Abstract
Here, we describe the improved antiarthritic properties of a nitric oxide-releasing derivative of prednisolone that includes a sparing of the effects on bone. Glucocorticoids are widely used in the treatment of chronic inflammatory pathologies, but their use is often accompanied by side effects, including osteoporosis. Recently, a new steroid able to release low levels of nitric oxide showed potent inhibition of leukocyte trafficking and chemokine generation in models of acute inflammation. The objective of this study was to assess the anti-inflammatory activity of this nitric-oxide releasing glucocorticoid, nitro-prednisolone (NCX-1015), in parallel with the parent compound prednisolone and a control molecule lacking an NO group, (NCX-1016), in a model of rat collagen-induced arthritis. Dosing of rats with NCX-1015 (0.4–4 μmol/kg, i.p.) greatly reduced all parameters of inflammation. A significant but inferior anti-inflammatory effect also was obtained with prednisolone. Collagen-induced arthritic rats had elevated pyridinoline values (>60% over naïve rats), indicating bone and cartilage erosion; this increase was prevented by NCX-1015 but not by prednisolone or NCX-1016 treatment. In vitro, prednisolone (1 nM), but not NCX-1015, elevated bone resorbing activity of rat primary osteoclasts. In conclusion, NCX-1015 is a steroid derivative with a potential for the treatment of chronic inflammatory pathologies and that has milder side effects anticipated on the bone compartment.
Glucocorticoid drugs (GCs) are potent immunosuppressive and anti-inflammatory agents that are used therapeutically in several inflammatory pathologies. Long-term therapy with GCs is often necessary to control the symptoms of rheumatoid arthritis and other rheumatic conditions. Recent evidence shows that GCs may have a disease-modifying effect in addition to their well documented anti-inflammatory actions (1). However, therapeutic management of long-term pathologies with steroids is often linked to a series of unwanted side effects involving the hypothalamus-pituitary-adrenal axis, the cardiovascular system, as well as fat and bone metabolism (2). Secondary osteoporosis is one of the major problems associated with long-term GC therapy in rheumatoid arthritis (RA) patients (3). Several mechanisms are implicated in GC-induced bone loss, including direct effects on bone-tissue cells and modulation of cytokine and growth-factor production (4, 5).
The molecular mechanisms responsible for the anti-inflammatory effects of GCs are complex and may vary with the target cell. GCs transactivate genes including those that control the synthesis of anti-inflammatory mediators (e.g., annexin 1) and β2 adrenoreceptor (6). Steroids interfere with the function and expression of several transcription factors, affecting in this manner the induction of numerous inflammatory genes (for review, see refs. 7 and 8).
Because GCs are the best anti-inflammatory agents available to date, therapeutic use would benefit greatly from a reduced burden of side effects, particularly those affecting the bone compartment. We have described recently a derivative of prednisolone, nitro-prednisolone or NCX-1015 (9), which releases nitric oxide (NO) and nitrate species in biological fluids. NCX-1015 was more potent than the parent compound prednisolone in in vitro assays of GC-receptor activation. This treatment resulted in a 2- to 10-fold higher activity in a model of acute inflammation, with a greatly reduced neutrophil extravasation, cytokine and chemokine formation, and expression of inducible pro-inflammatory enzymes (9).
In the present study, we have assessed the effect of NCX-1015 in a model of arthritis [collagen II-induced arthritis (CIA) in the rat] and evaluated its efficacy in comparison to prednisolone. Besides monitoring the classical signs of inflammation, some parameters of bone metabolism were measured and complemented by direct in vitro analysis of this compound on osteoclast activity.
Materials and Methods
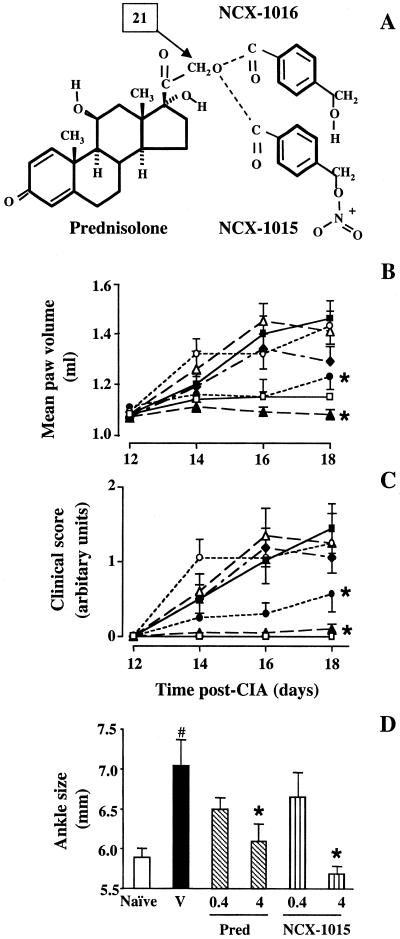
NCX-1015 {prednisolone 21-[(4′-nitrooxymethyl)benzoate], or nitro-prednisolone, molecular weight (Mw) 539.6} was synthesized as described (9). The negative control compound, deprived of the nitrooxy group, NCX-1016 (Mw 494.6) was synthesized at NicOx laboratories in Milan, Italy, as follows: prednisolone (33.3 mmol in tetrahydrofurane) was added to 49.9 mmol of 4-(hydroxymethyl)benzoyl chloride and triethylamine and stirred for 24 h at room temperature. The residue was purified by silica gel chromatography and crystallized. The final product, (prednisolone 21-[(4′-hydroxymethyl)benzoate]) was a white powder with an Mw of 494.6 and a melting point of 227–233°C. The structures of NCX-1015 and NCX-1016 were confirmed by NMR (with 1H and 13C) and infrared analyses (Fig. 1A).
