Abstract
Objective
The bed nucleus of the stria terminalis (BNST) is involved in feeding, reward, aversion, and anxiety-like behavior. We identify BNST neurons defined by the expression of vesicular glutamate transporter 3, VGluT3.
Methods
A combination of in situ hybridization, tract tracing, ex vivo whole-cell electrophysiology, in vivo recording, optogenetic, and behavioral approaches were used.
Results
VGluT3 neurons were localized to anteromedial BNST, were molecularly distinct from accumbal VGluT3 neurons, and co-express vesicular GABA transporter (VGaT). BNST VGluT3 neurons projected to arcuate nucleus (ARC) and paraventricular nucleus of the hypothalamus (PVN), regions critical for feeding and homeostatic regulation. Most single BNST VGluT3 neurons projected to either PVN or ARC and a subset projected to both. BNST VGluT3 neurons functionally transmit GABA to both ARC and PVN, with rare glutamate co-transmission to ARC. In vivo, VGluT3 BNST neurons showed greater neuronal activity in response to sucrose consumption while sated compared with fasted. When fasted, optogenetic stimulation of BNST VGluT3 neurons decreased sucrose consumption using several stimulation conditions but not when stimulation occurred prior to sucrose access, suggesting that BNST VGluT3 activation concurrent with consumption in the fasted state reduces feeding. BNST VGluT3 activation had no effect on anxiety-like behavior in several paradigms (novelty-suppressed feeding, open field, and elevated zero maze). BNST VGluT3 activation also did not result in real-time place preference or aversion.
Conclusions
We interpret these data such that VGluT3 BNST neurons represent a unique cellular population within the BNST that provides inhibitory input to hypothalamic regions to decrease sucrose consumption.
Keywords: VGluT3, Feeding, GABA, Glutamate, Internal state, Co-transmission
Highlights
-
•
BNST VGluT3 neurons largely co-express VGaT and reside in anteromedial BNST.
-
•
BNST VGluT3 neurons synapse within BNST and feeding-related hypothalamic targets.
-
•
BNST VGluT3 neurons transmit GABA, rarely glutamate, to hypothalamus targets.
-
•
BNST VGluT3 neurons state-dependently signal sucrose consumption.
-
•
BNST VGluT3 activation reduces sucrose consumption when fasted.
1. Introduction
In the late 1970s, George Alheid embarked on what was intended to be a “brief excursion into neuroanatomy” before investigating the functionality of the extended amygdala [1]. Over a half century later, the subdivisions and cell-types of the extended amygdala remain not wholly understood, as it continues to be discovered that its neuroanatomy and functionality are diverse, particularly the bed nucleus of the stria terminalis (BNST).
Historically, the neuroanatomy of the BNST was subdivided according to medial-lateral, dorsal-ventral, and anterior–posterior axes based on tract tracing and immunohistochemical expression [[2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13]]. The anterior BNST has dense projections to the paraventricular nucleus of the hypothalamus (PVN) [6,9,[14], [15], [16]]. The anterior BNST can also be further subdivided into the anteromedial and anterolateral BNST. The anteromedial BNST has projections to the nucleus accumbens, arcuate nucleus (ARC), central amygdala, supramammillary and tuberomammillary nuclei, ventral tegmental area (VTA), and ventrolateral periaqueductal gray [6,9,16].
Some mental health disorders, such as anorexia nervosa, have a behavioral pathology that exists at the intersection of eating habits and anxiety. The BNST, including the anteromedial BNST, has a strong role in feeding regulation [[17], [18], [19], [20], [21], [22], [23]], as well as in the expression of anxiety-like behavior. Early studies of pharmacologically-induced anorexia showed high expression of immediate early gene markers reflective of BNST activation [[24], [25], [26]]. Studies using BNST lesions or optogenetic manipulations demonstrated its importance in the expression of anxiety-like behavior through associative learning [[27], [28], [29], [30]] or through non-associative tasks, such as elevated mazes [[31], [32], [33], [34]]. How BNST cell-types may affect both feeding and anxiety-like behavior, such as foraging under threat, is less clear. Both foraging [35,36] and novelty-suppressed feeding studies [37,38] have shown that the BNST can be involved. By studying the BNST using both feeding and anxiety tasks, we may disentangle distinct roles of BNST cell-types preferentially to feeding, anxiety, or both.
With novel tools to probe the transcriptomics of neuroanatomically distinct brain regions, it was discovered that the human and rodent BNST have a high level of transcriptional diversity [39,40]. Single-cell RNA sequencing data from mouse BNST revealed 41 transcriptionally distinct cell-types with 86% of cells expressing GABAergic genetic markers and the remaining 14% expressing glutamatergic genetic markers [40]. Parcellating the behavioral functionality of the BNST is not trivial, with several studies suggesting dichotomous, even opposing roles in feeding, anxiety, and emotional valence depending on cell-type and circuit involvement [17,[41], [42], [43], [44]]. While BNST is primarily a GABAergic brain region, glutamatergic BNST populations may have different functions from other BNST cell-types [18,40]. Based on these data, we hypothesized that glutamatergic BNST neurons may be a distinct target for the regulation of feeding and/or anxiety-like behavior.
We identified a unique cell-type within the anteromedial BNST that expressed the vesicular glutamate transporter type 3 (VGluT3), a molecular marker for the vesicular loading of glutamate into synaptic vesicles. BNST VGluT3 neurons were distinct from striatal VGluT3 neurons due to the absence of the cholinergic marker choline acetyltransferase that classify striatal VGluT3-expressing cholinergic interneurons [[45], [46], [47]]. VGluT3 BNST neurons mostly resided in the anteromedial BNST region and most single BNST VGluT3 neurons projected to either PVN or ARC with a subset that projected to both hypothalamic regions. In contrast to initial prediction, BNST VGluT3 neurons send dense GABAergic projections to hypothalamic PVN and ARC. These VGluT3 BNST neurons are sensitive to internal state, such that their neuronal activity is greatest when consuming sucrose in the sated state compared with the fasted state. Optogenetic stimulation of BNST VGluT3 neurons was sufficient to reduce sucrose consumption in the fasted state. However, optogenetic VGluT3 activation had no effect in classic rodent paradigms of anxiety-like behavior. Together, we interpret these data such that VGluT3 BNST neurons may represent a unique target for manipulating the neuronal regulation of food consumption or energy balance.
2. Materials and methods
2.1. Animals
VGluT3-IRES∷Cre knock-in mice (4–5 months old; B6; 129S-Slc17a8tm1.1(cre)Hze/J; Stock #028534), VGaT-IRES::Cre knock-in mice (4–5 months old; Slc32a1tm2(cre)Lowl/J; Stock #016962), VGaT-IRES::FlpO (B6.Cg-Slc32a1tm1.1(flpo)Hze/J, Stock #029591), and wildtype C57Bl6/J mice (Stock #000664) were purchased from The Jackson Laboratory (Bar Harbor, ME). Ai193 mice were received from The Allen Institute and crossed with double transgenic VGluT3::Cre/VGaT::Flp mice to create triple transgenic Ai193/VGluT3::Cre/VGaT::Flp mice. These mouse lines were bred and maintained at the University of Colorado Boulder and the University of Colorado Anschutz Medical Campus. Wildtype C57Bl6/J mice were used for the in situ hybridization experiment. All mice were group-housed by sex (5 mice/cage) under a reversed 12hr:12hr light/dark cycle (lights on at 22:00) with access to water ad libitum. All behavior experiments were performed during the dark phase of the light cycle. The experiments described were conducted in accordance with the regulations by the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee at the University of Colorado Boulder as well as at the University of Colorado Anschutz Medical Campus.
2.2. Surgery
Mice were anesthetized in a gasket-sealed induction chamber at 3% isoflurane gas. After confirming a surgical plane of anesthesia, isoflurane was continuously delivered at 1–2% concentration while the mouse was secured in the stereotactic instrument. All injections were performed using an UltraMicroPump, Nanofil syringes, and 35-gauge needles (Micro4; World Precision Instruments, Sarasota, FL). The injection rate was 100 nl/min with a 400 nL injection volume. Syringes were left in place for 10 min following injections and slowly withdrawn. BNST coordinates were +0.6 mm anteroposterior, +0.6 mm mediolateral, −4.2 mm dorsoventral coordinates. Mice were provided with 3 days of postoperative care with 5 mg/kg carprofen (IP) and allowed 3–4 weeks of recovery before experimentation. For synaptophysin histology, VGluT3-IRES::Cre mice were unilaterally injected with AAV1-hSyn-FLEX-GFP-2A-Synaptophysin-mRuby (Stanford Vector Core, 5 × 1012 titer) into the BNST. For retrograde tracing histology, VGluT3-IRES::Cre mice were unilaterally injected with AVVrg-hSyn-DIO-eGFP (Addgene #50457-AAVrg, 6.5 × 1012 titer) into the PVN (−0.22 mm anteroposterior, +0.15 mm mediolateral, −4.75 mm dorsoventral coordinates) and with AAVrg-hSyn-DIO-mCherry (Addgene #50459-AAVrg, 6.5 × 1012) into the ARC (−1.46 mm anteroposterior, +0.15 mm mediolateral, −5.85 mm dorsoventral coordinates). For optogenetic stimulation experiments, AAV8-hSyn-FLEX-CoChR-GFP (UNC Vectore Core) or AAV8-hSyn-DIO-GFP (Addgene) were injected bilaterally (5 × 1012 titer) into the BNST. Optogenetic bilateral fibers were implanted at a 10° angle slightly dorsal to the BNST. For whole-cell patch-clamp electrophysiology experiments, both VGluT3-IRES::Cre and VGaT-IRES::Cre mice were injected with AAV8-hSyn-DIO-CoChR-GFP into the BNST. For in vivo monitoring of calcium-dependent neural signaling, VGluT3-IRES::Cre mice were unilaterally injected with AAV1-hSyn-FLEX-GCaMP6m (Addgene) into the BNST. Fiber photometry optic fibers (400-μm core diameter, 0.66 NA, Doric Lenses) were implanted slightly dorsal to the BNST and slowly lowered in discrete increments over the course of 25 min. All implants were secured with skull screws and dental cement.
2.3. Histology – viral verification, synaptophysin, and retrograde tracing
For viral verification, synaptophysin, and retrograde tracing, mice were anesthetized with isoflurane and perfused transcardially with 0.1M phosphate buffer followed by 4% (w/v) paraformaldehyde in 0.1M phosphate buffer, pH 7.3. Brains were extracted and cryoprotected in 18% sucrose solution in 0.1M phosphate buffer at 4 °C overnight. Brains were cryosectioned to obtain coronal slices with BNST (30 μm) and mounted onto gelatin-coated slides. For viral verification, slides were imaged for GFP fluorescent expression on a Zeiss widefield Axioscope. For synaptophysin and retrograde tracing histology, slides were imaged on a Nikon A1R confocal (20X) with 3 μm steps z-stacked images at 512 × 512 pixel resolution. For each z-stacked image, the maximum intensity of each z-stack was measured and collapsed across all stacks using FIJI software. For retrograde tracing histology, images of BNST somata were both manually and automatically counted in FIJI software, then cross-verified for comparison. There were no statistical differences between manual counts and automated counts and therefore automated counts were used. For automated cell counting in FIJI, appropriate regions of interest were drawn that included the entire BNST, and the Moments threshold method was used. Cell bodies were defined as particles that were over 20 μm2 in area and 0.40–1.00 in circularity. All reproductions of the mouse brain atlas for histology were derived from the Franklin and Paxinos [48] atlas. Mice with off-target or lack of expression were excluded from analysis.
2.4. Histology – in situ hybridization
For in situ hybridization, C57BL6/J mice (8–12 weeks old) were anesthetized with isoflurane and perfused transcardially with RNase-free 0.1M phosphate buffer followed by 4% (w/v) paraformaldehyde in 0.1M phosphate buffer, pH 7.3. Brains were rapidly extracted and flash-frozen with isopentane. Brains were cryosectioned to obtain coronal slices (12 μm) from +1.10 mm to +0.02 mm bregma and were immediately mounted to Fisher SuperFrost Plus slides. Sections were then heat-treated and exposed to protease digestion followed by hybridization with target probes to mouse VGluT3 and VGaT mRNA (ACDBio). Sections were further treated with Opal dyes specific to each probe. Slides were coverslipped in Prolong Diamond with DAPI (ThermoFisher) and imaged on a Nikon A1R confocal (20X) with 3 μm steps z-stacked images at 512 × 512 pixel resolution. Images were further analyzed in FIJI, such that the Moments threshold method was used, and suitable regions of interest were defined as particles with an area over 5 μm2. Particles that overlapped in expression for VGluT3 and VGaT were considered to be co-expressing VGluT3 and VGaT.
2.5. Histology – immunohistochemistry
Frozen brain sections were thawed and washed 3 times for 10 min each with 0.1M phosphate buffer. Sections were then incubated in blocking buffer (4% bovine serum albumin and 0.3% TritonX-100 in 0.1M phosphate buffer, pH 7.3) for 1 h at room temperature. For Ai193/VGluT3::Cre/VGaT::Flp mice, the primary antibodies were goat anti-ChAT (Invitrogen PA518518), rabbit anti-tdTomato (Takara 632496), and mouse anti-GFP (Takara JL-8). Sections incubated in primary antibodies overnight at 4 °C and then washed in 0.1M phosphate buffer (3 × 10 min). Secondary antibodies were donkey anti-rabbit Alexa594 (Jackson Immunoresearch #711585152) and donkey anti-goat Alexa647 (Jackson Immunoresearch #705605147), diluted at 1:200 in blocking buffer. Sections incubated for 2 h in antibody solutions and were washed again with 0.1 M PB (3 × 10 min). These sections were mounted onto gelatin-coated slides and coverslipped with ProLong diamond mounting medium. Slides were imaged with a Nikon A1R confocal microscope (20X air objective).
2.6. Whole-cell patch-clamp electrophysiology
For slice preparation, mice were anesthetized with isoflurane and transcardially perfused with ice-cold cutting solution containing (in mM): 75 NaCl, 2.5 KCl, 6 MgCl2, 0.1 CaCl2, 1.2 NaH2PO4, 25 NaHCO3, 2.5 d-glucose, 50 sucrose. Coronal slices (240 μm) with the BNST, ARC, and PVN were cut in the same cutting solution that was used for transcardial perfusion. Slices were maintained at 32 °C in aCSF containing (in mM): 126 NaCl, 2.5 KCl, 1.2 MgCl2, 1.2 NaH2PO4, 2.5 CaCl2, 21.4 NAHCO3, 11.1 d-glucose and 10 μm MK-801. After at least 30 min of incubation, slices were transferred to a recording chamber and continually perfused with 34 ± 2 °C aCSF at a rate of 2 ml/min. All solutions were always bubbled with 95% O2, 5% CO2. When appropriate, picrotoxin (100 μM) was either bath applied or in the aCSF. DNQX (10 μM) was bath applied when appropriate.
