Abstract
Glutamate, by activating N-methyl-d-aspartate (NMDA) receptors, alters the balance between dopamine D1 and D2 receptor signaling, but the mechanism responsible for this effect has not been known. We report here, using immunocytochemistry of primary cultures of rat neostriatal neurons, that activation of NMDA receptors recruits D1 receptors from the interior of the cell to the plasma membrane while having no effect on the distribution of D2 receptors. The D1 receptors were concentrated in spines as shown by colocalization with phalloidin-labeled actin filaments. The effect of NMDA on D1 receptors was abolished by incubation of cells in calcium-free medium and was mimicked by the calcium ionophore ionomycin. Recruitment of D1 receptors from the interior of the cell to the membrane was confirmed by subcellular fractionation. The recruited D1 receptors were functional as demonstrated by an increase in dopamine-sensitive adenylyl cyclase activity in membranes derived from cells that had been pretreated with NMDA. These results provide evidence for regulated recruitment of a G protein-coupled receptor in neurons, provide a cell biological basis for the effect of NMDA on dopamine signaling, and reconcile the conflicting hyperdopaminergic and hypoglutamatergic hypotheses of schizophrenia.
Increasing evidence indicates that interactions between different types of neurotransmitter receptors represent a major mechanism of neuronal plasticity. A variety of physiological and pharmacological stimuli alter the efficacy of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor-mediated synaptic transmission (1, 2). These changes in signaling have been accounted for in large part by changes in the distribution of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors between spines and the interior of the cell (3, 4). Although signaling in the dopaminergic pathway also shows a great deal of plasticity, little information is available about its cellular basis. In the present study, we examined the possibility that an alteration in the distribution of dopamine receptors might contribute to the ability of N-methyl-D-aspartate (NMDA) to alter the balance between D1 and D2 receptor signaling pathways.
Materials and Methods
Cell Culture.
Cultures of striatal neurons were prepared from 18- to 19-day rat embryos. The cells were plated on poly-D-lysine-coated glass coverslips and cultured in Eagle's MEM plus F-12 (1:1). The cultures were grown in the presence of 5% FBS, penicillin, or streptomycin (100 mg/ml). To suppress the growth of glial cells, FBS was equilibrated with N2 (1%) 2 days after plating, and cytosine arabinoside (5 μM) was added for 48 h to the culture medium on day 5. Cells were maintained in culture for 2–3 weeks before experiments; at that time, virtually all cells from embryonic rat striatum seemed to be mature neurons. They resembled typical medium spiny neurons and stained positively for neuronal markers (5).
Immunostaining.
Cells were fixed for 20 min in ice-cold 2% paraformaldehyde, permeabilized with 0.1% saponin, blocked with 7% normal goat or donkey serum, and then incubated overnight at 4°C with primary antibody in PBS containing 2.8% serum and 0.1% saponin. The cells were washed in PBS and 0.1% saponin, incubated with fluorescent secondary antibody in PBS with 2.8% serum and 0.1% saponin, and washed again. For actin labeling, the cells were incubated for 45 min with phalloidin-Alexa488 (Molecular Probes) (1/350) at room temperature. Finally, the cells were washed with PBS and mounted in Prolong antifade (Molecular Probes). D1 subclass dopamine receptors were probed with polyclonal rabbit affinity-purified anti-human D1 dopamine receptor antibody (1:100) (6). D2 subclass dopamine receptors were probed with polyclonal goat affinity-purified anti-human D2 dopamine receptor antibody (1:200) (Santa Cruz Biotechnology). Donkey-anti-goat Cy3, goat-anti-rabbit Cy5 (Jackson ImmunoResearch), and goat-anti-rabbit Alexa 546 (Molecular Probes) were used as secondary antibodies (all 1:200).
Confocal Microscopy.
The immunolabeled cells were recorded with a Leica TCS SP inverted confocal scanning laser microscope using 40×/1.25 N.A. and 20×/0.75 N.A. objectives. Green fluorescence was excited at 488 nm and detected at 492–535 nm. Red fluorescence was excited at 543 nm and detected at 570–650 nm. Preparations in which the primary antibody was omitted from the staining protocol were used as negative controls.
NMDA Treatment of Striatal Slices.
