Abstract
The aim of this study is to estimate prevalence, investigate potential risk factors and determine the antimicrobial sensitivity of Staphylococcus aureus in dairy farms of purposively selected towns in western Oromia: Bako, Nekemte and Gimbi. A cross-sectional study was conducted from November 2020 to June 2021. A total of 347 samples consisting of 255 raw milk, 49 milkers’ hand swab and 43 milking bucket swabs were collected and examined for Staphylococcus aureus using bacteriological culture and biochemical tests. Dairy animals were selected by simple random sampling. Risk factors for the occurrence of Staphylococcus aureus in dairy farms were assessed through interviews and personal observation. The isolates were tested for drug sensitivity using disk diffusion techniques. The overall prevalence of S. aureus was 18.44% (95% CI: 14.69–22.90). The proportion of isolation was 17.25% (95% CI: 13.07–22.43), 30.61% (95% CI: 19.03–45.29) and 11.63% (95% CI: 4.74–25.79) from raw milk, swabs of milkers’ hand and milking bucket, respectively showing insignificant difference (P < 0.05). Prevalence of Staphylococcus aureus within the breed, parity, lactation stage, hand washing at the milking interval and hygiene of the barn were also significant. The Antimicrobial test showed the highest susceptibility to ciprofloxacin (100%), gentamicin (90%), vancomycin (85%) and chloramphenicol (85%) but the highest resistance to penicillin G (85%), ampicillin (75%) and tetracycline (50%). None of the isolates showed multidrug resistance. Such Staphylococcus aureus prevalence with also the antibiotic resistant isolates suggests a public health risk thus the need to address this issue to protect both human and animal health.
Keywords: Antimicrobial sensitivity, Dairy farms, Prevalence, Risk factors, Western oromia
Introduction
Staphylococcus aureus is a ubiquitous, versatile and highly adaptive pathogen that colonizes the skin and mucous membrane of the anterior nostrils, the gastrointestinal tract, the perineum, the urogenital tract and the pharynx [1]. It is also found in the environment and in various foods of animal origin [2, 3]. Staphylococcus aureus is a facultative anaerobic, non-motile, fermentative and non-spore-forming bacteria [4]. They are often found in clusters that resemble a bunch of grapes when observed under a light microscope after Gram staining. The name ‘Staphylococcus’ was derived from the Greek words, Staphyle (bunch of grapes) and kokkos (berry) [5]. The term aureus is derived from Latin, which refers to the color of gold, as colonies (often) have a golden color when growing on solid media [6].
The main niche and the largest reservoir of Staphylococcus aureus is human nares. On average, every third person is colonized with this facultative pathogen [7]. Although the largest reservoir is human nares, the second largest may well be cows [8]. The skin, mucous membranes, teats and udder of milking animals are important reservoirs [9]. However, the main reservoirs in dairy herds are infected udders and teat skin [8]. Staphylococcus aureus infected purchased animals and chronically infected animals are a major source of new Staphylococcus infections within a farm [9].
Staphylococcus aureus causes a diverse array of diseases [10]. In animals, it is most commonly reported as the cause of mastitis in milk producing animals and bumble foot in chickens [11, 12]. In pigs, Staphylococcus aureus causes exudative dermatitis (greasy pig disease) [13]. In dairy cattle, Staphylococcus aureus causes one of the most common forms of chronic mastitis. Although some cows may flare up clinical mastitis, the infection is usually subclinical and causes increased somatic cell counts but no detectable changes in milk or udder [14]. The primary mode of transmission of Staphylococcus aureus is cow-to-cow during milking, when milk from an infected cow comes into contact with the teat end of an uninfected cow [15, 16].
In Ethiopia, several studies have reported the prevalence of Staphylococcus aureus in dairy farms in recent years. For instance [17], reported a prevalence of 24% for udder milk in Central Ethiopia, l [18] reported a prevalence of 20% for udder milk and 23.3% bucket swab in Jigiiga City [19], reported in and around Asella a prevalence of 11.9%, 0.00%, 11.1%, 33.3% for udder milk, bucket swab, milkers hand swab and milkers nasal swab respectively, In Addis Ababa [20] reported a prevalence of 20% from udder milk, 25% from bucket swab, 25% from milkers swab [21]. reported from Gondar the prevalence of 18.33% from udder milk 18.33% and 25% from bucket swab [22], reported from Mukaturi and Sululta the prevalence of 15.3% from udder milk, 20% from bucket swab and 25% from milk swab [23], reported from Mekelle the prevalence of 17% from udder milk.
Various potential risk factors are involved in the occurrence of Staphylococcus aureus in dairy farms. The host risk factors include age, parity, stage of lactation, somatic cell count, breed, anatomy of the mammary glands/morphology of the udder and teat, udder positioning, relative distance between teats, milk fat content and immunity [24, 25]. Environmental risk factors include the proper functioning of the milking machine, udder trauma, hygiene, climate, nutrition, management, season, and housing conditions. The warm, humid, and moist climate favors the growth of bacteria and increases the likelihood of intramammary infection [26]. Pathogen risk factors include the number of bacteria, virulence, frequency of exposure (dirty farm floor, dirty milking machine, and dirty towel for drying teats frequently exposed to pathogens), ability to resist flushing out of the glands by milk and resistance to antimicrobials [27].
Staphylococcus aureus is a highly adaptive pathogen that is constantly developing resistance to most of the antibiotics available [28]. Over the past few decades, antibiotic resistance of Staphylococcus aureus has gradually increased due to the evolution of bacteria and the abuse of antibiotics [29]. In the past, beta-lactam antibiotics have shown potent activity against Staphylococcus aureus, which, along with good safety profiles, makes them the antibiotics of choice for the treatment of Staphylococcal infections [30]. However, penicillin-resistant strains of Staphylococcus aureus emerged shortly after the antibiotic was introduced in the early 1940s [31]. Vancomycin resistance in S. aureus strains is a global issue and the resistance is linked to van genes which code for different resistance phenotypes and have the same name as the corresponding genes [32, 33]. Resistance rates of S. aureus to vancomycin are extremely high as noted by previous reports [32, 34]. The mechanisms involved in S. aureus resistance to tetracyclines are ribosomal protection by elongation of proteins, and activation of the efflux pump [33]. Acquisition of resistance to Macrolides and Lincosamides in S. aureus strains that includes modification of the target site by mutation or methylation of 23s rRNA subunit encoded, and activation of an efflux pump [35, 36].
To the best of the researchers’ knowledge, historically, Staphylococcus aureus has not been extensively studied in western Ethiopia and no such studies have been conducted to investigate its epidemiology in dairy farms of Bako, Nekemte and Gimbi towns. So, the objective of this study is to estimate the prevalence of Staphylococcus aureus in raw milk of lactating cows, swabs of milkers’ hands and milking bucket, to investigate the potential risk factors associated with its occurrence and to determine antimicrobial sensitivity of the isolated Staphylococcus aureus isolates in dairy farms of Bako, Nekemte and Gimbi towns.
Materials and methods
Study area description
The study was carried out in three purposively selected towns (Bako, Nekemte and Gimbi) based on their proximity to Ambo University Zoonoses and Food Safety Research Laboratory, the presence of large number of dairy farms and infrastructure accessibility. The towns are located in western part of Oromia Regional State, Ethiopia. Bako town is located in the west Shoa Zone of Oromia Regional State. The town is located at latitude and longitude of 09°08’N and 37o03’E, and at an altitude of 1743 m above sea level [37]. Nekemte town is located in the east Wollega Zone of Oromia Regional State. The town has latitude and longitude of 9°5′N and 36°33′E. It is located at an elevation of 2088 m above sea level [38]. Gimbi town is located in the west Wollega Zone of Oromia Regional State. The town has a latitude and longitude of 9°10’N and 35°50’E and is located at an altitude between 1845 and 1930 m above sea level [39]. The map of Ethiopia depicting the location of the study areas is shown in Fig. 1.
