Abstract
The cerebellum is considered a brain structure in which memories for learned motor responses (e.g., conditioned eyeblink responses) are stored. Within the cerebellum, however, the relative importance of the cortex and the deep nuclei in motor learning/memory is not entirely clear. In this study, we show that the cerebellar cortex exerts both basal and stimulus-activated inhibition to the deep nuclei. Sequential application of a γ-aminobutyric acid type A receptor (GABAAR) agonist and a noncompetitive GABAAR antagonist allows selective blockade of stimulus-activated inhibition. By using the same sequential agonist and antagonist methods in behaving animals, we demonstrate that the conditioned response (CR) expression and timing are completely dissociable and involve different inhibitory inputs; although the basal inhibition modulates CR expression, the conditioned stimulus-activated inhibition is required for the proper timing of the CR. In addition, complete blockade of cerebellar deep nuclear GABAARs prevents CR acquisition. Together, these results suggest that different aspects of the memories for eyeblink CRs are encoded in the cerebellar cortex and the cerebellar deep nuclei.
Keywords: learning‖memory‖cerebellar deep nuclei‖cerebellar cortex‖ γ-aminobutyric acid
Because of the highly organized cytoarchitecture of its numerous neurons, the cerebellum has long been considered as a neuronal substrate for some forms of learning and memory (1–4). Accumulating evidence supports this view by demonstrating the critical roles of the cerebellum in motor learning (5–10). On the basis of empirical results and theoretical models, the basic neural circuitries underlying motor learning, such as classical conditioning and vestibulo-ocular-reflex adaptation, have been delineated (6, 8, 9). For example, in classical eyeblink conditioning, mossy fibers (mf) and climbing fibers (cf) are thought to convey conditioned stimulus (CS) and unconditioned stimulus (US) signals, respectively, to both the cerebellar cortex and the deep nuclei. Various types of synaptic plasticity have been demonstrated in these regions, including parallel fiber-Purkinje cell long-term depression (pf-PC LTD) (11, 12) and long-term potentiation (pf-PC LTP) (13), pf-PC γ-aminobutyric acid (GABA)-transmitting rebound potentiation (14), PC-deep nuclear neuron (DNN) LTD (15, 16), and LTP of extracellular responses in the cerebellar deep nuclei (17). These different types of plasticity may be involved in supporting learning in both the cerebellar cortex and deep nuclei (10, 18, 19).
Although the early theoretical models only concerned the cerebellar cortex, recent studies implicated both the cortex and the deep nuclei in motor learning. Discrete lesions of the cerebellar interpositus nucleus (electrolytically, chemically, reversibly) consistently and completely prevent the acquisition and permanently and completely abolished retention/expression of classically conditioned eyeblink responses in rabbits, rats, and mice (20–28). Cortical lesions affect various aspects of learning including learning rate, asymptotic learning level, learning-dependent response timing, and immediate retention, but do not completely and permanently abolish learning (refs. 25 and 29–34, but see ref. 35). Thus, the synaptic plasticity underlying eyeblink conditioning seems to occur in both the cortex and the deep nuclei (8, 9, 10, 36).
The cerebellar DNN receives converging inhibitory GABAergic inputs from up to 30 PCs (37), each of which spontaneously discharges action potentials (APs) at frequencies up to 100 Hz (38, 39). Thus, the DNNs receive constant basal inhibition from PCs. When activated by a CS through the mf-pf-PC pathway, PCs change discharge rate in a variety of temporal patterns (40, 41). During learning, cortical plasticity such as pf-PC LTD is thought to reduce synaptic input to a subset of PCs upon which the pf and cf inputs are coincidental (42), and alter the temporal patterns of the cortical inhibition on the DNNs.
In this study, we first examined some basic physiological properties of the DNNs in slices prepared from the mouse cerebellum. We showed that perfusing the slices with muscimol (a GABAergic agonist) maximally hyperpolarized DNNs and occluded synaptic GABAA responses. Subsequent picrotoxin (PTX, a GABAergic antagonist) applications gradually depolarized DNNs without recovering synaptic GABAA responses. Thus, sequential applications of muscimol and PTX allow gradual adjustment of tonic inhibition on the DNNs while completely blocking synaptic inhibition from cerebellar Purkinje cells. This approach was then used in behaving rabbits to dissect the tonic and stimulus-activated inhibition on the DNNs in classical eyeblink conditioning.
Experimental Procedures
Electrophysiological Recording.
C57BL6 mice (male, 10–15 days old, The Jackson Laboratory) were decapitated, and the cerebellum was extirpated, placed in ice-cold artificial cerebrospinal fluid (ACSF; 124 mM NaCl/3 mM KCl/1.25 mM KH2PO4/3 mM CaCl2/1 mM MgCl2/26 mM NaHCO3/10 mM glucose, saturated with 5% CO2 and 95% O2), and sliced coronally (400 μm) by using a vibrotome. The slices were incubated in ACSF for at least 30 min before recording. All experiments were conducted at room temperature (25°C).
