Abstract
Binding of Zn2+ to the endogenous Zn2+ binding site in the human dopamine transporter leads to potent inhibition of [3H]dopamine uptake. Here we show that mutation of an intracellular tyrosine to alanine (Y335A) converts this inhibitory Zn2+ switch into an activating Zn2+ switch, allowing Zn2+-dependent activation of the transporter. The tyrosine is part of a conserved YXXΦ trafficking motif (X is any residue and Φ is a residue with a bulky hydrophobic group), but Y335A did not show alterations in surface targeting or protein kinase C-mediated internalization. Despite wild-type levels of surface expression, Y335A displayed a dramatic decrease in [3H]dopamine uptake velocity (Vmax) to less than 1% of the wild type. In addition, Y335A showed up to 150-fold decreases in the apparent affinity for cocaine, mazindol, and related inhibitors whereas the apparent affinity for several substrates was increased. However, the presence of Zn2+ in micromolar concentrations increased the Vmax up to 24-fold and partially restored the apparent affinities. The capability of Zn2+ to restore transport is consistent with a reversible, constitutive shift in the distribution of conformational states in the transport cycle upon mutation of Tyr-335. We propose that this shift is caused by disruption of intramolecular interactions important for stabilizing the transporter in a conformation in which extracellular substrate can bind and initiate transport, and accordingly that Tyr-335 is critical for regulating isomerization between discrete states in the transport cycle.
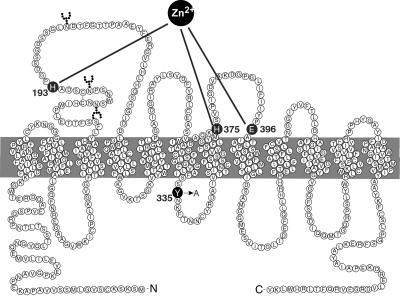
Specific transport proteins tightly control the availability of the monoamine neurotransmitters, dopamine, norepinephrine, and serotonin, in the synaptic cleft by mediating rapid reuptake of the released transmitters to presynaptic nerve terminals (1–3). Three distinct transporters have been identified for monoamines: the dopamine transporter (DAT), the norepinephrine transporter (NET), and the serotonin transporter (SERT) (1–3). Importantly, these transporters are the targets for the action of many psychoactive compounds including both antidepressants and widely abused psychostimulants, such as cocaine and amphetamine (1–3). The DAT, NET, and SERT belong to the family of Na+/Cl−-dependent transporters, which are characterized by a topology with 12 putative transmembrane segments (Fig. 1) (1, 2). The topology has been supported by several studies (4–8), but high-resolution structural information is not yet available. Only recently, the first series of proximity relationships has been described in the tertiary structure of the DAT (6–8).
Figure 1.
Two-dimensional representation of the hDAT. The intracellular Tyr-335 was mutated to an alanine (Y335A). The residues previously shown to be involved in Zn2+ binding to hDAT (His-193, His-375, and Glu-396) are also indicated (6–8).
It is a general assumption that Na+/Cl−-dependent transporters operate by an alternating access mechanism, where the transporter interchanges between an “outward facing” conformation, in which the substrate binding site is accessible to the extracellular medium, and an “inward facing” conformation, in which the binding site is accessible to the intracellular environment (9). The translocation process is energetically coupled to the transmembrane sodium gradient, and it is believed that the initial event in the translocation cycle is binding of sodium ions to the transporter (9). The model predicts that the transporter, in the presence of sodium but in the absence of substrate, primarily resides in the outward facing conformation, ready to bind extracellular substrate. Binding of substrate initiates translocation, causing transition of the transporter to the inward facing conformation followed by release of substrate and sodium to the intracellular environment.
