Abstract
Eyeblink classical conditioning is a relatively simple form of associative learning that has become an invaluable tool in our understanding of the neural mechanisms of learning. When studying rabbits in this paradigm, we observed a dramatic modification of learning rate by conducting training during episodes of either hippocampal theta or hippocampal non-theta activity as determined by on-line slow-wave spectral analysis. Specifically, if animals were given trials only when a computer analysis verified a predominance of slow-wave oscillations at theta frequencies (3–8 Hz), they learned in half as many trials as animals trained during non-theta hippocampal activity (58 vs. 115). This finding provides important evidence from awake, behaving animals that supports recent advances in our knowledge of (i) brain sites and neurobiological mechanisms of learning and memory, specifically hippocampus and theta oscillations, (ii) the biological plausibility of current models of hippocampal function that posit important roles for oscillatory potentials, and (iii) the design of interfaces between biological and cybernetic (electronic) systems that can optimize cognitive processes and performance.
Classical conditioning of the rabbit's nictitating membrane/eyeblink response has become a successful model system for investigating the neural substrates of associative learning. Eyeblink conditioning is a highly controlled and well-characterized paradigm that has been adapted for use in a variety of species (e.g., mice, rats, ferrets, rabbits, cats, monkeys, and humans). Striking parallels have emerged among species in terms of both behavioral learning and the neural mechanisms involved, making data obtained with animals generalizable to humans and vice versa. For example, lesion and elecrophysiological experiments have revealed major contributions from the hippocampus and cerebellum during learning and performance of this task (see refs. 1–3). To summarize, the cerebellum apparently contains the critical neural circuitry required for learning the basic association between the conditioned stimulus and unconditioned stimulus (US; refs. 4–6), and the hippocampus plays an important modulatory role (7–10; although the hippocampus becomes essential as the task demands are increased, 11–14).
The basic paradigm involves presenting a neutral conditioned stimulus (tone or light) followed 250 ms later by a noxious US (corneal air puff or peri-orbital shock). Initially, the organism produces only a reflexive unconditioned response (eyeblink) to the US. However, after repeated pairings of the conditioned stimulus and US, the conditioned stimulus begins to elicit anticipatory, conditioned responses (CRs), similar to the response originally produced only by the US.
We have reported a strikingly predictive relationship between the state of hippocampal slow waves and the rate of eyeblink conditioning in the rabbit (8). Just before the start of a delay-conditioning procedure, a free-running 2-min sample of slow-wave activity was recorded. Spectral analysis of these samples revealed that rabbits exhibiting a large proportion of slow-wave frequencies in the theta range (2–8 Hz) learned at a faster rate than those that had higher frequencies (8–22 Hz). The correlation between a ratio index of hippocampal frequencies (8–22 Hz/2–8 Hz) and trials to criterion was 0.72. These findings indicate that an important determinant of eyeblink acquisition rate is a preexisting brain state that can be assessed by differences in hippocampal slow waves. Accompanying these slow-wave differences were significantly higher levels of conditioning-related unit activity in animals displaying theta (15).
Consistent with this relationship are data showing that lesions and drug treatments capable of disrupting the hippocampal theta rhythm retard acquisition in this task (refs. 16–25; see also refs. 26–29). For example, medial septal lesions, which disrupt the pacemaker for the theta rhythm but leave lateral septal output pathways intact, slow acquisition of the conditioned response without completely preventing it. Pharmacological blockade of cholinergic systems, known to disrupt the low-frequency theta rhythm, has a similar effect on acquisition rate.
This pattern of results suggests that an optimal “brain state” exists for eyeblink conditioning that is accurately reflected in hippocampal slow waves, and, when disrupted, is associated with impaired acquisition. A more conclusive demonstration, however, would be the facilitation of learning/memory by direct modification of such brain states, thereby yielding evidence of superior or optimal function. A few studies with other behavioral paradigms have used electrical stimulation of the medial septum to artificially “drive” or block hippocampal theta activity as a means of studying its role in learning. In general, theta-driving stimulation yielded better acquisition and/or retention than either theta-blocking or no stimulation (30–33). Unfortunately, this situation is not an ideal one in which to investigate the functional significance of the endogenous theta rhythm. The nonphysiological nature of the approach inevitably weakens conclusions regarding the role of theta in learning and memory processes in the intact, behaving animal. It would be more informative if naturalistic approaches (7) were used to elicit theta or if the animal were given the opportunity to produce this activity spontaneously with no invasive experimental treatments (e.g., drugs or electrical stimulation). Therefore, the current experiment monitored hippocampal activity “on-line” and initiated individual behavioral training trials contingent on the explicit presence or absence of theta activity, assessing the role that naturally occurring theta plays in learning.
