Abstract
Background
Chronic stroke patients often experience persistent motor impairments, and current rehabilitation therapies rarely achieve substantial functional recovery. Sensory feedback during movement plays a pivotal role in driving neuroplasticity. This study introduces a novel multi-modal sensory feedback brain-computer interface (Multi-FDBK-BCI) system that integrates proprioceptive, tactile, and visual stimuli into motor imagery-based training. We aimed to explore the potential therapeutic efficacy and elucidate its neural mechanisms underlying motor recovery.
Methods
Thirty-nine chronic stroke patients were randomized to either the Multi-FDBK-BCI group (n = 20) or the conventional motor imagery therapy group (n = 19). Motor recovery was assessed using the Fugl-Meyer Assessment (primary outcome), Motor Status Scale, Action Research Arm Test, and surface electromyography. Functional MRI was used to examine brain activation patterns during upper limb tasks, while Granger causality analysis and machine learning evaluated inter-regional connectivity changes and their predictive value for recovery.
Results
Multi-FDBK-BCI training led to significantly greater motor recovery compared to conventional therapy. Functional MRI revealed enhanced activation of high-order transmodal networks—including the default mode, dorsal/ventral attention, and frontoparietal networks—during paralyzed limb movement, with activation strength positively correlated with motor improvement. Granger causality analysis identified a distinct information flow pattern: signals from the lesioned motor cortex were relayed through transmodal networks to the intact motor cortex, promoting interhemispheric communication. These functional connectivity changes not only supported motor recovery but also served as robust predictors of therapeutic outcomes.
Conclusions
Our findings highlight the Multi-FDBK-BCI system as a promising strategy for chronic stroke rehabilitation, leveraging activity-dependent neuroplasticity within high-order transmodal networks. This multi-modal approach holds significant potential for patients with limited recovery options and sheds new light on the neural drivers of motor restoration, warranting further investigation in clinical neurorehabilitation.
Trial registration
All data used in the present study were obtained from a research trial registered with the ClinicalTrials.gov database (ChiCTR-ONC-17010739, registered 26 February 2017, starting from 10 January 2017).
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s12916-025-04214-8.
Keywords: Stroke, Hemiplegia, Multi-modal sensory feedback, Brain-computer interface, Brain pasticity
Background
Stroke is a leading cause of long-term disability among adults, with more than 70% experiencing chronic disability, carrying a very high social and economic burden [1–3]. Hemiplegia is the most common functional impairments after stroke [4]. Despite intensive rehabilitation, up to 50% of chronic stroke patients experience persistent moderate-to-severe upper extremity paresis, significantly limiting their functional independence and ability to perform daily tasks, even after regaining walking ability through spontaneous recovery and compensatory strategies during the acute phase [5–7]. During the chronic phase of stroke, the efficacy of conventional motor rehabilitation therapies declines due to limitations in harnessing brain plasticity [8, 9]. The sensory system plays a crucial role in the execution and regulation of movement, and the preservation of sensory integrity following the stroke is essential for motor function improvement [10, 11]. Sensory feedback therapy has the potential to enhance neural plasticity and shows promise as an effective therapeutic approach during the chronic phase of stroke recovery [12, 13].
The sensory system relays multifaceted sensory information, including visual, tactile, auditory, and proprioceptive signals, to various brain regions, providing feedback that aids movement regulation. Diverse sensory feedback stimulation therapies have demonstrated substantial efficacy in facilitating motor recovery post-stroke, underscoring their integral role in neurorehabilitation. Visual feedback training can enhance the upper limb motor function, daily activity ability, and spatial visualization ability of stroke patients [14]. Tactile discrimination feedback has the potential to induce reorganization of the sensorimotor cortex in stroke patients, leading to improvements in the deep sensation and motor function on the affected hand [15]. Integrating multimodal sensory feedback training, such as proprioceptive and visual feedback, has been shown to effectively enhance the Fugl-Meyer assessment of the affected upper limb in stroke patients and improve their ability to perform daily activities [16]. However, a key gap remains in current research: no study has fully integrated proprioceptive, tactile, and visual feedback simultaneously within a single motor imagery brain-computer interfaces (MI-BCI) system for post-stroke neurorehabilitation.
Non-invasive brain-computer interfaces, a novel treatment strategy, can detect neuronal activity via electroencephalography and control exoskeletons or functional electrical stimulation (FES) devices, thereby facilitating motor rehabilitation and sensory feedback training in post-stroke patients [17–21]. Motor imagery (MI), a cognitive process involving mental rehearsal of motor tasks without physical execution, has been shown to activate the sensorimotor system and induce cortical changes similar to those observed during actual physical practice [22–24]. For stroke patients with limited mobility, MI reinforced by feedback may serve as an alternative to physical practice, as it engages neural activity patterns comparable to those elicited by overt movements, potentially facilitating motor recovery without the need for excessive physical exertion [25, 26]. Sensory feedback-based MI-BCI have demonstrated significant efficacy in enhancing fine motor function, such as grasp and pinch, in the affected limb of stroke patients, irrespective of the severity of impairment or the chronicity of the condition [20, 27–31]. Notably, the observed motor function recovery correlates with increased ipsilesional intrahemispheric connectivity within the affected hemisphere during movement of the impaired limb, suggesting that this innovative approach promotes adaptive neural plasticity and facilitates neurorehabilitation.
Different sensory feedback modalities demonstrate varying neurophysiological effects when integrated into MI-BCI systems. Compared to visual feedback, proprioceptive feedback induces greater desynchronization in the somatosensory-motor area and significantly enhances the brain connectivity pattern associated with motor imagery [32]. While both feedback types generate coordinated neural oscillations supporting functional connectivity, proprioceptive feedback uniquely enhances sensorimotor self-regulation [33]. This mechanism enhances activation of the motor cortex and optimizes the processing efficiency of descending motor commands, contributing significantly to motor recovery in stroke patients [26]. MI-BCI systems incorporating multiple sensory feedback modalities demonstrate superior performance compared to single-feedback systems, significantly enhancing overall effectiveness and optimizing rehabilitation potential [34]. Visual-proprioceptive feedback MI-BCI have the potential to facilitate information exchange between brain regions involved in motor commands and higher cognitive functions, while simultaneously reducing the cognitive demands on sensory regions and fostering positive emotions [34, 35]. Despite these encouraging findings, there remains a significant shortage of clinical studies evaluating the efficacy of multi-sensory feedback BCI (Multi-FDBK-BCI) systems in chronic stroke rehabilitation.
Despite a diverse range of available rehabilitation strategies, achieving optimal upper limb motor recovery following stroke remains a significant clinical challenge. Conventional rehabilitation approaches frequently prioritize isolated motor execution, often inadequately addressing the essential integration of sensory feedback with motor control [36]. Furthermore, many sensory-based interventions tend to employ repetitive passive stimulation, which may limit active patient participation and often lack structured integration with cognitive processes crucial for motor learning [37]. Concurrently, emerging technologies like BCI have been applied in the rehabilitation medicine. The invasive BCI have achieved remarkable results. But the non-invasive BCI predominantly targeted individuals with relatively preserved motor function (Fugl-Meyer Assessment scores exceeding 30 points), largely excluding severely impaired patients [38, 39]. This creates a critical therapeutic gap, leaving an underserved population—those with profound motor deficits who could potentially derive substantial benefit from advanced neurorehabilitation—with limited options. Furthermore, existing mechanistic studies often have a narrow scope, focusing solely on EEG data without investigating the direct influence of sensory feedback on motor function or the changes in different brain network connections.
To address these gaps, our study aims to investigate a novel Multi-FDBK-BCI that integrates proprioceptive, tactile, and visual stimulation into a single platform. Unlike previous studies, we employ functional magnetic resonance imaging (fMRI) to thoroughly investigate the impact of multimodal sensory feedback on brain network connectivity, elucidating its neural mechanisms. We hypothesize that, Multi-FDBK-BCI, by coupling sensory feedback with cortical activity, can induce functionally activity-dependent brain plastic changes in neural networks, which alter functional connectivity between different brain networks and lead to clinically functional improvements in patients with chronic stroke, with effects superior to those of classical motor imagery (CMI) rehabilitation.
To test our hypothesis, we designed a Multi-FDBK-BCI training system that emphasizes the critical role of sensory input in motor recovery. The system functions by first detecting the patient’s intention to perform wrist flexion or extension through EEG signals. Upon detecting this intention, the system simultaneously activates three feedback mechanisms: an exoskeleton that generates corresponding movements (providing proprioceptive feedback), a strategically positioned brush that stimulates the hand (delivering tactile feedback), and a virtual reality (VR) device that presents visual representations of the intended movement. This coordinated multi-modal sensory experience creates a rich feedback environment that enhances neural responses during motor imagery tasks.
Our findings suggested that the Multi-FDBK-BCI training might have the potential to improve the recovery of motor function in chronic stroke patients. Moreover, functional MRI results reveal that this intervention significantly activates transmodal regions in bilateral hemispheres during paralyzed limb movement, and the level of activation exhibited a positive correlation with movement recovery. Further causal analysis results indicate that this training strategy can improve the functional information interaction between transmodal regions, including the default mode network, ventral and dorsal attention networks, and the frontoparietal network, using the high-order network connections as bridges to transmit movement related information from the lesion motor cortex to the normal motor cortex, thereby increasing the compensatory ability of the healthy hemisphere for the impaired function on the lesion hemisphere. The magnitude of change in functional information interaction can be utilized to predict patients’ functional recovery, providing compelling evidence that Multi-FDBK-BCI training can induce activity-dependent neuroplastic changes, promoting adaptive neural reorganization and facilitating motor recovery in stroke survivors.
