Abstract
Even during well-calibrated cognitive tasks, successive brain responses to repeated identical stimulations are highly variable. The source of this variability is believed to reside mainly in fluctuations of the subject's cognitive “context” defined by his/her attentive state, spontaneous thought process, strategy to carry out the task, and so on … As these factors are hard to manipulate precisely, they are usually not controlled, and the variability is discarded by averaging techniques. We combined first-person data and the analysis of neural processes to reduce such noise. We presented the subjects with a three-dimensional illusion and recorded their electrical brain activity and their own report about their cognitive context. Trials were clustered according to these first-person data, and separate dynamical analyses were conducted for each cluster. We found that (i) characteristic patterns of endogenous synchrony appeared in frontal electrodes before stimulation. These patterns depended on the degree of preparation and the immediacy of perception as verbally reported. (ii) These patterns were stable for several recordings. (iii) Preparatory states modulate both the behavioral performance and the evoked and induced synchronous patterns that follow. (iv) These results indicated that first-person data can be used to detect and interpret neural processes.
Framework
When a subject is stimulated during an experiment, his/her brain is not idle or in a state of suspension but is engaged in cognitive activity. The brain response is derived from the active interaction between this cognitive background and the stimulation that disturbs it: the neural response is “shaped” by the ongoing activity (refs. 1–5; see ref. 6 for review). As this ongoing state has not been carefully monitored, most of the brain response is not understood: successive exposure to the same stimulus elicits highly variable responses, and this variability is treated as unintelligible noise (6). Although it is common to control, at least indirectly, for some of the factors that condition this ongoing state, such as attention, vigilance, or motivation (for reviews, see refs. 7 and 8), the ongoing activity has not yet been analyzed systematically. One strategy would be to precisely describe the ongoing cognitive activity by obtaining refined verbal reports from human subjects. These should reveal subtle changes in the subject's experience (conditioned, for instance, by his/her cognitive strategy, attention level, and inner speech). This type of qualitative first-person data is usually omitted from brain-imaging studies. We show that if methodological precautions are taken when gathering first-person data, they can indeed be used to shed light on cognition via a joint analysis with quantitative measures of neural activity.
Collection of First-Person Data: Phenomenological Clusters (PhCs).
It is not easy to collect reports about inner experience, because verbal reports can be biased or untrue (9). The definition of a precise and rigorous method to collect first-person data is at the core of the active ongoing research program from which this study is derived (10, 11). To enable us to combine these data with neuroimaging data, we had to find recurrent patterns in the subjects' reports after multiple repetitions of the same experimental situation.
Subjects were trained extensively with a well-known illusory depth perception task (12). They underwent the task until they found their own categories to describe the phenomenological context in which they performed it and the strategies they used to carry it out. We chose this paradigm because the perception of a three-dimensional (3D) object arising from an autostereogram triggers a vivid phenomenal experience with identified neurobiological mechanisms (13). This practice session was used to improve the perceptual discrimination (14) and accuracy of the verbal report. After this training, we recorded both the electrical brain activity and the subject's own report of each trial. A few phenomenological classes were described a posteriori on the basis of the subject's own descriptions. These classes were used to divide the trials into groups called PhCs.
Integration of First-Person Data with Electroencephalogram (EEG) Data: Mutual Constraints.
We focused on the integration of first-person data with neuroimaging data as an attempt to explore the mutual constraints between these two types of description (this research program is referred to as a “neurophenomenology;” see ref. 10). The instantiation of this program is highly dependent on defining the adequate neural counterpart of the subject's experience. We chose the dynamic description of the transient patterns of local and long-distance synchrony occurring between oscillating neural populations as a dynamical neural signature (DNS). We focused on synchrony because of its putative role in the constitution of the transient networks that integrate distributed brain processes into highly ordered cognitive functions (as reviewed in refs. 6 and 15). Increasing evidence indicates that such coherent temporal patterns occur during the ongoing activity related to top-down factors such as attention, vigilance, or expectation. These factors can modulate the temporal structure of the neural responses to sensory stimulation (refs. 3–5; for review, see ref. 6). Such patterns of synchrony can be found in various brain rhythms (16) [theta (4–8 Hz), alpha (8–12 Hz), beta (12–30 Hz), and gamma (30–70 Hz)], suggesting that DNS should be studied in a wide frequency range.
We assumed that distinct cognitive contexts, described in the subjects' verbal reports, would translate into distinct DNSs before the stimulation, and that these DNSs would then differentially condition the behavioral and neural response to the stimulus. To test this hypothesis, we analyzed the behavioral and EEG (DNS) data for each PhC separately.
We found that the behavioral responses of the subjects (reaction time) and the DNSs before and after the stimulus differed among PhCs. Furthermore, characteristic patterns of phase synchrony, which were stable through several recordings, were recorded by the frontal electrodes before the stimulus. These patterns depended on the degree of preparation as reported by subjects. The preparation states modulated the subsequent evoked and induced synchronous responses. Therefore, we have shown that information about the ongoing cognitive context can partly be accessed in trained subjects by means of verbal reports, and that this information can be used to constrain the DNS analysis and account for an important amount of variability found therein.
