Abstract
The parathyroid hormone 2 (PTH2) receptor's anatomical distribution suggests that, among other functions, it may be involved in modulation of nociception. We localized PTH2 receptor protein to spinal cord lamina II and showed that it is synthesized by subpopulations of primary sensory neurons and intrinsic spinal cord dorsal horn neurons. Tuberoinfundibular peptide of 39 residues (TIP39) selectively activates the PTH2 receptor. Intraplantar microinjection of TIP39 caused a paw-withdrawal response and intrathecal injection caused scratching, biting, and licking, a nocifensive response. Intrathecal administration of a TIP39 antibody decreased sensitivity in tail-flick and paw-pressure assays. Intrathecal administration of TIP39 potentiated responses in these assays. We determined the sequence of TIP39's precursor and found that mRNA encoding TIP39 and TIP39-like immunoreactivity is concentrated in two brainstem areas, the subparafascicular area and the caudal paralemniscal nucleus. Cells in these areas project to the superficial dorsal horn of the spinal cord. Our data suggest that TIP39 released from supraspinal fibers potentiates aspects of nociception within the spinal cord.
The parathyroid hormone 2 (PTH2) receptor was originally identified in a screen for novel G-protein-coupled receptors (1). It is distinguished both pharmacologically and anatomically from the PTH1 (or PTH/PTHrP) receptor (2), which mediates the calcium-regulating functions of parathyroid hormone (PTH), a classic endocrine hormone, and the effects of the distinct peptide PTH-related peptide, which regulates development, remodeling, and epithelial transport in several tissues (3–5).
The biological roles of the PTH2 receptor are not yet established. It is present at greatest levels in the nervous system, and unlike the PTH1 receptor, it is found at low density and in only a few cells in kidney and bone (1, 6, 7). PTH-related peptide has low affinity for PTH2 receptors, and does not significantly activate them (8). Unlike the human PTH2 receptor, which was initially characterized and led to the receptor's name, rat and zebrafish PTH2 receptors are poorly activated by PTH (9, 10). We recently used selective activation of the PTH2 receptor as an assay to purify a previously undescribed peptide from bovine hypothalamus (11). This peptide, tuberoinfundibular peptide of 39 residues (TIP39), has a structure and functional architecture similar to PTH and PTH-related peptide (12), but at most nine residues in common with PTH from any species (13). TIP39 potently activates human, rat, and zebrafish PTH2 receptors and has no effect on PTH1 receptors (10, 11). It may be the physiological ligand for PTH2 receptors.
Detailed mapping of the PTH2 receptor's anatomical distribution suggests several possible biological functions (6, 7, 14). The receptor is expressed at high levels in the hypothalamus (15), and recent experiments support a role for it in regulation of hypothalamic functions (16). It is also synthesized by a population of dorsal root ganglion (DRG) cells and by neurons within the dorsal horn of the spinal cord (14). A PTH2 receptor selective antibody intensely labels nerve fibers and terminals in the superficial dorsal horn of the spinal cord, and the corresponding caudal part of the spinal trigeminal nucleus. These areas contain most of the central terminals of nociceptive primary afferents. TIP39 increases cAMP in F-11 cells (11), which are a DRG-neuroblastoma hybrid cell line that possesses some of the properties of peptidergic nociceptors (16). Other agents that increase cAMP in DRG neurons potentiate nociception (17).
We have now used local administration of TIP39 and a TIP39-sequestering antibody to explore the involvement of the PTH2 receptor and TIP39 in nociception. We also used double-label immunohistochemistry to define the distribution of the PTH2 receptor in the spinal cord more precisely, and determined the locations of TIP39 synthesis. Our data show that TIP39 causes or potentiates nocifensive responses, that sequestering TIP39 with an antibody decreases withdrawal responses, and that TIP39 is synthesized by neurons in areas that have projections to the sensory trigeminal and spinal cord regions rich in PTH2 receptors. Thus, TIP39 may be a modulator of nociception.
Materials and Methods
Animals.
Male ddY mice weighing 20–22 g were used in physiological experiments. Procedures were approved by Nagasaki University Animal Care Committee and complied with the recommendations of the International Association for the Study of Pain (18). Anatomical experiments were approved by the National Institute of Mental Health Animal Care Committee.
Materials.
