Abstract
Theories of embodied cognition suggest that after an initial visual processing stage, emotional faces elicit spontaneous facial mimicry and that the accompanying change in proprioceptive facial feedback contributes to facial emotion recognition. However, this temporal sequence has not yet been properly tested, given the lack of methods allowing to manipulate or interfere with facial muscle activity at specific time points. The current study (N = 51, 28 women) investigated this key question using EEG and facial neuromuscular electrical stimulation (fNMES)—a technique offering superior control over which facial muscles are activated and when. Participants categorised neutral, happy and sad avatar faces as either happy or sad and received fNMES (except in the control condition) to bilateral zygomaticus major muscles during early visual processing (−250 to +250 ms of face onset), or later visual processing, when mimicry typically arises (500–1000 ms after face onset). Both early and late fNMES resulted in a happiness bias specific to neutral faces, which was mediated by a reduced N170 in the early window. In contrast, a modulation of the beta-band (13–22 Hz) coherence between somatomotor and occipital cortices was found in the late fNMES, although this did not predict categorisation choice. We propose that facial feedback biases emotion recognition at different visual processing stages by reducing visual processing load.
Subject terms: Neuroscience, Psychology
Facial muscle activity can shape how we recognise emotions. Using electrical stimulation and EEG, the study found that activation of smiling muscles makes people more likely to see neutral faces as happy, by reducing visual processing load.
Introduction
According to theories of embodied cognition1, emotion recognition and affective judgments are supported by humans’ ability to simulate, both in the brain and body, observed emotional states2–4. In line with this, emotional faces automatically trigger somatomotor activity in the brain5,6, and spontaneous facial mimicry in the face7, while interference with either can impair emotion recognition and affective judgments8–10.
Spontaneous facial mimicry may play an instrumental role in shaping our interpretation of expressions11. Indeed, the movement of facial muscles provides afferent proprioceptive/somatosensory feedback to the brain12–14, feedback that could assist in resolving visual ambiguities. In support of this idea, a number of studies demonstrate that blocking or interfering with facial mimicry in neurotypical participants15, and in those with congenital facial paralysis16, is associated with reduced emotion recognition ability. However, empirical evidence is mixed, and it remains controversial what role facial mimicry, and its accompanying changes in facial feedback, play in emotion recognition17.
A likely reason for at least some of the inconsistencies in the literature is that past studies investigating the issue were limited in their ability to precisely control which muscles were activated or inhibited to what degree. More importantly, the methods used by scholars to study the effects of altering facial mimicry and facial feedback offer only poor temporal control, and therefore cannot isolate effects on emotion recognition from other possible confounding factors, such as the effects of sustained effort of holding a pen between the lips (a commonly used technique to prevent facial mimicry). In order to overcome these limitations, we have recently started using computer-controlled facial neuromuscular electrical stimulation (fNMES) to activate specific facial muscles in a more controlled manner. For an overview of this method see18.
Using fNMES, we have demonstrated that bilateral activation of the zygomaticus major (ZM) muscle (the main muscle involved in smiling) increases the valence of self-reported emotion19 and the likelihood of perceiving emotionally ambiguous facial expression as happy20. These findings support the notion that facial feedback contributes to felt and perceived emotion, and are in line with the facial feedback hypothesis21,22. Importantly, by using fNMES we were able to isolate the ZM muscles and precisely control the intensity, symmetry, onset and duration of their activation during the simultaneous presentation of facial expressions (i.e. for the duration of a presented face).
Current experimental methods of manipulating proprioceptive facial feedback (e.g. holding a pen between the lips) offers poor temporal control. That is, the induced feedback occurs prior to, and for the duration of a number of cognitive operations, and in some cases, over many trials. In contrast, one can use fNMES to manipulate facial feedback at much smaller timescales, which allows to investigate the chronology of visual and proprioceptive events during embodied emotion recognition. For example, one can target the period of spontaneous facial mimicry, which typically arises around 500 ms after a face is presented23 or target periods of early visual processing. In doing so, one can elucidate the relative influence of facial feedback occurring at different times relative to the onset of a visual stimulus.
fNMES in combination with the high temporal resolution of EEG allows for the study of facial feedback effects at specific neural processing stages. We have previously demonstrated that fNMES artefacts can be removed from EEG, and accurate measurements of event-related potentials (ERPs) can be obtained24,25. Moreover, we showed that fNMES reduces the amplitude of a number of visual ERPs in response to facial expressions20, although these reductions were not specific to a particular emotion. An ERP of particular interest when investigating embodied emotion recognition in faces is the N170. This is an early visual ERP related to the structural encoding of faces26,often found to be larger for emotional relative to neutral faces24,27–29. Several studies have demonstrated that facial feedback contributes to emotion recognition and affects early visual processing (even before the typical onset time of spontaneous facial mimicry). For example, N170 amplitude for neutral faces becomes more similar to that for happy faces when participants pose a smile30. This suggests that facial feedback can indeed modulate early visual processing, and is not just instrumental from 500 ms after face onset onwards, when spontaneous facial mimicry typically occurs.
Emotional face perception likely relies on the combination of visual and somatosensory information. Somatosensory signals will influence perception more in cases where the visual signal is ambiguous. In line with this, Achaibou et al.31 reported smaller N170 amplitudes in trials resulting in greater facial mimicry. Moreover, individuals with congenital facial palsy (with partial to extended facial muscle paralysis) demonstrate reduced connectivity (beta-band coherence) between somatosensory and visual areas during emotional face perception32. Coherence in the beta-band has been suggested to represent long-distance EEG connectivity for the processing of stimuli with affective value33.
Modulations in N170 amplitude due to changes in facial feedback can also be contextualised within the framework of predictive coding34. The brain continuously generates predictions about sensory inputs and compares these with actual incoming sensory data35. If there is a mismatch, it results in a prediction error signal, which indicates that something unexpected has occurred. The internal model is then adjusted, so as to perpetually minimise these prediction errors. Indeed, N170 amplitude has previously been demonstrated to be sensitive to unexpected perceptual events36, and the degree to which expectations are violated has a graded effect on N170 amplitude (the larger the violation, the larger the N170). Activations of ZM may therefore provide a certain context in which a happy face is predicted. If a different face, for example, is then presented (a violation of this prediction), then the resulting prediction error may manifest as an increase in N170 amplitude30.
The current preregistered study (https://tinyurl.com/4wjy78nb) used fNMES in order to investigate whether facial feedback from ZM available during early visual processing (i.e. immediately prior to and during low level visual processing) or later visual processing (i.e. at the time spontaneous facial mimicry typically becomes measurable) has differential effects on the emotion categorisation (happy, sad) of neutral, happy, and sad facial expressions. EEG was recorded throughout to identify neural correlates of these effects. Prior to the main experiment, we ran two pilot studies (see Supplementary Materials) in order to select the best stimulus set, and to establish the onset time of spontaneous facial mimicry, to confirm the timing of late fNMES. In the main experiment, faces were presented for 1 s and fNMES was delivered at 70 Hz during early (−250 ms to 250 ms relative to face onset) or late (500 ms to 1000 ms relative to face onset) visual face processing, or not at all (off condition).
We expected (H1) that both early and late fNMES would significantly increase the likelihood of labelling neutral faces as happy, relative to no fNMES. We also expected (H2) this effect to be larger for the late fNMES period, which overlaps with the time period when spontaneous facial mimicry to emotional faces typically arises (as also confirmed in our EMG pilot experiment) and therefore should be the most natural timing for facial feedback changes to occur during emotion processing. Moreover, we expected (H3) that early fNMES would reduce the amplitude of the N170 in response to all faces, as was observed in our previous study20 and that this reduction would predict how faces were eventually labelled. Finally, we performed exploratory analyses on beta-band coherence between somatomotor and visual areas of the brain, in order to identify potential neural correlates of the effects of late fNMES on choice.
Methods
We have provided a full account of our sample size determination, justifications for data exclusion and comprehensive descriptions of all measures used within our research. The methods and hypotheses were registered in November 2023, before data acquisition started. The pre-registration, stimuli, tasks, analysis scripts and data are openly accessible at Open Science Framework (https://tinyurl.com/4wjy78nb). The analysis scripts are also available on Zenodo37.
