Abstract
Changes in the morphology of dendritic spines are correlated with synaptic plasticity and may relate mechanistically to its expression and stabilization. Recent work has shown that spine length can be altered by manipulations that affect intracellular calcium, and spine length is abnormal in genetic conditions affecting protein synthesis in neurons. We have investigated how ligands of group 1 metabotropic glutamate receptors (mGluRs) affect spine shape; stimulation of these receptors leads both to calcium release from intracellular stores and to dendritic protein synthesis. Thirty-minute incubation of cultured hippocampal slices and dissociated neurons with the selective group 1 mGluR agonist (S)-3,5-dihydroxyphenylglycine (DHPG) induced a significant increase in the average length of dendritic spines. This elongation resulted mainly from the growth of existing spines and was also seen even in the presence of antagonists of ionotropic receptors, indicating that activation of these receptors by mGluR-induced glutamate release was not required. Prolonged antagonism of group 1 mGluRs with (S)-α-methyl-4-carboxyphenylglycine (MCPG) did not result in shorter average spine length. Elongation of dendritic spines induced by DHPG was blocked by calcium chelation and by preincubation with the protein synthesis inhibitor puromycin. The results suggest that in vivo activation of group 1 mGluRs by synaptically released glutamate affects spine shape in a protein synthesis-dependent manner.
Many conditions that elicit enduring functional changes at glutamatergic synapses also result in morphological alterations in dendritic spines, the highly specialized actin-based protrusions on dendritic processes that constitute the postsynaptic element at the majority of these synapses. Long-term potentiation (LTP) and depression (LTD) at glutamatergic synapses both depend on calcium as a biochemical trigger and on protein synthesis for lasting expression. Cytoskeletal and other proteins with putative structural functions at the synapse are required for LTP. These proteins are locally synthesized during induction of LTP (1), and the organization of these proteins is regulated in a calcium-dependent manner (see ref. 2). These findings support the longstanding view that structural modifications underlie the stable expression of plasticity (3).
Recent data on the relationship between spine length and levels of postsynaptic calcium raise the possibility that multiple receptor systems linked to calcium mobilization may be involved in the control of spine dimensions. Bidirectional changes in spine length in cultured neurons have been correlated with the degree of calcium mobilization during iontophoretic application of glutamate (4). Intense synaptic activation of N-methyl-d-aspartate (NMDA)-type glutamate receptors in particular (5, 6) has been correlated with spine lengthening and sprouting; however, NMDA receptor activation has also been correlated with spine retraction (7, 8). Calcium released from intracellular stores has also been shown to affect spine length (9). Group 1 metabotropic glutamate receptors (mGluRs, subunits 1 and 5) modulate postsynaptic calcium release from internal stores (10, 11), suggesting that they may be able to trigger changes in spine length. As with activation of NMDA receptors (12), stimulation of group 1 mGluRs also leads to postsynaptic protein synthesis (13–15). Calcium mobilization and protein synthesis triggered by group 1 mGluRs are involved both in LTD (16, 17) and in the priming of hippocampal synapses for subsequent induction of stable LTP (15).
Our working hypothesis is that group 1 mGluR activation may regulate spine shape through mechanisms that are also involved in the production of synaptic plasticity. We assayed the effects of treating hippocampal neurons with the group 1 mGluR-selective agonist (S)-3,5-dihydroxyphenylglycine (DHPG) on the length of dendritic spines. Two hippocampal culture preparations commonly used for the study of dendritic spine dynamics were tested. Brief treatments with DHPG resulted in elongation of dendritic spines of dentate gyrus granule cells in cultured slices and in neurons maintained in dissociated cultures. (S)-α-methyl-4-carboxyphenylglycine (MCPG), an antagonist of group 1 and 2 mGluRs, blocked the effects of DHPG, but had no net effect on spine length when added alone, even on longer treatment. A calcium chelator and puromycin, an inhibitor of mRNA translation, both prevented these effects of mGluR stimulation. Our results favor a model in which metabotropic and ionotropic receptors can have convergent or opposing influences on spine length through modulation of at least two common variables: calcium levels and protein synthesis.
Methods
Culture Preparations.