Methods
Protocol.
Subjects.
We recorded four male subjects (S1, 28 years old; S2, 55 years old; S3, 53 years old; and S4, 28 years old) with normal or corrected-to-normal vision.
Task.
Trained subjects sat alone in a soundproof room and were shown random-dot static images subtending 18 × 26 cm on a digital monitor (refresh rate, 70 Hz) located 50 cm in front of them. The task began when the subjects fixed a dot pattern containing no binocular disparity (Fig. 1I). After an auditory signal, the subjects were asked to fuse two little squares at the bottom of the screen and to remain in this eye position for 7 sec. At the end of this preparation period, the random-dot pattern was changed to a slightly different random-dot pattern with binocular disparities (autostereogram; ref. 12). Subjects were readily able to see a 3D illusory geometric shape (depth illusion). They were instructed to press a button with their right hand as soon as the shape had completely emerged. This response ended the trial, after which the subjects gave a brief verbal report of their experience.
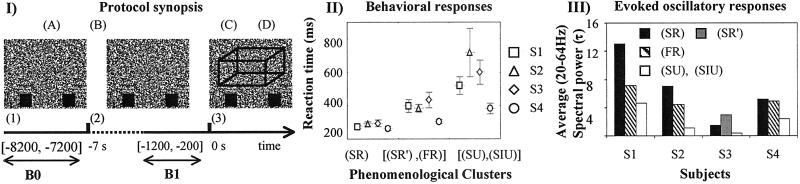
Figure 1.
(I) Protocol. Tasks: (A) Fixation of the center of the screen; (B) fusion of the two dots and refixation of the center of the screen; (C) motor response; and (D) phenomenological report. Events: (1) Presentation of an image without binocular disparities; (2) auditory warning at the beginning of B; (3) presentation of the autostereogram. (II) Reaction times. Mean reaction times between (3) and the motor response (D) with two standard errors. PhCs: SR and SR′, FR, SU, and SIU. (III) Evoked oscillatory responses. For each subject and each PhC, time-frequency power of evoked potential was normalized compared with baseline B1 and average across electrodes, time intervals [50, 150 ms], and frequencies (20–64 Hz).
Training of subjects.
To train the subjects, open questions were asked to try to redirect their attention toward their own immediate mental processes before the recordings were taken (10, 17). For example: Experimenter, “What did you feel before and after the image appeared?” Subject S1, “I had a growing sense of expectation but not for a specific object; however, when the figure appeared, I had a feeling of confirmation, no surprise at all;” or subject S4, “It was as if the image appeared in the periphery of my attention, but then my attention was suddenly swallowed up by the shape.” Subjects were reexposed to the stimuli until they found their own stable experiential categories (10) to describe the main elements of the cognitive context in which they perceived the 3D shapes. The categories were used to divide the individual trials into several PhCs.
Recordings.
EEG signals and phenomenological reports were recorded for the four subjects in two or three subsequent sessions. The number of trials ranged from 200 to 350 trials per subject, depending on the number of sessions needed to gather at least 40 trials for each PhC. EEG signals were collected from 62 electrodes at standard extended 10/20 positions and referenced to linked earlobes. They were analogically band-pass filtered between 0.16 and 160 Hz and sampled at 500 Hz. Horizontal and vertical eye movements and blinks were monitored by using bipolar electrodes (electro-oculogram, EOG).
Artifacts.
All results showing eye movements in EOG and EEG channels were excluded from the study. Ballistocardiographic artifacts were removed by using the Independent Component Analysis (18).
Data Analysis.
The synchronization of neural populations can be observed in the EEG at two complementary levels: either (i) “locally,” in the signal of a single electrode, or (ii) over a longer distance, between the signals of two electrodes.
(i) Local synchronization (time-frequency power emission) occurs when neurons recorded by a single electrode transiently oscillate at the same frequency with a common phase: their local electric fields add up to produce a burst of oscillatory power in the signal reaching the electrode. By averaging such emissions across successive responses to repeated stimulations, we can estimate the latencies and frequencies at which bursts are likely to occur. Such peaks of energy in average time-frequency maps are called “induced” synchronization patterns (for a review, see ref. 19). If the phase of these local oscillatory bursts remains constant across the trials, in other words is locked to the stimulus, then the synchronized oscillations are called “evoked” (for methods, see ref. 20). We measured evoked responses for each electrode by calculating the Pseudo Wigner-Ville transform of the evoked potential. This map was then z-transformed for each frequency and electrode by use of the mean and standard deviation of the map during the interval B1 (−1,200 ms, −200 ms); this is referred to as “normalization relative to B1′”). The induced responses were obtained for each electrode by averaging the Pseudo Wigner-Ville time-frequency map of all of the trials in a PhC normalized relative to B1. Statistical analyses were performed on the average over occipitoparietal electrodes (PO7-IZ) during [200, 400 ms]. We also quantified endogenous power emission (synchronous activity occurring before the stimulus) by use of the same technique as for the induced response but with a more distant baseline B0 = (−8,200, −7,200). They were normalized relative to the average activity over all trials in the interval preceding preparation B0. Statistical analyses were run on the average activity in each cluster of trials calculated during two intervals (B0 and B1) and in two groups of electrodes (posterior electrodes from TP7 to IZ and selected anterior electrodes from FP1 to FT8).
