Abstract
T lymphocytes are defective in cystine uptake and thus require exogenous thiols for activation and function. Here we show that monocyte-derived human dendritic cells (DCs) release cysteine in the extracellular space. Cysteine generation is increased by lipopolysaccharide and tumor necrosis factor α, and by contact with T cells specifically recognizing soluble or alloantigens. These stimuli also induce thioredoxin (TRX) accumulation in DCs. However, only the contact with antigen-specific T cells triggers TRX secretion by the antigen-presenting cells. Fewer extracellular thiols are recovered after DC–T cell interactions when cystine uptake or TRX activity are inhibited. In addition, glutamate (Glu) and anti-TRX-inactivating antibodies inhibit antigen-dependent T lymphocyte proliferation. These findings indicate that, during antigen presentation, DCs uptake cystine and release cysteine and TRX, thus providing a reducing microenvironment that facilitates immune response.
Gluthatione (GSH) is the major intracellular redox buffer and plays an essential role in protecting cells against oxidant damage (1). In addition, changes in the intracellular GSH levels modulate the expression of several genes involved in the control of cell growth and differentiation (2, 3). In T lymphocytes, intracellular GSH is critical for the proliferative response to mitogens or antigens (4–7). Cysteine (Cys) is a rate-limiting precursor for GSH synthesis; because extracellular fluids contain very low concentrations of Cys but substantial amounts of cystine (Cys2) (8), the latter is taken up by cells equipped with a Cys2 transporter and reduced intracellularly to Cys. However, lymphocytes lack an efficient system of Cys2 import, whereas they easily take up free thiols (9, 10). Therefore, to sustain lymphocyte activation and proliferation, exogenous thiols must somehow be generated in the microenvironment of an immune response (10). Extracellular thioredoxin (TRX) has been proposed to exert a synergistic activity on the mitogen- or cytokine-induced proliferation of lymphocytes (11, 12). TRX is a cytosolic enzyme with a redox-active disulfide/dithiol within the conserved active site sequence Cys-Gly-Pro-Cys (13). Despite its major intracellular localization and function, TRX can be secreted by certain cell types, especially upon activation (14, 15). Because surface receptor(s) for this protein have not been found on lymphocytes (16), it is possible that the effects on lymphocyte proliferation depend on its ability to generate extracellularly small thiol compounds that in turn can be used by T cells. Macrophages have been shown able to transport Cys2 intracellularly through a Cys2 transporter whose expression is induced by various stimuli (17) and to release Cys upon activation (10, 18, 19). However, it is not known whether dendritic cells (DCs), the professional antigen-presenting cells that are central in the development of the immune response (20), can generate the extracellular reducing milieu required for T lymphocyte activation. Furthermore, the role played by intracellular or secreted TRX in these processes is unclear. We thus investigated the ability of DCs to reduce the external medium and to modulate TRX synthesis and secretion after maturation or antigen presentation to T cells.
Materials and Methods
Antibodies.
Two IgG1 anti-human TRX mAbs generated in mice (2B1 and 9G9) were tested for their effects on the redox activity of TRX by using the insulin-reduction assay described by Arner et al. (21). In brief, 5 μg of recombinant TRX was incubated with increasing doses (5–200 μg) of pure anti-TRX 2B1 and 9G9 in 0.1 M sodium phosphate/1 mM EDTA, pH 7.5. As controls, the same amount of TRX was incubated in the absence of antibody or the presence of equivalent doses of a mouse anti-glyceraldehyde-3-phosphate dehydrogenase mAb of the same isotype (IgG1). After overnight incubation at 4°C, samples were warmed to room temperature, insulin (Sigma Aldrich) was added to a final concentration of 2 mg/ml, and the reaction was initiated by adding 0.5 mM DTT. The lag phase and rate of change in OD650 were recorded. The mAb 9G9 inhibited the reductase activity of TRX in a dose-dependent manner, complete inhibition being achieved at ≥100 μg/ml. In contrast, mAb 2B1 had only a slight effect on the redox activity of TRX with a maximum of 10% inhibition of activity observed at 100 μg/ml (means of triplicate determinations). Human recombinant TRX was produced and isolated as described (22).
A rabbit polyclonal anti-TRX antibody was produced by Primm Laboratories (Milan, Italy) after four cycles of immunization with human recombinant TRX.
Generation of DCs from Peripheral Blood Monocytes.
DCs were obtained by culturing adherent cells from peripheral blood mononuclear cells from healthy donors in RPMI 1640 medium (Biochrom, Berlin) supplemented with 2 mM glutamine, 100 units/ml penicillin, 100 μg/ml streptomycin (Biochrom), 10% heat-inactivated FCS (PAA Labour, Linz, Austria), 40 ng/ml recombinant granulocyte-monocyte colony-stimulating factor (Schering-Plough), and 10 ng/ml IL-4 (Preprotech, London) as described (23–25). Media were endotoxin free as shown by the Limulus lysate colorimetric assay (PBI, Milan, Italy). After 8 days, DCs were CD14−, CD80+, CD86+, and intensely positive for HLA-DR. Lipopolysaccharide (LPS, 1 μg/ml, Sigma Aldrich), or tumor necrosis factor α (TNF-α, 50 ng/ml, Genzyme) was added for different times as indicated to induce DC maturation. Mature DCs showed a strong up-regulation of CD80, CD86, and HLA-DR (23–26).