Methods for the preparation, maintenance, and transfection of organotypic and dissociated cultures of hippocampus have been described in ref. 18. Briefly, transverse sections of hippocampus (400 μm) were explanted to millicell-CM culture inserts (Millipore) and maintained in interface culture for a minimum of 10 days before transfection and pharmacological treatments. The culture medium contained MEM (GIBCO), 22.5% horse serum (heat treated 56°C for 30 min; Gemini Biological Products, Calabasas, CA), with additions to bring the following components to the indicated final concentrations (in mM): 2.5 MgSO4, 2 CaCl2, 25 glucose, 3 glutamine, 20 Hepes, and 5 NaHCO3 (pH 7.30). Dissociated neurons were prepared from the hippocampi of E18 rats and maintained in a medium composed of Neurobasal (GIBCO) with B27 supplement (GIBCO) and 3 mM glutamine for 3 weeks before use.
Plasmid Construction and Transfection.
Organotypic slices and dissociated neurons were transiently transfected with either of two constructs coding for the expression of fluorescent reporters, one coded for the expression of the enhanced yellow fluorescent protein (EYFP) whereas the other coded for a fusion protein of EYFP and the cyan variant, ECFP (18). Both constructs contained a PDZ cognate domain from the Shaker Kv1.7 potassium channel to facilitate retention of the fluorophores at synaptic sites.
Transfection of reporter plasmids into cultured neurons used two techniques. For organotypic slices, plasmid DNA was introduced by biolistic transfection with the Gene Gun (Bio-Rad) according to methods recommended by the manufacturer. Plasmid DNA was precipitated onto 1.6-μm gold microcarriers at a ratio of 2 μg DNA to 1 mg gold. Coating of DNA-gold complexes onto tefzel tubing used polyvinylpyrrolidine at a final concentration of 0.02 mg/ml. Transfection of dissociated neurons was done by calcium phosphate precipitation as described (18). For each culture system, pharmacological treatments were initiated between 2 and 4 days after transfection.
Pharmacological Treatments of Cultured Slices and Neurons.
Pharmacological manipulation of ionotropic and metabotropic glutamate receptors used selective agonists and antagonists. Treatment of cultures involved exchanging the medium in which they were maintained with an aliquot containing agonists and/or antagonists diluted from stocks. For slice cultures, treatments were started with an initial application of 400 μl of drug-containing media on top of the culture insert to facilitate entry of the drug into slices. Stimulation of group 1 mGluRs used the selective agonist DHPG at 100 μM applied for times ranging between 10 and 30 min. The antagonist MCPG was used at a concentration of 250 μM (30 min) to block group 1 mGluRs as an acute control condition and also to test the effects of tonic inhibition of these receptors over longer periods (2–3 days). In experiments including the calcium chelator 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetate (BAPTA; 20 mM) or the protein synthesis inhibitor puromycin (100 μM), the agents were added by medium exchange 30 min before addition of the mGluR ligands, and were then included with the mGluR reagents. Cultures that were untreated or coincubated with the NMDA-type and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)-type ionotropic receptor antagonists d(-)-2-amino-5-phosphonopentanoic acid (AP5, 50 μM) and 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, 20 μM) served as treatment controls. These antagonists were also included in some of the experiments with DHPG to control for potential indirect effects of mGluR stimulation on ionotropic currents. Cultures were processed for imaging by first fixing them on ice with 4% paraformaldehyde in PBS for 10 min (dissociated cells) or 30 min (slice cultures), after which they were washed, and mounted with Prolong or Slowfade antifade reagents (Molecular Probes).
Image Acquisition and Analysis.
Images were acquired by using a Zeiss Axioplan 2 fluorescence microscope fitted with a Cooke Sensicam charge-coupled device (CCD) camera (12-bit), a mercury lamp, and controlled by the slidebook software package from Intelligent Imaging Innovations (Denver, CO). Filter cube specifications for the EYFP fluorescent channel, which was the only channel used, were as follows (excitation, emission, beam splitter): EYFP (500/20, 535/30, 515; Chroma Technology, Brattleboro, VT). Images in the EYFP channel were taken by using a ×63 oil immersion objective. Spine length measurements were made from volumetric renderings of 20–30 successive planar images captured at z-increments of 0.30 μm in the EYFP channel and subsequently deconvolved with the constrained iterative algorithm by using point spread functions determined from three-dimensional images of Biobeads (Molecular Probes) mounted in the appropriate medium. Measurement of spine length was done manually with ruler tools in slidebook. The analysis focused on spines along secondary dendrites of granule cells in slice cultures and on dissociated hippocampal neurons. The radial distance from spine head to dendritic shaft was measured for at least 50 spines per neuron. The average spine length was determined for 2–3 slices, or chambers of dissociated neurons, per experiment; stated n values indicate the number of experiments.
