Abstract
Background
Autophagy is essential for removing damaged organelles and intracellular materials as well as invasive pathogens. The autophagic degradation of intracellular lipids plays a key role in maintaining cellular homeostasis. However, the mechanism of lipid metabolism regulated by autophagy, as well as whether or how lipid metabolites affect autophagy, remain unclear.
Results
RNAi of the key autophagy-related (Atg) genes, notably Atg1 and Atg8, suppressed autophagy, while overexpression of these Atg genes facilitated lipid degradation in both Bombyx mori and Drosophila melanogaster. In addition, disrupting autophagosome-lysosome fusion by chloroquine treatment inhibited lipid degradation during both metamorphosis and starvation. LC-MS/MS analysis showed that overexpression of DmAtg1:DmAtg13 mainly degraded glycerolipids, while DmAtg1 mutation predominantly accumulated glycerophospholipids. Notably, the significantly upregulated GPs following autophagy blockage, including C24H50NO7P (LPE, 19:0), C25H52NO7P (LPC, 0:0/17:0), C27H56NO7P (LPC, 0:0/19:0), and C28H58NO7P (LPC, 20:0/0:0), exerted a suppressive effect on autophagy occurrence mainly through the downregulation of AMPK signaling.
Conclusions
Autophagosome and autolysosome formations are both critical for lipid degradation. Conversely, the metabolites accumulated due to dysfunctional autophagy inhibit autophagy occurrence by downregulation of AMPK signaling, thereby forming a regulatory loop in insects. Collectively, our results provide valuable insights into applications for beneficial insects and pest management, while also present potential chemicals applied on human diseases related to autophagy or lipid metabolism.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12915-025-02274-z.
Keywords: Autophagy, Bombyx mori, Drosophila melanogaster, Lipid degradation, Metabolites
Background
Autophagy is essential for removing damaged organelles and intracellular materials as well as invasive pathogens to maintain cellular homeostasis [1]. Macroautophagy (hereafter referred to as autophagy) necessitates a series of autophagy-related (Atg) proteins for the initiation, extension, and maturation phases of autophagosome formation. Key components in this process mainly encompass the protein complexes Atg1/ULK1-Atg13/ATG13, BCEN1/Atg6-PI3C3C, Atg5-Atg12–Atg16, and LC3/Atg8–PE [2–4]. After maturation, autophagosomes subsequently fuse with lysosome, leading to the extensive degradation of encapsulated substrates within an acidified environment [5]. Of note, autophagy exerts regulatory control over lipid metabolism [6]. Studies have demonstrated that treatment of 3-methylladenine, an autophagy inhibitor targeting class III PI3K activity, results in enhanced cholesterol accumulation in hepatocytes subsequent to cholesterol supplementation [7]. Furthermore, knockout of Atg5 significantly elevated cellular triglyceride (TG) content in mouse hepatocytes subjected to a high-fatty acid diet [8]. Similarly, hepatocyte-specific knockout of Atg7 decreased autophagy occurrence and impeded starvation-induced lipid degradation in murine hepatocytes [9].
Autophagy facilitates the catabolism of stored lipids into free fatty acids, and in turn, several metabolites have been reported to affect autophagy occurrence, thereby establishing a feedback loop between lipid lipolysis and autophagy [10]. Phospholipids (PLs) constitute the primary structural components of cellular membranes. Notably, α-glycerylphosphorylethanolamine, the metabolite of phosphatidyl ethanolamine, induces autophagy occurrence accompanied by the release of membrane phospholipids and the acetylcholine neurotransmitter, showing its protective effect in the amyloid-injured astrocytes and aging model of human brain [11]. Similarly, treatment with docosahexaenoic acid monoglyceride (MAG-DHA) inhibits the growth of breast cancer cells, through the induction of autophagy triggered by MAG-DHA-induced ER stress [12]. Palmitic acid (PA), a ubiquitous saturated long-chain fatty acid found in food, induces apoptosis of human osteoblast-like Saos-2 cells via the upregulation of ER stress and ER stress-dependent autophagy [13]. In contrast, a high-fat diet (HFD) promotes autophagy to facilitate the renovation of intracellular membranes in kidney proximal tubular cells (PTECs). However, this process subsequently imposes a burden on lysosomes and impairs their acidification, ultimately suppressing autophagy and leading to the accumulation of phospholipids in mice. Notably, treatment with eicosapentaenoic acid (EPA) in the HFD-fed mice restores lysosomal function and autophagic flux, demonstrating that EPA functions in a manner dependent on autophagy to mitigate lipotoxicity [14].
Autophagy is governed by a multifaceted signaling, with a particular emphasis on the synergistic modulation exerted by the nutrient sensor MTOR and the energy sensor AMPK [15]. In insects, during molting and metamorphosis, a surge in the steroid hormone 20-hydroxyecdysone (20E) triggers autophagy via transcriptional upregulation of nearly all Atg and V-ATPase (vacuolar-type H + -adenosine triphosphatase) genes, while concurrently inhibiting MTOR signaling to activate the Atg1-Atg13 protein complex [16–18]. Furthermore, cholesterol, an essential precursor for 20E biosynthesis, upregulates autophagy by inducing the dephosphorylation of histone deacetylase 1, thereby facilitating the deacetylation of Atg proteins from BmAtg8–phosphatidylethanolamine (PE) ubiquitin-like system [19]. In Drosophila, the developmental profiles of membrane lipids, including glycerophospholipids (GPs), sphingolipids (SPs), and sterols, show that phosphatidylethanolamine, ceramide, phosphatidylserine, and phosphatidylglycerol are significantly reduced at the end of the larval stage [20]. However, the intricate interplay between metabolites and autophagy, as well as their reciprocal influences, remains largely unexplored in insects. In this study, we validated the physiological roles of autophagy in lipid degradation in Bombyx mori and Drosophila melanogaster and conducted a comprehensive analysis of metabolites in Drosophila fat body following the inhibition or enhancement of autophagy. Remarkably, we identified specific metabolites that, in turn, influence autophagy occurrence, thereby shedding lights on the intricate interplay between metabolites and autophagy. In addition, the identified lipid metabolites could provide molecular targets for the utilization of beneficial insects and pest management, while simultaneously presenting valuable research directions for addressing human diseases associated with autophagy and lipid metabolism.
Results
Formation of autophagosome and autolysosome affects lipid homeostasis in Bombyx fat body
Autophagy has been demonstrated to be dramatically induced at the end of larval stages in B. mori [17]. Thus, RNAi treatment of the key Bombyx Atg genes including BmAtg1, BmAtg5, and BmAtg8 was performed at the last day of 5th larval instar. Notably, BmAtg1 RNAi resulted in a ~ 40% delay in pupation and ultimately led to 20% abnormal metamorphosis and prepupal mortality (Fig. 1A, B and S1A, B). Additionally, the BmAtg1 knockdown group exhibited 22% pupal mortality and 8% defective eclosion. In comparison, there was only ~ 2% larval mortality in the control group. Similarly, BmAtg5 and BmAtg8 RNAi both led to substantial lethality at larval-pupal transition, as well as at pupal and eclosion stages, showing abnormal development after knockdown of Atg genes in silkworms (Fig. 1B). Subsequently, the loss of body weight from larvae prior to RNAi treatment to 1-day-old pupa, both in females and males, was reduced after BmAtg RNAi treatment. Specifically, BmAtg5 RNAi predominantly affected females, whereas BmAtg1 RNAi had the most significant impact on males (Fig. 1C). Consequently, lysosomal acidification was significantly blocked after knockdown of BmAtg1, BmAtg5, and BmAtg8, as indicated by LysoTracker Red staining (Fig. 1D and S1C). Compared to the reduction of autophagosome and autolysosome formation, lipid droplets (LDs) were accordingly accumulated in the fat body following knockdown of BmAtg genes, as observed by TEM (Fig. 1E and S1C). Therefore, further investigation was conducted to assess whether autophagy influences energy metabolism in B. mori. BODIPY staining demonstrated an accumulation of neutral lipids in the fat body following knockdown of BmAtg genes (Fig. 1F and S1C). Correspondingly, the concentration of triacylglycerol (TAG) in the fat body increased, while diacylglycerol (DAG) in the hemolymph decreased (Fig. 1G and H). In summary, knockdown of BmAtg genes in B. mori blocked autophagy and lipid metabolism, as well as larval-pupal transition.
Fig. 1.
