Abstract
We used the hypomorphic Egfrwa2 allele to genetically examine the impact of impaired epidermal growth factor receptor (Egfr) signaling on the ApcMin mouse model of familial adenomatous polyposis. Transfer of the ApcMin allele onto a homozygous Egfrwa2 background results in a 90% reduction in intestinal polyp number relative to ApcMin mice carrying a wild-type Egfr allele. This Egfr effect is potentially synergistic with the actions of the modifier-of-min (Mom1) locus. Surprisingly, the size, expansion, and pathological progression of the polyps appear Egfr-independent. Histological examination of the ilea of younger animals revealed no differences in the number of microadenomas, the presumptive precursor lesions to gross intestinal polyps. Pharmacological inhibition with EKI-785, an Egfr tyrosine kinase inhibitor, produced similar results in the ApcMin model. These data suggest that normal Egfr activity is required for establishment of intestinal tumors in the ApcMin model between initiation and subsequent expansion of initiated tumors. The role of Egfr signaling during later stages of tumorigenesis was examined by using nude mice xenografts of two human colorectal cancer cell lines. Treatment with EKI-785 produced a dose-dependent reduction in tumor growth, suggesting that Egfr inhibitors may be useful for advanced colorectal cancer treatment.
Epidermal growth factor receptor (Egfr) is the prototypical member of the Erbb family of ligand-activated receptor tyrosine kinases (1). Mice homozygous for the targeted null Egfrtm1Mag allele show strain-dependent lethality (2). Genetic backgrounds supporting survival of Egfrtm1Mag homozygous mutants to term demonstrate the importance of Egfr for epithelial homeostasis (2–4); neonatal mice lacking Egfr maintain a robust proliferative compartment but develop disorganized cryptal architecture of the lower gastrointestinal (GI) tract (2) and hemorrhagic enteritis (4). The hypomorphic Egfrwa2allele contains a single nucleotide mutation producing a valine to glycine amino acid substitution in the kinase domain, resulting in up to a 90% reduction in kinase activity (5, 6). Unlike Egfrtm1Maghomozygotes, mice homozygous for Egfrwa2are fully viable, although they manifest skin epithelium and mammary gland defects. Upon perturbation, Egfrwa2homozygotes exhibit subtle GI phenotypes; Egfrwa2 mice exhibit delayed intestinal adaptation and reestablishment of epithelial homeostasis after intestinal resection (7) as well as increased susceptibility to dextran sulfate-induced colitis (8). Furthermore, ectopic Egfr activation promotes increased intestinal epithelial cell proliferation and crypt size but a decrease in crypt fission rates (9).
Overexpression of Egfr, the most commonly observed cancer-associated misregulation in Egfr signaling, correlates with poor prognosis in a number of cancers including breast, ovarian, and head and neck (10–12). Because Egfr activation can promote proliferation and maintain survival, amplification of receptor signaling by means of overexpression may promote tumor growth and resistance to apoptosis. Egfr signaling up-regulates its cognate ligands, creating autocrine loops that maintain and amplify levels of Egfr activity (13). For instance, although Egfr activity is not required for the initiation of squamous papillomas derived from rasHa-transformed keratinocytes, an Egfr autocrine loop is essential for maintenance of papilloma growth and prevention of terminal differentiation of dysplastic cells (14, 15). Similarly, mice with impaired Egfr signaling are resistant to skin papillomas induced by ectopic expression of the downstream Egfr pathway member Sos1 (16). In the GI tract, expression of Egfr and its ligands is often higher in tumors than in surrounding normal tissue (17). Furthermore, the level of Egfr expression generally correlates with colon cancer progression and metastatic potential (18–20). In tumor cells Egfr polarity may be lost, providing an additional avenue for altered Egfr action influencing abnormal cell growth (18).
Because in part of evidence implicating hyperactivity of Egfr in a variety of human disease states, a number of Egfr inhibitors have been developed as potential therapeutic agents (21). One such agent is EKI-785, a small molecule inhibitor that irreversibly binds the ATP-binding region of Egfr, efficiently suppressing Egfr kinase activity (22). EKI-785 has been used to reduce severity of polycystic kidney disease in mouse models (23) and to reduce polyp number in the ApcMin mouse model of familial adenomatous polyposis (24). Interestingly, a conflicting report showed no effect on polyp multiplicity in the ApcMin model by using a similar Egfr inhibitor, N-[4-(3-chloro4-fluorophenylamino)-quinazolin-6-yl]-acrylamide (CFPQA) (25). Mice heterozygous for the ApcMin truncation mutation exhibit tens to hundreds of intestinal adenomas, primarily in the small bowel (26). Tumor multiplicity in ApcMin animals is highly influenced by genetic background. For example, the Mom1 locus accounts for ≈50% of strain variability in the ApcMin phenotype and contains at least two genes capable of altering ApcMin tumor biology (27). Polyps arising in ApcMin mice exhibit strong nuclear β-catenin immunoreactivity, a molecular hallmark of the majority of human colorectal adenomas and carcinomas (28, 29). We have used a combination of genetic and pharmacological approaches to resolve conflicting pharmacological reports and to directly examine the temporal dependency on Egfr signaling during adenomatous polyposis coli (Apc)-mediated intestinal tumorigenesis.
Materials and Methods
Mice.