All whole-cell recordings were performed using an Axopatch 200B amplifier (Molecular Devices). Data were acquired using an ITC-18 interface (Instrutech) and Axograph X software (Axograph Scientific) at 10 KHz and filtered to 2 KHz. Neurons were visualized on a BX51WI microscope (Olympus) with a LED and filter cube (ThorLabs). Samples that showed GFP fluorescence both at BNST cell bodies and fiber expression in the ARC and PVN were selectively used for whole cell recordings. Samples that did not show GFP fluorescence at BNST cell bodies nor fiber expression at the ARC or PVN were excluded. Widefield activation of CoChR was achieved with collimated light from a LED (470 nm) through the 40x water immersion objective. For whole-cell voltage-clamp recordings, cells were held at a voltage of 0 mV and either −50mV or −60mV for the ARC and PVN, respectively. For the ARC, only cells with a series resistance less than 30 MΩ and an input resistance of more than 1 GΩ were recorded, as previously reported [49,50]. For the PVN, only cells with a series resistance less than 20 MΩ and an input resistance of at least 500 MΩ were recorded, as previously reported [51]. Episode intervals were 30s with a single pulse of a 1 ms width occurred at 2500 ms within a 3000 ms timebase. For CoChR validation, BNST cells were current-clamped, and a single pulse with a 1 ms width occurred at 300 ms in a 500 ms timebase with episode intervals of 30s. Patch pipettes (2.5–3 MΩ) were pulled from borosilicate glass (World Precision Instruments). The internal pipette solution for all voltage-clamp experiments contained (in mM): 150 CsMeSO4, 10 HEPES (K), 0.1 EGTA, 0.1 CaCl2, 2 MgCl2, 0.2 Na2-GTP, 2 Na2-ATP, 4.6 Na-phosphocreatine, and 5 QX-314 bromide (pH 7.3, 280 mOsm). The internal pipette solution for current-clamp experiments contained (in mM): 135 KCl, 10 HEPES (K), 0.1 EGTA, 0.1 CaCl2, 2 MgCl2, 0.2 Na2-GTP, 2 Na2-ATP, and 4.6 Na-phosphocreatine (pH 7.3, 280 mOsm). All data processing occurred using Axograph (1.7.6). A baseline subtraction was applied based on a 100 ms range prior to the event. A sharp low-pass filter was applied with a cutoff frequency of 2000Hz. The maximum peak was detected after the stimulation event.
2.7. Calcium fiber photometry recordings
GCaMP6m was excited at two wavelengths (465 nm and 405 nm isosbestic control) with amplitude-modulated signals from two light-emitting diodes reflected off dichroic mirrors and then coupled into an optic fiber [[52], [53], [54]]. The GCaMP signal and the isosbestic control signal were returned through the same optic fiber and acquired using a femtowatt photoreceiver (Newport, Irvine, CA), digitized at 1 kHz, and then recorded by a real-time signal processor (Tucker Davis Technologies). For analysis of calcium fiber photometry recordings, custom-written MATLAB scripts were used and are available at www.root-lab.org/code. The isosbestic signal (405 nm) and the GCaMP signal (465 nm) were downsampled (10x) and peri-event time histograms were created surrounding events of interest (e.g., sucrose consumption, shock). For each trial, data were detrended by regressing the 405 nm signal on the 465 nm signal. The generated linear model was used to create a predicted 405 nm signal that was subtracted from the 465 nm signal to remove movement, photo-bleaching, and fiber bending artifacts [52].
2.8. Fiber photometry - sucrose consumption
Mice were recorded in response to consuming 8% sucrose either in the sated state (free fed) or fasted state (fasted to 85–90% free-feeding body weight). Mice were trained to consume sucrose within a 10-minute time period for 5 days before recordings. The apparatus (Med-Associates) was outfitted with a spout for a 250 ml sipper bottle (Allentown, LLC). Mice were habituated to the fiber photometry cable at least 48 h before recordings. The order of recordings during either the fasted or sated state were counter-balanced. A lick bout was defined as the occurrence of at least 2 consecutive licks under 400 ms interlick interval that was preceded by at least 3 s in which no licks occurred.
2.9. Fiber photometry - shock exposure
Mice were recorded in response to five electric footshocks while in the fasted or sated state, the order of which was counter-balanced. In the fasted state, mice were limited to 85–90% of their original body weight, while they were given food ad libitum in the sated state. Mice were administered 0.5 mA 500 ms electric foot shocks in a novel environment with an average inter-trial interval of 60s.
2.10. Fiber photometry – Pavlovian reward conditioning
Mice were fasted to 85–90% body weight and trained to consume 8% sucrose within a 100-minute time period in a Pavlovian conditioning task. The apparatus (Med-Associates) was outfitted with a custom 3D-printed reward magazine, which can be found on www.root-lab.org/code. A single-speed syringe pump (Med-Associates) was used to deliver 30 μl sucrose to the reward magazine. The task consisted of 40 presentations of a 10s conditioned stimulus (CS+) that co-terminated with sucrose reward delivery, which was 1.5s long. The task also included 40 presentations of a 10s conditioned stimulus (CS-) that had no programmable changes. The average inter-trial interval was 90s. Cue onset was defined as the time CS + or CS- started sounding. Reward retrieval onset was defined as the time mice entered the magazine during cue presentation.
2.11. Optogenetic stimulation – sucrose consumption
Mice were fasted to 85–90% of their original body weight and trained to consume 8% sucrose within a 40-minute time period for 7 days before stimulation experiments began. The apparatus (Med-Associates) was outfitted with a spout for a 250 ml sipper bottle (Allentown, LLC). Mice were habituated to the fiber optic cable at least 48 h before stimulation experiments began. All stimulation conditions had a pulse width of 5 ms and a frequency of 20Hz. Light intensity at the end of the tip was set to be approximately 10 mW. Stimulation conditions were determined based on previously published research [[55], [56], [57]]. For open-loop stimulation, stimulation occurred in two 10-minute time blocks within the 40-minute time period, such that there was no stimulation in the first 10 min and from 20 to 30 min. For the calculated sucrose ratio, licks that occurred during the 10-minute blocks of stimulation were divided by the number of licks during the non-stimulation 10-minute blocks. For closed-loop stimulation, a stimulation train was only triggered by 3 licks with an inter-lick interval less than 1s. For the calculated sucrose ratio, licks during closed-loop stimulation were divided by licks from the preceding day when no stimulation occurred. For prior stimulation, stimulation occurred continuously for 20 min in a novel environment before being placed in the behavior apparatus for sucrose consumption. For the calculated sucrose ratio, licks after prior stimulation were divided by licks from the preceding day when no stimulation occurred. For tonic stimulation, stimulation occurred continuously throughout the 40-minute session. At least 24 h of no stimulation separated each stimulation condition. For the calculated sucrose ratio, licks during tonic stimulation were divided by licks from the preceding day when no stimulation occurred.
2.12. Novelty-suppressed feeding
Mice were food-deprived for 24 h before the start of the novelty-suppressed feeding task. For the novelty-suppressed feeding task, mice were placed in an open field box (40 cm length x 40 cm width x 35 cm height, Stoelting) in which the center was illuminated at 40 lux. The center of the box contained a petri-dish weighed down by batteries. A standard food biscuit (approximately 5g) was attached to the petri-dish using a rubber band, in order to prevent mice from pulling the food biscuit away from the center. Photo-stimulation immediately began when mice were placed into the open field and lasted for 10 min. Light intensity at the end of the tip was set to be approximately 10 mW. Photo-stimulation was performed at 20 Hz with 5 ms pulse width using blue laser light (473 nm, CNI Laser). The center of the box was defined as 5% of the total area, while the periphery was defined as 50% of the total area and was limited to the edges of the box. Food approach latency was measured as the time it took the mice to approach the center with food. Novelty-suppressed feeding behavior was video recorded and quantified using ANY-Maze software.
2.13. Open field
Mice were fasted to 85–90% of their original body weight before being placed in an open field box (40 cm length x 40 cm width x 35 cm height, Stoelting). Photo-stimulation immediately began when mice were in the box and lasted for 20 min. Photo-stimulation parameters were the same as previously described. The center of the box was defined as 5% of the total area, while the periphery was defined as 50% of the total area and was limited to the edges of the box. Approach latency was defined as the time it took for the center of the mice to reach the center of the box. Open field behavior was video recorded and quantified using ANY-Maze software.
2.14. Elevated zero maze
Mice were fasted to 85–90% of their original body weight before being placed on an elevated zero maze apparatus (78 cm height, 40 cm diameter, 5 cm track width, 30 cm wall height, 31 cm closed arm length, Accuscan Instruments). The apparatus was adjusted such that the original black circular track was covered with white Plexiglas for better video tracking, and the clear walls of the closed arms were taped with black light-blocking tape. Mice were placed in the center of one of the closed arms, and photo-stimulation immediately began. Photo-stimulation lasted for 20 min, and parameters were the same as previously described. Enter latency was defined as the time it took for the mice to reach the other closed arm, in which all four paws were in the other closed arm. Elevated zero maze behavior was video recorded and quantified using ANY-Maze software.
2.15. Real-time place preference
The real-time place preference box (40 cm length x 40 cm width x 35 cm height, Stoelting) consists of a stripe chamber (18 cm × 20 cm), solid chamber (18 cm × 20 cm), and a connecting antechamber. The solid chamber had been modified with a peel-and-stick window film to prevent reflection. Mice were acclimated to the tethers during baseline real-time place preference with no photo-stimulation and with ad libitum food access. The next day, mice were subjected to 15-minute photo-stimulation on both the solid and stripe sides with the sides counter-balanced between mice. Photo-stimulation parameters were the same as previously described. The same experiment was repeated when mice were fasted to 85–90% of baseline body weight. Real-time place preference behavior was video recorded, and time spent in each side by seconds was quantified using ANY-Maze software. The time spent in the stripe side was subtracted from the time spent in the solid side, such that a positive value indicates more time spent in the striped side, and a negative value indicates more time spent in the solid side.
2.16. Statistical analysis
Values are reported as mean ± standard error with bars depicted as shaded areas in fiber photometry traces. All statistical tests were performed with R (4.0.5). Results were subject to a two-way ANOVA analysis to test the effect of holding voltage (−50 mV, 0 mV), drug (baseline, picrotoxin), group (GFP, CoChR), and state (fasted, sated), where appropriate. Comparisons were made by paired or non-paired, two-tailed t-test with Bonferonni corrections. Tukey’s HSD test was used for post-hoc analysis when appropriate. For analysis of latency from novelty-suppressed feeding, open field, and the elevated zero maze, hazard ratios were calculated from a Cox-proportional hazards regression model using the R package survival [58].
3. Results
3.1. Co-localization of vesicular markers of glutamate and GABA within the anteromedial BNST
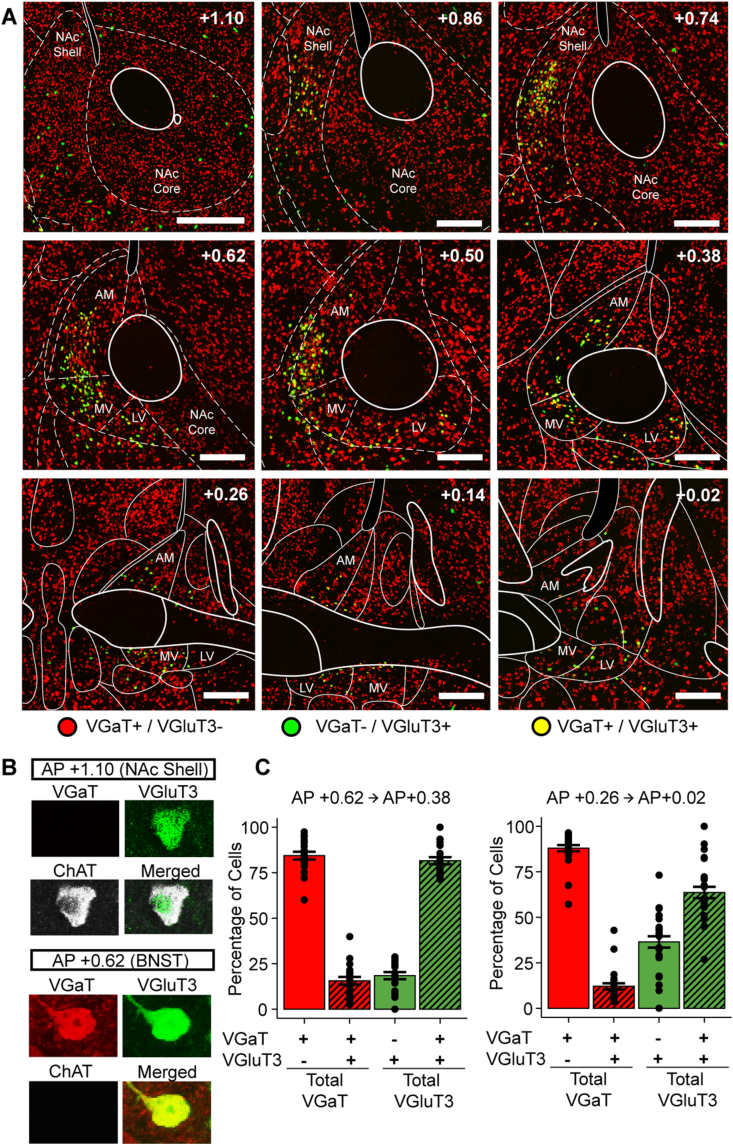
To identify glutamatergic and GABAergic BNST neurons, we used RNAscope in situ hybridization to label VGluT3 and VGaT mRNA in wildtype mice. Expression of VGaT mRNA was abundant throughout BNST while VGluT3 mRNA expression appeared mostly confined to the anteromedial BNST from AP +0.62 mm to AP +0.38 mm (Figure 1A). To test whether BNST VGluT3 neurons were distinct from striatal VGluT3 cholinergic interneurons [46,59], we generated triple transgenic Ai193/VGluT3::Cre/VGaT::Flp mice where VGluT3-Cre neurons expressed GFP and VGaT-Flp neurons expressed tdTomato. We found that BNST VGluT3 neurons lacked ChAT immunolabeling while striatal VGluT3 neurons co-expressed ChAT (Figure 1B), demonstrating that BNST VGluT3 neurons belong to a different class of neurons than striatal VGluT3 cholinergic interneurons.
