Abstract
This study aimed to develop and validate prognostic nomogram for predicting 3-month, 6-month, and 12-month overall survival in canine multicentric lymphoma. A total of 163 cases from January 2019 to December 2024 were used as a training set, and 50 cases from January 2017 to January 2019 served as a validation set. Predictors were identified using LASSO regression and univariate/multivariate Cox regression analyses, leading to the development of the nomogram: ATRAS2, incorporating age, red blood cell count at diagnosis, changes in red blood cell count after chemotherapy, and albumin-to-globulin ratio. Internal validation showed areas under the receiver operating characteristic curve for ATRAS2 of 0.991, 0.932, and 0.919 for 3-month, 6-month, and 1-year overall survival, respectively. Temporal validation yielded concordance indices of 0.708, 0.701 and 0.783 for 3-month, 6-month, and 1-year overall survival with calibration plots demonstrating strong agreement between predicted and observed overall survival. Decision curve analysis further confirmed the clinical utility of both models for 3-month, 6-month, and 12-month overall survival. The nomogram offers a tool for personalized survival evaluation of canine multicentric lymphoma.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12917-025-04857-y.
Keywords: Canine multicentric lymphoma, Overall survival, Prognostic nomogram, Retrospective study
Introduction
Canine multicentric lymphoma is the most common hematopoietic malignancy in dogs, accounting for approximately 80% of all lymphoma cases [1–3]. Comparative studies have revealed overlapping gene expression and mutational profiles between canine lymphoma and human leukemia, highlighting both shared and distinct features across human and canine disease subsets [4]. Notably, canine lymphoma share many characteristics with human non-Hodgkin lymphomas (NHLs), including similarities in pathogenesis, clinical manifestations, and disease progression [5–8]. In human oncology, various prognostic indices have been developed and validated to facilitate disease risk stratification and guide therapeutic decision-making [9–14]. However, prognostic tools tailored to canine lymphomas remain scarce.
The International Prognostic Index (IPI) remains a cornerstone in assessing the prognosis of human patients with lymphoma [15, 16]. Over time, its utility has been refined through integration with additional clinical and molecular parameters, such as quality of life assessments [17], tumor bulk measurements [18], neutropenia [19], and findings from flow cytometry, immunohistochemistry, and molecular profiling [20]. These advancements underscore the evolving complexity of lymphoma prognosis. Beyond the tumor itself, the tumor microenvironment (TME) has emerged as a critical factor influencing disease progression in human lymphoma [21–23]. The TME encompasses a dynamic interplay of inflammatory states, tumor metabolism [24], immune cell composition [25], microRNAs, and autophagic processes [26]. Inflammatory states within the TME, often driven by antigenic stimulation, autoimmune disorders, or environmental factors, play a pivotal role during lymphomagenesis [27]. Inflammatory biomarkers, including C-reactive protein (CRP), absolute lymphocyte count (ALC), and albumin levels, have been validated as significant prognostic indicators in DLBCL patients [28, 29]. These findings highlight the potential of leveraging systemic inflammatory markers, in combination with tumor and TME characteristics, to refine prognostic models and guide therapeutic strategies. This integrated approach may hold promise for improving prognostic accuracy and tailoring treatments in lymphoma management.
To elucidate the clinical significance of case-specific factors in the progression of canine lymphomas, we systematically analyzed cases from cohorts in Beijing, culminating in the development of prognostic nomogram tailored for estimating the overall survival (OS) of canine multicentric lymphoma. These nomograms were designed to address a critical gap in veterinary clinical oncology, where predictive tools specific to canine lymphomas remain limited. By integrating key clinical and biochemical parameters, the study establishes robust and user-friendly prognostic model to assist pet doctors in evaluating the survival prognosis of canine multicentric lymphoma.
Result
Clinical characteristics
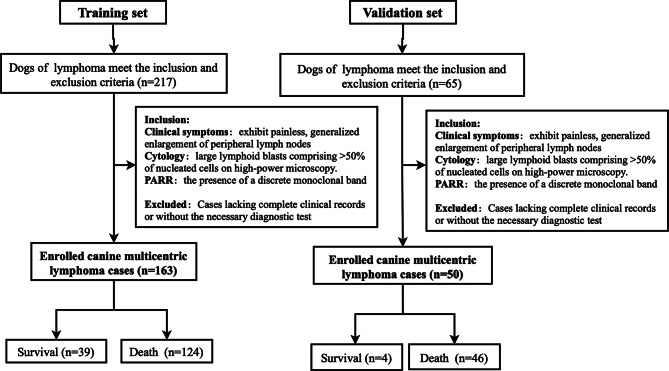
This study included 213 canines diagnosed multicentric lymphoma at the Animal Hospital between 2017 and 2024. Using a time‑based stratification approach, the cohort was partitioned into a training set and a validation set, 163 cases diagnosed from 2019 to 2024 were utilized for developing the predictive model (Training set ), while 50 cases diagnosed between 2017 and 2019 were allocated for external validation (Validation set) (Fig. 1). Baseline characteristics of the cohort are detailed in Table 1. The median age of the entire cohort was 8 years (interquartile range [IQR], 5–11 years), and the median survival time was 142 days (IQR, 78–267 days). Clinical stage distribution included 39 cases (19%) in stage III, 146 cases (70%) in stage IV, 23 cases (11%) in stage V, cases of stage I and II were not collected. Immunophenotyping revealed that 134 cases (79%) were B-cell lymphomas, while 35 cases (21%) were T-cell lymphomas.
