Abstract
Targeting of class II major histocompatibility complex molecules to endocytic compartments is mediated by their association with the invariant chain (Ii). Although the identity of certain sorting signals located in Ii's cytoplasmic tail is known, proteins that interact with Ii's cytoplasmic tail in living cells remain to be identified. Synthesis of a biotinylated trimeric Ii cytoplasmic tail allowed the retrieval of two proteins that interact with this domain. We identify one of them as the 70-kDa heat-shock cognate protein (hsc70), the uncoating ATPase of clathrin-coated vesicles, and the other as its mitochondrial homologue, the glucose-regulated protein grp75. Expression of Ii in COS cells results in the formation of large endocytic compartments. We observe extensive colocalization of hsc70 with Ii in these macrosomes. Expression of a dominant-negative (K71M) green fluorescent protein-tagged version of hsc70 counteracted the ability of Ii to modify the endocytic pathway, demonstrating an interaction in vivo of Ii with hsc70 as part of the machinery responsible for macrosome formation.
Keywords: heat shock cognate protein‖endocytic vesicles
The function of MHC class II molecules is to present peptides to the T cell receptor on CD4+ lymphocytes (1). The biosynthesis and transport of the MHC class II molecules is a tightly regulated process: it involves the intersection of the secretory and endocytic pathways to load newly synthesized MHC molecules with peptides produced by the proteolysis of foreign antigen in endocytic compartments (2, 3). MHC class II molecules are composed of an αβ heterodimer that associates in the endoplasmic reticulum with a third polypeptide, the invariant chain (Ii). The functions of Ii are multiple. It facilitates the proper folding of the αβ subunits, prevents premature binding of peptide to αβ, and targets the MHC-II complex to the endosomal/lysosomal system (4, 5). This specific targeting is mediated by two leucine-based signals located in the Ii cytoplasmic tail (6, 7). The cytoplasmic tail of Ii also affects the architecture of the endocytic pathway when expressed at high level (6, 8, 9), an attribute that may play a role in antigen processing and/or presentation. To date, the cytosolic factors capable of interacting with Ii and involved in these trafficking events remain unknown. The multimerization of Ii is required for proper delivery to the endosomal compartment (10) and for the formation of the large endosomal structures induced by Ii (11), which suggests the possibility of interaction of the trimerized cytoplasmic tail of Ii with components of the molecular machinery involved in membrane traffic. To our knowledge, attempts at identifying interacting partners by genetic means have not been successful, most probably because of the requirement for trimerization of Ii.
To identify cytosolic partners capable of interacting with Ii, we synthesized an affinity matrix in which the trimerization of the Ii cytoplasmic tail is forced chemically. The identification of the 70-kDa heat-shock cognate protein (hsc70) as one of the cytosolic partners allowed us to implicate the interaction between hsc70 and Ii in the remodeling of the endocytic compartments. Our results emphasize the important relationship between the presence of Ii, its removal by proteolysis, and the structure of the endocytic pathway.
Materials and Methods
Cells.
HOM-2 and COS-1 cells were maintained respectively in RPMI medium 1640 and DMEM containing 10% (vol/vol) FCS.
Antibodies and Reagents.
We used the rabbit Scyt antiserum (kindly provided by P. Benaroch, Institut Curie, Paris) and the mouse mAb Pin-1 to label the N-terminal region of Ii. The rat anti-hsc70 mAb 1B5 and the mouse mAb 13D3 were purchased from StressGen Biotechnologies (Victoria, Canada) and Maine Biotechnology (Portland, ME), respectively. The mouse mAb anti-75-kDa glucose-regulated protein (grp75) was purchased from StressGen Biotechnologies. The rabbit polyclonal serum anti-PDI was generated in our lab. The mouse mAb specific for Lamp-1 was purchased from PharMingen.
Expression Plasmids and Transfection.
The human Ii expression vector pSV5 was used previously (12). The cDNA encoding for wild-type (wt) hsc70 and hsc70K71M (13) were subcloned in the pEGFP-C1 vector (CLONTECH). COS-1 cells were transfected by using a DEAE-Dextran procedure. In cotransfection experiments, a plasmid ratio of 1:5 (Ii:GFP-construct) was used.
Ii Trimer and Scaffold Synthesis.