Methods
Participants
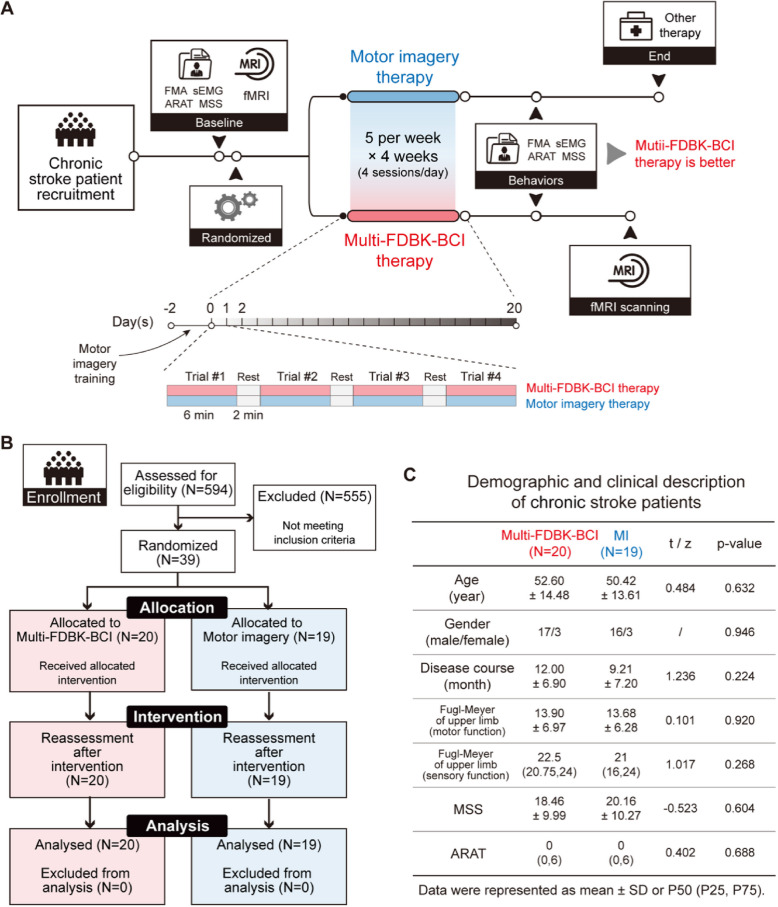
Participants in this study were chronic stroke patients with severe upper limb motor impairment who received rehabilitation therapy at the Department of Rehabilitation Medicine, Huashan Hospital Fudan University, from March 2017 to October 2021. The sample size was determined based on our previous results with the significance level of 0.05 and power of 85%. A total of 594 stroke patients were screened. Among them, 555 patients were excluded due to not meeting the inclusion criteria or meeting the exclusion criteria, leaving 39 eligible participants who were subsequently enrolled in the study and randomly assigned to either the Multi-FDBK-BCI group or the CMI group using random number sequence at a 1:1 ratio. All the participants had previously received rehabilitation therapy but still suffered from severe upper limb motor impairment. The duration of the rehabilitation time for each patient is listed in Additional File 1: Table S1. The baseline characteristics of the included participants are shown in Fig. 1C.
Fig. 1.
Diagram of study design. A Flow chart of the study protocol. Patients who meet the inclusion criteria underwent behavioral, electrophysiological and fMRI assessment. Then they were randomized into two groups, either received classical motor imagery therapy or Multi-FDBK-BCI therapy. When all the interventions were finished after 20 days, all the participants were reassessed. FMA, Fugl-Meyer assessment; sEMG, surface electromyography; ARAT, action research arm test; MSS, motor status scale. B Flow diagram of participant recruitment and participation in the study. C Demographic and clinical description of including participants at baseline
The inclusion criteria were as follows: (1) first ever cerebrovascular accident confirmed by CT or MRI, unilateral cortical lesion (left or right hemisphere) and/or subcortical lesion.; (2) severe wrist motor impairment with Manual Muscle Testing of wrist extension 0–1; (3) severe upper extremity paralysis with a FMA-UE (Fugl-Meyer Motor Score Assessment-Upper Extremity) score < 31 points; (4) age between 18 to 75 years old, male or female; (5) without auditory or visual impairment; (6) minimum 6 months from stroke; (7) vital signs stable, well controlled blood glucose and blood pressure (<140/90 mmHg), and no arrhythmia.
The exclusion criteria were as follows: (1) Motor and Visual Survey Questionnaire score [40] less than 25 indicating unable to perform motor imagery tasks; (2) Montreal Cognitive Assessment [41] less than 26 indicating cognitive impairment; (3) presence of metallic implants in the skull or devices such as a cardiac pacemaker that precludes transcranial magnetic stimulation examination; (4) poor compliance, unable to cooperate in completing the trial; (5) participation in other clinical trials.
The trial was conducted at Huashan Hospital-Department of Rehabilitation Medicine. This study was approved by the Ethics Committee of Huashan Hospital (2017-016). All patients provided informed consent prior to participating in the trial. The study adhered to the principles of the declaration of Helsinki and was prospectively registered in a publicly accessible clinical trials registry (https://www.chictr.org.cn/, identifier: ChiCTR-ONC-17010739).
Multi-FDBK BCI training system
BCI system setup
The system used in this study is a BCI training system providing real-time visual and proprioceptive feedback (nBETTER, BCI-O-24-CY, NCC Electrophysiology). The training system included an EEG signal amplifier, a continuous passive motion (CPM) device for wrist, a computer stored with motor imagery analysis software and a feedback display screen. The EEG signal amplifier consists of a transformer, EEG main control box, EEG amplification box, and electrodes.
A 24-channel EEG acquisition system was applied, with electrode placement following the international 10-20 system standard, including 'F3', 'F4', 'FC3', 'FC4', 'C3', 'C4', 'CP3', 'CP4', 'P3', 'P4', 'FT7', 'FT8', 'T3', 'T4', 'TP7', 'TP8', 'Fz', 'Oz', 'FCz', 'Cz', 'CPz', 'Pz'. The ground electrode is fixed on the medial side of the frontal cortex, and the reference electrodes are fixed on the mastoids bilaterally, with a sampling rate of 256 Hz.
On the basis of nBETTER, a brush was used on the back of the hand to enhance with light touch stimulation. The brush is manually controlled by the therapist during the training together with the activation of the CPM device.
EEG classification
According to previous researches, the typical spectrum feature of EEG in MI tasks included α (8−13 Hz) and β (14−30 Hz) rhythms [42, 43]. Specifically, imagery of hand movement would induce event-related desynchronization (ERD) of 8−30 Hz band in the contralateral hemisphere, and even-related synchronization (ERS) in the ipsilateral hemisphere.
In order to acquire motor imagery-related cortical activities, the raw EEG signal were preprocessed by a band filter of 8−30 Hz and then segmented into several epochs (−1.0–2.5 s, locking to the symbols display event). Power spectrum density (PSD), which was calculated by fast Fourier transforming, was used to extract the activities of α and β rhythms.
Rehabilitation assessment
Behavioral and neuroelectrophysiological assessments were conducted at the baseline and after the intervention. All the assessments were done by an experienced physiatrist in this study, who did not know the allocation of the participants.
Behavioral assessment
Behavioral assessment included assessments of motor and sensory functions of the paretic upper limb. The motor function was assessed using the Fugl-Meyer Assessment (motor part) of the upper limb and the Motor Status Scale (MSS). Sensory function was evaluated using the sensory part of the Fugl-Meyer Assessment. The Action Research Arm Test (ARAT) was used to assess upper extremity performance (coordination, dexterity, and functioning).
Neuroelectrophysiological assessment
Surface electromyography (sEMG) was utilized to collect electromyographic signals (mean amplitude and area under the curve) during voluntary contraction of the paretic wrist extensor muscles using MyoMove-EOW (Nuocheng Medical, Shanghai, China). The sEMG collecting was conducted in accordance with the SENIAM principles.
We used MEG-TD (Wuhan Yiruide Medical Equipment New Technology Co., Ltd., Wuhan, China) to assess motor-evoked potential (MEP). Electromyography (EMG) data were recorded from the abductor pollicis brevis using standard Ag/AgCl electrodes and a ground electrode positioned on the wrist. The EMG signals were amplified with a band pass filter of 10 Hz to 2 kHz. Participants were asked to sit quietly and relax their limbs. The M1 area on the affected side was stimulated, gradually increasing the stimulus intensity. If no waveform was observed even when the stimulus intensity reached 100%, it could be concluded that the patient’s MEP on the affected side could not be detected.
Rehabilitation intervention
Comprehensive rehabilitation training
In this study, all participants underwent comprehensive rehabilitation training. The comprehensive rehabilitation training was designed individually based on the functional impairments of the patients and included physical therapy, occupational therapy, speech therapy, physical modalities, etc. All training sessions were conducted once a day, five times a week, for a total of 4 weeks.
Multi-sensory feedback BCI training
As shown in the Fig. 2A, all patients underwent training in a separate, quiet room to minimize external interference during the training. An experienced physiatrist was responsible for the training. Before the intervention, all the participants were introduced to the entire training process to ensure they understand the whole process. Then, they had 2 days to practice motor imagery for wrist extension/flexion on the paretic side to ensure they could complete the motor imagery training for wrist extension/flexion during the formal training. The training consists of four sessions, each lasting 6 min, with a 2-min rest interval between sessions to ensure participants maintain focused attention. During the training, the environment should be kept as quiet as possible, and participants were instructed to avoid unnecessary movements, such as turning their heads, speaking, or making large movements with their forearms and shoulders. If participants experience any discomfort such as dizziness or tinnitus, they could request to end the training. The Multi-Sensory FDBK training is conducted once a day for 30 min, five times a week for 4 weeks, 20 sessions in total.
Fig. 2.
Multi-FDBK-BCI therapy improved motor functions than motor imagery in chronic stroke patients. A Schematic of the experimental setup of Multi-FDBK-BCI therapy, including visual, tactile and proprioceptive feedbacks. B-E Significant enhancement of motor functions after Multi-FDBK-BCI therapy (N = 20) compared to those after motor imagery therapy (N = 19), including FMA (B) and sEMG (C). No significant changes were observed in the MSS (D) and ARAT (E) function for chronic stroke patients. Statistical significance was determined using the (B-D) two sample t-test (two tails) or (E) Mann-Whitney U test for inter-group comparison depending on the distribution of the data. FMA, Fugl-Meyer assessment; sEMG, surface electromyography; ARAT, action research arm test; MSS, motor status scale. Each dot represented an individual patient. The box showed the first and third quartiles; inner line was the median over sessions; whiskers represented minimum and maximum values
Participants sit on a chair or wheelchair, with the paretic upper limb properly fixed on the wrist CPM, which was set to a passive range of motion from 30° wrist flexion to 40° wrist extension. The brush providing sensory feedback was manually controlled by the physiatrist during the training process in coordination with the passive movement of the wrist CPM. The brush touches the dorsal hand along with the finger axis.