Investigation of B. mori after Egfp, BmAtg1, BmAtg5, and BmAtg8 RNAi treatment. A and B Morphological observation after BmAtg1 RNAi (A) and lethality of B. mori after RNAi treatment of BmAtg genes (B), Egfp RNAi was used as the control group. n ≥ 42. C Pupal weight of both female and male after BmAtg genes RNAi. n = 3. Each group from control and RNAi groups were 15 larvae, totally 45 larvae, respectively. D LysoTracker Red staining of the fat body after BmAtg1 RNAi. E TEM observation of the fat body after BmAtg1 RNAi. F BODIPY staining of the fat body after BmAtg1 RNAi. G and H Lipid content in the fat body (G) and in the hemolymph (H) following RNAi of BmAtg genes. Data are presented as means ± SD; P values were determined by unpaired two-tailed Student’s t-test. Significance test was performed between Egfp and BmAtg gene RNAi group. *P < 0.05, **P < 0.01, ***P < 0.001. ns, no significant difference
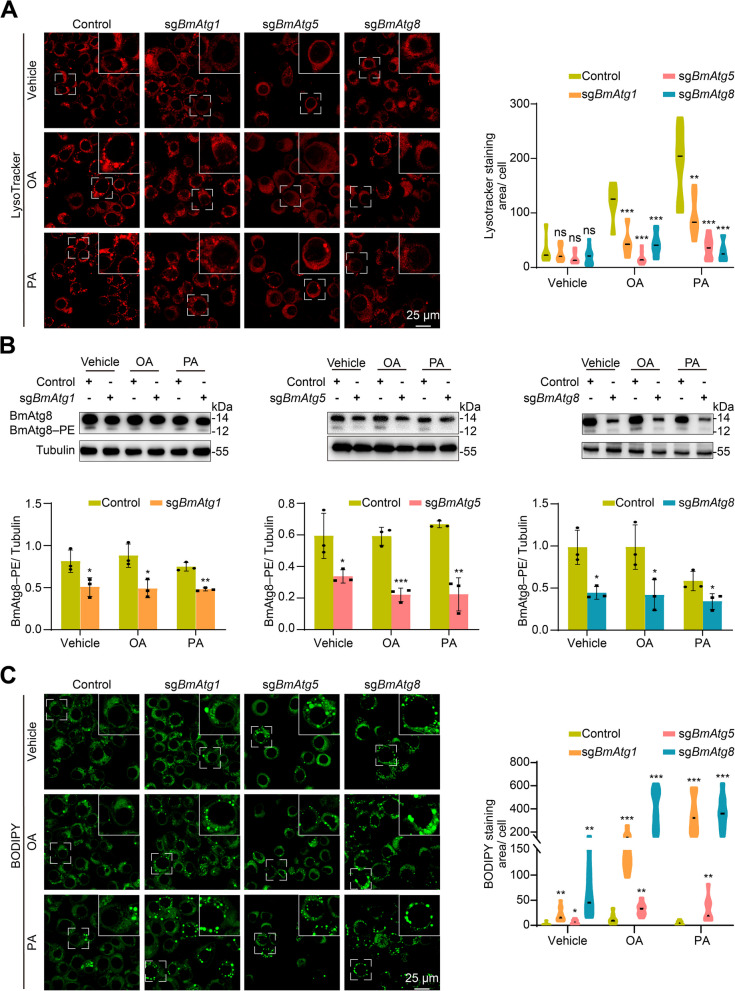
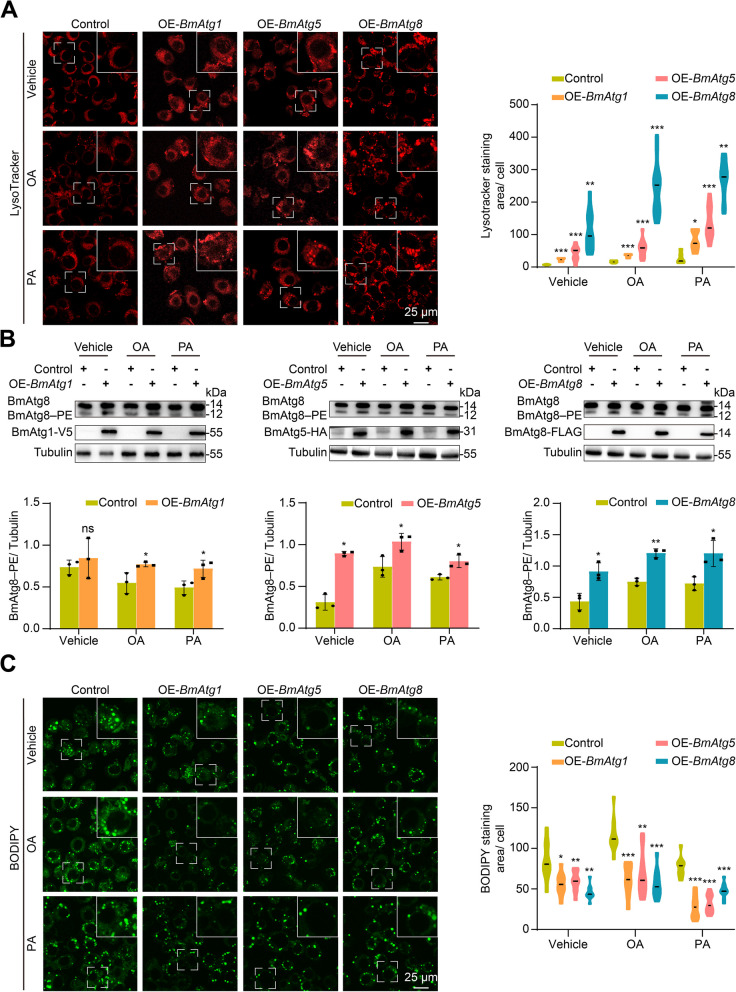
Similarly, knockout of BmAtg1, BmAtg5, and BmAtg8 in BmN cells partially blocked lysosomal acidification following a 24-h supplement with free fatty acid oleic acid (OA) or palmitic acid (PA) (Fig. 2A). Monitored by BmAtg8–PE formation, autophagy was notably reduced after functional loss of BmAtg genes (Fig. 2B). In addition, the blockage of autophagy in BmN cells resulted in a significant accumulation of neutral lipids, as indicated by BODIPY staining, particularly after treatment with OA or PA (Fig. 2C). In contrast, the overexpression of BmAtg1, BmAtg5, or BmAtg8 significantly promoted lysosomal acidification in BmN cells after a 24-h addition of OA or PA (Fig. 3A). Autophagy was notably upregulated after overexpression of BmAtg genes in BmN cells, as monitored by BmAtg8–PE formation (Fig. 3B). Moreover, functional gain of above BmAtg genes also significantly reduced the accumulation of neutral lipids following OA or PA treatment (Fig. 3C). Collectively, inhibition of autophagy accumulates while premature autophagy reduces lipids in B. mori, demonstrating the involvement of autophagy in lipid homeostasis in insects.
Fig. 2.
Autophagy and lipid detection in BmN cells after knockout of BmAtg1, BmAtg5, and BmAtg8. A-C LysoTracker Red staining (A), western blots and protein quantification (B), and BODIPY staining (C) after treatment with vehicle (10% BSA), OA (50 μM), or PA (50 μM) for 24 h in BmAtg gene knockout cells, pB-CRISPR-knockout cells was used as the control group. Data are presented as means ± SD; P values were determined by unpaired two-tailed Student’s t-test. Significance test was performed between pB-CRISPR and BmAtg gene knockout cells. *P < 0.05, **P < 0.01, ***P < 0.001. ns, no significant difference
Fig. 3.
Autophagy and lipid detection in BmN cells after overexpression of BmAtg1, BmAtg5, and BmAtg8. A-C LysoTracker Red staining (A), western blots (B), and BODIPY staining (C) after treatment with vehicle (10% BSA), OA (50 μM), or PA (50 μM) for 24 h in BmAtg gene overexpressing cells, transfection with pIEx-4 vector was used as control. Data are presented as means ± SD; P values were determined by unpaired two-tailed Student’s t-test. Significance test was performed between control and BmAtg gene overexpressing cells. *P < 0.05, **P < 0.01, ***P < 0.001
Autophagosomes finally fuse with lysosome for bulk degradation of substrates. Therefore, the necessity of lysosomal acidification for lipid metabolism was investigated. Larvae from the last day of larval stage (day 7 of 5th larval instar) were treated with various doses of chloroquine (CQ; 10, 25, 50, or 100 μg/larvae), an inhibitor of lysosomal acidification, for 24 h. LysoTracker Red staining revealed that lysosomal acidification was intensely inhibited and progressively decreased with increasing doses in the fat body (Fig. 4A). Consistently, TAG content in the fat body increased, while DAG content in the hemolymph decreased following the blockage of lysosomal acidification (Fig. 4B and C). In addition, TEM observation showed that treatment with 50 μg/larvae CQ dramatically reduced the formation of autolysosome, while increased the area of LDs in the fat body. Similarly, BODIPY staining confirmed the accumulation of neutral lipids in fat body after CQ treatment (Fig. 4D). Consequently, the content of TAG in the fat body and DAG in the hemolymph was respectively increased and decreased after CQ (50 μg/larvae) treatment (Fig. 4E and F). Subsequently, the feeding larvae from day 2 of 5th larval instar (5L2D), when autophagy and lysosomal acidification are rare, were injected with 25 μg/larvae CQ. After rearing for 6 h under normal conditions, the larvae were further starved for 12 h or 24 h. LysoTracker Red staining showed that CQ treatment significantly reduced starvation-induced lysosomal acidification (Fig. 4G). As expected, CQ treatment also inhibited starvation-induced degradation of neutral lipids in the fat body, as indicated by BODIPY staining (Fig. 4H). Accordingly, the content of TAG in the fat body and DAG in the hemolymph was respectively increased and decreased after CQ treatment in response to starvation for both 12 h and 24 h (Fig. 4I and J).