Fig. 1.
Map of the study area. The study was conducted in the West Wollega zone in Gimbi town, East Wollega zone in Nekemte town and West Shoa zone in Bako town
Study population
The study population was lactating local Zebu and crossbred cows (Holstein Friesian cross indigenous Zebu and Jersey cross indigenous Zebu) in Bako, Nekemte and Gimbi town dairy farms which are kept under intensive, semi-intensive and extensive management systems. In addition to the cows, the milkers’ hand and milking buckets were part of the study.
Study design
A cross-sectional study design was conducted from November 2020 to June 2021. A structured questionnaire and personal observation were also used to assess the milking hygienic practices and risk factors associated with the occurrence of Staphylococcus aureus.
Sampling technique
The dairy farms found in each town were selected using a simple random sampling technique. The lactating cows in the selected farm were sampled by simple random sampling technique. The proportionality of sampling dairy cows was applied according to the number of dairy farms in the town and the population size of each herd. Before the formal study, the researcher and Artificial Inseminators of the respective Woredas carried out preliminary visits to obtain the consent of the farmers, to localize the farms and to give each respondent a brief description of the study.
Sample size determination
The sample size was calculated according to the formula by [40]. Using 95% confidence interval, 5% desired level of precision and with expected prevalence of Staphylococcus aureus 16.6% in dairy farms of Mukaturi and Sululta towns [22].
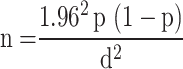 |
d.
Were,
n = sample size of the study population.
p = expected prevalence in the study area.
d = desired precision.
Accordingly, the calculated sample size for the current study was 214 animals; however, to increase precision the sample size was inflated to 255. Thus 255 animals were selected for milk sampling. In addition, 49 hand swabs from the milkers and 43 swabs from the milking buckets were purposively sampled. Therefore, a total of 347 samples were considered for the present study.
Data collection and laboratory investigation
Active observational survey
During the farm visit, personal observation was made to look for various hypothesized risk factors (body condition scores, presence of teat lesions, tick infestation, age, floor type and barn hygiene). The animal’s body condition score was categorized as poor, medium and good based on the criteria of [41]. The hygienic condition of the barn was classified as poor if there were bad smells, the feed trough and channel (for the waste drainage) were dirty, and when the flanks, udder and belly of the animals were soiled and rated good if none of the above defects were found [42].
Collection of samples
Milk samples were collected according to the National Mastitis Council procedure [43]. First the quarters were washed with clean water and wiped dry. The end of the teat was wiped with cotton wool soaked in 70% ethyl alcohol for disinfection. Approximately, 10 ml of raw milk was collected in a sterile universal bottle. The milk from all four quarters was collected in the same tube. To reduce the contamination of the teat ends during sampling, the close teats were sampled first and then followed by the far ones. The sample bottles were capped, labeled and stored in an ice-packed cool box and transported within 24 h to the Zoonoses and Food Safety Research Laboratory of Ambo University. Any presence of teat lesions and tick infestations on the udder of lactating cows were recorded during the process.
Swabs from the hands of milkers’ and milking buckets were collected with sterile, cotton-tipped swabs. Swab samples were taken from the hand of the milkers by rotating the swab 360° between the finger bases of both hands and the base of the milking bucket. The swab samples were transported in a sterilized universal bottle with 9 ml of peptone water. Upon arrival at the laboratory, all samples were stored in a refrigerator at 4 °C until processing.
Isolation and identification of Staphylococcus aureus
The collected samples were cultured directly on a blood agar base enriched with 7% defibrinated sheep blood. The inoculated plates were incubated aerobically at 37 °C and examined after incubation for 24 h. Incubation was extended to 48 h if no growth was observed within 24 h. Growth of the colonies (smooth and circular), the morphology (creamy, white/yellow) and the hemolytic properties were observed and recorded. Suspected colonies were then sub-cultured on nutrient agar plates and incubated for 24–48 h at 37 °C to obtain a pure culture. Gram’s staining was then used to identify the Gram reaction, cell shape and cell arrangement. Staphylococcus aureus is a gram-positive bacteria (staining purple by Gram staining) that is cocci-shaped and tends to cluster in what is described as grape-like. Suspected pure colonies from the nutrient agar plate were inoculated into nutrient slants and incubated at 37 °C for 24–48 h under aerobic culture conditions. The pure isolates in the culture medium were preserved and kept at 4oC for further biochemical tests [4].
The final identification of Staphylococcus aureus was based on biochemical tests (catalase test, mannitol fermentation, 1% maltose fermentation and coagulase test). Pure cultures of the isolates to be tested for catalase were picked up from the nutrient agar plate using a bacteriological loop and mixed with a drop of 3% hydrogen peroxide on a clean slide. When mixed with 3% H2O2, catalase-positive organisms create oxygen bubbles that are visible to the naked eye, while catalase-negative organisms do not. The catalase test is important to distinguish Streptococci (catalase-negative) from Staphylococci, which are catalase-positive. Catalase positive colonies were selected and streaked on mannitol salt agar plates incubated at 37oC and examined after 24 h. Mannitol Salt Agar is used as a selective and differential medium for the isolation and identification of Staphylococcus aureus from clinical and non-clinical specimens. Mannitol, the fermentable carbohydrate, the fermentation of which leads to acid production, is detected by the phenol red indicator, helps in the differentiation of Staphylococcal species. Staphylococcus aureus produces yellow colonies and a surrounding yellow medium [4].
To differentiate Staphylococcus aureus, a purple agar base with the addition of 1% maltose was used. The suspected colonies were inoculated with 1% maltose on a purple agar base media plate and incubated at 37 °C for 24 h. Staphylococcus aureus rapidly ferments maltose, changing the medium and colonies to yellow. Finally, a tube coagulase test is performed which is the standard test for the routine identification of Staphylococcus aureus. The tube coagulase test was performed in sterile tubes by adding 0.1 ml of selected isolates of Staphylococcus aureus grown on tryptone soya broth for 24 h at 37 °C to 0.5 ml of fresh rabbit plasma. The mixture within the tube was evaluated at 30 min intervals for the first 4 h of the test and then after 24 h of incubation, if any degree of clotting was visible when tilted, as Staphylococcus aureus produces clots [4].
Antimicrobial sensitivity test
A total of 20 randomly selected Staphylococcus aureus isolates were subjected to the antimicrobial sensitivity test using the Kirby-Bauer disc diffusion method according to the protocol of the Clinical and Laboratory Standards Institute [44]. The lists of antimicrobials tested were as follows; vancomycin (30 µg), penicillin G (10IU), tetracycline (30 µg), ciprofloxacin (5 µg), nalidixic acid (30 µg), ampicillin (10 µg), chloramphenicol (30 µg), gentamicin (10 µg), nitrofurantoin (30 µg) and ceftriaxone (30 µg). An inhibition zone diameter of each antimicrobial was then measured and interpreted as resistant, intermediate and susceptible by comparing it with recorded diameters of a control organism. Multiple antimicrobial resistance was recorded for isolates showing resistance to three or more antimicrobial classes [45].