Figure 1.
Modulation of CIA in the rat by prednisolone and the GC derivatives. (A) Chemical structure of prednisolone and the chains added to position 21 to yield either NCX-1015 or NCX-1016. (B–D) Rats were treated daily i.p. with NCX-1015 (▴, 4 μmol/kg; ▵, 0.4 μmol/kg), prednisolone (●, 4 μmol/kg; ○, 0.4 μmol/kg), NCX-1016 (♦, 4 μmol/kg) or vehicle (■, 100 μl peanut oil) from day 12 to day 18 after collagen II injection. A group of naïve rats (□) also was used. During the development of the arthritis, plethysmometry was used to assess paw volume (B), and rats' paws and ankles were individually assessed and scored (C), as described in Materials and Methods. After killing the animals (day 18), anklebone thickness was measured with calipers (D). Data are expressed as means ± SEM (n = 10 per group). *, P ≤ 0.05 vs. vehicle-treated group and #, P ≤ 0.05 vs. naïve animals.
Collagen II-Induced Arthritis.
Female Lewis rats (150 ± 20 g body weight; Harlan Olac, Bicester, U.K.) were fed on a standard chow pellet diet, had free access to water, and were maintained on a 12-h light/12-h dark cycle. Animal work was carried out under license from the Home Office in accordance with the Animals (Scientific Procedures) Act, 1986. Bovine nasal collagen II (4 mg/ml; Sigma) was dissolved in acetic acid (0.01 M) and then emulsified with the same volume of ice-cold Freund's incomplete adjuvant (Sigma). On day 0, rats were anesthetized with halothane, and the base of the tail was shaved and injected intradermally with collagen II/adjuvant suspension (400 μg of collagen II per rat). The first signs of arthritis were evident between days 11 and 13, with maximal inflammation observed at day 18 (longer time points were not allowed by the Home Office regulations).
During the course of the study, the total incidence of disease was 95%, 97.5%, and 100% for three separate series of experiments (n = 10 rats per group). Drugs were administered i.p. or orally daily at doses of 0.4–4 μmol/kg, therapeutically from day 12 to day 18. Two control groups were included: rats that received collagen II and GC vehicle (vehicle group: 0.5 ml/kg, i.p., or 2.5 ml/kg, orally) and rats without CIA-induced inflammation (naïve group). For i.p. administration, the vehicle was peanut oil (Sigma), and drugs were suspended in polyethylene glycol for oral gavage.
CIA-induced inflammation was confined to ankle joints and footpads of the hind legs (with digit involvement in severe cases). Hind ankles were scored clinically on an arbitrary scale, ranging from 0 (no inflammation) to three (severe inflammation, involving ankles, footpads and digits). In addition, between days 0 and 18, hind-paw volumes were measured with a plethysmometer (Ugo Basile, Varese, Italy) and values were averaged to give a measurement of inflammation for each animal. On day 18, animals were anesthetized with halothane, blood was removed by cardiac puncture, and sera were prepared. Animals were then killed by cervical dislocation. As an additional measurement of inflammation of the bone, overlying skin was removed from ankle joints of hind paws, and their diameter was measured with calipers. Spleens and thymuses were collected, and wet weight was recorded (μg of tissue per g of body weight). The feet and ankles were removed and placed in buffered formal saline (10% formalin in 0.01 M PBS, pH 7.4).
Histological analysis was commissioned to an external contractor (Centre Internationale de Toxicologie, Evereux Cedex, France). Examination of the rat paw articulation was performed after decalcification of paws, as described (10). A pathologist carried out blind examination of sections (n = 9 per treatment group). Individual groups were given a mean grading related to the severity of changes seen at the level of articulation and other periarticular tissue. Scoring ranged from not affected (0) to severe (3), depending on the degree of inflammation. The mean gradings for each group was calculated.
Rat Serum Biochemistry.
Clinical chemistry.
Sera were analyzed for several biochemical parameters by an external contractor, by using conventional autoanalyzer technology-performed analyses (VetLab Services, Southwater, Sussex).
Measurement of cytokine levels.
Serum levels of IL-1β and IL-6 were quantified with commercially available ELISAs (R & D Systems), according to manufacturer's protocol.
Measurement of collagen II antibodies.