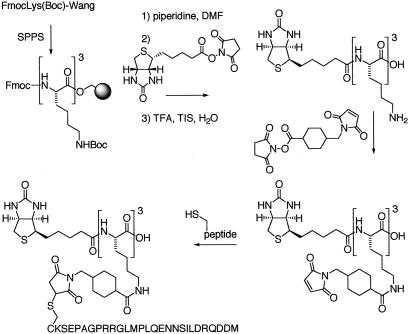
The synthesis is presented in Figure 1. Biotinylated-KKK peptide (scaffold) was synthesized by solid phase peptide synthesis by using Wang resin and Fmoc chemistry. After trifluoroacetic acid cleavage, Iicyt peptides or free cysteine were cross-linked to the side chain of lysine residues by using sulfosuccinimidyl 4(N-maleimidomethyl)cyclohexane-1-carboxylate. The “naked” scaffold results from the attachment of single cysteine residues. The modified scaffolds were purified by reverse HPLC, and their purity and identity were established by mass spectrometry. All peptides were synthesized on a peptide synthesizer model 440 (Advanced ChemTech) and purified by reverse HPLC; their identity was verified by mass spectrometry.
Figure 1.
Synthesis of invariant chain trimer. See Methods for details.
Affinity Purification.
HOM-2 cells were metabolically labeled for 5 h in DMEM methionine-cysteine-free supplemented with 10% (vol/vol) FCS/2 mM glutamine/0.5 mCi/ml [35S]methionine-cysteine (1 Ci = 37 GBq). After washes in PBS, cells were solubilized in Nonidet P-40 buffer (20 mM Tris⋅HCl, pH 7.1/140 mM KCl/20 mM NaCl/0.5 mM MgCl2/0.5% Nonidet P-40/1 mM PMSF/1 μg/ml leupeptin/1 μg/ml aprotinin). Lysates were precleared with streptavidin-agarose, and the equivalent of 5 × 106 cells were incubated with 0.5 nmol Ii trimer or naked scaffold for 2 h at 4°C. For competition experiments, lysates were preincubated for 30 min with a 30-fold molar excess of the indicated free peptide. Complexes were retrieved by using streptavidin-agarose. After extensive washes of the beads in lysis buffer, proteins were analyzed on an SDS/12% PAGE.
Protein Identification.
Proteins bound to Ii trimer were separated on an SDS/7.5% PAGE and silver-stained. The bands of interest were excised and digested with trypsin. Sequence analysis was performed by micropillary reverse-phase HPLC nano-electrospray tandem mass spectrometry (μLC/MS/MS) on a Finnigan LCQ quadrupole ion-trap mass spectrometer at the Harvard Microchemistry Facility (Harvard Univ., Cambridge). The MS/MS spectra then were correlated with known sequences by using the algorithm SEQUEST (14, 15).
Indirect Immunofluorescence.
Cells (2 × 104) were plated 24 h before labeling. To label endocytic compartments, cells were incubated for 1 h at 37°C in DMEM/0.2% BSA-25 μg/ml transferrin BODIPY-FL (Molecular Probes). After fixation for 20 min in −20°C methanol [or PBS/3.7% (vol/vol) formaldehyde for labeling of G-actin], cells were permeabilized for 20 min in PBS/0.05% Saponin/1% normal goat serum. The same solution was used for antibody dilutions. Slides were analyzed with a Bio-Rad MRC 1024 confocal laser scanning microscope. The merge images were analyzed with the colocalization program provided by the manufacturer. The size of Ii-expressing vesicles was evaluated with Adobe PHOTOSHOP 5.5. A total of 100 double-positive cells [green fluorescent protein- (GFP) and Ii-expressing] were randomly analyzed for each transfection condition. Cells containing at least one vesicle with a diameter ≥ to 5, 10, or 15 μm (depending on the threshold chosen) were considered as macrosome-positive cells.
Cell Sorting and Immunoblotting.
After transfection (48 h), GFP-expressing cells were sorted and lysed in Nonidet P-40 buffer. Total protein (1 μg) was separated on an SDS/12% PAGE and transferred onto a poly(vinylidene difluoride) membrane. The membrane was blocked in TBS/5% (vol/vol) BSA, incubated with the mAb of interest and with the appropriate secondary antibody. Immunoreactive proteins were detected with enhanced chemiluminescence.