The detailed training procedure is described below: After the system is activated, an upward/downward arrow appears on the screen, indicating wrist extension/flexion. Then the patient needs to perform wrist extension/flexion motor imagery according to the arrow prompts. If the motor imagery intention is detected, the wrist CPM would move toward the direction of the arrow to provide proprioceptive feedback and the virtual hand on the screen provide visual feedback. Meanwhile, the physiatrist manually controlled the brush to provide light touch feedback. If the motor imagery intention was not detected, the above feedbacks were not provided, and the training proceeds to the next round. If the patient fails to succeed three times, automatic feedback would be triggered to ensure that the patient could actively participate in the training.
Classic motor imagery training
Classic Motor imagery training is conducted by a therapist in a quiet and comfortable room. The training is divided into four steps:
First, patients are instructed to close their eyes and imagine themselves lying or sitting in a warm and relaxing place (such as a familiar living room sofa, bedroom, etc.), and then guide the patients to relax their whole body for 3–5 min.
Second, patients are instructed to imagine the movement of each joint of the paretic upper limb, including flexion and extension of the shoulder, elbow, and wrist, the pronation and supination of the forearm, and the grasping and extending the fingers, lasting 10–15 min.
Third, patients are instructed to engage in the imagination of more familiar daily upper limb practical activities, such as “Imagine you are now lifting your affected hand and wrist, raising and lowering it,” “Imagine you are grasping a big red apple in front of you with your affected hand, and then slowly putting it down,” “Imagine you are picking up a glass of water from the table with your affected hand, drinking the water, and then slowly putting the glass down,” etc. The content and guidance of the imagery content are appropriately adjusted by the therapist, mainly in combination with the patient’s daily occupational therapy, lasting 15–20 min.
After completing the above training, under the guidance of the therapist, the patient relaxes the whole body again, and the therapist slowly counts down from “10” to “1”. When counting to 1, the patients were asked to slowly open their eyes, and the training ends. The training time and frequency are the same as the multi-sensory feedback group. It is conducted once a day for 30 min, five times a week for 4 weeks, 20 sessions in total. During the training, to monitor whether the patient is performing effective motor imagery tasks, the therapist can ask the patient about the vividness and clarity of the imagery tasks to help the patient better enter the state of imagination.
fMRI acquisition
All MRI data were acquired with a 3.0T Siemens MAGNETOM Prisma whole body 60-cm bore human scanner with an 80-mT/m gradient at Shanghai University of Sport. An eight-channel head coil was used for transmission and reception. A T1-weighted structural image was also acquired based on magnetization-prepared 2 rapid acquisition gradient echoes (MP2RAGE) sequence, which was used for co-registration with following parameters: repetition time (TR) = 3130 ms, echo time (TE) = 2.98 ms, inversion time (TI) = 450 ms, flip angle = 12°, field of view = 256 × 256 mm2, matrix size = 256 × 256, slice thickness = 1 mm, 166 contiguous slices with whole brain coverage. For each participant, two sessions of functional data were collected using the echo planar imaging (EPI) sequence with the following parameters: TR = 2000 ms, TE = 30 ms, flip angle = 90°, field of view = 192 ×192 mm2, matrix size = 64 × 64, 206 volumes, slice thickness = 3 mm, 42 contiguous slices.
In this study, participants were asked to fixate on a back-projection screen viewed through a mirror during scanning and perform the motor imaging tasks, i.e., wrist flexion and extension. Here, we used a block design with three conditions: (A) motor imaging of the left hand, (B) motor imaging of the right hand, and (C) rest. During the rest period, subjects were instructed to focus their eyes on a fixation mark at the screen center and try not to think of anything. Each condition lasted for 20 s. The three conditions were conducted in the order “ABC BCA CAB,” and such sequence was repeated three times (Fig. 3A).
Fig. 3.
Significant activations in human transmodal regions evoked by impaired hand movement after Multi-FDBK-BCI therapy for chronic stroke patients. A Schematic setup of fMRI task. L, left hand movement; R, right hand movement. B No significant difference (post- v.s. pre- Multi-FDBK-BCI therapy) of whole-brain activations under healthy hand movement (FDR corrected p < 0.05). N = 20 patients. C As in (B) but for impaired hand movement. Significant BOLD activations were observed in transmodal brain regions (FDR corrected p < 0.05). D Functional parcels based on Yeo et. al. [74] seven cortical networks. E Quantitative comparison of BOLD signal changes between pre- and post- Multi-FDBK-BCI therapy across somatomotor, dorsal attention, ventral attention, frontoparietal and default mode networks in both lesion and healthy hemispheres. Error bar, standard error of the mean (SEM). N = 20 patients. Statistical significance was determined by one-way ANOVA with Tukey-Kramer's test for post hoc multiple comparisons. *, p < 0.05; n.s., no significance
fMRI processing
For each participant, we applied a conventionally used preprocessing pipeline, which was performed utilizing custom scripts in MATLAB 2020a (MathWorks, Natick, MA) and SPM12 (http://www.fil.ion.ucl.ac.uk/spm/). The pipeline involved following steps: (1) flipping the anatomical and functional images about the mid-sagittal line for stroke patients with lesions on right hemisphere, (2) removal of the first 10s to achieve steady-state magnetization, (3) slice-timing correction, (4) realignment for subsequent de-spiking processing, (5) normalization to Montreal Neurological Institute (MNI-152) standard space (2 mm isotropic resampling resolution), and (6) spatial smoothing with a 6-mm isotropic gauss kernel. Furthermore, blood-oxygen-level-dependent (BOLD) signals were regressed by “6 rp + 6 Δrp” nuisance signals to minimize the motion effects, in which “6 rp + 6 Δrp” signals represented 6 realignment parameters and their 1st-order first derivatives. Then, a high pass filter (1/128 Hz) was applied to whole brain BOLD signals.
General linear model (GLM)-based statistical analysis was conducted for individual EPI scans, i.e., first level analysis. The periods of healthy and lesion hand movement were set as two predictors, respectively. For the group-wise analysis, the one-sample t-test and paired t-test were conducted to generate the activation maps from “healthy or lesion head movement” (Fig. 3B,C) and “healthy v.s. lesion hand movent” (Fig. 3E), respectively. All BOLD activation maps were shown with FDR corrected p < 0.05.
Granger causality analysis across human functional networks
As the HRF variability distributed across the human brain could alter causal connections estimates from fMRI, thus we introduced a hemodynamic deconvolution approach [44] which used the linear time invariant system to model the relationship between neural event and BOLD response. The hemodynamic response represents such dynamic process; the resting-state BOLD signal in a given voxel (or region) at time , satisfies the following equation:
where is the hemodynamic response function, is the measurement noise which is uncorrelated with the unknown neural activity , and is the number of time bins spanning the desired length of the HRF.
Then, the estimated neural activity was used to estimate directed inter-network interactions using the multivariate Granger Causality analysis. For both healthy and lesion hand movement, we only focused on the neural activity during the “8 s rest – 20 s hand grasp – 20 s rest” period (Fig. 5A,B), so that the interaction period of alternating hand grasps was excluded.
Fig. 5.
Multi-FDBK-BCI therapy strengthened the functional compensation of ipsi-lateral motor cortex through transmodal regions for chronic stroke patients. A Computational pipeline of granger causality changes across human functional networks between post- and pre- multi-FDBK-BCI therapy for chronic stroke patients’ impaired hand movement. B Significant difference of granger causalities between post- and pre- Multi-FDBK-BCI therapy for chronic stroke patients’ lesion hand movements (paired t-test, two tails). C Mechanism summary of motor function recovery for chronic stroke patients after multi-FDBK-BCI therapy. For impaired hand movement, multi-FDBK-BCI therapy enhanced the information interaction among transmodal regions and thus achieved the motor function compensation of ipsilateral motor cortex
Statistics
For the clinical phenotype, data were analyzed using the SPSS 20.0. Quantitative data conforming to a normal distribution were expressed as mean ± standard deviation, while those not conforming to a normal distribution were represented using P50 (P25, P75). Count data were represented by frequency and percentage. For quantitative data conforming to a normal distribution, independent samples t-tests were used for group comparisons, and paired samples t-tests were used for pre- and post-intervention comparisons (Fig. 2B–D). Effect sizes were calculated using Hedges’ g, which corrects for small sample bias, with 95% confidence intervals. Hedges’ g values of 0.2, 0.5, and 0.8 were interpreted as small, medium, and large effect sizes, respectively. For groups not conforming to a normal distribution, the Mann-Whitney U test was used for comparisons between groups (Fig. 2E), and the non-parameter Dunn test was used for pre- and post-intervention comparisons (Supplementary Figure 2). The chi-square test was used for count data. The level of significance was set at α = 0.05.
For the fMRI results, statistical analyses were performed based on Matlab (Mathworks, Natick, MA). We used SPM12 (http://www.fil.ion.ucl.ac.uk/spm/) to calculate the three-dimensional BOLD activation maps by paired Student’s t test for comparisons between pre- and post- Multi-FDBK therapy. Statistical significance of ROI-wise results was determined by one-way ANOVA with Tukey-Kramer’s test for comparisons among multiple groups (Fig. 3E). Correlation between BOLD activations and behaviors was tested by Pearson’s correlation. Statistical details, including p values and sample numbers, were described in the relevant methods sections or figure legends.
Results
Clinical outcomes
Multi-FDBK-BCI promotes the recovery of upper limb motor function in chronic stroke patients
To further investigate the efficacy of the Multi-FDBK-BCI in improving upper limb motor function in chronic stroke patients, we designed a study to compare the behavioral, neuroelectrophysilogical, and imaging changes after Multi-FDBK-BCI training and the classic motor imagery training (Fig. 1A, Additional File 1: Figure S1). We screened 594 stroke patients and ultimately included 39 chronic stroke patients with severe unilateral upper limb motor impairment who met the inclusion criteria (Fig. 1B,C, Additional File 1: Table S1). These patients were randomly assigned to either the Multi-FDBK-BCI group (N = 20) or the CMI group (N = 19). All the 39 participants finished all the interventions and assessments without side effects. Both groups underwent comprehensive rehabilitation training. Notably, participants in the Multi-FDBK-BCI group received motor imagery-based brain-computer interface training combined with multi-sensory feedback (light touch, proprioception, and visual feedback), while participants in the CMI group received classical motor imagery training under the guidance of a therapist (Fig. 2A).