TIP39 was synthesized by Midwest Biomolecules (Waterloo, IL). Other reagents were from standard commercial suppliers. Intrathecal antibody injections were performed with IgG, purified from a rabbit immunized with bovine (b) TIP39 coupled by glutaraldehyde to keyhole limpet hemocyanin, or from preimmune serum. Immunohistochemistry used affinity-purified antibody from a rabbit immunized with mouse (m) TIP39 coupled by 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide to keyhole limpet hemocyanin. Titers (50% maximum binding to immobilized peptide) of anti-bTIP39 IgG and affinity-purified anti-mTIP39 were 800 and 6 ng/ml against bTIP39 and 900 and 3 ng/ml against mTIP39. Both antibodies exhibited ≈1% cross-reactivity with PTH and no detectable cross-reactivity with other peptides tested including PTH-related peptide, calcitonin, substance P, vasoactive intestinal peptide, glucagon, and calcitonin gene-related peptide (CGRP).
In Vivo Testing.
Evaluation of flexor responses.
Experiments were performed as described (19, 20). Mice were held in a suspended cloth sling. Test agents were delivered through two polyethylene cannulae inserted into the plantar surface of the right hindlimb, which was connected to an isotonic transducer/recorder. For normalization the largest spontaneous response occurring immediately after cannulation was considered the maximal withdrawal force for each animal. Nociceptive activity, measured after complete recovery (20–30 min) from light ether anesthesia, was expressed as the ratio of the test elicited force to the maximal force in each mouse. Intraplantar TIP39 injections were made at 5-min intervals through one cannula. In the dose-response experiments increasing doses were given at 5-min intervals, with each dose administered twice.
Evaluation of nocifensive responses.
Animals were adapted to individual plastic cages for 1 h. Five microliters of test agent was injected intrathecally between lumbar disks 5 and 6 (21), each mouse was returned to its transparent cage, and the time spent exhibiting characteristic nociceptive behaviors, such as reciprocal hindlimb scratching, and caudally directed scratching, biting, and licking during 20 min of observation was measured (22–24).
Tail-flick test.
Animals were gently restrained by hand, and a light beam adjusted for 10- to 12-sec latency in naive mice was focused onto the blackened dorsal surface of the tail. Latency up to a cut-off time of 30 sec was measured (25).
Paw-pressure test.
Mice were placed in a Plexiglas chamber on a wire mesh grid and allowed to accommodate for 1 h. A mechanical stimulus was then delivered onto the middle of the plantar surface of the right hindpaw by using a 0.8- to 0.9-mm-diameter filament connected to an automatic Transducer Indicator (model 1601, IITC, Woodland Hills, CA). The filament used produces 10 × g of force at 5 sec, when paw withdrawal is elicited in naive mice. A 20-sec cut-off time was used to avoid tissue damage.
Hargreaves' test.
A thermal beam was focused on the hindlimb footpads of mice placed on a glass surface and the withdrawal-response latency measured, with a 20-sec cut-off time, as described by Hargreaves et al. (26).
Sequence Identification, Reverse Transcription–PCR, in Situ Hybridization, and Immunohistochemistry.
Genomic sequences of mouse (GenBank no. AC073740) and human TIP39 (GenBank no. AC068670) were identified in public high-throughput gene sequence by tBLASTn (27) search with the amino acid sequence of bovine TIP39. cDNA sequences were predicted by using GENE (28). Fragments corresponding to amino acids −55 to 37 and −18 to 37 were amplified from total mouse brain cDNA with primers incorporating RNA polymerase recognition sites, 35S-labeled antisense and sense riboprobes were synthesized, and hybridized to cryostat sections as described on the World Wide Web (http://intramural.nimh.nih.gov/lcmr/snge/Protocols/ISHH/ISHH.html). The two probes produced equivalent hybridization patterns. For the tissue distribution survey ≈1 μg of DNase-treated RNA prepared from rat tissues was reverse-transcribed and then PCR amplified (35 cycles) by using primers (5′-GGAGACCTGCCAGATGTCCA and 5′-GTCCAGTAGCAACAGCTT) in different TIP39 exons. Immunolabeling with a PTH2 receptor selective antibody was performed as described (7, 14). Labeling with a TIP39 selective antibody, performed with free-floating 50-μm vibratome sections from paraformaldehyde-perfused animals, was detected by using Vectastain ABC reagents (Vector Laboratories) or by tyramide-mediated amplification (29). Incubation with TIP39 eliminated immunostaining. Some animals received an intraventricular injection of 80 μg of colchicine 48 h before euthanasia.
Statistics.
All data represent the mean ± SEM from a minimum of five separate experiments.