Figure 1.
Co-expression of vesicular markers of glutamate and GABA accumulation within the anteromedial BNST. (A) Example RNAscope histology from AP +1.10 mm to AP +0.02 mm with VGaT mRNA in red and VGluT3 mRNA in green. Scale bar is 200 μm. N = 4, 2 female, 2 male. (B) Nucleus accumbens shell (NAc shell) VGluT3 neurons express ChAT while BNST VGluT3 neurons co-express VGaT and lack ChAT. Magnification is 40X. (C) Quantification of VGluT3 and VGaT mRNA co-expression across AP axis of the BNST. N = 4 mice, approximately 20 sections/mouse. Outlines are based on the Franklin and Paxino mouse brain atlas. NAc Shell = nucleus accumbens shell, NAc Core = nucleus accumbens core, AM = anteromedial BNST, MV = ventromedial BNST, ventrolateral BNST. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
We next quantified the co-expression of VGaT and VGluT3 mRNA in BNST between AP +0.62 mm to AP +0.38 mm (anterior) and between AP +0.26 mm to AP +0.02 mm (posterior) (Figure 1C). Anteriorly, most VGluT3-expressing neurons co-expressed VGaT, and the VGluT3-expressing neurons were a small subpopulation out of the total VGaT-expressing population. There were fewer VGluT3-only neurons anteriorly compared with VGaT-only neurons. Posteriorly, most VGluT3-expressing neurons still co-expressed VGaT but were less than the anterior portion. Like anterior coordinates, most BNST neurons were VGaT-only and the percent of VGluT3-expressing neurons was low out of the total VGaT-expressing population.
3.2. VGluT3 neurons in the anteromedial BNST form synaptic projections with hypothalamic brain regions
After identifying the unique transcriptional and anatomical profile of VGluT3 BNST neurons, we next aimed to determine their efferent targets. Using AAV1-hSyn-FLEX-GFP-2a-Synaptophysin-mRuby that labels cell bodies and axons within GFP and the presynaptic vesicular protein synaptophysin with mRuby [60], we identified synaptophysin distribution of VGluT3 neurons (Figure 2A). Discrete sites of pre-synaptic vesicular expression were identified in the anterior BNST, lateral septum, septohypothalamic nucleus, paraventricular hypothalamus (PVN), lateral hypothalamic area (LHA), arcuate nucleus (ARC), and the ventral tegmental area (VTA) (Figure 2B). The densest mRuby expression that was not within the BNST itself was the ARC, followed by the PVN. Negligible to no expression was observed elsewhere, including lateral habenula that receives co-transmitted glutamate and GABA from VTA [61]. In summary, BNST VGluT3 neurons project strongly to hypothalamic regions, particularly the PVN and ARC.
Figure 2.
VGluT3 BNST neurons synapse with hypothalamic brain regions. (A) VGluT3-IRES::Cre mice were injected with FLEX-GFP-2A-Synaptophysin-mRuby into the BNST, labeling cell bodies and fibers in GFP and synaptophysin-expressing vesicles in mRuby. Scale bar is 10 μm. High magnification image scale is 50 μm. N = 6, 3 female, 3 male. (B) GFP and mRuby expression throughout the BNST, PVN, ARC, and VTA. Scale bar is 500 μm. Scale bar is 150 μm for VTA. LSV = lateral septum, Shy = septohypothalamic nucleus, AM = anteromedial BNST, MV = ventromedial BNST, PVN = paraventricular nucleus of the hypothalamus, LHA = lateral hypothalamic area, ARC = arcuate nucleus, VTA = ventral tegmental area. (C) VGluT3-IRES::Cre mice were injected with AAVrg-hSyn-DIO-eGFP into the PVN and with AAVrg-hSyn-DIO-mCherry into the ARC. Scale bar is 200 μm. N = 5, 3 female, 2 male. (D) The percentage of BNST neurons that were GFP-only, mCherry-only, and co-labeled. There was a significant difference in percentage expression (F(2, 12) = 4.2, p = 0.03, ANOVA). Further analysis showed a statistical difference between GFP-only neurons and co-labeled neurons (p = 0.03, Tukey’s HSD). (E) Pie chart of averaged proportion of PVN-projecting, ARC-projecting, and collateralizing BNST VGluT3 neurons. (F) Summary illustration of the heterogeneous synaptic projections to the PVN and ARC. Density of lines graphically reflect the percentage of expression.
We next investigated whether individual BNST VGluT3 neurons project to PVN alone, ARC alone, or both PVN and ARC. To determine this, in VGluT3-IRES:Cre mice the PVN was injected with AAVrg-DIO-eGFP, and the ARC was injected with AAVrg-DIO-mCherry. BNST VGluT3 somata that only project to the PVN expressed GFP, those that only project to the ARC expressed mCherry, and those that bifurcate to both co-expressed GFP and mCherry (Figure 2C). There was no observable spatial segregation within the BNST across these three populations that would suggest further neuronatomically-distinct subregions of the anteromedial BNST. The percentage of BNST VGluT3 neurons that were GFP-only, mCherry-only, or GFP + mCherry + out of the total labeled cell bodies was quantified. There was no statistical difference in the percentage of GFP-only and mCherry-only expressing BNST VGluT3 neurons. There was also no statistical difference between mCherry-only and GFP + mCherry + BNST VGluT3 neurons. However, there were significantly more GFP-only neurons compared with GFP + mCherry + BNST VGluT3 neurons (Figure 2D–E). While there was a greater proportion of PVN-projecting neurons (53.4%), this was not statistically different ARC-projecting neurons (31.6%). These results suggest that the majority of PVN-projecting BNST VGluT3 neurons do not bifurcate to the ARC. Further, while most ARC-projecting BNST VGluT3 neurons do not bifurcate to the PVN, a small proportion project to PVN as well (Figure 2F).
3.3. VGluT3 BNST neurons, while genetically expressing the capability to transmit glutamate, functionally transmit GABA and rarely transmit glutamate to the ARC
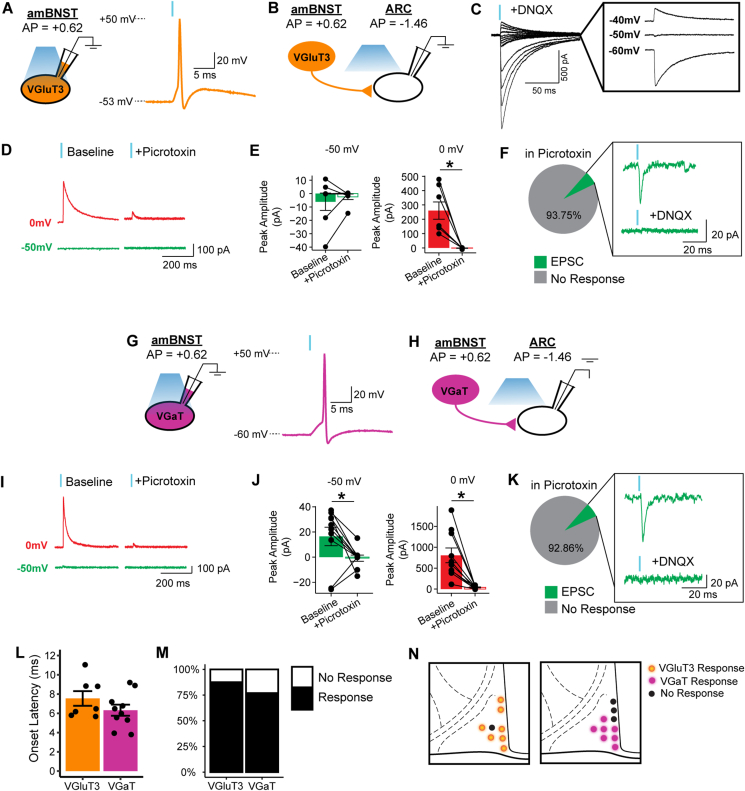
The co-expression of VGluT3 and VGaT mRNA in BNST neurons suggests BNST VGluT3 neurons may co-transmit glutamate and GABA. In order to investigate the synaptic functionality of VGluT3 and VGaT co-expression, VGluT3-IRES::Cre mice were injected with the Cre-dependent, high-photocurrent excitatory opsin CoChR [62,63] and BNST VGluT3 axons were optogenetically stimulated while performing whole-cell recordings in the ARC (Figure 3B). This optogenetic strategy to stimulate action potentials from BNST VGluT3 neurons was validated ex vivo (Figure 3A). The experimental design to obtain both a GABA-mediated inhibitory post-synaptic current and an AMPA-mediated excitatory post-synaptic current (EPSC) from the same cell necessitated an accurate reading of the chloride reversal potential. Thus, the chloride reversal potential was experimentally determined in the ARC while washing on the AMPA receptor antagonist, DNQX, which was −50 mV (Figure 3C). In order to determine if VGluT3 BNST neurons transmit both GABA and glutamate, ARC neurons were voltage-clamped at both 0 mV and −50 mV while the terminals were optogenetically stimulated. ARC neurons showed an optogenetic post-synaptic current at 0 mV, which was abolished following application of the GABAA receptor antagonist, picrotoxin; there were no differences in post-synaptic currents between baseline and picrotoxin at −50 mV (Figure 3D, E). Next, in the continued presence of picrotoxin in the aCSF, we investigated if any recorded ARC neurons demonstrated a DNQX-sensitive post-synaptic current. Only 6.525% of recorded neurons exhibited an optogenetic post-synaptic current at −50 mV, which was abolished with DNQX (Figure 3F). However, due to the continued presence of picrotoxin in the aCSF, it is undetermined if this percentage of recorded neurons that exhibited an EPSC would have also displayed an IPSC. In order to experimentally validate GABA transmission, VGaT-IRES::Cre were similarly injected with CoChR and whole-cell recordings in the ARC were performed (Figure 3H). The optogenetic strategy to stimulate action potentials from VGaT-expressing neurons was validated ex vivo (Figure 3G). Similar to VGluT3::Cre projections, ARC neurons illustrated an optogenetic post-synaptic current at 0 mV, which was abolished following application of the GABAA receptor antagonist, picrotoxin; at −50 mV, the optically-induced post-synaptic current was also abolished with picrotoxin, suggesting that this current is also mediated by GABAA receptor activation (Figure 3I, J). In the continued presence of picrotoxin in the aCSF, we investigated if any recorded ARC neurons demonstrated a DNQX-sensitive post-synaptic current that could be induced by input from VGaT BNST neurons. Only 7.14% of recorded neurons exhibited an optogenetic post-synaptic current at −50 mV, which was abolished with DNQX (Figure 3K). The peak amplitude onset latency was 7.54 ± 0.76 ms for VGluT3-stimulated ARC neurons and 6.32 ± 0.57 ms for VGaT-stimulated ARC neurons, suggestive of monosynaptic transmission (Figure 3L). The proportion of responsive ARC neurons was 87.5% for VGluT3 stimulated terminals and 76.9% for VGaT stimulated terminals (Figure 3M). There were no observable patterns of ARC location between responsive and non-responsive ARC neurons (Figure 3N). Together, only a small minority of VGluT3 terminals functionally transmit a glutamate-mediated ionotropic current to the ARC, and it is only a subset of ARC neurons that receive this presynaptic input from BNST VGluT3 neurons.
Figure 3.
VGluT3 BNST neurons functionally transmit GABA and rarely transmit glutamate to the ARC. (A) Validation of optogenetic-induced action potential of a BNST VGluT3 neuron. (B) VGluT3 terminals were optogenetically stimulated while patching onto an ARC neuron. (C) Chloride reversal potential was experimentally determined of ARC neurons while washing on AMPA receptor antagonist, DNQX, which was −50 mV. (D) VGluT3 to ARC example traces of optogenetic post-synaptic currents under 0 mV and −50 mV at baseline and with GABAA receptor antagonist, picrotoxin, wash. (E) Picrotoxin wash application on VGluT3 optogenetic post-synaptic current at −50 mV and 0 mV. There was a significant effect of drug and voltage (F(1,24) = 19.1, p = 0.0002, ANOVA). Bonferonni-corrected pairwise comparison tests showed that at 0 mV, there was a significant difference in amplitude between baseline and picrotoxin wash (t(6.01) = 4.34, p = 0.004). N = 7 cells, 2 female, 2 male. (F) Percentage of recorded neurons that demonstrated a DNQX-sensitive post-synaptic current with picrotoxin in aCSF. N = 16 cells, 2 female, 2 male. (G) VGaT terminals were optogenetically stimulated while patching onto an ARC neuron. (H) Validation of optogenetic-induced action potential of a VGaT-expressing neuron. (I) VGaT to ARC example traces of optogenetic post-synaptic currents under 0 mV and −50 mV at baseline and with GABAA receptor antagonist, picrotoxin, wash. (J) Picrotoxin wash application on VGaT optogenetic post-synaptic current at −50 mV and 0 mV. There was a significant effect of drug and voltage (F(1, 36) = 17.766, p = 0.0001, ANOVA). Bonferonni-corrected pairwise comparison tests showed that at 0 mV and −50 mV, there was a significant difference in amplitude between baseline and picrotoxin wash (t(9.07) = 4.31, p = 0.001, 0 mV) (t(11.05) = 2.23, p = 0.04, −50 mV). N = 10, 2 female, 3 male. (K) Percentage of recorded neurons that demonstrated a DNQX-sensitive post-synaptic current with picrotoxin in aCSF. N = 14 cells, 2 female, 2 male. (L) Peak amplitude onset latency for ARC recordings in response to BNST VGluT3 and VGaT optogenetic stimulation. (M) Proportion of responsive and non-responsive ARC neurons. (N) Graphic representation of the location of responsive and non-responsive ARC neurons.
