Abstract
When males of the roundworm Caenorhabditis elegans come into association with their hermaphroditic counterparts they cease foraging behavior and begin to mate. Here we detail several assays used to demonstrate that a diffusible cue is correlated with this process. This cue is sexually dimorphic, given off only by the hermaphrodite and eliciting a response only in the male. Males are attracted to, reverse direction of movement frequently, and remain in regions of agar conditioned with hermaphrodites. From our studies we suggest a form of kinesis that works by attracting males to their mating partners from a distance and functions, once males arrive, in holding attracted males in close proximity. The hermaphrodite vulva is not required for the cue. Males from general sensory mutants osm-5 and osm-6 fail to respond to the cue, whereas male-specific mutants lov-1 and pkd-2 respond. Finally, that males from multiple isolates of C. elegans also respond similarly to this cue indicates that this cue is robust and has been maintained during recent evolution.
Keywords: sensory behavior‖mating behavior‖kinesis
Mate-location behavior depends on the ability of the nervous system to integrate incoming mate-specific cues and translate this information into appropriate responses in muscles and other effectors. Odorant cues have been studied extensively in fruit flies (1), other insects (2), fish (3, 4), and rodents (5), and less in reptiles (6, 7), birds (8), various mammals (9, 10), and primates including humans (11–13). Although mate-finding cues are common throughout Nematoda (14, 15) and have been found in the hermaphroditic trematodes (flukes; refs. 16–18), it remains odd that no chemical communication has been demonstrated in bringing together Caenorhabditis elegans mating partners.
Although it has been suggested that some nontactile cue may work as a catalyst to a series of stereotyped steps by males resulting in successful reproduction (J. Sulston and E. Jorgensen, personal communication; see also ref. 19), no assays to address such a mate-finding cue have come into common practice (E. Jorgensen and R. Horvitz, personal communications; C. Song, K. Liu, and P.W.S., unpublished results). Moreover, other than for the fruit fly (20–22), simple, straightforward model systems in which to study the role of sensory organs and the underpinning genetic and neurologic machinery for processing of such sex-related olfactory cues remain poorly described. Here we describe the design and results of several assays that provide evidence for a mate-finding cue in C. elegans. We also tested vulvaless hermaphrodites for their ability to condition agar and as well both general and specific male sensory mutants for their ability to respond. Last, we report cue-detection results from males of several C. elegans isolates from diverse locations.
Materials and Methods
Strains.
Unless indicated otherwise, all males come from the him-5(e1490) mutant, which segregates XO male progeny by X chromosome nondisjunction during meiosis (23). To construct cue source regions, the muscle mutant unc-52(e444), individuals of which become relatively motionless by the time they are young adults, was used. For the vulvaless experiment we constructed a let-23(sy1) unc-52(e444); dpy-20(e1282)lin-3(n378); him-5(e1490) strain (PS3980) (24, 25). lov-1(sy582Δ) and pkd-2(sy606Δ) have been described (26). The sensory mutants osm-5(p813) and osm-6(p811), as well as the diverse isolates CB4932 (Taunton, England), CB4555 (Pasadena, CA), and CB4856 (Hawaii) were obtained from the Caenorhabditis Genetics Center (St. Paul); isolates are described by Hodgkin and Doniach (27) and further characterized by de Bono and Bargmann (28). Similar to wild-type [Bristol N2; him-5(+)] strains, males from isolates occur spontaneously at an inconveniently low frequency in the self-progeny of hermaphrodites. To obtain a constant supply of males, isolates were heat shocked and maintained by backcrossing (29, 30). To test males from osm-6, him-8(e1489); osm-6(p811) strains were made, and the presence of osm-6 was verified by use of fructose avoidance and dye-fill assays (http://cobweb.dartmouth.edu/∼ambros/worms/16.html) (31). All animal stocks were stored at 18°C. All stocks and animals harvested for upcoming trials were grown on standard 5-cm diameter NG agar plates inoculated with the Escherichia coli strain OP50, grown in Luria–Bertani media (LB), as a food source (32).
Response Assay Protocol.