Nunc Maxisorb plates were coated with 100 μl of bovine nasal collagen II (5 μg/ml in 0.01 M PBS) overnight at 4°C. Serum samples were diluted 1:500, and 100 μl was added to the coated 96-well plate and incubated overnight at 4°C, followed by a 4-h incubation with an alkaline phosphatase-linked secondary antibody (monoclonal anti-rat κ and λ, Sigma). At every step, plates were washed three times with 0.01 M PBS containing 0.05% Tween 20 and then blocked (0.01 M PBS/0.05% BSA; this solution was used for all further dilutions) for 1 h at room temperature. Plates were developed by using a p-nitrophenyl phosphate substrate (Sigma) for alkaline phosphatase. Absorbance (mU) was read at 405 nm and values were expressed as means ± SEM.
Serum pyridinoline.
Serum pyridinoline was assayed and quantified by a commercially available competitive EIA kit (Metra Biosystems, Mountain View, CA), according to manufacturer's protocol.
Osteoclast Activity in Vitro.
Rat primary osteoclasts were prepared as described (11). Osteoclast resorbing activity was measured by using 24-well plates coated with calcium phosphate apatite (OAAS, OCT, Korea). The cell suspension was added to plates and allowed to adhere for 30 min (37°C); nonadherent cells were removed. Cells were incubated for 18 h with or without steroids and other compounds such as ODQ, 1H-(1, 2, 4)-oxadiazolo-[4,3-a]quinoxalin-1-one, or NOC-18, (z)-1-[(aminoethyl)-N-(2-amynoethyl)amino]diazen-1-ium-1, 2-diolate. Plates then were bleached with 10% (vol/vol) sodium hypochlorite solution to remove cells. Samples were analyzed with an inverted microscope (Diaphot TMD, Nikon) interfaced with an Argus-10 image-processing system (Hamamatsu Photonics, Enfield, U.K.). The resorbed area was calculated as the sum of the areas of individual excavations and expressed as a percentage of control values. Selected experiments were repeated with bovine cortical bone slices; analysis was carried out by scanning electron microscopy (Stereoscan 180, Cambridge Scientific Instruments, Cambridge, MA).
Statistics.
All values are expressed as means ± SEM of n rats per group. Comparisons of more than two experimental groups were calculated on original values by using one-way ANOVA followed by Bonferroni post hoc test. For histological analysis, the χ2 assay was used. In all cases, a P < 0.05 was considered significant.
Results
Antiarthritic Effects of NCX-1015 in CIA.
The first signs of CIA developed between 11 and 13 days, as seen by an increase in paw volume (Fig. 1B) and clinical score (Fig. 1C). This profile was mirrored by a significant increase in the ankle size (day 18) in arthritic rats compared with the vehicle group (Fig. 1D). Prednisolone treatment (i.p.) from day 12 significantly reduced the signs of CIA at the highest dose (4 μmol/kg), whereas a lower dose of prednisolone (0.4 μmol/kg, i.p.) was ineffective (Fig. 1 B–D). Similarly to the parent compound, NCX-1015 was active only at the highest dose tested (4 μmol/kg, i.p.). However, in this group of animals, the inflammatory response was almost ablated, with paw volumes (Fig. 1B), clinical scores (Fig. 1C), and ankle sizes (Fig. 1D) being similar to naïve rats. Interestingly, NCX-1016 was modestly active when administered at the equimolar dose of 4 μmol/kg, i.p. (Fig. 1 B and C).
Therapeutic administration of all drugs had no further effect on the reduction in body weight produced by CIA (−15%, n = 10). Thymus wet weight (expressed as percentage of body weight) was 0.296 ± 0.011 in CIA rats, and it was reduced by 37% and 43% after treatment with prednisolone at 0.4 or 4 μmol/kg, i.p., respectively (n = 10, P < 0.05). In the NCX-1015 groups, thymus weight was reduced only at the highest dose of 4 μmol/kg, i.p. (−53%, n = 10, P < 0.05), whereas it was not modified at 0.4 μmol/kg, i.p. (0.294 ± 0.07% body weight, n = 10). A similar pattern was observed for spleen wet weight (data not shown).
Histological Analysis.
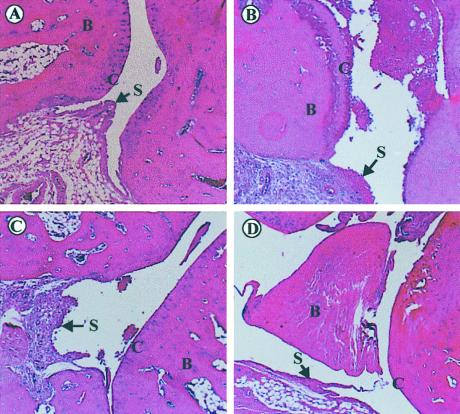
Microscopical analysis of phalangial joint articulations showed that the right and left paws of naïve animals were clear of any signs of inflammation, with a mean arthritic grading of 0.22 (n = 9; Fig. 2A). In contrast, the joint of vehicle-treated CIA rats had moderate to severe synovitis, considerable inflammatory cell infiltration into mineralized and nonmineralized tissue (Fig. 2B). Bone resorption in these animals was moderate, with minimal periosteal new bone formation, occasional hemorrhaging, and ulceration of cartilage (data not shown). This group of rats had a mean arthritic grading of 2.55 (n = 9). Prednisolone (4 μmol/kg, i.p.)-treated rats had moderate synovitis, minimal to moderate inflammatory-cell infiltration into tissues, and no signs of cartilage ulceration (Fig. 2C). Bone resorption was moderate, with a lower degree of periosteal new bone formation (data not shown). These animals had a mean arthritic grading of 2.0 (n = 9, not significant vs. vehicle). In rats treated with NCX-1015 (4 μmol/kg, i.p.), synovitis and inflammatory cell influx was minimal (Fig. 2D). Both bone resorption and periosteal bone formation was minimal, with no signs of cartilage ulceration or hemorrhaging of surrounding tissues and a mean arthritic grading of 1.11 (n = 9; P < 0.05).