(ii) Long-distance synchrony can occur when two neural populations recorded by two distant electrodes oscillate with a precise phase relationship that remains constant during a certain number of oscillation cycles. The emergence of such large-scale neural assemblies is believed to result from long-range interactions between neural populations and may mediate the large-scale integration between functionally distinct neural processes (15). The methods used to measure long-range synchronization have been described elsewhere (21). In brief, for each trial and electrode, the instantaneous phase of the signal was extracted at each frequency of the interval [8–64 Hz] (in 4-Hz steps) by using a convolution with Morlet Wavelets. For each trial and pair of electrodes, the stability in time of their phase difference was subsequently estimated in consecutive sliding windows adapted to frequency (from four cycles for lower frequencies to eight cycles for higher frequencies). This method provided a measure of the raw synchrony for a given electrode pair and trial. This measure was then compared with a distribution of synchrony values obtained for 300 pairs of independent surrogate white-noise signals. This comparison was used to determine the episodes significantly higher of synchrony than what would be expected to occur between independent signals (P < 0.05; see ref. 21). The number of pairs above threshold was computed and averaged in a given time window to obtain a probability density in this time interval. The statistical analyses were performed for each cluster on the probability density during B0 and B1. When the alignment is higher (or lower) than the baseline, this is known as relative phase locking (or phase scattering). To study phase scattering during the responses to stimulation, the raw long-distance synchrony between each pair was normalized relative to B1 by standard deviation. The analysis was performed only on the pairs between the parietooccipital electrodes (TP7-IZ) that were below two standard deviations on average for [200, 400 ms].
Statistics.
Separate ANOVAs were run within subjects on both local and long-distance synchrony before and after stimulus. Before stimulus, the between-trials factor was (i) the PhC, and the within-trials factors were (ii) frequency (from 8 to 64 Hz in 4-Hz steps) and (iii) the time interval in which synchrony was measured (B0 = [−8,200, −7,200 ms], B1 = [−1,200, −200 ms]). For the analysis of local synchrony before the stimulus, another within-trial factor was added: (iv) the recording site (posterior vs. anterior electrodes). After the stimulus, the between-trials factor was (i) the PhC, and the within-trials factor was (ii) frequency (from 8 to 64 Hz in 4-Hz steps).
Results
PhCs.
The verbal reports were classified according to the degree of preparation felt by the subject and the quality of his/her perception; we used this factor to cluster the trials, as listed below. Subcategories describing the unfolding of the visual perception for instance were found in individuals. They will not be studied in this report.
Steady readiness (SR).
In most trials, subjects reported that they were “ready,” “present,” “here,” or “well-prepared” when the image appeared on the screen, and that they responded “immediately” and “decidedly.” Perception was usually experienced with a feeling of “continuity,” “confirmation,” or “satisfaction.” These trials were grouped into a cluster SR, characterized by the subjects being in a state of “steady readiness.”
Fragmented readiness (FR).
In other trials, subjects reported that they had made a voluntary effort to be ready but were prepared either less “sharply” (because of a momentary “tiredness”) or less “focally” (because of small “distractions,” “inner speech,” or “discursive thoughts”). The emergence of the 3D image was experienced with a small feeling of surprise or “discontinuity.” These trials formed a second cluster FR corresponding to a state of “fragmented readiness.”
An intermediate cluster between SR and FR was defined for subject S3, corresponding to stable readiness SR′. This was described as a state of open attention without active preparation, unique to this subject, who found that this state contrasted sharply with that of prepared SR.
Unreadiness [spontaneous unreadiness (SU), self-induced unreadiness (SIU)].
In the remaining trials, subjects reported that they were unprepared, and that they saw the 3D image only because their eyes were correctly positioned. They were surprised by it and reported that they were “interrupted” by the image in the middle of a thought (memories, projects, fantasies, etc.). This state of distraction occurred spontaneously for S1 and S4, whereas S2 and S3 triggered it by either fantasizing or thinking about plans (subject 3) or by visualizing a mental image (subject 2). To separate passive and active distraction, these trials were divided in two different clusters: SU for S1 and S4, and SIU for S2 and S3.
Correlations Between First-Person and Behavioral Data.
Reaction times depended on the degree of preparation reported by the subjects. One-way ANOVA analyses within subjects revealed that PhCs had a significant effect on reaction time [F(2,316) = 70.4, P < 0.001); F(2,132) = 32.4, P < 0.001); F(2,194) = 45.9, P < 0.001); F(2,220) = 36.7, P < 0.001]. The reaction times were longer when the subjects were less prepared: {contrast analysis: SR vs. FR (F = [42.1; 7.4; 12.3], P < 0.001) FR vs. SU, SIU (F = [25.6; 11.0; 51; 38.3], P < 0.001) (Fig. 1II)}. To compare the clustering on the basis of verbal reports and to see whether the trials could be clustered differently on the basis of behavior, trials were also clustered into three groups (of size equivalent to the PhCs) on the basis of reaction time. We found an overlap ratio of 65% between the two types of clusters, significantly larger that would have been obtained by chance (33%).