Participants
We performed a power analysis for finding effects at the behavioural level (on neutral trials only) using data simulations in R. Drawing from the study by Efthimiou et al.20, we assumed a small effect size (b = 0.09) for the early condition, indicating a 9% increase in the likelihood of classifying facial expressions as happy. For the late condition, we expected this effect to double in size (b = 0.18). Using the simr package in R, we ran for various sample sizes 1000 simulations of generalised linear mixed effects models using a binomial distribution and including a full random effects structure with all individual slopes and intercepts. A sample size of 45 participants was found to provide an average statistical power of 87% (95% CI = 78.80% to 92.89%) to detect a significant effect of fNMES in the early condition.
The participants were 51 adults (28 females, 23 males, mean age = 22.9, SD = 3.65, range 18–33), with normal or corrected to normal vision, no current use of prescribed medication or history of illicit drug use, and no history of neurological or psychiatric illness. Participants were recruited through a number of channels (e.g. flyers, email lists, social media) and were financially compensated at a rate of £10 per hour. They gave written informed consent before taking part, and were debriefed at the end of the testing session. The study was approved by the local ethics committee (ETH1920-0847). The analyses concerning only neutral expressions (that is, comparisons between neutral faces labelled as happy with those labelled as sad), was carried out in 48 participants, as three participants had labelled all neutral faces as belonging to only one category.
Stimuli, task design and experimental procedure
The stimulus pool consisted of 48 greyscale images of computer-generated facial expressions from 16 different identities (8 female). Faces were generated with the FaceGen software (www.facegen.com), and their emotional expressions were created based on the Facial Action Coding System (FACS)38 using the FACSGen software39,40. This process involves manipulating Action Units (AUs), which correspond to specific muscle (group) movements that contribute to facial expressions. Each identity displayed a neutral, sad and happy expression, which were selected based on ratings from pilot study 1 (see Supplementary Materials). The expressions of happiness included AUs 6, 7 and 12, while sadness included AUs 1, 4, 7, 11 and 15. Each of the neutral expressions was repeated six times, and each happy and sad expression was repeated four times, in each fNMES condition (off, early, late; see below). This resulted in a total of 288 neutral faces, 192 happy faces and 192 sad faces being displayed to each participant. Five additional images were used for practice trials at the onset of the experiment. Scrambled faces (generated by scrambling the face area of the neutral face from each identity) were also presented immediately following each face on every trial to supress afterimages.
Participants completed six blocks of trials, each lasting ~6 min. Trials included neutral, happy, or sad faces during three different fNMES conditions (off, early, late). An additional 50 trials with fNMES but no face presentation were included in the task, but discarded from analyses, as a double-subtraction method was instead used to further remove fNMES noise in the EEG (see “Methods and Results”). More details on fNMES parameters are presented below. Participants completed 722 trials in total, which were presented in a pseudo-random order, whereby no more than four trials of the same fNMES type and no more than four trials of the same emotion type were presented consecutively. Importantly, two thirds of all trials included a 500 ms train of 70 Hz fNMES at motor threshold (MT) intensity to bilateral ZM (early and late conditions), with the other third of trials without stimulation (off condition). Specifically, in the early fNMES condition, stimulation started 250 ms prior to and was maintained until 250 following the onset of the face. In the late fNMES condition, an identical stimulation was delivered from 500 to 1000 ms after the onset of the face. The time window selected for the late fNMES conditions was based on the literature, as well as on pilot study 2 (see Supplementary Materials), where statistically significant facial mimicry to happy vs neutral faces was found from 500 ms onwards, using facial EMG.
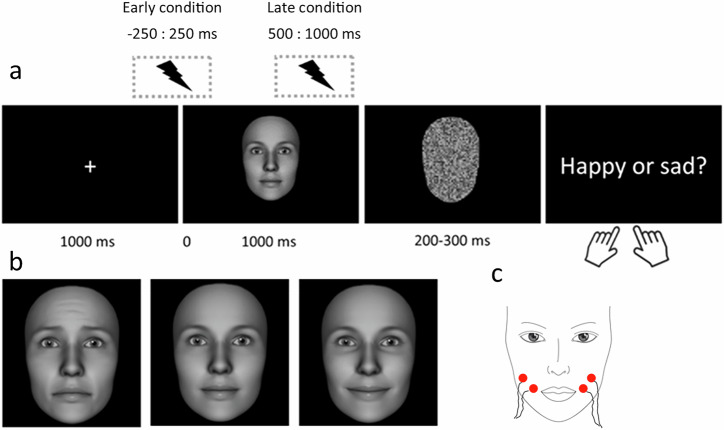
The experiment was programmed in PsychoPy v2021.1.441 .Each trial (see Fig. 1) began with a centrally presented white fixation cross (horizontal = 0.47° and vertical = 0.47° of visual angle) on a black background for 1000 ms, which was then followed by a face stimulus for 1000 ms (horizontal = 14° and vertical = 20° of visual angle). Thereafter, a scrambled face was presented for a jittered duration of 200–300 ms, followed by a final screen displaying the words ‘happy or sad?’ that invited participants to provide a button press on a number keyboard (4 or 6, button assignment counterbalanced across participants) in order to label the previously presented face as either happy or sad.
Fig. 1. Trial structure and example stimuli.
a each trial began with a fixation cross which was followed by either a neutral, happy, or sad face, displayed for 1000 ms. Following a scrambled face, participants responded via keypress as to whether they had perceived the face as happy or sad. fNMES was either delivered in an early period (−250 to 250 ms around face onset), late period (500–1000 ms post face onset), or not at all (off condition). b example of a sad (left), neutral (middle) and happy (right) facial expression from a female identity. c schematic of electrode placement over the participant’s bilateral zygomaticus major muscles.
Participants were first welcomed into the lab and were provided with consent forms and study information. Participants then provided their gender (choosing between the options: ‘male’, ‘female’, ‘other’, ‘prefer not to say’) and completed the PANAS questionnaire42 in order to measure positive and negative affect prior to the experiment (t1). Information on participants’ race/ethnicity was not collected. Head measurements were then taken before an appropriately sized EEG cap was fitted to the participant’s head. Following the application of conductive gel (signaGel), participants sat in a comfortable chair in a sound-attenuated booth ~60 cm away from a 24.5 inch screen (Alienware aw2521h) with a resolution of 1920 × 1080 pixels and a 60 Hz refresh rate. Prior to the onset of the experiment, a calibration of the fNMES equipment was performed. The experimenter cleaned the skin of the participants’ cheeks using 70% isopropyl alcohol wipes. Two pairs of disposable electrodes were placed over the bilateral ZM muscles, following electromyography (EMG) guidelines43,44. To identify the best positioning of the electrodes and ensure that a weak smile could be induced comfortably, fNMES intensity was gradually increased until visible muscle contractions were observed (the motor threshold, or MT). Participants’ faces were recorded throughout the testing session using a webcam mounted on top of the stimulus-displaying screen. This was to ensure the correct placement of stimulation electrodes, and to record the intensity of muscle movement on each trial. Finally, on completion of all trials, participants again completed the PANAS questionnaire (t2).
fNMES parameters and procedure
The delivery of fNMES to the bilateral ZM muscle was achieved using two constant-current electrical stimulators (Digitimer, DS5 https://tinyurl.com/yta3wa3a), each interfaced with a digital to analogue converter25. A 500-ms long train of biphasic square pulses (100-µs biphasic pulse width and 14-ms delay between biphasic pulses) was delivered at 70 Hz using disposable Ag/AgCl electrodes measuring 16 × 19 mm (Ambu BlueSensor BRS). Average stimulation intensity was 22.60 mA (SD = 3.62, range: 14.25–33.75), and current density per electrode area (M = 0.62, range 0.39–0.92) was well below the 2 RMS mA/cm2 safety threshold18.