Results
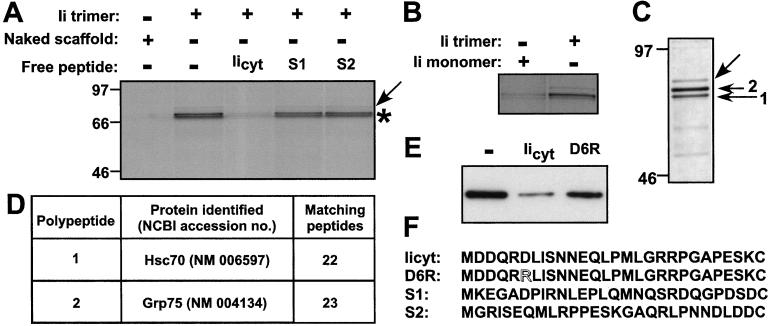
To mimic the trimeric disposition of Ii's tail, we synthesized an imidoester-derivatized scaffold that allows the attachment of a single or three copies of the Ii tail via the C-terminal cysteine of the Ii tail (Fig. 1). The scaffold further allows the attachment, via an appropriate spacer, of a biotin molecule to facilitate retrieval of proteins that bind to the different versions of this scaffold. The scaffolds lack the hydrophobic transmembrane segment of Ii and thus would be expected not to bind micelles of detergent, unlike intact purified Ii. The biotinylated Ii trimer scaffold was used to retrieve proteins that bind to it from cell extracts prepared from [35S]methionine/cysteine-labeled B lymphoblastoid cells. By SDS/PAGE and autoradiography, we identified a closely spaced doublet of polypeptides (molecular mass range of 70–75 kDa) that bound specifically to the trimeric scaffold and not to the naked scaffold (Fig. 2A, asterisk). This polypeptide doublet was recovered in only trace amounts when we used the scaffold conjugated to a single Ii tail peptide (Fig. 2B). In addition, a polypeptide of approximately 80 kDa was retrieved that bound to the scaffold regardless of the extent of its substitution (Fig. 2A, arrow). This binding was, therefore, considered nonspecific, and the identity of this polypeptide was not pursued further. The interacting proteins were bound in a rather stable fashion, as they resisted extensive washing in lysis buffer.
Figure 2.
Identification of proteins specifically bound to Ii trimer. (A) Profile of proteins specifically bound to Ii trimer. Lysates from biosynthetically labeled HOM-2 cells were incubated with Ii trimer or the naked scaffold in the presence or absence of a 30-fold molar excess of Iicyt, S1 or S2 peptides. Bound proteins were retrieved with streptavidin-agarose and subjected to SDS/12% PAGE and autoradiography. (B) Lysates from biosynthetically labeled HOM-2 cells were incubated with Ii monomer or trimer. Bound proteins were retrieved with streptavidin-agarose and subjected to SDS/12% PAGE and autoradiography. (C) Silver-stain profile of proteins bound to Ii trimer and subjected to SDS/7.5% PAGE. (D) Sequence analysis of proteins specifically bound to the Ii trimer scaffold. (E) Competition assay assessed by immunoblot by using anti-hsc70 antibodies. A competition assay was performed as described in Materials and Methods by using the trimeric scaffold as a retrieval matrix in the absence of free peptide (lane 1) or in the presence of Iicyt (lane 2) or Ii cytoplasmic tail mutant D6R (lane 3); see F for peptide sequence. Bound proteins were retrieved with streptavidin-agarose and subjected to SDS/12% PAGE and immunoblot anti-hsc70. Densitometry analysis reveals 76% competition for Iicyt and 20% for D6R. Data are representative of three independent determinations. (F) Competitor peptides. Iicyt peptide includes residues 1–28 of human Ii; D6R is a point mutant of Ii cytoplasmic tail (Asp-6 is exchanged with Arg); S1 and S2 are scrambled sequences with identical amino acid content.
We addressed the specificity of interaction by competition experiments. Monomeric Ii tail peptide competes effectively for binding but does so at a 30-fold molar excess, whereas similar concentrations of two distinct peptides of the identical (but scrambled in sequence) amino acid composition (Fig. 2F) failed to compete for binding to the scaffold (Fig. 2A). The need for inclusion of a molar excess of competitor peptides is best understood as the consequence of the avidity conferred by the trivalency of the Ii scaffold, whereas the competitor peptides are offered in monomeric form. Moreover, the interactions between components of the clathrin machinery, responsible for transport from the plasma membrane and the transGolgi network to the endosomal compartment (16), are characterized by low pairwise affinities (17). We conclude that binding of the 70–75 kDa polypeptides requires a trimeric Ii tail, and that such binding is sequence-specific.
Identification of hsc70 as an Ii-Interacting Protein.
The purification procedure was scaled up to obtain sufficient amounts of protein for sequence analysis. A silver-stained gel of the final preparation reveals the same set of polypeptides as those identified from biosynthetically labeled cells (Fig. 2C). The two polypeptides specifically retained on the Ii trimer were subjected to trypsin digestion followed by μLC/MS/MS sequence analysis. For band 1, this procedure allowed the unambiguous identification of 22 peptides, all of which were contained in the human hsc70 sequence (Fig. 2D). The identity of hsc70 was corroborated further by the reactivity of this polypeptide with the 1B5 antibody (anti-hsc70) in immunoblot (data not shown). The second polypeptide of the doublet (band 2) was identified by sequence as grp75 (Fig. 2D). Both proteins are constitutively expressed members of the Hsp70 protein family and are 67% homologous. Grp75 localizes predominantly to mitochondria (18) and is presumably released upon detergent extraction of the cells used to prepare the lysates, whereas hsc70 is both cytosolic and organelle-associated (19).
Colocalization of hsc70 and Ii in COS-Transfected Cells.