Kolmogorov-Smirnov test was used to test the normality of the behavior scores. Levene test was used to check the equal variance. At baseline, there were no significant differences between the two groups in upper limb motor function (Fugl-Meyer Motor Score Assessment and Motor Status Scale), upper limb sensory assessment (Fugl-Meyer Sensory Score), practical function of upper limb (ARAT), and the average amplitude of the wrist extensor muscles (P > 0.05) (Fig. 1C). After the intervention, the Fugl-Meyer Motor Score Assessment (FMA) score of the paretic upper limb was 18.30 ± 6.91 in the Multi-FDBK-BCI group and 15.89 ± 6.33 in the CMI group (p = 0.015), the MSS score of the paretic upper limb was 21.69 ± 10.24 in the Multi-FDBK-BCI group and 21.94 ± 9.94 in the CMI group (p = 0.046), and the average amplitude(μV) of the wrist extensor muscles was 6.11 ± 4.01 in the Multi-FDBK-BCI group and 5.53 ± 3.83 in the CMI group (p = 0.037).
We compared the pre- and post-intervention differences and found that improvements were more pronounced in the Multi-FDBK-BCI group (Fig. 2B–D). Specifically, the Multi-FDBK-BCI group demonstrated significantly greater improvement in FMA scores compared to the CMI group (mean difference: 4.40 ± 3.30 vs. 2.26 ± 1.63, p = 0.015), with a large effect size (Hedges’ g = 0.80, 95% CI [0.16, 1.44]). Similarly, the increase in average sEMG amplitude of wrist extensor muscles was significantly higher in the Multi-FDBK-BCI group than in the CMI group (2.41 ± 1.92 μV vs. 1.25 ± 1.36 μV, p = 0.037), demonstrating a medium-to-large effect size (Hedges’ g = 0.68, 95% CI [0.05, 1.31]). For MSS scores, the Multi-FDBK-BCI group also exhibited greater improvements compared to the CMI group (3.23 ± 2.45 vs. 1.78 ± 1.89, p = 0.046), with a medium-to-large effect size (Hedges’ g = 0.65, 95% CI [0.02, 1.28]). However, no significant differences were observed between the groups in upper limb sensory assessment and ARAT after the intervention (p = 0.304) (Fig. 2E, Additional File 1: Figure S2A).
Regarding paretic wrist range of motion, none of the participants were able to actively extend the affected wrist before the intervention. However, after the intervention, 7 participants in the Multi-FDBK-BCI group exhibited active wrist extension, while only 2 participants in the CMI group showed active wrist extension. All the participants have intact sensory function as assessed by the sensory portion of the FMA (Additional File 1: Figure S2B-C).
Concerning motor-evoked potential (MEP), no MEP could be elicited from the paretic side in any participants before the intervention. But after the intervention, MEP could be elicited from the paretic side in 7 participants in the Multi-FDBK-BCI group, whereas only 2 participants in the CMI group demonstrated elicitable MEP on the paretic side (Additional File 1: Figure S2D). This difference in MEP recovery represents a significant neurophysiological finding, as MEPs are direct indicators of corticospinal tract integrity and motor cortex excitability. The absence of MEPs at baseline in all participants indicates severe disruption of the descending motor pathways following stroke. The emergence of MEPs in 35% (7/20) of participants in the Multi-FDBK-BCI group compared to only 10.5% (2/19) in the CMI group suggests that the Multi-FDBK-BCI intervention more effectively facilitates reestablishment of functional connections between the motor cortex and spinal motor neurons. This neurophysiological improvement aligns with the observed clinical motor function gains and provides objective evidence for enhanced neural plasticity following Multi-FDBK-BCI therapy.
Neuroimaging findings
Multi-FDBK-BCI therapy enhances BOLD activations evoked by impaired hand movement
To gain a deeper understanding of the potential neural mechanisms underlying motor functional recovery in chronic stroke patients undergoing the Multi-FDBK-BCI therapy, we employed 3T fMRI to examine BOLD activity elicited by movements of both healthy and impaired hands (Fig. 3A). Our results indicated that movements of the healthy hand did not evoke significant changes in BOLD activations between the pre- and post-therapy assessments (Fig. 3B). However, following the Multi-FDBK-BCI therapy, movements of the impaired hand elicited significant changes in BOLD activations compared to the baseline results. These changes were observed in bilateral motor regions as well as high-order brain regions in both hemispheres. Specifically, we noted significant activations in the dorsal attention network, ventral attention network, frontoparietal network, and the default mode network both in the normal and lesion hemispheres (Fig. 3C,D). Additionally, we conducted a quantitative evaluation of functional activations elicited by movements of the healthy and impaired hands using a paired t-test (Fig. 3E). The analysis revealed significantly higher activations across the bilateral hemispheric cortices in response to movements of the impaired hand post-therapy compared to baseline measurements (Fig. 3E). These findings suggest that the recovery of motor function in chronic stroke patients after Multi-FDBK-BCI therapy may involve the engagement of multiple brain regions, including the frontoparietal, attention, and default mode networks. This multimodal brain region involvement highlights the complex neural adaptations contributing to the observed improvements in motor function, providing novel insights into the neuroplastic changes associated with motor recovery in chronic stroke patients undergoing the Multi-FDBK-BCI therapy.
Increased BOLD activations predicts improved clinical outcomes following Multi-FDBK-BCI therapy
To investigate whether the observed motor function recovery (Fig. 2) is related to the changes in BOLD activation between the pre- and post-Multi-FDBK-BCI training (Fig. 3), we calculated Pearson’s correlation coefficients between the changes in patients’ motor function performance and the functional activations in the motor cortex and high-order cerebral networks (Fig. 4A). Our analysis revealed significant correlations between behavioral measures, such as sEMG, FMA, and MSS, and the changes in fMRI activations within the human motor cortex and multimodal functional networks. However, no significant correlation was found between changes in the ARAT scores and the fMRI activation changes after the Multi-FDBK-BCI training. Notably, the changes in sEMG showed the highest correlations with BOLD activation changes compared to the other behavioral measures, including FMA, MSS, and ARAT (Fig. 4B–G, Additional File 1: Figure S3A-B). We speculate that this phenomenon may be attributable to the objective and unbiased nature of sEMG in evaluating motor function recovery in chronic stroke patients. In contrast, the FMA, MSS, and ARAT assessments highly rely on clinical judgment, which might introduce subjectivity. Furthermore, for chronic stroke patients, the changes in sEMG might serve as a potential biomarker for detecting incomplete or subtle motor function recovery, which might not be as readily apparent when using the commonly employed rating scales. This suggests that sEMG could provide a more sensitive measure for monitoring the nuanced progress of motor function recovery post-therapy.
Fig. 4.
Significant correlations between BOLD responses and motor function recovery after Multi-FDBK-BCI therapy for chronic stroke patients. A Correlation between impaired hand movements evoked BOLD responses and behavioral changes after Multi-FDBK-BCI therapy. Red lines were scaled according to the Pearson’s correlation coefficients between behavioral (upper) and BOLD signal (lower) changes with threshold p < 0.05. B-G Scatter plot for BOLD signal and sEMG changes (post- v.s. pre- Multi-FDBK-BCI therapy). Each dot indicated an individual chronic stroke patient (N = 20). The red lines represented the best linear fit. Scatter plots for BOLD signal and FMA (or MSS) changes were shown in Supplementary Figure 3
Information interactions among transmodal regions supports motor function recovery
Having demonstrated that changes in BOLD signals show significant correlations with behavioral changes in motor functions for chronic stroke patients, we next focused on understanding the involvement of human multimodal brain regions during motor function recovery. First, we selected the BOLD time series recorded during impaired hand movements, which included an 8-s rest period, a 20-s impaired hand movement period, and a subsequent 20-s rest period (Fig. 5A, upper left). Then, these BOLD signals were deconvolved using the human hemodynamic response function (HRF) to obtain the speculated neuronal activities evoked by hand movements across bilateral motor cortices, as well as the default mode, frontoparietal, dorsal attention, and ventral attention networks. Next, we applied Granger causality analysis to these speculated neuronal activities (Fig. 5A, right). This analysis allowed us to assess the directional influence of one brain region on another, thereby elucidating the causal relationships between different brain networks. Finally, we evaluated the differences in Granger causality evoked by impaired hand movements before and after Multi-FDBK-BCI training using a two-sample t-test (Fig. 5A, lower left).
Our results showed that, following Multi-FDBK-BCI training, impaired hand movements induced significantly higher causal influences from the motor cortex in the lesion hemisphere to multimodal regions, and ultimately to the ipsilateral motor cortex in the contralesional hemisphere (Fig. 5B). In other words, Multi-FDBK-BCI training strengthened the functional compensation of the ipsilateral motor cortex through enhanced information interactions among transmodal regions in chronic stroke patients (Fig. 5C). These findings suggest that the recovery of motor function after Multi-FDBK-BCI training is mediated by complex neural adaptations involving multiple brain networks. The strengthened causal interactions among these networks highlight the potential mechanisms through which this therapy facilitates motor function recovery.
Enhanced motor-to-multimodal information interaction predicts motor function recovery after Multi-FDBK-BCI therapy
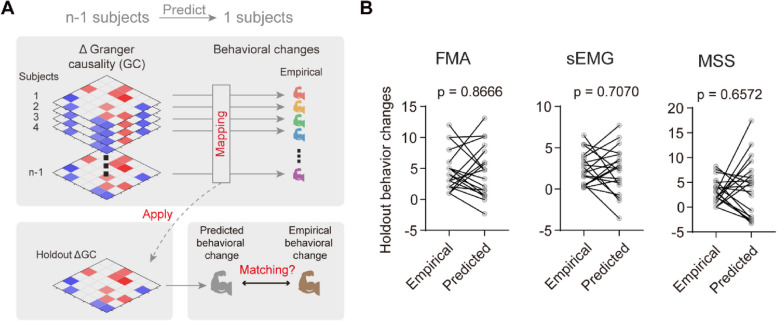
The above results of Granger causality analysis were derived from group data, which unfortunately precludes the ability to disentangle the contributions of individual patients. In contrast, subject-level predictions hold significant clinical importance, offering the potential to predict motor function recovery following multiple neurofeedback training sessions and other interventions. Our objective was to determine whether the differences in Granger causalities, referred to as ΔGranger causalities, are generalizable to the level of an individual patient.