Figure 2.
Histological analysis of joint morphology. Light micrographs of rat phalangial articulations in naïve (A) and CIA rats (B–D) after hematoxylin and eosin staining. (A) Naïve rat with normal articulation containing intact cartilage, bone, and synovium. (B) Representative CIA rat with ulceration of the cartilage and pronounced synovitis but intact bone compartment. (C) Effect of prednisolone (4 μmol/kg; 12–18 days) with reduced synovitis and cartilage ulceration, as seen at day 18 postCIA. (D) Articulation of a CIA rat treated with NCX-1015 (4 μmol/kg; 12–18 days) shows almost no sign of synovitis or adherent cell to the cartilage and appears essentially normal. B, bone; S, synovium; C, cartilage. Pictures are representative of nine distinct rats per group.
Drug Effect on Systemic Biochemical Indices During CIA.
CIA and all therapeutic treatments did not affect the serum levels of sodium, potassium, calcium, urea, or glutamic oxaloacetate transaminase. However, prednisolone 4 μmol/kg, i.p., produced a modest but significant reduction in total protein levels, whereas a significant reduction (≈30%) in glutamic pyruvate transaminase (GPT) was measured in all treatment groups (Table 1).
Table 1.
Effect of steroid treatment on rat serum indices at day 18 of CIA
| Serum marker | Naïve | Vehicle | Prednisolone
|
NCX-1015
|
||
|---|---|---|---|---|---|---|
| 0.4 | 4 | 0.4 | 4 | |||
| Total protein, g/liter | 72 ± 2 | 69 ± 2 | 65 ± 1 | 61 ± 1* | 67 ± 1 | 70 ± 1 |
| Sodium, mM | 140 ± 4 | 141 ± 1 | 145 ± 1 | 146 ± 2 | 143 ± 1 | 148 ± 1 |
| Potassium, mM | 5.6 ± 0.3 | 6.2 ± 0.4 | 6.3 ± 0.2 | 6.5 ± 0.7 | 6.6 ± 0.4 | 7.1 ± 0.5 |
| Calcium, mM | 3.4 ± 0.2 | 3.3 ± 0.1 | 3.0 ± 0.3 | 2.9 ± 0.2 | 2.8 ± 0.1 | 2.9 ± 0.1 |
| Urea, mM | 6.9 ± 0.5 | 6.1 ± 0.3 | 5.3 ± 0.2 | 5.3 ± 0.2 | 5.2 ± 0.2 | 5.4 ± 0.2 |
| AST (GOT), IU/liter | 134 ± 15 | 140 ± 8 | 125 ± 5 | 153 ± 30 | 139 ± 10 | 121 ± 10 |
| ALT (GPT), IU/liter | 109 ± 6 | 73 ± 5* | 63 ± 5* | 69 ± 5* | 70 ± 5* | 84 ± 6* |
Peanut oil (vehicle), prednisolone, or NCX–1015 were administered i.p. from day 12 to day 18 at the reported doses (in μmol/kg) to CIA rats. A group of naïve rats also was used. Blood was collected on day 18 under halothane anesthesia and was serum analyzed for the above parameters. Data are expressed as means ± SEM (n = 10 per group).
, P ≤ 0.05 vs. naïve animals. No statistical difference was obtained in CIA rats among vehicle- and drug-treated groups, with the exception of total protein levels in prednisolone top-dose group.