EEG data acquisition and signal processing
EEG data were acquired with a waveguard cap containing 64 Ag/AgCl electrodes in the international 10–20 configuration, and an eEGo sports amplifier (ANT neuro, Netherlands), at 512 Hz and digitised with 24-bit resolution. Data were referenced online to electrode CPz, with the ground electrode at AFz. For further analyses, EEG data were imported and processed using functions from the EEGLAB (v2022.1) environment45 for MATLAB (The Mathworks, Inc.). We followed a previously established procedure for the cleaning of fNMES-induced artefacts24. Continuous EEG were first filtered with a 0.5 Hz high pass and 80 Hz low pass filter, channels with excessive noise or artefacts were identified through visual inspection and interpolated, line noise was removed using Zapline and Cleanline, and data were epoched from 1000 ms before to 1200 ms after face stimulus onset. We performed independent component analysis (ICA) on the epoched data using the runica function in EEGLAB and removed components representing blinks and fNMES artefacts24. Following the removal of ICA components, trials were rejected if any values exceeded ±100 μV, resulting in an average of 72 trials per condition included in the final analysis. Baseline correction was then applied using the mean value between −500 and −260 ms pre-stimulus onset (i.e. a period of time not including fNMES). The data were finally filtered with a 40-Hz low pass filter and re-referenced to the common average.
Analysis of behavioural data
Statistical analyses were conducted in R (R Core Team, 2020), implementing (generalised) mixed models with the lme446 package, p values were computed using the lmerTest package47, approximations of Cohen’s d for generalised mixed effects models were computed using the formula d = beta/(pi/sqrt(3)), as suggested by48. Quality of the statistical models (including distribution of residuals) was verified with the performance package49. The first of our pre-registered models tested that fNMES would increase the percentage of participants’ choices of happiness in response to neutral faces. To this end, we used a Generalised Linear Mixed Effects model (GLMM) using a binomial distribution that included the categorical fixed effect of fNMES (off, early, late) and by-subject intercepts and slopes as random effects: Choice ~ fNMES + (fNMES | Participant). A second model included the additional fixed effect of emotion (neutral, happy, sad) to elucidate eventual interaction effects between fNMES and emotion on choice: Choice ~ emotion * fNMES + (1 | participant). Its random effects structure was simplified to prevent singular fits. Posthoc tests were carried out using the package emmeans50 with Bonferroni correction. For key non-significant effects Bayes factors were computed with the BayesFactor package and default priors. In addition, we also fitted the above model including two covariates: PANAS difference scores (negative and positive affect scores at t2 minus t1) in order to examine the potential influence of mood changes on behaviour, and the Point of Subjective Equality (PSE) values. PSE values were derived for each participant for each fNMES condition and represented the point on the emotion continuum (sad coded as −1, neutral coded as 0 and happy coded as 1) that participants were equally likely to choose happy or sad.
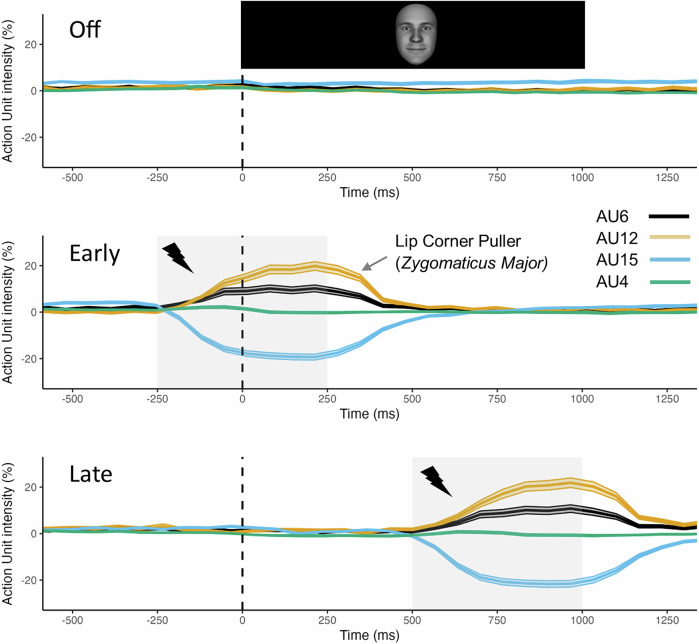
The degree of fNMES-induced smiling on each trial was captured with video recordings and estimated with automatic FACS coding38 implemented in OpenFace51. Firstly, videos of each trial were extracted for 46 participants (6 participants were not included given poor video data), which included 2 s before and after stimulation onset, or the face onset in the case of the off condition. Following baseline correction (using the first second of each video), AU intensity over time (% of baseline values) was calculated for AUs 6, 12, 15 and 4 (see Fig. 2) in each of the experimental conditions. Trials in each condition were then averaged together, and the mean intensity value for AU12 was calculated in the stimulation period. A linear model with the predictors fNMES (off, early, late) and emotion (sad, happy, neutral) was then used to compare smile intensity (AU12) in each condition.
Fig. 2. Automatic FACS coding.
Action unit intensity for AU6 (Orbicularis Oculi, i.e. Cheek Raiser), AU12 (Lip corner puller, i.e. Zygomaticus Major), AU15 (Lip corner depressor, i.e. Depressor Anguli Oris), and AU4 (Brow lowerer, i.e. Corrugator Supercilii) over time in the off (top), early (middle) and late (bottom) fNMES conditions. In both the early and late fNMES conditions and during the period of stimulation (grey boxes), AU12 intensity can be seen to increase relative to the off condition. Dotted line indicates the onset time for face stimuli. Shaded areas show 95% CI. N = 46.
Analysis of EEG
Time domain analyses
Pre-processed and epoched EEG data were first analysed in the time domain. A mass-univariate analysis approach52 was utilised in order to identify regions in time and space that presented fNMES-induced significant differences between happy and sad faces. The mass univariate approach involves computing multiple paired-sample t-tests across each time point for each channel for two different waveforms. Specifically, the following difference waveforms were computed and entered into the analysis. First, we subtracted sad face trials from happy face trials, in each of the three fNMES conditions. Following this first differencing procedure, we then directly compared these differences between the off and early fNMES conditions, and separately, the off and late fNMES conditions. The resulting derived waveforms therefore expressed the effects of fNMES on the difference between happy and sad faces. In using this approach, one can avoid directly comparing trials that contain fNMES with those that do not. Such differencing procedures can eliminate fNMES artefacts over and above our established procedure24.
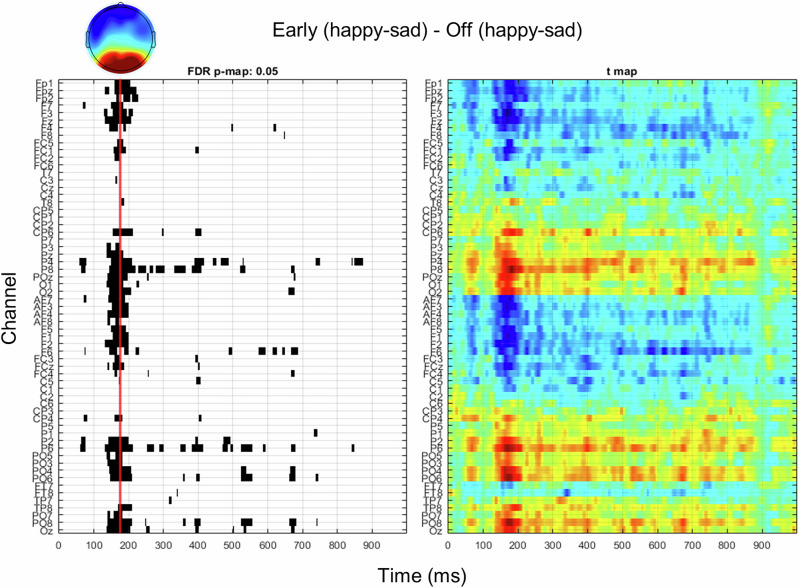
Visual inspection of the computed p-value maps (with false discovery rate correction) derived from comparing fNMES off and early conditions only revealed a significant bilateral occipito-temporal cluster from 150 to 200 ms post face onset, which we interpreted as the N170 component based on timing and topography. We derived N170 amplitude in each trial by averaging the identified significant channels (P5, P3, P1, Pz, P2, P4, P6, P8, PO7, PO5, PO3, POz, PO4, PO6, PO8, O1, Oz, O2) and deriving the mean value between 150 and 200 ms post face onset. A similar approach by comparing trials in the fNMES off and late condition revealed no significant differences (even at more liberal significance thresholds).
To examine whether N170 amplitude to neutral faces predicted how they were labelled in the early compared to the off condition, we used a GLMM with a binomial distribution with the categorical predictor fNMES (off, early), and the continuous predictor N170: Choice ~ fNMES * N170 + (fNMES * N170 | participant). A second model that included all face emotions was also fitted.