B6EiC3H-Egfrwa2 and C57BL/6J-ApcMin mice were obtained from The Jackson Laboratory. Egfrtm1Mag (previously designated Egfrtm1Cwr) was maintained on 129S6/SvEvTAC and CD1-mixed genetic backgrounds segregating Mom1s and Mom1r alleles. A line of mice segregating Egfrwa2, Egfrtm1Mag, and Mom1s, Mom1r was established by crossing their respective carriers. This line was then bred to the C57BL/6J-ApcMin line and the offspring intercrossed to generate progeny segregating alleles at each locus. Mice were given Purina Mills LabDiet 5010 and water ad libitum under specific pathogen-free conditions in an American Association for the Accreditation of Laboratory Animal Care-approved facility, and were killed by CO2 asphyxiation.
Genotyping.
Mice were genotyped for Egfrwa2 by PCR amplifying a 170-bp region (primers: 5′-CCCAGAAAGGGATATGCG-3′ and 5′-GCAACCGTAGGGCATGAG-3′) and digesting with FokI to produce an uncut 170-bp or cut 75- and 95-bp fragments diagnostic for wild-type (wt) Egfr and Egfrwa2 alleles, respectively. Mice were genotyped for Egfrtm1Mag and ApcMin alleles as reported (2, 30). Mice were genotyped for Mom1 status by PCR amplifying a 500-bp region (primers: 5′-GTCCAAGGGAACATTGCG-3′ and 5′-AGAACAGGTGATTTGGCCC-3′) and digesting with BamHI to produce diagnostic fragments of 100 and 400 bp for the Mom1r allele and 500 bp for the Mom1s allele.
Macroadenoma Counts.
The GI tract from pylorus to rectum was removed. Small intestine was cut into thirds, and the caecum and colon were separated. Segments were gently flushed with PBS to remove fecal material, cut longitudinally, splayed flat on Whatmann 3MM paper, and fixed overnight at 4°C in 4% paraformaldeyhyde. Polyp counts and diameter measurements were made under a dissection microscope with an in-scope micrometer, allowing detection of polyps >0.3 mm in diameter. Representative polyps were histologically confirmed by excision with surrounding normal tissue. The tissue was dehydrated, embedded in paraffin, and sectioned perpendicular to the plane of the GI tract. Hematoxylin and eosin (H&E) staining was used to examine tumor morphology.
Microadenoma Counts.
Ilea of 4-wk-old mice were dissected, gently flushed with PBS, splayed open, and rolled into a jelly roll before fixing in 4% paraformaldeyhyde. The processed ilea were embedded in paraffin and 7-μm sections cut at 50-μm intervals through 1,600 μm of tissue. Sections were stained with H&E and scored morphologically for microadenomas, characterized by alterations in cryptal architecture and nuclear cytology as described in results. Relative microadenoma score was expressed as the total number of microadenomas scored (nonoverlapping morphological and normalized nuclear β-catenin counts) divided by the number of slides for each case.
Immunohistochemistry.
Microwave antigen retrieval with citrate buffer and the Mouse-on-Mouse kit (Vector Laboratories) were used in conjunction with primary Ab for β-catenin (Transduction Laboratories, Lexington, KY, clone 14, 1:500 dilution) or Ki67 (NovoCastra, Newcastle, U.K., 1:100 dilution). Visualization was with diaminobenzidine substrate. Sections at 200-μm intervals were examined in a blinded fashion for nuclear β-catenin and compared to adjacent H&E sections.
Autoradiography.
Mice were injected i.p. 6 h before killing with 1 μCi methyl-[3H]thymidine/gm body weight in saline. Tissues were fixed as described above and sections were exposed to Kodak NTB2 emulsion for 4 wk before developing with Kodak D-19. Counterstain was 0.2% toluidine blue.
Pharmacologic Treatment.
EKI-785 obtained from Philip Frost (Wyeth-Ayerst) was suspended at 25 μg/μl in DMSO. Starting at 1 mo of age, mice were injected i.p. every other day with either 50 mg EKI-785 per kg body weight (treated cohort) or with an equivalent volume of DMSO alone (control cohort). Animals were killed at 3 mo of age and GI tracts were processed as described above.
Human Colon Cancer Cell Lines and Xenografts.
Cell lines HCA-7 Colony 29 and HCT-116 were obtained from Susan Kirkland (Imperial College, London; ref. 31) and American Type Culture Collection, respectively, and injected into dorsal s.c. tissue of athymic nude mice (Harlan Sprague–Dawley) as described (32). When tumors reached 150 mm3, mice received i.p. injections of EKI-785 or DMSO three times weekly. Tumor volume was determined by external measurement according to the equation: volume = length × width2 × 0.5.
Statistics.
The nonparametric Mann–Whitney U test was used to analyze all comparisons except polyp growth rate and the Mom1–Egfr interaction, analyzed with the paired Wilcoxon ranked sign test and ANOVA, respectively. One-sided P values are given.
Results
Egfr-Dependent Intestinal Adenoma Multiplicity.