Fig. 1.
Flowchart of canine lymphoma training and validation group cases screening
Table 1.
Clinical characteristics of canine multicentric lymphoma patients
| Characteristic | N = 2131 | Training cohort, N = 1631 | Validation cohort., N = 501 | p-value2 |
|---|---|---|---|---|
| Gender | 0.258 | |||
| Female | 92 (43%) | 74 (45%) | 18 (36%) | |
| Male | 121 (57%) | 89 (55%) | 32 (64%) | |
| Neuter | 0.628 | |||
| No | 102 (48%) | 76 (47%) | 26 (52%) | |
| Yes | 110 (52%) | 86 (53%) | 24 (48%) | |
| missing | 1 | 1 | 0 | |
| Age | 0.513 | |||
| Mean (SD) | 8.09 (3.75) | 8.09 (3.77) | 8.46 (3.44) | |
| Median (IQR) | 8.00 (5.00, 11.00) | 8.00 (5.00, 11.00) | 8.00 (6.00, 11.00) | |
| Range | 1.00, 16.00 | 1.00, 16.00 | 1.00, 15.00 | |
| Overall survival time | 0.089 | |||
| Mean (SD) | 206.34(214.21) | 196.21 (205.61) | 260.54 (238.15) | |
| Median (IQR) | 142.2 (78.0, 267.0) | 137.50 (70.00, 237.00) | 193.50 (106.50, 359.00) | |
| Range | 1.00, 1,421.00 | 1.00, 1,421.00 | 6.00, 1,104.00 | |
| missing | 1 | 1 | 0 | |
| Phenotype | 0.829 | |||
| B | 134 (79%) | 99 (79%) | 35 (81%) | |
| T | 35 (21%) | 27 (21%) | 8 (19%) | |
| missing | 44 | 37 | 7 | |
| Treatment | 0.738 | |||
| Combined chemotherapy | 167 (78%) | 127 (78%) | 40 (80%) | |
| Hormone therapy | 19 (8.9%) | 16 (9.8%) | 3 (6.0%) | |
| Single-agent chemotherapy | 27 (13%) | 20 (12%) | 7 (14%) | |
| WBC_AC | 76 (41%) | 0.495 | ||
| Lower | 108 (59%) | 55 (40%) | 21 (46%) | |
| Normal | 29 | 83 (60%) | 25 (54%) | |
| missing | 25 | 4 | ||
| RBC_AC | 0.101 | |||
| Lower | 84 (46%) | 59 (43%) | 25 (54%) | |
| Normal | 98 (54%) | 77 (57%) | 21 (46%) | |
| missing | 31 | 27 | 4 | |
| BW_AC | 0.97 | |||
| High | 26 (14%) | 20 (14%) | 6 (14%) | |
| Lower | 60 (33%) | 45 (33%) | 15 (35%) | |
| Normal | 95 (52%) | 73 (53%) | 22 (51%) | |
| missing | 32 | 25 | 7 | |
| Lung | 0.469 | |||
| No | 63 (33%) | 46 (31%) | 17 (38%) | |
| Yes | 129 (67%) | 101 (69%) | 28 (62%) | |
| missing | 21 | 16 | 5 | |
| Stage | 0.210 | |||
| 1 | 0 | 0 | 0 | |
| 2 | 0 | 0 | 0 | |
| 3 | 39 (19%) | 30 (19%) | 9 (19%) | |
| 4 | 146 (70%) | 109 (68%) | 37 (77%) | |
| 5 | 23 (11%) | 21 (13%) | 2 (4.2%) | |
| missing | 5 | 3 | 2 | |
| Substage | 0.419 | |||
| a | 105 (50%) | 83 (52%) | 22 (44%) | |
| b | 106 (50%) | 78 (48%) | 28 (56%) | |
| missing | 2 | 2 | 0 | |
| RBC | 0.857 | |||
| Lower | 63 (30%) | 48 (30%) | 15 (32%) | |
| Normal | 144 (70%) | 112 (70%) | 32 (68%) | |
| missing | 6 | 3 | 3 | |
| ALB | 0.055 | |||
| High | 2 (1.0%) | 1 (0.7%) | 1 (2.1%) | |
| Lower | 62 (32%) | 53 (36%) | 9 (19%) | |
| Normal | 131 (67%) | 94 (64%) | 37 (79%) | |
| missing | 18 | 15 | 3 | |
| A/G | 0.143 | |||
| High | 11 (5.5%) | 8 (5.3%) | 3 (6.4%) | |
| Lower | 46 (23%) | 40 (26%) | 6 (13%) | |
| Normal | 142 (71%) | 104 (68%) | 38 (81%) | |
| missing | 14 | 11 | 3 | |
| Spleen | 0.399 | |||
| No | 77 (39%) | 61 (41%) | 16 (33%) | |
| Yes | 122 (61%) | 89 (59%) | 33 (67%) | |
| missing | 14 | 13 | 1 |
1 n (%)
2 Fisher’s exact test; Welch Two Sample t-test
Prognosis of canine multicentric lymphoma
LASSO analysis identified age at diagnosis (Age), immune phenotype (Phenotype), treatment plan (Treatment), RBC changes after chemotherapy (RBC_AC), pulmonary metastasis (Lung), clinical stage (Stage), substage (Substage), albumin (ALB) and albumin-to-globulin ratio (A_G) as significant predictors of overall survival (OS) (Fig. 2). Further, Univariate and Multivariate Cox regression analysis highlighted Age, Treatment, RBC_AC, Stage, Substage, and A_G as independent prognostic factors associated with OS (Fig. 3).