Establishment of Tetanus Toxoid (tetox)-Specific T Cell Lines and Mixed Lymphocyte Reaction (MLR).
Purified T cells were obtained after two rounds of plastic adherence followed by immunodepletion of CD14+ and HLA-DR+ cells with immunomagnetic beads (Dynal, Milan, Italy) (24–26).
Tetox-specific or Dermatophagoides pteronissimus-specific T cell lines were obtained as described (25, 26) by stimulation of autologous peripheral blood mononuclear cells with tetox (10 μg/ml, Statens Serum Institut, Copenhagen) or D. pteronissimus (kind gift of F. Manca, S. Martino Hospital, Genoa, Italy) followed by Percoll (Amersham Pharmacia) gradient separation of T cell blasts after 7 days, culture in 10% T cell growth factor (Lymphocult, Biotest Diagnostics, Dreieich, Germany) and weekly restimulation with antigen. Allospecific T cell lines were obtained by coculturing for 1 week purified T cells with allogenic-irradiated (4,000 rad) peripheral blood mononuclear cells as described (24–26). The antigen specificity of autologous anti-tetox T cells (after three restimulations) and of 7-day alloreactive T cells was analyzed by [3H]thymidine AP Biotech, Milan, Italy) incorporation as described (24). The cytolytic activity of 7-day or, as a positive control, 18-day alloreactive T cells against DCs was tested in a 51Cr-release assay (27, 28). DCs were loaded with 51Cr and cocultured for 4 h with effector alloreactive T cells, at an effector/target ratio of 10:1. Results are expressed as percent cytotoxicity as described (28). T cells from 7-day MLR were also tested for their content in intracellular perforin by cytoplasmic immunofluorescence by using the antiperforin mAb G9 (PharMingen), before or after 6-h contact with DCs (28).
Culture Conditions.
Immature or mature DCs obtained as above were cultured for 6 h at 37°C in RPMI medium 1640 supplemented with 1% Nutridoma HU (Roche Molecular Biochemicals) in the presence or absence of allospecific T cells from a 7-day parallel primary MLR (or nonspecific T cells, activated in MLR with different donors as control), or with autologous tetox-specific or D. pteronissimus-specific T cell lines, all at a 10:1 ratio. In other experiments, DCs were challenged with CD40L transfected cells (29) (kind gift of P. Lane, Basel Institute for Immunology, Basel) at a 1:1 ratio for 6 h. When indicated, DCs were pretreated for 20 min and treated during the whole period of incubation with T cells, with glutamate (Glu, 2 mM, Sigma), 1,3-bis(2-chloroethyl)-1-nitrosurea (BCNU, 50 μM, Sigma), 1-chloro-2,4-dinitrobenzene (DCNB, 100 μM, Sigma), L-cysteine (50 μM, Sigma), and/or mAbs 9G9 or 2B1 (5 μg/ml). Glutamate is a specific inhibitor of the cystine transporter (17), whereas BCNU (9) and DCNB (30) inhibit glutathione reductase and TRX reductase, respectively. In other experiments, DCs were fixed for 10 min with 1% glutaraldehyde (Sigma) before coculture with alloreactive T cells (28).
Determination of Thiols in Culture Media.
To quantify extracellular thiols, supernatants (100 μl, corresponding to 7 × 103 DCs) from different cultures were added in a 96-well flat-bottom culture plate to 100 μl of 10 mM EDTA/100 mM Tris⋅HCl, pH 8.2. 5,5′-Dithiobis-(2-nitrobenzoic acid) (DTNB; 10 mM; Sigma) was then added and the absorption was measured at 412 nm (9). Cys (Sigma) was used as a standard; results are expressed in micromolar. In some experiments, acid-soluble thiols were determined after trichloroacetic acid precipitation (5% final concentration) and neutralization of supernatants with phosphate buffer and NaOH as described (19). Under these conditions, the amount of free thiols detected by DTNB was consistently similar to that detectable in the unprocessed supernatants. To quantify reduced and oxidized glutathione (GSH and GSSG), Cys and Cys2, culture media were collected, acidified with metaphosphoric acid (5% final concentration), and centrifuged at 22,300 × g for 30 min. Soluble thiols were then assayed upon formation of S-carboxymethyl derivatives with iodoacetic acid followed by conversion of free amino groups to 2,4-dinitrophenyl derivates by reaction with 1-fluoro-2,4-dinitrobenzene as described (31). Low molecular thiols were finally separated by HPLC with a Bondapak NH2 column (Warters, Milford, MA). GSH, GSSG (Roche Molecular Biochemicals), Cys and Cys2 (Sigma) were used as external standards. Results are expressed in micromolar.
Western Blot Analysis.