A prerequisite for this model is the existence of both external and internal “gates,” i.e., protein domains that are capable of occluding access to the substrate binding site from the extracellular and intracellular environment, respectively. In serotonin transporter and NET, it has been suggested that the outer part of transmembrane segment 3 could be part of an external gating domain (10), but otherwise little is known about such domains. The molecular mechanisms governing the cooperative function of the putative gating domains also remain unknown. It would be predicted, however, that stabilization of the transporter in the outward facing conformation requires a network of constraining intramolecular interactions, possibly in the gating domains themselves, which is released upon substrate binding to the transporter and thus controls the conformational equilibrium of the translocation cycle. It follows that mutation of residues that are part of this stabilizing network of intramolecular interactions may cause spontaneous changes in the distribution between different conformational states in the translocation cycle. In principle, such mutations would be analogous to constitutively activating mutations in G protein-coupled receptors (11, 12). In these mutants, it is believed that the agonist-independent shift in the equilibrium between inactive and active states of the receptor is caused by the release of constraining intramolecular interactions important for maintaining the receptor preferentially in its inactive state in the absence of agonist (11, 12).
Here we show data indicating that mutation of a conserved tyrosine residue (Tyr-335) in the third intracellular loop of the DAT (Fig. 1) spontaneously alters the distribution between different conformational states in the translocation cycle. Most importantly, this conclusion is based on the observation that mutation of the tyrosine completely reverts the effect of Zn2+ at the previously identified endogenous Zn2+ binding site in the human DAT (hDAT) (refs. 6 and 7; Fig. 1) from potent inhibition of transport to potent stimulation of transport. Furthermore, we observe substantial decreases in the apparent affinities for cocaine and several other inhibitors whereas the apparent affinities for substrates are markedly increased.
Experimental Procedures
Site-Directed Mutagenesis.
The cDNA encoding the hDAT was kindly provided by Marc G. Caron (Duke University, Durham, NC). All mutations including FLAG-hDAT (hDAT tagged at the amino terminus with the FLAG epitope, Sigma) were generated by PCR-derived mutagenesis using Pfu polymerase (Stratagene). The appropriate fragments were cloned into the eukaryotic expression vectors pRC/CMV (13, 14), pcDNA-3 (FLAG-tagged constructs) or pCIN-4 (15) containing enhanced green fluorescent protein (EGFP)-hDAT (kindly provided by Jonathan Javitch, Columbia University, New York). The constructs were verified by restriction enzyme mapping and DNA sequencing.
Expression in COS-7 Cells.
COS-7 cells were maintained as described and transiently transfected by the calcium phosphate precipitation method (16, 17).
Expression in Human Embryonic Kidney (HEK)-293 Cells.
HEK-293 cells were maintained as described (18). The hDAT and hDAT-Y335A tagged at the amino terminus with EGFP in the polycistronic expression vector pCIN4 were stably transfected into HEK-293 cells with Lipofectamine (Life Technologies, Gaithersburg, MD), and stably transfected pools were selected with geneticin as described (18).
[3H]Dopamine Uptake Experiments.
Uptake assays were performed as described (6–8), modified from Giros et al. (13) by using [2,5,6-3H]dopamine (7–21 Ci/mmol) (Amersham Pharmacia).
Confocal Microscopy.
HEK-293 cells stably expressing EGFP-hDAT or EGFP-hDAT-Y335A were grown in phenol red-free medium for 48 h in 8-well Lab-Tek II glass chamber slides (Nalge) before analysis. A Zeiss LSM 410 confocal laser scanning microscope equipped with a 150-mW Ar-Kr laser was used for excitation of EGFP at 488 nm, and the emitted light passed through a 520-nm long-pass filter.
Surface Biotinylation Experiments.