Coherence analyses
In addition to analysing N170 amplitude, we performed an exploratory analysis that examined whether fNMES would modulate spectral coherence between somatomotor and occipital areas during trials in which neutral faces were labelled as happy relative to when they were labelled as sad. To this end, we derived time-frequency resolved coherence coefficients between central and occipital regions of interest (ROIs) for neutral faces labelled as happy or sad for each fNMES condition. First, we derived coherence estimates between 0 and 1 s post-stimulus and between 5 and 40 Hz from the power and cross-spectra using a frequency resolution of 0.5 Hz, a time resolution of 10 ms and, for each given frequency, hanning windows with a length corresponding to 5 cycles between a select number of electrode pairs. Specifically, each electrode in the central ROI (Cz, C1, C2, C3, C4) was paired with each electrode in three separate occipital regions (left: P1, P3, P5, P7, PO3, PO5, PO7, middle: Pz, POz, Oz and right: P2, P4, P6, P8, PO4, PO6 and PO8). We then averaged the resulting coherence values between central and left occipital electrodes, central and middle occipital electrodes and central and right occipital electrodes, resulting in three coherence maps for each fNMES condition (off, early, late) for neutral faces labelled as happy and those labelled as sad.
Afterwards, we statistically assessed the categorisation choice (happy or sad) by fNMES interaction by performing a Monte Carlo based cluster permutation test based on a dependent samples t-test (two-tailed, 2000 permutations, cluster alpha = 0.01 and final alpha = 0.05) separately for each ROI on the difference in coherence between neutral faces rated as happy and sad in the early fNMES condition, compared to the difference for neutral faces labelled as happy and sad in the off condition. This was also performed to contrast the late fNMES and off conditions. This resulted in two clusters that showed that both early and late fNMES modulated the difference in coherence between neutral faces labelled as happy vs. labelled as sad. Both of these identified clusters expressed fNMES-induced changes in beta-band coherence from 650 ms post stimulus onset between the central and left-occipital region. Finally, both significant clusters were integrated, which generated a mask that included coherence values for frequencies and time points that expressed significant differences in the early-off and late-off contrasts, in order to extract mean coherence measures in each condition separately.
To first test whether coherence differed between neutral faces labelled as happy vs. sad in each of the fNMES conditions, we implemented a 3 (fNMES: off, early, late) x 2 (choice: happy, sad) repeated measures ANOVA. Posthoc analyses were performed using Bonferroni corrected paired-sample t-tests. In order to test whether coherence during trials containing neutral faces was predicted by fNMES condition, we used a linear model with the fixed factor fNMES (off, early, late), with off serving as the reference level. As such, the model formula was: Coherence ~ fNMES. Given that coherence estimates are not derived at the single-trial level (e.g. based on averages across conditions), the investigation of the relationship between coherence and choice was performed at the condition-average level. As such, we computed an average behavioural measure (percentage of neutral trials labelled as happy) in each fNMES condition to include in the following models. First, we fitted a linear model that predicted percent choice happy for neutral faces by coherence. Second, we fitted a model that also included fNMES (off, early, late) as a categorical predictor (percent choice happy ~ fNMES + coherence), with the fNMES off condition serving as the reference level.
Results
fNMES induces smiling as confirmed by automatic FACS coding
In order to confirm that fNMES activated AU12 (Lip Corner Puller; Zygomaticus Major), we performed automatic FACS coding on video footage captured with a webcam (see Fig. 2). Mean intensity values (% change relative to baseline) were computed for AU12 during the stimulation period in each condition. We implemented a linear model with the predictors fNMES (off, early, late) and emotion (happy, sad, neutral), which revealed significant differences between fNMES early (M = 0.137, SE = 0.01) vs. off (M = 0.002, SE = 0.01) [b = 0.12, 95% CI (0.06, 0.18), SE = 0.03, p < 0.001] and between fNMES late (M = 0.156, SE = 0.01) vs. off [b = 0.15, 95% CI (0.09, 0.21), SE = 0.03, p < 0.001], while fNMES early and late did not differ from each other [b = 0.02, 95% CI (−0.03, 0.08), SE = 0.03, p = 0.42]. Converting this to a type 3 anova model using the car package revealed a medium sized main effect of fNMES (ηp2 = 0.06). To summarise, fNMES successfully increased AU12 intensity during the stimulation periods, confirming a smiling expression.
fNMES increases the likelihood of labelling neutral faces as happy, regardless of the timing
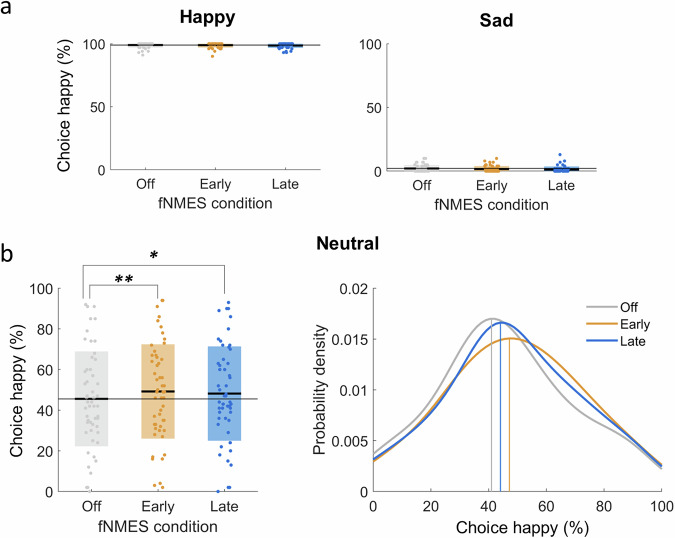
Our first pre-registered analysis concerned only neutral faces and was to predict choice (happy or sad) by fNMES condition (off, early, late), with off serving as the reference level (conditional R2 = 0.36, marginal R2 = 0.001). A significant main effect of early fNMES was found [b = 0.17, z = 3.84, 95% CI (0.09, 0.27), SE = 0.046, p < 0.001, d = 0.10], as was a significant main effect of late fNMES [b = 0.133, z = 2.88, 95% CI (0.04, 0.22), SE = 0.04, p = 0.003, d = 0.07], indicating, as predicted, that neutral faces were more likely to be categorised as happy when fNMES was delivered to ZM, regardless of the timing of fNMES (Fig. 3b). We had hypothesised that fNMES delivered in the late period (i.e. during the natural timing of spontaneous facial mimicry) would have a greater effect than when it was delivered in the early period, however, posthoc tests revealed that this was not the case [b = 0.04, 95% CI (−0.07, 0.15), z = 0.96, SE = 0.05, p = 1.00]. A Bayesian paired t-test with a standard Cauchy prior revealed moderate evidence in favour of the null hypothesis (BF10 = 0.27), suggesting no significant difference between early and late effects on neutral faces.
Fig. 3. The effects of fNMES on choice.
a Box plots showing the percentage of choosing happy for happy (left) and sad (right) faces in each fNMES condition. Regardless of the fNMES condition, participants had high accuracy and consistently labelled happy faces as being happy, and sad faces as being sad. b Box plot (left) and probability density plot (right) showing the percentage and probability of choosing happy for neutral faces in each fNMES condition. Both early and late fNMES conditions significantly increased the likelihood of choosing happy for neutral faces. Thick black lines in the box plot show condition means with coloured boxes showing standard deviation. The grey line spanning the plot shows the mean in the off condition. Vertical lines in the probability density plot show mean probability of choosing happy in each fNMES condition for neutral faces. ** p = 0.002, * p = 0.032. N = 51.