Spines were also classified with regard to “spine type” according to the following scheme: type 1 spines, also called “stubby protuberances,” were ≤0.5 μm in length, lacked a large spine head, and did not appear to have a neck; type 2 spines, or “mushroom shaped” spines, were between 0.5 and 1.25 μm long and were characterized by a short neck and large spine head; type 3 spines, or “thin class” spines, ranged between 1.25 and about 3.0 μm and had elongated spine necks with small heads; type 4 spines, or “filapodial extensions,” were long filamentous protrusions that lacked a discernible spine head. Frequency distributions and other data manipulations were done by using Graph Pad prism software (GraphPad, San Diego). Spine densities were estimated by counting the number of spines along 50- to 100-μm segments of secondary dendrite on 2–3 dendrites/neuron; densities were expressed as spines per 50 μm. Data sets were compared statistically by using the Mann–Whitney two-tailed U test.
Results
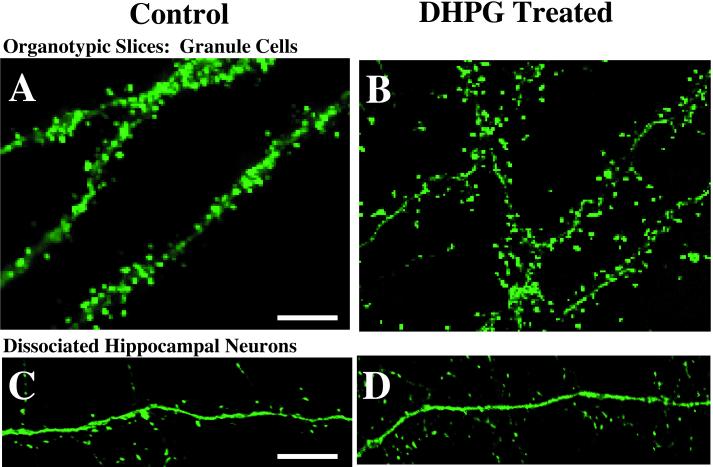
Organotypic slices of the hippocampus cultured for at least 10 days exhibited mature dendritic arbors and dendritic spine populations as imaged by EYFP-based fluorescent reporters introduced biolistically (Fig. 1A). Three weeks after plating, dissociated neurons exhibited dense spine populations and long dendritic and axonal arbors (Fig. 1C). These observations are consistent with previously published characterizations of the two hippocampal culture systems (19, 20) and provided a basis for the timing of transfections, pharmacological manipulations, and analyses used in this study.
Figure 1.
Examples of the effect of mGluR stimulation on the length of dendritic spines in two hippocampal culture systems. Slices (A and B) and dissociated neurons (C and D) were fixed after treatment with vehicle control (Left) or 100 μM DHPG (Right). Control and treated cultures prepared from the same animals were processed in parallel. Scale bar = 5 μm.
Brief Treatment with DHPG Increases the Average Length of Dendritic Spines.
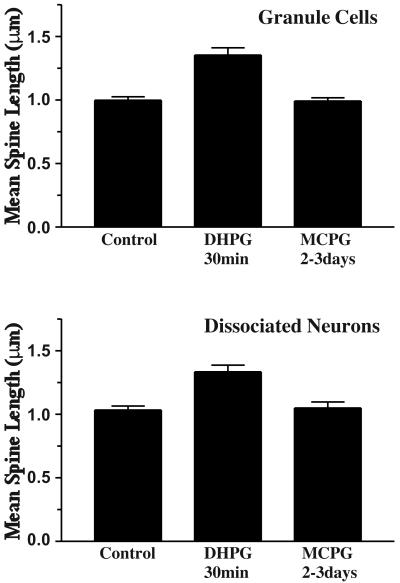
The average length of spines on the dendrites of dentate gyrus granule cells in hippocampal slice cultures and neurons grown in dissociated culture was increased by brief incubation with the group 1 mGluR agonist DHPG (Fig. 1). Increases in spine length were evident after 30 min of incubation with the mGluR agonist (100 μM), and in some experiments were seen as early as 10 min after treatment. The average radial distance from the spine head to the dendritic shaft increased relative to controls by 35.4 ± 5.7% (n = 10; P < 0.01) after a 30-min incubation with DHPG (Fig. 2 Upper). In dissociated neurons, the effect was similar in magnitude (29.0 ± 5.0%; n = 9; P < 0.01; Fig. 2 Lower). Cultures in which the mGluR antagonist MCPG was added for 30 min were not different from untreated controls. Prolonged incubation with MCPG (2–3 days) did not result in statistically significant changes in spine length overall (Fig. 2), although in some experiments it appeared that there was a trend toward reduced lengths.
