Abstract
Organ specific drug targeting was explored in mice as a possible alternative to surgery to treat prostate diseases. Peptides that specifically recognize the vasculature in the prostate were identified from phage-displayed peptide libraries by selecting for phage capable of homing into the prostate after an i.v. injection. One of the phage selected in this manner homed to the prostate 10–15 times more than to other organs. Unselected phage did not show this preference. The phage bound also to vasculature in the human prostate. The peptide displayed by the prostate-homing phage, SMSIARL (single letter code), was synthesized and shown to inhibit the homing of the phage when co-injected into mice with the phage. Systemic treatment of mice with a chimeric peptide consisting of the SMSIARL homing peptide, linked to a proapoptotic peptide that disrupts mitochondrial membranes, caused tissue destruction in the prostate, but not in other organs. The chimeric peptide delayed the development of the cancers in prostate cancer-prone transgenic mice (TRAMP mice). These results suggest that it may be possible to develop an alternative to surgical prostate resection and that such a treatment may also reduce future cancer risk.
Diseases affecting the prostate have gained major significance clinically and economically, primarily because of the increasing average age of the male population in the industrialized countries. Benign prostate hyperplasia affects to some degree most elderly men. Even more serious, the prostate is a frequent site of cancer. Some autopsy studies find that most men older than 70 have occult or overt cancer in the prostate (1). The surgical therapies of prostate hypertrophy and prostate cancer are associated with serious side effects, such as incontinence and impotence.
We have sought to develop a strategy that would provide a less traumatic treatment for prostate disease than is currently available. Our strategy is based on identification of peptides that home to specific sites in the vasculature by in vivo screening of intravenously injected phage libraries. These studies have revealed a surprising degree of specialization in the endothelia of various normal tissues (2, 3). Screening phage libraries for tumor homing has yielded a collection of peptides that home to tumor vasculature (4). We and others have used these tumor-homing peptides to direct therapies into tumors in mice (4, 5). We report here the identification of peptides that home to the vasculature of the prostate and the use of one of these homing peptides to deliver a proapoptotic peptide to the prostate.
Materials and Methods
Materials.
Peptides were synthesized to our specifications by AnaSpec (San Jose, CA) or by our Peptide Synthesis Facility. The peptides were purified by HPLC and their identity was confirmed with mass spectrometry.
Apotag Kit for TUNEL staining was purchased from Intergen (Purchase, NY). Testosterone pellets (12.5 mg) and control pellets were from Innovative Research of America (Sarasota, FL), and controlled release pumps from Alzet (Mountain View, CA). The pumps were loaded with peptides following the manufacturer's instructions.
Mice.
CD-1 male mice (The Jackson Laboratories) were used for phage screening at an age of 2–4 months. Transgenic adenocarcinoma of the mouse prostate (TRAMP) mice, kindly provided by Norman Greenberg, Baylor College of Medicine, Houston) were bred at our Animal Facility.
Phage Libraries and Library Screening.
The phage libraries were prepared in the fUSE5 vector as described (6, 7). The primary library contains about 5 × 109 individual recombinant phage. For the library screening, CD-1 mice were anesthetized with Avertin (0.015 ml/g) and injected intravenously (tail vein) with phage libraries containing 109 transducing units diluted in 200 μl of DMEM. The phage was rescued from tissues by bacterial infection (2), and about 300 individual colonies were grown separately. The bacterial cultures were then pooled and the amplified phage were injected into mice as described above. To test individual phage for homing, 109 colony-forming units (cfu) (fUSE5) or 1010 plaque-forming units (pfu) (T7), diluted in 200 μl of PBS, were injected. The SMSIARL insert and its scrambled variant were cloned to the T7 phage (T7select415–1 vector; Novagen), and the resulting phage was tested as described (8).