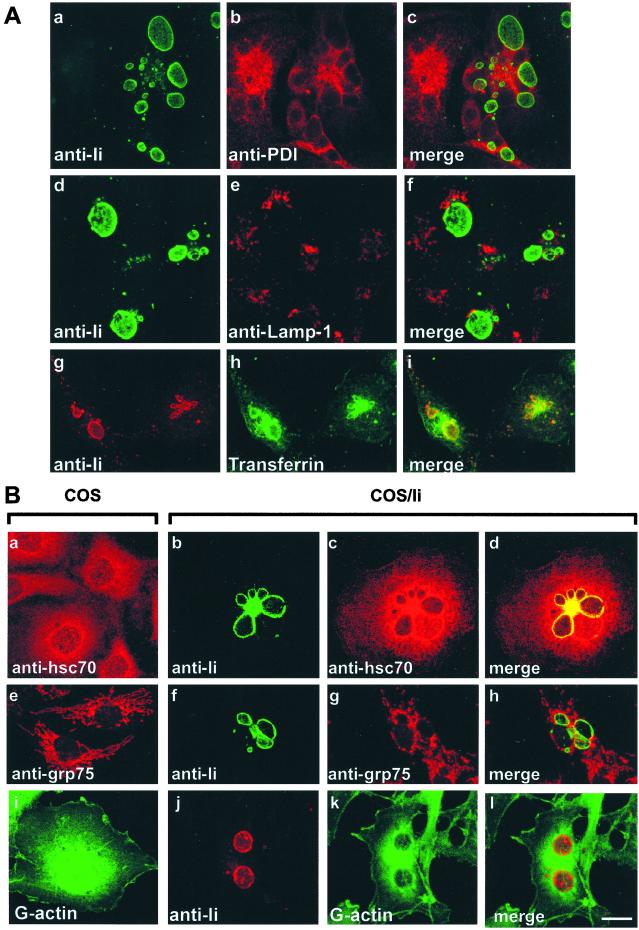
Hsc70 was identified initially as the uncoating ATPase that dissociates clathrin triskelions from clathrin coated-vesicles (20); additionally, a positive role of hsc70 in vesicle formation has been described (13, 21). This trait led us to investigate further a possible function for hsc70 in intracellular disposition of Ii. Indeed, expression of Ii can modify the organization of the endocytic compartment, culminating in the formation of large vesicular structures called macrosomes (6, 8). The above observations suggested the possibility that the generation of macrosomes, first seen in COS cells transfected with full length Ii, might result from an imbalance in fission and fusion of the vesicles that deliver Ii to the target endocytic organelle. If vesicle fusion (delivery) were to outpace fission (retrieval of vesicles), then the target organelle should increase in size. When overexpressed, the ability of the Ii trimer to recruit excess uncoating ATPase activity might cause premature uncoating and thereby either make early coated endosomal vesicles more fusogenic or inhibit subsequent fission reactions essential for retrieval of vesicular constituents. This recruitment would shift the balance toward fusion and lead to an increase in the size of the target organelle carrying Ii. We examined the subcellular localization of hsc70 in COS cells and Ii transfectants by confocal microscopy. COS cells transfected with full-length Ii showed the clear and unequivocal presence of macrosomes revealed by labeling of Ii (Fig. 3A a, d, and g; Fig. 3B b, f, and j). To identify the large compartments, staining of these cells for protein disulfide isomerase (PDI) as an ER marker (Fig. 3Ab), Lamp-1 for lysosomes (Fig. 3Ae), or transferrin as an endocytic tracer (Fig. 3Ah) was performed. PDI (Fig. 3Ac) and Lamp-1 (Fig. 3Af) show no colocalization with Ii, but the macrosomes clearly show transferrin accumulation (Fig. 3Ai). These observations demonstrate that macrosomes are endosomal-derived, which is in agreement with the earlier publications (8, 9). As expected, hsc70 shows punctate staining over the entire cytoplasm of COS cells (Fig. 3B a and c). In view of the role of hsc70 in uncoating, and given its demonstrated role in endocytic traffic control, this protein also shows colocalization with transferrin-accessible compartments (data not shown). In COS cells transfected with full-length Ii, hsc70 and Ii colocalize to these large endosomal compartments (Fig. 3Bd). As expected, grp75 shows a mitochondrial distribution pattern (Fig. 3Be) and does not significantly colocalize with Ii (Fig. 3Bh). For these immunofluorescence studies, cells were fixed in cold methanol, which eliminates the cytosolic fraction (22). However, as hsc70 is abundant and constitutes 1–2% of total cellular protein, a partial washout of the cytosolic fraction cannot be excluded. To rule out the possibility that the colocalization between Ii and hsc70 is fortuitous and caused by the abundance of the latter, we performed a control experiment by using monomeric actin (G-actin) as a cytosolic marker (Fig. 3B i and k). Indeed, G-actin failed to show significant colocalization with Ii (Fig. 3Bl).