Figure 2.
The effect of DHPG on the average length of dendritic spines of dentate gyrus granule cells in slice culture and dissociated hippocampal neurons. A 30-min incubation with 100 μM DHPG resulted in a significant increase in the average length of dendritic spines (1.35 ± 0.06 μm) relative to controls acutely treated with AP5/CNQX (0.99 ± 0.03 μm; n = 10 experiments, 2–3 granule cells per experiment, ≥50 spines per cell; P < 0.001, two-tailed Mann–Whitney test). Similar effects were obtained in dissociated neuronal culture (control 1.03 ± 0.03, DHPG 1.33 ± 0.05; P < 0.001). Prolonged incubation with MCPG did not alter the average length of dendritic spines (dentate granule cells, 0.99 ± 0.03; dissociated neurons, 1.05 ± 0.05).
These DHPG-dependent changes were also obtained when a combination of the NMDA- and AMPA-type ionotropic receptor antagonists AP5 and CNQX was included in the media (data not shown). Spine lengths in cultures treated with AP5/CNQX in the absence of DHPG were not different from those in non-treated controls. These results argue for a mechanism that requires direct activation of postsynaptic group 1 metabotropic receptors, rather than an indirect effect through postsynaptic ionotropic receptors triggered by glutamate release following DHPG binding to presynaptic receptors (21).
DHPG Shifts the Distribution of Spine Lengths with Minimal Changes in Spine Density.
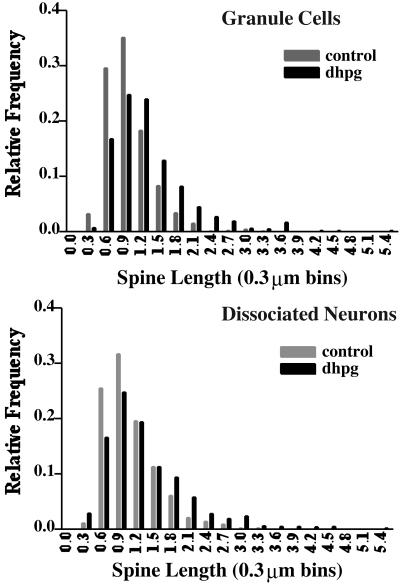
Changes in the length of dendritic spines induced by mGluR stimulation were also reflected in frequency distribution analyses of spine length and type. Fig. 3 shows the relative frequencies of dendritic spines grouped into 0.3-μm bins. The distribution of spine lengths was skewed toward longer spines with DHPG treatment for both dentate granule cells (Fig. 3A) and dissociated neurons (Fig. 3B). However, measurements of spine density were slightly different between control (70.9 ± 4.1 spines per 50 μm, n = 7) and DHPG-treated cultures (82.6 ± 5.1 spines per 50 μm, n = 9; P = 0.071 Mann–Whitney U test, two-tailed). The quantitative results suggest that the observed changes result mainly from the growth of existing spines.
Figure 3.
Frequency distribution histograms of dendritic spine length in control and DHPG-treated cultures. (Upper) The length of spines on the dendrites of dentate granule cells in slice culture were measured and segregated into bins of 0.3 μm. The composite frequency distribution from 10 experiments shows that the occurrence of longer spines increases whereas that of shorter spines decreases. (Lower) The same effect is evident in composite frequency distributions of spine lengths in dissociated neuronal cultures.
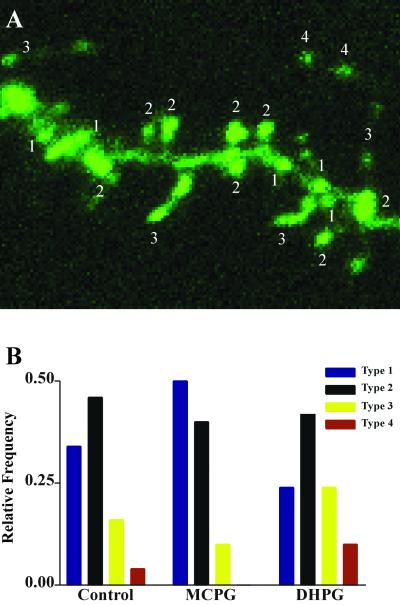
An analysis of spine types, based on a classification scheme that takes into account overall length and spine head and neck shape characteristics (see Methods) revealed a greater proportion of spines resembling filapodial extensions and of non-“mushroom” type spines. In Fig. 4, examples of four spine types discriminated by using this classification scheme are labeled throughout an image of a DHPG-treated dendrite. In the Lower panel, analysis of the spine types shows that the greatest effect of DHPG is on the number of thin spines (type 3) and on those resembling filapodial extensions (type 4); a slight decrease in the proportion of type 1 spines seems to accompany these effects. The relative abundance of the classical mushroom-shaped spines (type 2) was similar to that in cultures treated acutely with AP5 + CNQX or with MCPG for 2–3 days.