Methods
Surgery.
Subjects were 10 (3–6 months old) experimentally naive New Zealand White rabbits (Oryctolagus cuniculus). Stainless steel microelectrodes (size 00 insect pins insulated with Epoxylite except for 50–70 μm at the tip) were implanted in stratum oriens or pyramidale of CA1 in the dorsal hippocampus according to stereotaxic coordinates (4.5 mm posterior to bregma, 5.5 mm lateral to the midline, and ≈3.0 mm ventral to dura) as well as by monitoring the characteristic pattern of neural activity from the electrode tip during penetration.
Training.
A 1-kHz, 80-dB tone was presented for 350 ms and coterminated with a 3-psi, 100-ms corneal air puff (250-ms interstimulus interval). Daily sessions lasted 1 h and 45 min with a minimum intertrial interval (ITI) of 30 s. Animals were trained to a behavioral criterion of 8 CRs in any nine consecutive trials and for a minimum of 3 days. A CR was defined as at least 0.5-mm movement of the nictitating membrane occurring after tone onset but before air onset. Slow-wave activity from the electrode tip was filtered (25-Hz low pass) and digitized at 100 Hz by a PowerMac 7100 with a National Instruments I/O board (Lab-NB). LABVIEW software (National Instruments, Austin, TX) sampled slow-wave data in 640-ms segments, performed a fast Fourier transform, and computed a power spectrum. A “theta index” reflecting proportional power in the theta range was calculated from the output of the power spectrum (3.5–8.5 Hz activity in the numerator and 0.5–3.5 Hz and 8.5–22 Hz activity in the denominator). To assess theta continuity, a sliding window continued to sample the incoming slow wave in 160-ms increments and recalculated the ratio (480 ms previous data and 160 ms new data). One group of rabbits (T+; n = 5) was given trials when this ratio exceeded 1.0 for two consecutive repetitions (total, 800 ms) and another group (T−; n = 5) was given trials only when this ratio fell below 0.3 for two consecutive repetitions. The upper and lower values were determined empirically during pilot testing to reflect clear theta (or its absence) and to allow trials to occur at reasonable intertrial intervals.
Because the ITI and number of training trials per day were free to vary (i.e., they were determined by experimental rabbits' slow waves), it was possible that the two groups would differ on these two variables. Both have affected acquisition rates in nictitating membrane/eyeblink conditioning (34–36). Therefore, to rule out the possibility of these two factors contributing to any observed learning rate differences between the experimental groups, two groups of yoked control animals were run (n = 5 per group). One was matched to the T+ group, averaging 60 trials per day and an ITI of 110 s; the other replicated the ITIs (mean = 41 s) and trials per day (mean = 140) of the T− group. The slow-wave state of these subjects at the moment each trial started was not assessed, so these groups served as controls for training variables (ITI and number of trials per day), irrespective of brain-wave activity.
Histology.
At the conclusion of training, animals were given an overdose of sodium pentobarbital. A small marking lesion was made at the tip of each electrode by passing a DC current (200 μA for 10 s, Grass Stimulator model SD-9) through the electrode. Animals were perfused intracardially with 0.9% saline followed by a 10% formalin solution. Brains were fixed in a solution of formalin for at least 1 week before being placed in a 20%-ethanol solution for 48 h. Frozen coronal sections were taken at 56-μm intervals and embedded on gelatin-coated slides. A Prussian blue stain was used to mark the displaced iron from the electrode tip. A safranin counterstain was used to mark cell bodies. Electrode location was verified by examination with a compound microscope (magnification ×10, Nikon), and it was confirmed that all electrode placements were in either stratum oriens or stratum pyramidale of CA1.