Figure 3.
(A) Invariant chain-induced macrosomes are endosome-derived. Cells were methanol-fixed 48 h after transfection and permeabilized and processed for double indirect immunofluorescence by using anti-Ii antibodies (green, a and d; red, g) and anti-PDI (red, b) or anti-anti-Lamp-1 (red, e). To label early endocytic compartments, cells were pulsed for 1 h with transferrin-BODIPY-FL before fixation (green, h). Images c, f, and i were obtained by merging the green image (FITC or BODIPY-FL) and the red image (Cy3). (Bar = 20 μm.) (B) Colocalization of Ii and hsc70 in COS-1 cells transiently expressing Ii. Cells were methanol-fixed 48 h after transfection and permeabilized and processed for double indirect immunofluorescence by using anti-Ii antibodies (green, b and f; red, j), anti-hsc70 antibodies (red, a and c), anti-grp75 antibodies (red, e and g), or Alexa fluor 488-Dnase I (G-actin green, i and k). Images d, h, and l result from the colocalization analysis for Ii and hsc70, Ii and grp75, and Ii and G-actin, respectively. Colocalization is seen as yellow. (Bar = 20 μm.)
Mutations or deletions within the cytoplasmic tail of Ii failed to induce macrosome formation (8, 23), as exemplified in particular by the single amino acid Asp-6 → Arg (D6R) mutant (T. F. Gregers and O. Bakke, unpublished data). Indeed, as further evidence for a specific interaction between the cytoplasmic tail of Ii and hsc70, we find that a peptide that carries the Asp-6 → Arg mutation (D6R), like the scrambled peptides S1 and S2, fails to compete for binding of hsc70 to the trimeric scaffold (Fig. 2E), thus further correlating our biochemical data with in vivo results.
A Dominant-Negative Mutant of hsc70 Counteracts the Formation of Macrosomes.
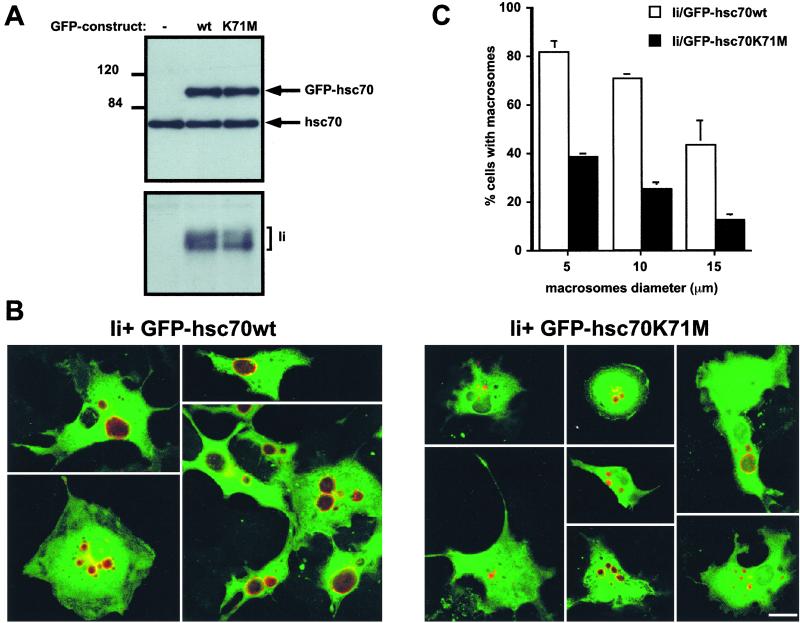
The activity of hsc70 itself can be manipulated through the introduction of point mutations. Notably, substitution of Lys-71 for Met inhibits the ATPase (24) and uncoating (13) activity in vitro. Moreover, this substitution generates a dominant-negative hsc70 mutant that interferes with proper endocytic trafficking (13). We generated enhanced green fluorescent protein (EGFP) fusions of hsc70, such that EGFP was fused in-frame at its COOH terminus with hsc70wt or with the mutant hsc70 K71M. The morphology of COS was unaltered after the transfection of these EGFP constructs: GFP-hsc70wt and GFP-hsc70K71M molecules exhibited broad intracellular distribution and ER and lysosomal staining were indistinguishable for untransfected and transfected COS cells (not shown). These EGFP constructs then were cotransfected with Ii into COS cells. Cells expressing EGFP-hsc70 were enriched by sorting for green fluorescent cells and analyzed by immunoblot by using antibodies against hsc70 to assess the level of expression of the fusion proteins relative to the endogenous hsc70. In the sorted cells, the expression level of both mutant and wild-type EGFP-hsc70 was comparable to that seen for endogenous hsc70 (Fig. 4A Upper). Immunoblotting with an antibody directed against Ii revealed that both hsc70wt and hsc70K71M transfectants expressed similar levels of Ii (Fig. 4A Lower). When analyzed for the presence and distribution of Ii, cells that received wild-type EGFP-hsc70 showed clear evidence of macrosome formation (Fig. 4B Left). In contrast, in cells that coexpress EGFP-hsc70 K71M and Ii, the distribution of Ii was confined to vesicles of far smaller average size (Fig. 4B Right). Most (82%) of the cells coexpressing Ii and EGPP-hsc70wt contain large Ii-containing vesicles with a diameter equal or superior to 5 μm compared with 38% of the cells that coexpress Ii and EGFP-hsc70 K71M (Fig. 4C). Setting the size threshold at 10 or 15 μm for vesicle size further accentuates this difference. We conclude that expression of EGFP-hsc70K71M indeed inhibits macrosome formation, consistent with the characterization of the K71M mutation as dominant-negative.