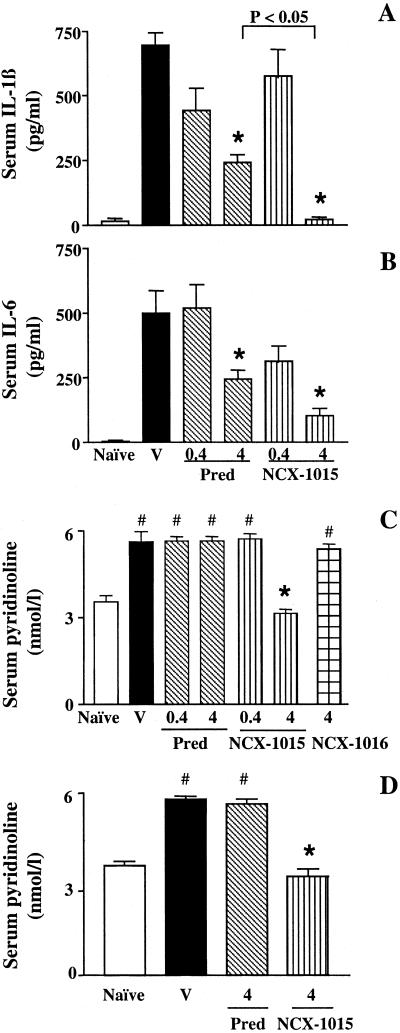
Serum levels of the pro-inflammatory cytokines IL-1β and IL-6 were greatly augmented after CIA (Fig. 3 A and B). Prednisolone (4 μmol/kg, i.p.) significantly reduced both cytokine levels. Interestingly, NCX-1015 had a more pronounced effect upon reducing circulating IL1β levels to those measured in naïve rats (Fig. 3 A and B). An immunosuppressive effect of NCX-1015 (4 μmol/kg, i.p.), but not of prednisolone, was observed in CIA-treated rat [total anti-collagen II antibodies: 1,240 ± 210 milliunits (mU) for vehicle group, and 920 ± 80 and 690 ± 40 mU for prednisolone and NCX-1015 4 μmol/kg, i.p., respectively; n = 10 rats per group; P < 0.05 vehicle vs. NCX-1015].
Figure 3.
Effect of CIA and GC treatment on serum levels of cytokines and pyridinoline. (A–C) Prednisolone (Pred), NCX-1015, NCX-1016, or vehicle (V) treatment was the same as in Fig. 1 (i.p. from day 12 at the indicated doses in μmol/kg), whereas in D, drugs or vehicle (V) were given orally. A group of naïve rats also was used. (A) IL-1β, (B) IL-6 and (C and D) pyridinoline concentration as measured by using specific ELISA. Data are expressed as means ± SEM (n = 10 per group). *, P ≤ 0.05 vs. vehicle-treated group and #, P ≤ 0.05 vs. naïve animals.
A highly reproducible increment in pyridinoline (a metabolite that is indicative of cartilage disruption and bone resorption) was measured after CIA (Fig. 3C) treatment of rats; the high dose of NCX-1015 abrogated the increase in pyridinoline produced by CIA, and the lower dose had no effect. No changes in serum pyridinoline were measured in CIA rats treated with NCX-1016 or prednisolone (4 μmol/kg, i.p., in either case) with values similar to those quantified in the vehicle group (Fig. 3C).
Oral Effect of Prednisolone and NCX-1015 in CIA.
Treatment of rats with prednisolone or NCX-1015 (4 μmol/kg) reduced to a similar degree paw volume (≈30%) and clinical score (≈70%) induced by CIA. Neither drug significantly reduced the level of circulating collagen II antibodies (data not shown). Importantly, oral NCX-1015, but not prednisolone, abrogated the CIA-induced rise in serum pyridinoline (Fig. 3D).
Analysis of NCX-1015 Effect on Rat Bone Cells.
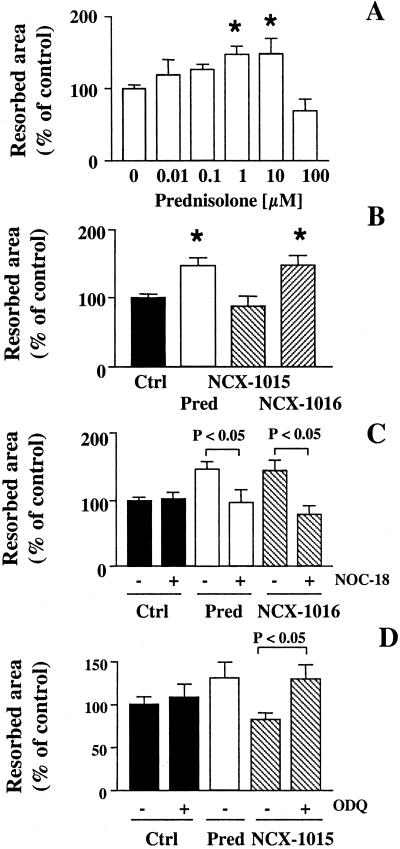
Because NCX-1015 reduced circulating pyridinoline concentrations in vivo, we investigated the effect of this drug on osteoclast activity in vitro. Prednisolone increased osteoclast-resorbing activity at 1 and 10 nM, producing a bell-shaped concentration–response curve (Fig. 4A). This effect was maintained by NCX-1016 (1 nM), whereas NCX-1015 was unable to augment osteoclast resorptive activity (Fig. 4B). This finding also was confirmed on cortical bone slices (data not shown).
Figure 4.