Questionary survey
A structured questionnaire was used to collect data about milkers’ demography, milking practices and potential risk factors through interviewing farm owners or workers. Different risk factors thought to influence the occurrence of Staphylococcus aureus in the dairy herds (type of farm, management system, lactation stage, parity, average milk production, barn cleaning frequency and milking hygiene) were collected.
The variables were categorized into various categories. According to [46], the dairy farms were classified into large scale (> 30 dairy cows), medium scale (6–30 dairy cows) and small scale (≤ 5 dairy cows). However, no large-scale dairy farm was observed in the study area. The age of the cows examined was determined based on recorded birth dates, and owners’ information and by using the age determination described by [47]. The study cows were then categorized into young (3–6 years), adult (7–9 years) and old (> 9 years) [48]. The parity was categorized as cows that gave birth to one calf, two calves, three calves and ≥ four calves according to [49]. The lactation stage was classified as early (≤ 3 months), medium (4–6 months), and late (≥ 7 months) and the average daily milk yield was categorized as ≤ 7 l (low), between 8 and 15 l (medium) and > 15 l (high) [50].
Data management and analysis
All collected data was entered into a computer using Microsoft Excel and transferred to STATA version 14.0 (Stata Corp. College Station, TX, USA) for analysis. Descriptive statistics were used to calculate the prevalence of Staphylococcus aureus and to describe the antimicrobial sensitivity. Logistic regression was used to test the significance of the effect of various risk factors on the prevalence of Staphylococcus aureus. Univariable logistic regression was used in which the level of the risk factors for the occurrence of Staphylococcus aureus was compared using an odds ratio (OR). Variables that have P < 0.25 were investigated using a correlation matrix to assess collinearity and all non-collinear variables were offered to multivariable logistic regression. After checking for confounders, variables with a significant association (P < 0.05) with the dependent variable were retained in the final model. The model validity and predictive ability were assessed using the Hosmer-Lemeshow goodness-of-fit test and Receiver Operating Characteristic curve (ROC curve), respectively.
Results
Prevalence of Staphylococcus aureus
From the total of 347 samples collected and processed, the overall prevalence of Staphylococcus aureus was 18.44% (95% CI: 14.69–22.90). The percentage of isolation from raw milk of lactating cows, milkers’ hand and milking bucket swabs are listed in Table 1.
Table 1.
Percentage of Staphylococcus aureus isolates from Raw milk and swab samples
| Sample type | Total samples examined | Number of positive samples (%) | (95% CI) |
|---|---|---|---|
| Raw milk | 255 | 44 (17.25) | 13.07–22.43 |
| Milkers’ hand swab | 49 | 15 (30.61) | 19.03–45.29 |
| Milking bucket swab | 43 | 5 (11.63) | 4.74–25.79 |
Risk factors associated with Staphylococcus aureus
Intrinsic risk factors
In the present study, the univariable logistic regression analysis showed that breed, age, parity, lactation stage and daily average milk yield of the cows were statistically significantly associated (P < 0.05) with the isolation rate of Staphylococcus aureus. Accordingly, the likelihood of isolation of Staphylococcus aureus from crossbred cows (OR = 2.27, CI:1.09–4.74, P = 0.028) was statistically significantly higher than that of local breeds. Similarly, older cows (OR = 3.34, CI: 1.39–7.97, P = 0.007) and adult cows (OR = 2.13, CI:1.00-4.51, P = 0.048) had statistically significantly higher odds of contracting Staphylococcus aureus than young cows. Cows that gave birth to one calf were statistically significantly less likely than cows that gave birth to three calves (OR = 4.47, CI:1.46–13.68, P = 0.009) and ≥ 4 calves (OR = 4.89, CI:1.69–14.15, P = 0.003) to be affected by Staphylococcus aureus. No statistically significant differences were observed between cows that gave birth to one calf and two calves.
The odds of Staphylococcus aureus in cows in the late lactation stage (OR = 3.48 CI: 1.44–8.43, P = 0.006) were statistically significantly higher than in cows in mid-lactation stage. Cows that produce medium milk (8–15 l/day) had significantly higher odds of Staphylococcus aureus (OR = 2.76, CI: 1.29–5.91, P = 0.009) than those that produce low milk (≤ 7 l/day). No statistically significant differences were seen between other milk production levels. The result of the univariable logistic regression analysis of Staphylococcus aureus with intrinsic risk factors is described below (Table 2).
Table 2.
Univariable logistic regression analysis of S. aureus with intrinsic risk factors
| Risk Factors | No of examined | No of positive (%) | OR (95% CI) | P-value |
|---|---|---|---|---|
|
Breed Local Cross |
102 153 |
11(10.78) 33(21.57) |
Ref 2.27 (1.09–4.74) |
0.028** |
|
Body condition score Poor Medium Good |
99 119 37 |
19(19.19) 20(16.81) 5(13.51) |
1.52 (0.52- 4.42) 1.29 (0.49- 3.72) Ref |
0.442 0.634 |
|
Age Young Adult Old |
146 73 36 |
17(11.64) 16(21.92) 11(30.56) |
Ref 2.13(1.00-4.51) 3.34(1.39–7.97) |
0.048** 0.007** |
|
Parity 1 2 3 ≥4 |
74 67 49 65 |
5(6.76) 10(14.93) 12(24.49) 17(26.15) |
Ref 2.42(0.78- 7.49) 4.47 (1.46–13.68) 4.89 (1.69–14.15) |
0.125 0.009** 0.003** |
|
Lactation stage Early Mid Late |
89 87 79 |
13(14.61) 10(11.49) 21(26.58) |
1.89 (0.76- 4.74) Ref 3.48(1.44–8.43) |
0.169 0.006** |
|
Daily average milk yield Low Medium High |
102 108 45 |
11(10.78) 27(25.69) 6(13.33) |
Ref 2.76 (1.29–5.91) 1.27 (0.44-3.69) |
0.009** 0.657 |
OR = odds ratio, CI = confidence interval, ** significant
Extrinsic risk factors
In the current study, the occurrence of Staphylococcus aureus in raw milk showed a statistically significant association (P < 0.05) with regard to hand washing at milking interval, the type of floor, the frequency of floor cleaning and the barn hygiene. Accordingly, the odds of Staphylococcus aureus in cows that were milked without hand washing at milking intervals (OR = 3.01, CI:1.33–6.80, P = 0.005) was statistically significantly higher than those milked after washing hand at milking intervals. The likelihood of occurrence Staphylococcus aureus were statistically significantly higher in cows kept on the earthen floor (OR = 2.32, CI:1.11–4.84, P = 0.025) than those kept on concrete floor. In addition, there were statistically significantly higher odds of Staphylococcus aureus in cows from herds where the floor of the barn was cleaned more than once per week (OR = 3.80, CI:1.86–7.79, P = 0.001), once per week (OR = 4.44, CI:1.01–19.38, P = 0.047) and less than once per week (OR = 5.33, CI:1.17–24.29, P = 0.031) than those barns where floor was cleaned daily, as well there was also statistically significantly higher odds in cows kept in poor barn hygiene (OR = 2.77, CI:1.39–5.53. P = 0.004) than those kept in good barn hygienic condition. The result of the univariable logistic regression analysis of Staphylococcus aureus with extrinsic risk factors is described below (Table 3).
Table 3.
