Abstract
Dye-sensitized solar cells (DSSCs) have drawn attention in recent years for their cost-efficient, green, and convenient means of harnessing solar power. The function of DSSCs is designed around the counter electrode (CE), which is traditionally composed of platinum (Pt) for its good catalytic activity and good electric conductivity. However, Pt is expensive and in short quantity, and extensive studies have gone in search of substitute materials. Among them, materials in the system of chalcogenides, such as sulfides, selenides, and tellurides, have drawn considerable attention as potential candidates. These materials have various unique advantages, such as their capability to modulate their electronic property, good catalytic activity, and good resistance to chemicals, and therefore have good potential for optimizing function of DSSCs. This review presents an in-depth overview of where the situation stands in chalcogenide-based CEs, critically evaluating their synthetic routes, electrocatalytic activity, and stability. We compare and contrast various synthetic routes, such as hydrothermal, solvothermal, electrodeposition, chemical vapor deposition, atomic layer deposition, and solution-based synthesis, employed to alter the nanostructure and topography of such materials. Sulfide- and selenide-based materials have displayed competing power conversion efficiencies and favorable charge transfer behavior, but tellurides have potential through their exceptionally good electric conductivity. Despite such breakthroughs, limitations such as corrosion by the electrolyte, phase instability, and scalability of the routes of fabrication persist and serious bottlenecks persist. This review suggests possible strategies such as doping, composite formation, and formation of the protective layer to overcome such limitations and to ensure cost-efficient, high-performance DSSCs.
1. Introduction
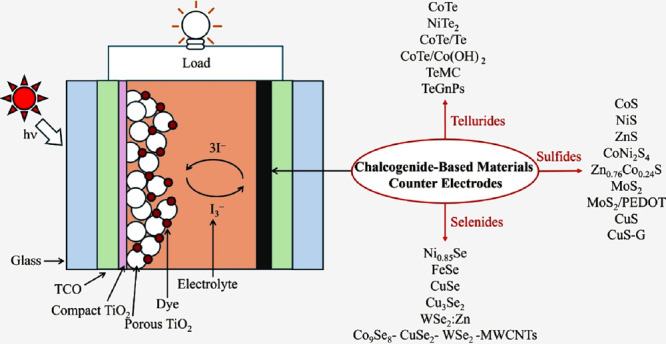
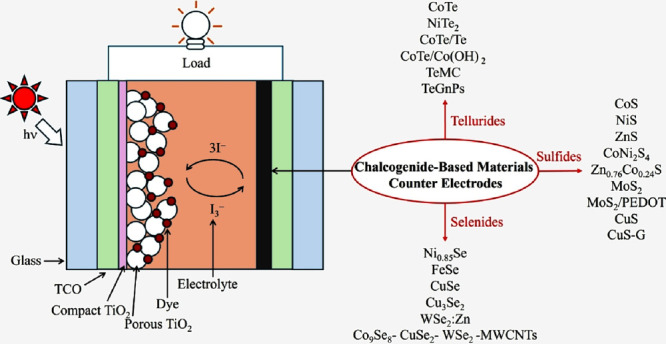
Dye-sensitized solar cells (DSSCs) have proved to be favorable photovoltaic technology in light of their low cost of fabrication, their simple mode of fabrication, and their mode of production that is benign. Essentially, DSSC is made up of three components (Figure ): dye-sensitized photoanode, redox electrolyte, and counter electrode (CE) for redox couple reduction, typically, for instance, for triiodide (I3 –) to iodide (I–). ,
1.
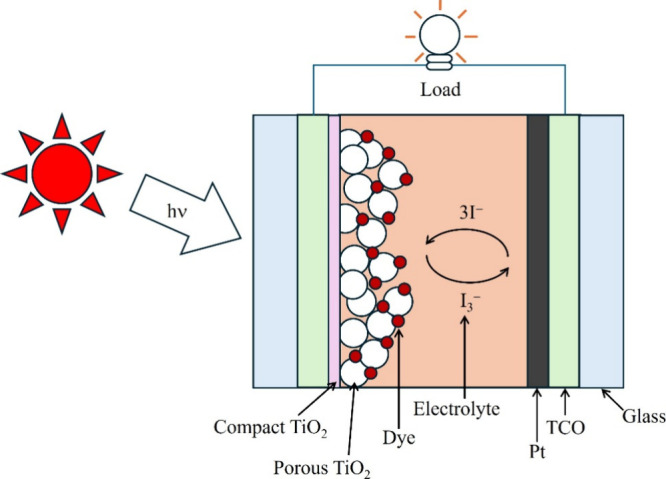
Structural illustration of the conventional DSSCs.
The state-of-the-art DSSCs have a focus on dyes and electrolytes. − Ruthenium-based dyes, such as N3, initially offered high PCE but high cost and environmental impacts. Thus, organic D-π-A dyes and porphyrin-based molecules are interesting developments due to their broad absorption, molecular tunability, and long-term stability. Simultaneously, the enhancement of DSSC’s performance requires synergy across all components. The production of better CEs can meet the requirements of new redox systems, providing affordable, stable, and high-performance alternatives instead of platinum. The CE is crucial for redox mediator regeneration and efficient transport of charges and hence directly influences the power conversion efficiency (PCE) of the device. Platinum (Pt) has conventionally been used for CEs, due to their excellent catalysis and excellent electric conductivity. However, Pt is rare and expensive; thus, there is an extensive search for substitute materials capable of performing similarly, but for a cheaper cost, such as graphene nanoplatelets (GnPs) cofabricated with halogen (F, I) or metalloids (Se, Te, and Sb), and carbon-based materials. − For example, a tellurium-doped carbon nanomaterial TeMC(P) synthesized via soft-templated carbonization was demonstrated as an efficient electrocatalytic CE for improving DSSC’s performance. The PCE increased to 12.69%, which is due to the low charge transfer resistance (R ct).
In this regard, materials in the category of chalcogenides, such as sulfides, selenides, and tellurides, have attracted considerable attention. Such materials have tunable electronic behavior, good catalysis, and built-in stability, and such favorable properties make them good candidates for replacing Pt in DSSCs.
Sulfide-based materials, such as cobalt sulfide (CoS), nickel sulfide (NiS), and molybdenum disulfide (MoS2) have been researched in-depth. Their efficient charge transfer capacity in addition to stable catalytic performance has made these materials efficient in producing PCEs that match those produced by Pt-based materials. , These materials utilize full leverage of various synthetic routes, including hydrothermal and solvothermal routes, to prepare nanostructured film materials with extensive surface area to realize optimum electrocatalyst performance. Parallel to sulfides, selenide materials such as nickel selenide (Ni0.85Se) and iron selenide (FeSe) possess superior capacity to absorb sunlight and superior charge transfer capacity. These materials’ capabilities, in particular, favor bifacial DSSC configurations, where light collection on both sides of the cell must be maximized. , In contrast, telluride compounds, though less developed, exhibit exceptionally good electric conductance and encouraging catalytic activity. Early reports have revealed that telluride-based CEs may rival or surpass standard Pt electrodes under optimal conditions. , Despite such encouraging breakthroughs, several limitations persist in the use of chalcogenide materials in CEs. Long-term stability is likely to be the most serious challenge. The corrosive environment inflicted by the I3 –/I– redox system may stimulate oxidation, phase transitions, and, in extreme conditions, dissolutions. For instance, sulfide electrodes, in spite of their excellent initial activity, undergo oxidation and local dissolutions, leading to gradually weakened catalytic activity. Conversely, selenides and tellurides also undergo degradations, such as insulating oxide layer formation, hindering efficient charge transport, and ultimately degrading device efficiency and lifespan. Aside from stability, scalability in the synthetic routes is also an insurmountable challenge. Synthesis techniques such as hydrothermal synthesis, solvothermal synthesis, electrodeposition, atomic layer deposition (ALD), and chemical vapor deposition (CVD) have been shown to have the potential to yield good-quality chalcogenide films having defined structure and composition. These, however, have limitations in their feasibility for large-scale industrial applications in the sense that they may require strict control of reaction conditions, elevated processing temperatures, or specialized equipment. An assessment of the available literature critically reveals that laboratory-scale devices have excellent figures of merit, but there is always an ongoing discrepancy between such data and commercial viability requirements. Chalcogenide materials such as Bi2S3 can be synthesized at a significantly lower cost, approximately $7 per gram, which is cheaper than Pt. Moreover, several studies demonstrate that certain chalcogenides, such as MoS2 and WS2, deliver competitive or superior PCEs in DSSCs when compared to Pt-based systems. Therefore, this analysis reinforces the practical value of chalcogenides for scalable and cost-effective DSSC applications.
This review aimed to offer an in-depth overview of chalcogenide materials for DSSC CEs. The recent achievements in materials synthesis, surface engineering, and optimization of their function have been synthesized while critically evaluating trade-offs between stability, catalytic activity, and scalability. In detail mechanisms of degradation, such as corrosion and oxidation by the electrolyte, and innovative strategies, such as doping, composite formation, and formation of the protective layer, this article tries to identify realistic pathways to overcome current limitations. Finally, more profound insights into such parameters will be beneficial in driving forward cost-efficient, robust, and high-performance DSSCs to meet future demands for energy.
2. Chalcogenide Materials for Counter Electrodes
Chalcogenide materials have extensively been under investigation for their excellent electronic behavior, excellent catalysis, and excellent stability and, hence, have good potential for advanced power applications, including DSSCs. Generally, sulfides, selenides, and tellurides have excellent advantages such as excellent charge transfer kinetics, low resistance to charge transferring, and tolerance to various doping species. Their innate properties result in excellent improvement in catalysis activity and stability of CEs, which are crucial components in DSSCs. The following is an extensive overview of applications of sulfides, selenides, and tellurides in DSSC CEs in dopants or active materials.
2.1. Sulfides
The search for low-cost, efficient substitute materials for Pt CEs in DSSCs has driven studies on sulfide materials due to their excellent electrocatalytic activity, excellent electric conductivity, and stability. CoS, NiS, ZnS, CoNi2S4, Zn0.76Co0.24S, MoS2, and composites of CuS have been synthesized through various routes including hydrothermal, solvothermal, and chemical deposition to enhance efficiencies in DSSCs. These materials have excellent catalytic activity toward reduction of the I3 –/I– redox couple and therefore have excellent prospects to replace Pt.
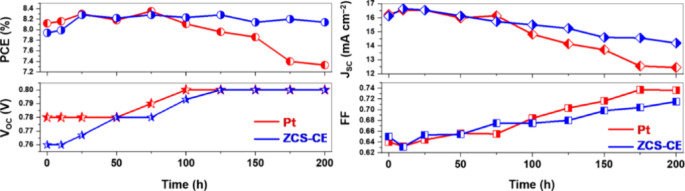
Subalakshmi et al. synthesized CoS, NiS, and ZnS, and CoNi2S4 and Zn0.76Co0.24S using a single-step hydrothermal technique. Among them, CoNi2S4 displayed better electrocatalysis, yielding a PCE of 4.03% comparable to Pt-DSSCs (4.59%). The excellent electrochemical behavior through cyclic voltammetry (CV), Tafel polarization, and electrochemical impedance spectroscopy (EIS) proved good charge transfer behavior and low dark current, minimizing recombination and stabilizing DSSCs. The structure revealed that lichen-like nanostructures of CoNi2S4 have an enhanced surface area (9 m2/g) for better catalysis. The aggregated spherical structure of Zn0.76Co0.24S proved to have good efficiency (PCE ∼ 3.52%), while CoS, having a flower-like structure, displayed good catalysis but low efficiency (2.4%). In comparative studies, Tashenov et al. synthesized Zn0.76Co0.24S (ZCS) through a single-step solvothermal technique, having good crystallinity in cubic-phase structure and homogeneous distribution of Zn, Co, and S. The electrochemical studies proved good redox activity through 0.38 V of peak-to-peak separation (E pp) comparable to Pt and 14.97 Ω·cm2 of charge transfer resistance (R ct). Though having greater R ct than Pt (4.18 Ω.cm2), ZCS-DSSCs proved to have a comparable PCE of 7.94%, 2.2% less than that of Pt-DSSCs (8.12%). Importantly, ZCS proved to have good long-time stability for 200 h, maintaining a PCE of 8.14%, while Pt-DSSCs declined to 7.33% through deactivation of Pt by corrosion of the electrolyte, as seen in Figure .
2.
PCE, J sc, V oc, and FF as a function of time for ZCS-based CE DSSCs. Reprinted with permission from ref (CC-BY). Copyright 2023 The Authors, Published by MDPI.