Fig. 2.
Feature selection using the least absolute shrinkage and selection operator (LASSO) cox regression model. (A) LASSO coefficient profiles of the 16 features for OS. (B) Tuning parameter (lamda) selection in the LASSO model used 10-fold cross-validation via minimum criteria for OS
Fig. 3.
(A) Univariate Cox regression analysis showing that canine multicentric lymphoma prognosis-related factors were Age, Treatment, RBC_AC, Lung, Stage, Substage, ALB and A_G. (B) Multivariate Cox regression analysis confirmed that Age, Treatment, RBC_AC, Lung, Stage, Substage, ALB and A_G were associated with prognosis of canine multicentric lymphoma
Kaplan-Meier (KM) analysis, combined with log-rank tests, revealed statistically significant associations between several predictors—Age, Treatment, RBC_AC, Stage, Substage, and A_G—and the prognosis of canine multicentric lymphoma (Fig. 4). These findings provide critical insights into the key factors influencing survival outcomes in canine multicentric lymphoma.
Fig. 4.
Kaplan-Meier analysis of overall survival of canine multicentric lymphoma in the training cohort was stratified by (A) substage (Substage), (B) age at diagnosis (Age), (C) RBC changes after chemotherapy (RBC_AC), (D) treatment plan (Treatment), (E) clinical stage (Stage), (F) albumin-to-globulin ratio (A_G). We calculated p values using the unadjusted log-rank test and hazard ratios using a univariate Cox regression analysis. HR = hazard ratio
Nomogram development
Through comprehensive analysis using LASSO and Cox regression models, independent predictors from the training cohort were integrated into nomogram for overall survival prediction. LASSO regression analysis effectively refined the initial 16 variables to 9 key factors: Age, Treatment, Phenotype, RBC_AC, Lung, Stage, Substage, ALB and A_G. After preliminary variable screening using LASSO analysis, we proceeded with Cox regression analysis to conduct further survival prognostic evaluation and selection of 9 key factors.
Further, Cox regression analysis identified 8 variables (Age, Treatment, RBC_AC, Lung, Stage, Substage, ALB and A_G) with p < 0.05 through univariate analysis. Subsequent multivariate analysis further distilled these to 6 independent predictors: Age, Treatment, RBC_AC, Stage, Substage and A_G. These predictors formed the basis of the ATRAS2 nomogram.
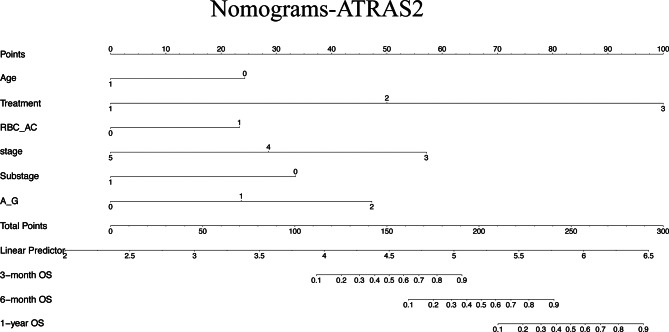
The resulting ATRAS2 (6 key features) nomogram were developed as practical tool for predicting OS in canine multicentric lymphoma (Fig. 5). While LASSO regression analysis and univariate analysis revealed associations between OS and variables such as Phenotype, ALB and Lung, these factors did not demonstrate significance in multivariate analysis. These findings underscore the robustness of the selected predictors in informing survival outcomes.
Fig. 5.