Univariable logistic regression analysis of S. aureus with extrinsic risk factors
| Risk factors | No of examined | No of positive (%) | OR (95% CI) | P-value |
|---|---|---|---|---|
|
Farm location Nekemte Bako Gimbi |
101 82 72 |
21(20.79) 13(15.85) 10(13.89) |
1.63 (0.72 − 3.70) 1.19 (0.49-2.85) Ref. |
0.733 0.256 |
|
Type of farm Small scale Medium Scale |
70 185 |
10(14.29) 34(18.38) |
Ref. 1.35(0.63 − 2.90) |
0.441 |
|
Management system Intensive Semi-intensive Extensive |
28 146 81 |
2(7.14) 29(19.86) 13(16.05) |
Ref. 2.48(0.76-11.78) 3.22(0.72-14.36) |
0.265 0.251 |
|
Presence of teat lesions Yes No |
53 202 |
13(24.53) 31(15.35) |
1.79(0.86-3.73) Ref. |
0.119 |
|
Tick infestation Yes No |
84 171 |
17(20.24) 27(15.79) |
1.35(0.69-2.65) Ref. |
0.378 |
|
Pre-milking hand washing Yes No |
237 18 | 40(16.88) 4(22.22) | 1.4(0.44-4.49) Ref. | 0.565 |
|
Washing hands at milking interval Yes No |
94 161 | 8(8.51) 35(21.75) | Ref. 3.01(1.33–6.80) | 0.005** |
|
Pre-milking udder washing Yes No |
76 179 | 11(14.47) 33(18.44) | Ref. 1.34(0.64 − 2.80) | 0.445 |
|
Post-milking udder washing Yes No |
53 202 | 5(9.43) 39(19.31) | Ref. 2.27 (0.86-6.15) | 0.098 |
|
Towel use for udder drying Yes No |
76 179 | 9(11.84) 35(19.55) | Ref. 1.80 (0.82-3.98) | 0.140 |
|
Post-milking teat dipping Yes No |
37 218 | 2(5.41) 42(19.27) | Ref. 4.18(0.96-18.06) | 0.056 |
|
Type of floor Earthen Concrete |
152 103 | 33(21.71) 11(10.68) | 2.32(1.1–4.84) Ref. | 0.025** |
|
Frequency of floor cleaning Daily More than once/week Once/week Less than once/week |
168 70 9 8 | 25(14.9) 13(18.6) 3(33.4) 3(37.5) | Ref. 3.80(1.86–7.79) 4.44(1.01–19.38) 5.33(1.17–24.29) | 0.001** 0.047** 0.031** |
|
Barn hygiene Poor Good |
122 133 | 30(24.59) 14(10.53) | 2.77(1.39–5.53) Ref. | 0.004** |
OR = odds ratio, CI = confidence interval, ** significant
Risk factors that were P < 0.25 in the univariable analyses (breed, age, parity, lactation stage, daily average milk yield, presence of teat lesions, hand washing at milking interval, post-milking udder washing, use of towel for udder drying, post-milking teat dipping, type of floor, frequency of floor cleaning and barn hygiene) were investigated using correlation matrix in order to assess collinearity. The independent variables were considered collinear when r > 0.5. Thus, the variables collinear with each other were parity and age (r = 0.8), average daily milk yield and breed (r = 0.9), post milking udder washing and post milking teat dipping (r = 0.8), post milking teat dipping and type of floor (r = 0.7) and floor cleaning frequency and barn hygiene (r = 0.7). Use of towel for udder drying and post milking teat dipping were removed from the model due to confounding. Finally, breed, parity, lactation stage, washing hands at the milking interval, presence of teat lesions and barn hygiene were subjected to multivariable logistic regression analysis.
The result of the multivariable logistic regression analysis showed that breed, parity, stage of lactation, hand washing at milking interval and barn hygiene were statistically significantly associated (P < 0.05) with the occurrence of Staphylococcus aureus. Accordingly, crossbred cows had statistically significantly (OR = 3.05, CI:1.47–18.07, P = 0.006) higher odds to contract Staphylococcus aureus compared to local breeds. Cows that gave birth to three calves (OR = 4.71 CI:1.47–15.07, P = 0.009) and ≥ 4 calves (OR = 4.34, CI:1.44–13.14, P = 0.009) were statistically significantly more likely to have Staphylococcus aureus positive result than cows that gave birth to one calf. The odds of Staphylococcus aureus in cows in late lactation stage (OR = 3.05, CI:1.19–7.82, P = 0.037) were statistically significantly higher than those in mid lactation stage. The odds of Staphylococcus aureus in dairy cows that were milked without washing hands at milking intervals (OR = 3.71, CI:1.94–11.46, P = 0.001) were statistically significantly higher than those cows that are milked after washing hands at the milking intervals. Similarly, the occurrence of Staphylococcus aureus in dairy cows that were kept in poor hygienic condition barns (OR = 2.53, CI:1.04–6.14, P = 0.040) were statistically significantly higher than those cows that have been kept in a good hygienic condition.
Hosmer-Lemeshow goodness-of-fit test result showed that the model fits the data reasonably well (P = 0.7998, χ2 = 43.29). ROC curve (0.7658) indicated that the model had good predictive ability. The result of multivariable logistic regression analysis of Staphylococcus aureus with various risk factors is depicted in Table 4.
Table 4.
Multivariable logistic regression analysis of S. aureus with various risk factors
| Risk factors | No of examined | No of positive (%) | OR (95% CI) | P-value | |||||
|---|---|---|---|---|---|---|---|---|---|
|
Breed Local Cross |
102 153 |
11(10.78) 33(21.57) |
Ref 3.05(1.47–18.07) |
0.006** | |||||
|
Lactation stage Early Mid Late |
89 87 79 |
15(14.61) 8(11.49) 21(26.58) |
1.69 (0.63-4.59) Ref 3.05(1.19–7.82) |
0.297 0.037** |
|||||
|
Parity 1 2 3 ≥4 |
74 67 49 65 |
5(6.76) 10(14.93) 12(24.49) 17(26.15) |
Ref 2.33(0.72 − 7.5) 4.71(1.47–15.07) 4.34(1.44–13.14) |
0.157 0.009** 0.009** |
|||||
|
Washing hands at milking interval Yes No |
94 161 |
8(8.51) 35(21.75) |
Ref 3.71 (1.94–11.46) |
0.001** | |||||
|
Barn hygiene Poor Good |
122 133 |
30(24.59) 14(10.53) |
2.53(1.04–6.14) Ref |
0.040** | |||||
|
Presence of teat lesions Yes No |
53 202 |
13(24.53) 31(15.35) |
2.23(0.91-5.04) Ref. |
0.09 | |||||
OR = odds ratio, CI = confidence interval, ** significant
Antimicrobial Sensitivity Pattern of Staphylococcus aureus
In the current study, 20 randomly selected Staphylococcus aureus isolates were subjected to 10 types of antimicrobials for an antimicrobial sensitivity test. Accordingly, the susceptibility pattern of the tested Staphylococcus aureus isolates was; ciprofloxacin (100%), followed by, gentamicin (90%), vancomycin (85%), chloramphenicol (85%), nitrofurantoin (75%), nalidixic acid (70%), ceftriaxone (60%), tetracycline (50%), ampicillin (20%) and penicillin (15%). Antimicrobial resistance was observed most frequently to penicillin G (85%), followed by ampicillin (75%), tetracycline (50%), ceftriaxone (30%), nalidixic acid (25%), nitrofurantoin (10%), vancomycin (10%) and chloramphenicol (5%). From the total isolates tested, 65.5% were susceptible, 5.5% were intermediate and 29% were resistant to the antimicrobials. The result is shown in Fig. 2.
Fig. 2.