In order to explore if fNMES effects were specific to neutral faces, a second model included all emotions (happy, sad, neutral, with neutral faces serving as the reference level). This model (conditional R2 = 0.81, marginal R2 = 0.74) revealed expected significant main effects of happy faces [b = 5.50, z = 29.22, 95% CI (5.14, 5.87), SE = 0.18, p < 0.001, d = 3.03] and sad faces [b = −4.23, z = 29.72, 95% CI (−4.52, −3.96), SE = 0.14, p < 0.001, d = −2.34], and significant main effects of both early and late fNMES (which did not differ from each other, as identified in the previous model and the Bayesian t-test). Posthoc tests that examined the effects of early and late fNMES for each emotion indicated that the impact of fNMES on choice was significant for neutral faces [Early fNMES: b = −0.17, 95% CI (−0.28, −0.06), z = −3.78, SE = 0.04, p < 0.001], Late fNMES: b = −0.13, 95% CI (−0.23, −0.02), z = −2.84, SE = 0.04, p = 0.013], but was not significant for happy faces [Early fNMES: b = 0.26, 95% CI (−0.31, 0.83), z = 1.07, SE = 0.24, p = 0.84], Late fNMES: b = 0.52, 95% CI (−0.02, 1.06), z = 2.31, SE = 0.22, p = 0.06] or sad faces [Early fNMES: b = 0.16, 95% CI (−0.32, 0.65), z = 0.81, SE = 0.20, p = 1.00], Late fNMES: b = 0.41, 95% CI (−0.10, 0.93), z = 1.93, SE = 0.21, p = 0.16]. The pattern of results remained the same when including as covariates the PANAS difference scores or the PSEs (although the model with the PSEs resulted in singular fits and posthoc tests no longer showed a significant difference between fNMES conditions for neutral faces, probably because of the overlap between the fNMES and PSE variables).
To summarise, fNMES to bilateral ZM in both the early and late time windows increased the likelihood of labelling neutral faces as happy to a similar degree.
fNMES decreases N170 amplitude and moderates the relationship between N170 and choice
Our second preregistered analysis was to examine whether N170 amplitude in interaction with fNMES predicted choice for neutral faces. Electrodes and time points to be included in this analysis were selected based on a mass-univariate analysis (Fig. 4, see ‘Methods’ section for more details).
Fig. 4. Mass-univariate analysis were used to derive effects of fNMES on the difference between happy and sad faces (impacting the N170).
Sad trials were first subtracted from happy trials in the fNMES early condition, which was then compared to the same difference in the off condition. p-value map (left) with false-discovery rate correction showing significant effects of early fNMES on happy-sad differences, with the corresponding t-values (right). Topographic map showing the voltage difference for the same contrast between 150 and 200 ms post face onset. N = 51.
We used a model that included the categorical predictor fNMES (off, early, with off serving as the reference level) and the continuous predictor N170 (marginal R2 = 0.003). A main effect of fNMES was observed [b = 0.14, z = 2.42, 95% CI (0.03, 0.27), SE = 0.06, p = 0.01, d = 0.08], indicating again that early fNMES increases the likelihood of labelling neutral faces as happy. Importantly, a significant interaction between fNMES and N170 amplitude was also observed [b = 0.02, z = 1.99, 95% CI (0.01, 0.04), SE = 0.01, p = 0.04, d = 0.01]. In the off condition, a negative slope (−0.01, SE = 0.01, z = −1.44, p = 0.15) revealed that a larger N170 amplitude (more negative value) increased the likelihood of labelling a neutral face as happy (see Fig. 5). In contrast, during early fNMES, the opposite was observed, in which a positive slope (0.01, SE = 0.01, z = 1.18, p = 0.24) revealed that a smaller N170 (more positive value) increased this likelihood. By itself, neither slope significantly differed from zero, as shown by simple slope analyses. In order to assess whether the same pattern was present for all faces (i.e. if N170 in interaction with fNMES predicts choice of all faces), we utilised the same model including all trials in the early and off conditions (marginal R2 = 0.002). This again revealed a significant interaction between fNMES and N170 [b = 0.02, z = 4.91, 95% CI (0.02, 0.04), SE = 0.01, p < 0.001, d = 0.01] that showed the same pattern as when analysing only neutral faces. A simple slope analysis revealed that both the negative slope in the off condition [b = -0.02, z = −3.72, SE = 0.01, p < 0.001] and the positive slope in the early condition [b = 0.01, z = 2.89, SE = 0.01, p < 0 0.001] were significantly different from zero.
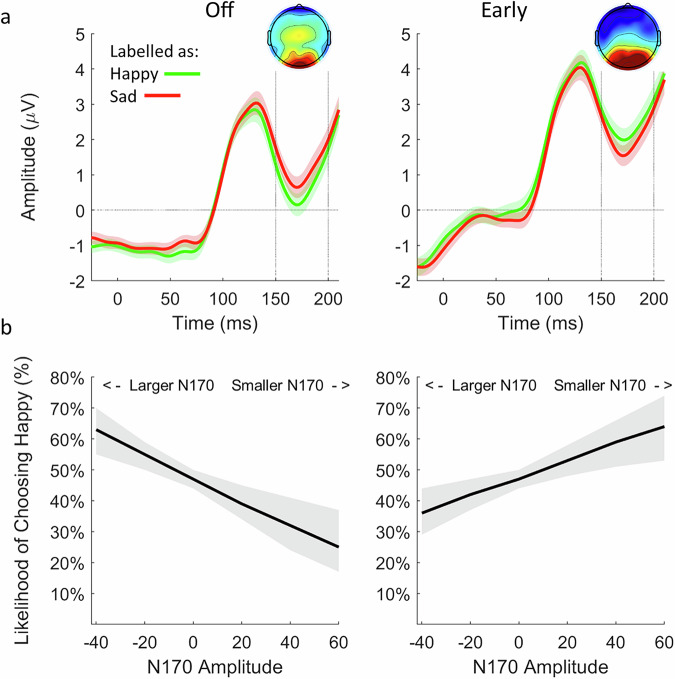
Fig. 5. Relationship between N170 amplitude and choice.
a N170 for all faces categorised as happy (green) and sad (red) in the off (left) and early (right) fNMES conditions. Waveforms and topographic maps show the average of channels identified in the mass-univariate analysis, between 150 and 200 ms after face onset. Shaded areas show standard error. b Derived marginal means from a model predicting choice of each trial by fNMES and N170 amplitude in the off (left) and early (right) fNMES conditions. In the off condition, a larger N170 amplitude increased the likelihood of labelling a face as happy. In contrast, in the early fNMES condition, a smaller N170 increased this likelihood. Shaded areas show 95% confidence intervals. N = 51.
To summarise, early fNMES is associated with a decrease in N170 amplitude, which is linked to an increased probability of labelling faces as happy (Fig. 5).
fNMES modulates beta-band coherence for neutral faces
Cluster based permutation tests resulted in two clusters showing significant fNMES-induced modulations of beta-band (13–22 Hz) coherence between central and left occipital regions from 650 ms post stimulus onset (see Fig. 6a). We derived a mask that incorporated both of these clusters in order to derive mean coherence estimates for neutral faces labelled as happy and those labelled as sad, in each fNMES condition.
Fig. 6. Beta coherence between central and left occipital region in response to neutral faces.
a Coherence maps showing coherence for neutral faces labelled as happy vs. sad in the off condition (left) and for early-off (middle) and late-off (right) contrasts. Clusters (values encased in green boundaries) show significant differences in beta-band coherence from 650 ms post stimulus onset identified from the contrasts. b Mean coherence values for neutral faces labelled as happy (green) and sad (red) in each fNMES condition. In the off condition, neutral faces labelled as happy presented greater coherence than those labelled as sad. During late fNMES, however, the reverse was observed. A similar trend was also present for early fNMES. Error bars show standard error. p values are Bonferroni corrected. ** p < 0.01, *p < 0.05, o trend (p = 0.072). N = 48.
In order to test whether beta-band coherence differed between neutral faces labelled as happy vs. those labelled as sad in each fNMES condition, we first implemented a 3 (fNMES: off, early, late) x 2 (choice: happy, sad) repeated measures ANOVA. There were no significant main effects of fNMES [F (1.91, 89.59) = 1.34, p = 0.267, ηp2 = 0.028, 95% CI (0, 1)] or choice, [F (1, 47) = 0.12, p =0.735, ηp2 = 0.002, 95% CI (0, 1)], but a significant interaction between fNMES and choice [F (1.92, 90.45) = 19.58, p <0.001, ηp2 = 0.294, 95% CI (0.17, 1)] was observed. Posthoc tests revealed that in the off condition, beta-band coherence was greater for neutral faces labelled as happy (M = 0.340, SE = 0.016), than those labelled as sad (M = 0.316, SE = 0.016) [t (47) = 2.95, 95% CI (0.008, 0.04), p = 0.004]. In contrast, in the late fNMES condition, coherence was greater for those labelled as sad (M = 0.332, SE = 0.016) than as happy (M = 0.318, SE = 0.015) [t (47) = −2.24, 95% CI (−0.02, −0.001), p = 0.029]. A similar pattern was observed in the early fNMES condition, however this was not significant [t (47) = −1.84, 95% CI (−0.03, 0.001), p = 0.072].