Cerebellar DNNs were recorded with whole-cell patch pipette (3–7 MΩ when filled with internal solutions) as described (16). The “blind” technique was used to form tight (>1 GΩ) seals (43). The recording pipette was lowered to the cerebellar deep nuclei that were identified with a dissecting microscope. Neurons were identified by their spontaneous APs (44) or voltage-activated currents. A K-Gluconate-based internal solution (140 mM K-Gluconate/4.5 mM MgCl2/9 mM EGTA/24 mM Hepes/4 mM ATPNa2/0.3 mM GTPNa3, pH 7.3) was used to record excitatory and inhibitory postsynaptic potentials (EPSPs and IPSPs, respectively) with whole-cell configuration current-clamp mode, and a CsCl-based internal solution (140 mM CsCl/2 mM MgCl2/5 mM EGTA/10 mM Hepes/4 mM ATPNa2/0.3 mM GTPNa3, pH 7.3) was used to record IPSCs with whole-cell configuration voltage-clamp mode and cell discharges with cell-attached configuration voltage-clamp mode. Seal resistance (in cell-attached mode) or input and access resistance (in whole-cell mode) were monitored frequently with a 10-ms, 5-mV hyperpolarizing step during the entire experiment. For cell-attached recordings, the holding potentials were adjusted so that the holding current was zero. To evoke synaptic responses, biphasic stimulation was delivered through a coaxial electrode placed on the white matter close to the recording site. Kynurenic acid (2 mM), an ionotropic glutamate receptor antagonist, was used to isolate GABAergic synaptic responses. To record EPSPs and IPSPs in the same cells, the cells were current-clamped either at Cl− reversal potential, or at reversal potential of glutamatergic synaptic responses. Cell discharges or synaptic responses were amplified with Axopatch-1D (Axon Instruments, Foster City, CA), digitized with TL-1 (Axon Instruments), and stored (10 kHz) and analyzed with PCLAMP6 (Axon Instruments).
Surgery.
New Zealand White rabbits (2.0–2.4 kg) were initially anesthetized with Xylazine (8.0 mg/kg) and ketamine (60 mg/kg), and subsequently maintained on 1% halothane during the surgery. The head of the rabbit was positioned onto a stereotaxic device so that bregma was 1.5 mm higher than lambda. A guide cannula (22 gauge) was lowered to the left dorsal anterior cerebellar interpositus nucleus (0.8 mm anterior, 5.2 mm lateral, 14.5 mm ventral from lambda) through a burr hole drilled on the skull. The cannula was held in place with dental acrylic anchored to the skull with stainless steel skull screws. A small receptacle designed to hold a minitorque potentiometer was also attached the skull. All rabbits were allowed 1-week recovery after surgery.
Behavioral Training.
The general procedures for rabbit eyeblink conditioning have been described in detail (26). In brief, 1 day after a 1-h adaptation to restraint and apparatus, rabbits were given daily training of 100 trials grouped into 10 blocks. Each block consisted of a CS alone (first) trial, a US alone (sixth) trial, and eight CS-US paired trials. The CS was a tone (350 ms, 85 dB, 1 kHz), and the US was a corneal airpuff (100 ms, 3 psi) to the left eye, ipsilateral to the cannula implant. In the paired CS-US trial, the CS preceded the US by 250 ms, and coterminated with the US. The eyeblink responses were recorded with a minitorque potentiometer attached to the rabbit's left nictitating membrane through a suture loop. The training, data acquisition, and analysis were accomplished by using a custom-made computer system (45). A greater than 0.5-mm nictitating membrane movement during the 250-ms CS-US interstimulus interval was registered as a CR. The CR onset was defined as the first moment at which the CR amplitude exceeded 0.5 mm. The CR peak latency was defined as the time at which the CR amplitude was maximal.
Two groups of rabbits (n = 7 for experimental group, n = 6 for control group) were trained until they reached 90% CRs. On the next day, the rabbits were first given two blocks of training to assess the predrug CR baseline. Then, all rabbits were infused with 10 nmol muscimol in 1 μl ACSF (at a rate of 0.13 μl/min) into their left cerebellar interpositus nucleus. After the muscimol infusion, the training was resumed for another two blocks to assess CRs. After the second two blocks, for the experimental group, PTX (10 nmol/μl) was continuously infused (0.13 μl/min) into the interpositus nucleus during the remaining six blocks. For the control group, ACSF was continuously infused instead during the remaining six blocks. A separate group of rabbits (n = 5) underwent training similar to that of the experimental group for CR timing analysis.
To assess the effects of PTX on the acquisition of the eyeblink CR, we trained a group of rabbits (n = 11) with PTX infusions (10 nmol/μl, 0.13 μl/min) into ipsilateral interpositus nucleus for 5 training days. Afterward, animals received additional training without PTX infusions for 3 days. Subsequently, PTX was infused again to test its effect on the expression of the CRs. Finally, the rabbits were trained again without PTX infusions to determine any potential long-term effects associated with PTX. A control group (n = 5) received identical training except that ACSF was infused.
On termination of behavioral testing, a lesion mark was made by passing a 0.1-mA anodal current through a stainless steel electrode (inserted into the guide cannula) for 10 s. Standard histological examination indicated that three subjects in the experimental group had inaccurate guide cannula placement; data from these rabbits were analyzed separately.
Results
Basal and Evoked GABAergic Inputs to the Cerebellar Nuclear Neurons.