Fig. 4.
Lipid detection in B. mori after blockage of lysosomal acidification. A-C LysoTracker Red staining of fat body (A), lipid content in the fat body (B), and hemolymph (C) after treatment with different dose of chloroquine (CQ; 10, 25, 50, and 100 μg/larva) at wandering stage. D LysoTracker Red staining, TEM observation, and BODIPY staining of fat body after treatment with 50 μg/larvae CQ at wandering stage. E and F Lipid content in the fat body (E) and hemolymph (F) after treatment with 50 μg/larvae CQ. G and H LysoTracker Red staining (G) and BODIPY staining (H) of the fat body after treatment with 25 μg/larvae CQ followed by starvation for 12 h or 24 h, compared to normal feeding or starved larvae without CQ treatment at 5L2D stage. I and J Lipid content in the fat body (I) and hemolymph (J) after treatment with 25 μg/larvae CQ followed by starvation for 12 h or 24 h. Data are presented as means ± SD, n = 3. P values were determined by unpaired two-tailed Student’s t-test. *P < 0.05, **P < 0.01, ***P < 0.001
Autophagy affects lipid degradation in Drosophila fat body
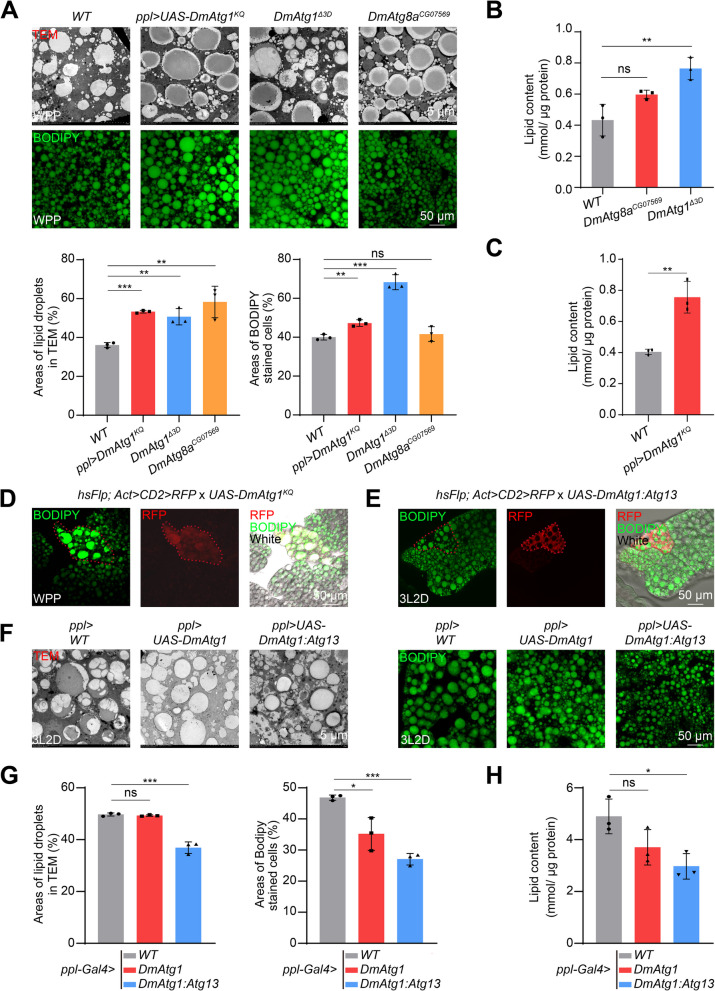
The physiological function of autophagy in lipid degradation was further verified in D. melanogaster. Fat body from DmAtg1△3D, DmAtg8CG07569 mutant, or ppl-GAL4:UAS-DmAtg1KQ (expressing a kinase-dead form of DmAtg1) line was collected at white prepupa (WPP) stage and subjected to BODIPY staining and TEM observation. As results were similar in WT1118 and ppl-GAL4 > WT1118 groups (Fig. S2A), thus only WT1118 was used as control hereafter. Monitored by TEM and BODIPY staining, the area occupied by LDs and the number of large LDs were significantly increased in the fat body of flies after inhibiting autophagy (Fig. 5A). Consistently, TAG content in the fat body was accumulated after mutation of DmAtg1, DmAtg8, or overexpression of DmAtg1KQ (Fig. 5B and C). Moreover, UAS-DmAtg1KQ clone cells in the fat body showed stronger BODIPY staining and more LDs, highlighting the necessity of autophagy for lipid metabolism in D. melanogaster (Fig. 5D). In contrast, overexpression of DmAtg1 or DmAtg1:DmAtg13 with ppl-GAL4 impaired the formation of LDs with the global reduction of size; accordingly decreased the content of TAG significantly in the fat body at day 2 of 3rd larval instar (3L2D) (Fig. 5F–H). Notably, the neutral lipids were degraded in UAS-DmAtg1:DmAtg13 clone cells, thereby resulting in smaller cells compared to the surrounding wild-type cells (Fig. 5E). In general, mutation of DmAtg1 and DmAtg8 inhibited, while overexpression of DmAtg1:DmAtg13 promoted autophagy and lipid metabolism in Drosophila, which are evolutionarily conserved with those observed in B. mori.
Fig. 5.
Lipid detection in D. melanogaster fat body with blocked or premature autophagy. A BODIPY staining and TEM observation of the fat body in ppl-GAL4 × UAS-DmAtg1KQ, DmAtg1△3D, and DmAtg8CG07629 larvae at WPP stage, the WT1118 was used as control. B Lipid content in the fat body of DmAtg1△3D and DmAtg8CG07629 larvae. C Lipid content in the fat body of ppl-GAL4 × UAS-DmAtg1KQ larvae at WPP stage. D BODIPY staining of the fat body after UAS-DmAtg1KQ at WPP stage using flip-out lines. RFP indicates DmAtg1KQ overexpressing clone cells. E BODIPY staining of the fat body after UAS-DmAtg1:DmAtg13 at 3L2D using flip-out lines. RFP indicates DmAtg1:DmAtg13 overexpressing clone cells. F BODIPY staining and TEM observation of fat body from ppl-GAL4 × UAS-DmAtg1 or ppl-GAL4 × UAS-DmAtg1:DmAtg13 larvae at 3L2D. G Quantification of BODIPY staining and TEM observation in F. H Lipid content in the fat body of ppl-GAL4 × UAS-DmAtg1 or ppl-GAL4 × UAS-DmAtg1:DmAtg13 larvae at 3L2D. Data are presented as means ± SD, n = 3. P values were determined by unpaired two-tailed Student’s t-test. *P < 0.05, **P < 0.01, ***P < 0.001. ns, no significant difference
Lipid metabolites are altered by premature or inhibited autophagy in Drosophila
Fat body-specific overexpression of DmAtg1:DmAtg13 or DmAtg1 mutated (DmAtg1△3D) was collected from wandering (W), white prepupa (WPP), and 3 h after white prepupa (3WPP) for widely targeted metabolomic analysis. Wild-type animals from above stages were used as controls. The biological repeats within each group clustered tightly and were distinctly separated from other groups (Fig. S2B). There were a total of 90 significantly affected metabolites (SAMs) with 55 downregulated and 35 upregulated after DmAtg1:DmAtg13 overexpression, and a total of 77 SAMs with 52 downregulated and 25 upregulated in DmAtg1 mutant at W stage. In comparison, there were a total of 53 SAMs with 44 downregulated and 9 upregulated after DmAtg1:DmAtg13 overexpression, while 72 SAMs with 27 downregulated and 45 upregulated in DmAtg1 mutant at WPP. When at 3WPP stage, there were only 29 SAMs with 25 downregulated and only 4 upregulated after DmAtg1:DmAtg13 overexpression, while a total of 54 SAMs with 14 downregulated and 40 upregulated in DmAtg1 mutant (Fig. S2C–J, Table S1). KEGG pathway analysis showed that these SAMs were enriched in several common pathways in DmAtg1:DmAtg13 overexpressed and DmAtg1 mutant larvae at W, WPP, and 3WPP stage (Fig. S3). The Venn diagram demonstrated that there were 13 pathways enriched in DmAtg1:DmAtg13 overexpressed larvae during all three stages (Fig. 6A), with 7 pathways contain at least 5 SAMs, including glycerolipid metabolism, cholesterol metabolism, lipid and atherosclerosis, vitamin digestion and absorption, fat digestion and absorption, regulation of lipolysis in adipocytes, and insulin resistance. Here, the SAMs within these 7 pathways mainly comprised glycerolipids (GLs, more than 97%), glycerophospholipids (GPs), fatty acyls (FAs), and sphingolipids (SPs) (Fig. 6B). Similarly, KEGG and Venn diagram analyses showed that these SAMs were involved in 8 identical pathways in DmAtg1 mutant larvae across all three stages (Fig. 6C), including linoleic acid metabolism, alpha-linolenic acid metabolism, arachidonic acid metabolism, choline metabolism in cancer, retrograde endocannabinoid signaling, (gpi)-anchor biosynthesis, autophagy-animal, and autophagy-other, whereas the SAMs within these 8 pathways mainly included GPs (more than 99%) instead of GLs, and only 1 FA in linoleic acid metabolism (Fig. 6D).