Antibiotic resistance profile of the Staphylococcus aureus in dairy farms of Bako, Nekemte and Gimbi towns
Multidrug Resistance Pattern of Staphylococcus aureus
Of the 20 Staphylococcus aureus isolates examined, none of them were resistant to three or more antimicrobial classes.
Discussion
Considering the overall prevalence of Staphylococcus aureus in the selected dairy farms of Bako, Nekemte and Gimbi towns; the finding is comparable with reports of [51] around Addis Ababa (17.5%) [52], in Sebata (19.9%) and [53] in Mekelle (17.3%) dairy farms in Ethiopia. The present finding is lower when compared with a study conducted by [17] in central Ethiopia (25%) and [54] in Arsi Negele town (25.47%) dairy farms. However, it is higher than reports of [55] in Bishoftu town (8.25%) and [56] in Addis Ababa (5.2%) dairy farms. These disparities in Staphylococcus aureus prevalence could be related to differences in management systems, sample size, the environment of the sampled area, and farm owners’ and milkers’ awareness.
Our S. aureus prevalence in raw milk is relatively similar to the findings of [57] in the Hawassa area (17.9%) [37], around Addis Ababa (16.2%) and [58] in and around Addis Ababa (17.2%) dairy farms. This finding is lower than reports of [59] in Mekelle (46.4%) [60], in Alage TVET College dairy farm (28.2%) and [23] in Mekelle (26.3) dairy farms. However, this finding is higher than that of [61] in Holeta (13.8%) and [20] in and around Asella (11.9%) dairy farms. Although the prevalence of Staphylococcus aureus has been reported to vary with various factors, the high presence of Staphylococcus aureus in milk in the present study areas might be due to poor hygienic and sanitary conditions during milking.
Our S. aureus prevalence in swabs of milker’s hand is relatively in line with [52] in Sebata (32%) and [53] in Mekelle (31%) dairy farms. This finding is higher when compared with a study previously conducted by [19] in and around Asella (11.1%) [20], in Addis Ababa (25%) and [62] in Bishoftu (26.7%) dairy farms. However, this finding is slightly lower than the report of [54] who reported 34.28% at Arsi Negele town dairy farms. This high prevalence of Staphylococcus aureus could be related to the fact that at least 30% of people carry the bacterium in their nasal passages, and throats, and contaminate their hands by coughing or sneezing, implying that milkers could be possible sources. The variation in the isolation rate could be attributable to differences in hand-washing practices in the study areas.
Considering the prevalence of S. aureus in swabs of milking buckets; our finding is in agreement with reports of [63] in Ambo and Guder (9%) and [52] in Sebata (11.1%) dairy farms. However, the present finding is lower than that of [18] in Jigjiga (23.3%) and [20] in Addis Ababa (25%) dairy farms. In contrast to this finding [19], reported 0% in and around Asella town dairy farms. This variation might be due to variations in washing practices of milking buckets and mixing of milk from Staphylococcus aureus positive cows with others.
In the present study, the presence of Staphylococcus aureus was statistically significantly influenced by breed. The odds of finding a cross breed cow with a positive Staphylococcus aureus result were 3.05 times higher than in the local breed. This is in agreement with the findings of previous works by [64] in Wolaita Sodo and [65] in and around Asella town dairy farms. This variation of Staphylococcus aureus occurrence in breeds’ level could be due to the influence of some inheritable characteristics such as the capacity of milk production, teat characteristic and udder conformation [66]. However, this finding is in disagreement with [51] in and around Addis Ababa and [67] in Holeta area dairy farms who reported that there was no significant association between breed and occurrence of Staphylococcus aureus.
The current study revealed that the prevalence of Staphylococcus aureus significantly varied with the stage of lactation which was highest in the late stage of lactation. Evidence on the trend of prevalence increment at the late lactation stage was also produced in previous studies by [68] in Addis Ababa and [58] in and around Asella town dairy farms. The increased risk of Staphylococcus aureus in the late lactation stage can be due to the accumulated exposure to the pathogen that cows undergo throughout lactation. However, this finding is in disagreement with [51] in and around Addis Ababa and [22] in Mukaturi and Sululta dairy farms who reported no significant association between lactation stage and occurrence of Staphylococcus aureus.
The prevalence of Staphylococcus aureus was statistically significantly lower in primiparous. The odds of Staphylococcus aureus occurrence were 4.71 times higher in cows that gave birth to three calves and 4.34 higher in cows that gave birth to more than four calves than the primiparous cows. The current finding conforms to reports of [69] in Dire-Dawa and [70] in and around Asella town dairy farms. The increasing prevalence of Staphylococcus aureus along with parity could be due to multiparous cows in the studied herds might have had cumulatively several exposures to Staphylococcus aureus from poor hygiene during milking. Poor integrity of the teat canal due to aging, decreased immunity, or a more pendulous udder prone to injury in older cows might all have increased the susceptibility of the older animals [71, 72]. This result is disagreeable with a report of [48] in Adama smallholder dairy farms in which there was no statistically significant variation among levels of parity and prevalence of Staphylococcus aureus.
This study revealed that there is a statistically significant association between the occurrence of Staphylococcus aureus and hand washing at the milking interval. The odds of Staphylococcus aureus occurrence in cows that are milked without washing hands at the milking interval were 3.71 times higher than those that are milked after washing hands at the milking interval. This finding is in line with the reports of [73] in Bahir Dar and [74] in and around Gonder dairy farms. This implies that Staphylococcus aureus could be transmitted easily from infected to uninfected udder quarters or cows through the milkers’ hands as infected udder is the primary reservoir [75]. asserted that Staphylococcus aureus is well adapted to survive in the udder and usually establishes a mild subclinical infection of long duration, from which it is shed in milk, facilitating transmission to healthy animals, mainly during the milking process.
This study also revealed the odds of Staphylococcus aureus occurrence were statistically significantly 2.53 times higher in cows kept in poor hygiene barns than those kept in good hygienic condition barn. This finding is in agreement with [73] in Bahir Dar and [66] in Holeta area dairy farms. Keeping cows under unhygienic conditions increases the bacterial load and potential transmission of mastitis-causing organisms, to enter the udder through the teat orifice [50]. In the studied areas, the majority of the dairy farms do not wash the udder before milking and this might be one factor for the spread of Staphylococcus aureus. Furthermore, poor hygiene will make control methods such as teat dipping and sanitization ineffective [76].
The antimicrobial sensitivity test carried out in this study indicated that Staphylococcus aureus isolates displayed differences in the antimicrobial susceptibility patterns. The highest susceptibility of Staphylococcus aureus observed in our study is slightly in agreement with other studies carried out by [54] who reported, vancomycin (92.7%), gentamicin (95.3%) [77], reported, vancomycin (100%), gentamicin (100%) and chloramphenicol (81.8%) and [78] reported ciprofloxacin (100%), gentamicin (100%) and vancomycin (100%). The high susceptibility observed in these antimicrobials might be due to these antibiotics being the least frequently used in the study area in veterinary services and also might imply that such antibiotics can be used to treat infections caused by Staphylococcus aureus.
The highest resistance of Staphylococcus aureus towards penicillin G and ampicillin observed in the current study is comparable with the findings of [19, 22] who reported 93.1%, 97.6% and 95.5% resistance to penicillin G, respectively and [64, 66] who reported 55.1% and 66.7% resistance to ampicillin respectively. This high level of resistance to penicillin might be due to penicillin are the most commonly used antibiotic in the study areas for veterinary use and Staphylococcus aureus produces a penicillinase enzyme (a type of β-lactamase) that hydrolyses the beta-lactam ring of penicillin. It is believed that around 50% of mastitis causing Staphylococcus aureus strains produce β-lactamase [29].