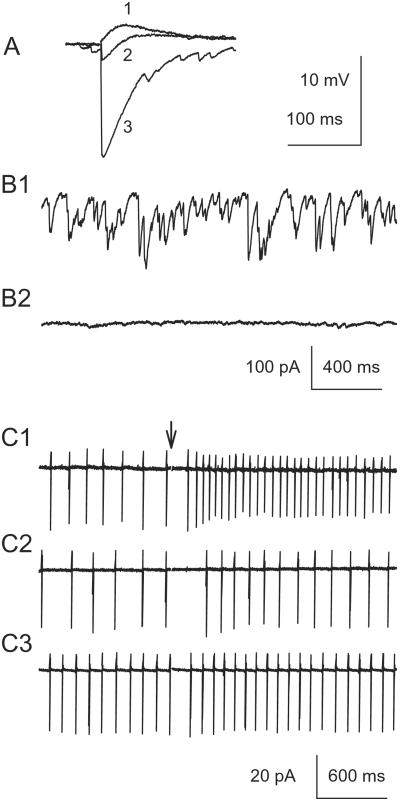
Neurons in the cerebellar deep nuclei were measured in vitro by using whole-cell configuration and current-clamp mode. Stimulation of white matter surrounding the recorded cells elicited large IPSPs, whereas EPSPs were small (Fig. 1A, n = 3). At resting potentials, cells exhibited a biphasic response: a hyperpolarizing response followed by a depolarizing response (Fig. 1A, trace 2). The IPSPs were mediated by GABAA receptors, and the EPSPs by ionotropic glutamate receptors because they were blocked by PTX and kynurenic acid, respectively (data not shown), as has been shown (15). DNNs (n = 3) also exhibited spontaneous GABAergic synaptic currents (Fig. 1B), which were completely blocked by PTX (40 μM), a GABAA antagonist, but not by 2 mM kynurenic acid, a broad-spectrum excitatory amino acid antagonist. These spontaneous synaptic GABAA currents may arise from spontaneous PC discharge, or from AP-independent GABA release (46). It is conceivable that the in vivo frequency of the spontaneous GABAA currents may be much higher than those presented here because many innervating PCs were severed from the slices. In addition, GABAA receptors may also be activated by residual GABA accumulated when GABA release outpaces its reuptake. Such GABAA currents appear as current noise and background conductance of the cells (see Fig. 2). Background conductance accounts for up to 99% of the bicuculline-sensitive charge transfer in some cases (47). We also examined the firing of the nuclear cells by using cell-attached recordings. Most nuclear cells (4 of 5; Fig. 1 C1) discharged spontaneously at 1–15 Hz, frequencies that are much lower than those reported in vivo (48). This discrepancy was probably caused by the differences in the experimental conditions (e.g., temperature, presence of the cell bodies of the excitatory input). Bath application of kynurenic acid (2 mM) did not consistently affect the basal firing rate of the DNNs (Fig. 1 C1 vs. C2), presumably because the excitatory mfs and cfs were severed from their cell bodies and their contributions to the excitatory synaptic drive were minimal. Application of PTX slightly increased spontaneous AP frequency (Fig. 1 C1 vs. C3, before stimulation), suggesting that the basal GABAergic input reduces the spontaneous firing. Electrical stimulation of the white matter surrounding the DNNs produced an increase in the discharge of the DNNs, which was largely blocked by kynurenic acid (n = 5, Fig. 1 C1 vs. C2). The remaining slight increase in discharge rate in the presence of kynurenic acid indicates that GABAergic input may cause slight rebound activation. This rebound activation was virtually eliminated when PTX was applied.
Figure 1.
Regulation of cerebellar DNN discharges by GABAergic input. (A) IPSPs and EPSPs recorded in IP neurons at different membrane potentials: trace 1, −70 mV (Cl− reversal potential); trace 2, 0 mV (glutamate receptor reversal potential); trace 3, −57 mV (resting potential). (B1) Spontaneous IPSCs recorded with [Cl−]in = 144 mM at the resting membrane potential. (B2) Application of PTX completely abolished the sIPSCs. (C1) Stimulation (arrow applies to C1–C3) of the surrounding white matter increased DNN action potential frequency recorded with the cell-attached mode. (C2) Kynurenic acid reduced stimulation-activated APs without affecting the basal AP frequency. (C3) Further application of PTX completely eliminated stimulation-activated APs, but increased basal AP frequency.
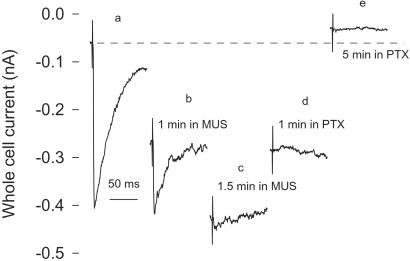
Figure 2.
Effects of sequential applications of a GABAA receptor agonist and an antagonist on basal and stimulation-activated GABAergic currents. Muscimol application increased holding currents, but gradually eliminated synaptically activated responses. Subsequent application of PTX restored the whole-cell holding current, but never recovered the synaptic responses. MUS, muscimol.
Effects of Sequential Applications of an Agonist and an Antagonist of GABAA Receptors.
Whole-cell recordings revealed that muscimol (100 μM), a high-affinity competitive GABAA agonist, opens Cl− channels (Fig. 2 a and b), thereby occluding further Cl− channel opening by synaptically released GABA (Fig. 2c). Additional application of 30 μM PTX gradually blocked Cl− channels, and reduced whole-cell holding currents, but never recovered the synaptic GABAA response (Fig. 2 c–e). A background PTX-sensitive current under control condition was also revealed by a reduction in baseline whole-cell currents by PTX (Fig. 2 a vs. e). Thus, sequential applications of muscimol and PTX in the cerebellar deep nuclei shift the nuclear cells from hyperpolarized to depolarized states, but totally blocking the synaptic GABAA input.