Figure 4.
Example of spine types and analysis of shifts in the relative abundance of these types accompanying DHPG treatment. (A) Maximum intensity projection of an image stack taken from a DHPG-treated dentate granule cell. Four spine types are labeled, classified as described in Methods and differentiated with respect to length and shape characteristics. (B) Relative abundance of spine types in control slices and in slices treated acutely with DHPG or chronically for 2–3 days with MCPG (control and DHPG treatments also included AP5 + CNQX). Spines identified as type 3 or 4, characterized by longer, thinner profiles with smaller spine heads, are increased in frequency with DHPG treatment whereas shorter (“nubbin”) spines, type 1, are decreased. A slight shift in the opposite direction with chronic MCPG is seen in this experiment, but this result did not occur reliably enough to influence the average spine length. The proportion of type 2 spines, the classic mushroom-shaped profiles, did not change with DHPG treatment.
Perturbation of Calcium Mobilization and of mRNA Translation Inhibits the Effects of DHPG.
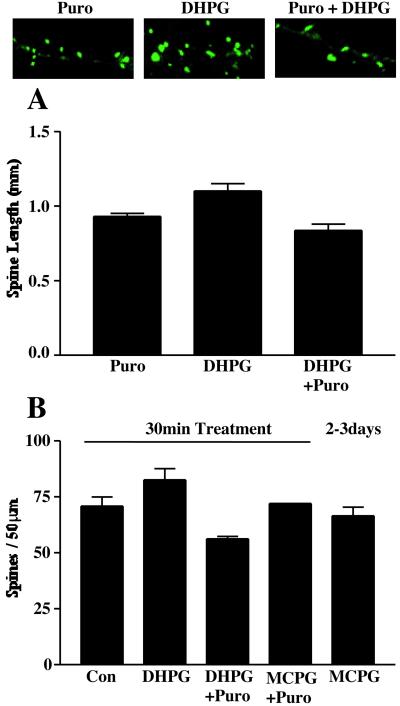
Attenuation of calcium mobilization and protein synthesis diminished the effects of DHPG on spine length. Chelation of internal calcium by treating slice cultures with BAPTA blocked the DHPG-induced increases in spine length (DHPG 1.29 ± 0.04 μm; DHPG + chelator 0.98 ± 0.05 μm; Controls 1.00 ± 0.04 μm). Inhibition of protein synthesis by preincubation of slices with puromycin (100 μM) for 30 min resulted in a slight decrease in average spine length following subsequent coincubation with DHPG (Fig. 5A). Treatment with puromycin alone did not change the average length of dendritic spines on dentate granule cell dendrites.
Figure 5.
(A) The effect of puromycin on DHPG-induced spine lengthening in hippocampal slice cultures. Mean spine lengths in slices treated with puromycin (Puro), DHPG, and DHPG in the presence of puromycin (DHPG + Puro) are graphed with sample images from each treatment above. (B) The effect of simultaneous manipulation of group 1 mGluRs and protein synthesis on spine density. The average number of dendritic spines per 50-μm segment of secondary dendrites of dentate granule cells was not significantly different from controls (70.9 ± 4.1, n = 7) after treatment with DHPG (82.6 ± 5.1, n = 9) or after 2–3 days of incubation with MCPG (66.5 ± 3.9, n = 11). Addition of puromycin simultaneously with either drug also did not significantly change the density of spines, although a trend toward fewer spines was seen with DHPG + puromycin (56.3 ± 1.0, n = 3; P = 0.06, Mann–Whitney U test, two-tailed).
To characterize further the nature of the changes induced by metabotropic receptor activation, the density of dendritic spines along secondary dendrites of dentate granule cells and dissociated hippocampal neurons was measured. Although the data suggested a small increase in the density with DHPG treatment (Fig. 5B), these changes were not statistically significant and may have reflected the influence of increased length on our ability to discern existing postsynaptic elements.