Nomogram predicting the 3-month, 6-month, and 1-year overall survival in canine multicentric lymphoma. Each risk factor corresponded to a point by drawing a line straight upward to the points axis. The sum of the points located on the total points axis represented the probability of the 3-month, 6-month, and 1-year overall survival by drawing a line straight down to the survival axis. (a) In the ATRAS2 nomogram, the predictors are Age, Treatment, RBC_AC, Stage, Substage and A_G
Internal validation of the nomogram
The prognostic performance of the ATRAS2 nomogram for predicting overall survival in canine lymphomas was assessed using multiple metrics. In the training cohort, the C-index for OS prediction was 0.854 (95% confidence interval [CI]: 0.831–0.873). For 3-month, 6-month, and 1-year OS, the area under the curve (AUC) values were 0.991, 0.932, and 0.919, respectively, indicating superior performance of the ATRAS2 nomogram across all time points (Fig. 6).
Fig. 6.
The ROC curves represented the discrimination of the model measured by the AUC in the training cohort. (A) For 3-month overall survival. (B) For 6-month overall survival. (C) For 12-month overall survival
Calibration curves demonstrated that ATRAS2 nomogram were well-calibrated for predicting 3-month, 6-month, and 1-year OS, with predictions closely aligned with observed outcomes. (Fig. 7).
Fig. 7.
Calibration curves of the model in the training cohort of canine multicentric lymphoma. (A) Prediction of 3-month overall survival in training cohort. (B) Prediction of 6-month overall survival in training cohort. (C) Prediction of 12-month overall survival in training cohort
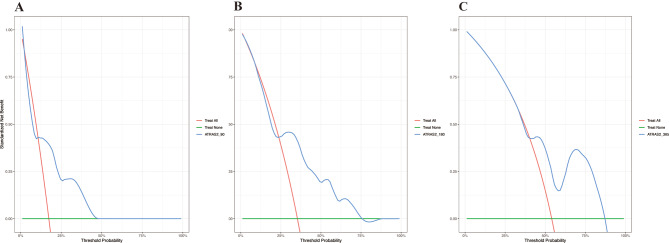
Decision curve analysis (DCA) further validated the clinical utility of the models. The ATRAS2 nomogram exhibited robust net benefits for predicting 3-month and 6-month OS. However, the utility in predicting 1-year OS was relatively lower (Fig. 8).
Fig. 8.
The DCA curves represented the clinical value of the model in the training cohort of canine multicentric lymphoma. (A) 3-month overall survival in training cohort. (B) 6-month overall survival in training cohort. C) 12-month overall survival in training cohort
In summary, the ATRAS2 nomogram demonstrated strong discriminatory power and accuracy for survival prediction in canine multicentric lymphoma in the training cohort. The model provide valuable tool for individualized prognostic assessment in veterinary clinical oncology.
External validation of the nomogram
The C-index of OS in ATRAS2 nomogram were 0.680 (CI: 0.643–0.717) in the validation cohorts. For 3, 6, and 12 months OS, the AUC values were 0.708, 0.701, and 0.783, respectively (Fig. 9). The calibration curves indicated the predicted values of OS in ATRAS2 agreed with the observed ones for 3, 6, and 12 months (Fig. 10). In addition, DCA showed that the ATRAS2 nomogram exhibited robust net benefits for predicting 3, 6, and 12 months OS in the validation cohorts (Fig. 11).
Fig. 9.
The ROC curves represented the discrimination of the model measured by the AUC in the validation cohort. (A) For 3-month overall survival. (B) For 6-month overall survival. (C) For 12-month overall survival
Fig. 10.
Calibration curves of the model in the validation cohort of canine multicentric lymphoma. (A) Prediction of 3-month overall survival in the validation cohort. (B) Prediction of 6-month overall survival in the validation cohort. (C) Prediction of 12-month overall survival in the validation cohort
Fig. 11.
The DCA curves represented the clinical value of the model in the validation cohort of canine multicentric lymphoma. (A) 3-month overall survival in the validation cohort. (B) 6-month overall survival in the validation cohort. (C) 12-month overall survival in the validation cohort
These results show that ATRAS2 nomogram performed well in terms of discrimination and the accuracy of OS prediction in the validation cohorts.
Discussion
In this study, a cohort of canine multicentric lymphoma cases was analyzed, with the training set used to identify key prognostic variables. Through LASSO regression analysis, nine critical predictors (Age, Treatment, Phenotype, RBC_AC, Lung, Stage, Substage, ALB and A_G) were identified, further, Cox regression analysis revealed six independent prognostic factors (Age, Treatment, RBC_AC, Stage, Substage and A_G). These variables formed the basis for developing two prognostic model, ATRAS2. The model exhibited robust predictive accuracy for 3-month, 6-month, and 1-year overall survival in canine multicentric lymphoma, thereby enhancing prognostic precision and facilitating more informed clinical decision-making.
Canine multicentric lymphoma, originating from the lymphatic system, represent one of the most prevalent malignancies encountered in veterinary clinical practice. Given the increasing incidence and biological heterogeneity of canine multicentric lymphoma, there is a critical need to refine individualized survival predictions and tailor therapeutic strategies. This study successfully identified pivotal prognostic factors for canine multicentric lymphoma and developed validated nomogram for risk stratification and survival prediction. Comprehensive assessments using various validation methods confirmed the robust performance of these models, establishing their value as effective tools in veterinary clinical oncology.