At the end of the incubation, supernatants were collected and concentrated by 10% trichloroacetic acid (32); cells were detached by scraping and lysed in 1% Triton X-100-containing buffer. Aliquots of lysates corresponding to 7 × 104 DCs and the corresponding trichloroacetic acid-concentrated supernatants, were resolved on SDS-15% polyacrylamide gel under reducing conditions (32). Gels were electrotransferred onto Hybond ECL filters (AP Biotech), stained with Ponceau Red (Sigma) to confirm comparable protein loading (not shown), and destained before blocking overnight with 10% nonfat dry milk in PBS. Filters were then hybridized with the anti-TRX polyclonal antibody followed by a horseradish peroxidase-conjugated goat anti-rabbit IgG (Dako) and developed by ECL-Plus (AP Biotech) according to the manufacturer's instructions.
Determination of LDH Release.
Cell viability was evaluated by trypan blue exclusion and by measurement of LDH activity in culture supernatants by using a LDH colorimetric assay (Sigma) as described (33).
Proliferation Assay.
DCs seeded at 104/well in 96-well flat-bottom microtiter plates were loaded with 10 μg/ml tetox for 12 h, followed by addition of 2 × 104 autologous antitetox T cells or alloreactive T cells from a parallel 7-day MLR, or T cells from an unrelated 7-day MLR. DC-T cell cocultures were incubated 3 days under different conditions as indicated, pulsed with 1 μCi of [3H]thymidine per well for the last 18 h of culture, harvested, and the 3H-incorporation measured in a β-counter (Packard) (24). Tests were performed in triplicate and results expressed as mean counts per min ± SD.
Results
DCs Generate Cysteine After Maturation or Antigen Presentation.
DCs were derived from peripheral blood monocytes by culture in recombinant granulocyte-monocyte colony-stimulating factor and IL-4, and their ability to release extracellular thiols was investigated by DTNB colorimetric assay (Table 1). After 8 days of culture, DCs actively generate discrete amounts of thiols, ranging from 5 to 30 μM in different individuals (Exp. 1–3). Maturation results in increased thiol release that is maximal after 24 h of treatment with LPS or TNF-α, or of cross-linking of CD40 (2- to 4-fold with respect to immature DCs, Table 1) but is already evident after 6 h (1.5- to 2-fold, not shown). In contrast, no thiols are detectable in the supernatants of T cell lines, such as Jurkat or MOLT-4 T cells.
Table 1.
DC maturation induces thiol release
| Exp. 1 | Exp. 2 | Exp. 3 | |
|---|---|---|---|
| DC− | 5 | 30 | 15 |
| DC + LPS | 20 | 85 | 34 |
| DC + TNF-α | 20 | 70 | 40 |
| DC + CD40L | 15 | 80 | 40 |
| Jurkat | <2, 5 | <2, 5 | <2, 5 |
| Molt-4 | <2, 5 | <2, 5 | <2, 5 |
Eight-day DCs, treated with or without 1 μg/ml LPS or 50 ng/ml TNF-α for the last 30 h of culture, were washed and incubated with fresh serum free medium for 6 h. At the end of the incubation time, thiols present in the supernatants were evaluated by DTNB colorimetric assays. Three different experiments, with DCs from three different donors are shown. Experimental values are in μM.
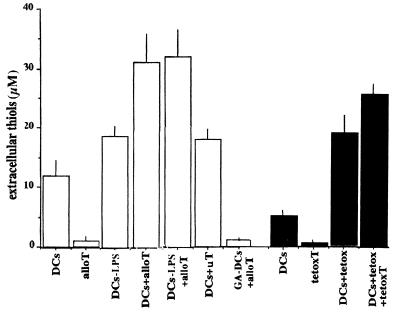
To investigate whether thiol release by DCs is modulated during specific interactions with T cells, DCs were cocultured for 6 h with alloreactive T lymphocytes generated in a 7-day primary MLR, and the presence of extracellular thiols was assessed at the end of incubations. As shown in Fig. 1, 3-fold more thiols were detected in the supernatant of DCs after coculture with alloreactive T cells (the increase varied from 2- to 5-fold in the different experiments). In contrast, T lymphocytes activated in MLR against an unrelated individual (uT) had only a marginal effect. When mature DCs (LPS- or TNF-α-treated) were coincubated with allospecific T cells, no significant increase on thiol release with respect to coculture of T cells with immature DCs was observed (Fig. 1). T cells alone did not produce detectable thiols; furthermore, no thiols were found in supernatants from cocultures of alloreactive T cells and glutaraldehyde-fixed DCs, indicating that exogenous thiols derive from DCs rather than from T cells (Fig. 1).
Figure 1.
Thiol release by DCs is induced by T cells. Eight-day DCs (7 × 104), untreated or treated for the last 24 h with LPS, were incubated 6 h alone (DCs; DCs-LPS) or with alloreactive T cells from a parallel primary 7-day MLR (DCs + alloT; DCs-LPS + alloT) or with T cells from a 7-day MLR between two uTs (DCs + uT) at a DC/T cell ratio of 1:10. As a control, DCs were fixed for 10 min with 1% glutaraldehyde before coculture with alloreactive T cells (GA-DCs + alloT). In a separate experiment (filled columns), DCs were cultured alone (DCs) or loaded overnight with tetox (DCs + tetox) and then incubated for 6 h with autologous tetox-specific T cells at the same DC/T cell ratio (DCs + tetox + tetoxT). At the end of the incubation, thiols present in the supernatants were evaluated by DTNB colorimetric assay. As a control, thiol release by the same number of alloreactive (alloT) or tetox-specific T cells (tetoxT) is shown. One representative experiment of six is shown ± SD.