Striatum from 40-day-old male Sprague–Dawley rats was rapidly dissected out, and 350-μm slices were prepared using a tissue chopper (McIlwain, Guildford, U.K.). The striatal slices were placed in cold Krebs medium on ice for 15 min. The tubes were transferred to a 30°C water bath under 5% CO2/95% O2 for 15 min, followed by 5 min under 5% CO2/95% O2 in Krebs medium without Mg. The slices were treated with 1 mM NMDA (Sigma) for 30 s at 30°C under 5% CO2/95% O2. To stop the reaction, the tubes were placed on dry ice, and the medium was rapidly removed. The samples were frozen for at least 15 min.
cAMP Assay.
Striatal slices were prepared and incubated as described above and immediately resuspended in a Tris buffer (50 mM Tris⋅HCl/5 mM EDTA, pH 7.4) and homogenized with a tissue grinder on ice. The homogenate was centrifuged at 45,000 × g at 4°C for 10 min, and the pellet was resuspended in Tris buffer on ice for 30 min. The samples were centrifuged at 45,000 × g at 4°C for 10 min. Final membrane preparations were stored at −80°C overnight.
Pellets were resuspended in 50 mM Tris on ice, and proteins were determined by using a Bio-Rad protein assay (Bradford method). A total of 450 μl of membrane was treated with SKF81297 at a final concentration of 1 μM, and 200 μl of reaction solution (25 mM creatine phosphatase/0.27 unit/μl creatine kinase/2.3 mM 3-isobutyl-1-methylxanthine/4 mM ATP/0.1 mM PMSF/25 μM GTP/50 mM Tris/10 mM MgCl/0.015% BSA) were added. Membrane preparations were incubated at 30°C for 2 min, and the reaction was stopped by incubating at 100°C for 10 min. The preparations were centrifuged at 70,000 × g at 4°C for 20 min. Supernatants were collected, an equal volume of cold 10% trichloroacetic acid was added, and residual proteins were precipitated by centrifugation at 2,400 × g at 4°C for 15 min. The supernatant was extracted four times with water-saturated ether, and the aqueous phase was evaporated to dryness. The residue was dissolved in cAMP assay buffer and used for cAMP assay. cAMP was determined by using an adenosine 3′5′-monophosphate kit from NEN Life Sciences Products. The assay was carried out under linear conditions (2-min incubation).
Subcellular Fractionation.
The frozen slices were thawed and incubated for 10 min in Harm's buffer (250 mM sucrose, 10 mM triethanolamine, acetic acid, 1 mM EDTA, pH 7.4) on ice, followed by homogenization. A sucrose gradient was prepared in a buffer containing 90 mM KCl/50 mM Hepes, pH 7.2. The gradient contained, from bottom to top, as follows: 1 ml, 65%; 2 ml, 40%; 2 ml, 30%; 2 ml, 20%, 2 ml, 10% sucrose. On top, 1 ml of sample was applied, and the gradient was centrifuged at 28,000 rpm for 16 h at 4°C. One-milliliter fractions were collected from the bottom of the tube. All fractions were subject to Western blot. To detect the fractions containing the plasma membrane, a mouse monoclonal antibody raised against the α-subunit of Na+-K+-ATPase (1:5,000) (a gift from C. Sweadner) was used. Ten fractions were obtained. The Na,K-ATPase immunosignal was strong in fraction 2, moderate in fraction 3, and absent in the remaining eight fractions. In subsequent studies, fraction 2 was used for evaluation of the abundance of D1 receptor in the plasma membrane. This plasma membrane fraction was subject to a second Western blot using a goat polyclonal affinity-purified anti-human D1 dopamine receptor antibody (1:500).