To achieve this goal, we tested the robustness of the group-level results using a “leave-one-subject-out” procedure (Fig. 6A). In this approach, we estimated behavioral changes based on the ΔGranger causalities from n-1 patients. We then conducted linear fitting using these data and employed the resulting coefficients to predict the behavioral change of the one left-out patient (Fig. 6A). This procedure was repeated for each patient, systematically holding out each one in turn, and we found no significant difference between the predicted and empirical behavioral changes, including those measured by FMA, sEMG, and MSS (Fig. 6B). Remarkably, using n-1 subjects to fit the model parameters and predict the left-out patient’s sEMG changes ensured that the optimal parameters were not unduly biased by any single subject.
Fig. 6.
Δ Granger causality had utility in predicting subject-level motor function recovery. A Schematic illustrating the ‘leave-one-subject-out’ procedure for predicting single-patient behavioral changes of motor function. Models were fit using the Δ Granger causality and behavioral changes from n-1 subjects to predict the behavioral change for the remaining 1 subject. B No significant difference between empirical and model predicted behavioral changes, illustrating the robustness of the model. N = 20 subjects; paired t-test, two tails. FMA, Fugl-Meyer assessment; sEMG, surface electromyography; MSS, motor status scale
Consequently, our results (Fig. 6B) revealed that, generally, we could predict individual patients’ recovery of motor function. These findings support our conclusion that Multi-FDBK-BCI training enhances the functional compensation of the ipsilateral motor cortex through interactions among transmodal regions. Furthermore, our findings suggest that the ΔGranger causalities could potentially serve as a non-invasive neuroimaging biomarker to guide clinical decision-making. This approach opens new avenues for personalized medicine, where individual predictions can inform tailored therapeutic strategies for motor function recovery in chronic stroke patients.
Summary
Taken together, our results suggested that Multi-FDBK-BCI therapy promoted motor recovery in chronic stroke patients by driving neuroplasticity across bilateral motor and high-order brain networks. Functional MRI showed increased BOLD activations during impaired hand movements post-therapy, involving attention, frontoparietal, and default mode networks, correlating with improved clinical outcomes (e.g., sEMG). Furthermore, the granger causality analysis demonstrated enhanced information flow from the lesioned motor cortex to transmodal regions, facilitating ipsilateral compensation. Critically, individual-level predictions confirmed that ΔGranger causalities robustly predicted motor recovery, suggesting their utility as biomarkers. Therefore, these findings suggested that Multi-FDBK-BCI harnessed network-level plasticity to restore function, offering insights for personalized rehabilitation.
Discussion
Main findings
Our study found that Multi-FDBK-BCI training could better improve upper limb motor function in chronic stroke patients with severe motor impairments compared to classical motor imagery training. This improvement was mediated by the activation of high-order brain networks (frontoparietal, dorsal/ventral attention, and default mode networks) and enhanced information exchange between these networks, which served as a bridge connecting bilateral motor cortices and compensating for the function of lesioned hemisphere.
Multi-FDBK-BCI system design
The Multi-FDBK-BCI training system monitors the patient's real-time mental imagery process and provides immediate multisensory feedback, including visual, tactile, and spatial changes, upon detecting the intention to mentally imagine movement. Throughout this process, we posit that real-time multisensory feedback plays a pivotal role in enhancing upper limb motor function. Feedback from sensory information can modulate motor control, and the analysis and integration of sensory information can be utilized to facilitate the execution of motor plans [45]. Multimodal sensory stimulation has the capacity to elicit purposeful autonomous movements [46], as sensory input can prompt changes in the motor cortex [47]. Peripheral sensory stimulation not only activates sensory input pathways but also triggers motor descending pathways through direct projections from the sensory cortex to the motor cortex [48]. This highlights the intricate relationship between sensory processing and motor function and underscores the potential for leveraging sensory feedback to enhance motor control and learning. However, in patients with chronic stroke, there is often abnormal sensorimotor integration, which correlates with impairment in motor function [49].
Our multi-sensory feedback, combined with an active motor imagery strategy, can simultaneously engage both sensory and motor brain regions. This coordinated activation likely strengthens the neural connections between these areas through a well-established learning principle called Hebbian plasticity—essentially, “neurons that fire together, wire together.” The repeated pairing of imagined movement with realistic sensory feedback helps rebuild communication pathways between brain regions [50, 51]. In our study, this intervention led to simultaneous activation of both sides of the motor cortex and higher-order networks during movement of the affected upper limb, suggesting that Multi-FDBK-BCI therapy can enhance neural plasticity and better integrate sensory-motor information during movement of the affected upper limb in chronic stroke patients. This enhanced neural plasticity and sensory-motor integration may be a key factor contributing to improved motor function in chronic stroke patients.
Comparative advantages of Multi-FDBK-BCI for clinical rehabilitation
Currently, non-invasive MI-BCI training is increasingly being utilized for rehabilitation intervention in stroke patients. During MI-BCI training, patients can receive proprioceptive, tactile, visual, and other sensory feedback through various external effectors, such as functional electrical stimulation, virtual reality feedback, exoskeleton robots, and vibrotactile feedback [52–55]. This approach effectively activates motor networks in the brain and facilitates the restoration of motor function [56, 57]. Previous clinical research indicates that MI-BCI training has a more pronounced impact on stroke patients in the early post-stroke period compared to those in the chronic phase [17, 58–61]. It is noteworthy that most existing MI-BCI training systems provide single sensory feedback, with only a limited number integrating two types of sensory feedback. Moreover, their application is primarily restricted to healthy subjects, with fewer studies focusing on its effectiveness for chronic stroke patients [34, 35]. In contrast, our Multi-FDBK-BCI training combines tactile, proprioceptive, and visual feedback stimuli simultaneously to activate multiple regions through different ascending pathways.
Although multi-sensory stimulation therapy itself can effectively improve motor function in paralyzed upper limbs of stroke patients, previous studies have primarily focused on chronic stroke patients with relatively high baseline function (FMA = 25.0 ± 14.6) [16]. When MI-BCI treatment was applied to subacute patients with poorer function (ARAT < 13), it improved corticospinal tract integrity but showed no significant difference in ARAT scores compared to control groups [20]. For chronic patients with better baseline function (FMA = 30.5 [17.0; 41.0]), MI-BCI treatment demonstrated significant motor improvements with an average FMA increase of 7.5, which exceeds our reported 4.4 improvement [30]. However, patients in those studies had substantially better baseline upper limb function than participants in our study (FMA = 13.9 ± 6.97). Our research specifically addresses this gap by providing a feasible rehabilitation approach for patients with relatively poor baseline upper limb function—a population often overlooked in previous BCI intervention studies.
Furthermore, the sensory feedback is induced by the patient’s active movement imagination, which enhances the realism and naturalness of their motor perception. By engaging in mental imagery of the desired movement, patients can trigger multisensory feedback that closely mimics the sensory consequences of real-world actions. This congruence between the imagined movement and the resulting feedback creates a more immersive and intuitive experience for the patient, potentially facilitating a stronger connection between the intended action and the corresponding neural activity. This multisensory feedback can increase β frequency energy in the corresponding brain regions, reduce cognitive fatigue, and fully engage the patients’ motivation during training [62, 63]. The real-time multisensory feedback induced by repeated intentional motor imagery serves to enhance sensory-motor interactions and stimulate brain plasticity for restoring upper limb motor function.
Neuroplastic mechanisms revealed by neuroimaging
After undergoing Multi-FDBK-BCI training, the patients demonstrated movement in the affected hand, accompanied by characteristic bilateral activation of the motor cortex. Additionally, significant activation was observed in many transmodal regions, also known as high-order brain network, including the frontoparietal network, dorsolateral attention network, ventral attention network, and the default mode network.
The frontoparietal networks serves as a critical hub for motor control and sensory integration. It helps plan and execute movements while integrating information from visual, touch, prefrontal, and auditory inputs [64], which enables better control of upper limb movements [65]. The ventral attention network primarily participates in directing attention and responding to novel stimuli, while the dorsal attention network is linked to sustained attention and processing goal-directed tasks. Furthermore, ventral and dorsal attention network are involved in processing sensory feedback (such as visual and tactile feedback) into motor commands to enhance motor control ability [66]. Although the default mode network primarily involves regions such as the medial prefrontal cortex, posterior cingulate cortex, and hippocampus, its support for overall brain function and neural networks is crucial for functional recovery in stroke patients [67]. Reconstructing or restoring the default mode network may facilitate the integration of functional connections between damaged and healthy brain regions to promote brain plasticity. Additionally, it also helps stroke patients learn new motor skills and strategies to compensate for impaired functions. Activation of these high-order brain networks can significantly contribute to sensory-motor integration [68], thus leading to improved restoration of upper limb motor function after stroke [69].
Our causality analysis and model predictions indicate that information transfer from the lesion motor cortex to the unaffected motor cortex, mediated by multiple high-order brain networks, is closely associated with motor function recovery in chronic stroke patients. All participants had severe unilateral upper limb motor dysfunction with significant brain damage, leaving their affected hemisphere unable to adequately support recovery alone. According to Di Pino’s Bimodal Balance Recovery theory [70], when brain damage is extensive, the affected hemisphere cannot sufficiently support recovery, requiring assistance from the unaffected hemisphere. Consequently, motor-related information originating from the affected hemisphere is transmitted through high-order brain networks to activate the unaffected motor cortex. This activation promotes further functional recovery through compensation by the contralateral hemisphere. The fMRI results confirm that compensatory mechanisms within the unaffected hemisphere play a crucial role in promoting functional recovery among chronic stroke patients. Enhancing multisensory stimulation can facilitate information exchange between affected and unaffected areas while fostering bilateral cooperation to improve overall motor function.