Results
Fig. 1 shows representative traces of slow-wave activity that met the triggering criteria during training for the two groups. One can clearly see the rhythmic character of the theta samples, whereas the non-theta traces are clearly non-rhythmic with mixed frequencies and are consistent with what others have described as large-amplitude irregular activity.
Figure 1.
Representative slow waves that met either the theta or the non-theta criterion for delivery of a training trial (A, theta; B, non-theta). Each trace is 800-ms long.
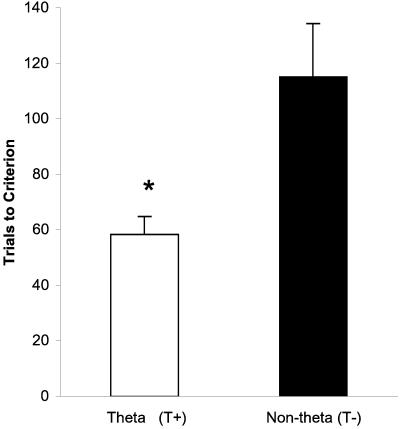
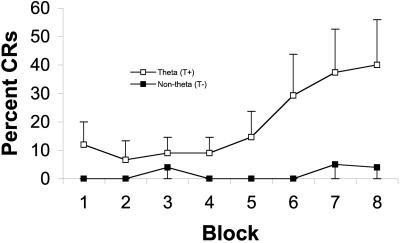
Our behavioral results revealed that triggering individual training trials on specific patterns of hippocampal activity had a dramatic impact on learning. Specifically, rabbits that received trials only when exhibiting hippocampal theta (T+) required half as many trials (58 vs. 115) to reach the learning criterion as animals receiving trials when explicitly not exhibiting theta (T−), F(1,8) = 7.88, P = 0.02 (Fig. 2). Analysis of pretraining baseline samples of hippocampal slow waves showed no differences in the prevalence of theta that could account, as in the Berry and Thompson (1978) study, for group differences in behavioral acquisition [t(8) = 0.63, P = 0.50]. An additional measure of acquisition and performance in this task, percent CRs, showed that T+ rabbits were highly consistent and gave a significantly larger percentage of CRs than did T− rabbits during the course of training [F(1,8) = 11.73, P = 0.009 (Fig. 3)]. A closer examination revealed that the theta-related differences emerged very early in training and were significant on day 1 [F(1,8) = 5.52, P = 0.047 (Fig. 4)], a result consistent with other data implicating the hippocampus in the earliest stages of acquisition (7, 8, 16, 25–27, 37–40). The groups of behavioral control animals that were assigned trials per day and ITIs matching those of the experimental groups did not differ in learning rate from each other [F(1,8) = 0.01, P = 0.92], indicating that the difference observed between T+ and T− groups was a result of the experimental manipulation (i.e., triggering trials on theta or non-theta states). In addition, as expected, their learning rates were intermediate (between T+ and T− groups) and somewhat variable. Finally, the presence of theta seemed to have its facilitatory effect early in training, as evidenced by a significant effect of block for the eight blocks of day 1 in the T+ group [F(7, 28) = 2.71, P = 0.03] that was not observed in the T+ yoked controls or in the first eight blocks for T− and T− yoked controls.
Figure 2.
Mean trials to criterion. Error bars indicate 1 SEM. *, Theta rabbits took significantly fewer trials to reach criterion than non-theta rabbits did (P < 0.05, n = 5 for each group).
Figure 3.
Percent CRs in five trial blocks across days of training. Rabbits in the theta condition gave a significantly larger percentage of CRs than rabbits in the non-theta condition (P < 0.01, n = 5 for each group). Error bars indicate 1 SEM.
Figure 4.
Percent CRs in five trial blocks on day 1. Rabbits in the theta condition gave a significantly larger percentage of CRs than rabbits in the non-theta condition (P < 0.05, n = 5 for each group). Error bars indicate 1 SEM.
Discussion
These findings demonstrate the existence of an important functional relationship between spontaneous or naturally occurring slow-wave activity in the hippocampus and concurrent behavioral learning. Unlike prior correlational studies (7, 8), theta-triggered training allowed slow waves to be used as the independent variable, producing group differences in learning rate depending on the state of the hippocampus. Specifically, the presence of theta seems to be beneficial, and its absence detrimental, to the rate of acquisition of eyeblink conditioning. As discussed next, this relationship seems to hold whether the difference is produced by theta disruption or by theta enhancement with artificial or naturalistic treatments.