Effect of PhC on Neurodynamical Measures Before Stimulus.
We found that local and long-range synchrony occurred at different frequencies before the stimulus depending on the degree of readiness reported by the subjects. For all subjects, there was a significant interaction between the PhC factor and the frequency factor of the average long-distance synchrony [F(28,4424) = 1.6, P < 0.02; F(28,1848) = 1.9, P < 0.005; F(28,2716) = 3.9, P < 0.001; F(28,3080) = 1.7, P < 0.01] and of power emission (F(28,4424) = 3.5, P < 0.001; F(28,1848) = 6.8, P < 0.001; F(28, 2716) = 2.4, P < 0.001; F(28, 3080) = 2.3, P < 0.001].
Trials in which the subjects reported a stable state of preparation were marked by a sustained and self-induced pattern of both local and global synchrony over the frontal electrodes. In clusters with SR, a contrast analysis of the time window factor of power emission revealed that the energy in the gamma band increased from B0 to B1 in all subjects [F(1,316) = 9.3, P < 0.01; F(1,132) = 7.2, P < 0.01; F(1,194) = 3.6, P < 0.05; F(1,220) = 3.9, P < 0.05], whereas it decreased in the lower (8- to 16-Hz) range [F(1,316) = 11.5, P < 0.001; F(1,132) = 14.9, P < 0.001; F(1,194) = 6.1, P < 0.02; F(1,220) = 6.5, P < 0.01]. This frontal pattern is presented for all subjects in Fig. 3, and the emergence of the long-distance synchrony pattern is illustrated in Fig. 2 for S1.
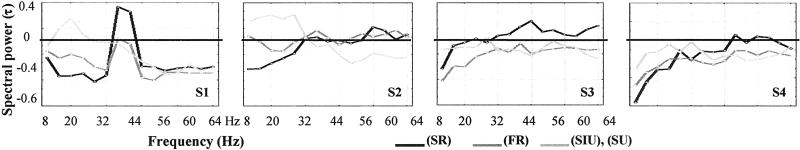
Figure 3.
Correlation between first-person data and time-frequency power emission. For each subject and PhC, spectral distribution of power emission was normalized compared with the activity for trials in B0 and averaged in time window B1 and for selected frontal electrodes
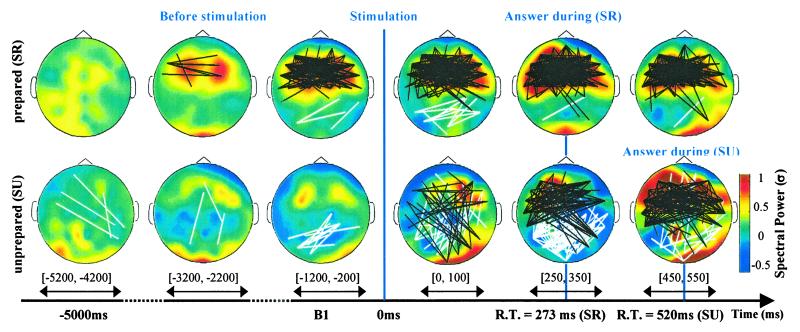
Figure 2.
DNS for S1 during readiness with immediate perception SR (154 trials) and SU with surprise during stimulation (38 trials). Color coding indicates scalp distribution of time-frequency gamma power around 35 Hz normalized compared with distant baseline B0 average for trials and for time windows indicated by an arrow. In prepared trials, gamma power in frontal electrodes (FP1-FT8) during B1 increased significantly (P < 0.01) compared with distant baseline B0 and was significantly higher (P < 0.005) than in the unprepared trials. Black and white lines correspond to significant increase and decrease in synchrony, respectively. For each pair of electrodes, the density of long-distance synchrony above a surrogate threshold was calculated (see Statistics). This measure was normalized compared with the distribution for trials in baseline B0. A significant threshold was estimated with white-noise surrogates (35).
This energy shift toward the gamma band was specific to the “prepared” PhCs. The energy in the gamma band was always higher during B1 for subjects in the prepared clusters than for subjects in the unprepared clusters [F(1,316) = 8.4 P < 0.005; F(1,132) = 4.7 P < 0.05; F(1,194) = 46 P < 0.001; F(1,220) = 6.4 P < 0.02], whereas it was lower for S1, S2, and S4 in the slower (8- to 16-Hz) band [F(1,316) = 8.3 P < 0.005; F(1,132) = 48 P < 0.001; F(1,220) = 12 P < 0.001]. These results suggest that the deployment of attention during the preparation strategy was characterized by an enhancement of the fast rhythms in combination with an attenuation of the slow rhythms.
Effect of PhC on the Neurodynamical Measurements During the Perception of the 3D Illusion.
Evoked oscillatory responses.