Bacterial lawns were grown on standard 5-cm diameter agar plates for all trials: three drops of OP50-inoculated LB, spread thinly, with a ≈0.25-centimeter gap between the edge of the lawns and the walls of the plates, to dissuade test animals from leaving trials. Plates were then stored at ≈22°C for 2 days until used for trials. Source and test animals were harvested daily at the fourth larval stage (L4), and stored at ≈18°C overnight with 10–20 animals per same-sex plate to be used the following day in trials as young adults. The muscle mutant unc-52 was used to condition agar plates for at least 3 and up to 8 h (see Fig. 1a). Five to 10 min before the onset of a particular trial, source animals were removed. Trials consisted of the introduction of a single test animal on to a single trial plate (see Fig. 1a), and movement of the animal was then documented for 5 min. Effort was made to orient animals in the direction of the 3 × 7-mm scoring regions. A motor-driven stage attached to a computer joystick was used to help videotape trials through a dissecting microscope. Trials were videotaped, and the number of reversals per crossing and the time animals spent in conditioned region were scored and are on record. Backwards movement equal to or greater than a body length was counted as “1 reversal”; backward movement less than a body length was counted as “0.5 reversal.” We used the frequency of reversals as indication that males detect a cue; it seems likely that when males pass over a mate-finding cue that they will move backwards, and by repeated reversals, home in on a cue's source. Equal numbers of unconditioned and conditioned agar trials were run in parallel on ≈5 plates each per day; moreover, trials were run blindly and interspersed at random. Isolate trials were run in parallel alongside trials of the laboratory strain (Bristol N2). Unconditioned agar trials were meant to parallel conditioned agar trials as closely as possible: (i) made from same day bacterial lawns, (ii) kept at the same temperature, and (iii) had bacteria picked on and off to mimic introduction of source animals as on conditioned agar trials. Average temperature during trials was ≈22°C.
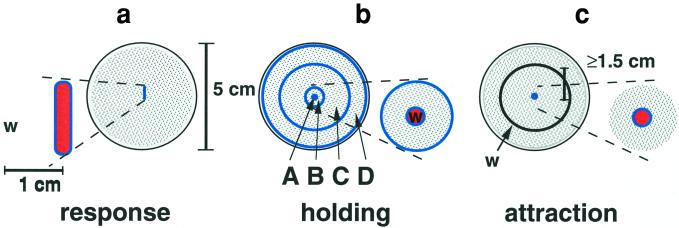
Figure 1.
Design of response, holding, and attraction assays. Trials were performed on 5-cm agar plates with 2-day-old bacterial lawns (gray, stippled area). Individual test animals (w) were introduced ≈1 cm from scoring region in the response assay (a), directly on area A in the holding assay, dispersing on their release (b), and more than 1.5 cm from conditioned point source in the attraction assay (c). Unconditioned or conditioned regions are denoted in orange, and blue lines demark scoring region and areas (A–B–C–D).
Holding Assay Protocol.
The holding assay was identical to the response assay with the following modifications: (i) source animals were introduced in a pile in area A, (ii) test animals were introduced directly on this conditioned spot (see Fig. 1b); (iii) in addition to area A, number of reversals was scored in outwardly stacked, concentric ring-areas (with radii of 1 mm, 5 mm, 16.5 mm, and ≈25 mm; unequal sized areas were normalized by dividing outer ring-areas by 24, 247, and 330, respectively); and (iv) movements of test animals were documented for 10 min.
Attraction Assay Protocol.
Design of the attraction assay was similar to the holding assay with the following modifications: (i) source animals were left to condition a point source for 24 h; (ii) at the onset of each trial, individual hermaphrodites were placed atop conditioned regions; and (iii) test males were introduced ≥1.5 cm from the conditioned point source/unconditioned scoring region (see Fig. 1c).
Results and Discussion
Evidence for a Kinesis Cue.
Response assay.