Results and Discussion
Both DRG and spinal cord dorsal horn neurons synthesize PTH2 receptors. The PTH2 receptors synthesizing DRG neurons seem to be of the smaller size class, and the spinal cord neurons are within the superficial layers of the dorsal horn (Fig. 1). We observed that a PTH2 receptor selective antibody labels nerve fibers and terminals much more strongly than cell bodies, suggesting that the receptor accumulates to much greater levels in the terminals and dendrites than in the soma of neurons (14). Fibers in the superficial layers of the dorsal horn of the spinal cord were particularly intensely labeled. We now performed double-labeling experiments, comparing the distribution of the PTH2 receptor with well studied markers, to define more precisely the PTH2 receptor's localization in the spinal cord (Fig. 2). Most PTH2 receptor labeling was ventral to the area of greatest substance P and CGRP labeling (marginal layers), and dorsal to the layer of labeling by an antibody to protein kinase C gamma (lamina III). The labeling was largely colaminar with a band of labeling by isolectin B4. On the basis of extensive studies of these markers (30–32), this labeling places the PTH2 receptor within lamina II. Partial overlap occurred in labeling by the PTH2 receptor and substance P and CGRP antibodies, but high magnification confocal microscopy showed no colocalization of substance P and the PTH2 receptor (not shown), and little colocalization of the PTH2 receptor with CGRP (Fig. 2b). There was also no apparent colocalization with isolectin B4 despite extensive laminar overlap (Fig. 2d). CGRP and isolectin B4 together label nearly all small-diameter primary afferent fibers (33). Relatively little PTH2 receptor synthesis occurs in supraspinal areas that project to the superficial dorsal horn (14). Thus, most PTH2 receptors present in the spinal cord seem to be on the processes of intrinsic dorsal horn neurons, which is consistent with the in situ hybridization pattern seen with the PTH2 receptor probe.
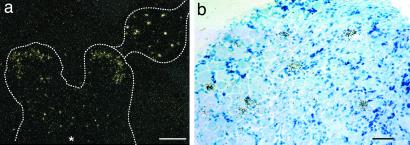
Figure 1.
In situ hybridization detection of PTH2 receptor mRNA in rat spinal cord and DRG. (a) Low-magnification dark-field micrograph. Part of the spinal cord gray matter at the thoracic level, and an attached DRG are outlined. Note hybridization in outer layers of the spinal cord dorsal horn, in scattered cells in deeper spinal cord layers, and in the DRG. (b) Higher-magnification bright-field image of a DRG. Note accumulation of silver grains (black) over some smaller neurons. Blue shows cellular counterstaining. * indicates central canal. [Bars = 1 mm (a), 100 μm (b).] Approximately 10–15% of DRG neurons are distinctly labeled by in situ hybridization. Antibody labeling (not shown) shows a continuum of intensity and numerical estimates are difficult to make.
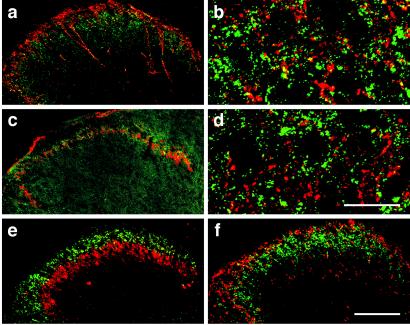
Figure 2.
Laminar localization of the PTH2 receptor in the dorsal horn of mouse spinal cord. PTH2 receptor immunoreactivity was detected with a green labeled secondary antibody and CGRP (a, b), isolectin B4 (c, d), protein kinase C gamma (e), and substance P (f), with red labeled secondary antibodies. High-power confocal microscopy indicates that there is virtually no colocalization between PTH2 receptor and CGRP (b) immunoreactivity or isolectin B4 labeling (d). [Bars = 100 μm (a, c, e, f), 20 μm (b, d).]