Nomogram, which integrate multidimensional risk factors, are widely recognized for their utility in cancer prognosis prediction. By visualizing results from Cox regression analysis, nomogram enhance prediction accuracy and facilitate clinical decision-making due to their interpretability. Despite these advantages, the application of nomogram to predict survival outcomes in canine multicentric lymphoma remains underexplored, largely due to challenges such as limited case numbers, incomplete clinical and prognostic data, and the dispersed nature of veterinary oncology cases.
This study addresses these gaps by analyzing 16 predictive factors derived from general clinical data, laboratory findings, imaging studies, and molecular analyses. For the first time, nomogram-based predictive model (ATRAS2) were developed and validated as effective tools for survival prediction in canine multicentric lymphoma.
Cox regression analysis identified Age, Treatment, RBC_AC, Stage, Substage and A_G as independent prognostic factors, with treatment emerging as the most influential determinant of survival. Interestingly, factors such as body weight and immunophenotype were not significantly associated with survival outcomes in this study, contrasting with previous findings [30]. These discrepancies may stem from the relatively small sample size. Furthermore, this study did not collect any canine lymphoma cases classified as stage I or II, resulting in missing of some data. Nonetheless, data from stages III to V were available and valuable for prognostic analysis. The study findings indicate that stages III to V are associated with the prognosis of canine lymphoma and were subsequently incorporated into the model. According to previous literature [31], stages I and II generally have a better prognosis compared to stages III to V. These underscore the need for further studies with larger and more diverse cohorts to validate and refine these findings.
A study involving 50 canines with multicentric lymphoma undergoing CHOP-based chemotherapy across 15 institutions in the United States demonstrated that canines experiencing chemotherapy-induced neutropenia had significantly longer remission durations and survival times compared to those without neutropenia [32]. In another investigation, substage (a and b) and immunophenotype (B and T) were found to be significantly associated with survival outcomes [33]. Furthermore, studies have suggested that platelet-to-neutrophil ratio (PNR) and lymphocyte-to-monocyte ratio (LMR) in peripheral blood may serve as valuable prognostic markers for CL progression [34, 35]. Additional research identified C-reactive protein (CRP), albumin levels, and LMR as three independent prognostic indicators for overall survival.
This study, while retrospective in design and potentially susceptible to selection bias, incorporated measures to minimize such risks. The multicentric nature of the research, along with the application of objective inclusion and exclusion criteria and minimal data loss, contributed to enhancing the reliability and validity of the findings.
In canine multicentric lymphoma, initial baseline characteristics and dynamic trends in clinical indicators play a crucial role in predicting the clinical course and short-term prognosis. The predictive model, ATRAS2, proposed in this study, demonstrated efficacy in forecasting the prognosis of canine multicentric lymphoma. However, prospective studies are warranted to further validate the predictive performance of these models. Our findings have the potential to assist veterinary clinicians in refining prognostic survival assessments for canine multicentric lymphoma.
Conclusions
This study developed and validated a prognostic nomogram, ATRAS2 to predict 3-month, 6-month, and 1-year overall survival in canine multicentric lymphoma. The model, based on factors such as Age, Treatment, RBC_AC, Stage, Substage and A_G, showed strong internal and external validation, demonstrating good predictive accuracy. The nomogram provides a quantitative tool for estimating individual survival probabilities in dogs with multicentric lymphoma, which assist veterinarians in prognostic assessment.
Materials and methods
Clinical sample collection
A total of 282 cases of canine lymphoma were collected from the teaching animal hospital of China Agricultural University between January 1, 2017, and December 30, 2024 (Fig. S1), of which 213 canine multicentric lymphoma cases met the inclusion criteria for this study. Specifically, 163 cases collected between 2019, and December 30, 2024, were utilized as the training and internal validation sets, while 50 cases collected between January 1, 2017, and 2019, served as the external validation set. All cases were independently reviewed and confirmed by two experienced veterinary clinicians.
Canine multicentric lymphoma diagnoses were established through clinical symptoms (Dogs must exhibit painless, generalized enlargement of peripheral lymph nodes, confirmed on physical examination), cytological evaluation (monoclonal population of large lymphoid blasts comprising > 50% of nucleated cells on high-power microscopy), PCR for Antigen Receptor Rearrangement (PARR, the presence of a discrete monoclonal band required to confirm lymphoma) or immunohistochemistry (IHC). Clinical staging is performed according to the WHO clinical staging system for canine lymphoma [1]. Ultrasound-guided cytological aspiration of the liver and spleen revealing more than 5% lymphoblasts is classified as stage IV, whereas bone marrow cytology demonstrating more than 2% lymphoblasts defines stage V [36, 37]. Comprehensive clinical, laboratory, and imaging examinations were performed for all subjects, with imaging results independently reassessed by two veterinary radiologists. Any discrepancies in interpretation were resolved through consensus.