Because mice lacking Egfr are nonviable, the Egfrwa2hypomorphic allele was used to test the importance of Egfr signaling during Apc-mediated intestinal tumorigenesis. ApcMin heterozygous mice (3-mo-old) exhibiting a waved coat, resulting from homozygosity for Egfrwa2 or compound heterozygosity for Egfrwa2/tm1Mag, developed on average 10-fold fewer macroscopic (>0.3 mm) polyps compared to nonwaved ApcMin littermates (4.6 ± 8.7 vs. 40.9 ± 27.2; P < 0.0001; Fig. 1A). The majority of ApcMin, Egfrwa2 animals had zero or only one detectable polyp. Histological analysis of polyps revealed no morphological differences related to Egfr genotype. The average polyp number was 4.6 for both ApcMin, Egfrwa2/wa2 (n = 25) and ApcMin, Egfrwa2/tm1Mag (n = 9) animals, proving that the reduction in Apc-mediated polyp number was specific to Egfr and not because of a linked chromosomal effect; the Egfrwa2allele is carried on a C57BL/6JEi chromosome whereas the Egfrtm1Magallele is carried on a 129S6/SvEvTAC chromosome. No differences in polyp number were observed between mice carrying one or two wt Egfr alleles. Polyp distribution along the length of the lower GI tract was not altered by Egfr genotype (data not shown); the Egfr-dependent, 10-fold reduction in polyp number was observed in all regions of the small intestine and colon.
Figure 1.
Intestinal lesion numbers in ApcMin mice vs. Egfr status. (A) Macroadenoma analysis. Each dot represents polyp number from a single 3-mo-old mouse with horizontal lines representing means. Egfr+ designates mice carrying a wt Egfr allele (genotypes Egfr+/+, Egfr+/wa2, and Egfr+/tm1Mag; n = 28); Egfrwa2 designates mice with the waved-2 phenotype (genotypes Egfrwa2/wa2 and Egfrwa2/tm1Mag; n = 37). (B) Microadenoma analysis. Each dot represents the ileal microadenoma score of a single, 1-mo-old mouse (Egfr+/wa2, n = 14; Egfrwa2/wa2, n = 10).
Interaction Between Egfr and Mom1.
The genetic background used in these experiments was segregating Mom1r and Mom1s, resistant and susceptible alleles of the Mom1 locus, respectively. As previously reported (33), when compared to Mom1s homozygotes, the semidominant Mom1r allele reduces polyp multiplicity ≈50% in ApcMin mice with a wt Egfr allele (Table 1). Interestingly, the Egfrwa2 background only reduces polyp number 7-fold in Mom1s homozygous animals whereas animals carrying a Mom1r allele exhibit a 14-fold reduction in polyp number. Furthermore, Mom1r and Egfrwa2 together would appear to have a much greater effect on the ApcMin phenotype than would be predicted from a simple additive effect of the two alleles; Mom1r, Egfrwa2 mice show greater than a 30-fold reduction in Apc-mediated polyp number over Mom1s homozygous mice on a wt Egfr background. However, ANOVA analysis shows this potential interaction to be statistically nonsignificant (P = 0.34). Precise quantitation of the combined Mom1r and Egfrwa2 affect on ApcMin phenotype will require measurement on isogenic backgrounds.
Table 1.
Mean polyp number in 3-mo-old ApcMin animals by Egfr and Mom1 genotype
| Genotype | Egfr+/+ and Egfrwa2/+ | Egfrwa2/wa2 and Egfrwa2/tm1Mag |
|---|---|---|
| Mom1s/s | 58.5 (100%)*, n = 14 | 7.9 (13.6%), n = 17 |
| Mom1r/r and Mom1r/s | 24.5 (41.8%), n = 13 | 1.8 (3.1%), n = 18 |
Percentages relative to Mom1s, Egfr+ genotype; P < 0.0001 for all genotype comparisons.
Egfr-Independent Intestinal Adenoma Initiation.
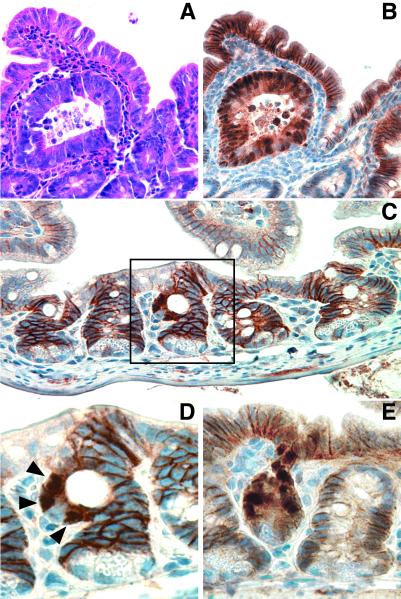
Previous reports have suggested that the majority of polyps in the ApcMin mouse arise between 1 and 3 mo of age (26, 33). Because polyp multiplicity at 3 mo of age highly depends on normal Egfr signaling, 1-mo-old ApcMin mice were examined to distinguish between Egfr-dependent effects on initiation and establishment of the polyps. H&E-stained ileal sections from ApcMin mice segregating Egfr alleles were analyzed for microadenomas based on crypt architecture and nuclear cytology (Fig. 2A). Adenomatous crypts were abnormally large and frequently cystically dilated or otherwise distorted, occasionally containing eosinophilic granular debris. Nuclear to cytoplasmic ratio was increased in adenomatous cells, nuclei were crowded and overlapping, and apoptotic bodies were increased. All microadenomas identified morphologically also exhibited strong nuclear β-catenin immunoreactivity (Fig. 2B), supporting their classification as adenomatous lesions and suggesting that impaired Egfr activity does not alter β-catenin transit into the nucleus, an early consequence of Apc loss (29, 34). To identify microadenomas not scored morphologically, β-catenin-labeled ileal sections were examined for clusters of cells exhibiting nuclear β-catenin localization in nondistorted crypts (Fig. 2 C–E). β-catenin-positive nuclei were located in clusters of contiguous cells above the proliferative zone in crypts but did not extend up the villus. On adjacent H&E-stained sections, these crypts exhibited only subtle architectural distortion with slight increases in nuclear to cytoplasmic ratio, apoptotic bodies, granular eosinophilic debris, and mixed inflammatory infiltrate in adjacent lamina propria. The difference in total microadenoma number (combined nonoverlapping morphological and normalized nuclear β-catenin counts) between wt Egfr and Egfrwa2mice was not statistically significant (0.42 ± 0.31 vs. 0.34 ± 0.32 microadenoma/slide, P = 0.50; Fig. 1B), suggesting that Egfr is required for intestinal polyp development after morphological initiation. Likewise, no difference in microadenoma architecture was seen based on Egfr status. No morphological microadenomas and a single β-catenin-positive nucleus were detected in ileal sections from wt Apc control littermates (n = 3), supporting the ApcMindependency of these lesions.