To investigate whether hermaphrodites discharge odorant cues that recruit mates, we compared the behavior of males placed in hermaphrodite-conditioned and unconditioned agar trials (Fig. 1a; representative examples, Fig. 2). In this response assay, we introduced individual males to trials conducted over several months and compared the number of reversals and the time spent in scoring regions. We found an ≈11-fold increase in mean number of reversals and an ≈2.5-fold increase in mean time spent in scoring regions between trials with unconditioned agar [0.50 (±0.14; SEM) reversals and 34.42 (±4.14) s; n = 86) and conditioned agar [5.48 (±0.83) reversals and 86.42 (±9.29) s; n = 90]. Statistical comparison of means (±SEM) between trials was performed by using a Mann–Whitney U test, number of reversals, P < 0.00001; time, P < 0.001; both 2α. Our findings indicate that a cue is released by hermaphrodites and detected by males.
Figure 2.
Time course of N2 males from representative film clips of unconditioned (−) and N2 hermaphrodite-conditioned (+) trials. On average, males moved directly through unconditioned regions, backing less than once and taking ≈30 s to cross through scoring regions. In contrast, during conditioned trials males reversed around six times while turning over scoring regions spending >90 s. Tracks from males are traced over in orange. Scoring regions are outlined in blue. Elapsed time (s) is included within each film frame. (Scale bar is 1 mm.)
Holding assay.
Do cues merely elicit a response when males pass randomly into distinct conditioned regions or do cues exhibit any additional, longer-range function? If cues work only proximally, then movement by males outside regions conditioned with hermaphrodites should not be affected (Fig. 1b). In a modified version of the response assay, we observed the extent and direction of movement at various distances, using outwardly concentric areas from A–D, with respect to a conditioned point (Fig. 1b). In unconditioned agar trials, 17% of the males returned to the starting area A, whereas over 58% of the males returned during conditioned agar trials (P = 0.0013; Fishers exact test, n = 61, 2α). During this experiment we asked whether males that moved from the outer ring C to ring B continued into area A, or returned back to ring C (Fig. 1b). In unconditioned agar trials 27% of the males continued on into the starting region as compared with 74% of the males in the conditioned agar trials (P = 0.0142; Fishers exact test, n = 34, 2α). Of the males returning to area A, 0% stayed through to the end of the trial period in unconditioned agar trials as compared with 72% in the conditioned agar trials (P = 0.0075; Fishers exact test, n = 23, 2α). To corroborate previous findings, we scored number of reversals and the time in region (now area A) in a manner similar to our response assay. We observed a 51-fold increase in mean number of reversals and a 25-fold increase in mean time spent in scoring regions between unconditioned [0.17 (±0.08) reversal and 3.6 (±1.15) s; n = 30) and conditioned agar trials [8.74 (±2.35) reversals and 91.10 (±22.97) s; n = 31]; P = 0.0004 and P = 0.0014, respectively; Mann–Whitney U test, (±SEM), both 2α. Results indicate that in conditioned agar trials, males return to a cue source and stay.
Attraction assay.
An odorant cue might function not only in holding mates, but also by attracting potential mates from a distance, increasing the likelihood of sexual encounters. To test whether the cue revealed in the above assays facilitate mate-finding, we observed whether males introduced at a distance (≥1.5 cm) found their hermaphrodite mates more efficiently when these hermaphrodites were placed on previously hermaphrodite-conditioned regions. More than 95% of males introduced to conditioned agar trials found their mates within 2 h; in contrast, only ≈77% of males introduced to unconditioned agar trials had found them at the same time point. Moreover, all of the males in conditioned agar trials found their mates, whereas ≈10% of males in unconditioned trials never found their mates within 5 h (P = 0.0031; Mann–Whitney U test; conditioned (n = 89) and unconditioned (n = 90) agar trials; 2α). Thus a cue given off by hermaphrodites helps males find their mates from a distance.
Evidence for a diffusible cue.