Discussion
Selective stimulation of group I metabotropic glutamate receptors resulted in an increase in the average length of dendritic spines present on the dendrites of hippocampal neurons maintained in organotypic and dissociated culture systems. The increases, which averaged over 30% in each system, took place within 30 min or less. The changes were likely due to growth of existing spines inasmuch as the distributions of spine lengths exhibited an overall shift from shorter to longer profiles and changes in spine density were minimal. Antagonism of iontropic receptors concomitant with DHPG application did not block the effect. Moreover, addition of the mGluR antagonist MCPG for 2–3 days did not cause opposite changes. Pharmacological disruption of either of two relatively rapid postsynaptic effects of group 1 mGluR stimulation (calcium mobilization from intracellular stores and protein synthesis) blocked the effects of DHPG on spine length. These results establish a link between the regulation of synaptic morphology and metabotropic glutamate receptor signaling. They take on added significance in the context of recent observations on the interrelationships between spine shape, synaptic efficacy, and dendritic protein synthesis (5–8, 22–27)
Stimulation of glutamatergic synapses has been found to result in both increased and decreased spine length (5–8, 22). This variation may result from different levels of calcium mobilization within spines and dendritic segments, as well as the relative balance of other mechanisms initiated with synaptic stimulation. In accord with this notion, iontophoretic application of glutamate in brief pulses triggered low levels of calcium mobilization and caused spine elongation, and subsequent longer pulses yielded high levels of free intracellular calcium and caused retraction of the same spines (4). Elongation of dendritic spines has also been linked to calcium mobilization from intracellular stores by using caffeine (9). All of these findings suggest that the level of intracellular-free calcium is a variable through which different receptor mechanisms may converge to produce opposing effects. Inasmuch as levels of spine calcium mobilized by mGluR stimulation are typically lower than levels accompanying intense NMDA receptor activation, our finding that spine length increases with DHPG treatment fits well with what might be expected from the effects of different levels of calcium mobilization on spine length.
It is noteworthy that the effects of DHPG were not obtained when protein synthesis was suppressed with puromycin. Stimulation of glutamatergic synapses has been shown to lead local translation of a number of proteins (28, 29), and blockade of protein synthesis prevents the stable expression of LTP (30–32). Activity-dependent induction and dendritic translation of proteins such as Arc, a putative actin-binding cytoskeletal protein required for late phase LTP, may participate in dendritic spine shape changes by reorganizing the actin-based cytoskeleton of spines. Pharmacological stimulation of mGluRs is known to induce dendritic translation (28, 29), but, unlike NMDA receptor activation, mGluR activation is not typically associated with the mobilization of high levels of calcium (10, 33, 34) or the rapid transcription of dendritically targeted mRNAs. The relative balance and temporal order of mGluR and NMDA receptor activation could thus be crucial in determining the type and direction of functional and morphological changes. It is of interest that burst stimulation, under conditions in which mGluR responses are isolated, primes hippocampal synapses for subsequent induction of the stable protein synthesis-dependent phase of LTP (15), whereas direct activation of mGluRs in multiple systems induces a form of LTD that depends on local translation (17).
Elongation of dendritic spines could have multiple functional consequences. Recent results suggest that longer spines induced by stimulation of group I mGluRs may provide a substrate for synaptic depression associated with this treatment. Ref. 23 reported that spine geometry and synaptic function are correlated such that thinner spines and filopodia have fewer functional AMPA receptors than shorter spines with larger heads. This observation supports the idea that longer, thinner spines constitute a less efficacious postsynaptic phenotype, and it is consistent with EM data on the relationship between synaptic AMPA receptor content and the geometry of the postsynaptic density (35). LTD induced by DHPG treatment was recently linked to internalization of ionotropic glutamate receptors (36), and this effect was shown to be sensitive to the translational inhibitor cyclohexamide. Taken together with our observation that DHPG causes spine elongation, it appears that the variables of spine length and AMPA receptor content are controlled in a coordinate manner by group I mGluRs to produce a weaker synaptic phenotype consistent with previous observations (23).