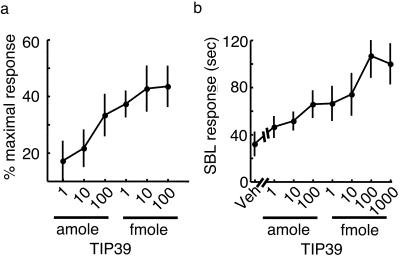
The localization of PTH2 receptor immunoreactivity and mRNA synthesis within the spinal cord and the small size of the PTH2 receptor expressing DRG cells suggest its possible involvement in nociception. Because many proteins synthesized by DRG neurons are transported in both their peripheral and central processes, we initially tested the effect of activating peripheral PTH2 receptors. Microinjection of TIP39 into the plantar surface of a mouse paw elicited a dose-dependent withdrawal response (Fig. 3a). The maximal effect occurred with approximately 10 fmol of peptide. By comparison, 1–10 fmol of nociceptin/orphanin FQ, 2 pmol of bradykinin, and 10 pmol of substance P cause maximal responses in this pain assay (19, 34). In situ hybridization histochemistry studies have shown that the PTH2 receptor is present at low levels on vascular endothelium (6, 7), so we cannot be absolutely certain that the effect of TIP39 results from activation of PTH2 receptors on the peripheral processes of sensory neurons. However, TIP39 has no effect on vascular tone in a perfused mesentery preparation (unpublished observations) and the labeling of DRG neurons is stronger than that of vasculature. We next examined the effects of central administration of TIP39. Intrathecal injection of TIP39 stimulated a dose-dependent nocifensive response, caudally directed scratching, biting, and licking (Fig. 3b).
Figure 3.
Responses to TIP39 administration. (a) Force of the paw-withdrawal response immediately after intraplantar administration of various doses of TIP39. (b) Time spent performing scratching, biting, and licking (SBL) behaviors during the 20 min after intrathecal administration of TIP39.
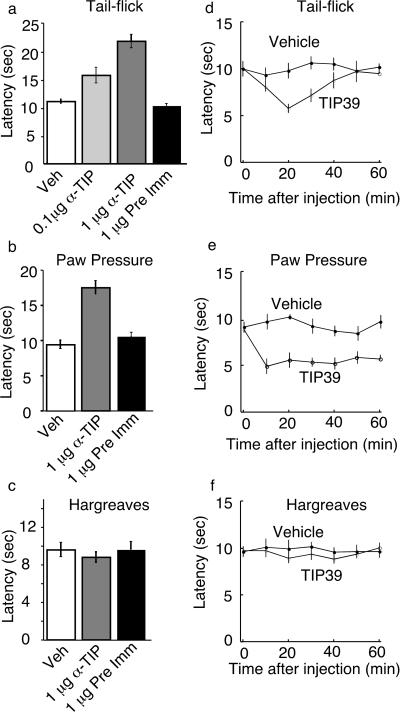
The responses to peripheral and central TIP39 administration show that it can activate nociceptive circuits. However, these observations do not address the effects of endogenous TIP39. Because no TIP39 antagonists were available, we sequestered TIP39 with an antibody, and asked what nociceptive responses were affected. Intrathecal injection of the TIP39 antibody increased the response latency in the thermal tail-flick assay (Fig. 4a). In the paw-pressure test, intrathecal administration of the TIP39 antibody increased the latency to paw withdrawal, corresponding to decreased pressure sensitivity (Fig. 4b). In contrast, intrathecal delivery of the TIP39 antibody did not significantly affect paw withdrawal latency in response to radiant heat [Fig. 4c; Hargreaves' assay (26)], and it did not affect the response to intraplantar injection of the chemoirritant capsaicin (not shown). Inhibition by the TIP39 antibody of responses in the thermal tail-flick and paw-pressure assays suggests that TIP39 may have a facilitatory role in the underlying circuits. We tested this possibility by performing the nociceptive assays after intrathecal administration of TIP39 (Fig. 4 d–f). Intrathecal TIP39 administration decreased the tail-flick and paw-pressure withdrawal latencies, but did not have a significant effect on withdrawal in response to paw heating. These effects are consistent with those predicted from the antibody sequestration experiments and with a facilitatory role of TIP39 in nociception. The tail-flick response is thought to be primarily a spinal reflex, whereas paw withdrawal from heat requires supraspinal mediation. The different effects of TIP39 and TIP39 sequestration on these responses may reflect differences in PTH2 receptor expression on neurons in the underlying circuits. However, the intrathecally applied reagents may also reach relevant sites with different efficiency.
Figure 4.
Modulation of nociceptive responses by intrathecal administration of an antibody to TIP39 or TIP39 itself. Tail-flick (a, d), paw pressure (b, e), and Hargreaves' (radiant heat directed to a paw; c, f) assays were performed as described in Materials and Methods. Responses after intrathecal delivery of vehicle (Veh), antibody to TIP39 (α-TIP), or preimmune serum (Pre Imm) are shown at 30 min after injection, the time at which the largest effect of the antibody was observed (a–c). Responses at various times after injection of vehicle or 100 fmol of TIP39 (TIP39) are shown (d–f).