Organisms use a variety of mechanisms to orient and navigate toward chemical cues in their environment (33). As opposed to a direct, taxis-like movement, our findings resemble a kinesis response in which animals move in an indirect manner toward a cue's source (2). To further understand this movement we reexamined video recordings from the previous holding experiment, and compared the number of reversals in equal-area regions from the four areas (A–B–C–D) (Fig. 1b). A graded response, falling off with distance, indicates that the hermaphrodite-derived cue is diffusible. On unconditioned agar, the mean number of reversals by males in all four areas remained constant. In contrast, on conditioned agar, the mean number of reversals increased 11-fold from area C to B and made a greater than 25-fold increase from area B to A. We used a two-factor ANOVA to examine the role of trial type, area, and any interaction between these variables on the response of reversals by males. Comparison of either all four areas (A–B–C–D) or consideration of only the more distant three areas (B–C–D; response in area A may be solely a holding phenomena, its magnitude biasing the statistical analysis), show effects caused by both variables (A–B–C–D, by trial, P < 0.001, by area, P < 0.001, and interaction, P < 0.000; B–C–D, by trial, P = 0.001, by area, P < 0.001, and interaction, P < 0.001; n = 61, 2α). Thus, during the same trials in which movement toward a conditioned source is observed, we see a correlation between the distance from source and response (reversals) in males.
Elucidation of Cue Specificity, both Within and Between Sexes, by Using Our Response Assay.
Cue source.
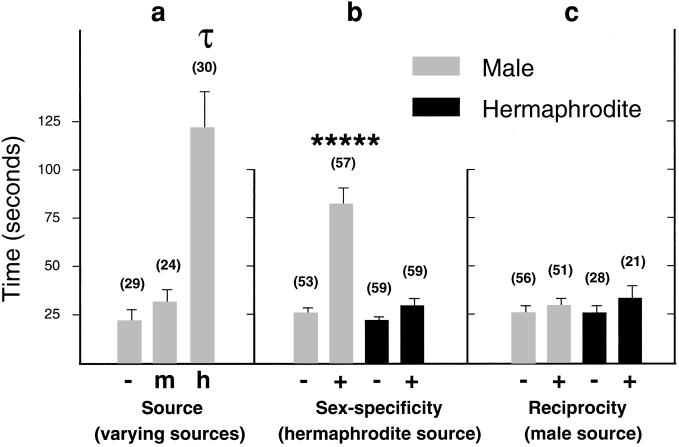
We determined the source of mate-finding cues by testing males on unconditioned agar plates and agar plates conditioned with hermaphrodites, and compared them with agar plates conditioned with males. We observed a 22-fold increase in the mean number of reversals and a greater than 5-fold increase in the mean time spent in scoring regions between unconditioned (0.34 reversals and 23 s) and hermaphrodite-conditioned agar trials (7.5 reversals and 123 s; Fig. 3a). We found no significant shift between unconditioned and male-conditioned agar trials, suggesting that the cue detected by males is given off exclusively by hermaphrodites (Fig. 3a).
Figure 3.
Effects of source and sex on cue response. Individual young-adult test animals were introduced (no. of animals in parentheses) to unconditioned (−) and conditioned (+) agar trials. Trial plates are compared for time in scoring region during a 5-min interval. Statistical comparison of means (error bars represent SEM) between trials in the source experiment were performed by using Tukey's honestly significant difference, τ significance level of 0.05 grouped unconditioned (−) and male-conditioned (m) trials together and found the hermaphrodite-conditioned (h) trial separate. Pairwise Mann–Whitney U tests were used for sex-specificity and reciprocity experiments; *****, P < 0.00001.
Cue sex-specificity.
We examined the sex-specificity of this hermaphrodite-derived cue by comparing response of males and hermaphrodites in regions conditioned with hermaphrodites. When we tested males we found an 8.5-fold increase in mean number of reversals and a greater than 3-fold increase in mean time spent in scoring regions between unconditioned (0.5 reversal and 26 s) and conditioned agar trials (>4 reversals and 83 s; Fig. 3b). In contrast, when we tested hermaphrodites, we found a negligible increase in mean number of reversals and no increase in mean time spent in scoring regions between trials (Fig. 3b). From these results we infer that the odorant cue given off by hermaphrodites does not elicit a response in hermaphrodites as it does in males.
Cue reciprocity.
To address the possibility that in addition to a male-specific cue given off by hermaphrodites there might be a reciprocal hermaphrodite-specific cue given off by males, we carried out experiments to assay the reciprocity of this mating system. We tested both sexes on agar plates conditioned with males. We found no difference in mean number of reversals or mean time spent in scoring regions between trials (Fig. 3c), suggesting that no cue is given off by males to attract or hold hermaphrodites.