Spine length is also known to influence calcium diffusion kinetics of the spine head relative to those of the dendritic shaft. This parameter could influence calcium-dependent events that affect synaptic efficacy (24, 25). Spine length could also change parameters such as synaptic membrane tension, which through biophysical effects could alter the properties or insertion of glutamate receptors. These effects of varying spine length may contribute to the behavioral deficits found in fragile X mental retardation. This syndrome is characterized neuroanatomically by a preponderance of abnormally long spines (26), and it results from the reduced expression of a dendritically localized mRNA binding protein, FMRP, that can function as a translational suppressor (37, 38). Experiments analogous to those reported here in which translation of synaptically localized mRNAs is blocked in hippocampal preparations could be particularly informative in regard to the roles of particular proteins in determining both spine shape and synaptic efficacy.
Acknowledgments
We thank Drs. Kathryn L. Crossin, Bruce A. Cunningham, and Joseph Gally for their constructive criticisms. This work was supported by United States Public Health Service Grants NS39837 (G.M.E.) and MH64036 (P.W.V.) as well as a grant from the G. Harold and Leila Y. Mathers Foundation. P.W.V. is partly supported by the Skaggs Institute of Chemical Biology.
Abbreviations
- LTP
long-term potentiation
- LTD
long-term depression
- NMDA
N-methyl-d-aspartate
- mGluR
metabotropic glutamate receptor
- DHPG
(S)-3,5-dihydroxyphenylglycine
- MCPG
(S)-α-methyl-4-carboxyphenylglycine
- EYFP
enhanced yellow fluorescent protein
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- AP5
d(-)-2-amino-5-phosphonopentanoic acid
- CNQX
6-cyano-7-nitroquinoxaline-2,3-dione
References
- 1.Guzowski J F, Lyford G L, Stevenson G D, Houston F P, McGaugh J L, Worley P F, Barnes C A. J Neurosci. 2000;20:3993–4001. doi: 10.1523/JNEUROSCI.20-11-03993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lynch G. Neurobiol Learn Mem. 1998;70:82–100. doi: 10.1006/nlme.1998.3840. [DOI] [PubMed] [Google Scholar]
- 3.Calverley R K, Jones D G. Brain Res Brain Res Rev. 1990;15:215–249. doi: 10.1016/0165-0173(90)90002-6. [DOI] [PubMed] [Google Scholar]
- 4.Korkotian E, Segal M. Neuroreport. 1999;10:2875–2877. doi: 10.1097/00001756-199909090-00032. [DOI] [PubMed] [Google Scholar]
- 5.Maletic-Savatic M, Malinow R, Svoboda K. Science. 1999;283:1923–1927. doi: 10.1126/science.283.5409.1923. [DOI] [PubMed] [Google Scholar]
- 6.Engert F, Bonhoeffer T. Nature (London) 1999;339:66–70. doi: 10.1038/19978. [DOI] [PubMed] [Google Scholar]
- 7.Lee K S, Schottler F, Oliver M, Lynch G. J Neurophysiol. 1980;44:247–258. doi: 10.1152/jn.1980.44.2.247. [DOI] [PubMed] [Google Scholar]
- 8.Hasbani M J, Schlief M L, Fisher D A, Goldberg M P. J Neurosci. 2001;21:2393–2403. doi: 10.1523/JNEUROSCI.21-07-02393.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Korkotian E, Segal M. Proc Natl Acad Sci USA. 1999;96:12068–12072. doi: 10.1073/pnas.96.21.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy S N, Miller R J. Proc Natl Acad Sci USA. 1988;85:8737–8741. doi: 10.1073/pnas.85.22.8737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fagni L, Chavis P, Ango F, Bockaert J. Trends Neurosci. 2000;23:80–88. doi: 10.1016/s0166-2236(99)01492-7. [DOI] [PubMed] [Google Scholar]
- 12.Scheetz A J, Nairn A C, Constantine-Paton M. Nat Neurosci. 2000;3:211–216. doi: 10.1038/72915. [DOI] [PubMed] [Google Scholar]