Data collected included demographic and clinical variables such as sex, neutering status, age at diagnosis, clinical stage, laboratory findings, imaging results, treatment modalities, and immunotyping. The primary outcome measure was overall survival, defined as the interval from diagnosis to death from any cause or the last known follow-up date with survival information. This study design ensured a comprehensive and detailed assessment of the prognostic factors associated with canine multicentric lymphoma.
Predictors
Compile and synthesize all clinical examination findings of canine multicentric lymphoma, a total of 16 variables were selected as candidate predictors for subsequent analyses. These included age (Age), neutering status, gender, clinical stage (Stage), substage (Substage), phenotype (Phenotype), treatment (Treatment), albumin (ALB), red blood cell (RBC) count at diagnosis, albumin-to-globulin ratio at diagnosis (A_G), white blood cell changes following therapy (WBC_AC), RBC changes after therapy (RBC_AC), body weight changes after therapy (BW_AC), presence of pulmonary metastasis (yes/no), presence of spleen honeycomb-like lesions (yes/no), and presence of pancreatitis (yes/no). The calculation methods for WBC_AC, RBC_AC, and BW_AC involve comparing the most recent test results obtained prior to medication with the test results obtained after the first medication. These variables were systematically analyzed to assess their prognostic value in canine multicentric lymphoma.
Transformation of the predictors
The continuous variables were categorized for analysis. Age at diagnosis was divided into two groups: ≤10 years and > 10 years. Tumor stage was classified into five stages (I, II, III, IV, and V) and substage was classified into two categories (a and b) according to the WHO clinical staging system for canine lymphoma [1]. Treatment modalities were categorized into three groups: hormone therapy (Prednisone), single-agent chemotherapy (doxorubicin, Carboplatin or Epirubicin), and combined chemotherapy (CHOP). Laboratory variables, including RBC (Normal range: 5.1–7.6 × 1012/L), WBC_AC (Normal range: 5.6–18.4 × 109/L), RBC_AC (Normal range: 5.1–7.6 × 1012/L), BW_AC (Normal range: ±10%), albumin-to-globulin ratio (A_G; Normal range: 0.9–1.9), and albumin (ALB; Normal range: 3.2–4.1 g/dL), were categorized into three levels: lower, normal, and high.
Predictor selection
All candidate predictors were subjected to LASSO regression and Cox regression analyses using the development cohort to identify potential survival predictors for canine multicentric lymphoma. To prevent overfitting, LASSO regression was applied with cross-validation, which penalizes the absolute values of regression coefficients. As the penalty increased, estimates of weaker factors were reduced toward zero, retaining only the most significant predictors. Through this process, nine variables (Age, Treatment, Phenotype, RBC_AC, Lung, Stage, Substage, ALB and A_G) were identified with non-zero coefficients and incorporated into next analysis.
Univariate Cox regression analysis was conducted on the imputed data, and predictors with a p-value of < 0.05 were considered for further evaluation using multivariate analysis. Multivariate Cox regression analysis, employing backward selection, was performed to assess the independent significance of each factor. Non-significant variables (p > 0.05) were excluded from the analysis. The final covariates included Age, Treatment, RBC_AC, Stage, Substage and A_G.
Statistical analysis
Categorical variables derived from continuous variables are presented as frequencies (percentages). Survival curves were generated using Kaplan-Meier (KM) analysis, with differences between groups assessed by the log-rank test. Independent prognostic factors for overall survival were identified through Cox proportional hazards (PH) regression analysis. Based on the results of the Cox analysis, nomogram for predicting 3-month, 6-month, and 1-year OS were developed. The discrimination between observed and predicted outcomes was evaluated using Harrell’s concordance index (C-index). Calibration plots were created to assess the agreement between predicted and actual survival outcomes. The accuracy of survival predictions was quantified by the area under the receiver operating characteristic (ROC) curve (AUC). The clinical utility of the constructed nomogram was further evaluated using decision curve analysis (DCA). Data analysis was performed using R software (version 3.6.1), with a p-value of less than 0.05 considered statistically significant.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors express their gratitude to the veterinarians and pet parents who generously contributed cases and valuable information to this study. Their invaluable contributions were instrumental in the successful completion of our research.
Author contributions
Z.Z performed all experiments, analyzed the data, and prepared all figures. Z.Z and Z.L wrote the manuscript. D.Z and J.D provided guidance throughout the study, contributed to data interpretation, and reviewed the manuscript critically for important intellectual content. All authors read and approved the final manuscript.
Funding
No financial support was provided for the conduct of this research.
Data availability
The datasets generated and/or analysed during the current study are not publicly available due to privacy and ethical restrictions concerning client-owned animals. The data were obtained from the case records of canine lymphoma patients at the College of Veterinary Medicine, China Agricultural University. Access to these data is available from the corresponding author upon reasonable request and with permission from the relevant institutional ethics committee.
Declarations
Ethics approval and consent to participate
This retrospective study was deemed exempt from ethical approval by the Laboratory Animal Welfare and Animal Experimental Ethics Committee of China Agricultural University (Issue No: AWF01305202-2-01). It relies solely on previous medical records provided by the university’s Teaching Animal Hospital and does not involve any additional experimental interventions. All case data involving client-owned dogs were handled in strict compliance with animal welfare and ethical guidelines.