Fig. 6.
Metabolomic analysis of D. melanogaster with blocked or premature autophagy. A The number of enriched pathways in KEGG analysis in DmAtg1:DmAtg13 overexpressed larvae at W, WPP, and 3WPP stages. B Classifications and numbers of SAMs within 13 overlapping pathways in A. C The number of enriched pathways in KEGG analysis in DmAtg1 mutant larvae at W, WPP, and 3WPP stages. D Classifications and numbers of SAMs within 8 overlapping pathways in C. E and F Venn diagram to show differential SAMs (≥ 2.0 folds or ≤ 0.5 folds) in DmAtg1:DmAtg13 overexpressed (E) and DmAtg1 mutant (F) larvae at W, WPP, and 3WPP stages
After further filtered with ≥ 2.0 folds or ≤ 0.5 folds, these differential metabolites were subjected to clustering analysis. In DmAtg1:DmAtg13 overexpressed larvae, there were 1 SP, 11 GPs, 21 GLs, 2 FAs upregulated and 15 GPs, 32 GLs, 8 FAs downregulated at W stage; 1 SP, 5 GPs, 2 GLs, 1 FA upregulated and 1 sterol lipid (ST), 2 SPs, 8 GPs, 32 GLs, 1 FA downregulated at WPP stage; and 4 GPs upregulated and 2 SPs, 5 GPs, 15 GLs, 3 FAs downregulated at 3WPP (Fig. S4A–C, Tables S2–S4). Notably, 1 SAM (PI(15:0_16:1), GP) was upregulated, and 20 SAMs (4 GPs, such as PC(16:1_16:2); 15 GLs, such as TG(8:0_16:0_18:3); 1 FA [carnitine C16:2]) were downregulated across all three stages (Fig. 6E). In comparison, in DmAtg1 mutant larvae, there were 1 SP, 15 GPs, 7 GLs, 2 FAs upregulated and 13 GPs, 26 GLs, 13 FAs downregulated at W stage; 29 GPs, 13 GLs, 3 FAs upregulated and 6 GPs, 17 GLs, 4 FAs downregulated at WPP stage; and 29 GPs, 9 GLs, 2 FAs upregulated and 11 GPs, 1 GL, 2 FAs downregulated at 3WPP stage (Fig. S4D–F, Tables S5–S7). The Venn diagram shows that 13 SAMs (3 GLs, such as TG(8:0_15:1_18:2); 10 GPs, such as LPC(0:0/17:0), LPC(0:0/19:0), LPC(20:0/0:0)) were upregulated and 7 SAMs (5 GPs, such as PG(16:0_16:0); 2 FAs [carnitine C21:0 and carnitine C10:1]) were downregulated across all three stages (Fig. 6F). Overall, GLs and GPs emerged as the primary metabolite forms detected in Drosophila. Premature autophagy primarily led to the degradation of GLs, whereas inhibition of autophagy mainly caused the accumulation of GPs during wandering and white prepupal stages, accompanied by GLs at 3 h after white prepupal stage. Notably, carnitine C16:2 was the most significantly downregulated metabolite in DmAtg1:DmAtg13 overexpressing larvae across all three stages, while LPE(19:0) was the most significantly upregulated metabolite in DmAtg1 mutant larvae at WPP and 3WPP stages.
Lipid metabolites suppress autophagy occurrence in Drosophila and Bombyx via inhibition of AMPK signaling
Compared with the control group, among the metabolites exhibiting consistent variation trends across at least two developmental stages (wandering, white prepupa, and 3 h after white prepupa) under the conditions of fat body-specific overexpression of DmAtg1:DmAtg13 or DmAtg1 gene mutation, we identified 29 SAMs that respond to autophagy inhibition. In contrast, premature autophagy in Drosophila resulted in alterations in 20 SAMs (Table S8). Thus, whether the increased metabolites after DmAtg1 mutation would affect autophagy occurrence was investigated. Of note, C24H50NO7P (LPE, 19:0) was accumulated by 1752 folds in DmAtg1 mutant at white prepupa and by 12.26 folds at 3 h after white prepupa. C25H52NO7P (LPC, 0:0/17:0) exhibited increases of 2.08, 2.97, and 3.19 folds at W, WPP, and 3WPP stages, respectively, compared to controls. C27H56NO7P (LPC, 0:0/19:0) was upregulated by 2.49, 3.48, and 5.11 folds, and C28H58NO7P (LPC, 20:0/0:0) was upregulated by 2.78, 4.94, and 5.19 folds at W, WPP, and 3WPP stages in DmAtg1 mutants (Table S8). Consequently, their effects on autophagy occurrence were investigated. As expected, the addition of LPE(19:0), LPC(0:0/17:0), LPC(0:0/19:0), or LPC(20:0/0:0) in the culture of Drosophila fat body from wandering stage all resulted in decreased lysosomal acidification, as indicated by LysoTracker Red staining (Fig. 7A). Correspondingly, their treatment also inhibited DmAtg8–PE conjugation (Fig. 7B), demonstrating their negative role on autophagy occurrence in D. melanogaster. Subsequently, whether these metabolites would affect autophagy in B. mori was investigated. Upon supplementing with LPE(19:0), LPC(0:0/17:0), LPC(0:0/19:0), or LPC(20:0/0:0), the starvation-induced autophagy, monitored by LysoTracker Red staining and DmAtg8–PE protein levels, was reduced in BmN cells (Fig. 7C–G). Notably, the autophagy-suppressive metabolites identified in both D. melanogaster and B. mori exhibited concurrent reduction of AMPK activation, but not MTOR activity, suggesting a potential link between their anti-autophagic effects and AMPK signaling attenuation (Fig. 7B, D–G and Fig. S5A, B).
Fig. 7.
Effects of metabolites on autophagy occurrence in Drosophila fat body and B. mori BmN cells. A and B LysoTracker Red staining (A), western blots and protein quantification of BmAtg8–PE and p-AMPKα (T172) (B) of Drosophila fat body at W stage after treatment with 40 μM LPE(19:0), LPC(0:0/17:0), LPC(0:0/19:0), or LPC(20:0/0:0) for 2 h, individually. C LysoTracker Red staining from nutritious and starved BmN cells after treatment with LPE(19:0), LPC(0:0/17:0), LPC(0:0/19:0), or LPC(20:0/0:0) (40 μM) for 2 h (N, normal nutrient; S, starvation). D-G Western blots and protein quantification of BmAtg8–PE and p-AMPKα (T172) after treatment with 40 μM LPE(19:0) (D), LPC(0:0/17:0) (E), LPC(0:0/19:0) (F), or LPC(20:0/0:0) (G) for 2 h in normal-nutrition and starved BmN cells (S, starvation). The solvent PBS was used as control. Data are presented as means ± SD; P values were determined by unpaired two-tailed Student’s t-test. Significance test was performed between control and each metabolite treatment. *P < 0.05, **P < 0.01, ***P < 0.001. ns, no significant difference
In general, Atg proteins involved in the key steps of autophagosome formation are required for autophagy-mediated lipid metabolism. Conversely, the metabolites accumulated due to dysfunctional autophagy inhibits autophagy occurrence by modulating AMPK signaling, thereby forming a regulatory loop in insects.