In order to test whether beta-band coherence in interaction with fNMES predicted choice for neutral faces, we used a model that included the categorical predictor fNMES (off, early, with off serving as the reference level) and the continuous predictor coherence. No main effects or interactions were observed (Early fNMES: b = 3.80, SE = 14.06, t = 0.27, 95% CI (−23.9, 31.5), p = 0.78; Late fNMES: b = 3.31, SE = 13.99, t = 0.24, 95% CI (−24.3, 30.97), p = 0.81; Beta Coherence: b = 7.83, SE = 28.71, t = 0.23, 95% CI (−48.9, 64.6), p = 0.79; Early fNMES x Beta Coherence: b = -0.27, SE = 41.18, t = -0.006, 95% CI (−81.6, 81.1), p = 0.99; Late fNMES x Beta Coherence: b = −1.50, SE = 40.70, t = −0.04, 95% CI (−81.9, 78.9), p = 0.97).
To summarise, although fNMES had differential effects on beta-band coherence for neutral faces eventually labelled as happy vs. sad, these fNMES-induced modulations did not predict the emotion categorisation of neutral faces.
Discussion
The aim of the current study was to examine the temporal dynamics of facial feedback effects in facial emotion recognition. Traditional manipulations of facial muscles afford very little temporal precision, whereby engagement/blocking of facial muscles persists through all stages of face processing. As such, it is unclear whether changes in facial feedback during early visual perception or relatively later stages (e.g. when spontaneous facial mimicry typically occurs) have differential effects on emotional face recognition. To this end, we manipulated facial feedback from the ZM muscle using fNMES in an early (−250 to +250 ms) and a late time window (+500 to +1000 ms) relative to the onset of neutral, happy and sad facial expressions. We examined how categorisation choice (happy or sad) for each face would be modulated by fNMES. In addition, we examined the neural correlates of the effects of fNMES on choice by identifying an emotion x fNMES effect on the N170 in a data-driven way (mass univariate analysis), and by computing beta-band coherence between somatomotor and occipital areas of the brain.
Our findings demonstrate, as predicted (H1), an induced bias for categorising neutral faces as happy when receiving stimulation to the bilateral ZM muscle. This is consistent with our previous study20 and provides clear evidence for the facial feedback hypothesis. It is important to emphasise, that fNMES modulated only the categorisation of neutral faces, and not of happy and sad faces. This was expected, given that multisensory integration is more likely to occur when stimuli in one or both sensory modalities are degraded or otherwise less informative53. In other words, in the absence of emotional information in neutral faces, participants tended to rely more on proprioceptive input. A similar pattern had been observed in our previous study that also showed a happiness bias in trials with fNMES to bilateral ZM muscles20, although there the emotion by fNMES interaction did not reach significance, probably due to the fact that the stimulus set included only emotional expressions that were all relatively ambiguous.
We had also expected (H2) that the facial feedback effect on categorisation choices would be more pronounced when fNMES was delivered in the late period. The reason is that the nervous system might be particularly sensitive to changes in facial feedback during the period in which facial mimicry typically occurs (500 ms and onwards following the presentation of a face). Facial mimicry is considered to provide additional (proprioceptive) information in resolving visual ambiguities during emotional face recognition11, and as such, changes in facial feedback accompanying facial mimicry do usually occur after the initial stages of visual face processing. However, in this experiment no differential effects of the timing of fNMES on categorisation choice were found. The reason we did not observe stronger effects in the later condition could be due to the fact that stimulation was not delivered in the optimal period (see “Limitations”).
The observation that stimulation delivered in both time periods (immediately prior to and during initial stages of visual face processing, and relatively later during the expected period of spontaneous facial mimicry) resulted in the same induced response bias, suggests that fNMES is modulating distinct neural operations. This seems also supported by the different neural effects of early and late fNMES. Regarding stimulation in the early period, we found that fNMES to ZM specifically reduced N170 amplitude. This is consistent with our previous study20, however, in the current study we found a pronounced reduction that was specific to both actual happy faces, and neutral faces categorised as happy (importantly, categorisation responses always occurred at the end of each trial). Moreover, the larger the fNMES-induced decrease of the N170, the more likely a face was to be categorised as happy. This suggests that the presence of facial feedback from the ZM muscle during early visual perception (e.g. prior to N170) modulates the succeeding cortical excitability of the visual system. That is, visual processing is ‘tuned down’, given that somatosensory information from smiling muscles informs the brain that the presented face is happy. As a result, the reduction in N170 (and the degree to which this reduction predicts categorisation choice), suggests that early visual face processes involved in unpacking the emotional content of the face (such as the N170), operate to a lesser degree in the context of salient facial feedback information. In other words, the workload of the visual system is offset due to convincing proprioceptive evidence that a perceived face is happy. The larger this offset, the more likely participants categorise faces as happy. This finding is consistent with a study that demonstrated a relationship between the magnitude of facial mimicry, recorded with EMG over the ZM and corrugator supercilii muscles, and the amplitude of the preceding N17031. Trials that had smaller N170 amplitudes in response to faces were associated with more facial mimicry. As such, when visual processing was operating to a lower degree, more facial mimicry was needed to decipher the content of the face. We suggest that our findings present a similar trade-off between somatosensory and visual inputs.
An alternative explanation for this modulation in N170 is provided by predictive coding, a theoretical framework aiming to explain how the brain processes sensory information34. Stimulation of ZM may lead to the prediction that a happy face is to be presented, and a prediction error would occur if the presented face violates this prediction. Indeed, during early fNMES, N170 was greater (more negative) to sad than happy faces (see Fig. S3 in Supplementary Materials). As such, the unexpected occurrence of a sad face (given the activation of the participant’s smiling muscles) resulted in a larger prediction error, reflected in a larger N170. Indeed, N170 amplitude has previously been demonstrated to be sensitive to unexpected perceptual events36, and the degree to which expectations are violated has a graded effect on N170 amplitude (the larger the violation, the larger the N170). When prediction errors are small or absent, the N170 amplitude will become relatively smaller, which is what we found in response to happy faces (and neutral faces labelled as happy) during fNMES to the ZM muscles (the perceived face was consistent with the provided facial feedback context).
In contrast, the categorisation response bias induced with fNMES in the late period cannot be explained by the aforementioned modulations in N170 amplitude. Indeed, in the late condition stimulation was delivered from 500 ms following the onset of the face, which is well after the period of the N170. As such, we suggest that modulation of different neural mechanisms resulted in the same induced bias across the early and late fNMES conditions. However, a mass univariate analysis contrasting the late and off fNMES conditions did not reveal any significant differences in the time domain. As such, we considered modulations in functional connectivity between somatomotor and visual areas as a potential neural marker of the induced bias due to fNMES delivered in the late period. Beta-band coherence, for example, has previously been shown to be attenuated in individuals with congenital facial paralysis32, potentially demonstrating limited information flow between somatomotor and visual areas during face processing. Coherence in the beta-band has been suggested to represent long-distance EEG connectivity for the processing of stimuli with affective value33.Our analysis revealed a modulation of beta-band coherence differences (between neutral faces labelled as happy vs. sad) from 650 ms onwards in the late fNMES conditions, relative to the off condition, in the left hemisphere. Specifically, beta-band coherence was greater for neutral faces labelled as happy than as sad in the off condition, however this relationship was reversed (i.e. greater for neutral faces labelled as sad) during late fNMES (with a similar non-significant trend observed in the early fNMES condition). These findings were surprising, as one might expect that coherence for neutral faces eventually labelled as happy should be greater during fNMES, indicating an increase in information flow between somatomotor and visual areas (given the magnitude of changes in facial feedback). Our findings are opposite to this expectation, whereby a decrease (relative to neutral faces labelled as sad) was observed. Importantly, however, this modulation of beta-band coherence did not predict how neutral faces were eventually labelled (possibly due to lower statistical power because analyses could no longer be carried out at the single trial level). As such, despite the effects of fNMES on beta-band coherence, such modulations did not influence choice in our study. Although our analyses identified fNMES effects specifically in the beta-band, future studies might wish to consider exploring modulations of coherence in other frequencies during facial feedback manipulations. For example, gamma band activity has been found to represent the binding of stimulus features with top-down information54.