Results
In vivo screening of a fUSE5 phage heptapeptide library for prostate-homing peptides (6) yielded two phage that accumulated selectively in the prostate. One of these phage, displaying the peptide SMSIARL (single letter code), homed to the prostate 15 times more than nonrecombinant control phage (Fig. 1a). The other prostate-selected phage (VSFLEYR) gave a prostate-homing ratio of ≈10. The homing of the SMSIARL phage to prostate tissue was inhibited when synthetic SMSIARL peptide was injected together with the phage, but not when an unrelated peptide was injected (Fig. 1a). The SMSIARL phage homed also to the rat prostate tissue (not shown). The SMSIARL peptide when cloned into the T7 phage (6) showed a similar homing specificity for the prostate.
Figure 1.
Specific homing of phage to the prostate. (a) Phage selected for prostate homing accumulates specifically in the prostate and the homing is inhibited by soluble peptide. The SMSIARL fUSE5 phage, identified by in vivo screening, was tested for prostate homing. This phage and an irrelevant control phage were injected intravenously to male mice [109 colony forming units (cfu) per mouse] and the phage were rescued from various tissues based on their ability to infect a host bacteria. As indicated, 200 μg of the SMSIARL peptide or a control peptide (CARAC) was included in the injection to test inhibition of SMSIARL phage homing. (b) The SMSIARL peptide directs specific homing of T7 phage to the prostate. The SMSIARL sequence was cloned to the coat protein of the T7. A 1:10 mixture of SMSIARL and nonrecombinant control T7 phage [1010 plaque-forming units (pfu)] was injected and allowed to circulate for 7 min. Phage was extracted from prostate and brain with buffer (PBS), or a detergent solution (0.5% Nonidet P-40 in PBS) and plated, and 32 colonies were randomly chosen for PCR. The PCR products of SMSIARL and control phage DNA were distinguished on the basis of a size difference in a 4% agarose gel. (Control tissue was brain.)
Phage displaying a scrambled variant of this peptide (LAMSRIS) showed no homing to the prostate. The T7 SMSIARL phage was not enriched in the brain (Fig. 1b), salivary gland, kidney, testis, thymus, pancreas, skeletal muscle, or lung (not shown). We also confirmed the homing specificity by co-injecting SMSIARL phage and nonrecombinant phage; the ratio of the two types of phage in the prostate was determined by PCR. The SMSIARL phage homed to the prostate 10–15 times more than the nonrecombinant phage. The recovery of the SMSIARL phage was more than 5-fold higher when the tissue was extracted with detergent rather than buffer alone. The brain as a control organ showed no enrichment with or without detergent (Fig. 1b). The greater phage recovery after lysis of the tissue with detergent suggests that the SMSIARL phage may have been taken up into cells.
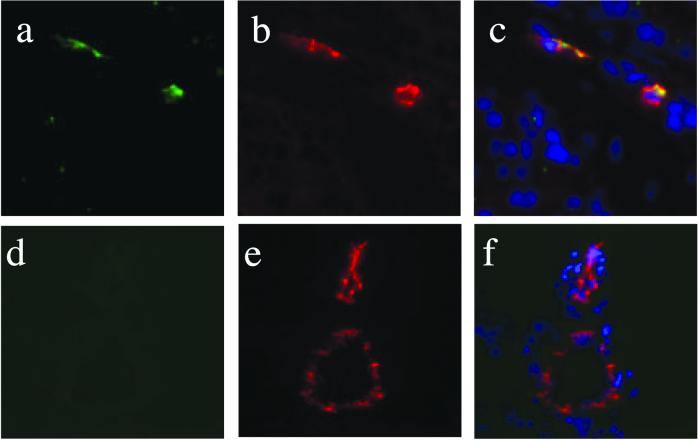
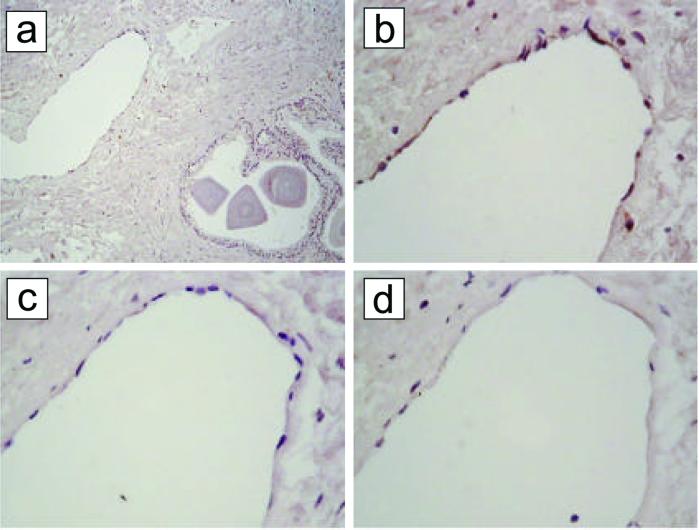