Discussion
Autophagy deficiency is closely associated with the occurrence of human diseases such as neurological disorders, cancer, and obesity. Proteins integral to autophagosome formation and autophagic flux hold significant potential in regulating lipid metabolism [21]. Inhibition of autophagy by 3-methyladenine or knockdown of Atg5 and Atg7 significantly increases TG levels in hepatocytes [8]. Moreover, immunogold labeling indicates that LC3-I, but not LC3-II, colocalizes with LDs after Atg7 knockout in hepatocyte, suggesting the dependence of Atg7 in formation of a limiting membrane initiated by LC3 on LDs [8]. Starvation and moderate fat supply enhance autophagy-mediated lipid degradation, termed as lipophagy, in hepatocytes, whereas prolonged fat stimulation inhibits lipophagy, suggesting that the alterations of fat content in liver following ATG knockdown may be more of a secondary effect rather than a direct consequence of autophagy deficiency [22]. These findings indicate that blockage of autophagy under different physiological status of organisms might result in different effects on lipid catabolism. In addition, lysosome activity also plays a pivotal role in lipid metabolism. Notably, inhibition of autophagosome and lysosome fusion by bafilomycin A1 treatment increases lipid accumulation in mice fed a diet supplemented with oleic acid [23]. Functional loss of the lysosomal LAMP1 and LAMP2 proteins strongly affects autophagy and cholesterol trafficking in mammals [24]. In contrast, Drosophila Lamp1 is required for sterols and diacylglycerols metabolism but without disrupting autophagic processes, suggesting broader functions of lysosome-associated proteins in insects [25]. In this study, we confirmed that key Atg proteins, such as Atg1 and Atg8, which are essential for autophagy occurrence and lysosomal acidification, are crucial for the breakdown of LDs during metamorphosis and the maintenance of lipid homeostasis under specific status, showing the evolutionarily conserved functions with their mammalian homologs. Nonetheless, it is worth noting that compared with Bombyx, Drosophila exhibits a more pronounced impact on lipid degradation following autophagy blockade. Despite inhibiting lysosomal acidification with varying concentrations of CQ, only minimal alterations in TAG accumulation were observed in fat body at the 50 μg/larvae. This observation suggests that TAG degradation is not exclusively reliant on autophagy, but rather involves coordinated activity with the classical lipolytic pathway mediated by cytosolic lipases such as adipose triglyceride lipase (ATGL) and hormone-sensitive lipase (HSL) [26].
Autophagy plays a pivotal role in lipid degradation; conversely, imbalances in lipid metabolism also exert feedback on autophagy occurrence, thereby maintaining lipid homeostasis. Several lipid metabolites have been reported to alert autophagy occurrence in mammals. Notably, eicosapentaenoic acid (EPA) supplementation could promote autophagic flux and alleviate renal lipotoxicity in mice under HFD [14]. Carnitine treatment upregulates LC3B–II, while decreases autophagic substrate sequestosome 1 (SQSTM1) protein levels in the skeletal muscle of mice subjected to HFD [27]. In addition, phospholipids or their derivatives can also affect autophagy occurrence. Lysophosphatidic acid (LPA) could induce macrophage autophagy in a dose-dependent manner in mice [28]. Phosphoinositides, the key lipids acting as tuners and regulators of organelle’s functions in eukaryotes, are crucial for each step of autophagosome formation [29]. Moreover, phosphatidylethanolamine (PE), the second most abundant glycerophospholipid in eukaryotic cells, is potential for the formation of LC3/Atg8–PE and mediates membrane dynamics during autophagy [30]. Sphingolipids, the major constituents of biological membrane, some of them serve as second messengers involved in the decision of cell fate. Bioactive sphingolipid ceramide and sphingosine 1-phosphate (S1P) can both trigger autophagy; however, ceramide promotes cell death, whereas S1P increases cell survival [31, 32]. Cholesterol, the precursor for the biosynthesis of the steroid hormones, bile acids, and vitamin D, induces autophagy occurrence in insects and mammals conservatively [19]. However, the feedback loop between autophagy and metabolites in insects is lack of investigation. While our study delineates metabolite-mediated suppression of autophagy in insects, this regulatory paradigm appears distinct from the positive feedback loops observed in mammalian systems, where specific metabolites (e.g., lipoxin-A4) can activate autophagy to restore metabolic homeostasis [33].
In this study, the function of Atg genes in lipid metabolism was confirmed both in B. mori and D. melanogaster, and the metabolites modulated by autophagy in Drosophila were elucidated through LC-MS/MS analysis. Metabolome profiling showed that blockage of autophagy by DmAtg1 mutation predominantly led to accumulation of GPs during the larval-pupal transition, whereas premature autophagy in the fat body mainly facilitated the degradation of GLs in feeding larvae. Carnitine C16:2 was the most significantly downregulated metabolite in the larvae exhibiting premature autophagy, while LPE (19:0) was the most significantly upregulated metabolite in Atg1 mutant during the larval-pupal transition. In addition, treatments with accumulated metabolites in autophagy-blocked fly, such as LPE(19:0), LPC(0:0/17:0), LPC(0:0/19:0), and LPC(20:0/0:0), inhibited autophagy in D. melanogaster fat body during autophagy occurrence. Intriguingly, the four candidate metabolites consistently correlated with AMPK signaling suppression across insect models (D. melanogaster and B. mori), hinting at a phylogenetically conserved but mechanistically unproven association between their autophagy-inhibitory activity and AMPK pathway modulation. Future studies employing AMPK loss/gain-of-function models are required to validate this putative axis. In Drosophila, three of the four metabolites, LPC(0:0/17:0), LPC(0:0/19:0), and LPC(20:0/0:0) treatments decreased MTOR activity, and in Bombyx cells, the three metabolites did not affect phospho-4EBP level in both nutritious and starved situations, implying these metabolites suppress autophagy through AMPK-dependent pathway rather than MTOR pathway. We validate the physiological functions of autophagy in mediating lipid degradation in insects and unveil the metabolites affected by induction or inhibition of autophagy. Especially, several GPs in turn affect autophagy occurrence in D. melanogaster and B. mori have been identified, providing potential chemical compounds for beneficial insect breeding and pest management, as well as for the treatment of autophagy-related human diseases.
Conclusions
In summary, our research highlighted the biological function of autophagy in lipid degradation and metabolism. In contrast, the metabolites accumulated as a result of dysfunctional autophagy inhibit the occurrence of autophagy by modulating AMPK signaling, thereby forming a regulatory loop in insects.
Methods
Cell line and tissue culture
Bombyx BmN cells were cultured at 28 °C in Grace’s Insect Medium (Sigma-Aldrich, G9771) supplied with 10% heat-inactivated fetal bovine serum (FBS) (AusGeneX, FBS500-S). D. melanogaster fat body collected from wandering stage were cultured in insect SIM SF medium (Sino Biological Inc, MSF1-1) supplemented with 5% FBS (AusGeneX, FBS500-S). Escherichia coli DH5α (TIANGEN, CB101-03) was used for cloning of recombinant DNA.
Animals
The B. mori (Dazao) were reared on fresh mulberry leaves in the laboratory at 25 °C under 14 h light/10 h dark cycles [19, 34]. Flies were reared on standard cornmeal food at 25 °C unless otherwise indicated. The WT1118 strain was used as the wild-type (WT) control. ppl-GAL4 (BS58768) and DmAtg8KG07569 (BS14639) were from Bloomington Stock Center, UAS-DmAtg1 (F003538) was from FlyORF, UAS-DmAtg13 was from our previous study [18], and DmAtg1Δ3D and UAS-DmAtg1KQ lines were presented by Dr. Thomas P. Neufeld. For gene RNAi or overexpression in vivo, UAS flies were crossed with ppl-GAL4; the progenies were cultured at 25 °C until they reached the indicated stages before analysis or dissection. To generate flip-out clone cells, UAS male flies were crossed with hsFlp; Act > CD2 > RFP female flies. At 24 h after egg laying, the progenies were subjected to a heat shock at 37 °C for 15 min. They were then cultured at 25 °C until reaching the indicated developmental stages, followed by dissection [35, 36].