Molybdenum disulfide (MoS2) is also found to be an effective Pt-free CE material. Khan et al. synthesized MoS2-based DSSCs, and their PCE reached 5.01%, J sc reached 13.48 mA/cm2, and V oc reached 0.73 V. Compared to Pt-based DSSCs (5.8%, 14.82 mA/cm2, 0.75 V), while inferior to them, MoS2 exhibited good redox activity and good charge transfer behavior in the I3 –/I– redox system. Its large surface area (57.5 m2/g) and lamellar sheet structure made efficient electron transport and catalysis performance. Moreover, MoS2 exhibited good stability and no noticeable degradation under prolonged operation. Surprisingly, MoS2 proved to have good prospects in electrochemical sensor applications for detecting thiabendazole (TBZ) and reached 0.1 μM sensitivity and 7.47 μA/μM·cm2 sensitivity, exhibiting good versatile electrocatalytic activity. Xu et al. enhanced MoS2 further by compositing it with PEDOT (MoS2/PEDOT) and utilizing good conductivity and light transmittance of PEDOT. The MoS2/PEDOT composites exhibited low R ct (5.55 Ω·cm2) compared to pure MoS2 (103.5 Ω·cm2), exhibiting good catalysis for reduction of I3 –. DSSCs fabricated with MoS2/PEDOT achieved a 7.00% PCE under the front light and 4.82% under the rear light and, in bifacial application, better than Pt-based DSSCs. In similarly, the composition of MoO3/MoS2-reduced graphene oxide (rGO) CEs facilitating superior electrocatalytic activity of the I3 –/I– redox couple was presented. The enhancement of charge transfer was performed by modifying MoO3 CEs with MoS2-rGO and screen-printed onto MoO2 films. The optimized MoO3/MoS2-rGO composite significantly improves MoO3’s electrocatalytic activity, reducing R ct by 2.6-fold and increasing limiting and exchange current densities by 2.2 and 2.9 times, respectively. This results in a PCE of 5.0%, which is 2.9 times higher than that of bare MoO3 and 1.1 times higher than that of conventional Pt-based CE’s DSSCs. Furthermore, the development of MoS2/graphene quantum dot (M/GQD) composites through a one-step hydrothermal method also revealed great catalytic activity and charge transfer efficiency. DSSCs fabricated with M/GQD CEs achieved a PCE of 5.79%, significantly outperforming MoS2-based DSSCs (3.11%). The synergistic interaction between MoS2 and GQDs resulted in abundant catalytic active sites, improved conductivity, and enhanced stability, facilitating an efficient charge transfer mechanism.
Copper sulfide (CuS) and its composites have also been examined for substitute CEs. Zheng and Zhang have examined the microtopography of successive ionic layer adsorption and reaction (SILAR) and hydrothermally synthesized CuS CEs in quantum dot-sensitized solar cells (QDSCs). The optimal SILAR cycle synthesized CuS displayed minimum R ct, much lower than that for hydrothermally synthesized CuS. QDSCs made using SILAR synthesized CuS achieved a 3.83% PCE, better than those of any other CuS materials, due to optimal charge transport. High SILAR cycles, however, increased resistance to the transport of electrons, impairing performance. Tafel polarization analysis found SILAR-derived CuS CEs to have enhanced catalytic activity for polysulfide electrolytes, hence presenting an excellent substitute for Pt-based QDSCs’ CEs. Mohammadnezhad et al. have upgraded CuS by adding graphene (CuS-G) to the CE structure. The CuS-G nanocomposite was synthesized by low-temperature solution treatment, optimizing the content of graphene (2–10 vol %) to advance electrocatalyst activity. Structure analysis found a hexagonal structure of CuS and homogeneous distributions of graphene and hence enhanced charge transports. The DSSCs using CuS-3G (3.3 vol % graphene) achieved a 4.83% PCE, 12% better than for only CuS CE (4.31%) and comparable to Pt-based DSSCs (5.14%). The J sc (10.33 mA/cm2) and V oc (708 mV) for the CuS-3G electrode were larger, thanks to enhanced catalytic activity. High content of graphene, however, yielded microcracks, reducing the efficiency to 3.96%. The EIS and Tafel polarization found CuS-3G to have excellent electrocatalyst activity, hence presenting a good low-cost substitute for Pt for DSSCs. S-based materials hold excellent prospects for cost-effective CEs in DSSCs listed in Table . These materials exhibit comparable PCEs, good electrocatalytic activity, and enhanced stability compared to their Pt-based CEs and thus hold prospects for large-scale application in solar power. The introduction of such materials in hybrid structures, such as in the case of MoS2/PEDOT, MoO3/MoS2-rGO, and CuS-G, enhances their catalytic activity, and their possible application is expanded in photovoltaics, electrochemical sensors, and multifunctional power conversion devices. In addition, a comparison of long-term stability in evaluating CE materials for DSSCs is shown in Table . Furthermore, chalcogenide-carbon composite material CEs can also be fabricated to modify catalytic activity for enhancing DSSC’s performance. − For example, the development of the N,Se-codoped porous carbon (NSeC) CEs in DSSCs showed the improved PCE of 9.79%, greater than the platinum-based DSSCs. This behavior is due to efficient electron transportation. Moreover, the synergistic effect of nitrogen and selenium dopants also enhances iodine adsorption.
1. Comparison of Some Sulfide-Based Materials for CE Applications in DSSCs Measured at 100 mW/cm2 .
| CE | electrolyte | active area (cm2) | Jsc(mA/cm2) | Voc (V) | FF | PCE (%) | ref. |
|---|---|---|---|---|---|---|---|
| CoS | I3 –/I– | 0.16 | 7.51 | 0.52 | 0.61 | 2.40 | |
| NiS | I3 –/I– | 0.16 | 5.72 | 0.57 | 0.63 | 2.06 | |
| ZnS | I3 –/I– | 0.16 | 5.39 | 0.46 | 0.45 | 1.12 | |
| CoNi2S4 | I3 –/I– | 0.16 | 11.24 | 0.54 | 0.66 | 4.04 | |
| Zn0.76Co0.24S | I3 –/I– | 0.16 | 10.49 | 0.55 | 0.61 | 3.52 | |
| Zn0.76Co0.24S | I3 –/I– | N/R | 16.12 | 0.76 | 0.65 | 7.94 | |
| MoS2 | I3 –/I– | N/R | 13.48 | 0.73 | 0.51 | 5.01 | |
| MoS2/PEDOT | I3 –/I– | N/R | 13.73 | 0.74 | 0.69 | 7.00 | |
| CuS | polysulfide | 0.2 | 16.34 | 0.64 | 0.38 | 3.83 | |
| CuS-G | I3 –/I– | N/R | 10.33 | 0.71 | 0.66 | 4.83 | |
| NSeC | I3 –/I– | N/R | 18.87 | 0.70 | 0.74 | 9.79 |
N/R: not reported.
2. Stability Comparison of Chalcogenide-Based CE in DSSCs.
| CE | PCE (%) | Pt reference PCE (%) | long-term device stability | reported issues or strengths | ref. |
|---|---|---|---|---|---|
| MoS2 | 7.59 | 6.50 | N/A | cost-effective alternatives to Pt | |
| WS2 | 7.73 | 6.50 | N/A | ||
| CoNi2S4 | 4.04 | 4.59 | over 50 cycles of cyclic voltammograms | cost-effective alternatives to Pt | |
| MoS2 | 5.01 | 5.8 | over 40 consecutive Linear sweep voltammetry cycles, | PCE of the MoS2 electrode is closely matching Pt | |
| WSe2:Zn | 8.19 | 7.66 | N/A | cost-effective alternatives to Pt | |
| MoS2–C | 7.69 | 6.74 | 100 cycle cyclic voltammetry | low charge transfer resistance |
N/A: not available.
2.2. Selenides
In recent years, selenide materials have displayed excellent prospects for novel applications, exhibiting excellent catalytic activity and better charge transport ability and stability. Selenide compounds, including nickel selenide (Ni–Se), iron selenide (FeSe), copper selenide (CuSe), Cu3Se2 nanosheets, and zinc-doped tungsten diselenide (WSe2:Zn), and multicomponent selenides have been studied to optimize DSSC performance. These materials exhibit better electrocatalyst activity, enhanced light-absorbing ability, and greater durability and thus have displayed excellent prospects for application in future-generation DSSCs. Despite their merits, stability under prolonged light exposure and scalability of their fabrication technique are serious drawbacks to overcome.
Duan et al. recorded an excellent PCE for bifacial DSSCs, where optimal Ni0.85Se materials achieved a 10.63% PCE under bifacial light. The gentle solution-processing technique made fine-tuning of the Ni/Se ratios possible, optimizing catalytic activity and transparencies. The EIS measurement revealed that Ni0.85Se exhibited a significantly low R ct (2.96 Ω·cm2) compared to Pt (7.23 Ω·cm2) to provide efficient electron transport. Moreover, optical measurement found excellent transparencies (89%), better than in Pt-based CEs (36%), thus enhancing the bifacial light collection and overall DSSC efficiencies. Iron selenide (FeSe) nanoporous alloys have also been shown to be effective CEs in bifacial DSSCs, fabricated by using dodecylbenzenesulfonic acid (DBSA) to yield a porous structure. The nanoporous structure facilitates electrolyte diffusivity and catalysis, leading to an R ct of 5.5 Ω·cm2, significantly lower than that for Pt. FeSe-based DSSCs yielded 9.16% PCE under the front light and 5.38% under the rear light. Its excellent rear-side light transmittance (>70%) also augmented bifacial DSSC operation. Long-time stability testing demonstrated FeSe-based CE stability, confirming their feasibility for scalable replacement of Pt. Meng et al. have developed copper selenide (CuSe) CEs for QDSCs in a net-like, highly porous structure, thus leading to greater infiltrations of the electrolyte and increased redox sites for redox reactions. Two-step solution-processed synthesized CuSe CEs exhibited ultralow R ct (0.45 Ω·cm2) in comparison to Cu2S (1.2 Ω·cm2) and an efficient Sn2–/S2– redox couple. As such, QDSCs employed using CuSe CEs achieved a PCE of 5.77%, greater than for Cu2S-based systems (5.06%). Incident photon-to-current collection spectra displayed greater collection of charges in the solar range, showing the potential for CuSe to act as an advanced CE. Further structure improvement of CuSe was developed by growing Cu3Se2 nanosheets through a combination of sputtering and ion-exchange processes. Controlled FTO-supported formation of Cu3Se2 layer-by-layer-by-layer depositions made the compact and homogeneous formation of nanosheets possible, exhibiting greater electrocatalyst activity than for CuS. The electrocatalyst activity of Cu3Se2 CE displayed lower R ct (22.92 Ω·cm2) in comparison to CuS (68.59 Ω·cm2), showing greater charge transport. As such, CdS/CdSe QDSSCs employed using Cu3Se2 achieved a PCE of 4.01%, greater than that for CuS-based systems (3.21%). The greater surface area and enhanced charge transport property by Cu3Se2 played an extra role in QDSCs’ performance.
In addition to two-phase selenides, Abbood et al. have also revealed zinc-doped tungsten diselenide (WSe2:Zn) to be an efficient and cost-efficient CE in DSSCs. Doping by Zn upgraded WSe2’s structure and electronic behavior, giving a significantly lower R ct and enhanced catalytic activity. DSSCs fabricated using WSe2:Zn CE achieved an optimal PCE of 8.19%, better than that of Pt-based DSSCs (7.66%). Incident photon-to-electron collection spectra revealed an enhanced charge collection, and stability testing revealed that WSe2:Zn retained more than 90% of the initial efficiency after prolonged cycling, indicating good durability and scalability. The most recent methodology to include using multicomponent selenides, such as Co9Se8–CuSe2–WSe2 hollow balls (Figure ), mixed with multiwall carbon nanotubes (Co9Se8–CuSe2–WSe2+MWCNTs) have been revealed by Liu et al. The composite CE material was synthesized by a two-step solvothermal method to obtain a highly porous nanostructure, improving electrolyte diffusivity and transport of electrons. EIS analysis revealed an exceptionally low R ct of 4.16 Ω.cm2), significantly lower than for Pt (6.49 Ω·cm2). DSSCs fabricated using such CE achieved an excellent PCE of 9.23% higher than that of Pt of 8.08%. Furthermore, stability testing revealed that the composite CE retained 89.23% of the initial PCE after 1000 h, better than Pt (71.73%), as presented in Figure . The comparison of such Se-based materials for CE application in DSSCs is shown in Table .
3.
Illustration of Co9Se8–CuSe2–WSe2 hollow balls. Reprinted with permission from ref . Copyright 2024 ACS.
4.
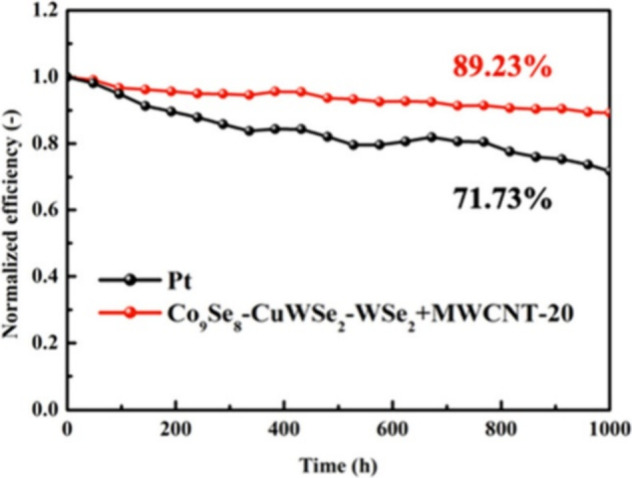
Normalized efficiency for 0–1000 h of DSSCs fabricated with Pt and illustration of Co9Se8–CuSe2–WSe2 + MWCNTs. Reprinted with permission from ref . Copyright 2024 ACS.