Limitations
We based our late stimulation period on a pilot study which only considered spontaneous facial mimicry to happy faces. As such, our late period might have been slightly ill-informed (i.e. did not reflect the timing of facial mimicry in the case of sad faces). Future studies should refine the late fNMES timing by testing alternative time windows (e.g. 400–800 ms, 600–1100 ms). Another possibility is that spontaneous facial mimicry manifests at a subthreshold level prior to being observable in facial EMG data. As such, although in a statistical sense facial mimicry occurred only after 500 ms following face onset, it is possible that undetectable state-changes in facial muscles already occur prior to this, and thus our late stimulation period was indeed too late. Future studies could investigate this further by providing fNMES, for example, from 200 ms onwards. The neural mechanisms underpinning the effects of late fNMES on choice requires further investigation as our analyses did not identify conclusive fNMES-induced modulations for this condition. Future studies might wish to focus solely on this time period, as only one third of the trials in the present study involved late stimulation. In doing so, one might achieve sufficient power to elucidate the neural correlates the effects of late fNMES on choice. It is also important to consider that avatar faces, although allowing for tight control of low-level visual features, might differ from real pictures of facial expressions in their eliciting of processes involved in emotional recognition. As such, future studies might wish to present real-life, even dynamic facial expressions so as to maximise the generalisability of our findings.
Conclusions
To conclude, the present study demonstrates that controlled changes in facial feedback from the smiling muscles either during early or relatively late visual processing can induce a happiness bias when labelling neutral facial expressions. Despite predicting that facial feedback effects would be more pronounced in a relatively late time window (during the period of facial mimicry), we found that proprioceptive information from facial muscles can exert an influence on behaviour both during this period, and to the same extent, during early visual processing stages. Notably, the observed bias introduced when delivering stimulation prior to and during early face processing can be explained by a reduction in N170 amplitude. We argue that the presence of salient somatosensory information from smiling muscles prior to the onset of a face relieved the visual system’s load in deciphering the emotional content of that face. Indeed, the larger this reduction, the more likely a face was labelled as happy. Late fNMES induced a modulation of beta band coherence between somatomotor and occipital areas, which however was not predictive of categorisation choices. As such, the neural correlates of the effects of late fNMES remain poorly understood, and should be addressed in future studies specifically focusing on that period. Finally, it would also be interesting to explore the effects of facial muscle activation, and other controlled somatosensory inputs, on the perception of emotion in non-visual stimuli—for example, Selosse et al.55 recently reported improved emotion recognition of fearful and angry voices, and modulation of early ERPS, when vocal cords were vibrated at emotion-congruent frequencies.
Supplementary information
Acknowledgements
This work was funded by a Stand Alone Grant by the Austrian Science Fund, awarded to SK, MM and AE (Grant number: P 32637-B). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author contributions
Joshua Baker: Conceptualisation, Data Curation, Formal Analysis, Investigation, Software, Visualisation, Writing—Original Draft Preparation. Hong-Viet Ngo-Dehning: Data Curation, Formal Analysis, Supervision, Writing—Review & Editing. Themis N. Efthimiou: Conceptualisation, Data Curation, Formal Analysis, Writing—Review & Editing. Arthur Elsenaar: Conceptualisation, Funding Acquisition, Writing—Review & Editing. Marc Mehu: Conceptualisation, Funding Acquisition, Writing—Review & Editing. Sebastian Korb: Conceptualisation, Formal Analysis, Funding Acquisition, Supervision, Writing—Review & Editing.
Peer review
Peer review information
Communications Psychology thanks Beatriz Calvo-Merino and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Troby Ka-Yan Lui. [A peer review file is available].
Data availability
All the data are openly accessible at Open Science Framework (https://tinyurl.com/4wjy78nb).
Code availability
All the code to run the experiment, preprocess and analyse the data are openly accessible at Open Science Framework (https://tinyurl.com/4wjy78nb).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
7/22/2025
A Correction to this paper has been published: 10.1038/s44271-025-00297-4
Contributor Information
J. Baker, Email: joshua.baker@essex.ac.uk
S. Korb, Email: sebastian.korb@essex.ac.uk
Supplementary information
The online version contains supplementary material available at 10.1038/s44271-025-00281-y.
References
- 1.Niedenthal, P. M. Embodying emotion. Science316, 1002–1005 (2007). [DOI] [PubMed] [Google Scholar]
- 2.Moody, E. J., Reed, C. L., Van Bommel, T., App, B. & McIntosh, D. N. Emotional mimicry beyond the face?: rapid face and body responses to facial expressions. Soc. Psychol. Personal. Sci.9, 844–852 (2018). [Google Scholar]
- 3.Peelen, M. V., Atkinson, A. P. & Vuilleumier, P. Supramodal representations of perceived emotions in the human brain. J. Neurosci.30, 10127–10134 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ross, P. & Atkinson, A. P. Expanding simulation models of emotional understanding: the case for different modalities, body-state simulation prominence, and developmental trajectories. Front. Psychol.11, 309 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keysers, C., Kaas, J. H. & Gazzola, V. Somatosensation in social perception. Nat. Rev. Neurosci.11, 417–428 (2010). [DOI] [PubMed] [Google Scholar]
- 6.Moore, A., Gorodnitsky, I. & Pineda, J. EEG mu component responses to viewing emotional faces. Behav. Brain Res.226, 309–316 (2012). [DOI] [PubMed] [Google Scholar]
- 7.Korb, S., Grandjean, D. & Scherer, K. R. Timing and voluntary suppression of facial mimicry to smiling faces in a Go/NoGo task—An EMG study. Biol. Psychol.85, 347–349 (2010). [DOI] [PubMed] [Google Scholar]
- 8.Korb, S. et al. Gender differences in the neural network of facial mimicry of smiles—an rTMS study. Cortex70, 101–114 (2015). [DOI] [PubMed] [Google Scholar]
- 9.Vilaverde, R. F. et al. Inhibiting orofacial mimicry affects authenticity perception in vocal emotions. Emotion. 10.1037/emo0001361 (2024). [DOI] [PubMed]
- 10.Wood, A., Lupyan, G., Sherrin, S. & Niedenthal, P. Altering sensorimotor feedback disrupts visual discrimination of facial expressions. Psychon. Bull. Rev.23, 1150–1156 (2016). [DOI] [PubMed]
- 11.Künecke, J., Hildebrandt, A., Recio, G., Sommer, W. & Wilhelm, O. Facial EMG responses to emotional expressions are related to emotion perception ability. PLoS ONE9, e84053 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cattaneo, L. & Pavesi, G. The facial motor system. Neurosci. Biobehav. Rev.38, 135–159 (2014). [DOI] [PubMed] [Google Scholar]
- 13.Cobo, J. L., Abbate, F., de Vicente, J. C., Cobo, J. & Vega, J. A. Searching for proprioceptors in human facial muscles. Neurosci. Lett.640, 1–5 (2017). [DOI] [PubMed] [Google Scholar]
- 14.Tereshenko, V. et al. Axonal mapping of the motor cranial nerves. Front. Neuroanat.17, 1198042 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borgomaneri, S., Bolloni, C., Sessa, P. & Avenanti, A. Blocking facial mimicry affects recognition of facial and body expressions. PLoS ONE15, e0229364 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sessa, P. et al. Degenerate pathway for processing smile and other emotional expressions in congenital facial palsy: an hdEEG investigation. Philos. Trans. R. Soc. B: Biol. Sci.377, 20210190 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hess, U. & Fischer, A. Emotional mimicry as social regulation. Pers. Soc. Psychol. Rev.17, 142–157 (2013). [DOI] [PubMed] [Google Scholar]