Consent for publication
The data in this manuscript have not been previously reported by the authors or considered for publication elsewhere. All authors participated, reviewed and approved the final submitted version of the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Valli VE, O, Myint MS, Barthel A, et al. Classification of canine malignant lymphomas according to the world health organization criteria [J]. Vet Pathol. 2011;48:198–211. [DOI] [PubMed] [Google Scholar]
- 2.Zandvliet M. Canine lymphoma: a review [J]. Veterinary Q. 2016;36:104–76. [DOI] [PubMed] [Google Scholar]
- 3.Vezzali E, Parodi AL, Marcato PS, et al. Histopathologic classification of 171 cases of canine and feline non-Hodgkin lymphoma according to the WHO [J]. Vet Comp Oncol. 2010;8(1):38–49. [DOI] [PubMed] [Google Scholar]
- 4.Mcdonald JT, Kritharis A, Beheshti A, et al. Comparative oncology DNA sequencing of canine T cell lymphoma via human hotspot panel [J]. Oncotarget. 2018;9:22693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fournel-Fleury C, Magnol JP, Bricaire P, et al. Cytohistological and immunological classification of canine malignant lymphomas: comparison with human non-Hodgkin’s lymphomas [J]. J Comp Pathol. 1997;117(1):35–59. [DOI] [PubMed] [Google Scholar]
- 6.Teske E. Canine malignant lymphoma: a review and comparison with human non-Hodgkin’s lymphoma [J]. Vet Q. 1994;16(4):209–19. [DOI] [PubMed] [Google Scholar]
- 7.Vail DM, Macewen EG. Spontaneously occurring tumors of companion animals as models for human Cancer [J]. Cancer Invest. 2000;18:781–92. [DOI] [PubMed] [Google Scholar]
- 8.Avery AC. The genetic and molecular basis for canine models of human leukemia and lymphoma [J]. Front Oncol, 2020;10. [DOI] [PMC free article] [PubMed]
- 9.Tetreault L, Kopjar B, Vaccaro AR, et al. A clinical prediction model to determine outcomes in patients with cervical spondylotic myelopathy undergoing surgical treatment: data from the prospective, multi-center aospine North America study [J]. J Bone Joint Surg Am Vol. 2013;95 18:1659–66. [DOI] [PubMed] [Google Scholar]
- 10.Tang X, Li Y, Liang S, et al. Development and validation of a gene expression-based signature to predict distant metastasis in locoregionally advanced nasopharyngeal carcinoma: a retrospective, multicentre, cohort study [J]. Lancet Oncol. 2018;19(3):382–93. [DOI] [PubMed] [Google Scholar]
- 11.Zhong H, Chen J, Cheng S, et al. Prognostic nomogram incorporating inflammatory cytokines for overall survival in patients with aggressive non-Hodgkin’s lymphoma [J]. EBioMedicine. 2019;41:167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun S, Wang W, He C. Development and validation of prognostic nomograms for patients with duodenal neuroendocrine neoplasms [J]. Med Sci Monitor: Int Med J Experimental Clin Res. 2020;26(1):e922613. - e922613-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ni X, Ma X, Qiu J, et al. Development and validation of a novel nomogram to predict cancer-specific survival in patients with uterine cervical adenocarcinoma [J]. Annals Translational Med. 2021;9(4):293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu ZZ, Zhang Y, Cao Y et al. A dynamic prediction model for prognosis of acute-on-chronic liver failure based on the trend of clinical indicators [J]. Sci Rep, 2021;11. [DOI] [PMC free article] [PubMed]
- 15.Shipp MA, Harrington DP, Anderson JR, et al. A predictive model for aggressive non-Hodgkin’s lymphoma [J]. N Engl J Med. 1993;329(14):987–94. [DOI] [PubMed] [Google Scholar]
- 16.Youjian H. Suitability study of the international prognostic index(IPI) in aggressive nonHodgkin’s lymphoma of Chinese [C], 1999.
- 17.Novik AA, Ionova TI, Kishtovich AV et al. Quality of Life (QoL) Impairment and International Prognostic Index (IPI) in New Aggressive Non-Hodgkin’s Lymphoma (ANHL) Patients [C], 2005.
- 18.Panwalkar AW, Loberiza FR, Vose JM et al. Addition of tumor bulk to the International Prognostic Index (IPI) does not improve prognostication in diffuse large B-cell Lymphoma (DLBCL) [J]. Journal of clinical oncology: official journal of the American Society of Clinical Oncology, 2006;24:18_suppl: 7585.