3.4. VGluT3 BNST neurons functionally transmit GABA but not glutamate to the PVN
The second major output of VGluT3 BNST neurons was the PVN. We investigated if transmission from VGluT3 BNST neurons was different between the ARC and the PVN. VGluT3-IRES::Cre mice were injected with CoChR and whole-cell recordings in the PVN was performed (Figure 4A). Due to the variability in chloride reversal potential between cell-types and the necessity of an accurate reading, the chloride reversal potential was experimentally determined in the PVN while washing on the AMPA receptor antagonist, DNQX, which was −60 mV (Figure 4B). In order to determine if VGluT3 BNST neurons transmit both GABA and glutamate, PVN neurons were voltage-clamped at both 0 mV and −60 mV while the terminals were optogenetically stimulated. PVN neurons showed an optogenetic post-synaptic current at 0 mV, which was abolished following application of the GABAA receptor antagonist, picrotoxin; there were no differences in post-synaptic current between baseline and picrotoxin at −50 mV (Figure 4C,D). Next, in the continued presence of picrotoxin in the aCSF, we investigated if any recorded PVN neurons demonstrated a DNQX-sensitive post-synaptic current. Compared to the ARC, no recorded neurons exhibited an optogenetic post-synaptic current at −60 mV (Figure 4E). VGaT-IRES::Cre were injected with CoChR while performing whole-cell recordings in the PVN (Figure 4F). PVN neurons showed an optogenetic post-synaptic current at 0 mV, which was abolished following application of the GABAA receptor antagonist, picrotoxin; at −50 mV, the optically-induced post-synaptic current was also abolished with picrotoxin, suggesting that this current is also mediated by GABAA receptor activation (Figure 4G, H). In the continued presence of picrotoxin in the aCSF, no recorded neurons exhibited a DNQX-sensitive post-synaptic current at −60 mV with stimulation from VGaT BNST terminals (Figure 4I). The peak amplitude onset latency was 6.98 ± 0.55 ms for VGluT3-stimulated PVN neurons and 5.18 ± 0.53 ms for VGaT-stimulated PVN neurons, suggestive of monosynaptic transmission (Figure 4J). The proportion of responsive PVN neurons was 47% for VGluT3 stimulated terminals and 72.7% for VGaT stimulated terminals (Figure 4K). There were no observable patterns of PVN location between responsive and non-responsive PVN neurons for VGaT stimulation, but responsive PVN neurons to VGluT3 stimulation may be more ventral (Figure 4L). Overall, different from the ARC, VGluT3 BNST neurons do not appear to functionally transmit a glutamate-mediated ionotropic current to the PVN. Additionally, the lower proportion of PVN responsive neurons as well as some spatial patterning of these responsive neurons suggests that BNST VGluT3 neurons preferentially synapse with a subset of PVN neurons.
Figure 4.
VGluT3 BNST neurons functionally transmit GABA but not glutamate to the PVN. (A) VGluT3 terminals were optogenetically stimulated while patching onto a PVN neuron. (B) Chloride reversal potential was experimentally determined of PVN neurons, which was −60 mV. (C) PVN example traces of VGluT3 optogenetic post-synaptic currents under 0 mV and −60 mV at baseline and with picrotoxin wash. (D) Picrotoxin wash application on VGluT3 optogenetic post-synaptic current.There was a significant effect of drug and voltage (F(1, 28) = 16.1, p = 0.0003, ANOVA). Bonferonni-corrected pairwise comparison tests showed that at 0 mV, there was a significant difference in amplitude between baseline and picrotoxin wash (t(7.03) = 3.79, p = 0.006). N = 8 cells, 2 female, 2 male. (E) With picrotoxin in aCSF, no recorded neurons demonstrated an excitatory post-synaptic current. N = 17 cells, 2 female, 2 male. (F) VGaT terminals were optogenetically stimulated while patching onto a neuron within the PVN. (G) PVN example traces of VGaT optogenetic post-synaptic currents under 0 mV and −60 mV at baseline and with picrotoxin wash. (H) Picrotoxin wash application on VGaT optogenetic post-synaptic current at −60 mV and 0 mV. There was a significant effect of drug and voltage (F(1, 28) = 30.2, p < 0.00001, ANOVA). Bonferonni-corrected pairwise comparison tests showed that at 0 mV and −50 mV, there was a significant difference in amplitude between baseline and picrotoxin wash (t(7.13) = 5.35, p = 0.001, 0 mV) (t(10.9) = -2.78, p = 0.01, −50 mV). N = 8 cells, 2 female, 2 male. (I) With picrotoxin in aCSF, no recorded neurons demonstrated an excitatory post-synaptic current. N = 22 cells, 2 female, 3 male.
3.5. Distinct VGluT3 neural signaling to sucrose reward in a state-dependent manner
Both the ARC and the PVN are hypothalamic regions that regulate homeostatic state. The ARC exerts influence over food intake [64,65] while the PVN is a critical node of the hypothalamic-pituitary-adrenal axis that also regulates food intake [66]. Thus, we first investigated calcium-dependent signaling of VGluT3 BNST neurons in response to sucrose consumption and aversive footshock when mice were sated or fasted. VGluT3-IRES::Cre mice were injected with Cre-dependent GCaMP6m into the BNST and implanted with an optic fiber (Figure 5A–B). Mice were recorded in response to sucrose consumption when fasted or sated, the order of which was counter-balanced between mice (Figure 5C). Sucrose consumption, as measured in licks, was significantly higher in the fasted state than the sated state (Figure 5D). In spite of higher sucrose consumption in the fasted state, BNST VGluT3 consumption-related neuronal activity was significantly higher when mice were sated compared with the fasted state (Figure 5E–F). Mice were also recorded in response to footshock when fasted or sated, the order of which was counter-balanced between mice (Figure 5G). In contrast to sucrose consumption, footshock increased VGluT3 neuronal activity in both the fasted and sated states (Figure 5H–I). We also assessed calcium-dependent VGluT3 activity during a Pavlovian conditioning task while mice were fasted to determine if reward learning had an impact on signaling. VGluT3 BNST neurons showed no signaling related to conditioned stimuli predicting sucrose delivery (CS+) or a stimulus predicting no sucrose delivery (CS-) (Figure 5J–K). When calcium-dependent signaling was time-locked to magazine entry to retrieve the sucrose reward during the cue, there was also no difference from baseline (Figure 5L-M). Overall, we interpret these results such that BNST VGluT3 neurons are sensitive to the interaction of palatable food reward and internal state, specifically when the subject is sated. However, BNST VGluT3 neurons are also sensitive to aversive stimuli that is not dependent on internal state.
Figure 5.
State-dependent BNST VGluT3 neuronal signaling of sucrose consumption. (A) VGluT3-IRES::Cre mice were injected with Cre-dependent GCaMP6m. Scale bar is 500 μm. (B) Histological verification of fiber placements. (C) While sated or fasted, mice were recorded in response to sucrose consumption. N = 11, 6 female, 5 male. (D) Sucrose consumption was higher in the fasted than the sated state (t(12.13) = -8.91, p < 0.0001, Bonferonni). (E) Averaged traces of VGluT3 calcium-dependent signaling in response to bouts of sucrose consumption between fasted and sated states. (F) Maximum peaks observed in response to sucrose of VGluT3 calcium-dependent signaling was higher in the sated than fasted state (t(10) = 4.06, p = 0.002, Bonferonni). (G) While sated and fasted, mice were recorded in response to electric footshock. N = 6, 3 female, 3 male. (H) Averaged traces of VGluT3 calcium-dependent signaling in response to electric footshock between fasted and sated states. (I) Maximum peaks observed in response to electric foot shock of VGluT3 calcium-dependent signaling between the sated and fasted states. (J) Averaged traces in response to a conditioned stimulus that predicted sucrose reward (CS+) and a neutral stimulus with no programmable effect (CS-). (K) Maximum peaks in response to CS+ and CS-. (L) Averaged traces of reward retrieval onset during cue presentation. (M) Maximum peaks of reward retrieval onset during CS+ and CS-.
3.6. Stimulation of VGluT3 neurons in the anteromedial BNST has homeostatic consequences
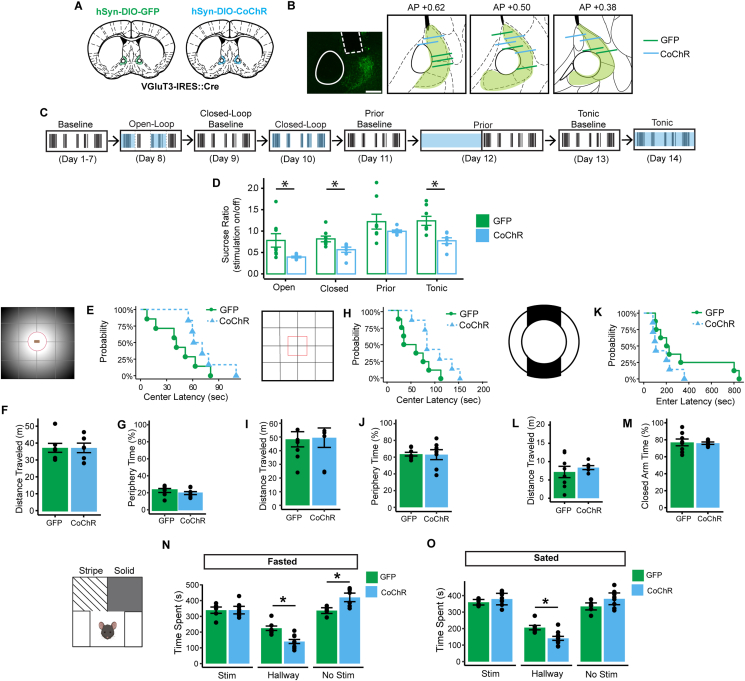
Based on larger BNST VGluT3 neuronal activity during sated sucrose consumption compared with fasted sucrose consumption, we hypothesized that imposing VGluT3 neuronal activation on fasted sucrose consumption would reduce intake. VGluT3-IRES::Cre mice were injected with Cre-dependent GFP or Cre-dependent CoChR-GFP and implanted with stimulating optic fibers (Figure 6A–B). Mice were habituated to a sucrose consumption environment before undergoing 4 different stimulation conditions: open-loop, closed-loop, prior, and tonic (Figure 6C). There was a significant effect of stimulation condition on the sucrose ratio, which was calculated as the number of licks when stimulation was on divided by the number of licks when stimulation was off (Figure 6D). Open-loop stimulation, closed-loop stimulation, and tonic stimulation significantly reduced sucrose consumption when VGluT3 BNST neurons were optogenetically stimulated. No difference was found between GFP and CoChR mice in response to prior stimulation, indicating that BNST VGluT3 stimulation reduces sucrose intake only when concurrent with consumption.
Figure 6.
Activation of VGluT3 BNST neurons rapidly reduced sucrose intake in a fasted state but had no effect on anxiety-like behavior nor had intrinsic rewarding or aversive properties. (A) VGluT3-IRES::Cre mice were injected with either Cre-dependent GFP or CoChR. (B) Fiber placement was verified through histology. Scale bar is 200 μm. (C) Illustration of the four stimulation conditions: open-loop, closed-loop, prior, and tonic. (D) The effect of four stimulation conditions on sucrose consumption (F(3, 52) = 19.6, p < 0.00001), ANOVA). Bonferonni-corrected pairwise comparisons revealed a significant reduction in sucrose ratio during CoChR stimulation, which was not observed in GFP mice, in response to open-loop stimulation (t(7.21) = 2.43, p = 0.04), closed-loop stimulation (t(12.9) = 2.81, p = 0.01), and tonic stimulation (t(11.7) = 3.69, p = 0.003). GFP = 8 (4 female, 4 male), CoChR = 7 (4 female, 3 male). Novelty-suppressed feeding’s (E) latency to approach the center, (F) distance traveled, and (G) periphery time. Open field’s (H) latency to approach the center, (I) distance traveled, and (J) periphery time. Elevated zero maze’s (K) latency to enter the opposite closed arm, (L) distance traveled, and (M) closed arm time. (N) In the fasted state, there was a statistically significant interaction of group and stimulation side (F (2, 24) = 8.276, p = 0.0018). Bonferonni-corrected tests: within chambers between groups (NonStimulated chamber t(10.43) = 3.93, p = 0.0026; Connecting chamber t(10.98) = 3.598, p = 0.0042; Stimulated chamber t(11.30) = 0.019, p = 0.98), within groups between stimulated versus nonstimulated chambers (CoChR t(7) = 2.537, p = 0.06; GFP t(5) = 0.1159, p > 0.05). GFP mice (N = 6, 3 female, 3 male), CoChR mice (N = 8, 4 female, 4 male). (O) In the sated state, there was a statistically significant interaction of group and stimulation side (F (2, 24) = 4.74, p = 0.0184). Bonferonni-corrected tests: within chambers between groups (NonStimulated chamber t(11.86) = 2.009, p = 0.067; Connecting chamber t(11.75) = 2.933, p = 0.0128; Stimulated chamber t(10.90) = 1.190, p = 0.259), within groups between stimulated versus nonstimulated chambers (CoChR t(7) = 0.059, p > 0.05; GFP t(5) = 1.544, p > 0.05). GFP mice (N = 6, 3 female, 3 male), CoChR mice (N = 8, 4 female, 4 male).
Given the BNST is involved in the expression of anxiety-like behavior [[27], [28], [29], [30], [31], [32], [33], [34]], we explored the role of VGluT3 BNST activation in classic anxiety-like behavior paradigms. The novelty-suppressed feeding task evaluates rodent approach-avoidance behavior [67]. In this task, 24-hour food deprivation acts as the source of motivation for approach, while the novel environment of an open field and bright light over the food source acts as the source for avoidance. Tonic optogenetic stimulation of GFP mice and CoChR mice during novelty-suppressed feeding had no effect on latency to approach the center (Figure 6E), distance traveled (Figure 6F), and time spent in the periphery (Figure 6G). The novelty-suppressed feeding task necessitates that subjects be fasted, so for appropriate comparison, mice were also fasted when tested in the open field and elevated zero maze, which are also traditional tasks for measuring anxiety-like behavior in rodents. Tonic stimulation for GFP mice and CoChR mice during open field had no group differences in latency to approach the center (Figure 6H), distance traveled (Figure 6I), and periphery time (Figure 6J). There were also no group differences between GFP and CoChR during elevated zero maze in latency to enter the other closed arm (Figure 6K), distance traveled (Figure 6L), and closed-arm time (Figure 6M).
Finally, the valence of VGluT3 BNST activation when sated or fasted was assessed using the real-time place preference task with the order of stimulation in each side counter-balanced. In the fasted state, there was a statistically significant interaction of group and stimulation chamber (Figure 6N). CoChR mice spent significantly more time in the nonstimulated chamber compared with GFP mice. However, this effect was driven by GFP mice spending significantly more time in the connecting chamber than CoChR mice and there were no differences in time spent in the stimulated chamber between groups. Further, there were no differences in time spent between the outer stimulated and nonstimulated chambers for both CoChR and GFP mice. In the sated state, there was a statistically significant interaction of group and stimulation chamber (Figure 6O). GFP mice again spent more time in the connecting chamber than CoChR mice and there were no differences in time spent between the outer stimulated and nonstimulated chambers for both CoChR and GFP mice. Thus, whether fasted or sated, activation of BNST VGluT3 neurons does not result in real-time place preference or aversion. Taken together, VGluT3 BNST neuron activation decreases sucrose consumption when fasted without affecting anxiety-like behavior or behavioral valence.