- 13.Weiler I J, Irwin S A, Klintsova A Y, Spencer C M, Brazelton A D, Miyashiro K, Comery T A, Patel B, Eberwine J, Greenough W T. Proc Natl Acad Sci USA. 1997;94:5395–5400. doi: 10.1073/pnas.94.10.5395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Angenstein F, Greenough W T, Weiler I J. Proc Natl Acad Sci USA. 1998;95:15078–15083. doi: 10.1073/pnas.95.25.15078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raymond C R, Thompson V L, Tate W P, Abraham W C. J Neurosci. 2000;20:969–976. doi: 10.1523/JNEUROSCI.20-03-00969.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kato N. Proc Natl Acad Sci USA. 1993;90:3650–3654. doi: 10.1073/pnas.90.8.3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huber K M, Kayser M S, Bear M F. Science. 2000;288:1254–1257. doi: 10.1126/science.288.5469.1254. [DOI] [PubMed] [Google Scholar]
- 18.Vanderklish P W, Krushel L A, Holst B H, Gally J A, Crossin K L, Edelman G M. Proc Natl Acad Sci USA. 2000;97:2253–2258. doi: 10.1073/pnas.040565597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muller D, Buchs P A, Stoppini L. Brain Res Dev Brain Res. 1993;71:93–100. doi: 10.1016/0165-3806(93)90109-n. [DOI] [PubMed] [Google Scholar]
- 20.Boyer C, Schikorski T, Stevens C F. J Neurosci. 1998;18:5294–5300. doi: 10.1523/JNEUROSCI.18-14-05294.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cartmell J, Schoepp D D. J Neurochem. 2000;75:889–907. doi: 10.1046/j.1471-4159.2000.0750889.x. [DOI] [PubMed] [Google Scholar]
- 22.Yuste R, Bonhoeffer T. Annu Rev Neurosci. 2001;24:1071–1089. doi: 10.1146/annurev.neuro.24.1.1071. [DOI] [PubMed] [Google Scholar]
- 23.Matsuzaki M, Ellis-Davies G C, Nemoto T, Miyashita Y, Iino M, Kasai H. Nat Neurosci. 2001;4:1086–1092. doi: 10.1038/nn736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Majewska A, Brown E, Ross J, Yuste R. J Neurosci. 2000;20:1722–1734. doi: 10.1523/JNEUROSCI.20-05-01722.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Majewska A, Tashiro A, Yuste R. J Neurosci. 2000;20:8262–8268. doi: 10.1523/JNEUROSCI.20-22-08262.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Comery T A, Harris J B, Willems P J, Oostra B A, Irwin S A, Weiler I J, Greenough W T. Proc Natl Acad Sci USA. 1997;94:5401–5404. doi: 10.1073/pnas.94.10.5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Irwin S A, Galvez R, Greenough W T. Cereb Cortex. 2000;10:1038–1044. doi: 10.1093/cercor/10.10.1038. [DOI] [PubMed] [Google Scholar]
- 28.Eberwine J, Miyashiro K, Kacharmina J E, Job C. Proc Natl Acad Sci USA. 2001;98:7080–7085. doi: 10.1073/pnas.121146698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steward O, Schuman E M. Annu Rev Neurosci. 2001;24:299–325. doi: 10.1146/annurev.neuro.24.1.299. [DOI] [PubMed] [Google Scholar]
- 30.Stanton P K, Sarvey J M. J Neurosci. 1984;4:3080–3088. doi: 10.1523/JNEUROSCI.04-12-03080.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deadwyler S A, Dunwiddie T, Lynch G. Synapse. 1987;1:90–95. doi: 10.1002/syn.890010112. [DOI] [PubMed] [Google Scholar]
- 32.Frey U, Krug M, Reymann K G, Matthies H. Brain Res. 1988;452:57–65. doi: 10.1016/0006-8993(88)90008-x. [DOI] [PubMed] [Google Scholar]
- 33.Frenguelli B G, Potier B, Slater N T, Alford S, Collingridge G L. Neuropharmacology. 1993;32:1229–1237. doi: 10.1016/0028-3908(93)90017-w. [DOI] [PubMed] [Google Scholar]
- 34.Wade S B, Oommen P, Conner W C, Earnest D J, Miranda R C. J Neurosci. 1999;19:6994–7006. doi: 10.1523/JNEUROSCI.19-16-06994.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takumi Y, Ramirez-Leon V, Laake P, Rinvik E, Otterson O P. Nat Neurosci. 1999;2:618–624. doi: 10.1038/10172. [DOI] [PubMed] [Google Scholar]
- 36.Snyder E M, Philpot B D, Huber K M, Dong X, Fallon J R, Bear M F. Nat Neurosci. 2001;4:1079–1085. doi: 10.1038/nn746. [DOI] [PubMed] [Google Scholar]
- 37.Laggerbauer B, Ostareck D, Keidel E M, Ostareck-Lederer A, Fischer U. Hum Mol Genet. 2001;10:329–338. doi: 10.1093/hmg/10.4.329. [DOI] [PubMed] [Google Scholar]
- 38.Li Z, Zhang Y, Ku L, Wilkinson K D, Warren S T, Feng Y. Nucleic Acids Res. 2001;29:2276–2283. doi: 10.1093/nar/29.11.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]