We next wanted to identify the source(s) of TIP39. We originally determined the amino acid sequence of TIP39 purified from bovine hypothalamus. We searched genomic databases with use of this sequence and identified homologous human and mouse sequences. From these we predicted sequences for TIP39's precursor: METC(R)QM(V)SRSPRE(V)RLLLLLLLLLL(V)VPWGT(V)G(R)P(T)*ASGVALPL(P)A(V)GVF(L)—SLRA(P)PGRAWAG(D)L(P)G(A)S(T)PL(R)S(P)RRSLALADDAAFRERARLLAALERRR(H)WLD(N)- SYMQ(H)KLLL(V)LDAP. {Bold letters indicate residues that differ between the mouse and human sequences, with the human residue in parentheses after the mouse residue. An asterisk indicates a predicted signal peptide cleavage site [http://www.cbs.dtu.dk/services/SignalP/; (35, 36)], and the dash indicates the location of an intron in the genomic sequences. Residues corresponding to the purified peptide are underlined.} We amplified cDNAs corresponding to this mRNA from mouse and rat hypothalamus, and isolated a clone containing this sequence from a human hypothalamic cDNA library. The deduced human TIP39 peptide sequence (underlined sequence above) is identical with purified bovine TIP39, whereas the predicted mouse peptide differs at 4 of the 39 residues. Sequences corresponding to the purified peptide were followed by a stop codon and preceded by nucleotides encoding two arginine residues. The entire predicted TIP39 precursor is 103 residues and includes a predicted signal peptide of 33 residues.
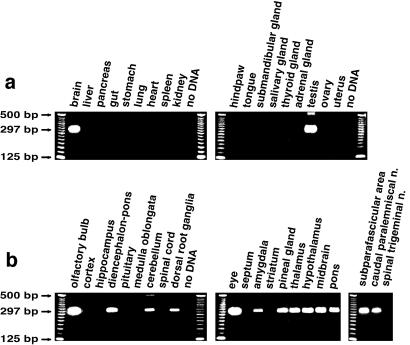
With reverse transcription–PCR we found TIP39 mRNA in rat brain, DRG, eye, and testis but not in the spinal cord or other tissues (Fig. 5). We determined the central nervous system distribution of TIP39 mRNA by in situ hybridization histochemistry (Fig. 6). No clear signal occurred after film autoradiography. After emulsion autoradiography two groups of intensely labeled cells were present in each of the nine animals examined. One was the subparafascicular area (Fig. 6 g and h; see ref. 37 for a detailed anatomical description of this area), in which TIP39-synthesizing cells were present both in the most rostral part of the central gray matter, medial to the fasciculus retroflexus at the diencephalic–midbrain junction, and in the parvicellular part of the subparafascicular nucleus (not shown). The dopaminergic A11 cell group is present in this area, but with adjacent sections it was clear that the TIP39 cells were tyrosine hydroxylase negative (not shown). The other group of intensely labeled cells was present in a well defined area of the lateral pons bordered by the oral reticular pontine, ventral lateral lemniscal, and Kölliker-Fuse nuclei (Fig. 6 b and c). We identified this latter area as the caudal paralemniscal nucleus on the basis of its topographical relation to the ventral nucleus of the lateral lemniscus and the rubrospinal tract (38–40). Significantly weaker hybridization signals were seen in cells scattered in the olfactory bulb, amygdala, hypothalamus, cerebellum, and the pineal gland (data not shown).
Figure 5.
PCR amplification of TIP39 cDNA from rat neuronal and peripheral tissues. The predicted size of the product amplified from cDNA is 297 bp; from genomic DNA it is 594 bp.
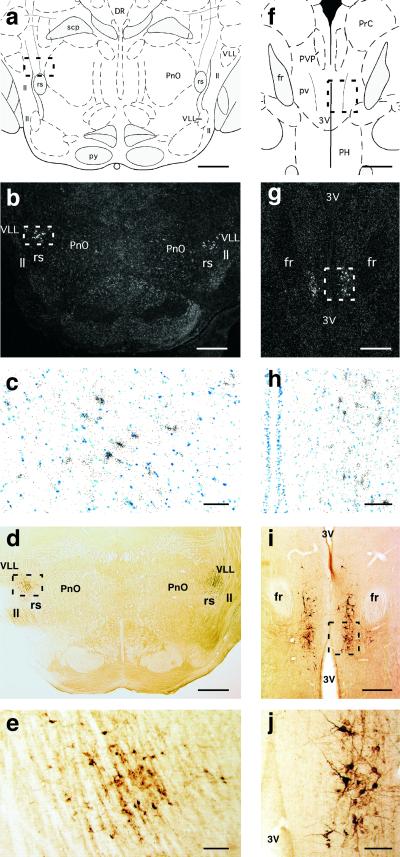
Figure 6.