Previous EEG studies have found that motor imagery BCIs can enhance functional connectivity between different sensory and motor areas in the lesion hemisphere at the β frequency bands [17, 28]. Similarly, fMRI studies have shown that motor imagery BCIs can enhance functional connectivity between both hemispheres, with stronger connections correlating with better motor recovery [71]. Sensory feedback training, such as visual feedback provided during BCI interventions, can also enhance functional connectivity between the bilateral sensorimotor cortices [72]. However, there have been fewer studies investigating the specific connections between the bilateral motor cortices. Previous research has shown that stronger connections between the sensorimotor cortex, subcortical structures, and higher-order networks correlate with better recovery in subcortical stroke patients [73]. Furthermore, this functional connectivity can be used to predict future motor function in subcortical stroke patients treated with motor imagery BCIs. The majority of our enrolled patients exhibited subcortical damage—15 of 20 patients in the Multi-FDBK-BCI group and 15 of 19 in the classic motor imagery group had pure subcortical damage. Despite having relatively intact motor cortices that could generate movement commands, damage to subcortical pathways prevented sensory feedback from reaching the cortex and movement commands from reaching the spinal cord. In this case, if the movement-related information from the lesion hemisphere is directly transmitted to the contralateral cortex via interhemispheric connections, the healthy side cannot receive enough sensory feedback from the affected upper limb and cannot integrate and process the movement commands of the lesion hemisphere well, making it difficult to effectively control the impaired arm.
Multi-FDBK-BCI training addresses this challenge by establishing new connections between the cortex and transmodal regions. This allows movement-related information from the lesion motor cortex to be integrated with sensory feedback from the affected upper limb in the transmodal regions and then transmitted to the motor cortex in the healthy side. With the help of the ipsilateral motor descending pathway in the healthy hemisphere, the affected upper limb can be effectively controlled, achieving functional recovery. This conceptual framework provides insights into our causal inference results: direct causality from the ipsilesional motor cortex to the contralesional motor cortex after Multi-FDBK-BCI training is reduced, but causality from the ipsilesional motor cortex through multiple high-order brain networks to the contralesional motor cortex is enhanced. Our investigation sheds light on significance attributed toward functional linkage originating at lesion motor cortex traversing through higher-order networks culminating at healthy motor cortex during post-stroke rehabilitation phase. These alterations observed within functional connectivity patterns hold potential as predictive biomarkers indicative toward therapeutic efficacy associated with Multi-FDBK-BCI interventions.
Limitations of the study
There are several important limitations to this study. Firstly, the sample size in our study was relatively small, which may limit the statistical power and generalizability of our findings. Secondly, assessments were only conducted before and immediately after the intervention, without long-term follow-up. This prevents us from determining whether the therapeutic effects were maintained over time. Additionally, the relatively short intervention period resulted in functional improvements that, while statistically superior in the Multi-FDBK-BCI group compared to the control group, were somewhat limited in magnitude. Furthermore, this study only included patients with severe upper limb motor dysfunction. Future research should compare the efficacy of Multi-FDBK-BCI therapy across stroke patients with varying degrees of motor impairment to identify which patient populations might benefit most from this intervention approach.
Due to the use of different rehabilitation strategies, the experiment could not be fully double-blinded. However, the evaluators and rehabilitation therapists were unaware of each other’s roles, ensuring independent and reliable evaluation results. Additionally, the MRI analysis was conducted independently from the evaluators, ensuring the reliability of the relationships between the recovery of function in the affected upper limb and high-order brain network activation, as well as the recovery of function in the affected upper limb and changes in functional connectivity.
This study primarily investigated brain regions activated during upper limb movement using fMRI, as well as the strength of functional connectivity and causality between different brain regions; however, it did not assess structural connectivity. Future studies may utilize diffusion tensor imaging (DTI) and other techniques to explore changes in fiber connections between the ipsilesional motor cortex and transmodal areas, among different transmodal areas, as well as between transmodal areas and the contralesional motor cortex following Multi-FDBK-BCI training.
Lastly, patients experienced head movement during fMRI evaluations, which may have introduced noise or artifacts into the imaging data. Although standard preprocessing steps, such as motion correction, were likely applied to minimize the impact of head movement on the results, it is essential to acknowledge this limitation and consider implementing additional strategies to reduce head motion during fMRI acquisitions in future studies.
Clinical implications and future research
Multi-FDBK-BCI therapy, as explored in this study, presents a potential new approach for enhancing upper limb motor function in individuals with chronic stroke. The intervention appears capable of facilitating improvements possibly linked to neuroplastic changes within the brain. Furthermore, investigating the neural mechanisms engaged by this therapy may help identify targets for complementary neuromodulation strategies, potentially refining ways to optimize rehabilitation outcomes. Building upon the current findings, future research should prioritize several key areas to further validate and refine Multi-FDBK-BCI therapy.
Longitudinal studies incorporating larger, more diverse patient cohorts are crucial to rigorously assess the long-term sustainability of therapeutic effects and enhance the generalizability of the findings. Furthermore, comparative effectiveness studies across distinct stroke populations (differentiated by lesion location, stroke type, severity, and chronicity) are warranted to delineate specific patient profiles most likely to respond favorably to this intervention. Critically, the inclusion of an active control group performing overt motor execution, rather than motor imagery alone, would help elucidate the unique contributions of the imagery-based BCI approach relative to direct motor practice.
From a translational perspective, the observed enhancement of activation and connectivity within higher-order cortical networks suggests promising avenues for synergistic interventions. Investigating the combination of Multi-FDBK-BCI therapy with cognitive-enhancing pharmacotherapies, or with neuromodulatory techniques such as pre-intervention repetitive transcranial magnetic stimulation (rTMS) aimed at priming relevant cognitive circuits, could potentially augment therapeutic outcomes. Finally, adapting the Multi-FDBK-BCI system for telerehabilitation represents a significant opportunity to broaden clinical accessibility. Real-time, remote electroencephalography (EEG) monitoring during home-based motor imagery practice, coupled with the delivery of multisensory feedback, could facilitate upper limb motor recovery while overcoming geographical and mobility barriers, thereby expanding the reach of specialized neurorehabilitation services.
Conclusions
To conclude, our study findings indicate that Multi-FDBK-BCI training enhances the motor function of chronic stroke patients who struggle to regain function through traditional rehabilitation. This improvement is associated with heightened information exchange between the bilateral motor cortices and high-order brain networks, with compensation from the contralateral hemisphere playing a pivotal role in this process. These findings have important implications for the refinement of existing non-invasive neural modulation protocols. By developing a bidirectional closed-loop BCI training system and optimizing multi-sensory feedback strategies, researchers, and clinicians can target the specific neural mechanisms identified in this study to promote greater plasticity in the injured brain and facilitate improved functional recovery for stroke patients. Future research should focus on further elucidating the complex interactions between various brain regions and networks in the context of motor recovery and investigating the long-term effects of Multi-FDBK-BCI training on neural reorganization and functional outcomes. By building upon the insights gained from this study, we can advance the development of more targeted and effective neurorehabilitation interventions that maximize the potential for recovery in individuals with chronic stroke and severe motor impairments.
Supplementary Information
Additional file 1: Figures S1-S3 and Tables S1. Figure S1-Demonstration of Multi-FDBK-BCI therapy. Figure S2-Behavioral Assessment and MEP for chronic stroke patients with Multi-FDBK-BCI therapy and CMI therapy. Figure S3-Correlations between BOLD signal changes and FMA (A) or MSS (B) changes for chronic stroke patients. Table S1-Patients’ characteristics, including lesion etiology.
Acknowledgements
We would like to express our sincere gratitude to Dr. Jie Wang for his valuable assistance in functional magnetic resonance imaging (fMRI) data analysis, to Dr. Shenghua Zhong from Shenzhen University for her expertise and assistance in electroencephalogram (EEG) data analysis, and to Dr. Guanghao Sun for his constructive guidance in manuscript preparation and revision.
Abbreviations
- Multi-FDBK-BCI
Multi-modal sensory feedback brain-computer interface
- MI-BCI
Motor imagery brain-computer interfaces
- FES
Functional electrical stimulation
- MI
Motor imagery
- fMRI
Functional magnetic resonance imaging
- CMI
Classical motor imagery
- VR
Virtual reality
- FMA-UE
Fugl-Meyer motor score assessment-upper extremity
- CPM
Continuous passive motion
- ERS
Even-related synchronization
- PSD
Power spectrum density
- MSS
Motor status scale
- ARAT
Action research arm test
- sEMG
Surface electromyography
- MEP
Motor evoked potential
- EMG
Electromyography
- TR
Repetition time
- TE
Echo time
- TI
Inversion time
- EPI
Echo planar imaging
- BOLD
Blood-oxygen-level-dependent
- GLM
General linear model
- FMA
Fugl-Meyer motor score assessment
- HRF
Hemodynamic response function
- DTI
Diffusion tensor imaging
- rTMS
Repetitive transcranial magnetic stimulation
- EEG
Electroencephalography
Authors' contributions
Conceptualization: Zhengrun Gao, Rongrong Lu. BCI intervention: Rongrong Lu, Tianhao Gao. Rehabilitative function assessment: Zhijie He. Rehabilitative training: Yiqian Hu. fMRI detection: Jie Zhuang. Writing—original draft: Rongrong Lu, Zhen Pang, Qin Zhang. Writing—review and editing: Zhengrun Gao.
Funding
This work was supported by the National Science Foundation of China (82271422, 82372570, T2394534), Shanghai Natural Science Foundation (22ZR1479000), Shanghai Municipal Health Commission (20234Y0043), and Medical Innovation Research Project funded by Shanghai Science and Technology Commission (23Y11900900).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Huashan Hospital (2017-016). All patients provided informed consent prior to participating in the trial. The study adhered to the principles of the declaration of Helsinki and was prospectively registered in a publicly accessible clinical trials registry (https://www.chictr.org.cn/, identifier: ChiCTR-ONC-17010739).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rongrong Lu and Zhen Pang these authors contributed equally to this work.