Figure 2.
Early ileal lesions in ApcMin mice. (A and B) Adjacent sections of an Egfr+ cystic microadenoma. (C) Egfr+ crypt, containing a cluster of three nuclear β-catenin-positive cells, flanked by normal crypts. (D) Close up of boxed crypt from C. (E) Egfrwa2 crypt with several β-catenin-positive nuclei. (A) H&E staining. (B–E) β-catenin immunohistochemistry. Arrowheads in D mark the β-catenin-positive nuclei.
Egfr-Independent Intestinal Adenoma Growth.
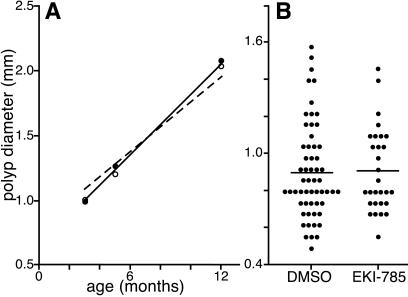
To assess the importance of Egfr on net tumor growth, polyp diameter was measured from a randomized set of 3-mo-old ApcMinmice on wt Egfr or Egfrwa2 backgrounds (Fig. 3A). Surprisingly, polyps forming on the Egfrwa2background were slightly larger than those forming on the wt Egfr background (1.10 ± 0.58 mm vs. 1.03 ± 0.77 mm; P = 0.024), suggesting that rate of polyp expansion was not hindered by reduced Egfr activity. Alternatively, these results could be attributed to Egfr-dependent differences in growth rate masked by temporal differences in initiation or establishment. To distinguish these two possibilities, polyp size was measured in a cohort of 4- to 12-mo-old mice segregating Egfr genotypes. A linear regression of polyp diameter vs. age was performed to detect Egfr-dependent differences in polyp growth rates; polyps from ApcMin mice have previously been reported to grow linearly (33). The linear fits of the two data sets show no significant difference in slope value (Egfrwa2/wa2 0.11 , Egfrwa2/+ 0.13; P = 0.59), suggesting that the reduced activity of the Egfrwa2-encoded receptor does not alter net growth rate of established polyps. Of interest, marker analysis suggests a slight expansion of the proliferative compartment in normal Egfrwa2 crypts compared to wt crypts (Ki67-labeled nuclei: Egfrwa2/wa223.9 ± 0.87, Egfrwa2/+ 20.0 ± 1.3, P = 0.014; [3H]thymidine incorporated nuclei: Egfrwa2/wa29.9 ± 2.2, Egfrwa2/+ 7.0 ± 1.0, P < 0.0001).
Figure 3.
Intestinal polyp sizes in ApcMin mice with reduced Egfr activity. (A) Genetic analysis. Solid and dashed lines indicate best linear fit of polyp diameter vs. age for 16 Egfr+ and 20 Egfrwa2 mice, respectively. Closed (Egfr+) and open (Egfrwa2) circles indicate diameter means at each time point. (B) Pharmacological analysis. Dots represent individual polyp diameters from DMSO-treated (controls; n = 5) and EKI-785-treated (treatment; n = 5) mice. Means are represented by horizontal lines.
Pharmaceutical Inhibition of Intestinal Adenoma Growth.
Previous conflicting reports used irreversible small molecule Egfr kinase inhibitors in the ApcMin mouse model. Although no effect on tumorigenesis was observed by using CFPQA (25), in a separate study EKI-785 was found to reduce ApcMin tumor multiplicity by ≈50% after treatment from 1 to 3 mo of age (24). To confirm the results obtained with the ApcMin, Egfrwa2 animals, we used a similar EKI-785 treatment regimen in ApcMin animals. Although we used i.p. rather than oral dosing, as previously reported, we saw a 60% reduction in polyp number in the EKI-treated cohort at 3 mo of age (treated: 6.3 ± 2.9; controls: 15.0 ± 6.3; P = 0.047; n = 5 mice/group). Supporting the genetic studies, no difference was observed in average polyp diameter between EKI-785-treated and control animals (0.89 ± 0.24 mm and 0.88 ± 0.28 mm; P = 0.76; Fig. 3B).
EGFR-Dependent Human Colon Cancer Xenograft Growth.
To address the role of EGFR signaling at later stages of tumor growth, EKI-785 was administered to athymic mice carrying 150-mm3 s.c. tumors derived from injection of two human colorectal cancer (CRC) cell lines, HCA-7 and HCT-116. Both lines exhibit constitutive EGFR phosphorylation under baseline conditions, suggesting the presence of an active EGFR autocrine loop. However, exogenous application of the EGFR ligand transforming growth factor α stimulates the in vitro growth of the HCA-7 line, whereas HCT-116 is resistant because of saturating levels of transforming growth factor α that are 22-fold higher than the HCA-7 line (E. Chung and R.J.C., unpublished data). Doses of EKI-785 as low as 25 mg/kg reduced the growth of HCA-7 cells, and a dose of 100 mg/kg prevented tumor growth entirely (Fig. 4A). Also, a dose of 50 mg/kg EKI-785 was effective at reducing growth of HCT-116 cells (Fig. 4B). These findings suggest that EGFR signaling, in addition to affecting the establishment phase of intestinal tumorigenesis, also contributes to late-stage tumor growth.