4. Discussion
The BNST is a cellularly diverse brain region linked with anxiety and stress disorders in humans [[68], [69], [70], [71], [72], [73]], as well as feeding [[17], [18], [19], [20], [21]], stress [[74], [75], [76], [77], [78], [79]], and anxiety [[27], [28], [29], [30], [31], [32], [33], [34]] in animal models. Here, we identified a unique BNST cell-type that transcriptionally co-expresses VGluT3 and VGaT. In spite of their transcriptional identity, BNST VGluT3 neurons functionally transmit GABA to the ARC and PVN with rare glutamate co-transmission to the ARC. BNST VGluT3 neurons are preferentially activated by sucrose consumption in the sated state compared with the fasted state and their activation reduces sucrose intake in the fasted state without affecting anxiety-like behavior nor resulting in rewarding or aversive processing.
BNST VGluT3 neurons were located in a distinct location within the anteromedial BNST, which is part of the extended amygdala. The term “extended amygdala” was derived from observations made through anterograde tract tracing methods that displayed a high degree of contiguity between the central amygdala, medial amygdala, and BNST [1,[80], [81], [82], [83], [84], [85]]. In the same tract tracing studies, the caudomedial end of the nucleus accumbens shell merges with the rostral portion of the BNST [80,[85], [86], [87]]. However, VGluT3 neurons observed within the anteromedial BNST VGluT3 neurons were more lateral compared to the caudomedial shell of the nucleus accumbens [88]. While striatal VGluT3 neurons, including those within the posterior nucleus accumbens shell, co-expressed ChAT immunolabeling [46,59], BNST VGluT3 neurons did not. As shown here and by others, including evidence from the Allen Brain Cell Atlas, BNST VGluT3 neurons do not co-express ChAT [89,90]. Thus, a lack of ChAT immunolabeling distinguishes BNST VGluT3 neurons from striatal ChAT-expressing VGluT3 neurons.
Our in situ hybridization data indicated that VGluT3 BNST neurons largely co-expressed VGluT3 and VGaT mRNA, extending earlier descriptions of VGluT3 mRNA in BNST [[91], [92], [93], [94], [95]]. The VGluT3 neurons were a small fraction of the total VGaT-expressing BNST population, indicating they are a unique subset of BNST GABA neurons. As a genetic homologue, VGluT3 shares ∼70% similarity to VGluT1 and VGluT2 and functions as a proton-dependent vesicular glutamate transporter [94,96,97]. Substrate selectivity for glutamate and not other neurotransmitters, including GABA, acetylcholine, and serotonin, was confirmed with its discovery [94,96,97]. VGluT3 is often co-expressed in neurons that co-transmit other neurotransmitters, such as in striatal cholinergic interneurons, raphe serotonin neurons, hippocampal GABA interneurons or retinal glycine neurons [93,94,[96], [97], [98], [99], [100]]. Thus, genetic evidence suggests VGluT3 BNST neurons could be capable of co-releasing glutamate and GABA but cannot be determined without functional validation through whole-cell electrophysiology.
Before testing whether VGluT3 BNST neurons co-transmit glutamate and GABA, we determined the projection targets of these neurons using Cre-dependent labeling of synaptophysin in VGluT3 BNST processes [60]. Consistent with results from PHA-L tracing studies from the anteromedial BNST [101], VGluT3 neurons in the anteromedial BNST projected to the nucleus accumbens, ventral aspect of the lateral septum, PVN, ARC, LHA, and VTA. We also found extensive mRuby expression throughout the BNST, indicating that BNST VGluT3 neurons provide significant input locally within the BNST. A novel projection that has not been previously observed was the septohypothalamic region, which is activated by stress [[102], [103], [104]] and also involved in feeding [105,106]. Minor synaptophysin labeling was found in the LHA but not as extensive as all BNST to LHA efferents [6], which suggests other non-VGlut3 anteromedial BNST neurons project to the LHA. While PHA-L tracing demonstrates that anteromedial BNST neurons also project to the central amygdala, supramammillary and tuberomammillary nuclei, and ventrolateral periaqueductal gray [6], we found no projections to these locations from VGluT3 neurons, indicating a selective projection from this unique cell-type. Presumably other anteromedial BNST cell-types synapse within the central amygdala, mammillary nuclei, and the periaqueductal gray. While synaptophysin labeling was observed in VTA, similar to previous neuroanatomy studies, the labeling from the anteromedial BNST was minor in comparison to their other targets [107].
After identifying the ARC and PVN as primary targets of BNST VGluT3 neurons, we next explored the synaptic functionality of BNST VGluT3 projections by optogenetic terminal stimulation and whole-cell patch-clamp recordings in the ARC and PVN. We found that while VGluT3 BNST neurons have the capability to accumulate glutamate into synaptic vesicles, they functionally transmit GABA, and a small proportion of projections co-transmit glutamate in the ARC. In contrast to the ARC, VGluT3 BNST neurons functionally transmit GABA alone to PVN, as we found no evidence that these neurons transmit glutamate in PVN. These results suggest similar but not identical mechanisms of neurotransmission from VGluT3 BNST neurons to hypothalamic regions. Indeed, neurotransmission from other VGluT3-expressing neurons is not equal across brain regions, with others documenting both GABA and glutamate co-transmission [108,109] or only glutamate transmission [110,111]. Our findings corroborate previous electron microscopy studies that revealed VGluT3-expressing terminals formed some asymmetrical synapses, but a large majority also formed symmetrical synapses, suggestive of inhibitory neurotransmission [93,97]. Another study also found a large proportion of VGaT protein expression that was absent of VGluT3 protein expression from BNST to VTA [91], suggesting the BNST VGluT3 pathway to VTA also transmits GABA. While we found no glutamate-mediated ionotropic currents, it is possible that glutamate is still released but acting on metabotropic receptors. Indeed, glutamate release can stimulate mGluR1, which would lead to the activation of TRP channel-mediated inward current [112,113]. However, we did not observe sufficient evidence of a slow inward current. Additionally, a growing body of literature from VGluT3 mouse knockout lines suggests that VGluT3 has a synergistic neuromodulatory effect by increasing rate of vesicular filling of non-glutamate neurotransmitters, which is driven by the co-expression of VGluT3 and the secondary transporter on the same vesicles, such as acetylcholine, dopamine, serotonin, and GABA [59,[114], [115], [116], [117]]. Whether VGluT3 shows vesicular synergy with VGaT in BNST hypothalamic projections requires further investigation. It is also possible that a state change by external environmental factors, such as circadian entrainment, may result in axonal reorganization that promotes glutamate transmission, as observed in median raphe nucleus neurons expressing VGluT3 [118]. Glutamate-mediated ionotropic current may become more observable in BNST VGluT3 projections with an internal state change but will require further research.
Based on the BNST VGluT3 projections to ARC and PVN that participate in homeostatic regulation, we used in vivo fiber photometry to observe calcium-dependent signaling of VGluT3 neurons to rewarding and aversive stimuli during fasted and sated states. BNST VGluT3 neurons were highly activated by aversive footshock stimuli and these signals did not differ between fasted versus sated states. However, VGluT3 neuronal activity was significantly higher in response to sucrose consumption when sated compared to when the mice consumed sucrose when fasted. This signaling pattern is different from the mesolimbic dopamine system where VTA and accumbens neurons show higher activation following reward consumption in the fasted state compared with the sated state [[119], [120], [121], [122]]. Others have shown that BNST VGaT neurons signal to chow or a palatable reward when sated and fasted [18,23], but this is the first evidence of a novel BNST cell-type that signals more highly to a palatable reward when sated rather than fasted. Whether BNST VGluT3 signals more or less for homeostatic feeding (consumption motivated by metabolic need) or hedonic feeding (consumption motivated by interoception or reward) [123,124] is unclear. Nevertheless, we also assessed whether BNST VGluT3 neurons were sensitive to Pavlovian predictors of sucrose delivery or non-delivery in the fasted state and found no modulation by the CS+ and CS-. Consistent with our free sucrose data in the fasted state, there was also no significant difference in signaling from baseline when the mice entered the magazine to consume the reward during CS + or CS-. Thus, VGluT3 BNST neurons do not appear to be involved in associative reward learning in the fasted state, potentially reflecting less influence of a hedonic impact on signaling. Together, VGluT3 neuronal activity is sensitive to internal state in response to a palatable reward but is sensitive to aversive stimuli regardless of internal state.
Based on BNST VGluT3 neuron signaling of state-dependent feeding as well as aversive stimuli, we tested whether optogenetic activation of BNST VGluT3 neurons results in changes in feeding, anxiety-like behavior or emotional valence. Activation of VGluT3 BNST neurons decreased feeding in a fasted state but had no effect on classical anxiety-like behavior paradigms: novelty-suppressed feeding, open field, and elevated zero maze. Additionally, stimulation prior to palatable reward access had no effect on consumption, indicating that BNST VGluT3 activation must be concurrent with consumption in the fasted state to reduce feeding. Thus, BNST VGluT3 neurons resulted in a specific behavioral functionality on feeding but not anxiety-like behavior. While previous work has shown that hunger stimulated by ARC agouti-related peptide (AgRP) neurons is aversive after several sessions [125], we did not find that acute activation of BNST VGluT3 neurons was aversive. Although our real-time place conditioning study showed that fasted BNST VGluT3 activated mice spent more time in the non-stimulated chamber than control mice, there was no difference in time spent in the chamber in which BNST VGluT3 neurons were stimulated between groups. Nor were there differences in time spent between the stimulated and non-stimulated chambers within the BNST VGluT3 activated mice. Thus, the activation of BNST VGluT3 neurons had no innate rewarding or aversive valence as assessed in the real-time place conditioning task. Altogether, we interpret the reduction of feeding observed in the fasted state following BNST VGluT3 activation may result from a reduced motivation to consume rather than influencing rewarding or aversive aspects of satiety [[126], [127], [128]]. Further supporting a lack of influence on rewarding or aversive processes, VGluT3 stimulation did not affect locomotor behavior which would be expected to enhance or reduce locomotor behavior depending on rewarding or aversive properties.
Like the BNST itself, the ARC is diverse in cell-types and not uniform in neurotransmitter release [[129], [130], [131]]. The two most well studied cell-types in the ARC are agouti-related peptide (AgRP) and pro-opiomelanocortin (POMC) neurons [132]. The ARC’s bifurcation into AgRP and POMC neuronal populations control hunger and satiety, respectively [64,65,[133], [134], [135]]. Activation of POMC neurons also increases heart rate and blood glucose [34,136]. Based on neuroanatomical retrograde tracing, the BNST equally provides input to POMC and AgRP neurons [137]. With our combined observations of a reduction in feeding by VGluT3 BNST activation and a largely inhibitory input to the ARC, we hypothesize that VGluT3 BNST neurons preferentially project to AgRP neurons. Future directions determining whether it is AgRP or POMC neurons being inhibited by the BNST VGluT3 neurons will provide valuable mechanistic insight of the extended amygdala to hypothalamus circuitry in the modulation of feeding.
The PVN, another output of the BNST VGluT3 neurons, is a critical node in the HPA-axis and plays a central role in stress regulation, as well as feeding [66,138]. It receives direct synaptic input from few, discrete brain regions within the hypothalamus, brainstem, circumventricular organs, and the BNST as well [[139], [140], [141]]. The PVN is divided into the parvocellular and magnocellular divisions [142,143], and unbiased transcriptional classification have further revealed that specific cell-types are more populated in discrete subregions of the PVN. For example, Gad2, a genetic marker of GABA synthesis, is comparatively more enriched in the anterior PVN than either the medial or posterior PVN [144]. This analysis likely includes the peri-PVN region, which is known to be a local GABAergic site that directly innervates the PVN [145]. Based on our synaptophysin tracing of BNST VGluT3 efferents, we hypothesize that BNST VGluT3 neurons project to this peri-PVN region due to the oval-shaped and anterior expression rather than the classic heart-shaped expression of the PVN itself. Like the ARC, the phenotypic identity of PVN neurons receiving input from BNST VGluT3 neurons will require further investigation due to the composition of both neuroendocrine and pre-autonomic neurons in the PVN that not only exerts influence on other brain regions but also direct hypophysiotropic effects. Furthermore, PVN cell-types are known to differentially respond to food and have different roles in body weight maintenance [138]. Determining the PVN cell-type that receives BNST VGluT3 input may reveal how GABAergic input from the BNST alters neuroendocrine or behavioral effects through PVN innervation.
With our retrograde tracing experiment, we were able to determine the proportion of BNST VGluT3 neurons that project to PVN-only, ARC-only, or both. While BNST VGluT3 neurons mostly segregated to either the PVN or ARC, a notable percentage bifurcated to both. The intersection of HPA-axis regulation and food intake is not novel – leptin can inhibit the CRH synthesis that is induced by food and glucose deprivation [146,147]. It has also been shown that ARC AgRP neurons activated by fasting synapse to the PVN where they pre-synaptically inhibit the GABAergic terminals of BNST neurons [148]. The existence of discrete BNST VGluT3 populations that separately innervate the PVN-only, ARC-only, and both suggest that these neurons are activated by discrete stimuli associated with feeding behavior that relates to HPA-axis activation. These data raise the possibility that subsets of BNST VGluT3 neurons may have unique functions depending on their targets.
In conclusion, we characterized a subset of GABAergic BNST neurons that express VGluT3 mRNA. BNST VGluT3 neurons transmit GABA in the PVN and ARC with rare glutamate co-transmission in the ARC. These neurons are activated by aversive stimuli and show a state-dependent activation following sucrose consumption in the sated state over the fasted state. Activation of BNST VGluT3 neurons reduces sucrose consumption in the fasted state and does not affect anxiety-like behavior or innate rewarding or aversive processing. GABAergic BNST input to the ARC has been previously found to reduce feeding [149]. Our results suggest that a subset of these GABAergic BNST neurons express VGluT3, but there are likely BNST VGluT3-negative neurons projecting to the ARC as well. This may also be the case for BNST projections to the PVN. Future work may utilize discrete cell-targeting methods, such as the INTRSECT viral strategy in order to target BNST VGluT3-positive and VGluT3-negative neurons that project to the ARC or PVN [150]. Nevertheless, the role of this newly-defined BNST VGluT3 input to the ARC and PVN may prove to be important in understanding the neural pathways involved in energy balance and feeding regulation.