An increased thiol release was also observed during soluble antigen presentation; coculturing tetox-primed DCs with autologous antitetox T cells led to enhanced production of extracellular thiols (Fig. 1). Also in this experimental system, DCs seemed to be the source of exogenous thiols. Little, if any, signal was detected in the supernatants of tetox-specific T lymphocytes.
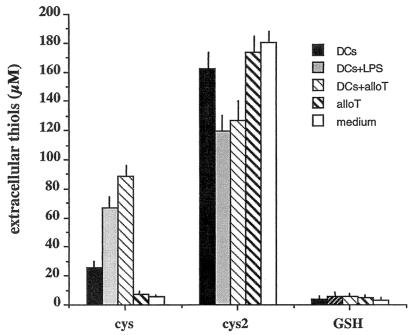
HPLC analyses revealed that Cys is the most represented thiol released by DCs (Fig. 2). The levels of extracellular GSH remained low even after stimulation of DCs with LPS or with antigen-specific T cells. The increase in extracellular Cys is paralleled by a decrease of Cys2, suggesting a partial Cys2 to Cys2 conversion in the medium.
Figure 2.
Cys is the major small thiol released by DCs. The supernatants from 5 × 105 nonstimulated (DCs), LPS-stimulated DCs (DCs + LPS), or DCs incubated with alloreactive T cells at a DC/T cell ratio of 1:10 (DCs + alloT), were analyzed for the presence of Cys, Cys2, or GSH by HPLC. Open columns show the amounts present in fresh medium.
TRX Accumulates Intracellularly in DCs upon Maturation and Is Secreted After Interaction with Antigen-Specific T Cells.
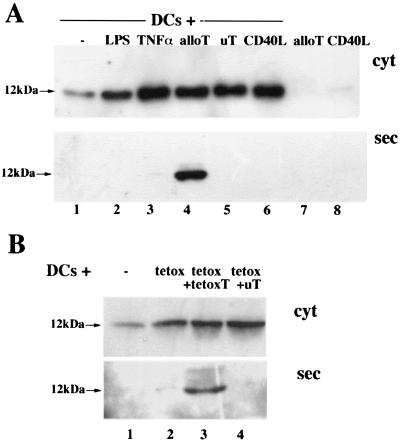
In addition to small thiols, secreted oxidoreductases (14, 15, 34, 35), and TRX in particular, may generate reducing conditions in the extracellular environment. Therefore, we investigated the synthesis and secretion of TRX by DCs, and their modulation by maturative agents or antigen-specific T cells (Fig. 3A). A 12-kDa TRX band was present in the lysates of immature DCs (Fig. 3A Upper, lane 1). LPS (Fig. 3A Upper, lane 2), TNF-α (Fig. 3A Upper, lane 3), or CD40L+ cells (Fig. 3A Upper, lane 6) induced an accumulation of TRX ranging from 2- to 10-fold in DCs from different donors. However, TRX secretion was barely detectable or undetectable (Fig. 3A Lower). In contrast, interaction with alloreactive T cells resulted in TRX secretion (Fig. 3A Lower, lane 4). The following lines of evidence indicated that T cell-induced TRX release was not caused by damage of DCs: (i) T lymphocytes from 7-day MLR were not cytotoxic, as evaluated in a 51Cr-release assay (27) (<5% of DC lysis at day 7 vs. >60% at day 18); (ii) in keeping with this result, we failed to detect secreted granzyme in 7-day MLR supernatants, confirming the immature state of CD8+ T cells, and (iii) >90% of CD8+ T cells were still positive for intracellular perforin after contact with DCs, as evaluated by cytoplasmic immunofluorescence (not shown; ref. 28); (iv) nonspecific T cells, activated in MLR against an uT, and thus functionally similar to alloreactive T cells, induced intracellular TRX but failed to trigger its secretion (Fig. 3A Lower, lane 5); and (v) the appearance of extracellular TRX was not paralleled by increase of the cytosolic enzyme lactate dehydrogenase in supernatants, which was consistently ≤3% of cellular LDH (not shown).
Figure 3.
Regulation of TRX synthesis and secretion in human DCs by LPS, TNF-α, or antigen-specific T cells. (A) Aliquots of the lysates (cyt, Upper) and supernatants (sec, Lower) from untreated DCs (lane 1), or DCs treated for the last 24 h with LPS (lane 2) or TNF-α (lane 3), cultured for 6 h alone (lanes 1–3) or with alloreactive (lane 4, alloT), or nonspecific T cells (lane 5, uT) or CD40L-transfected cells (lane 6, CD40L), were resolved under reducing conditions and transferred to nitrocellulose filters. Filters were decorated with polyclonal anti-TRX antibodies. Cell lysates and supernatants from the same number of alloreactive T cells (lane 7) and CD40L-transfected cells (lane 8) cultured alone are included as controls. (B) Lysates (cyt, Upper) and supernatants (sec, Lower) of 8-day DCs cultured alone for 6 h (lane 1), or loaded with tetox and incubated for 6 h alone (lane 2, tetox), with autologous anti-tetox T cells (lane 3, tetox + tetoxT) or with nonspecific activated T cells (lane 4, tetox + uT) were analyzed by Western blotting with anti-TRX as in A.