3. Comparison of Some Selenide-Based Materials for CE Applications in DSSCs Measured at 100 mW/cm2 .
| CE | electrolyte | active area (cm2) | Jsc(mA/cm2) | Voc (V) | FF | PCE (%) | ref. |
|---|---|---|---|---|---|---|---|
| Ni0.85Se | I3 –/I– | 0.25 | 24.34 | 0.74 | 0.59 | 10.63 | |
| FeSe | I3 –/I– | 0.25 | 17.72 | 0.72 | 0.72 | 9.16 | |
| CuSe | polysulfide | 0.1 | 18.88 | 0.52 | 0.59 | 5.77 | |
| Cu3Se2 | polysulfide | 0.1256 | 11.52 | 0.56 | 0.62 | 3.99 | |
| WSe2:Zn | I3 –/I– | 0.36 | 17.14 | 0.67 | 0.71 | 8.19 | |
| Co9Se8–CuSe2–WSe2+MWCNTs | I3 –/I– | 0.196 | 18.06 | 0.75 | 0.69 | 9.23 |
2.3. Tellurides
Tellurides have also been studied for their good electric conductivity and catalytic activity. Less than sulfides and selenides, metallic nanoparticle-doped tellurides have revealed considerable improvement in catalytic activity. The incorporation of telluride materials enhances electrocatalyst activity, charging and transferring capability, and stability and is a crucial parameter for PCE optimization in DSSCs. The current study presents a comprehensive evaluation of telluride materials, including nickel ditelluride (NiTe2), cobalt telluride (CoTe), and tellurium doping materials.
Guo et al. have studied the electrocatalytic activity of cobalt telluride (CoTe) and nickel ditelluride (NiTe2) nanostructures for application in platinum-free CE, in view of their ability to reduce I3 –. The nanostructures were synthesized using composite hydroxide-mediated (CHM) and subsequent annealing to develop crystallinity. High crystallinity is established by structure characterization by using X-ray diffraction (XRD) and scanning electron microscopy (SEM), indicating plate-like shapes between 300 nm and 1.2 μm. The electrochemical measurement by CV suggests an enhanced reduction process compared with standard electrodes made of Pt. The EIS observed apparently low R ct for both CoTe and NiTe2 compared to Pt and suggests enhanced electrocatalyst activity. CoTe and NiTe2 proved to have excellent PCE values of 6.92 and 7.21%, respectively, compared to 7.04% for Pt-based DSSCs. These facts suggest that NiTe2 is a good candidate for application in DSSCs because it has excellent charge transfer and enhanced electrocatalyst activity. Dong et al. synthesized CoTe and composite derivatives CoTe/Te and CoTe/Co(OH)2 in a heterogeneous system of water and oil and coated them on fluorine-doped tin oxide (FTO) substrates using spin coating. The structure, by means of XRD, found CoTe/Te to have excessive tellurium and CoTe/Co(OH)2 to have hydroxide impurities, both impairing the catalytic activity. The investigations using field emission SEM (FESEM) found CoTe to have good distributions of nanoparticles and nanobelts, whereas CoTe/Te and CoTe/Co(OH)2 have aggregated structures, hindering the efficient transport of charges. The electrochemical investigations found CoTe to have the lowest R ct (3.41 Ω·cm2) in comparison to the composites under investigation, performing better than CoTe/Te (12.16 Ω·cm2) and CoTe/Co(OH)2 (13.83 Ω·cm2). Tafel polarization found CoTe to have the highest J 0 value, confirming better catalytic activity. CoTe, in DSSCs, achieved an 8.59% PCE, performing better than CoTe/Te (7.68%), CoTe/Co(OH)2 (6.47%), and even standard Pt-based DSSCs (8.19%). The excellent efficiency of CoTe is credited to optimal structure, enhanced transport of charges, and enhanced electrocatalysis. The stability investigations proved to verify electrochemical stability of CoTe CEs in the long term, thus qualifying to substitute for Pt in DSSCs. Kim et al. developed tellurium-doped mesoporous carbon (TeMC) as a transparent CE for bifacial DSSCs, front and rear illuminations, as seen in Figure . The materials for TeMC were synthesized by stabilizing and carbonizing a block copolymer, polyacrylonitrile-block-poly(n-butyl acrylate) (PAN-b-PBA), optimizing doping levels for enhanced conductivity. The R ct (0.49 Ω·cm2) of TeMC was lower than that of Pt (0.62 Ω·cm2), while CV analysis revealed good redox capability for [Co(bpy)3 2+/3+] electrolyte. The 70% optical transmittance of TeMC made them perfect for bifacial DSSCs. Photovoltaic behavior revealed the PCE to be 9.43% for light on the front, better than for Pt-based DSSCs (9.23%), while the rear-side PCE of 8.06% remained comparable, though transmittance values were somewhat low. Surprisingly, TeMC CE revealed enhanced electrochemical stability for prolonged cycling with a small R ct increase of around four times compared with its original R ct. The increased R ct is very small when compared to that of Pt with increased R ct of approximately 22 times (Figure ). These reports make TeMC an ideal CE candidate for bifacial DSSCs, leading to green and cost-effective solar technology. In another doping host, Jeon et al. synthesized tellurium-doped graphene nanoplatelets (TeGnPs) by mechanochemical synthesis by planetary ball milling, where selective doping of Te happened on the graphene edge while maintaining crystallinity. The electrochemical analysis revealed an exceptionally low R ct of 0.15 Ω·cm2), better than for Pt-based CEs (R ct = 1.77 Ω·cm2). DSSCs using TeGnPs were revealed to have an excellent PCE of 11.58%, while Pt-based DSSCs had a PCE of 11.03%. The enhanced activity of TeGnPs was revealed to result in enhanced electrocatalytic activity, enhanced diffusion coefficient (1.84 × 10–6 cm2/s compared to 1.67 × 10–6 cm2/s for Pt), and enhanced charge transport. Furthermore, TeGnPs were revealed to have extreme stability in electrochemistry, maintaining their catalytic activity up to 1000 potential cycles, while for Pt CEs, serious degradation happened. Incident photon-to-electron conversion efficiency (IPCE) measurements revealed that DSSCs using TeGnPs have enhanced generation. These findings make TeGnPs an efficient and scalable alternative to Pt in high-performance DSSCs. The performance of DSSC fabricated using Te-based materials as CE is given in Table , indicating significant improvement compared to conventional Pt-based CE. The electrocatalytic activity, low resistance to charge transport, and enhanced photovoltaic behavior of NiTe2, CoTe, TeMC, and TeGnPs have revealed their prospects for application in DSSC. These materials have displayed encouraging outputs, and future investigations should focus on optimizing their structure, composite materials, and stability for a prolonged time to ensure their optimal application in reality. The improvement in telluride materials may provide prospects for efficient, strong, and cost-efficient DSSC technologies, thus enabling green technologies for energy.
5.
DSSCs fabricated with TeMC as a transparent CE for bifacial DSSCs. Reprinted with permission from ref (CC-BY). Copyright 2020 The Authors, Published by MDPI.
6.

Sequence EIS measurements of DSSCs fabricated with (a) Pt CE and (b) TeMC CE with inset figure of comparison of R ct for Pt and TeMC CEs with 10 EIS repeated measurements. Reprinted with permission from ref (CC-BY). Copyright 2020 The Authors, Published by MDPI.
4. Comparison of Some Telluride-Based Materials for CE Applications in DSSCs Measured at 100 mW/cm2 .
| CE | electrolyte | active area (cm2) | Jsc(mA/cm2) | Voc (V) | FF | PCE (%) | ref. |
|---|---|---|---|---|---|---|---|
| CoTe | I3 –/I– | N/R | 12.56 | 0.77 | 0.71 | 6.92 | |
| NiTe2 | I3 –/I– | N/R | 14.13 | 0.79 | 0.65 | 7.21 | |
| CoTe | I3 –/I– | 0.12 | 16.53 | 0.78 | 0.66 | 8.59 | |
| CoTe/Te | I3 –/I– | 0.12 | 15.98 | 0.76 | 0.34 | 7.68 | |
| CoTe/Co(OH)2 | I3 –/I– | 0.12 | 13.62 | 0.75 | 0.63 | 6.47 | |
| TeMC | SM315/Co(bpy)3 2+/3+ | 0.42 | 14.48 | 0.82 | 0.79 | 9.43 | |
| TeGnPs | Co(bpy)3 2+/3+ | 0.141 | 16.53 | 0.92 | 0.76 | 11.58 |
3. Synthesis Techniques for Chalcogenide-Based Materials
3.1. Hydrothermal Synthesis
The hydrothermal method is by far the most commonly employed bottom-up technique for the syntheses of nanomaterials due to its operational simplicity, cost, high purity, yield, low impact on the environment, and flexibility for the generation of diverse ranges of hybrid materials. The procedure usually involves reactions in sealed autoclaves under elevated pressures and autogenous temperatures. The unique strength of hydrothermal synthesis lies in fine-tuning of the resultant nanostructure’s morphology, crystallinity, purity, and composition by fine-tuning parameters such as temperature, pressure, pH, time, and precursor concentration. In hydrothermal conditions, precursor solubility is enhanced considerably, and thus efficient mechanisms for growth and nucleation take place, and well-defined nanostructures develop. The supersaturation levels and rate of a reaction determine the resultant particle geometry and size, and by incorporation of surfactants, mineralizers, or templating agents, fine-tuning is achieved. The technique is particularly favorable for the generation of metal oxides, hydroxides, sulfides, and complex nanocomposites, otherwise difficult to synthesize by solid-state or gas-phase routes. The technique is able to stabilize metastable phases, otherwise unavailable under atmospheric conditions, by utilizing unique solvothermal dynamics under elevated pressures. This ability is crucial for designing functional materials with electronic, optical, and catalytic behavior for applications in energy storage, photocatalysis, and bioapplications.
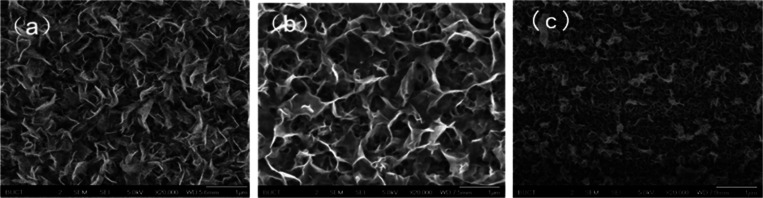
The process is mainly carried out in sealed reactors to obtain increased pressures. However, modifications, including open-vessel syntheses or the introduction of external pressures, can be carried out to push the reaction condition range and support the unique requirements of the material. As an example, in laboratory-scale hydrothermal WS2 or MoS2 synthesis, the process is commenced by dissolving precursor metals like ammonium molybdate ((NH4)6Mo7O24) in WS2 or sodium tungstate (Na2WO4) in WS2 in deionized water. The source of sulfur, e.g., thiourea (CH4N2S) or element sulfur (S), is added to trigger preferential growth toward metal sulfide compounds. For the development of target nanostructures, growth kinetics can be manipulated by including a surfactant (e.g., cetyltrimethylammonium bromide, CTAB) or a reducer (e.g., hydrazine hydrate) to prevent agglomeration. , Precursor solution is mixed violently at an appropriate time to obtain uniformity in mixture and homogeneity, and pH can be controlled by introducing acids (e.g., hydrochloric acid, HCl) or bases (e.g., sodium hydroxide, NaOH) to manipulate the reaction pathway and resultant structure. The homogeneous solution is transferred to a Teflon-lined stainless-steel autoclave, tightly sealed, and heated under regulated temperatures, usually 150 and 250 °C, for 12–24 h. High-pressure and superheat water under such conditions provides an exceptional environment for precursor dissolutions, recrystallization, and regulated nucleation and thus for crystalline nanostructural formation. The standard hydrothermal synthesis of MoS2 or WS2 is shown in Figure and WS2, MoS2, and composites of MoS2/WS2 nanosheets are presented in Figure . The examples of regulated growth mechanisms of MoS2 may be described in eqs –, and WS2 may be described in eqs and . ,
| 1 |
| 2 |
| 3 |
| 4 |
| 5 |
| 6 |
7.

Schematic diagram of the conventional hydrothermal synthesis.
8.
SEM images of (a) WS2, (b) MoS2, and (c) MoS2/WS2 nanosheets. Reprinted with permission from ref (CC-BY-NC). Copyright 2017 RSC. Copyright 2017 The Authors, Published by RSC.
After completion, the autoclave is allowed to cool to room temperature before it is opened naturally. The synthesized nanostructures are harvested by centrifugation or by filtration and multiple washing steps using deionized water and ethanol to ensure the elimination of any remaining contaminant, precursor, or organic residue. The purified product is subsequently dried in an oven to obtain a fine powder. In some cases, postsynthesis thermal treatment (annealing) in inert (argon or nitrogen) or in a reducing atmosphere (hydrogen) is performed to optimize crystallinity and electronic and electrocatalytic activity. Hence, such materials are suitable for application.
