cretaceous amber of Lebanon. Because rare or unique amber should never be subjected to destructive sampling, analysis of their microbiota must await the discovery of expendable specimens.
The biology, molecular sequence data, and our model for eukaryote origins (18) taken together lead us to interpret the over 300 species of the phylum Archaeprotista to be basal eukaryotes. Informal names of the classes of the phylum are mastigamoebids, diplomonads, and parabasalids. The latter includes trichomonads, devescovinids, and hypermastigotes. The extant members of all of these amitochondriate heterotrophic taxa we take as codescendants of the earliest nucleated cell lineages. Nuclei, in this view, evolved as organelles that emerged from the original chimera (the ancestral fusion between the archaebacterium and the eubacterium) (19). The membrane-bounded nucleus in the earliest eukaryote (the first protist) began as a part of the peculiar cytoskeleton conspicuous in most archaeprotists: the karyomastigont. The minimal karyomastigont, a robust organellar system, consists of a nucleus and the nuclear connector (rhizoplast) to the microtubule kinetosome. The morphology of live protists and the amber fossil images are explained by our concept that the nucleus evolved from a karyomastigont by severance of the connector. Nuclear release left akaryomastigonts with some karyomastigonts (e.g., in Calonympha) or akaryomastigonts with unadorned nuclei (e.g., Snyderella and Fig. 3B) (20). More than a dozen isolated protist nuclei were seen in the termite-gut amber samples. Most, attached to nuclear connectors, we interpret to be parabasalid karyomastigonts (Fig. 3 Insets A and C). Our most significant findings are protist nuclei and their connectors (karyomastigonts), the Arthromitus-like spore, and the Hollandina-like spirochete preserved at the electron microscopic level in amber.
Acknowledgments
We thank B. Afzelius, G. Brugerolle, J. Breznak, D. Callaham, B. D. Dyer, E. L. Davis, J. Ehleringer, L. Kent, S. Leschine, M. McMenamin, K. Nealson, H. Owen, L. To, R. Radek, J. Rikkinen and L. Yin, for their aid. D. Reppard, A. MacConnell, and J. Whiteside helped us with this work; however, we alone are responsible for our conclusions. Support for this work came from National Aeronautics and Space Administration Space Sciences, Planetary Biology Internship Program, the University of Massachusetts Graduate School, and the Lounsbery Foundation (to L.M.), Comision Interministerial de Ciencia y Tecnología Grant FEDER 2FD97-1649 (to R.G.), and Fundació la Caixa (Barcelona).
Abbreviation
- mya
million years ago
References
- 1.Lo N, Takuda G, Watanabe H, Rose H, Slaytor M, Maekawa K, Bandi C, Noda H. Curr Biol. 2000;10:801–804. doi: 10.1016/s0960-9822(00)00561-3. [DOI] [PubMed] [Google Scholar]
- 2.Nalepa C A, Lenz M. Proc R Soc London Ser B. 2000;267:1809–1813. doi: 10.1098/rspb.2000.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nel A, Paicheler J-C. Cahiers Paléontol. 1993. 103–179. [Google Scholar]
- 4.Thorne B L, Grimaldi D A, Krishna K. In: Termites: Evolution, Sociality, Symbiosis, and Ecology. Abe T, Bignell D E, Higashi M, editors. Dordrecht, The Netherlands: Kluwer; 2000. pp. 75–92. [Google Scholar]
- 5.Krishna, K. & Emerson, A. E. (1983) Am. Mus. Novit.2767.
- 6.Krishna, K. & Grimaldi, D. A. (1991) Am. Mus. Novit.3021.
- 7.Breznak J A, Brune A. Annu Rev Entomol. 1994;39:453–487. [Google Scholar]
- 8.Cleveland L R, Grimstone A V. Proc R Soc London Ser B. 1964;159:668–686. [Google Scholar]
- 9.Wier A, Ashen J, Margulis L. Internatl Microbiol. 2000;3:213–223. [PubMed] [Google Scholar]
- 10.Fröhlich J, Könîg H. Syst Appl Microbiol. 1999;22:249–257. doi: 10.1016/S0723-2020(99)80072-1. [DOI] [PubMed] [Google Scholar]
- 11.Grimaldi, D. A., Bonwich, E., Delannoy, M. & Doberstein, S. (1994) Am. Mus. Novit.3097.
- 12.Henwood A. Palaeontology. 1992;35:901–912. [Google Scholar]
- 13.Iturralde-Vincent M, MacPhee R D E. Science. 1996;273:1850–1852. [Google Scholar]
- 14.Donovan S E, Jones D T, Sands W A, Eggleton P. Biol J Linn Soc. 2000;70:467–513. [Google Scholar]
- 15.Thompson G J, Kitade O, Lo N, Crozier R H. J Evol Biol. 2000;13:869–881. doi: 10.1046/j.1420-9101.2003.00645.x. [DOI] [PubMed] [Google Scholar]
- 16.Krishna K, Grimaldi D A. In: Studies on Fossils in Amber, with Particular Reference to the Cretaceous of New Jersey. Grimaldi D A, editor. Leiden, The Netherlands: Backhuys; 2000. pp. 133–140. [Google Scholar]
- 17.French J R J, Turner G L, Bradbury J F. J Gen Microbiol. 1976;95:202–206. doi: 10.1099/00221287-95-2-202. [DOI] [PubMed] [Google Scholar]
- 18.Margulis L, Dolan M F, Guerrero R. Proc Natl Acad Sci USA. 2000;97:6954–6959. doi: 10.1073/pnas.97.13.6954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta R S. Microbiol Mol Biol Rev. 1998;62:1435–1491. doi: 10.1128/mmbr.62.4.1435-1491.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chapman M J, Dolan M F, Margulis L. Q Rev Biol. 2000;75:409–429. doi: 10.1086/393621. [DOI] [PubMed] [Google Scholar]