Also in soluble antigen presentation experiments, only antigen-specific T cells induced TRX secretion by DCs (Fig. 3B). Although both autologous (tetox-specific, Fig. 3B, lane 3) and unrelated (uT, D. pteronissimus-specific, Fig. 3B, lane 4) T lymphocytes increased the levels of TRX in cell lysates (Fig. 3B Upper), a 12-kDa extracellular TRX band was detected only after incubation of tetox-loaded DCs with specific T lymphocytes (Fig. 3B Lower, lane 3). Intracellular TRX increased slightly after incubation with tetox (Fig. 3B Lower, lane 2).
Mechanisms of Extracellular Medium Reduction by DCs.
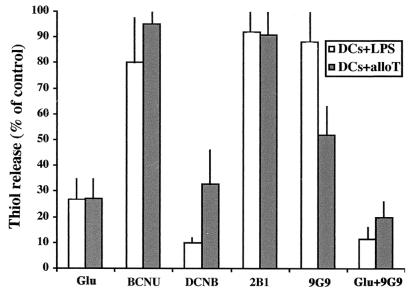
The conversion of Cys2 into Cys may occur intracellularly, upon uptake of Cys2, or extracellularly. To discriminate between these possibilities, we took advantage of the fact that the Cys2 membrane transporter is sensitive to Glu (16). Because the cytosolic content of Cys2 is very low, whereas the concentration of Glu is much higher in cells than in extracellular fluids, physiologic flows by this transporter are the entry of Cys2 and the exit of Glu. Raising the extracellular concentration of Glu results in competitive inhibition of Cys2 uptake (17). Addition of 2 mM Glu inhibited thiol generation by DCs induced by either LPS or alloreactive T cells (Fig. 4), suggesting that Cys2 import plays a major role in this process. If intracellular TRX were responsible for converting internalized Cys2 into Cys, inhibiting TRX reductase should effect thiol release by DCs. As shown in Fig. 4, the specific TRX-reductase inhibitor DCNB (30) caused a strong reduction in the amount of thiols recovered extracellularly. In contrast, inhibiting glutathione reductase with BCNU (9, 17) only slightly affected thiol release. Taken together, these findings suggested that stimulated DCs can uptake Cys2, convert it intracellularly by means of TRX-dependent reactions, and release it into the medium.
Figure 4.
Glutamate and antibodies inactivating TRX (9G9) inhibit the accumulation of extracellular thiols. LPS-stimulated DCs (7 × 104; DCs + LPS) were incubated alone, and the same number of immature DCs were incubated with alloreactive T cells (DCs + alloT). Incubations were performed 6 h in the presence of 2 mM Glu, 50 μM BCNU, 100 μM DCNB, 5 μg/ml of 2B1 or 9G9 mAb, or 2 mM Glu plus 5 μg/ml of 9G9 mAb. Thiols present in supernatants at the end of the culture period were determined by DTNB assay. Results are expressed as the percent of thiol release relative to untreated 6-h cultures of LPS-DCs (open columns) or DCs-alloreactive T cells (gray columns). One representative experiment of three is shown ± SD.
To determine whether secreted TRX played a role in the generation of extracellular thiols, we exploited 9G9, a mAb that specifically blocks the redox site of TRX (see Materials and Methods). Although the effects on LPS-stimulated DCs were minimal, 9G9 mAb significantly inhibited the release of thiols induced in DCs by coculture with alloreactive T cells, consistent with the observation that only in this condition TRX secretion is observed (Fig. 3). The simultaneous addition of Glu and 9G9 mAb results in abolition of thiol generation (Fig. 4). The mAb 2B1, directed against a nonredox-active epitope of the molecule, had negligible effects on thiol generation in cocultures of DCs with alloreactive T cell.
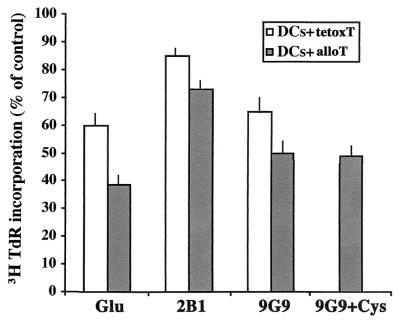
The Anti-TRX Antibody 9G9 Inhibits the Proliferation of Specific T Cells to Allogenic DCs or Autologous Tetox-Loaded DCs.