Results
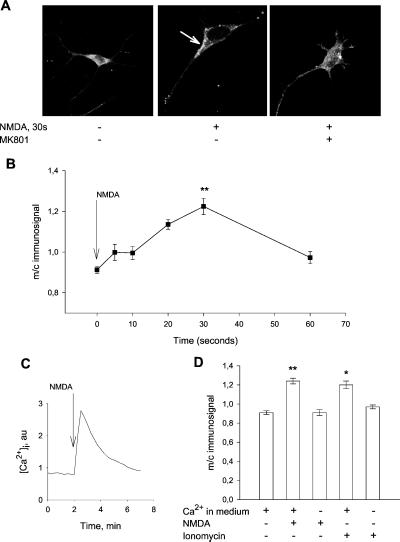
Incubation of primary neuronal cultures from rat striatum under basal conditions indicated that D1 receptors are, to a large extent, located in the cell body (Fig. 1A). To obtain a semiquantitative estimation of D1 receptor, we measured the intensity of the D1 receptor immunosignal in an area of the plasma membrane and an area of identical size in the cell body (Fig. 1B). The ratio between plasma membrane and cytosolic D1 receptor immunosignal was 0.91 ± 0.02. Upon addition of NMDA to the incubation medium, there was a movement of D1 receptors from the interior of the cell to the plasma membrane. A maximal accumulation of the D1 receptor at the plasma membrane was observed at 30 s of incubation, resulting in a membrane/cytosolic (m/c) ratio of the D1 receptor immunosignal of 1.24 ± 0.03 (P < 0.001 vs. control). This was followed by a redistribution of the D1 receptor to the interior of the cell. After 60 s of NMDA incubation, the m/c D1 receptor immunosignal ratio was 0.96 ± 0.03. The translocation effect of NMDA was abolished in the presence of the NMDA receptor antagonist MK801.
Figure 1.
(A) Confocal image of D1 receptor-labeled striatal neurons in primary culture. Cells were preincubated with the NMDA receptor antagonist, MK801, for 2 min and then exposed to NMDA (0.5 mM) for 30 s, as indicated. (B) Semiquantitative analysis of D1 receptor translocation over time. Cells were incubated with NMDA (0.5 mM) for the indicated times. The intensity of the immunosignal was measured in an area in the region of the plasma membrane and an area of identical size in the cell body subadjacent to the plasma membrane area. The ratio between the plasma membrane and cytosolic immunosignals was calculated. For each time-point, 28–64 cells were analyzed. Bars indicate SEM. (C) NMDA (0.5 mM) causes a transient increase in intracellular calcium in cultured striatal neuron. (D) NMDA-induced D1 receptor translocation is calcium dependent. Cells were treated with NMDA (0.5 mM) or with the calcium ionophore ionomycin (1 μM) in Ca2+ (1 mM) containing medium or in Ca2+ free EGTA (5 mM) containing medium. Removal of calcium from the medium in the absence of NMDA had no effect on the localization of D1 receptors (data not shown). Data represent mean values from 5–6 experiments. Bars indicate SEM. **, P < 0.001 for NMDA treatment; *, P < 0.01 for ionomycin treatment.
Because increased calcium influx is a main feature of NMDA-activated receptors, we examined whether D1 receptor translocation was a calcium-dependent process. Calcium imaging using the calcium sensitive dye, fura-2, showed a transient increase in intracellular calcium levels in response to NMDA (Fig. 1C). The ability of NMDA to recruit D1 receptor to the plasma membrane was abolished by removing calcium from the medium, and the calcium ionophore ionomycin caused a significant calcium-dependent increase in the m/c D1 receptor immunosignal (Fig. 1D). These findings are consistent with the concept that receptor-induced receptor recruitment requires calcium (7, 8).
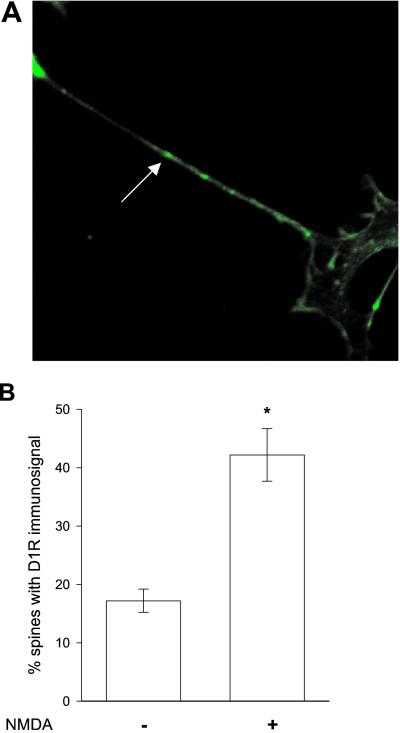
D1 receptors are known to be localized both in spines and in other regions of the plasma membrane. To determine whether the NMDA-induced redistribution of D1 receptors included recruitment to spines, we compared the localization of the D1 receptor with that of phalloidin (Fig. 2). Phalloidin has been used as a marker of spines by virtue of its ability to interact with f-actin, which is highly concentrated in spines. Cultured striatal neurons were incubated with NMDA for various intervals. In pilot studies, little effect was found on the D1 receptor localization in spines during the first few minutes after NMDA treatment. In contrast, after 15 min of NMDA treatment, a dramatic increase in D1 receptor-containing spines was observed. The number of spines that exhibited D1 receptor puncta was analyzed in five experiments by two independent observers, who were blind to the protocol. Values between the two observers never deviated by more than 6%. The fraction of spines expressing D1 receptors was between 16% and 22% in control cells and between 46% and 51% 15 min after NMDA treatment.