Previous work has shown that manipulations capable of disrupting hippocampal theta (e.g., medial septal lesions and cholinergic antagonists) have a deleterious effect on acquisition of this task (16–25). Animals are capable of learning the task, albeit at a slower rate (but see ref. 18), which suggests that the hippocampus may serve to modulate plasticity in critical circuits elsewhere (e.g., cerebellum). A complementary approach involving inducing or enhancing hippocampal theta activity has further supported this connection. Research with this paradigm has shown that manipulations that promote theta activity (e.g., water deprivation) produce enhanced hippocampal unit responses and faster rates of acquisition (7). Although not recorded here, increased pyramidal cell responsiveness has been documented for the nictitating membrane/eyeblink paradigm in animals showing high levels of theta activity (7, 15). Specifically, in the original study reporting a strong relationship between theta and subsequent learning rate (8), unit responses were monitored in the pyramidal cell layer of CA1, revealing significantly larger conditioned unit responses in animals displaying greater theta activity (15). Studies using rats in other behavioral learning paradigms have also shown relationships between theta and evoked neural activity, including conditioned unit responses (41) and place cell firing (42, 43).
Electrical stimulation studies have also shown beneficial effects of artificial theta driving by means of chronic macroelectrodes in the septal nuclei on behavioral acquisition and retention in various tasks (30–33), generalizing the theta-learning relationship to other species and to paradigms other than classical conditioning. At the basic, cellular neurophysiological level, studies have shown that high-frequency stimulation given in bursts at theta frequency produces much faster, larger amplitude, and more persistent long-term potentiation (44–47). Strong functional relationships have also been demonstrated between and among water deprivation, theta, long-term potentiation, and behavioral learning during contextual fear conditioning in rats (48). Although there is debate on whether long-term potentiation is the neural substrate or mechanism of behavioral learning/memory, these studies strongly suggest a beneficial impact on hippocampal and behavioral plasticity of phasic stimuli tied to hippocampal theta oscillations.
Computational and connectionist models of the hippocampal role in memory have posited an important role for oscillatory potentials (e.g., theta and gamma; refs. 49–53). In general, theta periodicities are thought to produce (or result from) neurobiological processes that play a strong, facilitatory role in neural and behavioral plasticity. Our findings provide necessary empirical support at the behavioral level for such a role for hippocampal theta. Under the training conditions in the current study, theta would optimize hippocampal processing of incoming conditioned stimulus, US, and perhaps contextual information by increasing pyramidal cell responsiveness to the conditioning stimuli and by enhancing plasticity in hippocampal circuits, ultimately resulting in faster behavioral acquisition. Moreover, the physiological impact of requiring trials to occur during theta makes the obtained behavioral results quite consistent with what has been predicted by some models of septohippocampal modulation of learning (51, 53). Thus, current and prior neurophysiological findings lend support to models that would predict the presence of theta during each trial in the present study should have beneficial effects on behavioral learning, perhaps by means of hippocampal modulation of activity in other areas (e.g., cerebellum) known to be essential for elaboration of an adaptive conditioned response (50, 54). The productive exchange of predictions and empirical data between developers of connectionist/computational models and laboratories studying the hippocampus and eyeblink conditioning can greatly accelerate our understanding of the neurobiology of learning and memory.
Our findings have potentially important applications to rapidly developing areas of cognitive neuroscience. Comprehensive theories of brain function (especially those explaining learning and memory) must continue to incorporate the significance of oscillatory potentials such as hippocampal theta and gamma (49–53) and the cortical gamma that has been related to perceptual binding and use-dependent plasticity (55). Our results demonstrate that it is possible to index optimal levels of such activity in a behaviorally significant manner. Future research can evaluate the relationship of our hippocampal theta index to processes such as arousal, attention, motivation, etc. that modulate memory formation and behavioral performance. The data reported here may be useful in elucidating the physiological and/or behavioral functions of neurobiological oscillations and ultimately lead to a better understanding of the neural mechanisms of learning and memory. In addition, given the connection between cholinergic mechanisms (of which 3- to 8-Hz hippocampal theta is one) and the memory impairments associated with normal aging and Alzheimer's disease, it seems plausible that a neurophysiological triggering approach similar to ours could have some diagnostic potential or ameliorative benefit for these populations. Existing noninvasive methods for recording human hippocampal theta (56–58) would allow direct application of our methods to humans. The conditionability (i.e., voluntary control) of specific patterns of cortical slow-wave activity in humans has been documented (59, 60). As demonstrated by our findings, initiating learning episodes contingent on specific, facilitatory patterns of brain activity has a dramatic effect on behavioral learning. Ultimately, it may be possible for teachers and students, being made aware of optimal brain states by way of neurofeedback, to modify and optimize learning experiences through technologies derived from those reported in the current study.