Figure 4.
Expression of a dominant-negative form of hsc70 impairs the formation of macrosomes. (A) Expression levels of EGFP-hsc70 fusion proteins (Upper) and Ii (Lower) in double-transfected COS-1 cells sorted for GFP expression were assessed by immunoblotting. (B) Confocal images of COS-1 cells cotransfected with cDNA encoding for Ii and GFP-hsc70wt (Left) or Ii and GFP-hsc70K71M (Right). Cells were stained with anti-Ii plus Cy3-conjugated anti-mouse IgG. Images were obtained by merging the green image (GFP) and the red image (Cy3). (Bar = 20 μm.) (C) Quantification of macrosomes in COS-1 cells coexpressing Ii and GFP-hsc70 fusion proteins based on an analysis of 100 double-positive cells (GFP+Ii+). See Methods for methods of measurement and calculation. The error bars represent the SD observed for three experiments.
Discussion
The invariant chain cytoplasmic tail plays multiple roles in the biogenesis of MHC class II molecules, including targeting of MHC molecules to the endocytic pathway and modification of the endocytic compartments. Despite considerable efforts, the molecular machinery interacting with this portion of Ii and the mechanisms involved in these functions remained unknown. Here, we demonstrate that the uncoating ATPase hsc70 interacts specifically with Ii tail and is responsible for the enlargement of the endocytic compartments when Ii is expressed in the absence of the Class II α and β chains.
To characterize the cytosolic partners of Ii, we engineered a new affinity matrix. This approach allowed us to show that hsc70 interacts preferentially with the trimerized but not the monomeric version of the Ii tail. This property is expected for a cytosolic partner of Ii, as its oligomerization is required for both proper targeting of MHC class II molecules (10) and macrosome formation (11). One explanation for the retrieval of grp75, the mitochondrial homologue of hsc70, is that this protein is released upon detergent extraction and then can bind to the affinity matrix. Notably, Bip, the endoplasmic reticulum-associated member of the Hsp70 family was not retrieved (25). Although Hsp70s have been shown to bind peptide sequence motifs enriched in hydrophobic and basic residues (26, 27), it is noteworthy that the Ii cytoplasmic tail is in fact quite hydrophilic. Furthermore, its mode of interaction with hsc70 seems to depend on the proper sequence of Ii rather than some general balance between polar and nonpolar residues, as revealed by the competition experiments with Ii tail peptide and peptides scrambled in sequence or with a point mutation. The exact manner in which hsc70 recognizes motifs in the Ii cytoplasmic tail remains to be determined. Different functional properties of hsc70 have been defined, not all of which are associated with the reported peptide-binding domain of hsc70 (28). In fact, it is likely that hsc70 is at the nexus of a substantial number of interactions, perhaps each involving a unique feature of hsc70's structure.
In the materials retrieved from the cell lysates, we did not observe any polypeptides that corresponded to subunits of the known adaptor protein (AP) complexes. Previous in vitro studies using purified Ii and purified AP complexes in detergent-containing solutions suggested that the sorting motifs of Ii can bind directly to both AP1 and AP2 (29). Such binding has yet to be demonstrated in living cells.