Brain localization of TIP39-containing cells. (a–e) caudal paralemniscal nucleus at −8.7 mm from the bregma level. (f–j) subparafascicular area at −4.4 mm from the bregma. Drawings from the atlas of Paxinos and Watson (39) indicate the location of the micrographs (a, f). The localization of TIP39 mRNA detected by in situ hybridization histochemistry is shown at low magnification in dark-field micrographs (b, g) and at greater magnification of the framed areas in bright field (c, h). The localization of TIP39 protein is demonstrated by peroxidase immunocytochemistry in colchicine-treated animals, shown at low magnification (d, i) and at greater magnification of the framed areas (e, j). DR, dorsal raphe nucleus; fr, fasciculus retroflexus; ll, lateral lemniscus; PH, posterior hypothalamic area; PnO, pontine reticular nucleus, oral part; PrC, precomissural nucleus, posterior; pv, periventricular fiber system; PVP, paraventricular thalamic nucleus, posterior part; py, pyramidal tract; rs, rubrospinal tract; scp, superior cerebellar peduncle; VLL, ventral nucleus of lateral lemniscus; 3V, third ventricle. [Bars = 1 mm (a, b, d), 100 μm (c, e, h, j), 500 μm (g, h, i).]
To test the highly discrete localization revealed by in situ hybridization histochemistry we performed reverse transcription–PCR on micro- and macrodissected tissue samples (Fig. 5b). Micropunches of the subparafascicular area and the caudal paralemniscal nucleus contained TIP39 mRNA. TIP39 mRNA was detectable in the hypothalamus, thalamus, pons, midbrain, olfactory bulb, amygdala, pineal gland, and the cerebellum but not in the cerebral cortex, hippocampus, striatum, pituitary, medulla oblongata (including microdissected spinal trigeminal nucleus), or spinal cord.
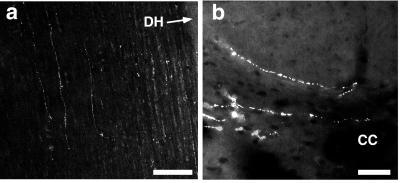
An antibody to TIP39 labels the same cell populations in rat (Fig. 6 d, e, i, j) and mouse (not shown) brain as detected by in situ hybridization. In the spinal cord, fine scattered fibers are found in the lateral funiculus, the lateral intermediate gray matter, central gray matter, and lamina II and III (Fig. 7). Most TIP39 fibers are in the lateral funiculus adjacent to the gray matter, an area that contains both descending and primary afferent fibers. We did not observe labeling of DRG neurons significantly over background by using either in situ hybridization or immunohistochemistry. The reverse transcription–PCR signal was consistently positive, but 35 cycles of amplification were used, suggesting that TIP39 may be present in DRG cells at a very low level. Because we have no evidence for TIP39 within spinal cord interneurons, the TIP39 in deep layers of the spinal cord is most likely within descending fibers. Thus, most of the TIP39 normally present in the spinal cord may be in descending fibers.
Figure 7.
TIP39-immunoreactive fibers in the spinal cord. (a) Horizontal section showing TIP39-immunoreactive fibers in craniocaudal orientation in the lateral funiculus. (b) Coronal section showing TIP39-immunoreactive fibers in lamina X and at the dorsal column-gray matter border. DH, dorsal horn; CC, central canal. [Bars = 200 μm (a), 50 μm (b).]
Cells within the areas of TIP39 concentration, the caudal paralemniscal nucleus (39, 40) and subparafascicular area (37) project to the dorsal horn, and to the caudal part of the sensory trigeminal nucleus (37, 38, 40), the medullary equivalent of the spinal dorsal horn. These areas have a high level of PTH2 receptor expression. Studies have implicated the caudal paralemniscal nucleus in nociception (40). These matches support the suggestion that TIP39 is a physiological ligand for the PTH2 receptor. DRG neurons also project to these areas. Thus, TIP39 synthesized by brainstem neurons and possibly TIP39 synthesized by primary afferent neurons are likely to contribute to modulation of spinal nociceptive information. Testis is the only peripheral tissue from which we amplified TIP39 mRNA, so it is not clear whether PTH2 receptors on the peripheral terminals of primary afferents are used physiologically, or represent nonselective transport by DRG neurons. The relative roles in the dorsal horn of the spinal cord of TIP39 released from primary afferents and from the supraspinal regions we have identified will be the subject of future investigation, as will the relative roles of pre- and postsynaptic PTH2 receptors. Future studies should also suggest whether blockade of the PTH2 receptor is a useful strategy for pain management.