Elucidation of Cue Source Within Hermaphrodites by Using Our Response Assay: Vulvaless Mutant.
The cellular localization of cue production/secretion is quite variable throughout Nematoda: from the entire epidermis to specifically the postanal or vulva regions (34). To garner insight on the source of this cue we built a highly penetrant, vulvaless mutant (see Materials and Methods; 100% of animals examined under Nomarski optics were completely vulvaless, i.e., not only have no opening to the outside, but have no vulval tissue; n = 50). We then compared male response between unconditioned, vulvaless-conditioned, and wild-type-conditioned agar. Response by males from unconditioned agar trials to vulvaless-conditioned trials increased by ≈5-fold in mean number of reversals and ≈3-fold in mean time spent in scoring regions, unconditioned (1.3 reversals and 20 s) and vulvaless-conditioned agar trials (>6 reversals and 64 s; Table 1); there was no significant change in response between vulvaless-conditioned, dpy-20-conditioned, and wild-type-conditioned trials (see Table 1). Thus the production of the cue is not limited to vulva tissue and is not secreted solely through the vulva.
Table 1.
Response: Vulvaless hermaphrodities and sensory mutants
| Strain | Trial type | n | Mean (SEM)
|
|
|---|---|---|---|---|
| Reversals per crossing | Time, s | |||
| Male response to vulvaless hermaphrodite-derived cue | ||||
| him-5 | − | 29 | 1.32 (0.37) | 20.14 (3.00) |
| vul (dpy) | 30 | 6.28 (1.32) | 64.13 (12.63) | |
| + (dpy) | 30 | 6.81 (1.33) | 62.00 (10.87) | |
| + | 25 | 7.26 (1.30) | 98.12 (16.21) | |
| Male sensory mutant response to hermaphrodite-derived cue | ||||
| him-5 | − | 28 | 0.33 (0.13) | 29.71 (3.65) |
| + | 29 | 2.09 (0.71) | 68.55 (10.87) | |
| osm-5 | − | 28 | 1.69 (0.36) | 50.25 (8.52) |
| + | 29 | 2.11 (0.51) | 53.79 (11.16) | |
| him-8 | − | 29 | 2.49 (0.92) | 31.41 (5.04) |
| + | 29 | 4.90 (1.26) | 63.21 (14.58) | |
| osm-6 | − | 30 | 4.41 (0.76) | 49.50 (8.87) |
| + | 30 | 5.16 (1.06) | 49.83 (9.91) | |
| him-5 | − | 25 | 0.44 (0.09) | 31.52 (4.38) |
| + | 28 | 2.92 (0.94) | 68.39 (12.78) | |
| lov-1 | − | 26 | 0.64 (0.19) | 28.58 (5.04) |
| + | 31 | 5.44 (0.95) | 104.06 (11.71) | |
| him-5 | − | 27 | 0.60 (0.13) | 33.85 (4.62) |
| + | 28 | 4.00 (0.74) | 99.36 (13.16) | |
| pkd-2 | − | 29 | 1.07 (0.31) | 31.86 (5.04) |
| + | 28 | 3.41 (1.10) | 62.76 (10.86) | |
Individual males (n) from wild type and sensory mutants were introduced to both unconditioned (−) and conditioned (+) agar trials. Trials were compared for reversals per crossing and time in scoring regions during a 5-min interval. Statistical comparison of means (SEM) between trials in the vulvaless experiment were performed using Tukey's honestly significant difference; all three conditioned trials were found to be distinct from the unconditioned negative control trial at a significance level of 0.05. For sensory mutant trials, pairwise Mann–Whitney U tests were used for osm-5, P = 0.878 and P = 0.632 and P < 0.001 and P = 0.001; t tests (one-tailed) for osm-6, P = 0.285 and P = 0.490 and P = 0.064 and P = 0.024; Mann–Whitney U tests for lov-1, P < 0.001 and P < 0.001 and P = 0.057 and P = 0.064; and pkd-2, P = 0.009 and P = 0.069 and P < 0.001 and P < 0.001; reversals-per-crossing and time-in-scoring regions, significance levels for mutant and corresponding him-5 or him-8 control trials respectively. Because the mutant dpy-20(e1282) was necessary in constructing the vulvaless hermaphrodites source strain, in addition to wild-type control trials, dpy-20(e1282) control trials were run.