These effects of competitive agonist and noncompetitive antagonist of GABAA on the neuronal membrane excitability are determined by their differential interactions with GABAA receptor and are not unique to the species or the slice preparations. Therefore, we next used similar manipulations in behaving animals to alter the excitability of the DNNs selectively, but completely blocking the GABAergic cortical input.
Basal GABAergic Input Modulates CR Expression.
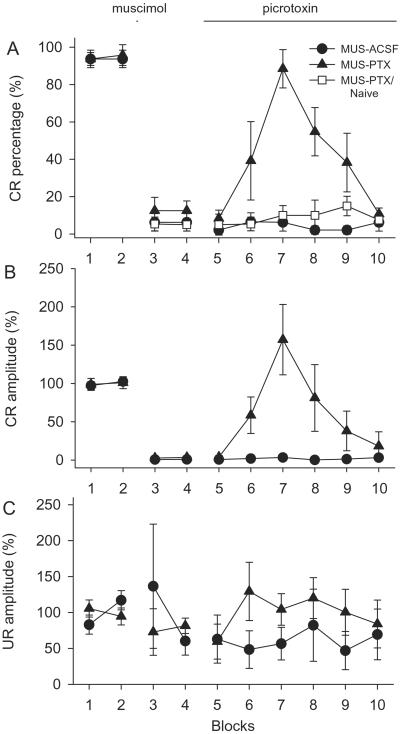
In rabbits well trained with tone-airpuff classical eyeblink conditioning, the tone CS reliably produced conditioned eyeblink responses (CRs) in more than 90% of the trials (Fig. 3A, n = 6 for the MUS-ACSF group, n = 7 for the MUS-PTX group). Muscimol (10 nmol in 1 μl ACSF) infusions into cerebellar interpositus nucleus completely abolished the CRs for more than 1 h. Infusions (0.13 μl/min) of PTX (10 nmol/μl) after muscimol restored the CRs (Fig. 3A, seventh block). However, further infusions of PTX gradually and completely abolished the CRs. The recovery of CRs after PTX infusion was not caused by the washout of muscimol by subsequent infusion because ACSF infusions did not restore the muscimol-blocked CRs. These results indicate that CR expression requires a critical level of DNN excitability, which is influenced by the GABAergic input mainly from cortical PCs. The CRs recovered by PTX (Fig. 3B, session 7) showed larger amplitudes than those before muscimol infusions (P < 0.05).
Figure 3.
Effects of muscimol and PTX on CR expression. (A) CR percentage. (B) CR amplitude normalized to the average CR amplitude during the first two blocks. (C) UR amplitude normalized to the average UR amplitude during the first two blocks. MUS, muscimol.
We next examined whether reduction in inhibition through the mf-pf-PC-DNN pathway is sufficient to support behavioral learning by testing naïve animals with sequential applications of muscimol and PTX as described. The sequential infusions of muscimol and PTX, which abolished and then recovered CRs in well-trained animals, did not produce any sign of CRs in naïve animals (Fig. 3A, open square, n = 5). The infusions should have modulated the PC output to DNNs in the naïve animals in a manner similar to that in the well-trained animals, effectively overriding the learning effects from the cortex. The observed CR expression in the well-trained animals but not in naïve animals indicates that some memory trace must have been established outside the cerebellar cortex (i.e., in the cerebellar deep nuclei) in well-trained animals. Here, we term it the primary memory trace in the sense that it is necessary for even the simplest forms (e.g., not properly timed and/or amplitude-reduced) of eyeblink CRs.
We also measured UR amplitudes (Fig. 3C) to assess whether the modulation of CR expression was correlated with the animal's ability to perform the behavior (49). Because of considerable variations of the URs across the animals, all URs were normalized to average baseline responses before muscimol infusions. Sequential muscimol and PTX infusions did not significantly change the UR amplitude for the entire course of the experiment (Fig. 3C, P > 0.05). Moreover, no reliable correlation exists between the CR percentage and UR amplitude (r = −0.047, P > 0.5).
The effects of combined muscimol and PTX on GABA type A receptor (GABAAR)-associated channel depend on the sequence of their applications. If PTX is applied first and blocks the pores of GABAAR-associated Cl− channels, subsequent binding of muscimol to GABAAR will not have any effect on Cl− conductance. In behaving rabbits, PTX infusions completely blocked CR expression, and the CRs were not recovered by subsequent muscimol infusions (n = 3, data not shown).
CS-Activated GABAA Input Is Necessary for CR Timing.
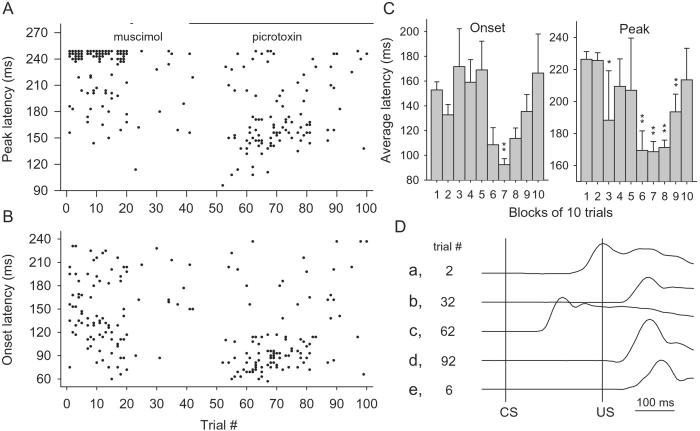
Fig. 4 shows the latencies of the CRs. In well-conditioned rabbits (n = 5), the CRs peaked around the onset of the US (Fig. 4A, trials 0–20). However, when muscimol and PTX were sequentially infused, the CR peak latency shifted from 226 ± 3.4 ms during the first two blocks to 169 ± 6.4 ms during the seventh block (Fig. 4C, P < 0.001). The onset latency also decreased from 143 ± 5.4 to 92.4 ± 4.9 ms (P < 0.001).