Acknowledgments
We gratefully acknowledge Makoto Inoue and Saori Kondo for contributions to the behavioral experiments, Tianlun Wang for contributing to preliminary anatomical work, and Carolyn Smith for confocal microscopy. We appreciate assistance from Milan Rusnak with in situ hybridization, and the advice and comments of Éva Mezey, Tom Bonner, and Mike Brownstein. Support was provided by the National Institute of Mental Health Intramural Research Program and Special Coordination Funds of the Science and Technology Agency of the Japanese Government and Human Frontier Science Program.
Abbreviations
- TIP39
tuberoinfundibular peptide of 39 residues
- DRG
dorsal root ganglion
- PTH
parathyroid hormone
- CGRP
calcitonin gene-related peptide
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Usdin T B, Gruber C, Bonner T I. J Biol Chem. 1995;270:15455–15458. doi: 10.1074/jbc.270.26.15455. [DOI] [PubMed] [Google Scholar]
- 2.Jüppner H, Abou-Samra A B, Freeman M, Kong X F, Schipani E, Richards J, Kolakowski L F, Hock J, Potts J T, Kronenberg H M, Segre G V. Science. 1991;254:1024–1026. doi: 10.1126/science.1658941. [DOI] [PubMed] [Google Scholar]
- 3.Brown E M, Segre G V, Goldring S R. Bailliere's Clin Endocrinol Metab. 1996;10:123–161. doi: 10.1016/s0950-351x(96)80346-6. [DOI] [PubMed] [Google Scholar]
- 4.Kronenberg H M, Lanske B, Kovacs C S, Chung U I, Lee K, Segre G V, Schipani E, Juppner H. Recent Prog Horm Res. 1998;53:283–301. [PubMed] [Google Scholar]
- 5.Wysolmerski J J, Stewart A F. Annu Rev Physiol. 1998;60:431–460. doi: 10.1146/annurev.physiol.60.1.431. [DOI] [PubMed] [Google Scholar]
- 6.Usdin T B, Bonner T I, Harta G, Mezey É. Endocrinology. 1996;137:4285–4297. doi: 10.1210/endo.137.10.8828488. [DOI] [PubMed] [Google Scholar]
- 7.Usdin T B, Hilton J, Vertesi T, Harta G, Segre S, Mezey É. Endocrinology. 1999;140:3363–3371. doi: 10.1210/endo.140.7.6855. [DOI] [PubMed] [Google Scholar]
- 8.Gardella T J, Luck M D, Jensen G S, Usdin T B, Juppner H. J Biol Chem. 1996;271:19888–19893. doi: 10.1074/jbc.271.33.19888. [DOI] [PubMed] [Google Scholar]
- 9.Hoare S R J, Bonner T I, Usdin T B. Endocrinology. 1999;140:4419–4425. doi: 10.1210/endo.140.10.7040. [DOI] [PubMed] [Google Scholar]
- 10.Hoare S R, Rubin D A, Juppner H, Usdin T B. Endocrinology. 2000;141:3080–3086. doi: 10.1210/endo.141.9.7645. [DOI] [PubMed] [Google Scholar]
- 11.Usdin T B, Hoare S R J, Wang T, Mezey É, Kowalak J A. Nat Neurosci. 1999;2:941–943. doi: 10.1038/14724. [DOI] [PubMed] [Google Scholar]
- 12.Piserchio A, Usdin T, Mierke D F. J Biol Chem. 2000;275:27284–27290. doi: 10.1074/jbc.M003869200. [DOI] [PubMed] [Google Scholar]
- 13.Usdin T B. Trends Pharmacol Sci. 2000;21:128–310. doi: 10.1016/s0165-6147(00)01455-3. [DOI] [PubMed] [Google Scholar]
- 14.Wang T, Palkovits M, Rusnak M, Mezey E, Usdin T B. Neuroscience. 2000;100:629–649. doi: 10.1016/s0306-4522(00)00282-7. [DOI] [PubMed] [Google Scholar]
- 15.Ward H L, Small C J, Murphy K G, Kennedy A R, Ghatei M A, Bloom S R. Endocrinology. 2001;142:3451–3456. doi: 10.1210/endo.142.8.8308. [DOI] [PubMed] [Google Scholar]