Acknowledgments
M.A.S. thanks the members of his dissertation committee (S.D.B., P. J. Best, K. A. Killian, and P. E. Simson) for helpful comments and input throughout this project. This research was supported by a Miami University Graduate School Dissertation Research Support Award (to M.A.S.) and a Miami University Undergraduate Summer Scholar Award (to E.S.C.). This research was submitted to the Department of Psychology, Miami University, in partial fulfillment by M.A.S. of the Doctor of Philosophy degree.
Abbreviations
- US
unconditioned stimulus
- CR
conditioned response
- ITI
intertrial interval
References
- 1.Berger T W, Berry S D, Thompson R F. In: The Hippocampus. Isaacson R L, Pribram K H, editors. Vol. 4. New York: Plenum; 1986. pp. 203–239. [Google Scholar]
- 2.Lavond D G, Kim J J, Thompson R F. Annu Rev Psychol. 1993;44:317–342. doi: 10.1146/annurev.ps.44.020193.001533. [DOI] [PubMed] [Google Scholar]
- 3.Steinmetz J E. In: Acquisition of Motor Behavior in Vertebrates. Bloedel J, Ebner T, Wise S, editors. Cambridge, MA: MIT Press; 1996. pp. 89–114. [Google Scholar]
- 4.Gruart A, Yeo C H. Exp Brain Res. 1995;104:431–448. doi: 10.1007/BF00231978. [DOI] [PubMed] [Google Scholar]
- 5.Krupa D J, Thompson J K, Thompson R F. Science. 1993;260:989–991. doi: 10.1126/science.8493536. [DOI] [PubMed] [Google Scholar]
- 6.McCormick D A, Lavond D J, Clark G A, Kettner R E, Rising C E, Thompson R F. Bull Psychon Soc. 1981;18:103–105. [Google Scholar]
- 7.Berry S D, Swain R A. Behav Neurosci. 1989;103:71–76. doi: 10.1037//0735-7044.103.1.71. [DOI] [PubMed] [Google Scholar]
- 8.Berry S D, Thompson R F. Science. 1978;200:1298–1300. doi: 10.1126/science.663612. [DOI] [PubMed] [Google Scholar]
- 9.Port R L, Mikhail A A, Patterson M M. Behav Neurosci. 1985;99:200–208. doi: 10.1037//0735-7044.99.2.200. [DOI] [PubMed] [Google Scholar]
- 10.Solomon P R, Vander Schaaf E R, Thompson R F, Weisz D J. Behav Neurosci. 1986;100:729–744. doi: 10.1037//0735-7044.100.5.729. [DOI] [PubMed] [Google Scholar]
- 11.Berger T W, Orr W B. Behav Brain Res. 1983;8:49–68. doi: 10.1016/0166-4328(83)90171-7. [DOI] [PubMed] [Google Scholar]
- 12.Moyer J R, Jr, Deyo R A, Disterhoft J F. Behav Neurosci. 1990;104:243–252. doi: 10.1037//0735-7044.104.2.243. [DOI] [PubMed] [Google Scholar]
- 13.Solomon P R. J Comp Physiol Psychol. 1977;91:407–417. doi: 10.1037/h0077330. [DOI] [PubMed] [Google Scholar]
- 14.Solomon P R, Moore J W. J Comp Physiol Psychol. 1975;89:1192–1203. doi: 10.1037/h0077183. [DOI] [PubMed] [Google Scholar]
- 15.Berry S D. In: Conditioning: Representation of Involved Neural Functions. Woody C D, editor. New York: Plenum; 1982. pp. 417–431. [Google Scholar]
- 16.Berry S D, Thompson R F. Science. 1979;205:209–211. doi: 10.1126/science.451592. [DOI] [PubMed] [Google Scholar]