The highest resistance of Staphylococcus aureus towards tetracycline observed in this study is relative to reports by [64] (50%) and [58] (82.2%) resistance to tetracycline. High tetracycline resistance could be associated with the fact this antibiotic is commonly used in the treatment of infections in the current study area. Lack of stringent regulation and monitoring in the dispensing and use of antimicrobials in the country also might contribute to the occurrence of high antimicrobial resistance to these drugs. In this study, none of Staphylococcus aureus isolates showed multi antimicrobial resistance to different classes of antimicrobials, this finding is similar with the report of [78].
Conclusion and recommendations
The present study, conducted in dairy farms of Bako, Nekemte and Gimbi towns, revealed a considerable presence of Staphylococcus aureus, posing potential public health and animal health concerns. The bacterium was isolated from raw milk, milkers’ hands, and milking buckets, highlighting the dairy production environment’s numerous sources of contamination. Breed type, parity, lactation stage, hand hygiene while milking, and barn cleanliness were risk factors that were strongly linked to the occurrence of Staphylococcus aureus, indicating both extrinsic and intrinsic factors that contribute to its presence. The antimicrobial susceptibility profile demonstrated that while Staphylococcus aureus isolates were highly sensitive to ciprofloxacin, gentamicin, vancomycin, and chloramphenicol, they showed high resistance to commonly used antibiotics such as penicillin G and ampicillin. Encouragingly, no multidrug-resistant strains were identified. These findings emphasize the urgent need for improved farm-level hygiene practices, responsible antibiotic use, and further molecular-level investigations to prevent the spread of antibiotic-resistant Staphylococcus aureus strains in the region. Overall, the study contributes valuable insights into the epidemiology of Staphylococcus aureus and calls for integrated efforts in training, surveillance, and intervention strategies to ensure safe milk production and safeguard public health.
Based on the above conclusion, the following recommendations were forwarded:
Training on proper milking hygiene, hygiene of the cow and barn area should be given to all actors involved in milk production by concerned body.
Close monitoring and rational use of antibiotics must be practiced to prevent antibiotic resistance from the pathogen.
Further work must address the factors influencing the occurrence of Staphylococcus aureus in dairy farms of the study area.
Further study on the molecular characterization of Staphylococcus aureus and detection of genes responsible for antimicrobial resistance needs to be carried out in the study area.
Limitation
The limitation of this study was the lack of molecular tests using Polymerase Chain Reaction (PCR) to confirm the presence of Staphylococcus aureus in dairy farms. The absence of PCR in this study might have underestimated the true prevalence of Staphylococcus aureus in dairy farms. In future studies, the use of molecular tests may increase the reliability and validity of the findings.
Acknowledgements
The authors would like to acknowledge the support and contribution of the Ambo University in this work.
Author contributions
Authors’ Contribution: (A) Kirubel Paulos Gutama: conceptualization; methodology; data curation; formal analysis; visualization; investigation; writing- original draft, writing–review and editing. (B) Manyazewal Anberber: supervision; visualization; data curation; formal analysis; writing original draft; writing–review and editing. All authors reviewed the manuscript.
Funding Declaration
.
Not applicable.
Data availability
All data supporting the findings of this study are available within the paper and its Supplementary Information files.
Declarations
Ethics approval and consent to participate
Informed consent was obtained from each participant. All the information obtained from the study participants was kept confidential.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Heijer C, Bijnen E, Van P, Pringle M, Goossens H, Bruggeman C, Schellevis F. Prevalence and resistance of commensal Staphylococcus aureus, including meticillin-resistant S. aureus, in nine European countries: a cross-sectional study. Lanc Infect Dis. 2013;13:409–15. [DOI] [PubMed] [Google Scholar]
- 2.Taylor T, Unakal C. Staphylococcus aureus. Treasure Island: StatPearls Publishing; 2020. [PubMed] [Google Scholar]
- 3.Hachemi A, Zenia S, Denia M, Guessoum M, Hachemi M, Ait-Oudhia K. Epidemiological study of sausage in algeria: prevalence, quality assessment, and antibiotic resistance of Staphylococcus aureus isolates and the risk factors associated with consumer habits affecting foodborne poisoning. Vet Worl. 2019;12:1240–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quinn P, Carter M, Markey B, Carter G. Veterinary microbiology microbial Diseases- bacterial causes of bovine mastitis. 8th ed. London: Mosby International Limited; 2002. [Google Scholar]
- 5.Licitra G, Staphylococcus. Emerg Infect Diseases: Etymolo. 2013;19:1553. [Google Scholar]
- 6.Liu G, Essex A, Buchanan J, Datta V, Hoffman H, Bastian J, Nizet V. Staphylococcus aureus golden pigment impairs neutrophil killing and promotes virulence through its antioxidant activity. J Experim Med. 2005;202:209–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moreillon P, Que A. Staphylococcus aureus (including Staphylococcal toxic shock). In: Mandell GL, Bennett JE, Dolin R, editors. Mandell, Douglas and bennett’s principles and practice of infectious diseases. 7th ed. New York: Elsevier; 2009. pp. 2321–51. In. [Google Scholar]
- 8.Sakwinska O, Giddey M, Moreillon M, Morisset D, Waldvogel A, Moreillon P. Staphylococcus aureus host range and human-bovine host shift. App Environ Microbiol. 2011;77:5908–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asperger H, Zangerl P, Roginski H, Fuquay J, Fox P. Staphylococcus aureus. Encyclopedia of Dairy Sciences; 2003.