Figure 4.
Growth curves of human CRC cell line xenografts in nude mice treated with EKI-785. Volumes of tumors derived from HCA-7 (A) and HCT-116 (B) cells. EKI-785 doses (mg/kg body weight) are given at the end of each growth curve.
Discussion
Although gain- or loss-of-function mutations in Egfr are not consistently found in specific epithelial tumor types, circumstantial evidence has accumulated suggesting that Egfr activity can modulate the initiation and progression of epithelial-derived tumors (17, 19). Indirect evidence derived from in vitro studies of GI cancer cell lines also suggest an important role for Egfr in intestinal tumorigenesis (18, 20). By placing the ApcMin mouse model of familial adenomatous polyposis on an Egfrwa2 background, we were able to examine intestinal polyp development in vivo in a genetic environment of reduced Egfr kinase activity. Despite a heterogeneous genetic background, the Egfrwa2 allele had a profound effect on polyp number in adult animals, reducing mean tumor number 90%. This reduction mirrors the reported 10-fold reduction in kinase activity in the Egfrwa2-encoded protein (5). In fact, the majority of Egfrwa2 mice had zero or only one intestinal tumor. Careful histologic examination of younger, 4-wk-old animals showed no significant difference between Egfr genotypes in the numbers of microadenomas or nuclear β-catenin-positive cell clusters, which are the earliest detectable morphologic and molecular lesions in Apc-mediated tumorigenesis. Interestingly, the size, growth rate, and morphology of the polyps examined in adult animals were not altered by Egfr status, a surprising result given that Egfr has been shown to mediate many of the cellular functions that are misregulated during tumorigenesis, including proliferation, differentiation, migration, and survival.
Our experiments are timely given recent contradictory reports on the requirement for Egfr signaling in intestinal tumor development (24, 25). Similar to the results reported here, Torrance, et al. (24) observed that two Egfr kinase inhibitors, EKI-785 and EKB-569, produced a significant reduction in ApcMin polyp number. In contrast, Ritland, et al. (25) did not observe a reduction in ApcMin polyp multiplicity by using the irreversible Egfr kinase inhibitor CFPQA. Experiments in both reports provided EKI treatment from 1 to 3 mo of age. Neither study examined the temporal requirement for Egfr in tumor development, and more importantly in relation to potential chemotherapeutic use of Egfr inhibitors, these reports did not address the continued dependency on Egfr signaling for tumor expansion. Furthermore, although EKI-785 and EKB-569 both have Egfr as their primary affinity target, they also are known to affect secondary targets such as Erbb2 and Src, and potentially may affect other, unknown targets as well (22, 24). Our genetic approach directly demonstrates that Egfr is required for intestinal tumor development, thus providing resolution to previous conflicting pharmaceutical studies and confirming Egfr as a valid therapeutic target. However, our results also pose considerable new questions that must be addressed, especially in light of clinical trials using Egfr inhibition as CRC treatment.
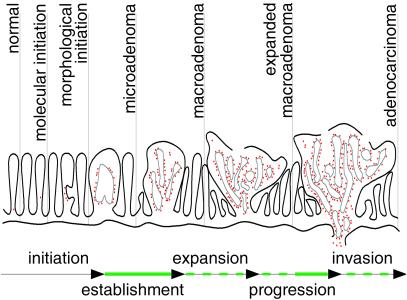
Our genetic results suggest that a threshold level of Egfr activity is transiently required after loss of Apc and morphological development of microadenomas but before microadenomas become established and expand into macroadenomas. This model defines a role for Egfr in an establishment stage during intestinal tumorigenesis when nascent tumors are highly susceptible to being lost (Fig. 5). Nascent microadenomas that are unable to pass through this establishment phase may senesce, be resorbed, or be lost into the intestinal lumen. Tumors on the Egfrwa2 background may become established when levels of Egfr activity stochastically breach the threshold level in cells that are already initiated by means of Apc loss. Once the threshold is achieved, downstream effects could maintain the threshold activity level, or alternatively, tumorigenic events induced by the threshold activity could continue independent of Egfr. This stochastic, Egfr activity-correlated model would predict that further reductions in Egfr activity would produce a similar reduction in polyp multiplicity.
Figure 5.
Model of Egfr activity requirements during intestinal tumorigenesis. Red dots represent nuclear β-catenin-positive cells. Solid green lines indicate evidence for Egfr activity during establishment and adenocarcinoma expansion. Dashed green lines indicate that a requirement for Egfr activity during adenoma expansion, progression, and invasion has yet to be demonstrated conclusively.
A strong alternative explanation for the profound Egfr-dependent reduction in polyp multiplicity may be that Egfr activity in Egfrwa2 mice is sufficiently below the required threshold such that stochastic fluctuations do not breach the required level; a possibility supported by the observation that Egfrwa2/wa2 mice develop the same number of macroadenomas as Egfrwa2/tm1Mag mice, even though the latter presumably have lower net Egfr activity. Rather, the few polyps that develop in Egfrwa2 mice, despite being pathologically similar to polyps developing in wt Egfr mice, may rely on perturbation of other signaling pathways for their growth and survival. The latter case is partially supported by the fact that the few polyps forming on the Egfrwa2 background seem to expand in an Egfr-independent manner. Albeit mechanistically different, a similar finding distinguished tumors arising on Mom1r and Mom1s backgrounds, where two molecularly different pathways for Apc loss have been identified that result in pathologically indistinguishable polyps (35).