- 16.Kusano K, Gainer H. J Neurosci Res. 1993;34:158–169. doi: 10.1002/jnr.490340203. [DOI] [PubMed] [Google Scholar]
- 17.Taiwo Y O, Bjerknes L K, Goetzl E J, Levine J D. Neuroscience. 1989;32:577–580. doi: 10.1016/0306-4522(89)90280-7. [DOI] [PubMed] [Google Scholar]
- 18.Zimmermann M. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 19.Inoue M, Kobayashi M, Kozaki S, Zimmer A, Ueda H. Proc Natl Acad Sci USA. 1998;95:10949–10953. doi: 10.1073/pnas.95.18.10949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ueda H. Jpn J Pharmacol. 1999;79:263–268. doi: 10.1254/jjp.79.263. [DOI] [PubMed] [Google Scholar]
- 21.Hylden J L, Wilcox G L. Eur J Pharmacol. 1980;67:313–316. doi: 10.1016/0014-2999(80)90515-4. [DOI] [PubMed] [Google Scholar]
- 22.Hylden J L, Wilcox G L. Brain Res. 1981;217:212–215. doi: 10.1016/0006-8993(81)90203-1. [DOI] [PubMed] [Google Scholar]
- 23.Hylden J L, Wilcox G L. J Pharmacol Exp Ther. 1983;226:398–404. [PubMed] [Google Scholar]
- 24.Inoue M, Shimohira I, Yoshida A, Zimmer A, Takeshima H, Sakurada T, Ueda H. J Pharmacol Exp Ther. 1999;291:308–313. [PubMed] [Google Scholar]
- 25.Ueda H, Fukushima N, Kitao T, Ge M, Takagi H. Neurosci Lett. 1986;65:247–252. doi: 10.1016/0304-3940(86)90269-7. [DOI] [PubMed] [Google Scholar]
- 26.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 27.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 28.Salamov A A, Solovyev V V. Genome Res. 2000;10:516–522. doi: 10.1101/gr.10.4.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hunyady B, Krempels K, Harta G, Mezey E. J Histochem Cytochem. 1996;44:1353–1362. doi: 10.1177/44.12.8985127. [DOI] [PubMed] [Google Scholar]
- 30.Molliver D C, Radeke M J, Feinstein S C, Snider W D. J Comp Neurol. 1995;361:404–416. doi: 10.1002/cne.903610305. [DOI] [PubMed] [Google Scholar]
- 31.Malmberg A B, Chen C, Tonegawa S, Basbaum A I. Science. 1997;278:279–283. doi: 10.1126/science.278.5336.279. [DOI] [PubMed] [Google Scholar]
- 32.Polgar E, Fowler J H, McGill M M, Todd A J. Brain Res. 1999;833:71–80. doi: 10.1016/s0006-8993(99)01500-0. [DOI] [PubMed] [Google Scholar]
- 33.Snider W D, McMahon S B. Neuron. 1998;20:629–632. doi: 10.1016/s0896-6273(00)81003-x. [DOI] [PubMed] [Google Scholar]
- 34.Ueda H, Matsunaga S, Inoue M, Yamamoto Y, Hazato T. Peptides (Tarrytown, NY) 2000;21:1215–1221. doi: 10.1016/s0196-9781(00)00262-x. [DOI] [PubMed] [Google Scholar]
- 35.Nielsen H, Engelbrecht J, Brunak S, von Heijne G. Int J Neural Syst. 1997;8:581–599. doi: 10.1142/s0129065797000537. [DOI] [PubMed] [Google Scholar]
- 36.Nielsen H, Engelbrecht J, Brunak S, von Heijne G. Protein Eng. 1997;10:1–6. doi: 10.1093/protein/10.1.1. [DOI] [PubMed] [Google Scholar]
- 37.Peschanski M, Mantyh P W. Brain Res. 1983;263:181–190. doi: 10.1016/0006-8993(83)90311-6. [DOI] [PubMed] [Google Scholar]
- 38.Zemlan F P, Kow L M, Morrell J I, Pfaff D W. J Anat. 1979;128:489–512. [PMC free article] [PubMed] [Google Scholar]
- 39.Andrezik J A, Beitz A J. In: The Rat Nervous System. Paxinos G, editor. Orlando, FL: Academic; 1986. pp. 1–28. [Google Scholar]
- 40.Martin G F, Holstege G, Mehler W R. In: The Human Nervous System. Paxinos G, editor. San Diego: Academic; 1990. pp. 203–220. [Google Scholar]