Figure 4.
Effects of muscimol and PTX on CR timing. (A) CR peak latency. (B) CR onset latency. (C) Average onset and peak latencies. (D) Examples of nictitating membrane responses. Trace a, a normal CR in well-trained animal (trial 2); trace b, a response after muscimol infusions (trial 32); trace c, a CR recovered by PTX (trial 62); trace d, a response after prolonged PTX infusions (trial 92); trace e, a normal UR recorded from a US alone trial (trial 6). (*, P < 0.05; **, P < 0.005 compared with the latencies during the second block).
This lack of proper CR timing after blockade of cerebellar cortical output suggests that the memory trace for learned response timing (ref. 32) is established in the cortex, which we refer to as the secondary memory trace.
In blocks 3–5 and 10 (trials 29–50 and 91–100), a few CRs exhibited latencies longer than those during blocks 6–9 (Fig. 4 A–C, trials 51–90), because these CRs were very small in amplitude (Fig. 4B), some of which were barely over the threshold (0.5 mm nictitating membrane movement). The latencies of these CRs cannot be measured reliably.
GABAA Antagonist Infusions Block CR Acquisition.
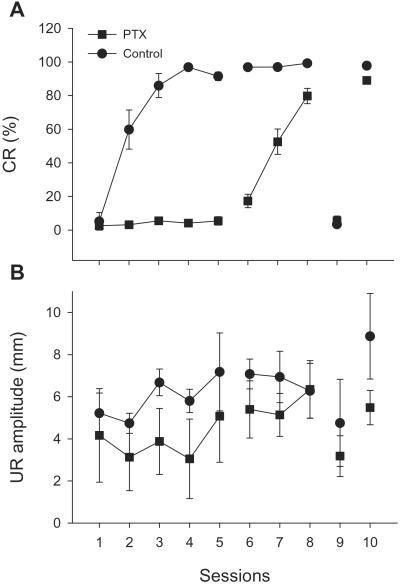
Studies have demonstrated that cortical lesions partially impair acquisition of the conditioned eyeblink response. However, the lesion-induced secondary effects in pontine nuclei, inferior olive, and cerebellar deep nuclei may complicate the interpretation of these effects. PTX infusions completely block the cerebellar corticonuclear projections, providing another approach to functional cortical lesions. We trained two groups (experimental and control) of rabbits for 5 days of acquisition. During 1-h daily training, the experimental group received continuous infusions of PTX, whereas the control group received vehicle (ACSF). Although the control group learned the CRs gradually, the rabbits in the experimental group did not show any sign of learning (Fig. 5A, sessions 1–5, P > 0.1). When PTX infusions were stopped, the experimental rabbits learned as if they were naïve rabbits (sessions 6–8 of experimental group compared with sessions 1–3 of the control group, P = 0.93). CR expression in both groups was completely blocked later by PTX infusions (session 9). PTX did not have a permanent effect on the CR expression; CRs recovered completely by the next day (session 10). Despite the complete blockade of CR acquisition and expression, PTX did not significantly alter URs during the 10 days of training (P > 0.1) or during the first 5 days of acquisition (P > 0.1).
Figure 5.
PTX infusions prevent learning of CRs. PTX completely blocked learning and expression of the eyeblink CRs with no significant effect on UR amplitude, or long-term effect on CR learning and expression. (A) CR percentage. (B) UR amplitude.
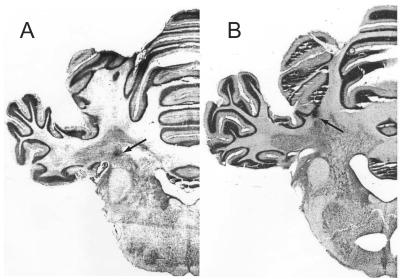
Posttraining histological examination indicated that the effective infusion sites were in the cerebellar medial dentate-lateral interpositus area (Fig. 6A). Slight deviation of the infusion sites (Fig. 6B) resulted in only partial blockade of learning (35 ± 11% average CRs, n = 3, data not shown). Therefore, the effects we observed apparently were not caused by spreading of PTX into cerebellar cortex.
Figure 6.
Typical effective and ineffective infusion sites. (A) An effective infusion site typically located in the close vicinity of the cerebellar interpositus nucleus; (B) an ineffective infusion site.
Discussion
In this study, we used sequential infusions of a GABAAR agonist and a noncompetitive antagonist to test the roles of the cerebellar cortex (i.e., its inhibitory output) in classical eyeblink conditioning. This method allows a gradual alteration of the inhibition on the DNNs while blocking the cerebellar cortical output. Under these conditions, the timing of the eyeblink CRs can be disrupted without reducing the CR amplitude. The fact that CR timing can be dissociated from its expression suggests that they are encoded and/or stored separately (36). In addition, we demonstrated that learning and expression of the conditioned eyeblink response requires some GABAergic input to cerebellar deep nuclei. Our results are in agreement with those of Mamounas et al. (50), which demonstrated a dose-dependent GABAergic modulation of the CR expression. Our results replicate our earlier finding (25) that the cerebellar cortex is necessary for adaptive timing of the CR (cerebellar cortical lesions result in shorter latency CRs) and agree with those of Mauk and associates indicating that GABAergic input to cerebellar deep nuclei is necessary for learned response timing (51, 52).