Hsc70, identified as the uncoating ATPase, has been shown to modulate the clathrin-coated vesicle cycle (13). Its recruitment by Ii could explain the morphological changes of the endocytic compartments induced by high expression levels of Ii. Indeed, we provide here in vivo and in vitro data that support a role for recruitment of hsc70 by Ii in induction of macrosomes: (i) in COS cells expressing a dominant-negative form of hsc70, transfection of Ii failed to induce the formation of macrosomes; and (ii) the mutant peptide D6R, unable to induce the formation of macrosome when expressed as part of a full length Ii, did not compete with Ii trimer for the binding of hsc70. We presume that, at normal levels of Ii expression, the balance between hsc70 recruitment and vesicle uncoating on the one hand and the retrieval of target organelle membranes on the other hand would ensure an ordered delivery of the Ii-containing complexes to the appropriate intracellular compartments. At high levels of expression of Ii, the morphology of endosomal compartments is strongly affected, as observed by the appearance of macrosomes in cell lines transfected with cDNA encoding for Ii in presence or absence of MHC class II molecules (6, 8, 9). Similarly, in cell lines accumulating Ii because of a defect in proteolysis, class II Ii complexes accumulate in large endocytic vesicles that contain lysosomal markers (30). In Langerhans cells, the only professional antigen-presenting cells that exhibit such large endosomal structures (31), the formation of macrosomes also correlates with a high rate of synthesis of Ii (32). Altogether, and along the lines of previous studies, our data establish an important role for the cytoplasmic tail of Ii in the biogenesis and morphology of the endocytic structures involved in antigen presentation. The identification of hsc70 as a protein recruited by Ii trimers can fully account for the generation of macrosomes, a process that had, thus far, defied a molecular explanation and underscores the important role of hsc70 in the maintenance of normal endocytic trafficking (13).
What could be the role of the recruitment of hsc70 by Ii in the context of the function of MHC class II molecules? The effects of Ii's cytoplasmic tail on the architecture of the endosomal pathway are associated with delayed traffic along this pathway (8, 33). Delayed access to lysosomes for internalized antigens could prevent their complete destruction in favor of the generation and loading of peptides onto MHC class II molecules. Whereas earlier studies postulated a role for hsc70 in antigen presentation (34, 35) and peptide delivery to MHC class II (35), this possibility seems less likely for topological reasons. In view of our findings, we suggest that hsc70's role extends to the maintenance of a properly organized endocytic compartment and, in this manner, contributes to MHC class II restricted antigen presentation.
Acknowledgments
The authors thank S. L. Schmid for helpful discussions and critical reading of the manuscript; A. M. Lennon-Dumenil for critical review of the manuscript; B. Kessler and C. Dahl for helpful advice with scaffold synthesis; and H. Overkleeft for assistance during manuscript preparation. We are grateful to M. Lowe for help with the laser-scanning microscope and B. Hekking for the synthesis of peptides. C.L.G. was supported by a postdoctoral fellowship from the Association pour la Recherche contre le Cancer (France). S.L.N was supported by the American Heart Association, Western States Affiliate. T.F.G. was supported by the Norwegian Cancer Society. This work was supported by grants to H.L.P. from the National Institutes of Health.
Abbreviations
- Ii
invariant chain
- hsc70
heat-shock cognate protein 70
- grp75
glucose-regulated protein 75
- GFP
green fluorescent protein
- EGFP
enhanced green fluorescent protein
- wt
wild type
References
- 1.Germain R N. Cell. 1994;76:287–299. doi: 10.1016/0092-8674(94)90336-0. [DOI] [PubMed] [Google Scholar]
- 2.Cresswell P. Annu Rev Immunol. 1994;12:259–293. doi: 10.1146/annurev.iy.12.040194.001355. [DOI] [PubMed] [Google Scholar]
- 3.Wolf P R, Ploegh H L. Annu Rev Cell Dev Biol. 1995;11:267–306. doi: 10.1146/annurev.cb.11.110195.001411. [DOI] [PubMed] [Google Scholar]
- 4.Bakke O, Dobberstein B. Cell. 1990;63:707–716. doi: 10.1016/0092-8674(90)90137-4. [DOI] [PubMed] [Google Scholar]