- 10.Plata K, Rosato A, Wegrzyn G. Staphylococcus aureus as an infectious agent: overview of biochemistry and molecular genetics of its pathogenicity. Act Biochim Pol. 2009;56:597–612. [PubMed] [Google Scholar]
- 11.McNamee P, Smyth J, Erratum. Bacterial chondronecrosis with osteomyelitis (‘femoral head necrosis’) of broiler chickens. Avi Pathol. 2000;29:477–95. [DOI] [PubMed] [Google Scholar]
- 12.Graveland H, Duim B, van Duijkeren E, Heederik D, Wagenaar J. Livestock-associated methicillin-resistant Staphylococcus aureus in animals and humans. Internat J Med Microbiol. 2011;301:630–4. [DOI] [PubMed] [Google Scholar]
- 13.Voss A, Loeffen F, Bakker J, Klaassen C, Wulf M. Methicillin-resistant Staphylococcus aureus in pig farming. Emerg Infec Dis. 2005;11:1965–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petersson-Wolfe C, Isis K, Mullarky G. Staphylococcus aureus mastitis: cause, detection, and control. Virg Coopera Ext. 2010;404:7. [Google Scholar]
- 15.Capurro A, Aspan A, Ericsson Unnerstad H, Persson Waller K, Artursson K. Identification of potential sources of Staphylococcus aureus in herds with mastitis problems. J Dairy Sci. 2010;93:180–91. [DOI] [PubMed] [Google Scholar]
- 16.Zadoks R, Middleton J, McDougall S, Katholm J, Schukken Y. Molecular epidemiology of mastitis pathogens of dairy cattle and comparative relevance to humans. J Mamm Glan Biol Neopl. 2011;16:357–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tigabu E, Asrat D, Kassa T, Sinmegn T, Molla B, Gebreyes W. Assessment of risk factors in milk contamination with Staphylococcus aureus in urban and peri-urban small-holder dairy farming in central Ethiopia. Zoon Public Heal. 2015;62:637–43. [DOI] [PubMed] [Google Scholar]
- 18.Reta M, Bereda T, Alemu A. Bacterial contaminations of Raw cow’s milk consumed at Jigjiga City of Somali regional state, Eastern Ethiopia. Inter J Food Contamin. 2016;3:1–9. [Google Scholar]
- 19.Abunna F, Abriham T, Gizaw F, Beyene T, Staphylococcus. Isolation, identification and antimicrobial resistance in dairy cattle farms, municipal abattoir and personnel in and around asella, Ethiopia. J Vet Sci Technol. 2016;07:1–7. [Google Scholar]
- 20.Beyene T, Hayishe H, Gizaw F, Beyi F, Abunna F, Mammo B, Abdi D. Prevalence and antimicrobial resistance profile of Staphylococcus in dairy farms, abattoir and humans in addis ababa,ethiopia. BMC Res Notes. 2017;10:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tegegne B, Tesfaye S. Bacteriological milk quality: possible hygienic factors and the role of Staphylococcus aureus in Raw bovine milk in and around gondar, Ethiopia. Inter J Food Contam. 2017;4:1–9. [Google Scholar]
- 22.Regasa S, Mengistu S, Abraha A. Milk safety assessment, isolation, and antimicrobial susceptibility profile of Staphylococcus aureus in selected dairy farms of Mukaturi and Sululta town, oromia region, Ethiopia. Vet Medic Internat. 2019;2019:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weldeselassie M, Gugsa G, Kumar A, Tsegaye Y, Awol N, Ahmed M, Taddele H. Isolation and characterization of Staphylococcus aureus from food of bovine origin in mekelle, tigray, Ethiopia. Open Microbiol J. 2020;14:234–41. [Google Scholar]
- 24.Grohn Y, Erb H, McCulloch C, Saloniemi H. Epidemiology of mammary gland disorders in multiparous Finnish Ayrshire cows. Prev Vet Med. 1990;8:241–252.
- 25.Sordillo L, Streicher K. Mammary gland immunity and mastitis susceptibility in the transition cow. J Mammary Gland Biol Neopl. 2002;7:135–146. [DOI] [PubMed]
- 26.Hogan J, Smith K. Occurrence of clinical and subclinical environmental streptococcal mastitis. In Symposium on udder health management for environmental streptococci. 1997.
- 27.Bradley J. Bovine mastitis: an evolving disease. Vet J. 2002;164:116–28. [DOI] [PubMed] [Google Scholar]
- 28.Ortega E, Abriouel H, Lucas R, Galvez A. Multiple roles of Staphylococcus aureus enterotoxins: pathogenicity, superantigenic activity, and correlation to antibiotic resistance. Tox. 2010;2:2117–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo Y, Song G, Sun M, Wang J, Wang Y. Prevalence and therapies of antibiotic-resistance in Staphylococcus aureus. Cell Infect Microbiol. 2020;10:1–11. [DOI] [PMC free article] [PubMed]
- 30.Nubel U, Roumagnac P, Feldkamp M, Song J, Ko K, Huang Y, Achtman M.. Frequent emergence and limited geographic dispersal of methicillin-resistant Staphylococcus aureus. Proceed Nat Acad Sci United States of America. 2008;105:14130–14135. [DOI] [PMC free article] [PubMed]
- 31.Walsh C, Wencewicz T, Antibiotics. Challenges, mechanisms, opportunities. Washington, DC: ASM. 2016. [Google Scholar]
- 32.Perichon B, Courvalin P. VanA-type vancomycin-resistant Staphylococcus aureus. Antimicrob Agen Chemother. 2009;53:4580–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramana K, Rao R. Significance of screening for colonization and Vancomycin resistance in Staphylococcus aureus isolated from anterior Nares of school going children. Online J Health Allied Scs. 2009;8:20. [Google Scholar]
- 34.Deyno S, Toma A, Worku M, Bekele M. (2017). Antimicrobial resistance profile of Staphylococcus aureus isolates isolated from ear discharges of patients at University of Hawassa comprehensive specialized hospital. BMC Pharma Toxicol. 2017;18:35. [DOI] [PMC free article] [PubMed]
- 35.Lina G, Quaglia A, Reverdy M, Leclercq R, Vandenesch F, Etienne J. (1999). Distribution of genes encoding resistance to macrolides, lincosamides, and streptogramin among Staphylococci. Antimicrob Agen Chemothera. 1999;43:1062–1066. [DOI] [PMC free article] [PubMed]
- 36.Mišic´ M, Ukic´ J, Vidanovic´ D, Šekler M, Matic´ S, Vukašinovic´ M. Prevalence of genotypes that determine resistance of Staphylococci to macrolides and Lincosamides in Serbia. Front Public Health. 2017;5:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bako town administration office (BTAO). Bako town administration office, report. Ethiopia: Bako. 2020. [Google Scholar]
- 38.Nekemte town administration office (NTAO). Nekemte town administration office, report. Ethiopia: Nekemte. 2020. [Google Scholar]
- 39.Gimbi town administration office (GTAO). Gimbi town administration office, report. Ethiopia: Gimbi. 2020. [Google Scholar]
- 40.Thrusfield M, Christley R. Veterinary Epidemiology. Elsevier 4th edition. Editor Wiley-Blackwell. Londres. 2018;276.
- 41.Mishra S, Kumari K, Dubey A. Body condition scoring of dairy cattle. J Vet Scie. 2016;2:1–8. [Google Scholar]
- 42.Meranga M, Study On Prevalence Of Mastitis And Associated Risk Factors With Isolation And Antimicrobial Susceptibility Of Major Pathogs. In Alage State Dairy Farm, Ethiopia. MSc thesis, Addis Ababa University, Addis Ababa, Ethiopia. 2012.
- 43.National Mastitis Control (NMC). Microbiological procedures for the diagnosis of bovine udder infection and determination of milk quality (4th ed). 2004.
- 44.The Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing. Thirteenth edition Informational Supplement M100,Wayne, PA. pp. 1-332. 2020.
- 45.Magiorakos A, Srinivasan A, Carey B, Carmeli Y, Falagas M. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clini Microbiol Infect. 2012;18:268–81. [DOI] [PubMed] [Google Scholar]
- 46.Kidane A, Delesa K, Mummed Y, Tadesse M. Production system characterization of large, medium and small scale dairy farms in ethiopia: implications for developing breeding objectives of Holstein Friesian and crossbreed dairy cattle. Internat J Adv Res Biol Scie. 2019;6:37–54. [Google Scholar]
- 47.Parish J, Karisch B. Estimating cattle age using dentition. USA: Mississippi State University; 2013. [Google Scholar]
- 48.Abera M, Demie B, Aragaw K, Regassa F, Regassa A. Isolation and identification of Staphylococcus aureus from bovine mastitic milk and their drug resistance patterns in Adama town, Ethiopia. J Vet Med Anim Healt. 2010;2:29–34. [Google Scholar]
- 49.Abebe R, Hatiya H, Abera M, Megersa B, Asmare K. Bovine mastitis: prevalence, risk factors and isolation of Staphylococcus aureus in dairy herds at Hawassa milk shed, South Ethiopia. BMC Vet Res. 2016;12:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mureithi D, Njuguna M. Prevalence of subclinical mastitis and associated risk factors in dairy farms in urban and peri-urban areas of Thika sub county, Kenya. Livest Res Rural Develop. 2016;28:1–9. [Google Scholar]
- 51.Mekuria A, Asrat D, Woldeamanuel Y, Tefera G. Identification and antimicrobial susceptibility of Staphylococcus aureus isolated from milk samples of dairy cows and nasal swabs of farm workers in selected dairy farms around addis ababa, Ethiopia. Afri J Microbiol Res. 2013;7:3501–10. [Google Scholar]
- 52.Ayele Y, Gutema D, Edao M, Girma R, Tufa B, Beyene J, Beyi F. Assessment of Staphylococcus aureus along milk value chain and its public health importance in sebeta, central oromia, Ethiopia. BMC Microbiol. 2017;17:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kalayu A, Woldetsadik D, Woldeamanuel Y, Wang S, Gebreyes W, Teferi T. Burden and antimicrobial resistance of S. aureus in dairy farms in mekelle, Northern Ethiopia. BMC Vet Res. 2020;16:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tsige E. Investigation of staphylococcus aureus along the milk value chain and assessment of its public health significance in Arsi Negelle town, Ethiopia. MSc Thesis, Addis Ababa University, Addis Ababa, Ethiopia. 2018.