Comparison of Sensory Mutants in Male by Using Our Response Assay.
General sensory mutants:
osm-5 and osm-6.
To better understand the sense modality used in the detection of the cue, we introduced males of the known general, ciliated sensory mutants osm-5 and osm-6, with corresponding him-5 or him-8 males as controls, to unconditioned and hermaphrodite-conditioned agar trials. We found no significant shift between unconditioned and male-conditioned agar trials in both osm-5 and osm-6 experiments (see Table 1). From these results we suggest that the cue may be detected through the modality of chemosensation.
Candidate mutants:
lov-1 and pkd-2.
Both lov-1 and pkd-2 are expressed in the adult male sensory neurons of the rays and hook, mediating response and vulva location, respectively (35). Because lov-1 and pkd-2 are also expressed in several head neurons found only in the male (cephalic companion cells or CEMs) these genes might play a role in chemotaxis toward hermaphrodites (35, 36). We thus tested whether lov-1 and/or pkd-2 play any role in the detection of the diffusible cue. We introduced lov-1, pkd-2, and him-5 (for control) males to unconditioned and hermaphrodite-conditioned agar trials. For lov-1, we observed an ≈9-fold increase in the mean number of reversals and a ≈4-fold increase in the mean time spent in scoring regions between unconditioned (0.6 reversal and 29 s) and hermaphrodite-conditioned agar trials (5.4 reversals and 104 s; see Table 1). For pkd-2, we observed a 3-fold increase in the mean number of reversals and a 2-fold increase in the mean time spent in scoring regions between unconditioned (1.1 reversals and 32 s) and hermaphrodite-conditioned agar trials (3.4 reversals and 63 s; see Table 1). We infer that neither gene plays a major role in the ability of males to detect any diffusible cue(s) given off by hermaphrodites.
Elucidation of Cue Response in Males from Diverse C. elegans Isolates by Using Our Response Assay.
To assay the extent, as well as variability, of male response to hermaphrodite-derived cues throughout the species of C. elegans, we examined males from three isolates in parallel to the standard laboratory strain (Bristol N2). We chose strains found both in a geographical local similar to the Bristol N2, the Taunton, England isolate (CB4932), and dissimilar, the Pasadena (CB4555) and Hawaiian isolates (CB4856) (27, 28). We introduced individual males from the four strains to four parallel trials and scored number of reversals and time spent in regions conditioned with hermaphrodites of the laboratory strain (Bristol N2). Response by males from the Bristol N2 strain increased by 9-fold in mean number of reversals and 3-fold in mean time spent in scoring regions between unconditioned (<1 reversal and ≈26 s) and conditioned agar trials (≈6 reversals and ≈88 s; Table 2). Isolates increased 3- to 8.5-fold in mean number of reversals and 1.5- to 3.5-fold in mean time spent in scoring regions between trials (Table 2). Thus, males from the various isolates elicit similar responses to the same cue, and the diffusion of this cue (from the hermaphrodite) and processing of this cue (in the male) has not degraded in the standard laboratory strain (Bristol N2).
Table 2.
Male response in diverse isolates to hermaphrodite-derived cue
| Strain | Trial type | n | Mean (SEM)
|
|
|---|---|---|---|---|
| Reversals per crossing | Time, s | |||
| N2 (Bristol) | − | 30 | 0.67 (0.14) | 25.9 (3.5) |
| + | 29 | 5.98 (1.42) | 87.9 (13.3) | |
| CB4555 (Pasadena) | − | 27 | 2.25 (0.67) | 53.5 (8.0) |
| + | 29 | 7.72 (1.15) | 118.2 (13.2) | |
| CB4932 (Taunton) | − | 29 | 0.51 (0.12) | 13.7 (1.8) |
| + | 29 | 4.37 (0.93) | 49.5 (7.5) | |
| CB4856 (Hawaii) | − | 30 | 0.41 (0.15) | 30.4 (5.7) |
| + | 29 | 3.42 (0.71) | 51.3 (8.0) | |
Individual males (n) were introduced to both unconditioned (−) and conditioned (+) agar trials. Trials were compared for reversals-per-crossing and time-in-scoring regions during a 5-min interval. Statistical comparisons of means (SEM) between the variables of trial type, strain, and corresponding interactions were examined using a two-factor ANOVA, by trial, P < 0.001 and P < 0.001, by strain, P = 0.001 and P < 0.001; and interaction between trial and strain, P = 0.356 and P = 0.028; reversals-per-crossing and time-in-scoring regions, respectively.