After we finished our study (53), Mauk and associates reported that PTX infusions recovered extinguished CRs (54). Blockade of CR expression by muscimol infusions in our study might be considered analogous to behavioral extinction of CRs. However, Mauk and associates failed to inhibit CRs with further GABA antagonist infusions, which may have been caused by insufficient infusion dose or inaccurate infusion sites.
It has been suggested that cerebellar LTD releases the cortical inhibition, thereby increasing the cerebellar output (1). Results from recent studies with genetically manipulated mice support some roles of cerebellar LTD in eyeblink conditioning (18, 55, 56) and vestibulo-ocular-reflex adaptation (57). However, a fundamental question remained whether release of inhibition is sufficient for the behavioral CRs. We now demonstrate that sequential muscimol and PTX infusions, which provided a large range of GABAergic inhibition to DNN, allow expression of behavioral CRs in well-conditioned rabbits, but not in naïve rabbits, arguing for plasticity in the mf-DNN pathway. Our results also indicate that the modification of DNN excitability through cortical GABAergic input is not sufficient to produce behavioral CRs in naïve animals. Instead, modification of mf-DNN synapses seems necessary for eyeblink conditioning.
The cerebellar deep nuclei are the final common output pathway of the cerebellum. CS information processed through cortical and nuclear pathways must be integrated at the DNNs. DNNs express functional N-methyl-D-aspartate and a-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors at mf-DNN and cf-DNN synapses (58), and GABAA and GABAB receptors at PC-DNN and local GABAergic synapses (59). They also possess a large variety of voltage-activated ion channels, which grant them complex ionic behaviors (44, 60). When mfs discharge, the DNNs receive both excitatory mf input and strong inhibitory mf-pf-PC input (37). Because GABAergic input strongly inhibits DNN discharges (59, 60), the DNNs are not optimally activated. In naïve rabbits, DNNs do not show long-lasting tone/CS-induced discharges (25). The cerebellar PCs respond to tone/CS in a variety of response patterns (40, 41). During eyeblink conditioning, the pf-PC synapses that coactivate with the cf-PC synapses may be selectively weakened as in cerebellar LTD (56, 61). Consequently, PCs may preferentially increase firing during early CS period, but decrease firing during late CS period, and indeed some do (41). This dynamically patterned inhibition from the cortex may shape the DNN discharge (25) and control CR timing (42). The release from inhibition also allows mf- and cf-activated DNN discharges, which may lead to activation of N-methyl-D-aspartate receptors and voltage-gated calcium channels (62), resulting in long-term synaptic plasticity in the cerebellar deep nuclei (15, 16, 63). The results of this study support the putative mechanisms above by directly demonstrating the critical roles of cortical inhibition in classical eyeblink conditioning.
In summary, the results from this study demonstrate that the acquisition, expression, and timing of the conditioned eyeblink response require properly timed discharges of the cerebellar DNNs, which are modulated by the cortical inhibitory inputs. Further, our results also indicate that multiple memory traces occur in the cerebellar cortex and the deep nuclei encoding different aspects of the learned eyeblink responses.
Acknowledgments
We thank Drs. M. Baudry, L. Byerly, and D. G. Lavond for comments on the manuscript. This work was supported by National Science Foundation Grant IBN-9215069, National Institute of Aging Grant AG-14751, National Institute of Mental Health Grant 5P20-MH52194, and a grant from Sankyo Company (to R.F.T.).
Abbreviations
- CS
conditioned stimulus
- US
unconditioned stimulus
- CR
conditioned response
- UR
unconditioned response
- mf
mossy fiber
- cf
climbing fiber
- PC
Purkinje cell
- DNN
cerebellar deep nuclear neuron
- LTD
long-term depression
- AP
action potential
- EPSP
excitatory postsynaptic potential
- IPSP
inhibitory postsynaptic potential
- IPSC
inhibitory postsynaptic current
- ACSF
artificial cerebrospinal fluid
- GABA
γ-aminobutyric acid