- 18.Efthimiou, T. N., Hernandez, M. P., Elsenaar, A., Mehu, M. & Korb, S. Application of facial neuromuscular electrical stimulation (fNMES) in psychophysiological research: practical recommendations based on a systematic review of the literature. Behav. Res.56, 2941–2976 (2023). [DOI] [PMC free article] [PubMed]
- 19.Efthimiou, T. N., Baker, J., Elsenaar, A., Mehu, M. & Korb, S. Smiling and frowning induced by facial neuromuscular electrical stimulation (fNMES) modulate felt emotion and physiology. Emotion.10.1037/emo0001408 (2024). [DOI] [PubMed]
- 20.Efthimiou, T. N. et al. Zygomaticus activation through facial neuromuscular electric stimulation (fNMES) induces happiness perception in ambiguous facial expressions and affects neural correlates of face processing. Soc. Cogn. Affect. Neurosci. nsae013 10.1093/scan/nsae013 (2024). [DOI] [PMC free article] [PubMed]
- 21.Coles, N. A. et al. A multi-lab test of the facial feedback hypothesis by the Many Smiles Collaboration. Nat. Hum. Behav. 1–12 10.1038/s41562-022-01458-9 (2022). [DOI] [PubMed]
- 22.Hatfield, E., Cacioppo, J. T. & Rapson, R. L. Emotional contagion. Curr. Dir. Psychol. Sci.2, 96–100 (1993). [Google Scholar]
- 23.Dimberg, U., Thunberg, M. & Elmehed, K. Unconscious facial reactions to emotional facial expressions. Psychol. Sci.11, 86–89 (2000). [DOI] [PubMed] [Google Scholar]
- 24.Baker, J. et al. Measurement of the N170 during facial neuromuscular electrical stimulation (fNMES). J. Neurosci. Methods393, 109877 (2023). [DOI] [PubMed] [Google Scholar]
- 25.Baker, J. et al. Computer-controlled electrical stimulation of facial muscles by facial neuromuscular electrical stimulation (fNMES): Hardware and software solutions. J. Neurosci. Methods411, 110266 (2024). [DOI] [PubMed] [Google Scholar]
- 26.Eimer, M. The face-specific N170 component reflects late stages in the structural encoding of faces. NeuroReport11, 2319 (2000). [DOI] [PubMed] [Google Scholar]
- 27.Blau, V. C., Maurer, U., Tottenham, N. & McCandliss, B. D. The face-specific N170 component is modulated by emotional facial expression. Behav. Brain Funct.3, 7 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hinojosa, J. A., Mercado, F. & Carretié, L. N170 sensitivity to facial expression: a meta-analysis. Neurosci. Biobehav. Rev.55, 498–509 (2015). [DOI] [PubMed] [Google Scholar]
- 29.Schindler, S., Bruchmann, M. & Straube, T. Beyond facial expressions: a systematic review on effects of emotional relevance of faces on the N170. Neurosci. Biobehav. Rev.153, 105399 (2023). [DOI] [PubMed] [Google Scholar]
- 30.Sel, A., Calvo-Merino, B., Tuettenberg, S. & Forster, B. When you smile, the world smiles at you: ERP evidence for self-expression effects on face processing. Soc. Cogn. Affect Neurosci.10, 1316–1322 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Achaibou, A., Pourtois, G., Schwartz, S. & Vuilleumier, P. Simultaneous recording of EEG and facial muscle reactions during spontaneous emotional mimicry. Neuropsychologia46, 1104–1113 (2008). [DOI] [PubMed] [Google Scholar]
- 32.Quettier, T., Maffei, A., Gambarota, F., Ferrari, P. F. & Sessa, P. Testing EEG functional connectivity between sensorimotor and face processing visual regions in individuals with congenital facial palsy. Front. Syst. Neurosci.17, 1123221 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aftanas, L. I., Varlamov, A. A., Pavlov, S. V., Makhnev, V. P. & Reva, N. V. Time-dependent cortical asymmetries induced by emotional arousal: EEG analysis of event-related synchronization and desynchronization in individually defined frequency bands. Int. J. Psychophysiol.44, 67–82 (2002). [DOI] [PubMed] [Google Scholar]
- 34.Friston, K. & Kiebel, S. Predictive coding under the free-energy principle. Philos. Trans. R. Soc. B364, 1211–1221 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brodski-Guerniero, A. et al. Information-theoretic evidence for predictive coding in the face-processing system. J. Neurosci.37, 8273–8283 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robinson, J. E., Breakspear, M., Young, A. W. & Johnston, P. J. Dose-dependent modulation of the visually evoked N1/N170 by perceptual surprise: a clear demonstration of prediction-error signalling. Eur. J. Neurosci.52, 4442–4452 (2020). [DOI] [PubMed] [Google Scholar]
- 37.Baker, J. & Korb, S. Analysis script in R. Zenodo 10.5281/zenodo.15364133 (2025).
- 38.Ekman, P., Friesen, W. V. & Hager, J. C. The Facial Action Coding System: A Technique for the Measurement of Facial Movement. San Francisco, CA: Consulting Psychologists Press (Consulting Psychologists Press, 2002).
- 39.Krumhuber, E. G., Kappas, A. & Manstead, A. S. R. Effects of dynamic aspects of facial expressions: a review. Emot. Rev.5, 41–46 (2013). [Google Scholar]
- 40.Roesch, E. B. et al. FACSGen: a tool to synthesize emotional facial expressions through systematic manipulation of facial action units. J. Nonverbal Behav.35, 1–16 (2011). [Google Scholar]
- 41.Peirce, J. et al. PsychoPy2: experiments in behavior made easy. Behav. Res.51, 195–203 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watson, D. & Clark, L. A. The PANAS-X: manual for the positive and negative affect schedule—expanded form. University of Iowa 10.17077/48vt-m4t2 (1994).
- 43.Chiappini, E., Silani, G., Lundström, J. N. & Korb, S. Facial electromyography in food research in a behavioral and MR setting. in Basic Protocols on Emotions, Senses, and Foods (ed. Bensafi, M.) 185–201. 10.1007/978-1-0716-2934-5_15 (Springer US, 2023).
- 44.Fridlund, A. J. & Cacioppo, J. T. Guidelines for human electromyographic research. Psychophysiology23, 567–589 (1986). [DOI] [PubMed] [Google Scholar]
- 45.Delorme, A. & Makeig, S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods134, 9–21 (2004). [DOI] [PubMed] [Google Scholar]
- 46.Bates, D., Mächler, M., Bolker, B. & Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Soft. 67, 1–48 (2015).
- 47.Kuznetsova, A., Brockhoff, P. B. & Christensen, R. H. B. lmerTest package: tests in linear mixed effects models. J. Stat. Softw.82, 1–26 (2017). [Google Scholar]
- 48.Borenstein, M., Hedges, L. V., Higgins, J. P. T. & Rothstein, H. R. Introduction to Meta‐Analysis. 10.1002/9780470743386 (Wiley, 2009).
- 49.Lüdecke, D., Ben-Shachar, M. S., Patil, I., Waggoner, P. & Makowski, D. Performance: an R package for assessment. Comp. Test. Stat. Models J. Open Source Softw.6, 3139 (2021). [Google Scholar]
- 50.Lenth, R. V. emmeans: estimated marginal means, aka least-squares means. 1.11.1 10.32614/CRAN.package.emmeans (2017).
- 51.Baltrusaitis, T., Zadeh, A., Lim, Y. C. & Morency, L.-P. OpenFace 2.0: facial behavior analysis toolkit. In Proc. 13th IEEE International Conference on Automatic Face & Gesture Recognition (FG 2018) 59–66. 10.1109/FG.2018.00019 (2018).
- 52.Groppe, D. M., Urbach, T. P. & Kutas, M. Mass univariate analysis of event-related brain potentials/fields I: a critical tutorial review: mass univariate analysis of ERPs/ERFs I: review. Psychophysiology48, 1711–1725 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stein, B. E. & Stanford, T. R. Multisensory integration: current issues from the perspective of the single neuron. Nat. Rev. Neurosci.9, 255–266 (2008). [DOI] [PubMed] [Google Scholar]
- 54.Widmann, A., Gruber, T., Kujala, T., Tervaniemi, M. & Schröger, E. Binding symbols and sounds: evidence from event-related oscillatory gamma-band activity. Cereb. Cortex17, 2696–2702 (2007). [DOI] [PubMed] [Google Scholar]
- 55.Selosse, G. et al. Neural dynamics of induced vocal tract vibrations during vocal emotion recognition. Preprint at 10.1101/2025.03.24.644945 (2025).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data are openly accessible at Open Science Framework (https://tinyurl.com/4wjy78nb).
All the code to run the experiment, preprocess and analyse the data are openly accessible at Open Science Framework (https://tinyurl.com/4wjy78nb).