Bone resorption measured in rat primary osteoclasts. A mixed population of rat bone cells was incubated overnight with prednisolone (Pred), NCX-1015, NCX-1016, or vehicle (V). Osteoclast activity was measured as described in Materials and Methods. (A) Osteoclast bone resorptive activity after 18-h incubation with increasing concentrations of prednisolone. *, P < 0.05 vs. vehicle (dose 0). (B) NCX-1016 mimicked prednisolone effect, whereas NCX-1015 was inactive. *, P < 0.05 vs. vehicle-treated cells. (C) Addition of the NO-donor NOC-18 (1 μM) to 1 nM prednisolone or NCX-1016 abolished the increase in bone resorption. (D) In the presence of the guanylate cyclase inhibitor (ODQ, 5 μM), 1 nM NCX-1015 stimulated osteoclast activity. All results are the means ± SEM of at least three to four experiments performed in triplicate.
The nitric oxide donor NOC-18 (1 μM) was unable to alter osteoclast activity by itself, but, in conjunction with prednisolone or NCX-1016, prevented the increasing effect of either drug (Fig. 4C). Conversely, the addition of the soluble guanylate cyclase inhibitor, ODQ, to NCX-1015 restored the increase of osteoclast activity displayed by prednisolone (Fig. 4D).
Discussion
Here, we describe the anti-inflammatory actions of a GC derivative, NCX-1015 or nitro-prednisolone, in an experimental model of rheumatoid arthritis. The more potent anti-inflammatory actions of NCX-1015 also were associated with a restoration of normal serum pyridinoline values. Although requiring confirmation in more focused studies, these results suggest that this GC may have bone sparing properties, at least in inflammatory situations.
Therapeutic administration of NCX-1015 suppressed the CIA-induced inflammatory reaction to a greater degree than prednisolone and NCX-1016 with regards to the three parameters under observation; paw volume, clinical score, and ankle size. At the microscopic level, NCX-1015 afforded protection against the increased pathology in the phalangial joints associated with CIA, as measured by inflammatory cell influx and bone and cartilage damage. This finding confirmed the increase in therapeutic index found in the acute peritonitis model (9). Based on these data and on those published (9), we propose that chemical modifications of prednisolone to produce NCX-1015 did not abrogate the anti-inflammatory properties of this GC, but rather augmented them. Importantly, oral administration of NCX-1015 maintained its anti-inflammatory efficacy in CIA. In preliminary pharmacokinetics analyses, no major differences between prednisolone and NCX-1015 were observed after oral administration of 4 μmol/kg at time 0. The maximal plasma concentration (as free prednisolone) had values of 84.65 and 105.35 ng/ml, and it was reached at 1.7 h and 3 h for prednisolone and NCX-1015, respectively.
NCX-1015 releases NO in biological fluids, as demonstrated in vitro and in vivo (9). Our data show that NO can clearly synergize with the GC moiety to produce a more potent anti-inflammatory effect. This finding is particularly true for the synthesis of inducible genes (6) that are under NF-κB regulation. GCs inhibit NF-κB actions through multiple mechanisms, depending on the cell target and the stimulus applied (7), with a similar effect also ascribed to NO (12, 13). In the present study, prednisolone and NCX-1015 were equipotent in reducing IL-6 levels, whereas the NO-releasing GC was more effective on circulating IL-1β levels. Interestingly, NCX-1015 is more potent than prednisolone in inhibiting IL-1β release stimulated by lipolysaccharide in human mononuclear cells (9). The potent inhibitory effect of NCX-1015 on IL-1β could be caused by a synergism between NO and prednisolone: both NO (14, 15) and prednisolone (16, 17) inhibit caspase-1 (or interleukin-1 converting enzyme) expression.
With the exception of total protein concentration, arthritic rats treated with either GC did not present altered serum biochemical indices among treatment groups. However, CIA-induced liver damage was evident because of the high glutamic pyruvate transaminase value, which remained unmodified in prednisolone- or NCX-1015-treated rats. NCX-1015, but not prednisolone, significantly reduced the levels of circulating total antibodies to collagen II. This result suggests that this GC may have potent immunomodulatory action, likely because of a synergism between NO and the immunosuppressive properties of prednisolone. The immunosuppressive effect of NCX-1015 clearly contributed to the overall anti-inflammatory profile displayed in CIA, because prophylactic administration of NCX-1015 (before the onset of inflammation) significantly reduced the incidence of CIA (unpublished data). This model of arthritis is clearly an adoptive immune-mediated experimental pathology (18); therefore, increased efficacy in the sensitization phase, together with the inhibition of antibody production, suggest that NCX-1015 can alter lymphocyte functions. Potential modulation of the T cell response is in line with recent reports of NO-linked nonsteroidal anti-inflammatory drugs (19, 20).