References
- 1.Collaborators GBDS. Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021;20(10):795–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.King D, Wittenberg R, Patel A, Quayyum Z, Berdunov V, Knapp M. The future incidence, prevalence and costs of stroke in the UK. Age Ageing. 2020;49(2):277–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duncan PW, Bushnell C, Sissine M, Coleman S, Lutz BJ, Johnson AM, Radman M, Pvru Bettger J, Zorowitz RD, Stein J. Comprehensive stroke care and outcomes: time for a paradigm shift. Stroke. 2021;52(1):385–93. [DOI] [PubMed] [Google Scholar]
- 4.Hatem SM, Saussez G, Della Faille M, Prist V, Zhang X, Dispa D, Bleyenheuft Y. Rehabilitation of motor function after stroke: a multiple systematic review focused on techniques to stimulate upper extremity recovery. Front Hum Neurosci. 2016;10:442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kajrolkar T, Yang F, Pai YC, Bhatt T. Dynamic stability and compensatory stepping responses during anterior gait-slip perturbations in people with chronic hemiparetic stroke. J Biomech. 2014;47(11):2751–8. [DOI] [PubMed] [Google Scholar]
- 6.Nikamp CDM, Hobbelink MSH, van der Palen J, Hermens HJ, Rietman JS, Buurke JH. A randomized controlled trial on providing ankle-foot orthoses in patients with (sub-)acute stroke: Short-term kinematic and spatiotemporal effects and effects of timing. Gait Posture. 2017;55:15–22. [DOI] [PubMed] [Google Scholar]
- 7.Li X, Lin YL, Cunningham DA, Wolf SL, Sakaie K, Conforto AB, Machado AG, Mohan A, O’Laughlin K, Wang X, et al. Repetitive transcranial magnetic stimulation of the contralesional dorsal premotor cortex for upper extremity motor improvement in severe stroke: study protocol for a pilot randomized clinical trial. Cerebrovasc Dis. 2022;51(5):557–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stinear CM, Lang CE, Zeiler S, Byblow WD. Advances and challenges in stroke rehabilitation. Lancet Neurol. 2020;19(4):348–60. [DOI] [PubMed] [Google Scholar]
- 9.Bernhardt J, Hayward KS, Kwakkel G, Ward NS, Wolf SL, Borschmann K, Krakauer JW, Boyd LA, Carmichael ST, Corbett D, et al. Agreed definitions and a shared vision for new standards in stroke recovery research: the Stroke Recovery and Rehabilitation Roundtable Taskforce. Neurorehabil Neural Repair. 2017;31(9):793–9. [DOI] [PubMed] [Google Scholar]
- 10.Shadmehr R, Smith MA, Krakauer JW. Error correction, sensory prediction, and adaptation in motor control. Annu Rev Neurosci. 2010;33:89–108. [DOI] [PubMed] [Google Scholar]
- 11.Ingemanson ML, Rowe JR, Chan V, Wolbrecht ET, Reinkensmeyer DJ, Cramer SC. Somatosensory system integrity explains differences in treatment response after stroke. Neurology. 2019;92(10):e1098–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mrachacz-Kersting N, Kristensen SR, Niazi IK, Farina D. Precise temporal association between cortical potentials evoked by motor imagination and afference induces cortical plasticity. J Physiol-London. 2012;590(7):1669–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pinardi M, Longo MR, Formica D, Strbac M, Mehring C, Burdet E, Di Pino G. Impact of supplementary sensory feedback on the control and embodiment in human movement augmentation. Commun Eng. 2023;2(1):64. [Google Scholar]
- 14.Ding L, Wang X, Guo X, Chen S, Wang H, Jiang N, Jia J. Camera-based mirror visual feedback: potential to improve motor preparation in stroke patients. IEEE Trans Neural Syst Rehabil Eng. 2018;26(9):1897–905. [DOI] [PubMed] [Google Scholar]
- 15.Kitai K, Odagiri M, Yamauchi R, Kodama T. Evaluation of intervention effectiveness of sensory compensatory training with tactile discrimination feedback on sensorimotor dysfunction of the hand after stroke. Brain Sci. 2021;11(10):1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He J, Li C, Lin J, Shu B, Ye B, Wang J, Lin Y, Jia J. Proprioceptive training with visual feedback improves upper limb function in stroke patients: a pilot study. Neural Plast. 2022;2022:1588090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Biasiucci A, Leeb R, Iturrate I, Perdikis S, Al-Khodairy A, Corbet T, Schnider A, Schmidlin T, Zhang H, Bassolino M, et al. Brain-actuated functional electrical stimulation elicits lasting arm motor recovery after stroke. Nat Commun. 2018;9(1):2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guggenberger R, Heringhaus M, Gharabaghi A. Brain-machine neurofeedback: robotics or electrical stimulation? Front Bioeng Biotechnol. 2020;8:639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang R, Feng S, Hu N, Low S, Li M, Chen X, Cui H. Hybrid brain-computer interface controlled soft robotic glove for stroke rehabilitation. IEEE J Biomed Health Inform. 2024;28(7):4194–203. [DOI] [PubMed] [Google Scholar]
- 20.Brunner I, Lundquist CB, Pedersen AR, Spaich EG, Dosen S, Savic A. Brain computer interface training with motor imagery and functional electrical stimulation for patients with severe upper limb paresis after stroke: a randomized controlled pilot trial. J Neuroeng Rehabil. 2024;21(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lo YT, Lim MJR, Kok CY, Wang S, Blok SZ, Ang TY, Ng VYP, Rao JP, Chua KSG. Neural interface-based motor neuroprosthesis in poststroke upper limb neurorehabilitation: an individual patient data meta-analysis. Arch Phys Med Rehabil. 2024;105(12):2336–49. [DOI] [PubMed] [Google Scholar]
- 22.Naros G, Naros I, Grimm F, Ziemann U, Gharabaghi A. Reinforcement learning of self-regulated sensorimotor beta-oscillations improves motor performance. Neuroimage. 2016;134:142–52. [DOI] [PubMed] [Google Scholar]
- 23.Bauer R, Fels M, Vukelic M, Ziemann U, Gharabaghi A. Bridging the gap between motor imagery and motor execution with a brain-robot interface. Neuroimage. 2015;108:319–27. [DOI] [PubMed] [Google Scholar]
- 24.Kilteni K, Andersson BJ, Houborg C, Ehrsson HH. Motor imagery involves predicting the sensory consequences of the imagined movement. Nat Commun. 2018;9(1):1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boe S, Gionfriddo A, Kraeutner S, Tremblay A, Little G, Bardouille T. Laterality of brain activity during motor imagery is modulated by the provision of source level neurofeedback. Neuroimage. 2014;101:159–67. [DOI] [PubMed] [Google Scholar]
- 26.Khademi F, Naros G, Nicksirat A, Kraus D, Gharabaghi A. Rewiring cortico-muscular control in the healthy and poststroke human brain with proprioceptive β-band neurofeedback. J Neurosci. 2022;42(36):6861–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ang KK, Guan C, Chua KS, Ang BT, Kuah C, Wang C, Phua KS, Chin ZY, Zhang H. Clinical study of neurorehabilitation in stroke using EEG-based motor imagery brain-computer interface with robotic feedback. Annu Int Conf IEEE Eng Med Biol Soc. 2010;2010:5549–52. [DOI] [PubMed] [Google Scholar]
- 28.Pichiorri F, Morone G, Petti M, Toppi J, Pisotta I, Molinari M, Paolucci S, Inghilleri M, Astolfi L, Cincotti F, et al. Brain-computer interface boosts motor imagery practice during stroke recovery. Ann Neurol. 2015;77(5):851–65. [DOI] [PubMed] [Google Scholar]
- 29.Remsik AB, van Kan PLE, Gloe S, Gjini K, Williams L Jr, Nair V, Caldera K, Williams JC, Prabhakaran V. BCI-FES with multimodal feedback for motor recovery poststroke. Front Hum Neurosci. 2022;16:725715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frolov AA, Mokienko O, Lyukmanov R, Biryukova E, Kotov S, Turbina L, Nadareyshvily G, Bushkova Y. Post-stroke rehabilitation training with a motor-imagery-based Brain-Computer Interface (BCI)-controlled hand exoskeleton: a randomized controlled multicenter trial. Front Neurosci-Switz. 2017;11:400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kruse A, Suica Z, Taeymans J, Schuster-Amft C. Effect of brain-computer interface training based on non-invasive electroencephalography using motor imagery on functional recovery after stroke - a systematic review and meta-analysis. BMC Neurol. 2020;20(1):385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Corbet T, Iturrate I, Pereira M, Perdikis S, Millán JDR. Sensory threshold neuromuscular electrical stimulation fosters motor imagery performance. Neuroimage. 2018;176:268–76. [DOI] [PubMed] [Google Scholar]
- 33.Vukelić M, Gharabaghi A. Oscillatory entrainment of the motor cortical network during motor imagery is modulated by the feedback modality. Neuroimage. 2015;111:1–11. [DOI] [PubMed] [Google Scholar]
- 34.Barsotti M, Leonardis D, Vanello N, Bergamasco M, Frisoli A. Effects of continuous kinaesthetic feedback based on tendon vibration on motor imagery BCI performance. IEEE Trans Neural Syst Rehabil Eng. 2018;26(1):105–14. [DOI] [PubMed] [Google Scholar]