Overexpression of Bombyx Atg genes in BmN cells
Full length of BmAtg1, BmAtg5, and BmAtg8 was fused with V5, HA, and FLAG tag, respectively, and constructed into pIEx-4 plasmid by multiple cloning sites. After planted into the 6-well plate for 24 h, the BmN cells were transfected with the recombinant plasmid for Atg gene overexpression using TransIT®-Insect Transfection Reagent (Mirus Biotech, MIR6100) according to the manufacturer’s instructions; transfection with pIEx-4 vector was used as control. After transfection for 48 h, the cells were further treated with 50 μM oleic acid or 50 μM palmitic acid for 24 h, and then cells were collected for BODIPY and LysoTracker Red staining, or western blot analysis.
CRISPR/Cas9 knockout in BmN cells
The CRISPR/Cas9 system was used for gene knockout in BmN cells, and sgRNAs targeted to BmAtg1, BmAtg5, and BmAtg8 were designed and inserted into the pB-CRISPR vector, respectively. Subsequently, the plasmid was co-transfected with A3-Helper vector encoding transposase with the ratio of 1:1 in BmN cells using TransIT®-Insect Transfection Reagent (Mirus Biotech, MIR6100); the pB-CRISPR knockout cells were used as control. After co-transfection for 48 h, the cells were selectively maintained with the addition of 200 μg/mL Zeocin™ Selection Reagent (Gibco, R25001) to remove the cells unsuccessfully integrated with CRISPR/Cas9 system in the genome for 1 month, and the BmN cells transfected with empty pB-CRISPR vector were used as control [37, 38]. The genomic segment containing the sgRNA sites for each Atg genes was amplified and sequenced. The successfully knockout cells were further treated with 50 μM oleic acid or 50 μM palmitic acid for 24 h, and then were collected for BODIPY and LysoTracker Red staining, or western blot analysis. The primers are listed in Table S9.
Chloroquine treatment
Different doses of chloroquine (CQ, 10, 25, 50, or 100 μg/larva) were injected into the larvae at day 7 of 5th larval instar. The fat body was collected and subjected to LysoTracker Red staining after CQ treatment for 24 h. According to the observation of LysoTracker Red staining, 50 μg/larva injection was chosen to detect the variation of autophagy and lipid content. Larvae at day 2 of 5th larval instar (5L2D) were injected with 25 μg/larva CQ; 6 h later, the larvae were further starved for 12 h or 24 h. The fat body and hemolymph were collected for further assay. The larvae injected with the same volume of DMSO dissolving CQ were used as control.
Metabolite treatments
In mouse TM3 cells, lysophosphatidylcholine (L-alpha-lysophosphatidylcholine, LPC (14:0)) concentrations ranging from 2.5 to 160 μM were shown to induce apoptosis (with maximum induction at 40 μM) and inhibit autophagy [39]. Consequently, the effects of LPE (19:0), LPC (0:0/17:0), LPC (0:0/19:0), or LPC (20:0/0:0) on autophagy occurrence were observed. The perivisceral fat body of D. melanogaster collected from wandering stage was cultured in SIM SF medium supplemented with 40 µM LPE (19:0), LPC (0:0/17:0), LPC (0:0/19:0), or LPC (20:0/0:0) (Avanti Research™, 855674P, 855676P, 855776P, and 855777P) for 2 h, respectively. After cultured in the 12-well plate for 24 h with Grace’s Insect Medium, the BmN cells were then starved with PBS for 2 h to induce autophagy, followed by treatment of above metabolites for further 2 h; the same volume of the solvent PBS was used as control. Finally, both the fat body and BmN cells were collected for BODIPY, LysoTracker Red staining, or western blot analysis.
RNAi treatment in B. mori larvae
The template for synthesizing double-stranded RNA (dsRNA) was amplified by PCR from the cDNA of Bombyx Atg genes and EGFP-N1 plasmid. dsRNA targeting Egfp, BmAtg1, BmAtg5, and BmAtg8 was generated using the T7 RiboMAX™ Express RNAi system (Promega, P1700) according to the manufacturer’s instructions. Each individual larva was injected with 30 μg dsRNA at 12 h before initiation of wandering (IW), and the injection of Egfp dsRNA (30 μg) was used as a control. The fat body was collected at 24 h post RNAi treatments for further bioassays [40].
Western blot analysis
Proteins were extracted from the cells and tissues using RIPA buffer (Beyotime Biotechnology, p0013b) supplemented with a cocktail of protease inhibitors (Merck KGaA, 11836170001). The protein extracts were subsequently separated by SDS-PAGE and transferred onto polyvinylidene difluoride membranes (BIO-RAD, 1620177), which were then sectioned based on the molecular weight of the target protein and incubated with primary and secondary antibodies. Visualization of protein bands was achieved through utilization of the Enhanced ECL Chemiluminescent Substrate WB detection system (Yeasen Biotechnology, 36222ES60). The primary antibodies for V5 (Cell Signaling Technology, 13202S; 1:3000), FLAG (Cell Signaling Technology, 14793S; 1:3000), HA (Santa Cruz Biotechnology, sc-7392; 1:2000), BmAtg8–PE (Abcam, ab109364; 1:4000), Phospho-AMPKα (Thr172) (Cell Signaling Technology, 50081; 1:3000), and Tubulin (Beyotime Biotechnology, AT819; 1:5000) were used for western blotting according to the standard procedure as previously described [17, 41]. ImageJ software version 1.46 (NIH, USA; http://rsbweb.nih.gov/ij) was used for the gray value quantification of protein bands.
LysoTracker Red staining
Perivisceral fat bodies near genital gland were newly collected from 10 animals and separated into small pieces by forceps and thoroughly washed with PBS (pH 7.0, 0.1 M). The pieced fat bodies and BmN cells were stained with LysoTracker Red DND-99 (Invitrogen, L7528) as previously described [17, 41]. Observation was monitored under confocal microscope (Olympus, FV3000, Japan). Three biological replicates were conducted and ImageJ software (NIH) was used to quantify the areas of LysoTracker Red staining.
Transmission electron microscopy (TEM) analysis
The perivisceral fat body from B. mori and D. melanogaster was collected and fixed in 2.5% glutaraldehyde for more than 24 h at 4 °C, and then samples were processed as previously described [17]. Specimens were observed for lipid droplets (LDs) under a TEM. Three independent biological repeats were conducted and ImageJ software was used to quantify the areas of autophagic compartments or LDs in the TEM image.
Neutral lipid detection by BODIPY staining
The lipid probe, BODIPY (4,4-difluoro-3a,4adiaza-s-indacene) 493/503 (Invitrogen, 3922), was used to stain the neutral lipid in the fat body or cells according to the manufacturer’s instruction. The stained lipids were observed under the confocal microscope (Olympus, FV3000, Japan). Three biological replicates were conducted and ImageJ software was used to quantify the areas of BODIPY staining.
Measurement of lipid content in the fat body or hemolymph
Perivisceral fat body near genital gland were newly collected, and hemolymph samples were collected into 1.5-mL Eppendorf tubes by cutting an abdominal leg of a silkworm. Content of triglyceride in the fat body and diglyceride in the hemolymph were measured by Triglyceride assay kit (Nanjing Jiancheng Bioengineering Institute, A110-1-1) according to the manufacturer’s instruction.
Widely targeted metabolome
The variation of metabolites in D. melanogaster were detected by widely targeted metabolome. The larvae were collected for more than 20 mg in each sample from WT1118, DmAtg1Δ3D, and ppl-GAL4 > UAS-DmAtg1:DmAtg13 at wandering (W), white prepupa (WPP), and 3 h after white prepupa (3WPP) stages. The Drosophila larvae in each sample were homogenized and extracted for lipids by 1 mL extract solution containing methyl tert-butyl ether and methanol in the volume ratio of 3:1 as well as international standard lipids. The supernatant was measured for metabolites by UPLC-MS/MS. The ultra-performance liquid chromatography (UPLC, ExionLC AD), equipped with Thermo Accucore™ C30 column (i.d. 2.1 × 100 mm, 2.6 μm) and maintained at 45 °C, was used for UPLC-MS/MS analysis (Wuhan Metware Biotechnology Co., Ltd., Wuhan, China).
Statistical analysis
Experimental data were analyzed in GraphPad Prism 8 software, differences between two groups were analyzed using unpaired two-tailed Student’s t-test, and two-way ANOVA with Tukey’s multiple comparisons test was applied for comparisons between more than two groups. Throughout the paper, the data are presented as the mean ± SD of 3 independent experiments, and statistically significant differences are represented as *P < 0.05, **P < 0.01, and ***P < 0.001.
Supplementary Information
Additional file 1: Fig. S1 Autophagy and lipid detection in B. mori fat body after BmAtg1, BmAtg5, and BmAtg8 RNAi and detection of BmAtg gene knockout efficiency in BmN cells.