Patients taking more than 7.5 mg/day of prednisolone (or its equivalent in other GC) have an accelerated bone loss and are at high risk for developing osteoporosis (5, 21). The reduction in bone mass is accompanied by a decrease in skeletal resistance and an increased incidence of fractures. Thus, up to 30–50% of patients on chronic steroid therapy develop fractures of the vertebrae and other trabecular sites (21, 22). In children, a marked retardation of growth also is observed (23). Rheumatoid arthritis is associated with bone loss (osteoporosis; refs. 24 and 25) as it is CIA (26). Periarticular and localized bone loss is related to the release of several mediators including IL-1, IL-6, prostaglandin E2, and osteoprotegerin ligand by cells in the inflamed synovial environment (3). Microscopic analysis of the inflamed joints indicated the presence of bone resorption, which was mirrored by a marked increase in serum pyridinoline, a marker of collagen breakdown (27). Prednisolone or NCX-1016 (4 μmol/kg) were ineffective in preventing the increase of serum pyridinoline induced by CIA, although they produced significant anti-inflammatory effects. In contrast, 5-day administration of NCX-1015 (4 μmol/kg) by all routes and dosing regimes abrogated the rise in serum pyridinoline, in addition to having an enhanced anti-inflammatory profile.
NO is a recognized mediator of bone cell metabolism, where it seems to regulate osteoblast and osteoclast activity (28–30). By using an in vitro assay of bone resorption, prednisolone and NCX-1016 were found to increase osteoclast activity at 1 nM. Prednisolone produced a bell-shaped response, a feature that is relatively common for GC biology and is likely caused by a combination of trans-activating and trans-repressing actions (6). In addition, GCs at high concentrations promote osteoclast apoptosis (31); therefore, the increase in bone resorption observed at low concentrations is lost. In this assay, NCX-1015 was inactive. This important property of NCX-1015 is related to its ability to release NO, because ODQ restored osteoclast activation. Conversely, addition of the slow releasing NO-donor NOC-18 (32) to the cell cultures was ineffective by itself but abrogated the stimulating action of prednisolone and NCX-1016. Therefore, NO could also account for the inhibitory effect of NCX-1015 on osteoclast resorptive activity and explain the reduction in serum pyridinoline observed in the CIA model. It is of interest that a study by Wimalawansa et al. (33) showed that the NO-donor nitroglycerin prevented bone loss induced by methylprednisolone, despite being inactive when administered alone.
In conclusion, the GC derivative NCX-1015 has potent anti-inflammatory activities in CIA, being more potent than prednisolone on some of the inflammatory parameters measured. In addition, our data suggest that NO species released by NCX-1015 may counteract prednisolone potentiation of osteoclast activity. More studies are urgently needed to confirm this important facet of the first nitro-steroid, NCX-1015.
Acknowledgments
We thank Dr. R. Foster (Centre Internationale de Toxicologie, Evereux Cedex, France) for performing the histomorphometric analyses and Dr. A. Moore (Celltech-Chiroscience, Slough, U.K.) for teaching us the collagen II antibody determination. Personal support to M.P. was from the Arthritis Research Campaign, United Kingdom. Support for L.M. was from the Special Trustees of St. Bartholomew's Hospital. R.J.F. is a Principal Research Fellow of the Wellcome Trust, United Kingdom.
Abbreviations
- GC
glucocorticoid
- RA
rheumatoid arthritis
- CIA
collagen-induced arthritis
- NCX-1015
nitro-prednisolone or prednisolone 21-[(4′-nitrooxymethyl)benzoate]
- NCX-1016
prednisolone 21-[(4′-hydroxymethyl)benzoate]
- NOC-18
(z)-1-[(aminoethyl)-N-(2-amynoethyl)amino]diazen-1-ium-1, 2-diolate
- ODQ
1H-[1,2,4]-oxadiazolo-[4,3-a]quinoxalin-1-one
References
- 1.Kirwan J R. N Engl J Med. 1995;333:142–146. doi: 10.1056/NEJM199507203330302. [DOI] [PubMed] [Google Scholar]
- 2.Bond W S. Am J Hosp Pharm. 1977;34:479–485. [PubMed] [Google Scholar]
- 3.Henderson N K, Sambrook P N. Curr Opin Rheumatol. 1996;8:365–369. doi: 10.1097/00002281-199607000-00015. [DOI] [PubMed] [Google Scholar]
- 4.Reid I R. Osteoporos Int. 1997;7:S213–S216. doi: 10.1007/BF03194375. [DOI] [PubMed] [Google Scholar]