- 17.Harvey J A, Gormezano I, Cool-Hauser V A. J Pharmacol Exp Ther. 1983;255:42–49. [PubMed] [Google Scholar]
- 18.Kaneko T, Thompson R F. Psychopharmacology. 1997;131:161–166. doi: 10.1007/s002130050279. [DOI] [PubMed] [Google Scholar]
- 19.Moore J W, Goodell N, Solomon P R. Physiol Psychol. 1976;4:395–399. [Google Scholar]
- 20.Powell D A, Hernandez L, Buchanan S L. Behav Neurosci. 1985;99:75–87. doi: 10.1037//0735-7044.99.1.75. [DOI] [PubMed] [Google Scholar]
- 21.Powell D A. Bull Psychon Soc. 1979;14:161–164. [Google Scholar]
- 22.Powell D A, Milligan W L, Buchanan S L. Physiol Behav. 1976;17:955–962. doi: 10.1016/0031-9384(76)90014-7. [DOI] [PubMed] [Google Scholar]
- 23.Salvatierra A T, Berry S D. Behav Neurosci. 1989;103:715–721. doi: 10.1037//0735-7044.103.4.715. [DOI] [PubMed] [Google Scholar]
- 24.Solomon P R, Gottfried K E. J Comp Physiol Psychol. 1981;95:322–330. doi: 10.1037/h0077779. [DOI] [PubMed] [Google Scholar]
- 25.Solomon P R, Solomon S E, Vander Schaaf E, Perry H E. Science. 1983;220:329–331. doi: 10.1126/science.6836277. [DOI] [PubMed] [Google Scholar]
- 26.Asaka Y, Seager M A, Griffin A L, Berry S D. Behav Neurosci. 2000;114:1068–1077. doi: 10.1037//0735-7044.114.6.1068. [DOI] [PubMed] [Google Scholar]
- 27.Seager M A, Asaka Y, Berry S D. Behav Brain Res. 1999;100:143–151. doi: 10.1016/s0166-4328(98)00123-5. [DOI] [PubMed] [Google Scholar]
- 28.Givens B. Alcohol Clin Exp Res. 1995;19:763–767. doi: 10.1111/j.1530-0277.1995.tb01580.x. [DOI] [PubMed] [Google Scholar]
- 29.Givens B, Olton D S. Neurobiol Learn Mem. 1995;63:269–276. doi: 10.1006/nlme.1995.1031. [DOI] [PubMed] [Google Scholar]
- 30.Deupree D, Coppock W, Willer H. J Comp Physiol Psychol. 1982;96:557–562. doi: 10.1037/h0077908. [DOI] [PubMed] [Google Scholar]
- 31.Galey D, Jeantet Y, Destrade C, Jaffard R. Behav Neural Biol. 1983;38:240–250. doi: 10.1016/s0163-1047(83)90240-6. [DOI] [PubMed] [Google Scholar]
- 32.Landfield P W. Physiol Behav. 1977;18:439–445. [Google Scholar]
- 33.Wetzel W, Ott T, Matthies H. Behav Biol. 1977;21:32–40. doi: 10.1016/s0091-6773(77)92231-3. [DOI] [PubMed] [Google Scholar]
- 34.Brelsford J, Theios J. Psychon Sci. 1965;2:81–82. [Google Scholar]
- 35.Kehoe E J, Gormezano I. Bull Psychon Soc. 1974;2:434–436. [Google Scholar]
- 36.Salafia W R, Mis F W, Terry W S, Bartosiak R S, Daston A P. Anim Learn Behav. 1973;1:109–115. [Google Scholar]
- 37.Berger T W, Alger B, Thompson R F. Science. 1976;192:483–485. doi: 10.1126/science.1257783. [DOI] [PubMed] [Google Scholar]
- 38.Prokasy W F. Learn Motiv. 1973;4:247–258. [Google Scholar]
- 39.Seager M A, Borgnis R L, Berry S D. Neurobiol Aging. 1997;18:631–639. doi: 10.1016/s0197-4580(97)00160-7. [DOI] [PubMed] [Google Scholar]
- 40.Seager M A, Borgnis R L, Berry S D. Neurobiol Aging. 1998;19:277–281. doi: 10.1016/s0197-4580(98)00061-x. [DOI] [PubMed] [Google Scholar]