Additional file 2: Fig. S2 The TEM and BODIPY results of WT1118 andppl-GAL4>WT1118,and principal component analysis (PCA) of metabolites in all groups, and volcano plot analysis of differential metabolites with blocked or premature autophagy.
Additional file 3: Fig. S3 KEGG enrichment of metabolites in D. melanogaster with blocked or premature autophagy.
Additional file 4: Fig. S4 Heatmap of metabolites in DmAtg1:DmAtg13 overexpressing and DmAtg1 mutant larvae at W, WPP, and 3WPP stages.
Additional file 5: Fig. S5 Effect of metabolite treatments on MTOR activity under normal or starvation conditions.
Additional file 6: Table S1 The total number of metabolites that are up- and downregulated in metabolomics.
Additional file 7: Table S2 The data provides information on metabolites that are up- and downregulated in DmAtg1:DmAtg13 overexpressed larvae at W stage.
Additional file 8: Table S3 The data provides information on metabolites that are up- and downregulated in DmAtg1:DmAtg13 overexpressed larvae at WPP stage.
Additional file 9: Table S4 The data provides information on metabolites that are up- and downregulated in DmAtg1:DmAtg13 overexpressed larvae at 3WPP stage.
Additional file 10: Table S5 The data provides information on metabolites that are up- and downregulated in DmAtg1 mutant larvae at W stage.
Additional file 11: Table S6 The data provides information on metabolites that are up- and downregulated in DmAtg1 mutant larvae at WPP stage.
Additional file 12: Table S7 The data provides information on metabolites that are up- and downregulated in DmAtg1 mutant larvae at 3WPP stage.
Additional file 13: Table S8 The data presents the fold change of metabolites after blocking or inducing autophagy in D. melanogaster.
Additional file 14: Table S9 The information of primers in this study.
Additional file 15: Original uncropped western blots.
Acknowledgements
We are grateful for Prof. San-Yuan Ma form Southwest University providing pB-CRISPR vector.
Abbreviations
- AMPK
AMP-activated protein kinase
- Atg
Autophagy-related
- CQ
Chloroquine
- DAG
Diacylglycerol
- 20E
20-Hydroxyecdysone
- FAs
Fatty acyls
- GLs
Glycerolipids
- GPs
Glycerophospholipids
- HFD
High-fat diet
- IW
The initiation of wandering stage
- 3L2D
Day 2 of 3rd larval instar
- 5L2D
Day 2 of 5th larval instar
- MTOR
Mechanistic target of rapamycin kinase
- OA
Oleic acid
- PA
Palmitic acid
- PE
Phosphatidylethanolamine
- PP
The prepupal stage
- SAM
Significantly affected metabolite
- SPs
Sphingolipids
- TAG
Triacylglycerol
- TEM
Transmission electron microscopy
- TG
Triglyceride
- Tubulin
Tubulin alpha 1a
- W
The wandering stage
- WPP
White prepupa
- 3 WPP
3 h after white prepupa
Authors’ contributions
K.L. and L.T. conceived and designed the experiments. L.T., Q.E.Z., and Y.B.Y. performed most of the experiments, analyzed the data and wrote the initial draft. Y.X., S.L., and K.L. reviewed and edited the manuscript. L.T., W.M.W., S.L., and K.L. provided the funding for this research. All authors read and approved the final manuscript.
Funding
This study was supported by the Natural Science Foundation of Guangdong Province (2022A1515010498 and 2024A1515011551 to L.T., 2024A1515013140 to K.L.) and the National Natural Science Foundation of China (NSFC31970463 to L.T., NSFC31930014 and 32220103003 to S.L., 32400410 to W.M.W.).
Data availability
Data will be made available on request. All data generated or analysed during this study are included in this published article, its supplementary information files and publicly available repositories. The original metabolomics data supporting this study are available on Figshare (10.6084/m9.figshare.29137676).
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Kang Li is the lead contact.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ling Tian, Qien Zhong and Yubei Yang contributed equally to this work.
Contributor Information
Yang Xiao, Email: xiaoyang@gdaas.cn.
Sheng Li, Email: lisheng@scnu.edu.cn.
Kang Li, Email: likang@m.scnu.edu.cn.
References
- 1.Chen X, Tsvetkov AS, Shen HM, Isidoro C, Ktistakis NT, Linkermann A, Koopman WJH, Simon HU, Galluzzi L, Luo S, et al. International consensus guidelines for the definition, detection, and interpretation of autophagy-dependent ferroptosis. Autophagy. 2024;20(6):1213–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ajazi A, Foiani M. Vps30/Atg6/BECN1 at the crossroads between cell metabolism and DNA damage response. Autophagy. 2022;18(5):1202–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Puente C, Hendrickson RC, Jiang X. Nutrient-regulated phosphorylation of ATG13 inhibits starvation-induced autophagy. J Biol Chem. 2016;291(11):6026–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao L, You W, Sun D, Xu H, You X, Xu H, Wu Z, Xie Z, Liang Y. Vps21 directs the PI3K-PI(3)P-Atg21-Atg16 module to phagophores via Vps8 for autophagy. Int J Mol Sci. 2022;23(17):9550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao YG, Codogno P, Zhang H. Machinery, regulation and pathophysiological implications of autophagosome maturation. Nat Rev Mol Cell Biol. 2021;22(11):733–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shin DW. Lipophagy: molecular mechanisms and implications in metabolic disorders. Mol Cells. 2020;43(8):686–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He Y, Lu H, Zhao Y. Development of an autophagy activator from class III PI3K complexes, Tat-BECN1 peptide: mechanisms and applications. Front Cell Dev Biol. 2022;10: 851166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, Tanaka K, Cuervo AM, Czaja MJ. Autophagy regulates lipid metabolism. Nature. 2009;458(7242):1131–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amir M, Zhao E, Fontana L, Rosenberg H, Tanaka K, Gao G, Czaja MJ. Inhibition of hepatocyte autophagy increases tumor necrosis factor-dependent liver injury by promoting caspase-8 activation. Cell Death Differ. 2013;20(7):878–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olzmann JA, Carvalho P. Dynamics and functions of lipid droplets. Nat Rev Mol Cell Biol. 2019;20(3):137–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daniele S, Mangano G, Durando L, Ragni L, Martini C: The Nootropic Drug Alpha-Glyceryl-Phosphoryl-Ethanolamine Exerts Neuroprotective Effects in Human Hippocampal Cells. Int J Mol Sci. 2020;21(3):941. [DOI] [PMC free article] [PubMed]
- 12.Wang TT, Yang Y, Wang F, Yang WG, Zhang JJ, Zou ZQ. Docosahexaenoic acid monoglyceride induces apoptosis and autophagy in breast cancer cells via lipid peroxidation-mediated endoplasmic reticulum stress. J Food Sci. 2021;86(10):4704–16. [DOI] [PubMed] [Google Scholar]
- 13.Yang L, Guan G, Lei L, Lv Q, Liu S, Zhan X, Jiang Z, Gu X. Palmitic acid induces human osteoblast-like Saos-2 cell apoptosis via endoplasmic reticulum stress and autophagy. Cell Stress Chaperones. 2018;23(6):1283–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamamoto T, Takabatake Y, Minami S, Sakai S, Fujimura R, Takahashi A, Namba-Hamano T, Matsuda J, Kimura T, Matsui I, et al. Eicosapentaenoic acid attenuates renal lipotoxicity by restoring autophagic flux. Autophagy. 2021;17(7):1700–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang T, He M, Zhang X, Guo Z, Wang P, Long F. Deciphering the impact of circRNA-mediated autophagy on tumor therapeutic resistance: a novel perspective. Cell Mol Biol Lett. 2024;29(1):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dai Y, Li K, Wu W, Wu K, Yi H, Li W, Xiao Y, Zhong Y, Cao Y, Tian L. Steroid hormone 20-hydroxyecdysone induces the transcription and complex assembly of V-ATPases to facilitate autophagy in Bombyx mori. Insect Biochem Mol Biol. 2020;116: 103255. [DOI] [PubMed] [Google Scholar]
- 17.Tian L, Ma L, Guo E, Deng X, Ma S, Xia Q, Cao Y, Li S. 20-Hydroxyecdysone upregulates Atg genes to induce autophagy in the Bombyx fat body. Autophagy. 2013;9(8):1172–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao H, Long S, Liu S, Yuan D, Huang D, Xu J, Ma Q, Wang G, Wang J, Li S, et al. Atg1 phosphorylation is activated by AMPK and indispensable for autophagy induction in insects. Insect Biochem Mol Biol. 2023;152: 103888. [DOI] [PubMed] [Google Scholar]