CRediT authorship contribution statement
Annie Ly: Writing – review & editing, Writing – original draft, Validation, Supervision, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Rachel Karnosky: Methodology, Investigation, Formal analysis. Emily D. Prévost: Writing – review & editing, Formal analysis. Hayden Hotchkiss: Investigation. Julianne M. Pelletier: Investigation. Robert L. Spencer: Writing – review & editing, Conceptualization. Christopher P. Ford: Writing – review & editing, Writing – original draft, Validation, Supervision, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. David H. Root: Writing – review & editing, Writing – original draft, Resources, Project administration, Investigation, Funding acquisition, Formal analysis, Conceptualization.
Declaration of competing interest
The authors report no conflicts of interest.
Acknowledgements
This research was supported by the Webb-Waring Biomedical Research Award from the Boettcher Foundation (DHR), Institute for Cannabis Research (DHR), AB Nexus from University of Colorado’s Boulder campus and Anschutz Medical Campus (DHR/CPF), National Institute on Drug Abuse DA047443 (DHR) & DA35821 (CPF), the National Institute on Mental Health MH13222 (AL), and the National Institute of Neurological Disorders and Stroke NS95809 (CPF). AL, DHR, and CPF contributed to experimental design and manuscript writing. AL, DHR, CPF, and RLS contributed to data interpretation. AL, DHR, RK, EDP, HH, and JP contributed to data collection. All authors edited and reviewed the manuscript for publication.
Data availability
Data will be made available on request.
References
- 1.Alheid G.F. Extended Amygdala and basal forebrain. Ann N Y Acad Sci. 2003;985(1):185–205. doi: 10.1111/j.1749-6632.2003.tb07082.x. [DOI] [PubMed] [Google Scholar]
- 2.Bota M., Sporns O., Swanson L.W. Neuroinformatics analysis of molecular expression patterns and neuron populations in gray matter regions: the rat BST as a rich exemplar. Brain Res. 2012;1450:174–193. doi: 10.1016/j.brainres.2012.02.034. [DOI] [PubMed] [Google Scholar]
- 3.Ju G., Swanson L.W., Simerly R.B. Studies on the cellular architecture of the bed nuclei of the stria terminalis in the rat: II. chemoarchitecture. J Comp Neurol. 1989;280(4):603–621. doi: 10.1002/cne.902800410. [DOI] [PubMed] [Google Scholar]
- 4.Ju G., Swanson L.W. Studies on the cellular architecture of the bed nuclei of the stria terminalis in the rat: I. cytoarchitecture. J Comp Neurol. 1989;280(4):587–602. doi: 10.1002/cne.902800409. [DOI] [PubMed] [Google Scholar]
- 5.Dong H.-W., Swanson L.W. Projections from bed nuclei of the stria terminalis, magnocellular nucleus: implications for cerebral hemisphere regulation of micturition, defecation, and penile erection. J Comp Neurol. 2006;494(1):108–141. doi: 10.1002/cne.20789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dong H.-W., Swanson L.W. Projections from bed nuclei of the stria terminalis, anteromedial area: cerebral hemisphere integration of neuroendocrine, autonomic, and behavioral aspects of energy balance. J Comp Neurol. 2006;494(1):142–178. doi: 10.1002/cne.20788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dong H.-W., Swanson L.W. Projections from bed nuclei of the stria terminalis, dorsomedial nucleus: implications for cerebral hemisphere integration of neuroendocrine, autonomic, and drinking responses. J Comp Neurol. 2006;494(1):75–107. doi: 10.1002/cne.20790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong H.-W., Swanson L.W. Projections from bed nuclei of the stria terminalis, posterior division: implications for cerebral hemisphere regulation of defensive and reproductive behaviors. J Comp Neurol. 2004;471(4):396–433. doi: 10.1002/cne.20002. [DOI] [PubMed] [Google Scholar]
- 9.Dong H.-W., Swanson L.W. Organization of axonal projections from the anterolateral area of the bed nuclei of the stria terminalis. J Comp Neurol. 2004;468(2):277–298. doi: 10.1002/cne.10949. [DOI] [PubMed] [Google Scholar]
- 10.Dong H.-W., Swanson L.W. Projections from the rhomboid nucleus of the bed nuclei of the stria terminalis: implications for cerebral hemisphere regulation of ingestive behaviors. J Comp Neurol. 2003;463(4):434–472. doi: 10.1002/cne.10758. [DOI] [PubMed] [Google Scholar]
- 11.Dong H.W., Petrovich G.D., Swanson L.W. Organization of projections from the juxtacapsular nucleus of the BST: a PHAL study in the rat. Brain Res. 2000;859(1):1–14. doi: 10.1016/s0006-8993(99)02246-5. [DOI] [PubMed] [Google Scholar]
- 12.Dong H.-W., Petrovich G.D., Watts A.G., Swanson L.W. Basic organization of projections from the oval and fusiform nuclei of the bed nuclei of the stria terminalis in adult rat brain. J Comp Neurol. 2001;436(4):430–455. doi: 10.1002/cne.1079. [DOI] [PubMed] [Google Scholar]
- 13.Krettek J.E., Price J.L. Amygdaloid projections to subcortical structures within the basal forebrain and brainstem in the rat and cat. J Comp Neurol. 1978;178(2):225–254. doi: 10.1002/cne.901780204. [DOI] [PubMed] [Google Scholar]
- 14.Gungor N.Z., Paré D. Functional heterogeneity in the bed nucleus of the Stria Terminalis. J Neurosci. 2016;36(31):8038–8049. doi: 10.1523/JNEUROSCI.0856-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moga M.M., Saper C.B. Neuropeptide-immunoreactive neurons projecting to the paraventricular hypothalamic nucleus in the rat. J Comp Neurol. 1994;346(1):137–150. doi: 10.1002/cne.903460110. [DOI] [PubMed] [Google Scholar]
- 16.Barbier M., González J.A., Houdayer C., Burdakov D., Risold P.Y., Croizier S. Projections from the dorsomedial division of the bed nucleus of the stria terminalis to hypothalamic nuclei in the mouse. J Comp Neurol. 2021;529(5):929–956. doi: 10.1002/cne.24988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang J., Wang L., Yang Y., Wang S., Huang C., Yang L., et al. Dissection of the bed nucleus of the stria terminalis neuronal subtypes in feeding regulation. Physiol Behav. 2023;271 doi: 10.1016/j.physbeh.2023.114333. [DOI] [PubMed] [Google Scholar]
- 18.Luskin A.T., Bhatti D.L., Mulvey B., Pedersen C.E., Girven K.S., Oden-Brunson H., et al. Extended amygdala-parabrachial circuits alter threat assessment and regulate feeding. Sci Adv. 2021;7(9) doi: 10.1126/sciadv.abd3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jennings J.H., Rizzi G., Stamatakis A.M., Ung R.L., Stuber G.D. The inhibitory circuit architecture of the lateral hypothalamus orchestrates feeding. Science. 2013;341(6153):1517–1521. doi: 10.1126/science.1241812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dodt S., Widdershooven N.V., Dreisow M.L., Weiher L., Steuernagel L., Wunderlich F.T., et al. NPY-mediated synaptic plasticity in the extended amygdala prioritizes feeding during starvation. Nat Commun. 2024;15(1):5439. doi: 10.1038/s41467-024-49766-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Worth A.A., Feetham C.H., Morrissey N.A., Luckman S.M. Paraventricular oxytocin neurons impact energy intake and expenditure: projections to the bed nucleus of the stria terminalis reduce sucrose consumption. Front Endocrinol. 2024;15 doi: 10.3389/fendo.2024.1449326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Betley J.N., Cao Z.F., Ritola K.D., Sternson S.M. Parallel, redundant circuit organization for homeostatic control of feeding behavior. Cell. 2013;155(6):1337–1350. doi: 10.1016/j.cell.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shrivastava K., Athreya V., Lu Y., Luis-Islas J., Han A., Kowalski T.F., et al. Energy state guides reward seeking via an extended amygdala to lateral hypothalamus pathway. Nat Commun. 2025;16(1):4474. doi: 10.1038/s41467-025-59686-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li B.H., Rowland N.E. Cholecystokinin- and dexfenfluramine-induced anorexia compared using devazepide and c-fos expression in the rat brain. Regul Pept. 1994;50(3):223–233. doi: 10.1016/0167-0115(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 25.Chait A., Suaudeau C., De Beaurepaire R. Extensive brain mapping of calcitonin-induced anorexia. Brain Res Bull. 1995;36(5):467–472. doi: 10.1016/0361-9230(94)00223-n. [DOI] [PubMed] [Google Scholar]
- 26.Li B.H., Rowland N.E. Peripherally and centrally administered bombesin induce Fos-like immunoreactivity in different brain regions in rats. Regul Pept. 1996;62(2–3):167–172. doi: 10.1016/0167-0115(96)00029-8. [DOI] [PubMed] [Google Scholar]
- 27.Sullivan G.M., Apergis J., Bush D.E., Johnson L.R., Hou M., Ledoux J.E. Lesions in the bed nucleus of the stria terminalis disrupt corticosterone and freezing responses elicited by a contextual but not by a specific cue-conditioned fear stimulus. Neuroscience. 2004;128(1):7–14. doi: 10.1016/j.neuroscience.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 28.Waddell J., Morris R.W., Bouton M.E. Effects of bed nucleus of the stria terminalis lesions on conditioned anxiety: aversive conditioning with long-duration conditional stimuli and reinstatement of extinguished fear. Behav Neurosci. 2006;120(2):324–336. doi: 10.1037/0735-7044.120.2.324. [DOI] [PubMed] [Google Scholar]
- 29.Duvarci S., Bauer E.P., Paré D. The bed nucleus of the Stria Terminalis mediates inter-individual variations in anxiety and fear. J Neurosci. 2009;29(33):10357–10361. doi: 10.1523/JNEUROSCI.2119-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davis M., Walker D.L., Miles L., Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35(1):105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ventura-Silva A.P., Pêgo J.M., Sousa J.C., Marques A.R., Rodrigues A.J., Marques F., et al. Stress shifts the response of the bed nucleus of the stria terminalis to an anxiogenic mode. Eur J Neurosci. 2012;36(10):3396–3406. doi: 10.1111/j.1460-9568.2012.08262.x. [DOI] [PubMed] [Google Scholar]
- 32.Mazzone C.M., Pati D., Michaelides M., DiBerto J., Fox J.H., Tipton G., et al. Acute engagement of Gq-mediated signaling in the bed nucleus of the stria terminalis induces anxiety-like behavior. Mol Psychiatr. 2018;23(1):143–153. doi: 10.1038/mp.2016.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williford K.M., Taylor A., Melchior J.R., Yoon H.J., Sale E., Negasi M.D., et al. BNST PKCδ neurons are activated by specific aversive conditions to promote anxiety-like behavior. Neuropsychopharmacology. 2023;48(7):1031–1041. doi: 10.1038/s41386-023-01569-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jia X., Chen S., Li X., Tao S., Lai J., Liu H., et al. Divergent neurocircuitry dissociates two components of the stress response: glucose mobilization and anxiety-like behavior. Cell Rep. 2022;41(6) doi: 10.1016/j.celrep.2022.111586. [DOI] [PubMed] [Google Scholar]
- 35.Ly A., Barker A., Hotchkiss H., Prévost E.D., McGovern D.J., Kilpatrick Z., et al. Bed nucleus of the stria terminalis GABA neurons are necessary for changes in foraging behaviour following an innate threat. Eur J Neurosci. 2023;58(7):3630–3649. doi: 10.1111/ejn.16137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Araujo Salgado I., Li C., Burnett C.J., Rodriguez Gonzalez S., Becker J.J., Horvath A., et al. Toggling between food-seeking and self-preservation behaviors via hypothalamic response networks. Neuron (Camb, Mass) 2023;18(111):2899–2917. doi: 10.1016/j.neuron.2023.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Louderback K.M., Wills T.A., Muglia L.J., Winder D.G. Knockdown of BNST GluN2B-containing NMDA receptors mimics the actions of ketamine on novelty-induced hypophagia. Transl Psychiatr. 2013;3(12) doi: 10.1038/tp.2013.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jaramillo A.A., Williford K.M., Marshall C., Winder D.G., Centanni S.W. BNST transient activity associates with approach behavior in a stressful environment and is modulated by the parabrachial nucleus. Neurobiol Stress. 2020;13 doi: 10.1016/j.ynstr.2020.100247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siletti K., Hodge R., Mossi Albiach A., Hu L., Lee K.W., Lönnerberg P., et al. Transcriptomic diversity of cell types across the adult human brain. Science. 2022;382(6667) doi: 10.1126/science.add7046. [DOI] [PubMed] [Google Scholar]
- 40.Welch J.D., Kozareva V., Ferreira A., Vanderburg C., Martin C., Macosko E.Z. Single-cell multi-omic integration compares and contrasts features of brain cell identity. Cell. 2019;177(7):1873–1887.e1817. doi: 10.1016/j.cell.2019.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giardino W.J., Eban-Rothschild A., Christoffel D.J., Li S.-B., Malenka R.C., de Lecea L. Parallel circuits from the bed nuclei of stria terminalis to the lateral hypothalamus drive opposing emotional states. Nat Neurosci. 2018;21(8):1084–1095. doi: 10.1038/s41593-018-0198-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim S.-Y., Adhikari A., Lee S.Y., Marshel J.H., Kim C.K., Mallory C.S., et al. Diverging neural pathways assemble a behavioural state from separable features in anxiety. Nature. 2013;496(7444):219–223. doi: 10.1038/nature12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jennings J.H., Sparta D.R., Stamatakis A.M., Ung R.L., Pleil K.E., Kash T.L., et al. Distinct extended amygdala circuits for divergent motivational states. Nature. 2013;496(7444):224–228. doi: 10.1038/nature12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Han R.W., Zhang Z.Y., Jiao C., Hu Z.Y., Pan B.X. Synergism between two BLA-to-BNST pathways for appropriate expression of anxiety-like behaviors in male mice. Nat Commun. 2024;15(1):3455. doi: 10.1038/s41467-024-47966-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fremeau R.T., Jr., Burman J., Qureshi T., Tran C.H., Proctor J., Johnson J., et al. The identification of vesicular glutamate transporter 3 suggests novel modes of signaling by glutamate. Proc Natl Acad Sci U S A. 2002;99(22):14488–14493. doi: 10.1073/pnas.222546799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gras C., Herzog E., Bellenchi G.C., Bernard V., Ravassard P., Pohl M., et al. A third vesicular glutamate transporter expressed by cholinergic and serotoninergic neurons. J Neurosci. 2002;22(13):5442–5451. doi: 10.1523/JNEUROSCI.22-13-05442.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schafer M.K., Varoqui H., Defamie N., Weihe E., Erickson J.D. Molecular cloning and functional identification of mouse vesicular glutamate transporter 3 and its expression in subsets of novel excitatory neurons. J Biol Chem. 2002;277(52):50734–50748. doi: 10.1074/jbc.M206738200. [DOI] [PubMed] [Google Scholar]