Discussion
Results from our response, holding, and attraction experiments indicate purposeful movement by males toward and to a diffusible, hermaphrodite-derived cue. These experiments, together with observations from the source, sex-specificity, and reciprocity experiments—indicating the existence of a sexually dimorphic cue(s) that is given off exclusively by hermaphrodites and eliciting a response specifically by males—define the first step of mating behavior in this organism. Moreover, use of the vulvaless mutant shows that this cue is not discharged solely from either vulval tissue or through the vulva. Whereas experiments carried out with the extant sensory mutants indicate that this cue is likely detected by a chemosensory organ in the male, response does not require lov-1 or pkd-2, which are required for male response to hermaphrodite contact. Furthermore, from our experiments with diverse isolates we suggest that within the species C. elegans the hermaphrodite-derived cue is pervasive; males from multiple isolate strains elicit a similar response. Last, it has been thought that males have no preference with regards to the sex of mating partners; in laboratory conditions, in addition to mating with hermaphrodites, males kept at high population densities exhibit mating behaviors with both themselves and other males. Likewise, hermaphrodites have been considered passive mating partners, playing no active role in mating. Our findings correct both misconceptions and demonstrate that males do have a preference in their mate selection and that hermaphrodites do contribute in mating.
A mate-finding cue in C. elegans and hence the presence and understanding of the sensory machinery implicated in cue processing have been elusive for over 20 years (37). Olfaction is an important mode of communication among soil organisms (2); our assay for response to mate-finding cue(s) by this soil nematode coupled with the extensive cellular, molecular, and genetic understanding of C. elegans might provide a useful system for elucidating the basis of genetically determined and ethologically relevant behavior.
Acknowledgments
We thank R. Garcia and N. Moghal for discussions, J. DeModena for experimental suggestions, S. Mukhtar for setting up the video-tracking system, and members of the Sternberg and Benzer laboratories for experimental guidance and reading drafts of our manuscript. The Caenorhabditis Genetics Center provided strains. This work was supported by the Howard Hughes Medical Institute, with which P.W.S. is an investigator.
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Jallon J. Behav Genet. 1984;15:441–478. doi: 10.1007/BF01065444. [DOI] [PubMed] [Google Scholar]
- 2.Shorey H. Annu Rev Entomol. 1973;18:349–380. doi: 10.1146/annurev.en.18.010173.002025. [DOI] [PubMed] [Google Scholar]
- 3.Dulka J. Brain Behav Evol. 1993;42:265–280. doi: 10.1159/000114166. [DOI] [PubMed] [Google Scholar]
- 4.Sorensen P, Christensen T, Stacey N. Curr Opin Neurobiol. 1998;8:458–467. doi: 10.1016/s0959-4388(98)80032-9. [DOI] [PubMed] [Google Scholar]
- 5.Bronson F. Biol Reprod. 1971;4:344–357. [PubMed] [Google Scholar]
- 6.Mason R, Fales H, Jones T, Pannell L, Chinn J, Crews D. Science. 1989;245:290–292. doi: 10.1126/science.2749261. [DOI] [PubMed] [Google Scholar]