As an indicator of the functional relevance of extracellular accumulation of Cys and TRX, we analyzed the proliferation of T lymphocytes (either alloreactive or tetox-specific) to antigen-presenting DCs in the absence or presence of Glu, 9G9 or 2B1 mAb (Fig. 5). The incorporation of [3H]thymidine by alloreactive T cells incubated 3 days with DCs was considerably reduced by treatment either with Glu or 9G9 mAb (to about 60% of controls), whereas it was only slightly decreased by 2B1 mAb. The addition of 50 μM L-cysteine did not revert the inhibition exerted by 9G9 mAb on alloreactive T cell proliferation. When the effects of Glu and anti TRX mAbs were investigated on tetox-specific T cells proliferation in response to autologous tetox-loaded DCs, similar results were obtained; Glu and 9G9 mAb inhibited [3H]thymidine incorporation by 40% and 35%, respectively, whereas 2B1 mAb had almost no effect on the response (Fig. 5).
Figure 5.
Glutamate and 9G9 inhibit T cell proliferation induced by antigen-presenting DCs. Eight-day DCs (5 × 104) were incubated with alloreactive T cells from a parallel primary 7-day MLR (DCs + alloT) or loaded with tetox and incubated with autologous tetox-specific T cells (DCs + tetoxT), at a DC/T cell ratio of 1:4, in the presence or absence of 2 mM Glu or 5 μg/ml of 9G9 or 2B1 mAb, or 5 μg/ml of 9G9 and 50 μM Cys (this condition was tested on DC + alloT cocultures only), as indicated. [3H]Thymidine incorporation was evaluated at day 3. Results are expressed as the percent of [3H]thymidine incorporation relative to control cocultures of DC-alloreactive T cells (filled columns, 55,826 ± 6,895 cpm) or of DCs-tetox-specific T cells (open columns, 66,318 ± 3,153 cpm). No significant differences in [3H]thymidine incorporation were observed when LPS-treated DCs were used (not shown). Mean of three different experiments ± SD.
Discussion
It has long been known that lymphocytes require a reducing milieu for optimal activation/proliferation (36). However, the mechanism(s) underlying the local generation of optimal redox conditions in lymphoid tissues and the cells involved are still unclear. Our results demonstrate that DCs can generate extracellular Cys by at least two mechanisms. First, they can take up Cys2 and convert it intracellularly into Cys. This mechanism seems to correlate with increased TRX synthesis and may occur in the absence of antigen-specific interactions with cognate T cells. The second strategy that DCs exploit to reduce the extracellular space (the secretion of TRX) is restricted to antigen-specific responses. This one is not the first case in which the secretion of a cytosolic protein is triggered by cognate-interacting T cells, because we reported that secretion of the leaderless secretory proteins IL-1β and IL-18 by DCs are similarly induced by antigen-specific, but not by unrelated T lymphocytes (24–26). The observation that cognate T lymphocytes induce TRX release by DCs supports the existence of a bidirectional cross-talk between DCs and T cells in the immunological synapse. On the one hand, DCs activate T lymphocytes, on the other, T cells specifically induce a secretory switch in DCs, which results in the release of TRX, IL18, and/or IL-1β.
Polyclonal activators can induce TRX synthesis and secretion in T lymphocytes (15). However, in our coculture systems, only DCs seem to be responsible for TRX secretion. Alloreactive or tetox-specific T cells produce minute amounts of TRX (Fig. 3). Furthermore, we failed to detect extracellular TRX after incubation of antigen-specific or alloreactive T lymphocytes with glutaraldehyde-fixed DCs (not shown), further supporting that extracellular TRX derives primarily from DCs.
Thiol generation by DCs is increased by LPS or TNF-α, or by interaction with activated T cells, in keeping with the stronger T cell activation capacity of mature DCs (37). CD40, expressed by activated T cells, seems to play an important regulatory role, because CD40 engagement by CD40L-transfected cells (29) results in increased thiol release by DCs. However, nonspecific DC contact with CD40L-bearing activated T cells, as well as CD40 cross-linking or LPS or TNF-α stimulation, result in a weaker release of thiols than contact with specific T cells. This finding suggests that other surface molecules engaged during antigen-specific interaction between DCs and T cells are involved. CD40 engagement does not lead to TRX secretion, further supporting that, unlike thiol release, secretion of TRX strictly depends on an antigen-specific interaction between DCs and T cells.
Glutamate inhibits the accumulation of extracellular Cys by DCs, suggesting that, as in monocytes (17), Cys2 is taken up by DCs by means of Cys2/Glu transporters to be converted intracellularly into Cys. GSH and TRX are the principal thiol-reducing systems operating in the cytosol. They are maintained in the reduced state by the NADPH-dependent enzymes glutathione reductase and TRX reductase. The specific inhibitor of glutathione reductase BCNU only slightly reduces the generation of extracellular thiols by both DCs (Fig. 4) and monocytes (19), indicating that the GSH/GSSG system plays a minor role. In contrast, inhibiting TRX reductase severely impairs thiol generation. Therefore, it seems that Cys is generated by the TRX/TRX-reductase pathway, because stimuli triggering the accumulation of extracellular Cys have been observed to increase intracellular TRX significantly in DCs.