- 48.Franklin K.B.J., Paxinos G. ed. Academic Press, an imprint of Elsevier; Amsterdam: 2013. Paxinos and Franklin’s the mouse brain in stereotaxic coordinates. [Google Scholar]
- 49.Yang Y., Atasoy D., Su H.H., Sternson S.M. Hunger states switch a flip-flop memory circuit via a synaptic AMPK-dependent positive feedback loop. Cell. 2011;146(6):992–1003. doi: 10.1016/j.cell.2011.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hentges S.T., Nishiyama M., Overstreet L.S., Stenzel-Poore M., Williams J.T., Low M.J. GABA release from proopiomelanocortin neurons. J Neurosci. 2004;24(7):1578–1583. doi: 10.1523/JNEUROSCI.3952-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luther J.A., Tasker J.G. Voltage-gated currents distinguish parvocellular from magnocellular neurones in the rat hypothalamic paraventricular nucleus. The Journal of Physiology. 2000;523(1):193–209. doi: 10.1111/j.1469-7793.2000.t01-1-00193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barker D.J., Miranda-Barrientos J., Zhang S., Root D.H., Wang H.L., Liu B., et al. Lateral preoptic control of the lateral habenula through convergent glutamate and GABA transmission. Cell Rep. 2017;21(7):1757–1769. doi: 10.1016/j.celrep.2017.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McGovern D.J., Ly A., Ecton K.L., Huynh D.T., Prévost E.D., Gonzalez S.C., et al. Ventral tegmental area glutamate neurons mediate nonassociative consequences of stress. Mol Psychiatr. 2024;29(6):1671–1682. doi: 10.1038/s41380-022-01858-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McGovern D.J., Polter A.M., Root D.H. Neurochemical signaling of reward and aversion to ventral tegmental area glutamate neurons. J Neurosci. 2021;41(25):5471–5486. doi: 10.1523/JNEUROSCI.1419-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aitken T.J., Ly T., Shehata S., Sivakumar N., Medina N.S., Gray L.A., et al. bioRxiv; 2023. Negative feedback control of hunger circuits by the taste of food. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen Y., Lin Y.-C., Zimmerman C.A., Essner R.A., Knight Z.A. Hunger neurons drive feeding through a sustained, positive reinforcement signal. eLife. 2016;5 doi: 10.7554/eLife.18640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.O’Connor Eoin C., Kremer Y., Lefort S., Harada M., Pascoli V., Rohner C., et al. Accumbal D1R neurons projecting to lateral hypothalamus authorize feeding. Neuron (Camb, Mass) 2015;88(3):553–564. doi: 10.1016/j.neuron.2015.09.038. [DOI] [PubMed] [Google Scholar]
- 58.Therneau T.M. A package for survival analysis in R, 3.3–1. R Package. 2022 [Google Scholar]
- 59.Gras C., Amilhon B., Lepicard È.M., Poirel O., Vinatier J., Herbin M., et al. The vesicular glutamate transporter VGLUT3 synergizes striatal acetylcholine tone. Nat Neurosci. 2008;11(3):292–300. doi: 10.1038/nn2052. [DOI] [PubMed] [Google Scholar]
- 60.Beier K.T., Steinberg E.E., DeLoach K.E., Xie S., Miyamichi K., Schwarz L., et al. Circuit architecture of VTA dopamine neurons revealed by systematic input-output mapping. Cell. 2015;162(3):622–634. doi: 10.1016/j.cell.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Root D.H., Barker D.J., Estrin D.J., Miranda-Barrientos J.A., Liu B., Zhang S., et al. Distinct signaling by ventral tegmental area glutamate, GABA, and combinatorial Glutamate-GABA neurons in motivated behavior. Cell Rep. 2020;32(9) doi: 10.1016/j.celrep.2020.108094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Klapoetke N.C., Murata Y., Kim S.S., Pulver S.R., Birdsey-Benson A., Cho Y.K., et al. Independent optical excitation of distinct neural populations. Nat Methods. 2014;11(3):338–346. doi: 10.1038/nmeth.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shemesh O.A., Tanese D., Zampini V., Linghu C., Piatkevich K., Ronzitti E., et al. Temporally precise single-cell-resolution optogenetics. Nat Neurosci. 2017;20(12):1796–1806. doi: 10.1038/s41593-017-0018-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Krashes M.J., Koda S., Ye C., Rogan S.C., Adams A.C., Cusher D.S., et al. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin Investig. 2011;121(4):1424–1428. doi: 10.1172/JCI46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aponte Y., Atasoy D., Sternson S.M. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci. 2011;14(3):351–355. doi: 10.1038/nn.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Spencer R.L., Deak T. A users guide to HPA axis research. Physiol Behav. 2017;178:43–65. doi: 10.1016/j.physbeh.2016.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Samuels B.A., Hen R. In: Mood and anxiety related phenotypes in mice: characterization using behavioral tests, volume II. Gould T.D., editor. Humana Press; Totowa, NJ: 2011. Novelty-suppressed feeding in the mouse; pp. 107–121. [Google Scholar]
- 68.Petranu K., Webb E.K., Tomas C.W., Harb F., Torres L., deRoon-Cassini T.A., et al. Investigating the bed nucleus of the stria terminalis as a predictor of posttraumatic stress disorder in black Americans and the moderating effects of racial discrimination. Transl Psychiatr. 2024;14(1):337. doi: 10.1038/s41398-024-03050-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Feola B., Flook E.A., Gardner H., Phan K.L., Gwirtsman H., Olatunji B., et al. Altered bed nucleus of the stria terminalis and amygdala responses to threat in combat veterans with posttraumatic stress disorder. J Trauma Stress. 2023;36(2):359–372. doi: 10.1002/jts.22918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mobbs D., Yu R., Rowe J.B., Eich H., FeldmanHall O., Dalgleish T. Neural activity associated with monitoring the oscillating threat value of a tarantula. Proc Natl Acad Sci U S A. 2010;107(47):20582–20586. doi: 10.1073/pnas.1009076107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brinkmann L., Buff C., Neumeister P., Tupak S.V., Becker M.P., Herrmann M.J., et al. Dissociation between amygdala and bed nucleus of the stria terminalis during threat anticipation in female post-traumatic stress disorder patients. Hum Brain Mapp. 2017;38(4):2190–2205. doi: 10.1002/hbm.23513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Buff C., Brinkmann L., Bruchmann M., Becker M.P.I., Tupak S., Herrmann M.J., et al. Activity alterations in the bed nucleus of the stria terminalis and amygdala during threat anticipation in generalized anxiety disorder. Soc Cognit Affect Neurosci. 2017;12(11):1766–1774. doi: 10.1093/scan/nsx103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Awasthi S., Pan H., LeDoux J.E., Cloitre M., Altemus M., McEwen B., et al. The bed nucleus of the stria terminalis and functionally linked neurocircuitry modulate emotion processing and HPA axis dysfunction in posttraumatic stress disorder. Neuroimage: Clinical. 2020;28 doi: 10.1016/j.nicl.2020.102442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hammack S.E., Richey K.J., Watkins L.R., Maier S.F. Chemical lesion of the bed nucleus of the stria terminalis blocks the behavioral consequences of uncontrollable stress. Behav Neurosci. 2004;118(2):443–448. doi: 10.1037/0735-7044.118.2.443. [DOI] [PubMed] [Google Scholar]
- 75.Roman C.W., Lezak K.R., Kocho-Schellenberg M., Garret M.A., Braas K., May V., et al. Excitotoxic lesions of the bed nucleus of the stria terminalis (BNST) attenuate the effects of repeated stress on weight gain: evidence for the recruitment of BNST activity by repeated, but not acute, stress. Behav Brain Res. 2012;227(1):300–304. doi: 10.1016/j.bbr.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zelikowsky M., Hui M., Karigo T., Choe A., Yang B., Blanco M.R., et al. The neuropeptide Tac2 controls a distributed brain state induced by chronic social isolation stress. Cell. 2018;173(5):1265–1279.e1219. doi: 10.1016/j.cell.2018.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Maita I., Roepke T.A., Samuels B.A. Chronic stress-induced synaptic changes to corticotropin-releasing factor-signaling in the bed nucleus of the stria terminalis. Front Behav Neurosci. 2022;16 doi: 10.3389/fnbeh.2022.903782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hu P., Liu J., Maita I., Kwok C., Gu E., Gergues M.M., et al. Chronic stress induces maladaptive behaviors by activating corticotropin-releasing hormone signaling in the mouse oval bed nucleus of the stria terminalis. J Neurosci. 2020;40(12):2519–2537. doi: 10.1523/JNEUROSCI.2410-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Radley J.J., Sawchenko P.E. Evidence for involvement of a limbic paraventricular hypothalamic inhibitory network in hypothalamic-pituitary-adrenal axis adaptations to repeated stress. J Comp Neurol. 2015;523(18):2769–2787. doi: 10.1002/cne.23815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Alheid G.F., Heimer L. New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: the striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neuroscience. 1988;27(1):1–39. doi: 10.1016/0306-4522(88)90217-5. [DOI] [PubMed] [Google Scholar]
- 81.de Olmos J.S., Heimer L. The concepts of the ventral striatopallidal system and extended amygdala. Ann N Y Acad Sci. 1999;877:1–32. doi: 10.1111/j.1749-6632.1999.tb09258.x. [DOI] [PubMed] [Google Scholar]
- 82.De Olmos J.S., Ingram W.R. The projection field of the stria terminalis in the rat brain. An experimental study. J Comp Neurol. 1972;146(3):303–334. doi: 10.1002/cne.901460303. [DOI] [PubMed] [Google Scholar]
- 83.Dong H.W., Petrovich G.D., Swanson L.W. Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Res Brain Res Rev. 2001;38(1–2):192–246. doi: 10.1016/s0165-0173(01)00079-0. [DOI] [PubMed] [Google Scholar]
- 84.Weller K.L., Smith D.A. Afferent connections to the bed nucleus of the stria terminalis. Brain Res. 1982;232(2):255–270. doi: 10.1016/0006-8993(82)90272-4. [DOI] [PubMed] [Google Scholar]
- 85.de Olmos S., Lorenzo A. Developing the theory of the extended amygdala with the use of the cupric-silver technique. J Hist Neurosci. 2023;32(1):19–38. doi: 10.1080/0964704X.2022.2133569. [DOI] [PubMed] [Google Scholar]
- 86.Woodhams P.L., Roberts G.W., Polak J.M., Crow T.J. Distribution of neuropeptides in the limbic system of the rat: the bed nucleus of the stria terminalis, septum and preoptic area. Neuroscience. 1983;8(4):677–703. doi: 10.1016/0306-4522(83)90003-9. [DOI] [PubMed] [Google Scholar]
- 87.Heimer L., Harlan R.E., Alheid G.F., Garcia M.M., de Olmos J. Substantia innominata: a notion which impedes clinical-anatomical correlations in neuropsychiatric disorders. Neuroscience. 1997;76(4):957–1006. doi: 10.1016/s0306-4522(96)00405-8. [DOI] [PubMed] [Google Scholar]
- 88.Zahm D.S. Is the caudomedial shell of the nucleus accumbens part of the extended amygdala? A consideration of connections. Crit Rev Neurobiol. 1998;12(3):245–265. doi: 10.1615/critrevneurobiol.v12.i3.50. [DOI] [PubMed] [Google Scholar]
- 89.Nickerson Poulin A., Guerci A., El Mestikawy S., Semba K. Vesicular glutamate transporter 3 immunoreactivity is present in cholinergic basal forebrain neurons projecting to the basolateral amygdala in rat. J Comp Neurol. 2006;498(5):690–711. doi: 10.1002/cne.21081. [DOI] [PubMed] [Google Scholar]
- 90.Yao Z., van Velthoven C.T.J., Kunst M., Zhang M., McMillen D., Lee C., et al. A high-resolution transcriptomic and spatial atlas of cell types in the whole mouse brain. Nature. 2023;624(7991):317–332. doi: 10.1038/s41586-023-06812-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kudo T., Uchigashima M., Miyazaki T., Konno K., Yamasaki M., Yanagawa Y., et al. Three types of neurochemical projection from the bed nucleus of the stria terminalis to the ventral tegmental area in adult mice. J Neurosci. 2012;32(50):18035–18046. doi: 10.1523/JNEUROSCI.4057-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Welch J.D., Kozareva V., Ferreira A., Vanderburg C., Martin C., Macosko E.Z. Single-cell multi-omic integration compares and contrasts features of brain cell identity. Cell. 2019;177(7):1873–1887 e1817. doi: 10.1016/j.cell.2019.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fremeau R.T., Burman J., Qureshi T., Tran C.H., Proctor J., Johnson J., et al. The identification of vesicular glutamate transporter 3 suggests novel modes of signaling by glutamate. Proc Natl Acad Sci. 2002;99(22):14488–14493. doi: 10.1073/pnas.222546799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schäfer M.K.H., Varoqui H., Defamie N., Weihe E., Erickson J.D. Molecular cloning and functional identification of mouse vesicular glutamate transporter 3 and its expression in subsets of novel excitatory neurons. J Biol Chem. 2002;277(52):50734–50748. doi: 10.1074/jbc.M206738200. [DOI] [PubMed] [Google Scholar]
- 95.Herzog E., Gilchrist J., Gras C., Muzerelle A., Ravassard P., Giros B., et al. Localization of VGLUT3, the vesicular glutamate transporter type 3, in the rat brain. Neuroscience. 2004;123(4):983–1002. doi: 10.1016/j.neuroscience.2003.10.039. [DOI] [PubMed] [Google Scholar]