Previous studies (24, 25) examined the effects of EKI treatment on the phenotype of C57BL/6J-ApcMin mice, which are homozygous for the Mom1s allele. The animals used in our genetic studies were segregating the Mom1r and Mom1s alleles in addition to the various Egfr alleles, and our data suggest potential synergy between the Egfrwa2 and Mom1r alleles. However, the mixed genetic background is likely segregating unknown modifiers of polyp multiplicity, suggested by the presence of two outliers in the Egfrwa2 class (Fig. 1A). These animals phenotypically resemble animals carrying a wt allele of Egfr, in part demonstrating the potential for multiple susceptibility alleles in the genetic background overcoming the effects of a strong modifier such as Egfrwa2. Because the Mom1r background is putatively more representative of the human population than the mouse-specific Mom1s background, any synergy between reduction of Egfr activity and Mom1r should further the efficacy of Egfr inhibition as therapy for human cancers.
A pattern of Egfr action is emerging from studies of epithelial–stromal interactions during organ development and growth. Tissue recombination experiments support a stromal requirement for Egfr activity in epithelial organ growth and patterning, whereas epithelial Egfr activity appears dispensable for these functions (36, 37). In this model, epithelial growth is regulated in part by means of Egfr-mediated signaling from the stromal compartment. Epithelial tumors may require an event such as spatial misexpression of Egfr or its ligands to uncouple epithelial proliferation and patterning from stromal control. Extensive morphological studies of polyp formation in ApcΔ716 mice reveal a complex architectural transition from early microadenoma to macroadenoma during the establishment phase of tumorigenesis (38, 39). The transition involves the invagination of dysplastic epithelium into the stromal center of a single villus, with the nascent microadenoma spreading into neighboring villi, growing under the normal gut epithelium. Thus the establishment stage of ApcMin polyp formation involves movement of the dysplastic cell population out of the lumen and into an environment of stromal interaction. Ability of the stroma to support epithelial survival and proliferation in this abnormal state may require a level of Egfr activity above that provided by the Egfrwa2-encoded receptor. Egfr activity has also been implicated in regulation of tumor cell adhesion by means of E-cadherin complexes (40, 41); it may be possible that wt Egfr activity is necessary to allow cell adhesion states that permit the complete and continued establishment of the microadenoma in the adjacent normal tissue. Outside the local environment the tumor may not survive, may be exposed to growth inhibitory factors, or may be lost into the lumen of the gut.
The proposition that reduction of Egfr activity affects early establishment of intestinal tumors, while seemingly having no affect on established polyp growth, seems to contraindicate use of Egfr inhibitors as chemotherapeutics for human CRC. To test the potential efficacy of Egfr inhibition for reduction of human CRC growth, mice carrying xenografts of human colon cancer cell lines were treated with EKI-785. We observed significant dose-dependent reduction in the growth rate and final tumor volume in treated mice relative to controls, and expansion of tumors seeded from the HCA-7 cell line was abrogated with an EKI-785 dose of 100 mg/kg. These cell lines represent more advanced tumors than adenomas forming in ApcMin mice. Thus Egfr activity may be required during later stages of intestinal tumor progression, a possibility supported by evidence correlating high levels of Egfr expression with invasive and metastatic cancer potential (18, 20, 42).
Although initially our pharmacologic data may seem to contradict our genetic data, there are major differences in the reduction of Egfr activity both temporally and in kind. The ApcMin, Egfrwa2 animals experience a reduction of Egfr activity from conception, whereas EKI-785 treatment reduces Egfr activity in xenografts of established, progressed tumor cell lines. Also, the mutant receptor encoded by the Egfrwa2 mutation has reduced signaling capacity because of a conformational alteration of the kinase domain. This is a very different situation than EKI-785 inhibition, which causes permanent inactivation of the receptors it targets; EKI-inhibited cells retain the ability to produce more wt receptors with full signaling capacity. It is likely that both normal and transformed cells would adapt very differently to these two forms of Egfr reduction. The effects of EKI-785 treatment seen in xenograft tumors may be caused by an established dependence on Egfr signaling. In ApcMin, Egfrwa2 animals, effects on polyp expansion and survival beyond establishment may not be observed because the subset of polyps forming on that background have by necessity arisen in an Egfr-independent manner. Perhaps further reduction of Egfr activity beyond that provided by the Egfrwa2 mutation could inhibit growth of even this subset of polyps.
Egfr kinase inhibition may prove to be a potent therapy in all stages of colon carcinogenesis; however, our data suggests judicious use at discrete stages. Furthermore, our results suggest that a subset of Apc-mediated intestinal polyps will not respond to Egfr inhibition. Also, long-term EKI treatment in humans may recapitulate the results seen in ApcMin, Egfrwa2 animals; that is, some tumors may adapt by becoming Egfr-independent, much as prostate tumors progress to androgen independency after surgical or chemical castration (43, 44). The latter concern would be especially relevant to the use of EKIs as extended preventative treatment for genetically predisposed individuals such as familial adenomatous polyposis kindreds. Continued elucidation of the mechanism by which Egfr signaling contributes to intestinal tumorigenesis, combined with the advent of genetic profiling of tumors, may allow the specific identification of those tumors that will respond to EKI treatment.