The amplitudes of the evoked responses were normalized relative to B1 and averaged over the fast rhythms ([20–64 Hz]) (as suggested in ref. 6). Evoked responses increased with the reported degree of preparation (Fig. 1III). They were higher in SR and SR′ than in SU or SIU. These data suggest that the evoked response is modulated by the cognitive context in which the stimulation occurs.
Induced oscillatory responses.
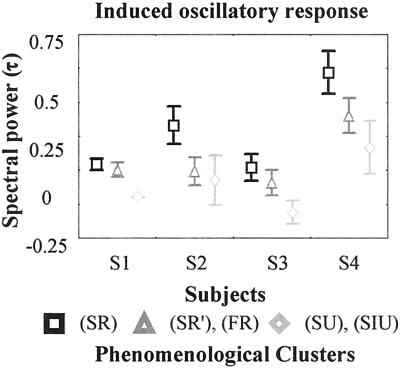
The topography (Fig. 2), frequency span, and time course (Fig. 2) of the induced responses differed between different PhCs. We found that the induced response in the gamma band recorded by the occipitoparietal electrodes (PO7-IZ) was modulated by the degree of preparation: a contrast analysis (Fig. 4) showed that the mean amplitude (normalized relative to B1) recorded by the posterior electrodes in the [200, 400 ms] × [30- to 64-Hz] window was larger in SR and SR′ than in SU and SIU [F(1,316) = 4.2 P < 0.05; F(1,132) = 17 P < 0.001; F(1,194) = 3.6 P < 0.05; F(1,220) = 3.5 P < 0.05].
Figure 4.
Mean induced oscillatory responses for all subjects and PhCs in occipitoparietal electrodes in gamma frequency band normalized compared with B1. Error bars represents one standard error (see Statistics).
We found that the phase-scattering recorded by the occipitoparietal electrodes (P7-IZ) was also modulated by the degree of preparation. There was a significant interaction between the PhC factor alone or the PhC factor and the frequency factor of the density of electrode pairs below two standard deviations compared with B1 for [200, 400 ms] [F(28,4424) = 1.5, P < 0.05; F(2,132) = 6.2, P < 0.01; F(2, 194) = 3.6, P < 0.05; F(28, 3080) = 1.6, P < 0.05].
Individual Differences and Their Stability Through Recordings.
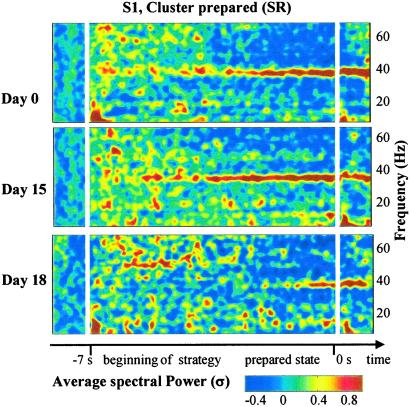
Apart from the patterns common to all of the subjects mentioned above, we found that the topography, frequency, and time course of the synchrony patterns during the preparation period varied widely across subjects. However, these variations should not be treated as “noise,” because they seem to indicate intrinsic differences between individuals that remained stable for several days. For example, the gamma emission (at 35 Hz) in the anterior electrodes of S1 was accompanied by a simultaneous decrease in higher frequencies (44–64 Hz) compared with B0 [contrast analysis: F(1,316) = 62.5, P < 0.001]. This pattern was not observed in the other subjects (see Fig. 3), but it remained stable throughout three recording sessions (Fig. 5) {contrast analysis: around 36 Hz, F(1,310) = [23.1, 32.1, 3.8], P < [0.001, 0.001, 0.05], in [44–64 Hz] F(1,310) = [8.7, 18.9, 8.7], P < [0.005, 0.001, 0.005]}.
Figure 5.
Stability of DNSs for recordings for S1 during SR in frontal electrodes (FP1 to FT8) with significant increase at 36 Hz and decrease between [44–64 Hz] during B1 for every recording (59, 60, and 35 trials, respectively) (see Results). S1 reported to be globally less focused during the third recording than during the second one. Color coding indicates scalp distribution of time-frequency gamma power normalized compared with distant baseline B0 averaged for trials and electrodes.
The DNSs were generally stable in each subject. As each subject was recorded two (S2 and S3) or three times (S1 and S4), we tested whether the day of recording interacted with the PhC. ANOVA analysis within subjects on local synchrony revealed no significant interactions during preparation [factors PhC, time interval (B0, B1), frequency, recording site, and day of recording] (Fig. 5).
These results suggest that DNSs are stable throughout several recordings and that strategies or aptitudes to do the task are characterized in intersubject variations.
Distant vs. Proximal Baseline.