- 41.Otto T, Eichenbaum H, Wiener S I, Wible C G. Hippocampus. 1991;1:181–192. doi: 10.1002/hipo.450010206. [DOI] [PubMed] [Google Scholar]
- 42.O'Keefe J, Recce M L. Hippocampus. 1993;3:317–330. doi: 10.1002/hipo.450030307. [DOI] [PubMed] [Google Scholar]
- 43.Skaggs W E, McNaughton B L, Wilson M A, Barnes C A. Hippocampus. 1996;6:149–172. doi: 10.1002/(SICI)1098-1063(1996)6:2<149::AID-HIPO6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 44.Holscher C, Anwyl R, Rowan M J. J Neurosci. 1997;17:6470–6477. doi: 10.1523/JNEUROSCI.17-16-06470.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Larson J, Wong D, Lynch G. Brain Res. 1986;368:347–350. doi: 10.1016/0006-8993(86)90579-2. [DOI] [PubMed] [Google Scholar]
- 46.Pavlides C, Greenstein Y J, Grudman M, Winson J. Brain Res. 1988;439:383–387. doi: 10.1016/0006-8993(88)91499-0. [DOI] [PubMed] [Google Scholar]
- 47.Thomas M J, Watabe A M, Moody T D, Makhinson M, O'Dell T J. J Neurosci. 1998;18:7118–7126. doi: 10.1523/JNEUROSCI.18-18-07118.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maren S, DeCola J P, Swain R A, Fanselow M S, Thompson R F. Behav Neurosci. 1994;108:44–56. doi: 10.1037//0735-7044.108.1.44. [DOI] [PubMed] [Google Scholar]
- 49.Borisyuk R M, Hoppensteadt F C. BioSystems. 1998;48:3–10. doi: 10.1016/s0303-2647(98)00044-6. [DOI] [PubMed] [Google Scholar]
- 50.Gluck M A, Granger R. Annu Rev Neurosci. 1993;16:667–706. doi: 10.1146/annurev.ne.16.030193.003315. [DOI] [PubMed] [Google Scholar]
- 51.Hasselmo M E, Schnell E. J Neurosci. 1994;14:3898–3914. doi: 10.1523/JNEUROSCI.14-06-03898.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lorincz A, Buzsaki G. Ann NY Acad Sci. 2000;911:83–111. doi: 10.1111/j.1749-6632.2000.tb06721.x. [DOI] [PubMed] [Google Scholar]
- 53.Myers C E, Ermita B R, Harris K, Hasselmo M, Solomon P, Gluck M A. Neurobiol Learn Mem. 1996;66:51–66. doi: 10.1006/nlme.1996.0043. [DOI] [PubMed] [Google Scholar]
- 54.Berger T W, Weikart C L, Bassett J L, Orr W B. Behav Neurosci. 1986;100:802–809. doi: 10.1037//0735-7044.100.6.802. [DOI] [PubMed] [Google Scholar]
- 55.Singer W. Neuron. 1999;24:49–65. doi: 10.1016/s0896-6273(00)80821-1. [DOI] [PubMed] [Google Scholar]
- 56.Tesche C D. Brain Res. 1997;749:53–60. doi: 10.1016/s0006-8993(96)01286-3. [DOI] [PubMed] [Google Scholar]
- 57.Tesche C D, Karhu J. J Cognit Neurosci. 1999;11:424–436. doi: 10.1162/089892999563517. [DOI] [PubMed] [Google Scholar]
- 58.Tesche C D, Karhu J. Proc Natl Acad Sci USA. 2000;97:919–924. doi: 10.1073/pnas.97.2.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Elbert T, Rockstroh B, Lutzenberger W, Birbaumer N. Electroencephalogr Clin Neurophysiol. 1980;48:293–301. doi: 10.1016/0013-4694(80)90265-5. [DOI] [PubMed] [Google Scholar]
- 60.Rosenfeld J P, Rudell A P, Fox S S. Science. 1969;165:821–823. doi: 10.1126/science.165.3895.821. [DOI] [PubMed] [Google Scholar]