Figure 2.
(A) Confocal image of phalloidin-labeled striatal neurons in primary culture. Spines, one of which is indicated by an arrow, were defined as small actin rich protrusions. (B) Percentage of spines exhibiting D1 receptor immunosignal in neurons 15 min after exposure to vehicle or NMDA (0.5 mM) for 30 s. Data represent mean values from two independent observers and five experiments; bars indicate SEM. *, P < 0.001. In total, 158 spines were examined.
It was previously demonstrated that D1 receptors expressed in the kidney can be recruited to the plasma membrane by dopamine. We have also tested the effects of dopamine on the mobility of the D1 receptors in striatal cultures. We did not find any increase in the m/c ratio of the D1 receptor immunosignal (data not shown).
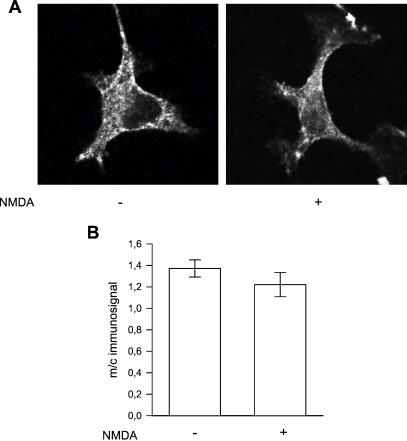
Recent studies from our laboratory have shown that D1 receptors and D2 receptors are colocalized in cultured striatal neurons (5). In control cells, D2 receptors were found to have a more prominent plasma membrane localization than D1 receptors; in these cells, the D2 receptor m/c immunosignal ratio, 1.37 ± 0.08, was significantly (P < 0.01) higher than the D1 receptor m/c immunosignal ratio. Upon preincubation with NMDA for 30 s, there was a slight but not significant decrease in the amount of D2 receptors in the plasma membrane relative to the cytosol (Fig. 3). Nor was there any significant difference in the m/c D2 receptor immunosignal ratio after 10 or 60 s of NMDA exposure (data not shown).
Figure 3.
(A) Confocal image of D2 receptor immunolabeled striatal neurons in primary culture. Cells were incubated with vehicle or NMDA (0.5 mM) for 30 s. (B) Semiquantitative analysis of m/c D2 receptor immunosignal after 30-s exposure to NMDA or vehicle. The D2 receptor m/c immunosignal was calculated as described in the Fig. 1 legend. Data represent mean values ± SEM from 78 cells.
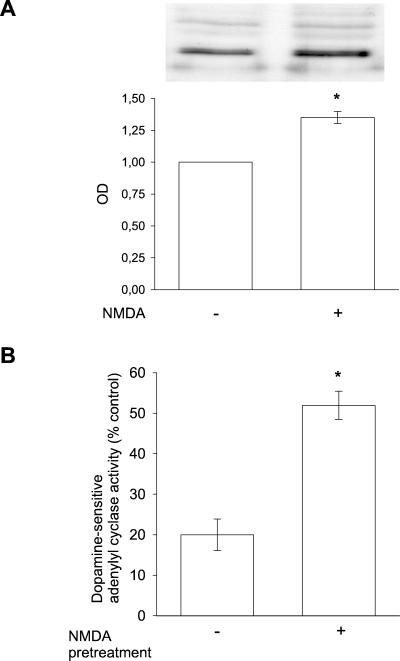
The translocation of D1 receptors from the interior of the cell to the plasma membrane was confirmed in subcellular fractionation experiments using slices of adult rat striatum. Using Na,K-ATPase as a marker for the plasma membrane, it was found that incubation of nerve cells with NMDA for 30 s caused a significant (1.4-fold) increase in the amount of D1 receptor in the fraction most highly enriched in plasma membrane (Fig. 4). This increase was in the same range as the NMDA-induced increase in the m/c D1 receptor immunosignal in cultured neurons.
Figure 4.