The use of the distant baseline, B0, revealed a stable and sustained oscillatory mode during preparation in subject S1 (Fig. 5). This baseline provided the opportunity to investigate the relationship between the prestimulation pattern and the brain responses in detail (Fig. 2). When the subject reported that he was prepared and that he immediately saw the illusion, a frontal pattern of synchrony gradually emerged in the gamma band several seconds before the stimulus. This contextual activity was still present during the perception and motor response and was mixed with the frontooccipital long-distance synchrony induced by the stimulus. In contrast, when the subject was unprepared and surprised by the arrival of the stimulus, there was no stable pattern in the gamma band before the stimulation (significant long-distance synchrony is directly displayed on the figure): transitory patterns of synchronies emerged after stimulation, in discontinuity with the prestimulation activity. The effect of surprise was associated with a different temporal structure in the neural response, combining phase-scattering (white lines) and an increase in synchrony (black lines). The motor response was accompanied by patterns of synchronies that were spatially similar to those observed during preparation but were delayed by 300 ms.
Discussion
A Coupling Between Three Levels of Description: Behavioral, Neurophysiological, and First-Person Data.
We have shown that (i) the verbal descriptions of the subjects' cognitive contexts were related to stable local and global synchrony patterns measured in their EEG recordings before the stimulus; (ii) the states of preparation and perception, as reported by the subjects, modulated both the behavioral responses and the dynamic neural responses after the stimulation; and (iii) although the precise shape of these synchrony patterns varied between subjects, they were stable in individual subjects throughout several recording sessions; they therefore seem to constitute a consistent signature of a subject's cognitive strategy. These results demonstrate a relationship between behavioral, neurophysiological, and first-person data.
What Is the Status of First-Person Data?
This study draws on the regaining of interest in so-called first-person methods in the study of cognition and consciousness (11). A number of novel methods derived from psychology, phenomenology (22), and other areas (23) have begun to extract data from subjective experiences. The objective is to pay more meticulous attention to the intimate and direct knowledge that a subject has about his/her experience and to access this knowledge in a sufficiently controlled manner so that it is compatible with more traditional methods for the collection of neural data.
We explored ways of using first-person data to understand EEG data better. Most EEG studies rely on averaging techniques—across trials and often across subjects. These techniques are very effective in finding the major components of neural activity. But averaging also cancels out the highly variable and spontaneous EEG. It is unintelligible because we cannot relate it to the well-identified cognitive processes involved in specific behavior in a predictable way. Most brain-imaging studies impose a strict experimental protocol to try to constrain exogenously the chain of cognitive processes that occur so that the neural data can be related to the assumed processes. Yet even very precise protocols do not fully constrain the brain's activity: fluctuations of the subject's emotional state, attention, and even strategy always occur and cannot be fully controlled. However, they can be reported and taken into account to some extent.
We have taken two steps in this direction by (i) keeping a trial-by-trial account of the subject's reports, and (ii) using the subject's own categories to organize the trials into clusters with similar experiential features. This strategy can be seen as an extension of the traditional procedure in cognitive science based on the use of verbal reports and questionnaires after the experiment (24). One important difference is that we collected data from subjects who had been trained to both perform the task and make a precise verbal report immediately after each trial. The challenge is to capture the experience “on the fly” to study the mutual constraints between the first person and the neurophysiological data (10, 25). In our study, mutual constraints were tested and instantiated in the implementation of PhCs to guide the analyses of neurophysiological data.
However, our study is clearly only a first step; further refinement is needed to capture the potential richness of even this simple perceptual experience. Thus, this study should be considered as an initial basic example in the context of the wider scope of this approach. The more ambitious goal is to find a rigorous way to integrate a more sustained and careful examination of subjective experience, including its temporal structure.
First-Person Clusters or Behavioral Clusters?
We found that the clusters obtained via verbal reports largely overlapped with the clusters based on reaction time alone. This observation left us with a choice of which data to use to group the trials into clusters. The reaction time is a quantitative but one-dimensional indirect measure, which furthermore may not solely depend on preparation. Conversely, the subject's own verbal report may be qualitative and introspective but provides multidimensional knowledge about the texture and structure of conscious experience that can effectively constrain the analysis and interpretation of neurodynamical data. We chose first-person data because our goal was not simply to characterize behavioral responses but also to find neural correlates of ongoing consciousness states during the task. This decision may be controversial for the estimation of readiness, but we believe it should impose itself in future studies trying to capture more sophisticated aspects of experience.
DNS as Dynamical Imaging.
These results were obtained by combining first-person data and powerful data analysis techniques. We introduced the DNS, which is defined as the mapping through time, space, and frequency of local and long-distance synchrony patterns in a specific PhC. This measure offers a novel dynamical view of both the endogenous neural activity preceding the stimulation and its modulation by the stimulation, in contrast to classical studies of brain responses (19). It seems particularly useful when calculated on the specific PhCs, adapted to each subject. In this case, it revealed multiband patterns that would have been lost by averaging. The direct averaging of all subjects and trials might blur the complex and variable balance between increases and decreases of self-induced activity inherent in each subject's strategy.
Even if an absolute baseline cannot be defined, the choice of baseline is important in the characterization of DNSs, because it allows one to contrast differently the context provided by the state of preparation. The proximal baseline B1 can enhance the contrast between the synchronous process immediately preceding the arrival of the stimulation and those triggered by the stimulation. In contrast, the distant baseline B0 can reveal resemblance in synchrony patterns between prestimulus activity and the response induced by stimulation.