Surface biotinylation was performed modified from ref. 19 on COS-7 cells transiently expressing FLAG-hDAT or FLAG-hDAT-Y335A. Upon biotinylation with Sulfo-NHS-Biotin (1.5 mg/ml, Pierce) the cells were lysed in lysis buffer (25 mM Tris, pH 7.5, containing 1 mM EDTA, 5 mM N-ethylmaleimide, 200 μM PMSF, and a protease inhibitor mixture tablet; Roche Diagnostics), harvested, and centrifuged at 1,000 × g for 5 min. The cell pellets were subsequently solubilized in lysis buffer supplemented with 150 mM NaCl and 0.1% Triton X. Solubilized protein was incubated with monomeric avidin beads (Pierce) washed four times with solubilization buffer and eluted with 50 μl loading buffer (31.25 mM Tris⋅HCl, pH 6.8/10% glycerol/1% SDS/2.5% β-mercaptoethanol/2.5% bromophenol blue). The eluate was analyzed by 10% SDS/PAGE, blotted with the M2 anti-FLAG antibody (10 μg/ml) (Sigma), and visualized by the ECL system (Amersham Pharmacia).
Calculations.
Uptake data were analyzed by nonlinear regression analysis using PRISM 3.0 (GraphPad, San Diego). KI values were calculated by using the equation KI = IC50/[1 + (L/KM)] (L = concentration of [3H]dopamine) (20). For the Y335A mutants, we performed uptake experiments on mock-transfected cells and subtracted the nonspecific uptake in these cells from the total uptake in Y335A expressing cells to achieve an accurate measure for the low specific transporter-mediated [3H]dopamine uptake. The nonspecific uptake was less than 25% of total uptake in Y335A.
Results
Mutation of an Intracellular Tyrosine-Based Motif in hDAT.
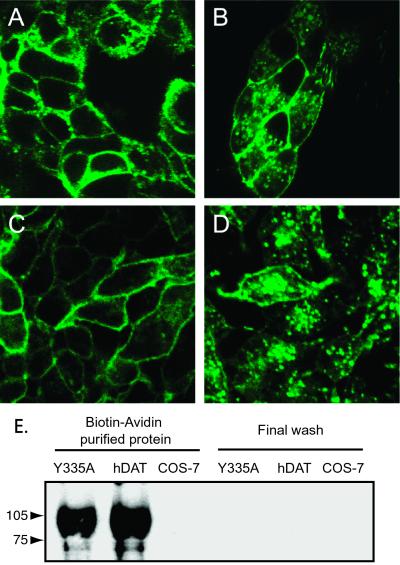
Tyr-335 in the hDAT is part of a tyrosine-based YXXφ trafficking motif (X is any amino acid and φ is a residue with a bulky hydrophobic side chain) (21) (Fig. 1). To investigate the functional importance of Tyr-335 we mutated the residue to an alanine (Y335A). In transiently transfected COS-7 cells, the mutant displayed a dramatic decrease in Vmax for [3H]dopamine uptake as compared with the wild type (WT) (Table 1). To assess whether the decreased uptake capacity was caused by decreased surface expression, the cDNA encoding EGFP was fused to the amino termini of the WT hDAT and Y335A. The EGFP-hDAT-Y335A displayed the same phenotype as hDAT-Y335A (data not shown); however, this decrease in uptake capacity (>99%) could not be accounted for by a similar decrease in apparent surface expression of EGFP-hDAT-Y335A. Confocal microscopy analysis of HEK-293 cells stably transfected with EGFP-hDAT-Y335A showed clear cell surface expression (Fig. 2). As previously reported for the WT (22), EGFP-hDAT-Y335A was also efficiently internalized in HEK-293 cells upon activation of protein kinase C with phorbol esters (Fig. 2). Surface biotinylation experiments on transiently transfected COS-7 cells confirmed that Y335A is efficiently expressed on the surface (80 ± 2% of WT, Fig. 2E). These data suggest that the dramatic loss of transport capacity in Y335A cannot be explained by impaired surface targeting, altered internalization properties, or enhanced degradation of the transporter.
Table 1.