(A) Determination of D1 receptor abundance in plasma membrane fraction from adult rat striatum. Slices from striatum were incubated with vehicle or NMDA (1 mM) for 30 s and subjected to subcellular fractionation (as described in Materials and Methods). NMDA-treated samples showed a more intense D1 receptor immunosignal than did vehicle-treated samples. Bars indicate semiquantitative values for D1 receptor signal in five experiments ± SEM. *, P < 0.005. (B) D1 receptor-dependent cAMP generation in plasma membranes prepared from adult rat striatum exposed to vehicle or NMDA (1 mM) for 30 s. Bars indicate the % cAMP increase ± SEM in membranes incubated with the D1 receptor agonist, SKF81297, versus membranes incubated with vehicle. *, P < 0.001; n = 6 experiments for each group.
Finally, we determined whether the recruitment of D1 receptors to the plasma membrane was associated with an increase in functional D1 receptors. For this purpose, slices of adult rat neostriatum were preincubated in the absence or presence of 1 mM NMDA for 30 s. The plasma membrane fraction was isolated and assayed for dopamine-sensitive adenylyl cyclase activity. By using the membrane preparation, we eliminated the risk of a direct effect of NMDA on cAMP production. There was no effect of preincubation with NMDA on the basal level of adenylyl cyclase activity assayed in the membrane fraction (10.6 ± 0.5 ng of cAMP/mg protein per min in control cells vs. 9.7 ± 0.9 in NMDA-exposed cells). In contrast, pretreatment with NMDA caused a significant, 2.6-fold increase in dopamine-sensitive adenylyl cyclase activity (Fig. 4B).
Discussion
We have carried out a number of studies, all of which uniformly indicate that activation of NMDA receptors in striatal neurons triggers the translocation of cytoplasmic D1 receptors to the plasma membrane and spines. These results demonstrate a positive interaction between NMDA and D1 receptors in single cells. The studies performed on subcellular fractions from striatal slices confirm the results obtained by using cells in primary culture.
There is now ample evidence that responses to neurotransmitters can be enhanced by recruitment of their receptors to functional sites. The up-regulation of functional α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors in hippocampal neurons, triggered by NMDA and calcium signaling, is a well documented example of this principle (1–4). Studies on this type of heterologous sensitization have generally been carried out on receptors that are ligand-operated ion channels. There has been virtually no information on whether such sensitization also applies to neuronal G protein-coupled receptors. One recent study has suggested that neurons might possess the machinery needed for regulated recruitment of cytoplasmic D1 receptor to the plasma membrane and to dendrites. Mice lacking dopamine transporters typically have high levels of extracellular dopamine. In these mice, destruction of dopaminergic neurons results in relocation of D1 receptors to the plasma membrane (9).
Our results provide a cell biological explanation for the well established observation that NMDA shifts the balance between D1 receptor signaling and D2 receptor signaling in favor of the D1 receptor pathway (10, 11). One major hypothesis for schizophrenia is that there is hyperactivity of dopaminergic neurotransmission (12, 13). An imbalance between D1 receptor and D2 receptor signaling may contribute to the symptoms of schizophrenia. Most currently used antipsychotic drugs include, in their pharmacological profile, the ability to act as antagonists at the D2 receptor (14). Conversely, it has been reported that D1 receptor antagonists exacerbate psychotic symptoms (15, 16).
The other major hypothesis for schizophrenia is that there is hypoactivity of glutamatergic neurotransmission (12, 13, 17). There is evidence that glutamate receptor deficiencies occur in schizophrenia (18), that administration of noncompetitive NMDA receptor antagonists and reduced NMDA receptor expression are associated with a broad range of schizophrenic-like symptoms (19, 20), and that drugs that enhance NMDA-receptor function reduce negative symptoms and cognitive deficits in chronic schizophrenics who are receiving neuroleptics (13). The ability of NMDA agonists to recruit D1 receptors to the plasma membrane and shift the balance of dopamine signaling toward the D1 receptor and away from the D2 receptor provides a plausible cellular basis for the antipsychotic activity of this class of pharmacological agents. Thus, these observations reconcile the conflicting hyperdopaminergic and hypoglutamatergic hypotheses of schizophrenia.
Acknowledgments
This work was supported by grants from the Swedish Research Council (to A.A. and H.B.) and the Märta and Gunnar V. Philipson Foundation and by U.S. Public Health Service Grants MH-40899 and DA-10044 (to P.G.).