It is clear that DNS, as proposed in this paper, is a first step in characterizing neural correlates of complex mental states. Further work is needed to improve this strategy. For example, the lack of spatial resolution of the EEG makes it difficult to interpret the synchrony patterns directly in terms of precise neural networks (26). Also, neural interactions may take multiple forms that are not detected by linear measures such as synchrony (27).
Despite these limitations, which may give a simplified view of the neural basis of experience, this strategy has already provided some insight into the large-scale integration problem. This problem deals with mechanisms that select and coordinate distributed brain activity to produce a flow of adapted and unified cognitive moments (15, 28).
Shaping of Endogenous Neural Activity During Preparation.
We found that the frontal spectral emissions and long-distance synchronies peak into specific frequencies that vary with the degree of preparation. These patterns were stable during the 1-sec interval preceding the stimulus. This frontal activation is consistent with the role of the prefrontal cortex in the top-down modulation of attention (29), action goals (30), or in the maintenance of spatial visual information in working memory (31).
Balance Between Phase-Locking and Phase-Scattering.
In contrast with the increase in synchrony between the frontal electrodes, we observed an active decrease in synchrony (phase-scattering) between some occipital electrodes after the stimulus, modulated by the preparation state of the subjects. Similar phase-scattering effects have already been observed in a face-perception task (20) that punctuated the transition between two episodes of synchrony corresponding to the actual perception and the motor response. Our study provides additional support that such phase scattering could be a necessary transition between two very distinct neural patterns, in particular during the adaptive response to a salient change in sensory flow.
We also recorded a decrease in emission during preparation in one subject in gamma (>45 Hz) in frontal electrodes associated with an increase in gamma at 35 Hz. This observation suggests that this active uncoupling occurred concomitantly with the phase synchrony in another high frequency, emphasizing the competitive side of emergence of synchronous assemblies (32).
Implications for Consciousness Studies.
We found that the perception of 3D stereograms was accompanied by an induced gamma response over the occipital and parietal electrodes, in agreement with Revonsuo et al. (33). We confirmed that this induced response is modulated by attention (6, 8). We also showed that when the subject was expecting the stimulus, this pattern in the gamma band was dynamically linked with the frontal electrodes synchronized before stimulus. In contrast, this occipitoparietal pattern was weaker and was interrupted by phase scattering when the subject was unprepared.
It seems appropriate, therefore, to redefine the temporal interval of interest for a neural correlate of a conscious act. The correlate of depth perception obviously occurs between the appearance of the stimulus and the motor response. Yet this moment of consciousness extends from a previous one, a horizon of anticipation and expectation (34), that cannot be seen as neutral. The characterization of both the ongoing activity preceding the stimulation and the activity following it appears necessary for a complete description of the dynamics of a moment of consciousness. In this respect, the brain's response and its phenomenological correlate must result from the intertwining of the endogenous activity with its corresponding phenomenological distinctions and the peripheral afferent activity evoked by the stimulation.
Acknowledgments
In Memoriam: A.L., J.-Ph.L., and J.M. dedicate this work to the memory of Francisco Varela, who profoundly shaped their ideas about the relationship between embodied conscious states and brain dynamics. For an obituary, see http://psyche.csse.monash.edu.au/v7/psyche-7-12-thompson.html. For helpful discussion, we are grateful to the neurodynamic team of LENA: N. Depraz, A. Cohen-Varela, and E. Thompson. We thank D. Rudrauf for advice on statistical analyses. This work was supported by the National French Research Center (CNRS) and the Fetzer Institute.