Antibody staining of the phage in tissue sections from mice injected intravenously with the T7 SMSIARL phage revealed staining in the prostate 7 min after an i.v. injection (Fig. 2). The phage staining colocalized with staining for the blood vessel marker CD31, indicating homing to blood vessels in the prostate. No specific staining was seen in control organs, or in prostate or control organs of mice injected with a nonrecombinant control phage. The phage staining appeared to be intracellular, supporting the detergent extraction results shown in Fig. 1b. Overlay of tissue sections from human prostate with the SMSIARL phage indicated that this phage also binds to the endothelium of human prostate blood vessels the same way it binds to the mouse prostate vessels (Fig. 3). Significantly, vessels in hypertrophic human prostate tissue bound the SMSIARL phage. No binding of this phage was detected in the blood vessels in several other human tissues. Similar localization results were obtained with the free SMSIARL peptide coupled to fluorescein (data not shown).
Figure 2.
Immunohistochemical staining of phage within prostate endothelial cells after i.v. injection into mice. SMSIARL-phage preparation was injected intravenously into mice. After 7 min circulation, animals were perfused with PBS, the prostate (a–c), brain (d–f), and various control organs were removed, processed for frozen sectioning, and stained with a polyclonal antibody against T7 phage (FITC; a and d) and CD31 (rhodamine; b and e). Merge with nuclear counterstain with DAPI (c and f). Control organs (kidney, spleen, lung; not shown) were negative for the phage, except for liver and spleen, where the reticuloendothelial tissue traps phage nonspecifically (4). (Magnification: ×400.)
Figure 3.
SMSIARL phage binds to endothelium in human prostate. A human prostate tissue section containing both normal and cancerous tissue was overlaid with the SMSIARL phage (109 cfu/ml) and the binding of the phage was detected with anti-M13 phage antibody and peroxidase staining. (a) An overview (×200); (b) a detail from a at a higher magnification (×400). Staining of the endothelium is seen. (c) Overlay with phage that contains no peptide insert produces no endothelial staining. (d) The SMSIARL-phage staining is inhibited when soluble SMSIARL peptide is included in the overlay at 0.3 mg/ml.
We next studied the ability of the SMSIARL peptide to deliver a biologically active compound to the prostate. D(KLAKLAK)2 is an amphipathic D-amino acid peptide that binds selectively to bacterial, but not eukaryotic cell membranes (9). It has antibacterial activity, but is relatively nontoxic to eukaryotic cells. We have previously shown that D(KLAKLAK)2, if delivered into mammalian cells, disrupts mitochondria (mitochondrial membranes resemble those of bacteria), initiating apoptosis (10). Conjugated through a G-G linker to a homing peptide that homes to tumor vasculature, D(KLAKLAK)2 yields a chimeric compound that is selectively cytotoxic to angiogenic endothelial cells and has antitumor activity in vivo (10). We used the same strategy to prepare a proapoptotic chimera that targets the vasculature of the normal prostate, and studied its ability to cause selective tissue destruction in the prostate.