3.2. Solvothermal Synthesis
Solvothermal synthesis is similar to hydrothermal synthesis but employs organic solvents instead of water, offering distinct advantages in reaction kinetics, precursor solubility, and nanostructural formation. The use of organic solvents, such as alcohols, amines, ketones, or ionic liquids, enables precise control over key processes, including dissolution, nucleation, and growth, making solvothermal reactions more advanced than hydrothermal systems. Additionally, different solvents significantly influence various material properties, such as morphology, crystallinity, phase stability, and polarity. The capability to sustain metastable phases and heterostructural complexities is also made possible by using nonaqueous solvents, enabling the formation of advanced heteromaterials of enhanced optical, electronic, and catalytic behavior. The technique also allows for the lowering of reaction temperatures, compared with conventional hydrothermal routes, through several organic solvents having low boiling points and enabling reactions under milder thermal conditions. Solvothermal strategies also provide greater flexibility in modulating precursor metals’ oxidation states through solvent-mediated redox and, thus, in the formation of metal chalcogenides, phosphides, nitrides, and other functional materials, whose formation is otherwise recalcitrant through aqueous routes. For example, in the case of the hydrothermal method, it is initiated by dissolving an adequate precursor metal, for example, molybdenum chloride (MoCl5) or tungsten chloride (WCl6), in an organic solvent, for example, ethanol, ethylene glycol, or dimethylformamide (DMF), capable of also serving as a structure-directing agent. The choice of solvent significantly influences the atmosphere of the reaction, for organic solvents may alter precursor solubility, diffusivity, and reactivity. The incorporation of a sulfur source, for example, thiourea, thioacetamide, or elemental sulfur, is required to favor the formation of compounds of metals and chalcogens. Surfactants or capping reagents may also be incorporated to control growing up, to limit aggregation, and to provide desirable shapes to nanostructures, for example, nanosheets, nanorods, nanoflakes, and spheres. −
3.3. Electrodeposition
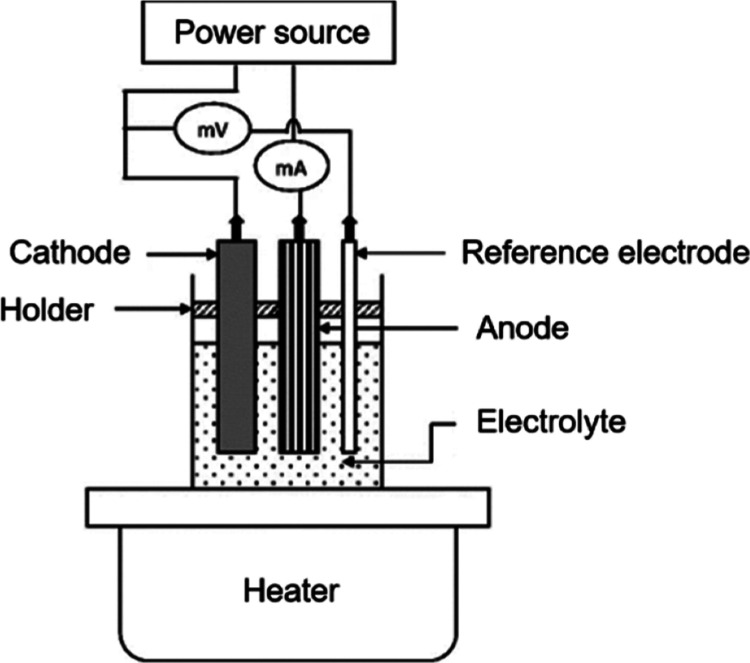
The electrodeposition (Figure ) is typically performed in an electrochemical cell, in which precursor salts’ metallic ions are reduced and deposited in a growing thin film under an electric field. The use of controlled deposition potential assures homogeneous nucleation and growth and is crucial for the formation of crystalline and adherent films having desirable optoelectronic behavior. The technique is also able to modulate deposited material’s stoichiometry and phase by modulating precursor concentration, pH of the electrolyte, and temperature, and thus metastable phases and heterostructured materials having augmented function. , Another advantage of electrodeposition over vacuum-based film-forming technologies is electrodeposition’s ability to function under atmospheric conditions and lack of any requirement for advanced equipment or high-energy inputs. Moreover, electrodeposition is able to coat large areas and produce a conformal film on substrates of complex geometry and is thus of special interest for application in photovoltaics, electrocatalysis, and electrodes for supercapacitors. In general, substrate cleaning is an initial operation for electrodeposition by ultrasonication in acetone, ethanol, deionized water, and oxygen plasma or UV-ozone treatment to promote wettability and film adhesion. An electrolyte solution is prepared by dissolving metal precursors (i.e., chloride and sulfate) in deionized water or mixed solvent system, and pH is regulated (in general 2–5) by using acids or bases to modulate deposition kinetics. The incorporation of complexing species such as EDTA or citrate is possible to stabilize metal ions and modulate nucleation. The electrodeposition is performed in a three-electrode setup, having in it the cleaned substrates as a working electrode, a carbon or platinum CE, and a Ag/AgCl type of reference electrode, and connected to a potentiostat. The deposition is either performed in potentiostatic mode (constant voltage, in general, −0.8 to −1.2 V versus Ag/AgCl), galvanostatic mode (constant current, 0.5–5 mA/cm2), or pulsed mode for improvement in film uniformity. The electrochemical reduction of precursor metals and chalcogens on the substrate surface gives rise to CoSe2 or NiS film formation, the crystallinity and rate of reaction of which are governed by deposition parameters and by the composition of the electrolyte. The deposited films, after their formation, undergo washing in deionized water and subsequent drying at approximately 80 °C and postannealing at 300–500 °C in an inert or reducing atmosphere to improve crystallinity and catalytic activity. Furthermore, treatments may also be performed depending on the CE thin film’s property requirements.
9.
Conventional schematic of electrodeposition. Reprinted with permission from ref (CC-BY). Copyright 2020 The Authors, Published by MDPI.
For CoSe2 electrodeposition, e.g., Na2SeO3 and Co(CH3COO)2 in electrolytes of LiCl, preparation by a formulation route to obtain CoSe2 electrode material can take two pathways through Se-induced reduction on the surface and nucleic reduction of Se through eqs and and eqs – independently, resulting in porous CoSe2 films.
| 7 |
| 8 |
| 9 |
| 10 |
| 11 |
| 12 |
3.4. Chemical Vapor Deposition
Chemical vapor deposition (CVD) is a commonly used technique to deposit ultrathin, pure, and highly crystalline films. − This is done by controlling the reaction between the gas-phase precursor in precisely controlled temperatures, pressures, and gas flow to deposit uniform film with desired structural, electronic, and optical properties. , Volatile precursor metals in traditional CVD systems decompose and react with heated substrate to initiate layer-by-layer growth by the subsequent extension of atomic layer growth through nucleation in two-dimensional (2D) material. Nucleation kinetics and subsequent extension of atomic layer growth are controlled by reaction conditions to determine film morphology, film thickness, and crystallinity. Factors including precursor concentration, carrier gas composition, growth temperatures, and substrate material control nucleation kinetics and subsequent extension of atomic layer growth. Choosing a precursor, varied between metal–organic chemicals like molybdenum hexacarbonyl to atomic sources like sulfur vapor, is required to modulate film stoichiometry and to keep defect concentration to minimal amounts. ,
CVD is particularly advantageous in growing transition metal dichalcogenides (TMDs) like MoS2 (Figure ) since it can achieve atomically thin monolayers with unparalleled uniformity over extensive areas. Layer-dependent bandgap properties in these monolayers make them incredibly desirable to apply in nanoelectronics, optoelectronics, and catalysis. Furthermore, scalability in CVD allows seamless incorporation into industrially feasible production processes to propel next-gen electronic and photovoltaic device development. Modified variants in the forms of plasma-enhanced CVD (PECVD) and metal–organic CVD (MOCVD) offer additional film growth process control through altered reaction energetics and precursor breakdown pathways. , PECVD uses a plasma environment to induce precursor disassociation below conventional temperatures to deposit over thermally sensitive substrates. Meanwhile, MOCVD utilizes metal–organic precursors to achieve the compositional specificity required to construct complicated heterostructures for doping purposes.
10.
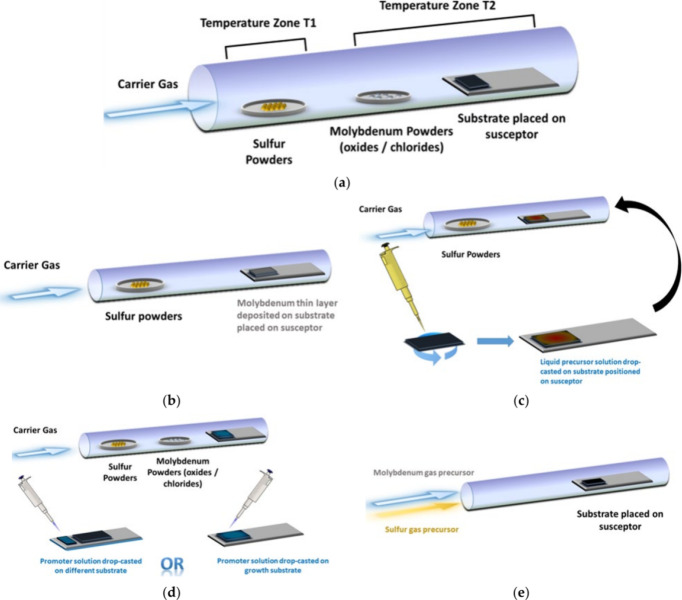
Synthesis of MoS2 flake through CVD using different deposit conditions: (a) sulfur and molybdenum powders deposited on substrates, (b) sulfur powders deposited on a molybdenum thin layer, (c) sulfur powders deposited on liquid molybdenum precursors, (d) sulfur and molybdenum powders and drop-casted promoters, and (e) sulfur and molybdenum gases deposited on substrates. Reprinted with permission from ref (CC-BY). Copyright 2021 The Authors, Published by MDPI.
Though advantageous in several aspects, CVD has limitations in precursor material choice, byproduct management, and substrate compatibility. Temperatures required to achieve growth, usually over 600 °C in TMDs, restrict substrate options and demand superior thermal management schemes. Additionally, layer thicknesses are controlled to demanding standards while minimizing grain boundaries. A typical example of growth of chalcogenide material by CVD is MoS2, SnS, and SnS2, in which precursor vapors react in thermally controlled temperatures and pressures. This is preceded by precursor selection in most examples in the form of sources of metals (such as molybdenum trioxide (MoO3) or tin chloride (SnCl4)) and sources of chalcogens (such as sulfur (S) or hydrogen sulfide (H2S)) in accordance with their reactivity and volatility. − Deposition chambers are heated to specific temperatures between 500 and 900 °C in MoS2 (Figure ) or between 300 and 600 °C in SnS or SnS2 to induce precursor breakdown and reaction.
11.
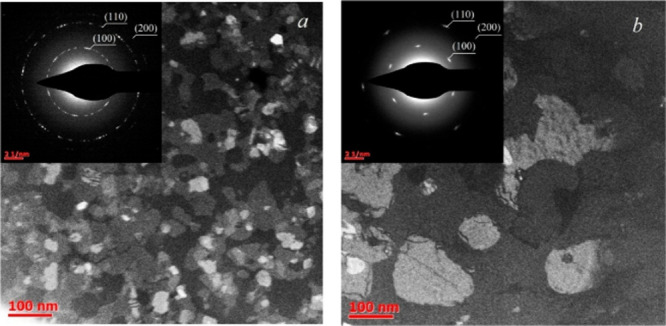
TEM images of MoS2 films with SAED patterns deposited at (a) 750 and (b) 850 °C. Reprinted with permission from ref (CC-BY). Copyright 2023 The Authors, Published by MDPI.
3.5. Atomic Layer Deposition
Atomic layer deposition (ALD) is an advanced film fabrication technique that offers atomic-level control over the layer composition and layer thickness in chalcogenide layers. Unlike in chemical vapor deposition (CVD), in ALD, sequence-by-sequence, self-limiting reaction steps between precursor gases and the substrate surface are used to obtain strongly conformal film growth over intricate, high-aspect-ratio geometries. , A deposition is done by a cyclic sequence of precursor introduction and purging steps: introduction of a metal precursor to a reaction chamber where chemisorption to the substrate surface results in a monolayer; subsequent purging by an inert gas to remove the byproduct and excess precursor; and introduction of a precursor to a chalcogen precursor to react with the chemisorbed layer to yield desired chalcogenide material. This process is repeated iteratively to deposit to desired film thickness to obtain film growth to an atomic level. ALD is conducted in relatively lower temperatures (150–400 °C) compared to conventional film growth to prevent heating substrate to destructive temperatures in specific substrate systems to achieve desired properties (e.g., smooth topology, Figure ). ,
12.
Smooth topology of Al2O3 films deposited at 80 °C (T80), 100 °C (T100), 150 °C (T150), and 250 °C (T250). Reprinted with permission from ref (CC-BY). Copyright 2022 The Authors, Published by Springer.
Films deposited by ALD possess exceptional uniformity, high purity, and better step coverage, making this process uniquely well-suited to deposit pinhole-free chalcogenide coatings. However, owing to its layer-by-layer growth process, ALD is relatively slow in comparison to CVD, and the exorbitant price of specialized equipment and precursor chemicals is a hindrance to mass production in large areas. However, ALD is an impactful process to employ in ultrathin, highly controlled requirements in electronic, photovoltaic, and catalytic device development. For instance, ultrathin MoS2 monolayers (Figure ) can be deposited by ALD using MoCl5 and H2S precursor sources. Deposition is carried out in sequence in a controlled manner: an initial heating in low pressure to 900 °C is followed by ramping to growth temperatures in the range of 700–900 °C to obtain optimum crystallinity while minimizing defects. MoCl5 is added in between during ALD cycles with nitrogen purges in between, followed by H2S introduction and final nitrogen purge to obtain layer-by-layer growth in a controlled manner over conventional CVD. Postannealing in an atmosphere of H2S at 900 °C optimizes crystallinity and optics further.