- 7.Thompson R, Moore F. Horm Behav. 2000;38:75–85. doi: 10.1006/hbeh.2000.1610. [DOI] [PubMed] [Google Scholar]
- 8.Bohnet S, Rogers L, Sasaki G, Kolattukudy P. J Biol Chem. 1991;266:9795–9804. [PubMed] [Google Scholar]
- 9.Marchlewska-koj A. Oxford Rev Reprod Biol. 1984;6:266–302. [PubMed] [Google Scholar]
- 10.Buck L. Cell. 2000;100:611–618. doi: 10.1016/s0092-8674(00)80698-4. [DOI] [PubMed] [Google Scholar]
- 11.Micheal R, Bonsall R, Zumpe D. Vitam Horm. 1976;34:137–186. doi: 10.1016/s0083-6729(08)60075-8. [DOI] [PubMed] [Google Scholar]
- 12.Hennessy D, Slotnick B, Goldfoot D. Science. 1979;203:1139–1140. [Google Scholar]
- 13.Savic I, Berglund H, Gulyas B, Roland P. Neuron. 2001;31:661–668. doi: 10.1016/s0896-6273(01)00390-7. [DOI] [PubMed] [Google Scholar]
- 14.Green C. Helminthol Abstr Ser B. 1980;49:327–339. [Google Scholar]
- 15.Haseeb M, Fried B. Adv Parasitol. 1988;27:169–207. doi: 10.1016/s0065-308x(08)60355-3. [DOI] [PubMed] [Google Scholar]
- 16.Sogandares-Bernal F. J Parasitol. 1966;52:701–703. [PubMed] [Google Scholar]
- 17.Fried B, Roberts T. J Parasitol. 1972;58:88–91. [PubMed] [Google Scholar]
- 18.Nollen P. Parasitology. 1983;86:99–120. doi: 10.1017/s0031182000050861. [DOI] [PubMed] [Google Scholar]
- 19.Liu K, Sternberg P. Neuron. 1995;14:79–89. doi: 10.1016/0896-6273(95)90242-2. [DOI] [PubMed] [Google Scholar]
- 20.Siddiqi O. Trends Genet. 1987;3:137–142. [Google Scholar]
- 21.Carlson J. Trends Neurosci. 1991;14:520–524. doi: 10.1016/0166-2236(91)90004-e. [DOI] [PubMed] [Google Scholar]
- 22.Stocker R. Cell Tissue Res. 1994;275:3–26. doi: 10.1007/BF00305372. [DOI] [PubMed] [Google Scholar]
- 23.Hodgkin J, Horvitz H, Brenner S. Genetics. 1979;91:67–94. doi: 10.1093/genetics/91.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferguson E, Horvitz H. Genetics. 1985;110:17–72. doi: 10.1093/genetics/110.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aroian R, Sternberg P. Genetics. 1991;128:251–267. doi: 10.1093/genetics/128.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barr M, DeModena J, Braun D, Nguyen C Q, Hall D H, Sternberg P W. Curr Biol. 2001;11:1341–1346. doi: 10.1016/s0960-9822(01)00423-7. [DOI] [PubMed] [Google Scholar]
- 27.Hodgkin J, Doniach T. Genetics. 1997;146:149–164. doi: 10.1093/genetics/146.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Bono M, Bargmann C. Cell. 1998;94:679–689. doi: 10.1016/s0092-8674(00)81609-8. [DOI] [PubMed] [Google Scholar]
- 29.Hodgkin J. Genetics. 1983;103:43–64. doi: 10.1093/genetics/103.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sulston J, Hodgkin J. In: The Nematode Caenorhabditis elegans. Wood W, editor. Plainview, NY: Cold Spring Harbor Lab. Press; 1988. pp. 591–592. [Google Scholar]
- 31.Culotti J, Russell R. Genetics. 1978;90:243–256. doi: 10.1093/genetics/90.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brenner S. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vickers N. Biol Bull. 2000;198:203–212. doi: 10.2307/1542524. [DOI] [PubMed] [Google Scholar]
- 34.Duggal C L. Nematologica. 1978;24:213–221. [Google Scholar]
- 35.Barr M, Sternberg P. Nature (London) 1999;401:386–389. doi: 10.1038/43913. [DOI] [PubMed] [Google Scholar]
- 36.Sulston J, Horvitz H. Dev Biol. 1977;56:110–156. doi: 10.1016/0012-1606(77)90158-0. [DOI] [PubMed] [Google Scholar]
- 37.Bone L, Shorey H. J Chem Ecol. 1978;4:595–612. [Google Scholar]