- 5.Manolagas S C, Weinstein R S. J Bone Miner Res. 1999;14:1061–1066. doi: 10.1359/jbmr.1999.14.7.1061. [DOI] [PubMed] [Google Scholar]
- 6.Barnes P J. Clin Sci. 1998;94:557–572. doi: 10.1042/cs0940557. [DOI] [PubMed] [Google Scholar]
- 7.Barnes P J, Karin M. N Engl J Med. 1997;336:1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- 8.Buttgereit F, Wehling M, Burmester G-R. Arthritis Rheum. 1998;41:761–767. doi: 10.1002/1529-0131(199805)41:5<761::AID-ART2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 9.Paul-Clark M, Del Soldato P, Fiorucci S, Flower R J, Perretti M. Br J Pharmacol. 2000;131:1345–1354. doi: 10.1038/sj.bjp.0703704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams R O, Feldmann M, Maini R N. Proc Natl Acad Sci USA. 1992;89:9784–9788. doi: 10.1073/pnas.89.20.9784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mancini L, Moradi-Bidhendi N, Brandi M L, MacIntyre I. Biochem Biophys Res Commun. 1998;243:785–790. doi: 10.1006/bbrc.1998.8175. [DOI] [PubMed] [Google Scholar]
- 12.Park S K, Lin H L, Murphy S. Biochem J. 1997;322:609–613. doi: 10.1042/bj3220609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katsuyama K, Shichiri M, Marumo F, Hirata Y. Arterioscler Thromb Vasc Biol. 1998;18:1796–1802. doi: 10.1161/01.atv.18.11.1796. [DOI] [PubMed] [Google Scholar]
- 14.Dimmeler S, Haendeler J, Nehls M, Zeiher A M. J Exp Med. 1997;185:601–607. doi: 10.1084/jem.185.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fiorucci S, Antonelli E, Santucci L, Morelli O, Miglietti M, Federici B, Mannucci R, Del Soldato P, Morelli A. Gastroenterology. 1999;116:1089–1106. doi: 10.1016/s0016-5085(99)70012-0. [DOI] [PubMed] [Google Scholar]
- 16.Lee S W, Tsou A P, Chan H, Thomas J, Petrie K, Eugui E M, Allison A C. Proc Natl Acad Sci USA. 1988;85:1204–1208. doi: 10.1073/pnas.85.4.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lew W, Oppenheim J J, Matsushima K. J Immunol. 1988;140:1895–1902. [PubMed] [Google Scholar]
- 18.Clague R B, Morgan K, Shaw M J, Holt P J. J Rheumatol. 1980;7:775–782. [PubMed] [Google Scholar]
- 19.Cicala C, Ianaro A, Fiorucci S, Calignano A, Bucci M, Gerli R, Santucci L, Wallace J L, Cirino G. Br J Pharmacol. 2000;130:1399–1405. doi: 10.1038/sj.bjp.0703449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fiorucci S, Santucci L, Antonelli E, Distrutti E, Del Sero G, Morelli O, Romani L, Federici B, Del Soldato P, Morelli A. Gastroenterology. 2000;118:404–421. doi: 10.1016/s0016-5085(00)70223-x. [DOI] [PubMed] [Google Scholar]
- 21.Laan R F, van Riel P L, van de Putte L B, van Erning L J, van't Hof M A, Lemmens J A. Ann Intern Med. 1993;119:963–968. doi: 10.7326/0003-4819-119-10-199311150-00001. [DOI] [PubMed] [Google Scholar]
- 22.Lane N E, Lukert B. Endocrinol Metab Clin North Am. 1998;27:465–483. doi: 10.1016/s0889-8529(05)70017-7. [DOI] [PubMed] [Google Scholar]
- 23.Reid I R. Eur J Endocrinol. 1997;137:209–217. doi: 10.1530/eje.0.1370209. [DOI] [PubMed] [Google Scholar]
- 24.Laan R F, Buijs W C, Verbeek A L, Draad M P, Corstens F H, van de Putte L B, van Riel P L. Ann Rheum Dis. 1993;52:21–26. doi: 10.1136/ard.52.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lane N E, Goldring S R. J Rheumatol. 1998;25:1251–1253. [PubMed] [Google Scholar]
- 26.Hoshino K, Hanyu T, Arai K, Takahashi H E. J Bone Miner Metab. 2001;19:76–83. doi: 10.1007/s007740170044. [DOI] [PubMed] [Google Scholar]
- 27.Knott L, Bailey A J. Bone. 1998;22:181–187. doi: 10.1016/s8756-3282(97)00279-2. [DOI] [PubMed] [Google Scholar]
- 28.Ralston S H. Br J Rheumatol. 1997;36:831–838. doi: 10.1093/rheumatology/36.8.831. [DOI] [PubMed] [Google Scholar]
- 29.Riancho J A, Salas E, Zarrabeitia M T, Olmos J M, Amado J A, Fernandez-Luna J L, Gonzalez-Macias J. J Bone Miner Res. 1995;10:439–446. doi: 10.1002/jbmr.5650100315. [DOI] [PubMed] [Google Scholar]
- 30.Mancini L, Moradi-Bidhendi N, Becherini L, Martineti V, MacIntyre I. Biochem Biophys Res Commun. 2000;274:477–481. doi: 10.1006/bbrc.2000.3164. [DOI] [PubMed] [Google Scholar]
- 31.Dempster D W, Moonga B S, Stein L S, Horbert W R, Antakly T. J Endocrinol. 1997;154:397–406. doi: 10.1677/joe.0.1540397. [DOI] [PubMed] [Google Scholar]
- 32.Shibuta S, Mashimoto T, Ohara A, Zhang P, Yoshiya I. Neurosci Lett. 1995;187:103–106. doi: 10.1016/0304-3940(95)11354-1. [DOI] [PubMed] [Google Scholar]
- 33.Wimalawansa S J, Chapa M T, Yallampalli C, Zhang R, Simmons D J. Bone. 1997;21:275–280. doi: 10.1016/s8756-3282(97)00125-7. [DOI] [PubMed] [Google Scholar]