Acknowledgments
We thank members of our laboratories for valuable suggestions and Dr. Fei Zou for statistical analysis of the Mom1–Egfr interaction. This research was supported by Grants CA092479, CA084239, and CA046413 from the National Cancer Institute.
Abbreviations
- Apc
adenomatous polyposis coli
- CRC
colorectal cancer
- Egfr
epidermal growth factor receptor
- EKI
Egfr kinase inhibitor
- GI
gastrointestinal
- H&E
hematoxylin and eosin
- Mom
modifier-of-min
- wt
wild type
References
- 1.Gullick W J. Biochem Soc Symp. 1998;63:193–198. [PubMed] [Google Scholar]
- 2.Threadgill D W, Dlugosz A A, Hansen L A, Tennenbaum T, Lichti U, Yee D, LaMantia C, Mourton T, Herrup K, Harris R C, et al. Science. 1995;269:230–234. doi: 10.1126/science.7618084. [DOI] [PubMed] [Google Scholar]
- 3.Sibilia M, Wagner E F. Science. 1995;269:234–238. doi: 10.1126/science.7618085. [DOI] [PubMed] [Google Scholar]
- 4.Miettinen P J, Berger J E, Meneses J, Phung Y, Pedersen R A, Werb Z, Derynck R. Nature (London) 1995;376:337–341. doi: 10.1038/376337a0. [DOI] [PubMed] [Google Scholar]
- 5.Luetteke N C, Phillips H K, Qiu T H, Copeland N G, Earp H S, Jenkins N A, Lee D C. Genes Dev. 1994;8:399–413. doi: 10.1101/gad.8.4.399. [DOI] [PubMed] [Google Scholar]
- 6.Fowler K J, Walker F, Alexander W, Hibbs M L, Nice E C, Bohmer R M, Mann G B, Thumbwood C, Maglitto R, Danks J A, et al. Proc Natl Acad Sci USA. 1995;92:1465–1469. doi: 10.1073/pnas.92.5.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Helmrath M A, Erwin C R, Warner M D. J Surg Res. 1997;69:76–80. doi: 10.1006/jsre.1997.5033. [DOI] [PubMed] [Google Scholar]
- 8.Egger B, Buchler M W, Lakshmanan J, Moore P, Eysselein V E. Scand J Gastroenterol. 2000;35:1181–1187. doi: 10.1080/003655200750056664. [DOI] [PubMed] [Google Scholar]
- 9.Park H S, Goodlad R A, Ahnen D J, Winnett A, Sasieni P, Lee C Y, Wright N A. Am J Pathol. 1997;151:843–852. [PMC free article] [PubMed] [Google Scholar]
- 10.Umekita Y, Ohi Y, Sagara Y, Yoshida H. Int J Cancer. 2000;89:484–487. doi: 10.1002/1097-0215(20001120)89:6<484::aid-ijc3>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 11.Dassonville O, Formento J L, Francoual M, Ramaioli A, Santini J, Schneider M, Demard F, Milano G. J Clin Oncol. 1993;11:1873–1878. doi: 10.1200/JCO.1993.11.10.1873. [DOI] [PubMed] [Google Scholar]
- 12.Tewari K S, Kyshtoobayeva A S, Mehta R S, Yu I R, Burger R A, DiSaia P J, Fruehauf J P. Gynecol Oncol. 2000;78:130–136. doi: 10.1006/gyno.2000.5837. [DOI] [PubMed] [Google Scholar]
- 13.Barnard J A, Graves-Deal R, Pittelkow M R, DuBois R, Cook P, Ramsey G W, Bishop P R, Damstrup L, Coffey R J. J Biol Chem. 1994;269:22817–22822. [PubMed] [Google Scholar]
- 14.Dlugosz A A, Hansen L, Cheng C, Alexander N, Denning M F, Threadgill D W, Magnuson T, Coffey R J, Yuspa S H. Cancer Res. 1997;57:3180–3188. [PubMed] [Google Scholar]
- 15.Hansen L A, Woodson R L, II, Holbus S, Strain K, Lo Y C, Yuspa S H. Cancer Res. 2000;60:3328–3332. [PubMed] [Google Scholar]
- 16.Sibilia M, Fleischmann A, Behrens A, Stingl L, Carroll J, Watt F M, Schlessinger J, Wagner E F. Cell. 2000;102:211–220. doi: 10.1016/s0092-8674(00)00026-x. [DOI] [PubMed] [Google Scholar]
- 17.Messa C, Russo F, Caruso M G, Di Leo A. Acta Oncol. 1998;37:285–289. doi: 10.1080/028418698429595. [DOI] [PubMed] [Google Scholar]
- 18.Tong W-M, Ellinger A, Sheinin Y, Cross H S. Br J Cancer. 1998;77:1792–1798. doi: 10.1038/bjc.1998.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayashi Y, Widjono Y W, Ohta K, Hanioka K, Obayashi C, Itoh K, Imai Y, Itoh H. Pathol Int. 1994;44:124–130. doi: 10.1111/j.1440-1827.1994.tb01696.x. [DOI] [PubMed] [Google Scholar]
- 20.Radinsky R, Risin S, Fan D, Dong Z, Bielenberg D, Bucana C D, Fidler I J. Clin Cancer Res. 1995;1:19–31. [PubMed] [Google Scholar]
- 21.Noonberg S B, Benz C C. Drugs. 2000;59:753–767. doi: 10.2165/00003495-200059040-00003. [DOI] [PubMed] [Google Scholar]