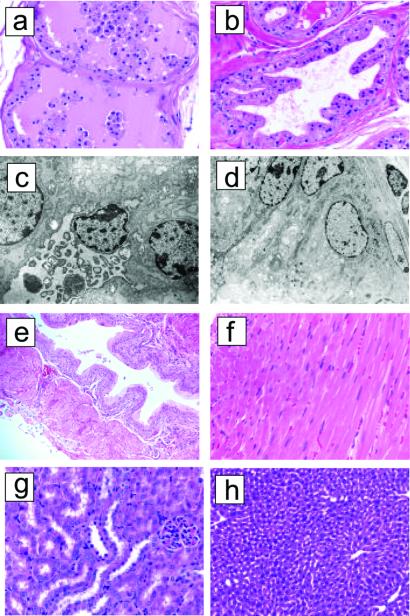
Mice were injected with 250 μg of the targeted SMSIARL-GG-D(KLAKLAK)2 chimeric compound and the prostates were collected after 1, 4, 8, 12, 16, 24, and 48 h, and after 7 days. Control groups received D(KLAKLAK)2 coupled to a nonhoming scrambled peptide (LAMSRIS), SMSIARL and D(KLAKLAK)2 as an uncoupled mixture, or buffer alone. A total of 62 mice treated with SMSIARL-GG-D(KLAKLAK)2 were evaluated. In prostates collected 16 h or later after the injection, histology revealed an unevenly distributed destruction of the prostate glandular epithelial cells that in some areas included epithelial shedding and destruction of entire glandular structures (Fig. 4 a and b). These changes were still present 7 days after the treatment and no mitotic figures were observed, suggesting sustained damage and poor regeneration (not shown). Electron microscopy showed extensive destruction of intracellular organelles in the SMSIARL-GG-D(KLAKLAK)2-treated, but not control-treated, mice (Fig. 4 c and d). Tissue damage was also evident from an increase in TUNEL-positive vascular and glandular cell nuclei in the prostates of mice treated with SMSIARL-GG-D(KLAKLAK)2 (not shown). The prostates of control animals displayed only rare degenerating epithelial cells and all other organs examined (brain, heart, liver kidney, lung, urothelium) were histologically normal during or after treatment with each of the compounds (Fig. 4 e–h).
Figure 4.
Targeted proapoptotic peptide to mouse prostate vasculature causes tissue damage in prostate but not in other tissues. Mice received an i.v. 250 μg injection of the SMSIARL-GG- D(KLAKLAK)2 or an equivalent dose of SMSIARL and D(KLAKLAK)2 as uncoupled peptides (control-treated mice). The mice were killed 24 h after the injection. Prostates were fixed in paraformaldehyde or glutaraldehyde solution and processed for light microscopy by staining with hematoxylin/eosin (H&E) or electron microscopy. Light microscopy showed focal loss of cell borders and epithelial shedding in the ventral lobe of prostates from the SMSIARL-GG- D(KLAKLAK)2 group. (a) H&E-stained micrograph shows massive glandular destruction with nearly complete shedding of the glandular epithelial cells into the lumen. (b) A representative micrograph of normal prostate tissue from a mouse treated with the uncoupled peptide mixture. (Magnification in a and b, ×400.) (c) An electron microscopic image of a single epithelial cell from a SMSIARL-GG-D(KLAKLAK)2-group prostate. The cell has sloughed off into the glandular lumen and massive destruction of its organelles is seen. (d) A representative micrograph of normal prostate shows intact cellular structure. (Magnification in c and d, ×6,000.) Light microscopy shows no damage to bladder (e; ×200), heart (f; ×400), kidney (g; ×400), or liver (h; ×400).
To effect sustained levels of the compounds used in the treatments, we used an implanted peristaltic pump for controlled release. Each pump was loaded with either SMSIARL-D(KLAKLAK)2 or an uncoupled mixture of SMSIARL and D(KLAKLAK)2. The animals were killed after 1 week, and their organs processed for histology. In another control experiment, we also implanted s.c. testosterone pellets to eliminate any variation in the sensitivity of prostate tissue caused by possible fluctuations in endogenous androgen levels (11). Seven days later, controlled release pumps loaded with the peptides were implanted on the peritoneal area opposite the pellets. SMSIARL-GG-D(KLAKLAK)2 consistently produced damage in the prostate (data not shown).