13.

MoS2 monolayers deposited via ALD. Adopted with permission from ref (CC-BY). Copyright 2021 The Authors, Published by AIP.
3.6. Solution-Based Synthesis and Spin Coating
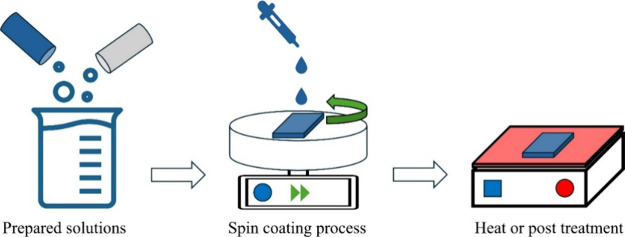
Solution-based deposition techniques provide tunable film composition control, an attribute that is not always accessible to vacuum methods, including thermal evaporation. Stoichiometric control is achieved to a high degree through adjustment of precursor concentration and solution factors in these methods, something desirable in intricate materials in particular. Additionally, since solution-based deposition is conducted in ambient pressure, vacuum equipment is not needed, significantly minimizing production costs. However, among this technique’s key limitations is contamination by residue solvents in the film matrix, something that can have detrimental effects on structural, optical, and electronic film qualities. Postdeposition thermal annealing is employed to alleviate these effects by enhancing film densification, evaporating residues of solvents, and ensuring phase purity to realize the optimum film quality ultimately. Extensively used solution routes such as sol–gel processing and spin coating offer facile yet efficient routes to prepare defect-free, uniform chalcogenide films such as Ge20Sb5S75/Ge20Sb5Se75/Ge20Sb5S75 (S/Se/S) and Ge20Sb5Se75/Ge20Sb5S75/Ge20Sb5Se75 (Se/S/Se), as depicted in Figure .
14.
Chalcogenide films of S/Se/S and Se/S/Se deposited via a spin coating. Reprinted with permission from ref (CC-BY). Copyright 2023 The Authors, Published by Springer.
Sol–gel processing is carried out by starting to prepare a colloidal dispersion (sol) of precursor metals, generally metals–salts such as copper acetate in the case of Cu2S or nickel nitrate in the case of NiS, mixing with a source of chalcogen such as thiourea or sulfur powder in an appropriate solvent such as ethanol or water. This sol is then hydrolyzed and polycondensed to obtain a networked gel that is deposited over a substrate by dip-coating or by spin-coating routes. For example, Ranjith et al. demonstrated a solution growth method to prepare the CdS spheres by mixing cadmium acetate dihydrate (Cd(CH3COO)2·2H2O) and polyvinylpyrrolidone (PVP) in ethylene glycol (EG) under stirring as the initial cadmium precursor. Then, addition of thiourea (CH4N2S) was performed by heating, centrifuging, and annealing to obtain CdS nanospheres. In spin coating, prepared solutions are dropped on a substrate, followed by a spin-coating process to obtain a uniform film, as presented in Figure . The deposition film is dried to remove residues of solvent and annealed in a reducing or inert atmosphere to initiate phase development and crystallization. Flexibility in controlling film thickness and morphology by changing the concentration in the solution, spinning speed, and annealing conditions offers a wide variety of solution methods. Solution methods prove to be especially advantageous in low-temperature processing, substrate compatibility with plastics, and scalability in film deposition over large areas.
15.
Illustration of the conventional spin-coating process.
3.7. Sulfurization and Selenization of Metal Precursors
The thermally treated methods comprising sulfurization and selenization have been instrumental in synthesizing chalcogenide material through the process of transforming precursor metals or metal oxide material to products like MoS2, WS2, CoSe2, and NiS. , Conversion processes involve treating predeposited metals or metal oxide film samples in an atmosphere whole of controlled amounts of sulfur or selenium gas heated to temperatures greater than ambient. The solid-state reaction by a thermally stimulated solid provides solid-phase incorporation of chalcogen in precursor material to obtain stoichiometric and crystallinity-controlled metal chalcogenide phases. The consistency and effectiveness of these reactions are determined by key factors such as precursor film thickness, auxiliary gases, vapor pressure, and temperature, each influencing the phase purity, material properties, and nucleation kinetics. Under an atmosphere composed of inert (argon or nitrogen) or reducing (hydrogen) gas, the precursor film is placed in a tube furnace.
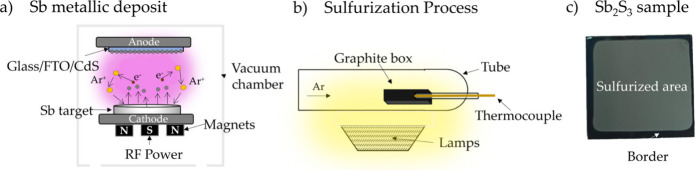
For sulfurization, sulfur sources such as elemental sulfur (S) powder, CdS, or hydrogen sulfide (H2S) are introduced at temperatures ranging 300–600 °C, allowing the reaction between sulfur and metal precursors to form crystalline metal sulfide layers. One example is the sulfurization of antimony sulfide (Sb2S3) thin films. Initially, CdS was deposited on an FTO substrate via RF sputtering. Subsequently, an antimony (Sb) layer was sputtered onto the CdS films, followed by sulfurization at 300 °C in an argon atmosphere, forming Sb2S3, as shown in Figure . Ai et al. also demonstrated the sulfurization of MoO3–x . The process was carried out using a CVD method, where sulfur powder was transported by N2 gas and deposited onto MoO3–x at 800 °C. This sulfurization process resulted in the formation of MoS2 nanoparticles, as depicted in Figure .
16.
Schematic sulfurization for Sb2S3 fabrication. Reprinted with permission from ref (CC-BY). Copyright 2024 The Authors, Published by MDPI.
17.

Sulfurization of MoS2 from MoO3–x . Adopted with permission from ref (CC-BY). Copyright 2025 The Authors, Published by ACS.
Similarly, selenization is conducted by converting metal or metal oxide precursors to metal selenides by treating them with an ample source of selenium vapor. This is employed to prepare substances, such as CoSe2, MoSe2, and NiSe. As an example, CoSe2/MoSe2 structures have been synthesized through the selenization of CoMoO4 nanosheets grafted onto carbon cloth. The process was carried out at 450 °C using selenium (Se) powder under transportation by an Ar/H2 atmosphere, resulting in the formation of CoSe2/MoSe2, as shown in Figure . The integration of CoSe2/MoSe2 structures reveals excellent electrocatalytic activity, as evidenced by their significantly lower R ct values compared to CoMoO4, CoSe2, and MoSe2 (Figure ), making them highly beneficial for water-splitting applications. A selenization of the NiSe2–CoSe2 composite was also successfully presented for efficient CEs. The synthesis was conducted by direct selenization of the nickel–cobalt hydroxide layer. The synthesized NiSe2–CoSe2 CEs explore excellent electrochemical catalytic activity for the I3 –/I– redox, which results in an achieved PCE of 8.21%, higher than Pt- and CoSe2-based CEs of 7.62 and 6.78%, respectively.
18.
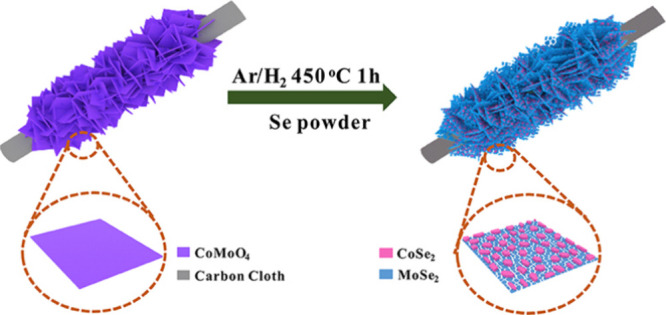
Selenization of CoMoO4 nanosheets using Se powder to form CoSe2/MoSe2. Reprinted with permission from ref (CC-BY). Copyright 2020 The Authors, Published by Frontiers.
19.

R ct analysis of CoMoO4, CoSe2/MoSe2, CoSe2, and MoSe2. Reprinted with permission from ref (CC-BY). Copyright 2020 The Authors, Published by Frontiers.
To evaluate these synthesis methods, a comparison is presented in Table , providing a summary of advantages and limitations for the synthesis methods. Moreover, a comparison of key factors, including electrocatalytic activity, electrical conductivity, chemical stability, material cost, material structure, and surface properties of chalcogenide materials based on the different synthesis methods, is also presented in Table .
5. Comparison of the Synthesis Methods for Chalcogenide Materials.
| synthesis method | advantages | limitations |
|---|---|---|
| hydrothermal | -morphology control | -long reaction times |
| -environmentally friendly | -high-pressure requirements | |
| -low-cost | -limited scalability | |
| -suitable for diverse nanomaterials | ||
| solvothermal | -solvent tunability | -toxicity solvents |
| -complex structure setup formation | -flammability risk | |
| electrodeposition | -ambient condition synthesis | -requires precise control of potential/current |
| -low-cost system | -requires conductive substrates | |
| -scalable on substrates | -limited to thin-film applications | |
| CVD | -produces ultrathin, high-purity, crystalline films | -high-temperature process |
| -scalable for industrial applications | -expensive equipment | |
| -atomic-layer control | -requires toxic gas management | |
| ALD | -enables atomic-level thickness control | -slow deposition rate |
| -conformal coating on high-aspect-ratio structures | -high cost for equipment/precursors | |
| -low-temperature process | ||
| solution-based/spin coating | -simple and low-cost processes | -solvent residue contamination |
| -vacuum-free | -requires post-treatment | |
| -flexible substrates | ||
| sulfurization/selenization | -simple conversion process | -requires precise control of gas flow, vapor pressure, and temperature |
| -high crystallinity | -not suitable for thermally sensitive substrates | |
| -easily adaptable to various substrates |
6. Comparison of Key Factors for Synthesized Chalcogenide Materials Based on the Different Synthesis Methods.
| synthesis method | electrocatalytic activity | electrical conductivity | chemical stability | material cost | material structure | surface properties | ref. |
|---|---|---|---|---|---|---|---|
| hydrothermal | N/A | semiconducting behavior | stable under ambient condition | low | nanoparticles | uniform distribution | |
| electrodeposition | moderate to high (depends on deposition control) | moderate to good | moderate to good | low | uniform thin films | microporous or nanotextured surfaces | |
| CVD | N/A | high conductivity in metallic TMDs (e.g., NbS2, VSe2) | monolayers sensitive to oxidation | moderate to low cost, but dependent on metal precursor (e.g., MoCl5, WCl6) | thin films, morphologies include triangles, ribbons, spirals, and hexagons | surface properties depend on morphology and crystal orientation | |
| ALD | high | N/A | stable | moderate | uniform | low roughness, smooth surface | |
| spin coating | N/A | N/A | significantly increased chemical resistance with annealing | very low | amorphous structure | smooth, crack-free surfaces |
4. Stability and Corrosion Resistance
The durability in operation and PCE in DSSCs are significantly influenced by stability over the long term as well as by corrosion resistance of CE material. Electrocatalytic performance in the triiodide/iodide (I3 –/I–) redox reaction is directly influenced by CE stability. Chalcogenide-derived materials, such as sulfides (S-based), selenides (Se-based), and tellurides (Te-based), are among those that have been in widespread attention because of superior electronic conductivity, superior catalytic performance, and economic viability. Their sensitivity to deterioration in electrolyte atmosphere in DSSCs is an additional challenge to these materials, influencing their electrochemical stability and practice applicability.
Among sulfide-based chalcogenides, MoS2, NiS, and CoS have been investigated in-depth, owing to their superior catalytic performance and electronic conduction. These sulfides exhibit efficient electron transfer and superior catalytic performance in enabling the I3 –/I– redox reaction. Thus, these sulfides have been regarded as potential alternatives to conventional Pt-based CEs. However, despite these desirable electrochemical characteristics, sulfide materials degrade significantly in stability in electrolyte solutions. Oxidation is sped up by the I3 –/I– redox couple in sulfides to cause phase changes in sulfides such as NiS transforming to NiS2 or MoS2 transforming to MoO3. Such phase changes significantly degrade catalytic performance over time by inhibiting electron transfer and reducing the electrochemical active area, ultimately leading to performance deterioration. Aside from oxidation, sulfides like NiS2 and CoS exhibit partial iodide electrolyte solubility, resulting in leaching, leading to morphological instability and ongoing consumption of the CE material. This not only lowers structural strength in the electrode but also forms undesirable secondary reactions in the electrolyte, driving further device deterioration further. Counter to these issues, researchers have studied various ways to stabilize these systems by protecting with carbonaceous material (such as graphene or carbon nanotubes), core–shell systems by passive layering by an inert material, and conductive polymer passivation to preserve catalytic functionality while inhibiting oxidative deterioration.