- 55.Mekonnen A, Pal M, Moses N. Isolation and identification of Staphylococcus species from Raw milk bovine milk in Denre zeit, Ethiopia. Vet Res. 2011;4:45–9. [Google Scholar]
- 56.Sani M, Gugsa G, Mekuria A, Ahmed M. Identification and antimicrobial resistance pattern of Staphylococcus aureus isolated from bovine Raw milk in addis ababa, Ethiopia. Abys J Scie Technol Identific. 2018;3:20–7. [Google Scholar]
- 57.Deresse D, G/sillasie S, Yihdego D. Antibiotic-resistance Staphylococcus aureus isolated from cow’s milk in Hawassa area, South Ethiopia. Afric J Microbiol Res. 2012;6:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gizaw F. Staphylococcus: epidemiology and its drug resistence in cattle, food chains and human in central Ethiopia. MSc Thesis. Addis Ababa University, Bishoftu, Ethiopia. 2014.
- 59.Enquebaher T, Siv S, Knut R, Taran S, Judith A. Staphylococcus aureus and other Staphylococcus species in milk and milk products from Tigray region, Northern Ethiopia. Afric J Food Scie. 2015;9:567–76. [Google Scholar]
- 60.Tessema D, Tsegaye S. Study on the prevalence and distribution of Staphylococcus aureus in Raw cow milk originated from Alage TVET college dairy farm, Ethiopia. J Nutri Food Scie. 2017;07:1–4. [Google Scholar]
- 61.Ayano A, Hiriko F, Simyalew M, Yohannes A. Prevalence of subclinical mastitis in lactating cows in selected commercial dairy farms of Holeta district. J Vet Medi Anim Healt. 2013;5:67–72. [Google Scholar]
- 62.Kassa M. The prevalence, distribution of staphylococcus and its antimicrobial susceptibility status of the isolates from meat and dairy milks of bishoftu, Ethiopia. Internat J Adv Res Biolog Scie. 2020;7:152–67. [Google Scholar]
- 63.Megersa L. Identification and antimicrobial susceptibility profiles of Staphylococcus species isolated from Raw milk, swabs of udders, milking utensils and milkers hands in small holder and dairy farms in Ambo and Guder town. Ethiopia: MScThesis, Addis Ababa University, Bishoftu; 2015. [Google Scholar]
- 64.Tessema F. Prevalence and drug resistance patterns of Staphylococcus aureus in lactating dairy cow’s milk in Wolayta sodo, Ethiopia. EC Vet Sci. 2016;2:226–30. [Google Scholar]
- 65.Kemal K, Tesfaye S, Ashanafi S, Muhammadhussien A. Prevalence, risk factors and multidrug resistance profile of Staphylococcus aureus isolated from bovine mastitis in selected dairy farms in and around Asella town, Arsi zone, South Eastern Ethiopia. Afri J Microbiol Res. 2017;11:1632–42. [Google Scholar]
- 66.Seykora A, McDaniel B. Genetics statistics and relationships of teat and udder traits, somatic cell counts, and milk production. J Dairy Sci. 1986;69:2395–407. [DOI] [PubMed] [Google Scholar]
- 67.Gebremeskel W, Tadesse T. Prevalence and antibiotic resistance of Staphylococcus aureus mastitis in Holeta area, Western Ethiopia. Glo Vet. 2016;16:365–70. [Google Scholar]
- 68.Garedew L, Melese B, Tesfaye R. Staphylococcus aureus in mastitic crossbreed cows and its associated risk factors in addis Ababa City. Ethio Vet J. 2015;19:107–16. [Google Scholar]
- 69.Disassa H, Zenebe T, Kebede G. A study on bovine mastitis, isolation and identification of staphylococcus species in dairy farms of dire Dawa city, Eastern Ethiopia. Glo Vet. 2016;16:222–30. [Google Scholar]
- 70.Seyoum B, Kefyalew H, Mukatr Y, Medicine V, Dawa P. Prevalence, associated risk factors and antimicrobial susceptibility of Staphylococcus aureus isolated from bovine mastitic milk in and around Asella town, Ethiopia. Adv Biol Res. 2017;11:295–301. [Google Scholar]
- 71.Awale M, Dudhatra B, Kumar A, Chauhan N, Kamani R, Modi M, Patel H, Mody S. Bovine mastitis: A threat to the economy. Open Access Rep Online. 2012;1:295. [Google Scholar]
- 72.Suleiman T, Karimuribo E, Mdegela R. Prevalence of bovine subclinical mastitis and antibiotic susceptibility patterns of major mastitis pathogens isolated in Unguja Island of zanzibar, Tanzania. Trop Anim Heal Prod. 2018;50:259–66. [DOI] [PubMed] [Google Scholar]
- 73.Sileshi S, Munees A. Prevalence and antibiotic susceptibility of Staphylococcus aureus from lactating cows milk in Bahir Dar dairy farms. Afri J Microbiol Res. 2016;10:1444–54. [Google Scholar]
- 74.Tegegne B, Tesfaye S. Bacteriological milk quality: possible hygienic factors and the role of Staphylococcus aureus in Raw bovine milk in and around gondar, Ethiopia. Internat J Food Contamin. 2017;4:1–9. [Google Scholar]
- 75.Radostits O, Gay C, Hinchcliff K, Constable P. Veterinary medicine: A text book of disease of cattle, horse, sheep, pig, and goats. 10thed. London. pp. 673–762. 2007.
- 76.Ndahetuye J. Mastitis in dairy cows in Rwanda: Prevalence, aetiology, antimicrobial resistance, molecular epidemiology and effects on milk quality. Doctoral Thesis. Swedish University of Agricultural Sciences, Uppsala, Rwanda. 2019.
- 77.Haftay A, Geberemedhin H, Belay A, Goytom E, Kidane W. Antimicrobial resistance profile of Staphylococcus aureus isolated from Raw cow milk and fresh fruit juice in mekelle, tigray, Ethiopia. J Vet Med Anim Heal. 2018;10:106–13. [Google Scholar]
- 78.Dabele D, Borena B, Admasu P, Gebremedhin E, Marami L. Prevalence and risk factors of mastitis and isolation, identification and antibiogram of staphylococcus species from mastitis positive Zebu cows in Toke kutaye, cheliya, and Dendi districts, West Shewa zone, oromia, Ethiopia. Infect Drug Resis. 2021;14:987–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data supporting the findings of this study are available within the paper and its Supplementary Information files.