The availability of extracellular TRX secreted by DCs after specific interaction with T cells may contribute to the generation of the reducing microenvironment required by T cells. When the activity of secreted TRX is blocked by specific antibodies (9G9), fewer thiols accumulate extracellularly and T cell proliferation to antigen-presenting DCs is partially inhibited. These data are in agreement with the findings that catalytically inactive TRX mutants do not sustain T cell growth (38). TRX is a leaderless protein, whose secretion avoids the classical endoplasmic reticulum–Golgi route (15); the low efficiency of nonclassical secretion may limit TRX bioactivity in lymphoid tissues, in keeping with the hypothesis of a local control of cell growth/differentiation for leaderless secretory proteins (39). Because only reduced TRX is able to convert Cys2 into Cys, its activity in the oxidizing conditions of the extracellular space may be limited in time. It will be of interest to determine whether professional antigen-presenting cells also secrete TRX reductase.
Addition of Cys did not revert the inhibitory effects of 9G9 mAb on alloreactive T cell proliferation, suggesting that secreted TRX has additional effects beyond the reduction of cystine. Although membrane receptors for TRX have not been detected, it is possible that secreted TRX reduces disulfides in soluble or membrane proteins modifying their folding, assembly, or oligomerization state, and thus modulates their signaling properties. Other extracellular oxidoreductases have been identified, including the T cell cytokine macrophage migration inhibitory factor (40) and protein-disulfide isomerase (34, 35). Therefore, the local redox control may play a wide role in the sites of inflammation and immune response. It will be of interest to identify the extracellular substrates of TRX and other secreted oxidoreductases.
In conclusion, we propose that DCs, upon cross-talk with T cells, are induced to release free Cys and TRX; both contribute to the generation of the extracellular reducing milieu required for T lymphocyte activation (9, 10, 36) and an efficient immune response.
Acknowledgments
We thank Drs. C. Andrei, A. T. Palamara, and M. R. Zocchi for helpful discussion, Dr. F. Manca for the kind gift of D. pteronissimus, and S. Trinca for secretarial assistance. This work was supported in part by grants from Consiglio Nazionale Ricerche (target project on biotechnology) and Associazione Italiana per la Ricerca sul Cancro.
Abbreviations
- DC
dendritic cell
- GSSG
oxidized glutathione
- GSH
reduced glutathione
- Cys2
cystine
- LPS
lipopolysaccharide
- MLR
mixed lymphocyte reaction
- TNF-α
tumor necrosis factor α
- TRX
thioredoxin
- DCNB
1-chloro-2,4-dinitrobenzene
- BCNU
1,3-bis(2-chloroethyl)-1-nitrosurea
- DTNB
5,5′-dithiobis-(2-nitrobenzoic acid)
- tetox
tetanus toxoid
- uT
unrelated individual
Footnotes
See commentary on page 1107.
References
- 1.Meister A. J Biol Chem. 1994;269:9397–9400. [PubMed] [Google Scholar]
- 2.Staal F J, Anderson M T, Staal G E, Herzenberg L A, Gitler C, Herzenberg L A. Proc Natl Acad Sci USA. 1994;91:3619–3622. doi: 10.1073/pnas.91.9.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hutter D E, Till B G, Greene J J. Exp Cell Res. 1997;232:435–438. doi: 10.1006/excr.1997.3527. [DOI] [PubMed] [Google Scholar]
- 4.Messina J P, Lawrence D A. J Immunol. 1989;143:1974–1981. [PubMed] [Google Scholar]
- 5.Suthanthiran M, Anderson M E, Sharma V K, Meister A. Proc Natl Acad Sci USA. 1990;87:3343–3347. doi: 10.1073/pnas.87.9.3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mihm S, Galter D, Droge W. FASEB J. 1995;9:246–252. doi: 10.1096/fasebj.9.2.7781927. [DOI] [PubMed] [Google Scholar]
- 7.Smyth M J. J Immunol. 1991;146:1921–1927. [PubMed] [Google Scholar]
- 8.Mansoor M A, Svardal A M, Ueland P M. Anal Biochem. 1992;200:218–229. doi: 10.1016/0003-2697(92)90456-h. [DOI] [PubMed] [Google Scholar]
- 9.Ishii T, Sugita Y, Bannai S. J Cell Physiol. 1987;133:330–336. doi: 10.1002/jcp.1041330217. [DOI] [PubMed] [Google Scholar]
- 10.Gmunder H, Eck H-P, Benninghoff B, Roth S, Droge W. Cell Immunol. 1990;129:32–46. doi: 10.1016/0008-8749(90)90184-s. [DOI] [PubMed] [Google Scholar]