- 96.Takamori S., Malherbe P., Broger C., Jahn R. Molecular cloning and functional characterization of human vesicular glutamate transporter 3. EMBO Rep. 2002;3(8):798–803. doi: 10.1093/embo-reports/kvf159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gras C., Herzog E., Bellenchi G.C., Bernard V., Ravassard P., Pohl M., et al. A third vesicular glutamate transporter expressed by cholinergic and serotoninergic neurons. J Neurosci. 2002;22(13):5442. doi: 10.1523/JNEUROSCI.22-13-05442.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Haverkamp S., Wässle H. Characterization of an amacrine cell type of the Mammalian retina immunoreactive for vesicular glutamate transporter 3. J Comp Neurol. 2004;468(2):251–263. doi: 10.1002/cne.10962. [DOI] [PubMed] [Google Scholar]
- 99.Johnson J., Sherry D.M., Liu X., Fremeau Jr., R.T., Seal R.P., Edwards R.H., et al. Vesicular glutamate transporter 3 expression identifies glutamatergic amacrine cells in the rodent retina. J Comp Neurol. 2004;477(4):386–398. doi: 10.1002/cne.20250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stensrud M.J., Chaudhry F.A., Leergaard T.B., Bjaalie J.G., Gundersen V. Vesicular glutamate transporter-3 in the rodent brain: vesicular colocalization with vesicular γ-aminobutyric acid transporter. J Comp Neurol. 2013;521(13):3042–3056. doi: 10.1002/cne.23331. [DOI] [PubMed] [Google Scholar]
- 101.Dong H.-W., Swanson L.W. Projections from bed nuclei of the stria terminalis, anteromedial area: cerebral hemisphere integration of neuroendocrine, autonomic, and behavioral aspects of energy balance. J Comp Neurol. 2006;494(1):142–178. doi: 10.1002/cne.20788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Campeau S., Watson S.J. Neuroendocrine and behavioral responses and brain pattern of c-fos induction associated with audiogenic stress. J Neuroendocrinol. 1997;9(8):577–588. doi: 10.1046/j.1365-2826.1997.00593.x. [DOI] [PubMed] [Google Scholar]
- 103.Day H.E., Masini C.V., Campeau S. The pattern of brain c-fos mRNA induced by a component of fox odor, 2,5-dihydro-2,4,5-trimethylthiazoline (TMT), in rats, suggests both systemic and processive stress characteristics. Brain Res. 2004;1025(1–2):139–151. doi: 10.1016/j.brainres.2004.07.079. [DOI] [PubMed] [Google Scholar]
- 104.Imaki T., Shibasaki T., Hotta M., Demura H. Intracerebroventricular administration of corticotropin-releasing factor induces c-fos mRNA expression in brain regions related to stress responses: comparison with pattern of c-fos mRNA induction after stress. Brain Res. 1993;616(1–2):114–125. doi: 10.1016/0006-8993(93)90199-w. [DOI] [PubMed] [Google Scholar]
- 105.Stratford T.R. Activation of feeding-related neural circuitry after unilateral injections of muscimol into the nucleus accumbens shell. Brain Res. 2005;1048(1–2):241–250. doi: 10.1016/j.brainres.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 106.Sweeney P., Yang Y. An inhibitory septum to lateral hypothalamus circuit that suppresses feeding. J Neurosci. 2016;36(44):11185–11195. doi: 10.1523/JNEUROSCI.2042-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zahm D.S., Williams E.A., Latimer M.P., Winn P. Ventral mesopontine projections of the caudomedial shell of the nucleus accumbens and extended amygdala in the rat: double dissociation by organization and development. J Comp Neurol. 2001;436(1):111–125. [PubMed] [Google Scholar]
- 108.Pelkey K.A., Calvigioni D., Fang C., Vargish G., Ekins T., Auville K., et al. Paradoxical network excitation by glutamate release from VGluT3+ GABAergic interneurons. eLife. 2020;9 doi: 10.7554/eLife.51996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Stinson H.E., Ninan I. Median raphe glutamatergic neuron-mediated enhancement of GABAergic transmission and suppression of long-term potentiation in the hippocampus. Heliyon. 2024;10(19) doi: 10.1016/j.heliyon.2024.e38192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang H.L., Zhang S., Qi J., Wang H., Cachope R., Mejias-Aponte C.A., et al. Dorsal raphe dual serotonin-glutamate neurons drive reward by establishing excitatory synapses on VTA mesoaccumbens dopamine neurons. Cell Rep. 2019;26(5):1128–1142 e1127. doi: 10.1016/j.celrep.2019.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Qi J., Zhang S., Wang H.L., Wang H., de Jesus Aceves Buendia J., Hoffman A.F., et al. A glutamatergic reward input from the dorsal raphe to ventral tegmental area dopamine neurons. Nat Commun. 2014;5:5390. doi: 10.1038/ncomms6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cai Y., Ford C.P. Dopamine cells differentially regulate striatal cholinergic transmission across regions through corelease of dopamine and glutamate. Cell Rep. 2018;25(11):3148–3157.e3143. doi: 10.1016/j.celrep.2018.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cai Y., Nielsen B.E., Boxer E.E., Aoto J., Ford C.P. Loss of nigral excitation of cholinergic interneurons contributes to parkinsonian motor impairments. Neuron (Camb, Mass) 2021;109(7):1137–1149.e1135. doi: 10.1016/j.neuron.2021.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Favier M., Martin Garcia E., Icick R., de Almeida C., Jehl J., Desplanque M., et al. The human VGLUT3-pT8I mutation elicits uneven striatal DA signaling, food or drug maladaptive consumption in male mice. Nat Commun. 2024;15(1):5691. doi: 10.1038/s41467-024-49371-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Amilhon B., Lepicard E., Renoir T., Mongeau R., Popa D., Poirel O., et al. VGLUT3 (vesicular glutamate transporter type 3) contribution to the regulation of serotonergic transmission and anxiety. J Neurosci. 2010;30(6):2198–2210. doi: 10.1523/JNEUROSCI.5196-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nelson A.B., Bussert T.G., Kreitzer A.C., Seal R.P. Striatal cholinergic neurotransmission requires VGLUT3. J Neurosci. 2014;34(26):8772–8777. doi: 10.1523/JNEUROSCI.0901-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Stensrud M.J., Sogn C.J., Gundersen V. Immunogold characteristics of VGLUT3-positive GABAergic nerve terminals suggest corelease of glutamate. J Comp Neurol. 2015;523(18):2698–2713. doi: 10.1002/cne.23811. [DOI] [PubMed] [Google Scholar]
- 118.Maddaloni G., Chang Y.J., Senft R.A., Dymecki S.M. Adaptation to photoperiod via dynamic neurotransmitter segregation. Nature. 2024;632(8023):147–156. doi: 10.1038/s41586-024-07692-7. [DOI] [PubMed] [Google Scholar]
- 119.McGovern D.J., Phillips A., Ly A., Prévost E.D., Ward L., Siletti K., et al. Salience signaling and stimulus scaling of ventral tegmental area glutamate neuron subtypes. J. 2025 doi: 10.1523/JNEUROSCI.1073-24.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Liu S., Borgland S.L. Regulation of the mesolimbic dopamine circuit by feeding peptides. Neuroscience. 2015;289:19–42. doi: 10.1016/j.neuroscience.2014.12.046. [DOI] [PubMed] [Google Scholar]
- 121.Wallace C.W., Loudermilt M.C., Fordahl S.C. Effect of fasting on dopamine neurotransmission in subregions of the nucleus accumbens in male and female mice. Nutr Neurosci. 2022;25(7):1338–1349. doi: 10.1080/1028415X.2020.1853419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hsu T.M., McCutcheon J.E., Roitman M.F. Parallels and overlap: the integration of homeostatic signals by mesolimbic dopamine neurons. Front Psychiatr. 2018;9:410. doi: 10.3389/fpsyt.2018.00410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rossi M.A., Stuber G.D. Overlapping brain circuits for homeostatic and Hedonic feeding. Cell Metab. 2018;27(1):42–56. doi: 10.1016/j.cmet.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Siemian J.N., Arenivar M.A., Sarsfield S., Aponte Y. Hypothalamic control of interoceptive hunger. Curr Biol. 2021;31(17):3797–3809.e3795. doi: 10.1016/j.cub.2021.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Betley J.N., Xu S., Cao Z.F.H., Gong R., Magnus C.J., Yu Y., et al. Neurons for hunger and thirst transmit a negative-valence teaching signal. Nature. 2015;521(7551):180–185. doi: 10.1038/nature14416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Swerdlow N.R., van der Kooy D., Koob G.F., Wenger J.R. Cholecystokinin produces conditioned place-aversions, not place-preferences, in food-deprived rats: evidence against involvement in satiety. Life Sci. 1983;32(18):2087–2093. doi: 10.1016/0024-3205(83)90096-6. [DOI] [PubMed] [Google Scholar]
- 127.Deutsch J.A., Gonzalez M.F. Food intake reduction: satiation or aversion? Behav Biol. 1978;24(3):317–326. doi: 10.1016/s0091-6773(79)90171-8. [DOI] [PubMed] [Google Scholar]
- 128.Olson B.R., Freilino M., Hoffman G.E., Stricker E.M., Sved A.F., Verbalis J.G. c-Fos expression in rat brain and brainstem nuclei in response to treatments that alter food intake and gastric motility. Mol Cell Neurosci. 1993;4(1):93–106. doi: 10.1006/mcne.1993.1011. [DOI] [PubMed] [Google Scholar]
- 129.Campbell J.N., Macosko E.Z., Fenselau H., Pers T.H., Lyubetskaya A., Tenen D., et al. A molecular census of arcuate hypothalamus and median eminence cell types. Nat Neurosci. 2017;20(3):484–496. doi: 10.1038/nn.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hentges S.T., Otero-Corchon V., Pennock R.L., King C.M., Low M.J. Proopiomelanocortin expression in both GABA and glutamate neurons. J Neurosci. 2009;29(43):13684–13690. doi: 10.1523/JNEUROSCI.3770-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Graebner A.K., Iyer M., Carter M.E. Understanding how discrete populations of hypothalamic neurons orchestrate complicated behavioral states. Front Syst Neurosci. 2015;9:111. doi: 10.3389/fnsys.2015.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chen Y., Knight Z.A. Making sense of the sensory regulation of hunger neurons. Bioessays. 2016;38(4):316–324. doi: 10.1002/bies.201500167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Luquet S., Perez F.A., Hnasko T.S., Palmiter R.D. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310(5748):683–685. doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- 134.Gropp E., Shanabrough M., Borok E., Xu A.W., Janoschek R., Buch T., et al. Agouti-related peptide–expressing neurons are mandatory for feeding. Nat Neurosci. 2005;8(10):1289–1291. doi: 10.1038/nn1548. [DOI] [PubMed] [Google Scholar]
- 135.Qu N., He Y., Wang C., Xu P., Yang Y., Cai X., et al. A POMC-originated circuit regulates stress-induced hypophagia, depression, and anhedonia. Mol Psychiatr. 2020;25(5):1006–1021. doi: 10.1038/s41380-019-0506-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Üner A.G., Keçik O., Quaresma P.G.F., De Araujo T.M., Lee H., Li W., et al. Role of POMC and AgRP neuronal activities on glycaemia in mice. Sci Rep. 2019;9(1) doi: 10.1038/s41598-019-49295-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang D., He X., Zhao Z., Feng Q., Lin R., Sun Y., et al. Whole-brain mapping of the direct inputs and axonal projections of POMC and AgRP neurons. Front Neuroanat. 2015;9:40. doi: 10.3389/fnana.2015.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Li C., Navarrete J., Liang-Guallpa J., Lu C., Funderburk S.C., Chang R.B., et al. Defined paraventricular hypothalamic populations exhibit differential responses to food contingent on caloric state. Cell Metab. 2019;29(3):681–694.e685. doi: 10.1016/j.cmet.2018.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ulrich-Lai Y.M., Herman J.P. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10(6):397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Herman J.P., Figueiredo H., Mueller N.K., Ulrich-Lai Y., Ostrander M.M., Choi D.C., et al. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo–pituitary–adrenocortical responsiveness. Front Neuroendocrinol. 2003;24(3):151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 141.Cullinan W.E., Ziegler D.R., Herman J.P. Functional role of local GABAergic influences on the HPA axis. Brain Struct Funct. 2008;213(1–2):63–72. doi: 10.1007/s00429-008-0192-2. [DOI] [PubMed] [Google Scholar]
- 142.Swanson L.W., Sawchenko P.E. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci. 1983;6(1983):269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- 143.Liposits Z. Ultrastructure of hypothalamic paraventricular neurons. Crit Rev Neurobiol. 1993;7(2):89–162. [PubMed] [Google Scholar]
- 144.Xu S., Yang H., Menon V., Lemire A.L., Wang L., Henry F.E., et al. Behavioral state coding by molecularly defined paraventricular hypothalamic cell type ensembles. Science. 2020;370(6514) doi: 10.1126/science.abb2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Roland B.L., Sawchenko P.E. Local origins of some GABAergic projections to the paraventricular and supraoptic nuclei of the hypothalamus in the rat. J Comp Neurol. 1993;332(1):123–143. doi: 10.1002/cne.903320109. [DOI] [PubMed] [Google Scholar]
- 146.Heiman M.L., Ahima R.S., Craft L.S., Schoner B., Stephens T.W., Flier J.S. Leptin inhibition of the hypothalamic-pituitary-adrenal axis in response to stress. Endocrinology. 1997;138(9):3859–3863. doi: 10.1210/endo.138.9.5366. [DOI] [PubMed] [Google Scholar]
- 147.Huang Q., Rivest R., Richard D. Effects of leptin on corticotropin-releasing factor (CRF) synthesis and CRF neuron activation in the paraventricular hypothalamic nucleus of obese (ob/ob) mice. Endocrinology. 1998;139(4):1524–1532. doi: 10.1210/endo.139.4.5889. [DOI] [PubMed] [Google Scholar]
- 148.Douglass A.M., Resch J.M., Madara J.C., Kucukdereli H., Yizhar O., Grama A., et al. Neural basis for fasting activation of the hypothalamic-pituitary-adrenal axis. Nature. 2023;620(7972):154–162. doi: 10.1038/s41586-023-06358-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Smith M.A., Choudhury A.I., Glegola J.A., Viskaitis P., Irvine E.E., de Campos Silva P.C.C., et al. Extrahypothalamic GABAergic nociceptin-expressing neurons regulate AgRP neuron activity to control feeding behavior. J Clin Investig. 2020;130(1):126–142. doi: 10.1172/JCI130340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Fenno L.E., Mattis J., Ramakrishnan C., Hyun M., Lee S.Y., He M., et al. Targeting cells with single vectors using multiple-feature Boolean logic. Nat Methods. 2014;11(7):763–772. doi: 10.1038/nmeth.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.