- 5.Lotteau V, Teyton L, Peleraux A, Nilsson T, Karlsson L, Schmid S L, Quaranta V, Peterson P A. Nature (London) 1990;348:600–605. doi: 10.1038/348600a0. [DOI] [PubMed] [Google Scholar]
- 6.Pieters J, Bakke O, Dobberstein B. J Cell Sci. 1993;106:831–846. doi: 10.1242/jcs.106.3.831. [DOI] [PubMed] [Google Scholar]
- 7.Odorizzi C G, Trowbridge I S, Xue L, Hopkins C R, Davis C D, Collawn J F. J Cell Biol. 1994;126:317–330. doi: 10.1083/jcb.126.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Romagnoli P, Layet C, Yewdell J, Bakke O, Germain R N. J Exp Med. 1993;177:583–596. doi: 10.1084/jem.177.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stang E, Bakke O. Exp Cell Res. 1997;235:79–92. doi: 10.1006/excr.1997.3617. [DOI] [PubMed] [Google Scholar]
- 10.Arneson L S, Miller J. J Cell Biol. 1995;129:1217–1228. doi: 10.1083/jcb.129.5.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gedde-Dahl M, Freisewinkel I, Staschewski M, Schenck K, Koch N, Bakke O. J Biol Chem. 1997;272:8281–8287. doi: 10.1074/jbc.272.13.8281. [DOI] [PubMed] [Google Scholar]
- 12.Bijlmakers M J, Benaroch P, Ploegh H L. J Exp Med. 1994;180:623–629. doi: 10.1084/jem.180.2.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newmyer S L, Schmid S L. J Cell Biol. 2001;152:607–620. doi: 10.1083/jcb.152.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yates J R d, Eng J K, McCormack A L, Schieltz D. Anal Chem. 1995;67:1426–1436. doi: 10.1021/ac00104a020. [DOI] [PubMed] [Google Scholar]
- 15.Chittum H S, Lane W S, Carlson B A, Roller P P, Lung F D, Lee B J, Hatfield D L. Biochemistry. 1998;37:10866–10870. doi: 10.1021/bi981042r. [DOI] [PubMed] [Google Scholar]
- 16.Schmid S L. Annu Rev Biochem. 1997;66:511–548. doi: 10.1146/annurev.biochem.66.1.511. [DOI] [PubMed] [Google Scholar]
- 17.Ungewickell E. Proc Natl Acad Sci USA. 1999;96:8809–8810. doi: 10.1073/pnas.96.16.8809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dahlseid J N, Lill R, Green J M, Xu X, Qiu Y, Pierce S K. Mol Biol Cell. 1994;5:1265–1275. doi: 10.1091/mbc.5.11.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mayer M P, Bukau B. Biol Chem. 1998;379:261–268. [PubMed] [Google Scholar]
- 20.Schlossman D M, Schmid S L, Braell W A, Rothman J E. J Cell Biol. 1984;99:723–733. doi: 10.1083/jcb.99.2.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang R, Gao B, Prasad K, Greene L E, Eisenberg E. J Biol Chem. 2000;275:8439–8447. doi: 10.1074/jbc.275.12.8439. [DOI] [PubMed] [Google Scholar]
- 22.Agarraberes F A, Terlecky S R, Dice J F. J Cell Biol. 1997;137:825–834. doi: 10.1083/jcb.137.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pond L, Kuhn L A, Teyton L, Schutze M P, Tainer J A, Jackson M R, Peterson P A. J Biol Chem. 1995;270:19989–19997. doi: 10.1074/jbc.270.34.19989. [DOI] [PubMed] [Google Scholar]
- 24.O'Brien M C, Flaherty K M, McKay D B. J Biol Chem. 1996;271:15874–15878. doi: 10.1074/jbc.271.27.15874. [DOI] [PubMed] [Google Scholar]
- 25.Gething M J. Semin Cell Dev Biol. 1999;10:465–472. doi: 10.1006/scdb.1999.0318. [DOI] [PubMed] [Google Scholar]
- 26.Fourie A M, Sambrook J F, Gething M J. J Biol Chem. 1994;269:30470–30478. [PubMed] [Google Scholar]
- 27.Takenaka I M, Leung S M, McAndrew S J, Brown J P, Hightower L E. J Biol Chem. 1995;270:19839–19844. doi: 10.1074/jbc.270.34.19839. [DOI] [PubMed] [Google Scholar]
- 28.James P, Pfund C, Craig E A. Science. 1997;275:387–389. doi: 10.1126/science.275.5298.387. [DOI] [PubMed] [Google Scholar]
- 29.Hofmann M W, Honing S, Rodionov D, Dobberstein B, von Figura K, Bakke O. J Biol Chem. 1999;274:36153–36158. doi: 10.1074/jbc.274.51.36153. [DOI] [PubMed] [Google Scholar]
- 30.Riberdy J M, Avva R R, Geuze H J, Cresswell P. J Cell Biol. 1994;125:1225–1237. doi: 10.1083/jcb.125.6.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stossel H, Koch F, Kampgen E, Stoger P, Lenz A, Heufler C, Romani N, Schuler G. J Exp Med. 1990;172:1471–1482. doi: 10.1084/jem.172.5.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kampgen E, Koch N, Koch F, Stoger P, Heufler C, Schuler G, Romani N. Proc Natl Acad Sci USA. 1991;88:3014–3018. doi: 10.1073/pnas.88.8.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gorvel J P, Escola J M, Stang E, Bakke O. J Biol Chem. 1995;270:2741–2746. doi: 10.1074/jbc.270.6.2741. [DOI] [PubMed] [Google Scholar]
- 34.Hoeger P H, Tepper M A, Faith A, Higgins J A, Lamb J R, Geha R S. J Immunol. 1994;153:3908–3916. [PubMed] [Google Scholar]
- 35.Panjwani N, Akbari O, Garcia S, Brazil M, Stockinger B. J Immunol. 1999;163:1936–1942. [PubMed] [Google Scholar]