- 11.Wakasugi N, Tagaya Y, Wakasugi H, Mitsui A, Maeda M, Yodoi J, Tursz T. Proc Natl Acad Sci USA. 1990;87:8282–8286. doi: 10.1073/pnas.87.21.8282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iwata S, Hori T, Sato N, Ueda-Taniguchi Y, Yamabe T, Nakamura H, Masutani H, Yodoi J. J Immunol. 1994;152:5633–5640. [PubMed] [Google Scholar]
- 13.Arner E S, Holmgren A. Eur J Biochem. 2000;267:6102–6109. doi: 10.1046/j.1432-1327.2000.01701.x. [DOI] [PubMed] [Google Scholar]
- 14.Di Trapani G, Perkins A, Clarke F. Mol Hum Reprod. 1998;4:369–375. doi: 10.1093/molehr/4.4.369. [DOI] [PubMed] [Google Scholar]
- 15.Rubartelli A, Bajetto A, Allavena G, Wollman E, Sitia R. J Biol Chem. 1992;267:24161–24164. [PubMed] [Google Scholar]
- 16.Powis G, Montfort W R. Annu Rev Pharmacol Toxicol. 2001;41:261–295. doi: 10.1146/annurev.pharmtox.41.1.261. [DOI] [PubMed] [Google Scholar]
- 17.Sato H, Tamba M, Ishii T, Bannai S. J Biol Chem. 1999;274:11455–11458. doi: 10.1074/jbc.274.17.11455. [DOI] [PubMed] [Google Scholar]
- 18.Watanabe H, Bannai S. J Exp Med. 1987;165:628–640. doi: 10.1084/jem.165.3.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sido B, Braunstein J, Breitkreutz R, Herfarth C, Meuer S C. J Exp Med. 2000;192:907–912. doi: 10.1084/jem.192.6.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu Y J, Pulendran B, Palucka K. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 21.Arner E S, Zhong L, Holmgren A. Methods Enzymol. 1999;300:226–239. doi: 10.1016/s0076-6879(99)00129-9. [DOI] [PubMed] [Google Scholar]
- 22.Clarke F M, Orozco C, Perkins A V, Cock I, Tonissen K T, Robins A J, Wells J R E. J Reprod Fertil. 1991;93:525–539. doi: 10.1530/jrf.0.0930525. [DOI] [PubMed] [Google Scholar]
- 23.Sallusto F, Lanzavecchia A. J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gardella S, Andrei C, Costigliolo S, Poggi A, Zocchi M R, Rubartelli A. J Leukocyte Biol. 1999;66:237–241. [PubMed] [Google Scholar]
- 25.Gardella S, Andrei C, Costigliolo S, Olcese L, Zocchi M R, Rubartelli A. Blood. 2000;95:3809–3815. [PubMed] [Google Scholar]
- 26.Gardella S, Andrei C, Poggi A, Zocchi M R, Rubartelli A. FEBS Lett. 2000;481:245–248. doi: 10.1016/s0014-5793(00)02015-9. [DOI] [PubMed] [Google Scholar]
- 27.Zocchi M R, Rubartelli A, Morgavi P, Poggi A. J Immunol. 1998;161:2938–2943. [PubMed] [Google Scholar]
- 28.Gardella S, Andrei C, Poggi A, Lotti L V, Torrisi M R, Zocchi M R, Rubartelli A. Blood. 2001;98:2152–2159. doi: 10.1182/blood.v98.7.2152. [DOI] [PubMed] [Google Scholar]
- 29.Lane P, Traunecker A, Hubele S, Inui S, Lanzavecchia A, Gray D. Eur J Immunol. 1992;22:2573–2578. doi: 10.1002/eji.1830221016. [DOI] [PubMed] [Google Scholar]
- 30.Arnér E S J, Bjornstedt M, Holmgren A. J Biol Chem. 1995;270:3479–3482. doi: 10.1074/jbc.270.8.3479. [DOI] [PubMed] [Google Scholar]
- 31.Reed D J, Babson J R, Beatty P W, Ellis W W, Potter D W. Anal Biochem. 1980;196:55–62. doi: 10.1016/0003-2697(80)90118-9. [DOI] [PubMed] [Google Scholar]
- 32.Hamon Y, Luciani M F, Becq F, Verrier B, Rubartelli A, Chimini G. Blood. 1997;90:2911–2915. [PubMed] [Google Scholar]
- 33.Rubartelli A, Cozzolino F, Talio M, Sitia R. EMBO J. 1990;9:1503–1510. doi: 10.1002/j.1460-2075.1990.tb08268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mezghrani A, Courageot J, Mani J C, Pugniere M, Bastiani P, Miquelis R. J Biol Chem. 2000;275:1920–1929. doi: 10.1074/jbc.275.3.1920. [DOI] [PubMed] [Google Scholar]
- 35.Lay A J, Jiang X M, Kisker O, Flynn E, Underwood A, Condron R, Hogg P J. Nature (London) 2000;408:869–873. doi: 10.1038/35048596. [DOI] [PubMed] [Google Scholar]
- 36.Ishii T, Hishinuma I, Bannai S, Sugita Y. J Cell Physiol. 1981;107:283–293. doi: 10.1002/jcp.1041070215. [DOI] [PubMed] [Google Scholar]
- 37.Sallusto F, Cella M, Danieli C, Lanzavecchia A. J Exp Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oblong J E, Berggren M, Gasdaska P Y, Powis G. J Biol Chem. 1994;269:11714–11720. [PubMed] [Google Scholar]
- 39.Rubartelli A, Sitia R. In: Unusual Secretory Pathways: From Bacteria to Man. Kuchler K, Rubartelli A, Holland B I, editors. Austin, TX: Landes; 1997. pp. 87–104. [Google Scholar]
- 40.Kleemann R, Kapurniotu A, Frank R W, Gessner A, Mischke R, Flieger O, Juttner S, Brunner H, Bernhagen J. J Mol Biol. 1998;280:85–102. doi: 10.1006/jmbi.1998.1864. [DOI] [PubMed] [Google Scholar]