- GABAAR
GABA type A receptor
- PTX
picrotoxin
References
- 1.Albus J S. Math Biosci. 1971;10:25–61. [Google Scholar]
- 2.Eccles J C. Brain Res. 1977;127:327–352. doi: 10.1016/0006-8993(77)90550-9. [DOI] [PubMed] [Google Scholar]
- 3.Ito M. Brain Res. 1972;40:81–84. doi: 10.1016/0006-8993(72)90110-2. [DOI] [PubMed] [Google Scholar]
- 4.Marr D. J Physiol (London) 1969;202:437–470. doi: 10.1113/jphysiol.1969.sp008820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ito M. Annu Rev Neurosci. 1982;5:275–296. doi: 10.1146/annurev.ne.05.030182.001423. [DOI] [PubMed] [Google Scholar]
- 6.Lisberger S G. Science. 1988;242:728–735. doi: 10.1126/science.3055293. [DOI] [PubMed] [Google Scholar]
- 7.Thach W T, Goodkin H P, Keating J G. Annu Rev Neurosci. 1992;15:403–442. doi: 10.1146/annurev.ne.15.030192.002155. [DOI] [PubMed] [Google Scholar]
- 8.Thompson R F. Science. 1986;233:941–947. doi: 10.1126/science.3738519. [DOI] [PubMed] [Google Scholar]
- 9.Thompson R F. Philos Trans R Soc London B. 1990;329:161–170. doi: 10.1098/rstb.1990.0161. [DOI] [PubMed] [Google Scholar]
- 10.Thompson R F, Bao S, Chen L, Cipriano B D, Grethe J S, Kim J J, Thompson J K, Tracy J A, Weninger M S, Krupa D J. Int Rev Neurobiol. 1997;41:151–189. doi: 10.1016/s0074-7742(08)60351-7. [DOI] [PubMed] [Google Scholar]
- 11.Ito M, Kano M. Neurosci Lett. 1982;33:253–258. doi: 10.1016/0304-3940(82)90380-9. [DOI] [PubMed] [Google Scholar]
- 12.Linden D J, Connor J A. Annu Rev Neurosci. 1995;18:319–357. doi: 10.1146/annurev.ne.18.030195.001535. [DOI] [PubMed] [Google Scholar]
- 13.Salin P A, Malenka R C, Nicoll R A. Neuron. 1996;16:797–803. doi: 10.1016/s0896-6273(00)80099-9. [DOI] [PubMed] [Google Scholar]
- 14.Kano M, Rexhausen U, Dreessen J, Konnerth A. Nature (London) 1992;356:601–604. doi: 10.1038/356601a0. [DOI] [PubMed] [Google Scholar]
- 15.Aizenman C D, Manis P B, Linden D J. Neuron. 1998;21:827–835. doi: 10.1016/s0896-6273(00)80598-x. [DOI] [PubMed] [Google Scholar]
- 16.Morishita W, Sastry B R. J Neurophysiol. 1996;76:59–68. doi: 10.1152/jn.1996.76.1.59. [DOI] [PubMed] [Google Scholar]
- 17.Racine R J, Wilson D A, Gingell R, Sunderland D. Exp Brain Res. 1986;63:158–162. doi: 10.1007/BF00235658. [DOI] [PubMed] [Google Scholar]
- 18.Kim J J, Thompson R F. Trends Neurosci. 1997;20:177–181. doi: 10.1016/s0166-2236(96)10081-3. [DOI] [PubMed] [Google Scholar]
- 19.Raymond J L, Lisberger S G, Mauk M D. Science. 1996;272:1126–1131. doi: 10.1126/science.272.5265.1126. [DOI] [PubMed] [Google Scholar]
- 20.Chen L, Bao S, Thompson R F. Behav Neurosci. 1999;113:204–210. doi: 10.1037//0735-7044.113.1.204. [DOI] [PubMed] [Google Scholar]
- 21.Clark R E, Zhang A A, Lavond D G. Behav Neurosci. 1992;106:879–888. doi: 10.1037//0735-7044.106.6.879. [DOI] [PubMed] [Google Scholar]
- 22.Krupa D J, Thompson J K, Thompson R F. Science. 1993;260:989–991. doi: 10.1126/science.8493536. [DOI] [PubMed] [Google Scholar]
- 23.Krupa D J, Thompson R F. Learn Mem. 1997;3:545–556. doi: 10.1101/lm.3.6.545. [DOI] [PubMed] [Google Scholar]
- 24.Lavond D G, Hembree T L, Thompson R F. Brain Res. 1985;326:179–182. doi: 10.1016/0006-8993(85)91400-3. [DOI] [PubMed] [Google Scholar]
- 25.McCormick D A, Thompson R F. Science. 1984;223:296–299. doi: 10.1126/science.6701513. [DOI] [PubMed] [Google Scholar]
- 26.Steinmetz J E, Lavond D G, Ivkovich D, Logan C G, Thompson R F. J Neurosci. 1992;12:4403–4426. doi: 10.1523/JNEUROSCI.12-11-04403.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steinmetz J E, Logue S F, Steinmetz S S. Behav Brain Res. 1992;51:103–114. doi: 10.1016/s0166-4328(05)80317-1. [DOI] [PubMed] [Google Scholar]
- 28.Yeo C H, Hardiman M J, Glickstein M. Exp Brain Res. 1985;60:87–98. doi: 10.1007/BF00237022. [DOI] [PubMed] [Google Scholar]
- 29.Chen L, Bao S, Lockard J M, Kim J K, Thompson R F. J Neurosci. 1996;16:2829–2838. doi: 10.1523/JNEUROSCI.16-08-02829.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lavond D G, Steinmetz J E, Yokaitis M H, Thompson R F. Exp Brain Res. 1987;67:569–593. doi: 10.1007/BF00247289. [DOI] [PubMed] [Google Scholar]
- 31.Lavond D G, Steinmetz J E. Behav Brain Res. 1989;33:113–164. doi: 10.1016/s0166-4328(89)80047-6. [DOI] [PubMed] [Google Scholar]