Materials in the form of selenide, e.g., diselenides in the forms of cobalt (CoSe2), nickel (NiSe2), and copper (Cu2Se), exhibit better performance in an electrocatalytic context owing to improved conduction of electricity and intense redox–catalyst interface interactions. When compared to sulfides, better charge conduction results in improved performance in an electrochemical context in DSSCs. However, stability is an issue in these materials owing mainly to sensitivity to liquid electrolyte-induced breakdowns by oxidation, primarily upon increased irradiance in sunlight and rising temperatures. NiSe2 is an illustration that changes to various forms through phase transformations similar to sulfides to inactivate active sites in addition to overall reductions in efficiency. This is further complicated by additional growth in insulative formations of selenium oxide that hinder the transfer of charge while reducing performance in an electrochemical context. Besides this, selenium-containing materials have been plagued by partial dissolution in iodide electrolytes, causing structural degradation and poor performance over time. Many methods have been proposed to address these issues. One potential method is to alloy selenides with other transition metals to increase phase stability and tolerance to oxidation. Encapsulation in stable matrices such as graphene or carbon nanotubes has been demonstrated to have promise in making them more durable in an electrochemical context. Another viable method is to prepare hybrid composites, e.g., CoSe2/MoS2 structures, where cooperative catalytic advantages of various chalcogenide species can be achieved while reducing each’s weaknesses.
Telluride-based CEs, including antimony telluride (Sb2Te3) and bismuth telluride (Bi2Te3), possess superior electronic conductance and catalytic performance and thus have potential in DSSCs. Their superior charge carrier mobility enables efficient charge transfer and intense redox interacting capabilities. Their stability over a longer period is a concern. Tellurium is prone to facile oxidation in both ambient and electrolyte environments to form nonconductive tellurium oxides that interfere with the transfer of charge while reducing electrocatalytic performance. Gradual performance degradation is brought about by loss of active site through oxidation over time.
In addition to problems related to oxidation, tellurium compounds experience leaching in iodide electrolytes to induce extensive morphological changes and reduce the overall life in electrodes. This process is detrimental to CE structural stability and contributes undesirable electrochemical side reactions to further degrade stability in DSSCs further. Many material design routes have been studied to mitigate these issues. Encapsulation in chemically stable matrices can prevent electrolyte exposure to tellurides, while the introduction of transition metals such as titanium (Ti) or vanadium(V) has been shown to inhibit oxidation. Hybridization between stable conductors and tellurides is another viable method, ensuring durability while maintaining catalytic effectiveness. ,
5. Future Challenges
Advancement in the area of DSSCs with chalcogenide-based CEs is hindered by significant limitations in scalability, cost-effectiveness, and material stability. Scalability is a key consideration because most methods of synthesis, including hydrothermal synthesis, CVD, and electrodeposition, turn out to be cumbersome when scaled to mass production. Hydrothermal synthesis is conducted under high-pressure and high-temperature conditions that turn out to be challenging to reproduce in an industrially viable process. Similarly, whereas exacting film quality is achieved by CVD, the processing cost is prohibitive and equipment requirements are complicated to realize in mass production. As alternatives to these limitations, researchers have been working to explore novel methods of fabrication through routes in solutions and roll-to-roll processing that have greater potential in reducing production costs while maintaining scalability. −
Though cheaper alternatives to Pt-based CEs in the form of chalcogenides can be achieved, material cost savings in precursor cost can be offset by the processing technique cost. Dopant incorporation to enhance performance in catalysis contributes to material-related cost aspects. Scaling down material costs by making precursor development cheaper, reducing synthesis routes to simplified forms, and recycling can support improving overall economic viability in chalcogenide-based DSSCs. Material stability is another essential consideration for long-term DSSCs. Chalcogenide-based CEs demonstrate the potential long-term applications comparable to Pt-based CEs for stability toward I3 –/I– redox electrolytes. Previous research has presented that excellent electrochemical stability is observed for chalcogenide-based CEs, such as FeSe2, TiS2, MoS2, and CoS2. Thus, it serves as a viable alternative to expensive, highly stable materials like Pt, as well as to low-cost materials with poor stability and weak corrosion resistance, such as carbon-based materials and conjugated polymers. In addition, cobalt-based electrolytes, such as Co2+/Co3+, enable high Voc in DSSCs due to their higher positive redox potential in comparison with the conventional I3 –/I– system. Recent research efforts have increasingly focused on metal chalcogenidesparticularly selenides and telluridesas promising candidates for cobalt-based electrolytes. There are reports indicating that materials such as FeS2 can function as CEs in DSSCs employing the Co2+/Co3+ redox couple; however, the PCE remains modest at approximately 6.3%. To date, detailed studies of the charge transfer kinetics and long-term electrochemical stability of FeS2 in cobalt-based systems are still lacking. Nevertheless, FeS2 is generally considered to possess a moderate corrosion resistance under typical DSSC electrolyte conditions.
Future development should focus on making production methods scalable, while maintaining material quality and cost-effectiveness. Inkjet printing and solution routes offer viable pathways to volume production, while novel doping constituents and mixed materials can achieve both structural stability and catalytic performance. Additionally, protective coats in the ALD film forms can work effectively to avoid environmental deterioration, ensuring long-term usability. Furthermore, advancing environmentally friendly technologies will be an essential consideration for sustainable goal development.
6. Conclusions
The potential to replace Pt in DSSCs has been established in chalcogenide materials by tunable electronic properties, better catalytic performance, and economic viability. Their possibility to match conventional CEs in PCEs while providing added value in light absorption and charge transfer is highlighted by this survey of sulfides, selenides, and tellurides. Enhanced material synthesis methods by hydrothermal synthesis, solvothermal synthesis, electrodeposition, chemical vapor deposition, and atomic layer deposition have been employed to obtain material morphology and composition with greater control to optimize the electrocatalytic performance. In spite of this breakthrough, several issues continue to hinder it. Issues related to stability, most notably electrolyte-induced corrosion-related stability and phase stability, continue to be significant impediments to the long-term performance of DSSCs with chalcogenide-based CEs. Additionally, while laboratory demonstrations have been efficient in proving efficacy, scalability and reproducibility continue to be problems to be tackled before these materials can gain market viability. The future direction should be to design stable composites, efficient doping processes, and protective layer development to avoid deterioration, while providing optimum catalytic performance. Research in these directions is required to achieve the full potential in chalcogenide materials to realize next-generation efficient, stable, and cost-effective DSSCs, meeting sustainable development.
The authors declare no competing financial interest.
References
- Najihah M. Z., Aizamddin M. F., Saaid F. I., Winie T.. Enhanced efficiency of dye-sensitized solar cells (DSSCs) with polyaniline-decorated FeCo2O4 counter electrodes: Synthesis, characterization, and performance analysis. Curr. Appl. Phys. 2025;72:28–38. doi: 10.1016/j.cap.2025.01.017. [DOI] [Google Scholar]
- Kamanja R., Wongrerkdee S., Rungsawang T., Wongrerkdee S., Krobthong S., Pimpang P., Kaewtrakulchai N., Manatura K.. Activated carbon films from water hyacinth waste for stable and sustainable counter-electrode application in dye-sensitized solar cells. Indones. J. Sci. Technol. 2025;10:133–144. doi: 10.17509/ijost.v10i1.80970. [DOI] [Google Scholar]
- Zhou H., Aftabuzzaman M., Masud, Kang S. H., Kim H. K.. Key Materials and Fabrication Strategies for High-Performance Dye-Sensitized Solar Cells: Comprehensive Comparison and Perspective. ACS Energy Lett. 2025;10:881–895. doi: 10.1021/acsenergylett.4c03579. [DOI] [Google Scholar]
- Masud, Kim H. K.. Advanced polymeric matrices for gel electrolytes in quasi-solid-state dye-sensitized solar cells: recent progress and future perspective. Mater. Today Energy. 2023;38:101440. doi: 10.1016/j.mtener.2023.101440. [DOI] [Google Scholar]
- Masud, Kim H. K.. Redox Shuttle-Based Electrolytes for Dye-Sensitized Solar Cells: Comprehensive Guidance, Recent Progress, and Future Perspective. ACS Omega. 2023;8:6139–6163. doi: 10.1021/acsomega.2c06843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aftabuzzaman M., Sarker S., Lua C., Kim H. K.. In-depth understanding of the energy loss and efficiency limit of dye-sensitized solar cells under outdoor and indoor conditions. J. Mater. Chem. A. 2021;9:24830–24848. doi: 10.1039/D1TA03309C. [DOI] [Google Scholar]
- Subalakshmi K., Kumar K. A., Paul O. P., Saraswathy S., Pandurangan A., Senthilselvan J.. Platinum-free metal sulfide counter electrodes for DSSC applications: Structural, electrochemical, and power conversion efficiency analyses. Sol. Energy. 2019;193:507–518. doi: 10.1016/j.solener.2019.09.075. [DOI] [Google Scholar]
- Kim C. K., Kim H. M., Aftabuzzaman M., Jeon I. Y., Kang S. H., Eom Y. K., Baek J. B., Kim H. K.. Comparative study of edge-functionalized graphene nanoplatelets as metal-free counter electrodes for highly efficient dye-sensitized solar cells. Mater. Today Energy. 2018;9:67–73. doi: 10.1016/j.mtener.2018.05.003. [DOI] [Google Scholar]
- Aftabuzzaman M., Lu C., Kim H. K.. Recent progress on nanostructured carbon-based counter/back electrodes for high-performance dye-sensitized and perovskite solar cells. Nanoscale. 2020;12:17590–17648. doi: 10.1039/D0NR04112B. [DOI] [PubMed] [Google Scholar]
- Kim C. K., Zhou H., Kowalewski T., Matyjaszewski K., Kim H. K.. Soft-Templated Tellurium-Doped Mesoporous Carbon as a Pt-Free Electrocatalyst for High-Performance Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces. 2019;11(2):2093–2102. doi: 10.1021/acsami.8b19223. [DOI] [PubMed] [Google Scholar]
- Ji J. M., Kim C. K., Kim H. K.. Well-dispersed Te-doped mesoporous carbons as Pt-free counter electrodes for high-performance dye-sensitized solar cells. Dalton Trans. 2021;50:9399–9409. doi: 10.1039/D0DT04372A. [DOI] [PubMed] [Google Scholar]
- Tashenov Y., Suleimenova D., Baptayev B., Adilov S., Balanay M. P.. Efficient one-step synthesis of a Pt-free Zn0.76Co0.24S counter electrode for dye-sensitized solar cells and its versatile application in photoelectrochromic devices. Nanomaterials. 2023;13:2812. doi: 10.3390/nano13202812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Y., Tang Q., He B., Li R., Yu L.. Transparent nickel selenide alloy counter electrodes for bifacial dye-sensitized solar cells exceeding 10% efficiency. Nanoscale. 2014;6:12601. doi: 10.1039/C4NR03900A. [DOI] [PubMed] [Google Scholar]