Abbreviations
- NMDA
N-methyl-d-aspartate
- m/c
membrane/cytosolic
References
- 1.Barria A, Muller D, Derkach V, Griffith L C, Soderling T R. Science. 1997;276:2042–2045. doi: 10.1126/science.276.5321.2042. [DOI] [PubMed] [Google Scholar]
- 2.Lee H K, Kameyama K, Huganir R L, Bear M F. Neuron. 1998;21:1151–1162. doi: 10.1016/s0896-6273(00)80632-7. [DOI] [PubMed] [Google Scholar]
- 3.Lüscher C, Xia H, Beattie E C, Carroll R C, von Zastrow M, Malenka R C, Nicoll R A. Neuron. 1999;24:649–658. doi: 10.1016/s0896-6273(00)81119-8. [DOI] [PubMed] [Google Scholar]
- 4.Shi S-H, Hayashi Y, Petralia R S, Zaman S H, Wenthold R J, Svoboda K, Malinow R. Science. 1999;284:1811–1816. doi: 10.1126/science.284.5421.1811. [DOI] [PubMed] [Google Scholar]
- 5.Aizman O, Brismar H, Uhlén P, Zettergren E, Levey A I, Forssberg H, Greengard P, Aperia A. Nat Neurosci. 2000;3:226–230. doi: 10.1038/72929. [DOI] [PubMed] [Google Scholar]
- 6.Brismar H, Asghar M, Carey R M, Greengard P, Aperia A. Proc Natl Acad Sci USA. 1998;95:5573–5578. doi: 10.1073/pnas.95.10.5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang L-Y M, Neher E. Neuron. 1996;17:135–145. doi: 10.1016/s0896-6273(00)80287-1. [DOI] [PubMed] [Google Scholar]
- 8.Maletic-Savatic M, Malinow R. J Neurosci. 1998;18:6803–6813. doi: 10.1523/JNEUROSCI.18-17-06803.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dumartin B, Jaber M, Gonon F, Caron M G, Giros B, Bloch B. Proc Natl Acad Sci USA. 2000;97:1879–1884. doi: 10.1073/pnas.97.4.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Konradi C, Leveque J-C, Hyman S E. J Neurosci. 1996;16:4231–4239. doi: 10.1523/JNEUROSCI.16-13-04231.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keefe K A, Ganguly A. Dev Neurosci. 1998;20:216–228. doi: 10.1159/000017315. [DOI] [PubMed] [Google Scholar]
- 12.Carlsson A, Hansson L O, Waters N, Carlsson M L. Br J Psychol. 1999;174, Suppl. 37:2–6. [PubMed] [Google Scholar]
- 13.Coyle J T. Harvard Rev Psychiatry. 1996;3:241–253. doi: 10.3109/10673229609017192. [DOI] [PubMed] [Google Scholar]
- 14.Cooper J R, Bloom F E, Roth R H. The Biochemical Basis of Neuropharmacology. Oxford: Oxford Univ. Press; 1996. pp. 480–510. [Google Scholar]
- 15.Den Boer J A, van Megen H J G M, Fleischhacker W W, Louwerens J W, Slaap B R, Westenberg H G M, Burrows G D, Srivastava O N. Psychopharmacology. 1995;121:317–322. doi: 10.1007/BF02246069. [DOI] [PubMed] [Google Scholar]
- 16.Karlsson P, Smith L, Farde L, Härnryd C, Sedvall G, Wiesel F-A. Psychopharmacology. 1995;121:309–316. doi: 10.1007/BF02246068. [DOI] [PubMed] [Google Scholar]
- 17.Tamminga C A. Crit Rev Neurobiol. 1998;12:21–36. doi: 10.1615/critrevneurobiol.v12.i1-2.20. [DOI] [PubMed] [Google Scholar]
- 18.Hirsch S R, Das I, Carey L J, de Belleroche J. Pharmacol Biochem Behav. 1997;56:797–802. doi: 10.1016/s0091-3057(96)00428-5. [DOI] [PubMed] [Google Scholar]
- 19.Mohn A R, Gainetdinov R R, Caron M G, Koller B H. Cell. 1999;98:427–436. doi: 10.1016/s0092-8674(00)81972-8. [DOI] [PubMed] [Google Scholar]
- 20.Jentsch J D, Roth R H. Neuropharmacology. 1999;20:201–224. [Google Scholar]