- 22.Discafani C M, Carroll M L, Floyd M B, Jr, Hollander I J, Husain Z, Johnson B D, Kitchen D, May M K, Malo M S, Minnick A A, Jr, et al. Biochem Pharmacol. 1999;57:917–925. doi: 10.1016/s0006-2952(98)00356-6. [DOI] [PubMed] [Google Scholar]
- 23.Sweeney W E, Chen Y, Nakanishi K, Frost P, Avner E D. Kidney Int. 2000;57:33–40. doi: 10.1046/j.1523-1755.2000.00829.x. [DOI] [PubMed] [Google Scholar]
- 24.Torrance C J, Jackson P E, Montgomery E, Kinzler K W, Vogelstein B, Wissner A, Nunes M, Frost P, Discafani C M. Nat Med. 2000;6:1024–1028. doi: 10.1038/79534. [DOI] [PubMed] [Google Scholar]
- 25.Ritland S R, Gendler S J, Burgart L J, Fry D W, Nelson J M, Bridges A J, Andress L, Karnes W E. Cancer Res. 2000;60:4678–4681. [PubMed] [Google Scholar]
- 26.Moser A R, Pitot H C, Dove W F. Science. 1990;247:322–324. doi: 10.1126/science.2296722. [DOI] [PubMed] [Google Scholar]
- 27.Cormier R T, Bilger A, Lillich A J, Halberg R B, Hong K H, Gould K A, Borenstein N, Lander E S, Dove W F. Oncogene. 2000;19:3182–3192. doi: 10.1038/sj.onc.1203646. [DOI] [PubMed] [Google Scholar]
- 28.Korinek V, Barker N, Morin P J, van Wichen D, de Weger R, Kinzler K W, Vogelstein B, Clevers H. Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- 29.Iwamoto M, Ahnen D J, Franklin W A, Maltzman T H. Carcinogenesis. 2000;21:1935–1940. doi: 10.1093/carcin/21.11.1935. [DOI] [PubMed] [Google Scholar]
- 30.Dietrich W F, Lander E S, Smith J S, Moser A R, Gould K A, Luongo C, Borenstein N, Dove W. Cell. 1993;75:631–639. doi: 10.1016/0092-8674(93)90484-8. [DOI] [PubMed] [Google Scholar]
- 31.Marsh K A, Stamp G W, Kirkland S C. J Pathol. 1993;170:441–450. doi: 10.1002/path.1711700407. [DOI] [PubMed] [Google Scholar]
- 32.Chinery R, Brockman J A, Peeler M O, Shyr Y, Beauchamp R D, Coffey R J. Nat Med. 1997;3:1233–1241. doi: 10.1038/nm1197-1233. [DOI] [PubMed] [Google Scholar]
- 33.Gould K A, Dietrich W F, Borenstein N, Lander E S, Dove W F. Genetics. 1996;144:1769–1776. doi: 10.1093/genetics/144.4.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sheng H, Shao J, Williams C S, Pereira M A, Taketo M M, Oshima M, Reynolds A B, Washington M K, DuBois R N, Beauchamp R D. Carcinogenesis. 1998;19:543–549. doi: 10.1093/carcin/19.4.543. [DOI] [PubMed] [Google Scholar]
- 35.Shoemaker A R, Moser A R, Midgley C A, Clipson L, Newton M A, Dove W F. Proc Natl Acad Sci USA. 1998;95:10826–10831. doi: 10.1073/pnas.95.18.10826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wiesen J F, Young P, Werb Z, Cunha G R. Development (Cambridge, UK) 1999;126:335–344. doi: 10.1242/dev.126.2.335. [DOI] [PubMed] [Google Scholar]
- 37.Hom Y K, Young P, Wiesen J F, Miettinen P J, Derynck R, Werb Z, Cunha G R. Endocrinology. 1998;139:913–921. doi: 10.1210/endo.139.3.5817. [DOI] [PubMed] [Google Scholar]
- 38.Oshima M, Oshima H, Kitagawa K, Kobayashi M, Itakura C, Taketo M. Proc Natl Acad Sci USA. 1995;92:4482–4486. doi: 10.1073/pnas.92.10.4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oshima H, Oshima M, Kobayanshi M, Tsutsumi M, Taketo M M. Cancer Res. 1997;57:1644–1649. [PubMed] [Google Scholar]
- 40.Hazan R B, Norton L. J Biol Chem. 1998;273:9078–9084. doi: 10.1074/jbc.273.15.9078. [DOI] [PubMed] [Google Scholar]
- 41.Roura S, Miravet S, Piedra J, Garcia de Herreros A, Dunach M. J Biol Chem. 1999;274:36734–36740. doi: 10.1074/jbc.274.51.36734. [DOI] [PubMed] [Google Scholar]
- 42.Gross M E, Zorbas M A, Danels Y J, Garcia R, Gallick G E, Olive M, Brattain M G, Boman B M, Yeoman L C. Cancer Res. 1991;51:1452–1459. [PubMed] [Google Scholar]
- 43.Galbraith S M, Duchesne G M. Eur J Cancer. 1997;33:545–554. doi: 10.1016/s0959-8049(96)00444-3. [DOI] [PubMed] [Google Scholar]
- 44.Theyer G, Hamilton G. Urology. 1998;52:353–359. doi: 10.1016/s0090-4295(98)00251-9. [DOI] [PubMed] [Google Scholar]