- Liu R. J., Chang L. Y., Lin F. S., Lee Y. H., Yeh M. H., Ho K. C.. Multifunctional structure-modified quaternary compounds Co9Se8–CuSe2–WSe2 mixed with MWCNT as a counter electrode material for dye-sensitized solar cells. ACS Appl. Mater. Interfaces. 2024;16:3476–3488. doi: 10.1021/acsami.3c16527. [DOI] [PubMed] [Google Scholar]
- Guo J., Shi Y., Chua Y., Ma T.. Highly efficient telluride electrocatalysts for use as Pt-free counter electrodes in dye-sensitized solar cells. Chem. Commun. 2013;49:10157. doi: 10.1039/c3cc45698f. [DOI] [PubMed] [Google Scholar]
- Dong J., Jia J., Cao B., Wu J.. Spin-coated cobalt telluride counter electrodes for highly efficient dye-sensitized solar cells. Mater. Res. Bull. 2019;115:65–69. doi: 10.1016/j.materresbull.2019.03.008. [DOI] [Google Scholar]
- Mohammadnezhad M., Selopal G. S., Alsayyari N., Akilimali R., Navarro-Pardo F., Wang Z. M., Stansfield B., Zhao H., Rosei F.. CuS/Graphene nanocomposite as a transparent conducting oxide and Pt-free counter electrode for dye-sensitized solar cells. J. Electrochem. Soc. 2019;166:H3065–H3073. doi: 10.1149/2.0121905jes. [DOI] [Google Scholar]
- Liu A., Zhu H., Zou T., Reo Y., Ryu G. S., Noh Y. Y.. Evaporated nanometer chalcogenide films for scalable high-performance complementary electronics. Nat. Commun. 2022;13:6372. doi: 10.1038/s41467-022-34119-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M., Wang Y., Lin X., Yu N., Wang L., Wang L., Hagfeldt A., Ma T.. Economical and effective sulfidecatalysts for dye-sensitized solar cells as counter electrodes. Phys. Chem. Chem. Phys. 2011;13:19298–19301. doi: 10.1039/c1cp22819f. [DOI] [PubMed] [Google Scholar]
- Khan M. Q., Ahmad K., Raza W., Khan R. A., Sutradhar M., Paul A.. Hydrothermally synthesized MoS2 as an electrochemical catalyst for the fabrication of thiabendazole electrochemical sensor and dye-sensitized solar cells. Catalysts. 2024;14:107. doi: 10.3390/catal14020107. [DOI] [Google Scholar]
- Xu T., Kong D., Tang H., Qin X., Li X., Gurung A., Kou K., Chen L., Qiao Q., Huang W.. Transparent MoS2/PEDOT composite counter electrodes for bifacial dye-sensitized solar cells. ACS Omega. 2020;5:8687–8696. doi: 10.1021/acsomega.0c00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivakumar V., Dharmajan N., Jayaram A., Mani N., Santhana Krishnan H.. Enhanced catalytic performance of MoO3/MoS2-rGO counter electrode towards a Pt-free dye-sensitized solar cell. Sol. Energy Mater. Sol. Cells. 2025;285:113496. doi: 10.1016/j.solmat.2025.113496. [DOI] [Google Scholar]
- Sun B., Wang X., Cai X., Yang C., Chen M., Lu S., Wang Y., Zhang X.. N, S double-doped GQDs intercalated nanoflower-like MoS2 as an efficient counter electrode catalyst for DSSCs. Carbon. 2025;234:119977. doi: 10.1016/j.carbon.2024.119977. [DOI] [Google Scholar]
- Zheng W., Zhang S.. The effect of CuS counter electrode microtopography on the properties of quantum dot-sensitized solar cells. Inorg. Chem. Commun. 2020;122:108294. doi: 10.1016/j.inoche.2020.108294. [DOI] [Google Scholar]
- Zou H., Meng X., Zhao X., Qiu J.. Hofmeister Effect-Enhanced Hydration Chemistry of Hydrogel for High-Efficiency Solar-Driven Interfacial Desalination. Adv. Mater. 2023;35:2207262. doi: 10.1002/adma.202207262. [DOI] [PubMed] [Google Scholar]
- Zhao X., Meng X., Zou H., Wang Z., Du Y., Shao Y., Qi J., Qiu J.. Topographic Manipulation of Graphene Oxide by Polyaniline Nanocone Arrays Enables High-Performance Solar-Driven Water Evaporation. Adv. Funct. Mater. 2023;33:2209207. doi: 10.1002/adfm.202209207. [DOI] [Google Scholar]
- Zhao X., Meng X., Zou H., Zhang Y., Ma Y., Du Y., Shao Y., Qi J., Qiu J.. Nano-enabled solar driven-interfacial evaporation: Advanced design and opportunities. Nano Res. 2023;16:6015–6038. doi: 10.1007/s12274-023-5488-2. [DOI] [Google Scholar]
- Ma Y., Meng X., Li K., Zhang L., Du Y., Cai X., Qiu J.. Scrutinizing Synergy and Active Site of Nitrogen and Selenium Dual-Doped Porous Carbon for Efficient Triiodide Reduction. ACS Catal. 2023;13:1290–1298. doi: 10.1021/acscatal.2c05024. [DOI] [Google Scholar]
- Abbood M. A., Saleh E. A. M., Kumar A., Rodrigues P., Askar S., Alawsi T., Alawadi A., Alsalamy A.. Enhancing dye-sensitized solar cell performance by employing an innovative WSe2:Zn counter electrode for improved electrocatalytic activity. Mater. Sci. Semicond. Process. 2024;171:108015. doi: 10.1016/j.mssp.2023.108015. [DOI] [Google Scholar]
- Yue G., Wu J., Xiao Y., Huang M., Lin J., Lin J. Y.. High performance platinum-free counter electrode of molybdenum sulfide–carbon used in dye-sensitized solar cells. J. Mater. Chem. A. 2013;1:1495–1501. doi: 10.1039/C2TA00860B. [DOI] [Google Scholar]
- Liu J., Tang Q., He B., Yu L.. Cost-effective, transparent iron selenide nanoporous alloy counter electrode for bifacial dye-sensitized solar cell. J. Power Sources. 2015;282:79–86. doi: 10.1016/j.jpowsour.2015.02.045. [DOI] [Google Scholar]
- Meng K., Liu Z., Wu L., Wang X., Xu Q., Hu Y., Xiu Z., Chen G.. Controllable formation of efficient CuSe counter electrodes for quantum dot-sensitized solar cells. J. Electrochem. Soc. 2017;164:F1566–F1571. doi: 10.1149/2.0521714jes. [DOI] [Google Scholar]
- Bai H., Shen T., Wang S., Li B., Cao G., Tian J.. Controlled growth of Cu3Se2 nanosheets array counter electrode for quantum dot-sensitized solar cell through ion exchange. Sci. China Mater. 2017;60:637–645. doi: 10.1007/s40843-017-9037-1. [DOI] [Google Scholar]
- Kim C. K., Ji J.-M., Zhou H., Lu C., Kim H. K.. Tellurium-doped, mesoporous carbon nanomaterials as transparent metal-free counter electrodes for high-performance bifacial dye-sensitized solar cells. Nanomaterials. 2020;10:29. doi: 10.3390/nano10010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon I.-Y., Kim H. M., Kweon D. H., Jung S.-M., Seo J.-M., Shin S.-H., Choi I. T., Eom Y. K., Kang S. H., Kim H. K., Ju M. J., Baek J.-B.. Metalloid tellurium-doped graphene nanoplatelets as ultimately stable electrocatalysts for cobalt reduction reaction in dye-sensitized solar cells. Nano Energy. 2016;30:867–876. doi: 10.1016/j.nanoen.2016.09.001. [DOI] [Google Scholar]
- Jiang X., Sun B., Song Y., Dou M., Ji J., Wang F.. One-pot synthesis of MoS2/WS2 ultrathin nanoflakes with vertically aligned structure on indium tin oxide as a photocathode for enhanced photo-assistant electrochemical hydrogen evolution reaction. RSC Adv. 2017;7:49309. doi: 10.1039/C7RA10762E. [DOI] [Google Scholar]
- Chaudhary A., Khan R. A., Almadhhi S. S., Alsulmi A., Ahmad K., Oh T. H.. Hydrothermal synthesis of La-MoS2 and its catalytic activity for improved hydrogen evolution reaction. Catalysts. 2024;14:893. doi: 10.3390/catal14120893. [DOI] [Google Scholar]
- Zhang X., Ma G., Wang J.. Hydrothermal synthesis of two-dimensional MoS2 and its applications. Tungsten. 2019;1:59–79. doi: 10.1007/s42864-019-00014-9. [DOI] [Google Scholar]
- Khatri R., Puri N. K.. Electrochemical study of hydrothermally synthesised reduced MoS2 layered nanosheets. Vacuum. 2020;175:109250. doi: 10.1016/j.vacuum.2020.109250. [DOI] [Google Scholar]
- Lee S.-J., Son Y.-S., Choi J.-H., Kim S.-S., Park S.-Y.. Morphology and catalytic performance of MoS2 hydrothermally synthesized at various pH values. Catalysts. 2021;11:1229. doi: 10.3390/catal11101229. [DOI] [Google Scholar]
- Duraisamy S., Ganguly A., Sharma P. K., Benson J., Davis J., Papakonstantinou P.. One-step hydrothermal synthesis of phase-engineered MoS2/MoO3 electrocatalysts for hydrogen evolution reaction. ACS Appl. Nano Mater. 2021;4:2642–2656. doi: 10.1021/acsanm.0c03274. [DOI] [Google Scholar]
- Xuan T. T., Long L. N., Van Khai T.. Effect of reaction temperature and reaction time on the structure and properties of MoS2 synthesized by hydrothermal method. Vietnam J. Chem. 2020;58:92–100. doi: 10.1002/vjch.2019000144. [DOI] [Google Scholar]
- Cao S., Liu T., Hussain S., Zeng W., Peng X., Pan F.. Hydrothermal synthesis of variety low dimensional WS2 nanostructures. Mater. Lett. 2014;129:205–208. doi: 10.1016/j.matlet.2014.05.013. [DOI] [Google Scholar]
- Jing X., Liu D., Zhang N., Zhao X., Wang J., Wang D., Ji W., She J., Meng L.. Engineering two-dimensional tungsten-doped molybdenum selenide transformed conformational nanoarchitectonics: Trimodal therapeutic nanoagents for enhanced synergistic photothermal/chemodynamic/chemotherapy of breast carcinoma. J. Colloid Interface Sci. 2025;678:646–657. doi: 10.1016/j.jcis.2024.09.155. [DOI] [PubMed] [Google Scholar]
- Wu J., Fu X.. A low-temperature solvothermal method to prepare hollow spherical WS2 nanoparticles modified by TOA. Mater. Lett. 2007;61:4332–4335. doi: 10.1016/j.matlet.2007.01.099. [DOI] [Google Scholar]
- Vattikuti P., Byon S. V., Venkata Reddy C., Venkatesh B., Shim J.. Synthesis and structural characterization of MoS2 nanospheres and nanosheets using solvothermal method. J. Mater. Sci. 2015;50:5024–5038. doi: 10.1007/s10853-015-9051-8. [DOI] [Google Scholar]
- Durai L., Kong C. Y., Badhulika S.. One-step solvothermal synthesis of nanoflake-nanorod WS2 hybrid for non-enzymatic detection of uric acid and quercetin in blood serum. Mater. Sci. Eng., C. 2020;107:110217. doi: 10.1016/j.msec.2019.110217. [DOI] [PubMed] [Google Scholar]
- Saha S., Johnson M., Altayaran F., Wang Y., Wang D., Zhang Q.. Electrodeposition fabrication of chalcogenide thin films for photovoltaic applications. Electrochem. 2020;1:286–321. doi: 10.3390/electrochem1030019. [DOI] [Google Scholar]
- Huang W., Li J., Xu Y.. Nucleation and growth of porous MnO2 coatings prepared on nickel foam and evaluation of their electrochemical performance. Materials. 2018;11:716. doi: 10.3390/ma11050716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- França E. L. T., Assis L. K. C. S., Castro-Lopes S., Abrão J. E., do Nascimento-Junior A. M., Padrón-Hernández E.. Effect of pH and precursor solution on the microstructural properties and ferromagnetic resonance of nickel nanowires. Mater. Lett. 2023;342:134360. doi: 10.1016/j.matlet.2023.134360. [DOI] [Google Scholar]
- Yamaguchi A., Kubo R., El Aisnada A. N., Adachi K., Lee J.-E., Kitadai N., Hashizume D., Nakamura R., Miyauchi M.. Temperature and pressure dependence of hydrothermal electrodeposition of molybdenum sulfide. ACS Appl. Energy Mater. 2024;7:2593–2599. doi: 10.1021/acsaem.3c02565. [DOI] [Google Scholar]
- Chat-Wilk K., Rudnik E., Włoch G., Osuch P.. Importance of anions in electrodeposition of nickel from gluconate solutions. Ionics. 2021;27:4393–4408. doi: 10.1007/s11581-021-04166-y. [DOI] [Google Scholar]
- Guo X., Xu R., Li D., Yang Y., Tian Q.. One-step electrodeposited CoSe2 nano-reticular with high electroconductivity and electrocatalytic as a counter electrode for dye-sensitized solar cell. J. Alloys Compd. 2020;831:154712. doi: 10.1016/j.jallcom.2020.154712. [DOI] [Google Scholar]
- Voronenkov V., Groven B., Medina Silva H., Morin P., De Gendt S.. Guiding principles for the design of a chemical vapor deposition process for highly crystalline transition metal dichalcogenides. Phys. Status Solidi A. 2024;221:2300943. doi: 10.1002/pssa.202300943. [DOI] [Google Scholar]
- Luo L., Hou L., Cui X., Zhan P., He P., Dai C., Li R., Dong J., Zou Y., Liu G., Liu Y., Zheng J.. Self-condensation-assisted chemical vapour deposition growth of atomically two-dimensional MOF single-crystals. Nat. Commun. 2024;15:3618. doi: 10.1038/s41467-024-48050-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L., Yuan G., Gao L., Yang J., Chhowalla M., Gharahcheshmeh M. H., Gleason K. K., Choi Y. S., Hong B. H., Liu Z.. Chemical vapour deposition. Nat. Rev. Methods Primers. 2021;1:5. doi: 10.1038/s43586-020-00005-y. [DOI] [Google Scholar]
- Choe M., Lee H., Choi H. C.. Vapor-phase synthesis of MOF films. Bull. Korean Chem. Soc. 2024;45:584. doi: 10.1002/bkcs.12854. [DOI] [Google Scholar]
- Ghorai S., Rajan A. G.. From molecular precursors to MoS2 monolayers: Nanoscale mechanism of organometallic chemical vapor deposition. Chem. Mater. 2024;36:2698–2710. doi: 10.1021/acs.chemmater.3c02675. [DOI] [Google Scholar]