- 19.Choi YW, Jeong SH, Ahn MS, et al. Patterns of neutropenia and risk factors for febrile neutropenia of diffuse large B-Cell lymphoma patients treated with Rituximab-CHOP [J]. J Korean Med Sci. 2014;29:1493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Talaulikar D, Shadbolt B, Dahlstrom JE, et al. Routine use of ancillary investigations in staging diffuse large B-cell lymphoma improves the international prognostic index (IPI) [J]. J Hematol Oncol. 2009;2:49–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dobaño-López C, Araujo-Ayala F, Serrat N et al. Follicular lymphoma microenvironment: an intricate network ready for therapeutic intervention [J]. Cancers, 2021;13. [DOI] [PMC free article] [PubMed]
- 22.Liu Y, Zhou X, Wang X. Targeting the tumor microenvironment in B-cell lymphoma: challenges and opportunities [J]. Journal of Hematology & Oncology; 2021;14. [DOI] [PMC free article] [PubMed]
- 23.Khurana A, Ansell SM. Role of microenvironment in Non-Hodgkin lymphoma [J]. Cancer J. 2020;26:206–16. [DOI] [PubMed] [Google Scholar]
- 24.Beielstein AC, Pallasch CP. Tumor metabolism as a regulator of Tumor–Host interactions in the B-Cell lymphoma Microenvironment—Fueling progression and novel brakes for therapy [J]. Int J Mol Sci, 2019;20. [DOI] [PMC free article] [PubMed]
- 25.Autio MI, Leivonen S-K, Brück O et al. Immune cell constitution in the tumor microenvironment predicts the outcome in diffuse large B-cell lymphoma [J]. Haematologica, 2020. [DOI] [PMC free article] [PubMed]
- 26.Matarrese P, Mattia G, Pagano MT et al. The Sex-Related interplay between TME and cancer: on the critical role of estrogen, MicroRNAs and autophagy [J]. Cancers, 2021;13. [DOI] [PMC free article] [PubMed]
- 27.Baecklund E, Smedby KE, Sutton L-A, et al. Lymphoma development in patients with autoimmune and inflammatory disorders–what are the driving forces? [J]. Sem Cancer Biol. 2014;24:61–70. [DOI] [PubMed] [Google Scholar]
- 28.Kim CS, Lee HS, Jo J-C, et al. Clinical usefulness of inflammatory factors based modified international prognostic index in diffuse large B cell lymphoma treated with rituximab combined chemotherapy [J]. Blood. 2016;128:4220–4220. [Google Scholar]
- 29.Sun F, Zhu J, Lu S et al. An inflammation-based cumulative prognostic score system in patients with diffuse large B cell lymphoma in rituximab era [J]. BMC Cancer, 2017;18. [DOI] [PMC free article] [PubMed]
- 30.Marconato L, Stefanello D, Valenti P, et al. Predictors of long-term survival in dogs with high-grade multicentric lymphoma [J]. J Am Vet Med Assoc. 2011;238(4):480–5. [DOI] [PubMed] [Google Scholar]
- 31.Valli VE, Kass PH, Myint MS, et al. Canine lymphomas: association of classification type, disease stage, tumor subtype, mitotic rate, and treatment with survival [J]. Vet Pathol. 2013;50(5):738–48. [DOI] [PubMed] [Google Scholar]
- 32.Wang SL, Lee J, Liao AT-C. Chemotherapy-induced neutropenia is associated with prolonged remission duration and survival time in canine lymphoma [J]. Vet J. 2015;205(1):69–73. [DOI] [PubMed] [Google Scholar]
- 33.Rassnick KM, Bailey DB, Kamstock DA, et al. Survival time for dogs with previously untreated, peripheral nodal, intermediate- or large-cell lymphoma treated with prednisone alone: the canine lymphoma steroid only trial [J]. J Am Vet Med Assoc. 2021;259(1):62–71. [DOI] [PubMed] [Google Scholar]
- 34.Henriques J, Felisberto R, Constantino-Casas F, et al. Peripheral blood cell ratios as prognostic factors in canine diffuse large B-cell lymphoma treated with CHOP protocol [J]. Veterinary and comparative oncology. 2020. [DOI] [PubMed]
- 35.Marconato L, Martini V, Stefanello D, et al. Peripheral blood lymphocyte/monocyte ratio as a useful prognostic factor in dogs with diffuse large B-cell lymphoma receiving chemoimmunotherapy [J]. Vet J. 2015;206(2):226–300. [DOI] [PubMed] [Google Scholar]
- 36.Joetzke AE, Eberle N, Nolte I, et al. Flow cytometric evaluation of peripheral blood and bone marrow and fine-needle aspirate samples from multiple sites in dogs with multicentric lymphoma. Am J Veterinary Res. 2012;73(6):884–93. [DOI] [PubMed] [Google Scholar]
- 37.Nerschbach V, Eberle N, Joetzke AE, et al. Splenic and hepatic ultrasound and cytology in canine lymphoma: effects of findings on stage migration and assessment of prognosis. Vet Comp Oncol. 2016;14(1):82–94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analysed during the current study are not publicly available due to privacy and ethical restrictions concerning client-owned animals. The data were obtained from the case records of canine lymphoma patients at the College of Veterinary Medicine, China Agricultural University. Access to these data is available from the corresponding author upon reasonable request and with permission from the relevant institutional ethics committee.