The tolerated dose of SMSIARLGG-D(KLAKLAK)2 was limited by acute toxicity of the compound; the dose could be increased by giving the injection slowly over several minutes. Mice injected with SMSIARL-GG-D(KLAKLAK)2, as well as those injected with equivalent amount of nonconjugated mixture of the homing peptide and proapoptotic peptide, showed marginal elevation of serum parameters of liver (ALT, AST, GGT) and kidney (creatinine and blood urea nitrogen) function. The levels returned to normal 1 week after the treatment. In one experiment, four mice that had been treated with four weekly injections of SMSIARL-GG-D(KLAKLAK)2 were allowed to mate. Vaginal plugs showed that mating had occurred and litters were born in each case. These results suggest that SMSIARL-GG-D(KLAKLAK)2 causes damage in the prostate, while other tissues are spared and the mice remain fertile.
We next analyzed the effect of a systemic SMSIARL-GG-D(KLAKLAK)2 treatment on the longevity of TRAMP mice (12). Two independent experiments gave similar results; one of the experiments is shown in Fig. 5. The SMSIARL-GG-D(KLAKLAK)2 survived significantly longer than the control groups that received the uncoupled peptides or buffer (P < 0.01 for both; Log Rank test).
Figure 5.
Survival of TRAMP mice treated with SMSIARL-GG-D(KLAKLAK)2 or control materials. The treatment was initiated at 12 weeks of age. Male mice (ten per group) received i.v. injections of SMSIARL-GG-D(KLAKLAK)2 peptide (200 μg per dose), or an equivalent dose of SMSIARL and (KLAKLAK)2 as uncoupled peptides (control-treated group). The injections were given once a week for a total of ten doses. The mice in the SMSIARL-GG- D(KLAKLAK)2 group survived significantly longer than the control mice treated with the uncoupled peptide mixture or with buffer.
Discussion
We show here that peptides selected from phage libraries for homing to the prostate vasculature reveal tissue-specific features in the blood vessels of the prostate. We also show that a peptide capable of homing to the blood vessels in the prostate can target a proapoptotic peptide to the prostate, and that systemic treatment with this targeted compound can cause destruction of prostate tissue and delay the development of prostate cancer in mice. Our results show that, like the vasculature of many other tissues analyzed in previous work (2–4), the vasculature of the prostate is biochemically distinct. The accumulation of the SMSIARL phage and fluorescein-labeled SMSIARL peptide in the prostate blood vessels after an i.v. injection indicates that this peptide binds selectively to the blood vessels in the prostate. The selective destruction of prostate tissue caused by targeting of a proapoptotic peptide to the prostate with the SMSIARL homing peptide supports this conclusion.
The molecular nature of the vascular specialization is incompletely understood. We have identified the receptor for a peptide that homes to lung vasculature as membrane dipeptidase (13). Others have shown that a modified von Willebrand factor promoter is activated in endothelial cells in a tissue-specific manner under the influence of the surrounding parenchymal tissue (14), providing one possible regulatory mechanism for the expression of tissue-specific endothelial markers. Perhaps prostate tissue induces receptors for SMSIARL in the resident endothelium. Although the molecule the SMSIARL peptide binds to in the prostate vasculature remains to be identified, our results suggest some practical applications.
The destruction of prostate tissue by the SMSIARL-targeted proapoptotic peptide is likely to be secondary to loss of blood vessels, the main target of the homing peptide. However, we cannot exclude a direct effect on prostate epithelial cells. The tissue damage was specific for the prostate, suggesting that it may be possible to develop a “medical prostatectomy” procedure based on this principle. Such a procedure could provide an alternative treatment for prostate hypertrophy. Furthermore, the proapoptotic peptide treatment postponed the development of prostate cancer in TRAMP mice. We attribute the effect in the TRAMP mice to a reduction in the number of target cells available for malignant transformation, because the SMSIARL peptide does not home to the vessels in the TRAMP tumors (W.H. and E.R., unpublished result). The lifespan extension in our treated TRAMP mice was 6–8 weeks, close to 20% of the lifespan, even though the treatment works against a tremendous oncogenic pressure in these transgenic mice (12, 15). In human terms, this would mean postponement of prostate cancer development for several years. A medical treatment that reduces the size of the prostate and at the same time delays the development of prostate cancer could be an extremely useful procedure.