- Seravalli L., Bosi M.. A review on chemical vapour deposition of two-dimensional MoS2 flakes. Materials. 2021;14:7590. doi: 10.3390/ma14247590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalt R. A., Arcifa A., Wäckerlin C., Stemmer A.. CVD of MoS2 single layer flakes using Na2MoO4-impact of oxygen and temperature-time-profile. Nanoscale. 2023;15:18871–18882. doi: 10.1039/D3NR03907B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bil A. S., Alexandrov S. E.. A study of the influence of process parameters of AP PECVD on the mechanical properties of silica-like films deposited on polycarbonate. Silicon. 2025;17:17–27. doi: 10.1007/s12633-024-03178-3. [DOI] [Google Scholar]
- Diao M., Li H., Hou R., Liang Y., Wang J., Luo Z., Huang Z., Zhang C.. Vertical heterostructure of SnS–MoS2 synthesized by sulfur-preloaded chemical vapor deposition. ACS Appl. Mater. Interfaces. 2020;12:7423–7431. doi: 10.1021/acsami.9b19495. [DOI] [PubMed] [Google Scholar]
- Romanov R. I., Zabrosaev I. V., Chouprik A. A., Yakubovsky D. I., Tatmyshevskiy M. K., Volkov V. S., Markeev A. M.. Temperature-dependent structural and electrical properties of metal-organic CVD MoS2 films. Nanomaterials. 2023;13:2712. doi: 10.3390/nano13192712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue G. H., Lin Y. D., Wen X., Wang L. S., Chen Y. Z., Peng D. L.. Synthesis and characterization of the SnS nanowires via chemical vapor deposition. Appl. Phys. A: Mater. Sci. Process. 2012;106:87–91. doi: 10.1007/s00339-011-6560-4. [DOI] [Google Scholar]
- Chen Y., Zhang M.. Large-area growth of SnS2 nanosheets by chemical vapor deposition for high-performance photodetectors. RSC Adv. 2021;11:29960–29964. doi: 10.1039/D1RA05779K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremers V., Puurunen R. L., Dendooven J.. Conformality in atomic layer deposition: Current status overview of analysis and modelling. Appl. Phys. Rev. 2019;6:021302. doi: 10.1063/1.5060967. [DOI] [Google Scholar]
- Madadi M., Heikkinen M., Philip A., Karppinen M.. Conformal high-aspect-ratio solid electrolyte thin films for Li-ion batteries by atomic layer deposition. ACS Appl. Electron. Mater. 2024;6:1574–1580. doi: 10.1021/acsaelm.3c01565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Lee S. H., Jo I. H., Seo J., Yoo Y. E., Kim J. H.. Influence of growth temperature on dielectric strength of Al2O3 thin films prepared via atomic layer deposition at low temperature. Sci. Rep. 2022;12:5124. doi: 10.1038/s41598-022-09054-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bui H., Nguyen A. P., Dang M. D., Dinh T. D., Kooyman P. J., Van Ommen J. R.. What could be the low-temperature limit of atomic layer deposition of platinum using MeCpPtMe3 and oxygen? Chem. Commun. 2024;60:14045–14048. doi: 10.1039/D4CC04679J. [DOI] [PubMed] [Google Scholar]
- Hoye R. L. Z., Muñoz-Rojas D., Sun Z., Okcu H., Asgarimoghaddam H., MacManus-Driscoll J. L., Musselman K. P.. Spatial atomic layer deposition for energy and electronic devices. PRX Energy. 2025;4:017002. doi: 10.1103/PRXEnergy.4.017002. [DOI] [Google Scholar]
- Gonzalez M. A., Pareek D., Büsing L., Beer M., Parisi J., Schäfer S., Gütay L.. Rapid formation of large-area MoS2 monolayers by a parameter resilient atomic layer deposition approach. APL Mater. 2021;9:051122. doi: 10.1063/5.0041042. [DOI] [Google Scholar]
- Zhou Y., Najar A., Zhang J., Feng J., Cao Y., Li Z., Zhu X., Yang D., Liu S. F.. Effect of solvent residue in the thin-film fabrication on perovskite solar cell performance. ACS Appl. Mater. Interfaces. 2022;14:28729–28737. doi: 10.1021/acsami.2c02525. [DOI] [PubMed] [Google Scholar]
- Bai S., Haase K., Andrade J. P., Hambsch M., Talnack F., Millek V., Prasoon A., Liu J., Arnhold K., Boye S., Feng X., Mannsfeld S. C. B.. Impact of thermal annealing on the dissolution of semiconducting polymer thin films. Adv. Electron. Mater. 2024;10:2300801. doi: 10.1002/aelm.202300801. [DOI] [Google Scholar]
- Jemelka J., Palka K., Janicek P., Slang S., Jancalek J., Kurka M., Vlcek M.. Solution-processed multi-layered thin films of Ge20Sb5S75 and Ge20Sb5Se75 chalcogenide glasses. Sci. Rep. 2023;13:16609. doi: 10.1038/s41598-023-43772-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zha Y., Waldmann M., Arnold C. B.. A review on solution processing of chalcogenide glasses for optical components. Opt. Mater. Express. 2013;3:1259–1272. doi: 10.1364/OME.3.001259. [DOI] [Google Scholar]
- Ranjith K. S., Mohammadi A., Raju G. S. R., Huh Y. S., Han Y. K.. Interfacial charge transfer on hierarchical synergistic shell wall of MXene/MoS2 on CdS nanospheres: Heterostructure integrity for visible light responsive photocatalytic H2 evolution. Nano Converg. 2024;11:51. doi: 10.1186/s40580-024-00454-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slang S., Janicek P., Palka K., Vlcek M.. Structure and properties of spin-coated Ge25S75 chalcogenide thin films. Opt. Mater. Express. 2016;6:1973–1985. doi: 10.1364/OME.6.001973. [DOI] [Google Scholar]
- Agarwal S., Vincent K. C., Turnley J. W., Hayes D. C., Uible M. C., Durán I., Canizales A. S. M., Khandelwal S., Panicker I., Andoh Z., Spilker R. M., Ma Q., Huang L., Hwang S., Kisslinger K., Svatek S., Antolin E., Bart S. C., Agrawal R.. Breaking barriers in chalcogenide perovskite synthesis: A generalized framework for fabrication of BaMS3 (M = Ti, Zr, Hf) materials. Adv. Funct. Mater. 2024;34:2405416. doi: 10.1002/adfm.202405416. [DOI] [Google Scholar]
- Agarwal S., Turnley J. W., Pradhan A. A., Agrawal R.. Moderate temperature sulfurization and selenization of highly stable metal oxides: An opportunity for chalcogenide perovskites. J. Mater. Chem. C. 2023;11:15817–15823. doi: 10.1039/D3TC02716C. [DOI] [Google Scholar]
- Uc-Canché S., Camacho-Espinosa E., Mis-Fernández R., Loeza-Poot M., Ceh-Cih F., Peña J. L.. Influence of sulfurization time on Sb2S3 synthesis using a new graphite box design. Materials. 2024;17:1656. doi: 10.3390/ma17071656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ai R., Chui K. K., Chen Y., Wang J.. Transformation of plasmonic MoO3‑x nanoparticles into dielectric MoS2 nanoparticles. J. Phys. Chem. C. 2025;129:4126–4133. doi: 10.1021/acs.jpcc.4c08803. [DOI] [Google Scholar]
- Xia L., Song H., Li X., Zhang X., Gao B., Zheng Y., Huo K., Chu P. K.. Hierarchical 0D–2D Co/Mo selenides as superior bifunctional electrocatalysts for overall water splitting. Front. Chem. 2020;8:382. doi: 10.3389/fchem.2020.00382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J., Jin J., Zheng D., Ye S., Cheng J., Li R., Wang Q., Fan S., Qian X.. NiSe2-CoSe2 hollow polyhedron as counter electrode catalyst for high-efficiency dye-sensitized solar cells. Appl. Surf. Sci. 2024;651:159176. doi: 10.1016/j.apsusc.2023.159176. [DOI] [Google Scholar]
- Das S., Sa K., Mahakul P. C., Raiguru J., Alam I., Subramanyam B. V. R. S., Mahanandia P.. Synthesis of quaternary chalcogenide CZTS nanoparticles by a hydrothermal route. IOP Conf. Ser.: Mater. Sci. Eng. 2018;338:012062. doi: 10.1088/1757-899X/338/1/012062. [DOI] [Google Scholar]
- Manivannan R., Victoria S. N.. Preparation of chalcogenide thin films using electrodeposition method for solar cell applications – A review. Sol. Energy. 2018;173:1144–1157. doi: 10.1016/j.solener.2018.08.057. [DOI] [Google Scholar]
- You J., Hossain M. D., Luo Z.. Synthesis of 2D transition metal dichalcogenides by chemical vapor deposition with controlled layer number and morphology. Nano Converg. 2018;5:26. doi: 10.1186/s40580-018-0158-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oke J. A., Olotu O. O., Jen T. C.. Atomic layer deposition of chalcogenide thin films: Processes, film properties, applications, and bibliometric prospect. J. Mater. Res. Technol. 2022;20:991–1019. doi: 10.1016/j.jmrt.2022.07.098. [DOI] [Google Scholar]
- Hara K., Wang Z. S., Cui Y., Furube A., Koumura N.. Long-term stability of organic–dye-sensitized solar cells based on an alkyl-functionalized carbazole dye. Energy Environ. Sci. 2009;2:1109–1114. doi: 10.1039/b907486d. [DOI] [Google Scholar]
- Lamkaouane H., Ftouhi H., Richard-Plouet M., Gautier N., Stephant N., Zazoui M., Addou M., Cattin L., Bernède J. C., Mir Y., Louarn G.. Efficient and facile synthetic route of MoO3:MoS2 hybrid thin layer via oxidative reaction of MoS2 nanoflakes. Nanomaterials. 2022;12:3171. doi: 10.3390/nano12183171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Gong F., Zhou G., Wang Z. S.. NiS2/reduced graphene oxide nanocomposites for efficient dye-sensitized solar cells. J. Phys. Chem. C. 2013;117:6561–6566. doi: 10.1021/jp401032c. [DOI] [Google Scholar]
- Tapa A. R., Xiang W., Zhao X.. Metal chalcogenides (MxEγ; E = S, Se, and Te) as counter electrodes for dye-sensitized solar cells: An overview and guidelines. Adv. Energy Sustain. Res. 2021;2:2100056. doi: 10.1002/aesr.202100056. [DOI] [Google Scholar]
- Huang Y. J., Lee C. P., Pang H. W., Li C. T., Fan M. S., Vittal R., Ho K. C.. Microemulsion-controlled synthesis of CoSe2CoSeO3 composite crystals for electrocatalysis in dye-sensitized solar cells. Mater. Today Energy. 2017;6:189–197. doi: 10.1016/j.mtener.2017.10.004. [DOI] [Google Scholar]
- Wu J., Lan Z., Lin J., Huang M., Huang Y., Fan L., Luo G., Lin Y., Xie Y., Wei Y.. Counter electrodes in dye-sensitized solar cells. Chem. Soc. Rev. 2017;46:5975–6023. doi: 10.1039/C6CS00752J. [DOI] [PubMed] [Google Scholar]
- Murugadoss V., Lin J., Liu H., Mai X., Ding T., Guo Z., Angaiah S.. Optimizing graphene content in a NiSe/graphene nanohybrid counter electrode to enhance the photovoltaic performance of dye-sensitized solar cells. Nanoscale. 2019;11:17579–17589. doi: 10.1039/C9NR07060E. [DOI] [PubMed] [Google Scholar]
- Singh E., Kim K. S., Yeom G. Y., Nalwa H. S.. Two-dimensional transition metal dichalcogenide-based counter electrodes for dye-sensitized solar cells. RSC Adv. 2017;7:28234–28290. doi: 10.1039/C7RA03599C. [DOI] [Google Scholar]
- Alami A. H., Aokal K., Zhang D., Taieb A., Faraj M., Alhammadi A., Ashraf J. M., Soudan B., El Hajjar J., Irimia-Vladu M.. Low-cost dye-sensitized solar cells with ball-milled tellurium-doped graphene as counter electrodes and a natural sensitizer dye. Int. J. Energy Res. 2019;43:5824–5833. doi: 10.1002/er.4684. [DOI] [Google Scholar]
- Gao H., Yang T., Dong A., Xing Y., Liu D., Ma Y., Zhu K.. Recent advances in transition metal chalcogenides electrocatalysts for oxygen evolution reaction in water splitting. Catalysts. 2025;15:124. doi: 10.3390/catal15020124. [DOI] [Google Scholar]
- Ran F., Hu M., Deng S., Wang K., Sun W., Peng H., Liu J.. Designing transition metal-based porous architectures for supercapacitor electrodes: A review. RSC Adv. 2024;14:11482–11512. doi: 10.1039/D4RA01320D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah K. W., Wang S. X., Zheng Y., Xu J.. Solution-based synthesis and processing of metal chalcogenides for thermoelectric applications. Appl. Sci. 2019;9:1511. doi: 10.3390/app9071511. [DOI] [Google Scholar]
- Kurley J. M., Pan J. A., Wang Y., Zhang H., Russell J. C., Pach G. F., To B., Luther J. M., Talapin D. V.. Roll-to-roll friendly solution-processing of ultrathin, sintered CdTe nanocrystal photovoltaics. ACS Appl. Mater. Interfaces. 2021;13:44165–44173. doi: 10.1021/acsami.1c08325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnley J. W., Agrawal R.. Solution processed metal chalcogenide semiconductors for inorganic thin-film photovoltaics. Chem. Commun. 2024;60:5245–5269. doi: 10.1039/D4CC01057D. [DOI] [PubMed] [Google Scholar]
- Liu T., Li M., Bo X., Zhou M.. Comparison study toward the influence of the second metals doping on the oxygen evolution activity of cobalt nitrides. ACS Sustainable Chem. Eng. 2018;6:11457–11465. doi: 10.1021/acssuschemeng.8b01510. [DOI] [Google Scholar]
- Özel F., Sarılmaz A., İstanbullu B., Aljabour A. A., Kuş M., Sönmezoğlu S.. Penternary chalcogenides nanocrystals as catalytic materials for efficient counter electrodes in dye-synthesized solar cells. Sci. Rep. 2016;6:29207. doi: 10.1038/srep29207. [DOI] [PMC free article] [PubMed] [Google Scholar]