- 19.Wu W, Luo M, Li K, Dai Y, Yi H, Zhong Y, Cao Y, Tettamanti G, Tian L. Cholesterol derivatives induce dephosphorylation of the histone deacetylases Rpd3/HDAC1 to upregulate autophagy. Autophagy. 2021;17(2):512–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guan XL, Cestra G, Shui G, Kuhrs A, Schittenhelm RB, Hafen E, van der Goot FG, Robinett CC, Gatti M, Gonzalez-Gaitan M, et al. Biochemical membrane lipidomics during Drosophila development. Dev Cell. 2013;24(1):98–111. [DOI] [PubMed] [Google Scholar]
- 21.Thelen AM, Zoncu R. Emerging roles for the lysosome in lipid metabolism. Trends Cell Biol. 2017;27(11):833–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schulze RJ, Drizyte K, Casey CA, McNiven MA. Hepatic lipophagy: new insights into autophagic catabolism of lipid droplets in the liver. Hepatol Commun. 2017;1(5):359–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim S, Kim N, Park S, Jeon Y, Lee J, Yoo SJ, Lee JW, Moon C, Yu SW, Kim EK. Tanycytic TSPO inhibition induces lipophagy to regulate lipid metabolism and improve energy balance. Autophagy. 2020;16(7):1200–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eskelinen EL. Roles of LAMP-1 and LAMP-2 in lysosome biogenesis and autophagy. Mol Aspects Med. 2006;27(5–6):495–502. [DOI] [PubMed] [Google Scholar]
- 25.Chaudhry N, Sica M, Surabhi S, Hernandez DS, Mesquita A, Selimovic A, Riaz A, Lescat L, Bai H, MacIntosh GC, et al. Lamp1 mediates lipid transport, but is dispensable for autophagy in Drosophila. Autophagy. 2022;18(10):2443–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zechner R, Zimmermann R, Eichmann TO, Kohlwein SD, Haemmerle G, Lass A, Madeo F. FAT SIGNALS–lipases and lipolysis in lipid metabolism and signaling. Cell Metab. 2012;15(3):279–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi JW, Ohn JH, Jung HS, Park YJ, Jang HC, Chung SS, Park KS. Carnitine induces autophagy and restores high-fat diet-induced mitochondrial dysfunction. Metabolism. 2018;78:43–51. [DOI] [PubMed] [Google Scholar]
- 28.Yang HL, Lai ZZ, Shi JW, Zhou WJ, Mei J, Ye JF, Zhang T, Wang J, Zhao JY, Li DJ, et al. A defective lysophosphatidic acid-autophagy axis increases miscarriage risk by restricting decidual macrophage residence. Autophagy. 2022;18(10):2459–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Claude-Taupin A, Morel E. Phosphoinositides: functions in autophagy-related stress responses. Biochim Biophys Acta Mol Cell Biol Lipids. 2021;1866(6): 158903. [DOI] [PubMed] [Google Scholar]
- 30.Ichimura Y, Kirisako T, Takao T, Satomi Y, Shimonishi Y, Ishihara N, Mizushima N, Tanida I, Kominami E, Ohsumi M, Noda T, Ohsumi Y. A ubiquitin-like system mediates protein lipidation. Nature. 2000;408(6811):488–92. [DOI] [PubMed] [Google Scholar]
- 31.Jiang W, Ogretmen B. Autophagy paradox and ceramide. Biochim Biophys Acta. 2014;1841(5):783–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lavieu G, Scarlatti F, Sala G, Levade T, Ghidoni R, Botti J, Codogno P. Is autophagy the key mechanism by which the sphingolipid rheostat controls the cell fate decision? Autophagy. 2007;3(1):45–7. [DOI] [PubMed] [Google Scholar]
- 33.Prieto P, Rosales-Mendoza CE, Terron V, Toledano V, Cuadrado A, Lopez-Collazo E, Bannenberg G, Martin-Sanz P, Fernandez-Velasco M, Bosca L. Activation of autophagy in macrophages by pro-resolving lipid mediators. Autophagy. 2015;11(10):1729–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li K, Tian L, Guo Z, Guo S, Zhang J, Gu SH, Palli SR, Cao Y, Li S. 20-Hydroxyecdysone (20E) primary response gene E75 isoforms mediate steroidogenesis autoregulation and regulate developmental timing in Bombyx. J Biol Chem. 2016;291(35):18163–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Long S, Cao W, Qiu Y, Deng R, Liu J, Zhang L, Dong R, Liu F, Li S, Zhao H, et al. The appearance of cytoplasmic cytochrome C precedes apoptosis during Drosophila salivary gland degradation. Insect Sci. 2024;31(1):157–72. [DOI] [PubMed] [Google Scholar]
- 36.Zhang J, Zhang W, Wei L, Zhang L, Liu J, Huang S, Li S, Yang W, Li K. E93 promotes transcription of RHG genes to initiate apoptosis during Drosophila salivary gland metamorphosis. Insect Sci. 2023;30(3):588–98. [DOI] [PubMed] [Google Scholar]
- 37.Chang J, Wang R, Yu K, Zhang T, Chen X, Liu Y, Shi R, Wang X, Xia Q, Ma S. Genome-wide CRISPR screening reveals genes essential for cell viability and resistance to abiotic and biotic stresses in Bombyx mori. Genome Res. 2020;30(5):757–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu J, Xie X, Ma Q, Zhang L, Li Y, Chen Y, Li K, Xiao Y, Tettamanti G, Xu H, et al. Identification of host molecules involved in the proliferation of nucleopolyhedrovirus in Bombyx mori. J Agric Food Chem. 2022;70(45):14427–38. [DOI] [PubMed] [Google Scholar]
- 39.Zeng L, Ma B, Yang S, Zhang M, Wang J, Liu M, Chen J. Role of autophagy in lysophosphatidylcholine-induced apoptosis in mouse Leydig cells. Environ Toxicol. 2022;37(11):2756–63. [DOI] [PubMed] [Google Scholar]
- 40.Wu W, Li K, Guo S, Xu J, Ma Q, Li S, Xu X, Huang Z, Zhong Y, Tettamanti G, et al. P300/HDAC1 regulates the acetylation/deacetylation and autophagic activities of LC3/Atg8-PE ubiquitin-like system. Cell Death Discov. 2021;7(1):128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xie K, Tian L, Guo X, Li K, Li J, Deng X, Li Q, Xia Q, Zhong Y, Huang Z, et al. BmATG5 and BmATG6 mediate apoptosis following autophagy induced by 20-hydroxyecdysone or starvation. Autophagy. 2016;12(2):381–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Fig. S1 Autophagy and lipid detection in B. mori fat body after BmAtg1, BmAtg5, and BmAtg8 RNAi and detection of BmAtg gene knockout efficiency in BmN cells.
Additional file 2: Fig. S2 The TEM and BODIPY results of WT1118 andppl-GAL4>WT1118,and principal component analysis (PCA) of metabolites in all groups, and volcano plot analysis of differential metabolites with blocked or premature autophagy.
Additional file 3: Fig. S3 KEGG enrichment of metabolites in D. melanogaster with blocked or premature autophagy.
Additional file 4: Fig. S4 Heatmap of metabolites in DmAtg1:DmAtg13 overexpressing and DmAtg1 mutant larvae at W, WPP, and 3WPP stages.
Additional file 5: Fig. S5 Effect of metabolite treatments on MTOR activity under normal or starvation conditions.
Additional file 6: Table S1 The total number of metabolites that are up- and downregulated in metabolomics.
Additional file 7: Table S2 The data provides information on metabolites that are up- and downregulated in DmAtg1:DmAtg13 overexpressed larvae at W stage.
Additional file 8: Table S3 The data provides information on metabolites that are up- and downregulated in DmAtg1:DmAtg13 overexpressed larvae at WPP stage.
Additional file 9: Table S4 The data provides information on metabolites that are up- and downregulated in DmAtg1:DmAtg13 overexpressed larvae at 3WPP stage.
Additional file 10: Table S5 The data provides information on metabolites that are up- and downregulated in DmAtg1 mutant larvae at W stage.
Additional file 11: Table S6 The data provides information on metabolites that are up- and downregulated in DmAtg1 mutant larvae at WPP stage.
Additional file 12: Table S7 The data provides information on metabolites that are up- and downregulated in DmAtg1 mutant larvae at 3WPP stage.
Additional file 13: Table S8 The data presents the fold change of metabolites after blocking or inducing autophagy in D. melanogaster.
Additional file 14: Table S9 The information of primers in this study.
Additional file 15: Original uncropped western blots.
Data Availability Statement
Data will be made available on request. All data generated or analysed during this study are included in this published article, its supplementary information files and publicly available repositories. The original metabolomics data supporting this study are available on Figshare (10.6084/m9.figshare.29137676).