Acknowledgments
We thank Dr. Norman Greenberg for providing TRAMP mice and Eva Engvall for comments on the manuscript. This work was supported by Grants DAMD17-99-1-8164 (to W.A.), DAMD17-98-8581 (to D.E.B.), and DAMD17-98-1-8562 (to E.R.) from the Department of Defense, research awards from CaP CURE (to W.A. and E.R.), and Grants CA74238 and CA82713 (to E.R.) and Cancer Center Support Grant CA30199 from the National Cancer Institute.
Abbreviation
- TRAMP
transgenic adenocarcinoma of the mouse prostate
References
- 1.Cotran R S, Kumar V, Collins T, editors. Robbins Pathological Basis of Disease. 6th Ed. Philadelphia: Saunders; 1999. [Google Scholar]
- 2.Pasqualini R, Ruoslahti E. Nature (London) 1996;380:364–366. doi: 10.1038/380364a0. [DOI] [PubMed] [Google Scholar]
- 3.Rajotte D, Arap W, Hagedorn M, Koivunen E, Pasqualini R, Ruoslahti E. J Clin Inv. 1998;102:430–437. doi: 10.1172/JCI3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arap W, Pasqualini R, Ruoslahti E. Science. 1998;279:377–380. doi: 10.1126/science.279.5349.377. [DOI] [PubMed] [Google Scholar]
- 5.Curnis F, Sacchi A, Borgna L, Magni F, Gasparri A, Corti A. Nat Biotechnol. 2000;18:1185–1190. doi: 10.1038/81183. [DOI] [PubMed] [Google Scholar]
- 6.Koivunen E, Wang B, Dickinson C D, Ruoslahti E. Methods Enzymol. 1999;245:346–369. doi: 10.1016/0076-6879(94)45019-6. [DOI] [PubMed] [Google Scholar]
- 7.Smith G P, Scott J K. Methods Enzymol. 1993;217:228–257. doi: 10.1016/0076-6879(93)17065-d. [DOI] [PubMed] [Google Scholar]
- 8.Hoffman J A, Laakkonen P, Porkka K, Ruoslahti E. In: Phage Display, a Practical Approach. Lowman H, Clarkson T, editors. Oxford: Oxford Univ. Press; 2002. , in press. [Google Scholar]
- 9.Javadpour M M, Lo W C, Bishop S M, Alberty J B, Corwell S M, Becker C L, McLaughlin M L. J Med Chem. 1996;39:3107–3113. doi: 10.1021/jm9509410. [DOI] [PubMed] [Google Scholar]
- 10.Ellerby H M, Arap W, Ellerby L M, Kain R, Andrusiak R, Del Rio G, Krajewski S, Lombardo C R, Ruoslahti E, Bredesen D E, Pasqualini R. Nat Med. 1999;5:1032–1038. doi: 10.1038/12469. [DOI] [PubMed] [Google Scholar]
- 11.Agus D B, Solde D W, Sgouros G, Bellanzrud A, Cordon-Cardo C, Scher H I. Cancer Res. 1998;58:3009–3014. [PubMed] [Google Scholar]
- 12.Gingrich J R, Barrios R J, Morton R A, Boyce B F, DeMayo F J, Finegold M J, Angelopoulou R, Rosen J M, Greenberg N M. Cancer Res. 1996;56:4096–4102. [PubMed] [Google Scholar]
- 13.Rajotte D, Ruoslahti E. J Biol Chem. 1999;274:11593–11598. doi: 10.1074/jbc.274.17.11593. [DOI] [PubMed] [Google Scholar]
- 14.Aird W C, Edelberg J M, Weiler-Guettlez H, Simmons W W, Smith T W, Rosenberg R D. J Cell Biol. 1997;138:1117–1124. doi: 10.1083/jcb.138.5.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsu C S, Ross B D, Chrisp C E, Derrowm S Z, Charles L G, Pienta K J, Greenberg N M, Zeng Z, Sandor M G. J Urol. 1998;160:1500–1505. [PubMed] [Google Scholar]