- 35.Phunruangsakao C, Achanccaray D, Bhattacharyya S, Izumi SI, Hayashibe M. Effects of visual-electrotactile stimulation feedback on brain functional connectivity during motor imagery practice. Sci Rep. 2023;13(1):17752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bolognini N, Russo C, Edwards DJ. The sensory side of post-stroke motor rehabilitation. Restor Neurol Neurosci. 2016;34(4):571–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Serrada I, Hordacre B, Hillier SL. Does sensory retraining improve sensation and sensorimotor function following stroke: a systematic review and meta-analysis. Front Neurosci. 2019;13:402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rustamov N, Souders L, Sheehan L, Carter A, Leuthardt EC. IpsiHand brain-computer interface therapy induces broad upper extremity motor rehabilitation in chronic stroke. Neurorehabil Neural Repair. 2025;39(1):74–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim MS, Park H, Kwon I, An KO, Kim H, Park G, Hyung W, Im CH, Shin JH. Efficacy of brain-computer interface training with motor imagery-contingent feedback in improving upper limb function and neuroplasticity among persons with chronic stroke: a double-blinded, parallel-group, randomized controlled trial. J Neuroeng Rehabil. 2025;22(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hayward KS, Schmidt J, Lohse KR, Peters S, Bernhardt J, Lannin NA, Boyd LA. Are we armed with the right data? Pooled individual data review of biomarkers in people with severe upper limb impairment after stroke. Neuroimage Clin. 2017;13:310–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Malouin F, Richards CL, Jackson PL, Lafleur MF, Durand A, Doyon J. The Kinesthetic and Visual Imagery Questionnaire (KVIQ) for assessing motor imagery in persons with physical disabilities: a reliability and construct validity study. J Neurol Phys Ther. 2007;31(1):20–9. [DOI] [PubMed] [Google Scholar]
- 42.Pfurtscheller G, Neuper C. Motor imagery activates primary sensorimotor area in humans. Neurosci Lett. 1997;239(2–3):65–8. [DOI] [PubMed] [Google Scholar]
- 43.Ramoser H, Müller-Gerking J, Pfurtscheller G. Optimal spatial filtering of single trial EEG during imagined hand movement. IEEE Trans Rehabil Eng. 2000;8(4):441–6. [DOI] [PubMed] [Google Scholar]
- 44.Wu GR, Colenbier N, Van Den Bossche S, Clauw K, Johri A, Tandon M, Marinazzo D. rsHRF: a toolbox for resting-state HRF estimation and deconvolution. Neuroimage. 2021;244:118591. [DOI] [PubMed] [Google Scholar]
- 45.Jager AP, Huntenburg JM, Tremblay SA, Schneider U, Grahl S, Huck J, Tardif CL, Villringer A, Gauthier CJ, Bazin PL, et al. Motor sequences; separating the sequence from the motor. A longitudinal rsfMRI study. Brain Struct Funct. 2022;227(3):793–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patel N, Jankovic J, Hallett M. Sensory aspects of movement disorders. Lancet Neurol. 2014;13(1):100–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Edwards LL, King EM, Buetefisch CM, Borich MR. Putting the “Sensory” into sensorimotor control: the role of sensorimotor integration in goal-directed hand movements after stroke. Front Integr Neurosc. 2019;13:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matyas F, Sreenivasan V, Marbach F, Wacongne C, Barsy B, Mateo C, Aronoff R, Petersen CCH. Motor control by sensory cortex. Science. 2010;330(6008):1240–3. [DOI] [PubMed] [Google Scholar]
- 49.Brown KE, Neva JL, Feldman SJ, Staines WR, Boyd LA. Sensorimotor integration in chronic stroke: baseline differences and response to sensory training. Restor Neurol Neuros. 2018;36(2):245–59. [DOI] [PubMed] [Google Scholar]
- 50.Schranz C, Vatinno A, Ramakrishnan V, Seo NJ. Neuroplasticity after upper-extremity rehabilitation therapy with sensory stimulation in chronic stroke survivors. Brain Commun. 2022;4(4):191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wilson RD, Page SJ, Delahanty M, Knutson JS, Gunzler DD, Sheffler LR, Chae J. Upper-limb recovery after stroke: a randomized controlled trial comparing emg-triggered, cyclic, and sensory electrical stimulation. Neurorehab Neural Re. 2016;30(10):978–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ren S, Wang W, Hou ZG, Liang X, Wang J, Shi W. Enhanced motor imagery based brain- computer interface via FES and VR for lower limbs. IEEE Trans Neural Syst Rehabil Eng. 2020;28(8):1846–55. [DOI] [PubMed] [Google Scholar]
- 53.Chen L, Gu B, Wang Z, Zhang L, Xu M, Liu S, He F, Ming D. EEG-controlled functional electrical stimulation rehabilitation for chronic stroke: system design and clinical application. Front Med. 2021;15(5):740–9. [DOI] [PubMed] [Google Scholar]
- 54.Ang KK, Chua KS, Phua KS, Wang C, Chin ZY, Kuah CW, Low W, Guan C. A randomized controlled trial of EEG-based motor imagery brain-computer interface robotic rehabilitation for stroke. Clin EEG Neurosci. 2015;46(4):310–20. [DOI] [PubMed] [Google Scholar]
- 55.Grigorev NA, Savosenkov AO, Lukoyanov MV, Udoratina A, Shusharina NN, Kaplan AY, Hramov AE, Kazantsev VB, Gordleeva S. A BCI-based vibrotactile neurofeedback training improves motor cortical excitability during motor imagery. IEEE Trans Neural Syst Rehabil Eng. 2021;29:1583–92. [DOI] [PubMed] [Google Scholar]
- 56.Belardinelli P, Laer L, Ortiz E, Braun C, Gharabaghi A. Plasticity of premotor cortico-muscular coherence in severely impaired stroke patients with hand paralysis. Neuroimage Clin. 2017;14:726–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guggenberger R, Kraus D, Naros G, Leão MT, Ziemann U, Gharabaghi A. Extended enhancement of corticospinal connectivity with concurrent cortical and peripheral stimulation controlled by sensorimotor desynchronization. Brain Stimul. 2018;11(6):1331–5. [DOI] [PubMed] [Google Scholar]
- 58.Liu X, Zhang W, Li W, Zhang S, Lv P, Yin Y. Effects of motor imagery based brain-computer interface on upper limb function and attention in stroke patients with hemiplegia: a randomized controlled trial. BMC Neurol. 2023;23(1):136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liao W, Li J, Zhang X, Li C. Motor imagery brain-computer interface rehabilitation system enhances upper limb performance and improves brain activity in stroke patients: a clinical study. Front Hum Neurosci. 2023;17:1117670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ramos-Murguialday A, Curado MR, Broetz D, Yilmaz Ö, Brasil FL, Liberati G, Garcia-Cossio E, Cho W, Caria A, Cohen LG, et al. Brain-machine interface in chronic stroke: randomized trial long-term follow-up. Neurorehabil Neural Repair. 2019;33(3):188–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Coscia M, Wessel MJ, Chaudary U, Millán JDR, Micera S, Guggisberg A, Vuadens P, Donoghue J, Birbaumer N, Hummel FC. Neurotechnology-aided interventions for upper limb motor rehabilitation in severe chronic stroke. Brain. 2019;142(8):2182–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Foong R, Ang KK, Quek C, Guan C, Phua KS, Kuah CWK, Deshmukh VA, Yam LHL, Rajeswaran DK, Tang N, et al. Assessment of the efficacy of EEG-Based MI-BCI with visual feedback and EEG correlates of mental fatigue for upper-limb stroke rehabilitation. IEEE Trans Biomed Eng. 2020;67(3):786–95. [DOI] [PubMed] [Google Scholar]
- 63.Halme HL, Parkkonen L. The effect of visual and proprioceptive feedback on sensorimotor rhythms during BCI training. PLoS ONE. 2022;17(2):e0264354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Whitlock JR. Posterior parietal cortex. Curr Biol. 2017;27(14):691–5. [DOI] [PubMed] [Google Scholar]
- 65.Rathelot JA, Dum RP, Strick PL. Posterior parietal cortex contains a command apparatus for hand movements. Proc Natl Acad Sci U S A. 2017;114(16):4255–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Veldsman M, Churilov L, Werden E, Li Q, Cumming T, Brodtmann A. Physical activity after stroke is associated with increased interhemispheric connectivity of the dorsal attention network. Neurorehabil Neural Repair. 2017;31(2):157–67. [DOI] [PubMed] [Google Scholar]
- 67.Wu CWW, Lin SHN, Hsu LM, Yeh SC, Guu SF, Lee SH, Chen CC. Synchrony between default-mode and sensorimotor networks facilitates motor function in stroke rehabilitation: a pilot fMRI study. Front Neurosci-Switz. 2020;14:548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mattos DJS, Rutlin J, Hong X, Zinn K, Shimony JS, Carter AR. The role of extra-motor networks in upper limb motor performance post-stroke. Neuroscience. 2023;514:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lam TK, Dawson DR, Honjo K, Ross B, Binns MA, Stuss DT, Black SE, Chen JJ, Levine BT, Fujioka T, et al. Neural coupling between contralesional motor and frontoparietal networks correlates with motor ability in individuals with chronic stroke. J Neurol Sci. 2018;384:21–9. [DOI] [PubMed] [Google Scholar]
- 70.Di Pino G, Pellegrino G, Assenza G, Capone F, Ferreri F, Formica D, Ranieri F, Tombini M, Ziemann U, Rothwell JC, et al. Modulation of brain plasticity in stroke: a novel model for neurorehabilitation. Nat Rev Neurol. 2014;10(10):597–608. [DOI] [PubMed] [Google Scholar]
- 71.Yuan K, Wang X, Chen C, Lau CC, Chu WC, Tong RK. Interhemispheric functional reorganization and its structural base after BCI-Guided upper-limb training in chronic stroke. IEEE Trans Neural Syst Rehabil Eng. 2020;28(11):2525–36. [DOI] [PubMed] [Google Scholar]
- 72.Tang Z, Wang H, Cui Z, Jin X, Zhang L, Peng Y, Xing B. An upper-limb rehabilitation exoskeleton system controlled by MI recognition model with deep emphasized informative features in a VR scene. IEEE Trans Neural Syst Rehabil Eng. 2023;31:4390–401. [DOI] [PubMed] [Google Scholar]
- 73.Cheng HJ, Ng KK, Qian X, Ji F, Lu ZK, Teo WP, Hong X, Nasrallah FA, Ang KK, Chuang KH, et al. Task-related brain functional network reconfigurations relate to motor recovery in chronic subcortical stroke. Sci Rep. 2021;11(1):8442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zöllei L, Polimeni JR, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106(3):1125–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figures S1-S3 and Tables S1. Figure S1-Demonstration of Multi-FDBK-BCI therapy. Figure S2-Behavioral Assessment and MEP for chronic stroke patients with Multi-FDBK-BCI therapy and CMI therapy. Figure S3-Correlations between BOLD signal changes and FMA (A) or MSS (B) changes for chronic stroke patients. Table S1-Patients’ characteristics, including lesion etiology.
Data Availability Statement
No datasets were generated or analysed during the current study.