- 32.Perrett S P, Ruiz B P, Mauk M D. J Neurosci. 1993;13:1708–1718. doi: 10.1523/JNEUROSCI.13-04-01708.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perrett S P, Mauk M D. J Neurosci. 1995;15:2074–2080. doi: 10.1523/JNEUROSCI.15-03-02074.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woodruff-Pak D S, Cronholm J F, Sheffield J B. NeuroReport. 1990;1:165–168. doi: 10.1097/00001756-199010000-00020. [DOI] [PubMed] [Google Scholar]
- 35.Yeo C H, Hardiman M J, Glickstein M. Exp Brain Res. 1985;60:99–113. doi: 10.1007/BF00237023. [DOI] [PubMed] [Google Scholar]
- 36.Steinmetz J E. Behav Brain Res. 2000;110:13–24. doi: 10.1016/s0166-4328(99)00181-3. [DOI] [PubMed] [Google Scholar]
- 37.Ito M. The Cerebellum and Neural Control. New York: Raven; 1984. [Google Scholar]
- 38.Berthier N E, Moore J W. Exp Brain Res. 1986;63:341–350. doi: 10.1007/BF00236851. [DOI] [PubMed] [Google Scholar]
- 39.Thach W T., Jr J Neurophysiol. 1967;30:675–696. doi: 10.1152/jn.1967.30.4.675. [DOI] [PubMed] [Google Scholar]
- 40.Katz D B, Steinmetz J E. Learn Mem. 1997;4:88–104. doi: 10.1101/lm.4.1.88. [DOI] [PubMed] [Google Scholar]
- 41.King D A, Krupa D J, Foy M R, Thompson R F. Int Rev Neurobiol. 2001;45:313–337. doi: 10.1016/s0074-7742(01)45017-3. [DOI] [PubMed] [Google Scholar]
- 42.Bullock D, Fiala J C, Grossberg S. Neural Netw. 1994;7:1101–1114. [Google Scholar]
- 43.Blanton M G, Lo Turco J J, Kriegstein A R. J Neurosci Methods. 1989;30:203–210. doi: 10.1016/0165-0270(89)90131-3. [DOI] [PubMed] [Google Scholar]
- 44.Jahnsen H. J Physiol (London) 1986;372:129–147. doi: 10.1113/jphysiol.1986.sp016001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lavond D G, Steinmetz J E. Behav Res Methods Instruments Computers. 1989;21:435–440. [Google Scholar]
- 46.Soltesz I, Smetters D K, Mody I. Neuron. 1995;14:1273–1283. doi: 10.1016/0896-6273(95)90274-0. [DOI] [PubMed] [Google Scholar]
- 47.Brickley S G, Cull-Candy S G, Farrant M. J Physiol (London) 1996;497:753–759. doi: 10.1113/jphysiol.1996.sp021806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Berthier N E, Moore J W. Exp Brain Res. 1990;83:44–54. doi: 10.1007/BF00232192. [DOI] [PubMed] [Google Scholar]
- 49.Welsh J P, Harvey J A. J Neurosci. 1989;9:299–311. doi: 10.1523/JNEUROSCI.09-01-00299.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mamounas L A, Thompson R F, Madden J T. Proc Natl Acad Sci USA. 1987;84:2101–2105. doi: 10.1073/pnas.84.7.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garcia K S, Mauk M D. Neuropharmacology. 1998;37:471–480. doi: 10.1016/s0028-3908(98)00055-0. [DOI] [PubMed] [Google Scholar]
- 52.Ohyama T, Mauk M. J Neurosci. 2001;21:682–690. doi: 10.1523/JNEUROSCI.21-02-00682.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bao S. Ph.D. dissertation. Los Angeles: Univ. of Southern California; 1999. [Google Scholar]
- 54.Medina J F, Garcia K S, Mauk M D. J Neurosci. 2001;21:4081–4089. doi: 10.1523/JNEUROSCI.21-11-04081.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aiba A, Kano M, Chen C, Stanton M E, Fox G D, Herrup K, Zwingman T A, Tonegawa S. Cell. 1994;79:377–388. [PubMed] [Google Scholar]
- 56.Shibuki K, Gomi H, Chen L, Bao S, Kim J J, Wakatsuki H, Fujisaki T, Fujimoto K, Katoh A, Ikeda T, et al. Neuron. 1996;16:587–599. doi: 10.1016/s0896-6273(00)80078-1. [DOI] [PubMed] [Google Scholar]
- 57.De Zeeuw C I, Hansel C, Bian F, Koekkoek S K, van Alphen A M, Linden D J, Oberdick J. Neuron. 1998;20:495–508. doi: 10.1016/s0896-6273(00)80990-3. [DOI] [PubMed] [Google Scholar]
- 58.Audinat E, Gahwiler B H, Knopfel T. Neuroscience. 1992;49:903–911. doi: 10.1016/0306-4522(92)90366-a. [DOI] [PubMed] [Google Scholar]
- 59.Mouginot D, Gahwiler B H. Neuroscience. 1995;64:699–712. doi: 10.1016/0306-4522(94)00456-f. [DOI] [PubMed] [Google Scholar]
- 60.Jahnsen H. J Physiol (London) 1986;372:149–168. doi: 10.1113/jphysiol.1986.sp016002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schreurs B G, Oh M M, Alkon D L. J Neurophysiol. 1996;75:1051–1060. doi: 10.1152/jn.1996.75.3.1051. [DOI] [PubMed] [Google Scholar]
- 62.Muri R, Knopfel T. J Neurophysiol. 1994;71:420–428. doi: 10.1152/jn.1994.71.1.420. [DOI] [PubMed] [Google Scholar]
- 63.Katz D B, Tracy J A, Steinmetz J E. Physiol Behav. 2001;72:499–510. doi: 10.1016/s0031-9384(00)00444-3. [DOI] [PubMed] [Google Scholar]