Abbreviations
- 3D
three-dimensional
- EEG
electroencephalogram
- DNS
dynamical neural signature
- PhC
phenomenological cluster
- SR
steady readiness
- FR
fragmented readiness
- SU
spontaneous unreadiness
- SIU
self-induced unreadiness
References
- 1.Arieli A, Sterkin A, Grinvald A, Aertsen A. Science. 1996;273:1868–1871. doi: 10.1126/science.273.5283.1868. [DOI] [PubMed] [Google Scholar]
- 2.Riehle A, Grun S, Diesmann M, Aertsen A. Science. 1997;278:1950–1953. doi: 10.1126/science.278.5345.1950. [DOI] [PubMed] [Google Scholar]
- 3.Fries P, Neuenschwander S, Engel P, Goebel R, Singer W. Nat Neurosci. 2001;4:194–200. doi: 10.1038/84032. [DOI] [PubMed] [Google Scholar]
- 4.Fries P, Reynolds J H, Rorie A E, Desimone R. Science. 2001;291:1560–1563. doi: 10.1126/science.1055465. [DOI] [PubMed] [Google Scholar]
- 5.Steinmetz P N, Roy A, Fitzgerald P J, Hsiao S S, Johnson K O, Niebur E. Nature (London) 2000;404:187–190. doi: 10.1038/35004588. [DOI] [PubMed] [Google Scholar]
- 6.Engel A K, Fries P, Singer W. Nat Rev Neurosci. 2001;10:704–716. doi: 10.1038/35094565. [DOI] [PubMed] [Google Scholar]
- 7.Desimone R, Duncan J. Annu Rev Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- 8.Kastner S, Ungerleider L G. Annu Rev Neurosci. 2000;23:315–341. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- 9.Hurlburt R, Heavey C. Trends Cognit Sci. 2001;5:400–403. doi: 10.1016/s1364-6613(00)01724-1. [DOI] [PubMed] [Google Scholar]
- 10.Varela F. J Consc Studies. 1996;3:330–350. [Google Scholar]
- 11.Varela F, Shear J. The View from Within. London: Imprint Academic; 1999. [Google Scholar]
- 12.Julesz B. Foundations of Cyclopean Perception. Chicago: Univ. of Chicago Press; 1971. [Google Scholar]
- 13.DeAngelis G C. Trends Cognit Sci. 2000;4:80–90. doi: 10.1016/s1364-6613(99)01443-6. [DOI] [PubMed] [Google Scholar]
- 14.Gold J, Bennett P, Sekuler A. Nature (London) 1999;402:176–178. doi: 10.1038/46027. [DOI] [PubMed] [Google Scholar]
- 15.Varela F, Rodriguez E, Lachaux J P, Martinerie J. Nat Rev Neurosci. 2001;230:229–239. doi: 10.1038/35067550. [DOI] [PubMed] [Google Scholar]
- 16.von Stein A L, Sarnthein J. Int J Psychophysiol. 2000;38:301–313. doi: 10.1016/s0167-8760(00)00172-0. [DOI] [PubMed] [Google Scholar]
- 17.Vermersch P. In: The View from Within. Varela F, Shear J, editors. London: Imprint Academic; 1999. pp. 17–42. [Google Scholar]
- 18.Makeig S, Jung T-P, Bell A J, Sejnowski T J. Proc Natl Acad Sci USA. 1997;94:10979–10984. doi: 10.1073/pnas.94.20.10979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tallon-Baudry C, Bertrand O. Trends Cognit Sci. 1999;3:151–161. doi: 10.1016/s1364-6613(99)01299-1. [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez E, George N, Lachaux J P, Martinerie J, Varela F J. Nature (London) 1999;397:340–343. doi: 10.1038/17120. [DOI] [PubMed] [Google Scholar]
- 21.Lachaux J P, Rodriguez E, Le Van Quyen M, Lutz A, Martinerie J, Varela F J. Int J Bifurc Chaos. 2000;12:2608–2622. [Google Scholar]
- 22.Depraz N. In: The View from Within. Varela F, Shear J, editors. London: Imprint Academic; 1999. pp. 95–110. [Google Scholar]
- 23.Wallace A. In: The View from Within. Varela F, Shear J, editors. London: Imprint Academic; 1999. pp. 175–187. [Google Scholar]
- 24.Ericsson K A, Simon H A. Protocol Analysis, Verbal Protocols as Sata. Cambridge, U.K.: MIT Press; 1993. [Google Scholar]
- 25.Thompson E, Varela F. Trends Cognit Sci. 2001;5:418–425. doi: 10.1016/s1364-6613(00)01750-2. [DOI] [PubMed] [Google Scholar]
- 26.Nunez P L, Srinivasan R, Westdorp A F, Wijesinghe R S, Tucker D M, Silberstein R B, Cadusch P J. EEG Clin Neurophysiol. 1997;103:499–515. doi: 10.1016/s0013-4694(97)00066-7. [DOI] [PubMed] [Google Scholar]
- 27.Kantz H, Schreiber T. Non-Linear Series Analysis. Cambridge, U.K.: Cambridge Univ. Press; 1997. [Google Scholar]
- 28.Dehaene S, Kerszberg M, Changeux J P. Proc Natl Acad Sci USA. 1998;95:14529–14534. doi: 10.1073/pnas.95.24.14529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dias R, Robbins T W, Roberts A C. Nature (London) 1996;380:69–72. doi: 10.1038/380069a0. [DOI] [PubMed] [Google Scholar]
- 30.Miller E K, Cohen J D. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 31.Rao S R, Rainer G, Miller E K. Science. 1997;276:821–823. doi: 10.1126/science.276.5313.821. [DOI] [PubMed] [Google Scholar]
- 32.Milner P M. Psychol Rev. 1974;81:521–535. doi: 10.1037/h0037149. [DOI] [PubMed] [Google Scholar]
- 33.Revonsuo A, Wilenius-Emet M, Kuusela J, Lehto M. NeuroReport. 1997;8:3867–3870. doi: 10.1097/00001756-199712220-00006. [DOI] [PubMed] [Google Scholar]
- 34.Varela F. J Consc Studies. 1999;6:111–140. [Google Scholar]
- 35.Le Van Quyen M, Foucher J, Lachaux J-Ph, Rodriguez E, Lutz A, Martinerie J, Varela F J. J Neurosci Methods. 2001;111:83–98. doi: 10.1016/s0165-0270(01)00372-7. [DOI] [PubMed] [Google Scholar]