Vmax and KM values for [3H]dopamine uptake in WT hDAT and Y335A with and without 10 μM Zn2+
| DAT | No Zn2+
|
+10 μM Zn2+
|
||||
|---|---|---|---|---|---|---|
| Vmax ± SE, fmol/min/105 cells | KM (DA), μM [SE interval] | n | Vmax ± SE, fmol/min/105 cells | KM (DA), μM [SE interval] | n | |
| hDATWT | 6,000 ± 900 | 2.6 [2.3–2.9] | 11 | 1,800 ± 400 | 4.5 [3.9–5.2] | 10 |
| Y335A | 16 ± 3 | 0.13 [0.11–0.17] | 11 | 390 ± 60 | 0.46 [0.43–0.50] | 9 |
| H193K + Y335A | 19 ± 3 | 0.19 [0.12–0.29] | 8 | 20 ± 5 | 0.17 [0.11–0.24] | 3 |
| H375A + Y335A | 45 ± 7 | 0.19 [0.13–0.28] | 7 | 33 ± 8 | 0.11 [0.08–0.16] | 3 |
| E396Q + Y335A | 25 ± 5 | 0.29 [0.21–0.40] | 7 | 56 ± 4 | 0.13 [0.10–0.17] | 3 |
The Vmax and KM values for [3H]dopamine (DA) uptake were calculated from nonlinear regression analysis of uptake data. The mean KM value is calculated from means of pKM, and the SE interval from the pKM ± SE.
Figure 2.
Evidence for surface expression of hDAT-Y335A. Confocal microscopy images of HEK-293 cells stably expressing (A and B) EGFP-hDAT or (C and D) EGFP-hDAT-Y335A. Cells were preincubated in the absence (A and C) and the presence (B and D) of 1 μM phorbol 12-myristate 13-acetate for 30 min. (Magnifications: ×125.) (E) Surface biotinylation of COS-7 cells expressing FLAG-hDAT, FLAG-hDAT-Y335A, or control (mock transfected). Surface biotinylated protein was purified by using monomeric avidin beads and analyzed by SDS/PAGE followed by immunoblotting with the M2 anti-FLAG antibody as described in Experimental Procedures. Immunoreactive bands were quantitated with image (Scion, Frederick, MD). The intensity of the Y335A band was 80 ± 2% of WT band (mean ± SE, n = 4).
Conversion of an Inhibitory Zn2+ Switch to an Activating Zn2+ Switch by Mutation of Tyr-335.
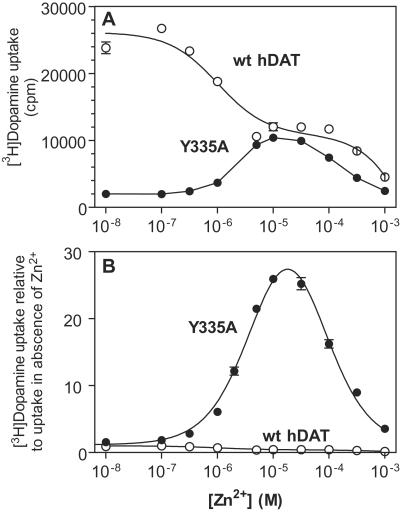
Zn2+ is a potent noncompetitive inhibitor of [3H]dopamine uptake in the hDAT (6). The inhibitory effect on hDAT of Zn2+ in micromolar concentrations is caused by the interaction of Zn2+ with a tridentate Zn2+ binding site in the transporter consisting of residues His-193, His-375, and Glu-396 (Fig. 1). In Y335A, Zn2+ did not inhibit [3H]dopamine uptake but instead caused an unexpected dose-dependent potentiation of [3H]dopamine uptake, reaching a maximum in the presence of ≈10 μM Zn2; at higher concentrations, the potentiation gradually decreased, resulting in a bell-shaped dose–response curve (Fig. 3). Likely, this decrease (or loss of potentiation) at high Zn2+ concentrations corresponds to the low affinity phase of the WT inhibition curve (6). A “raw” data experiment using a subsaturating concentration of [3H]dopamine is shown in Fig. 3A, and a normalized curve showing the relative change in uptake is shown in Fig. 3B. It should be noted that identical effects of Zn2+ were observed in the Y335A mutation in the presence of an inhibitor of catechol O-methyltransferase (U-05212) (23) excluding the possibility that putative intracellular metabolism of [3H]dopamine by catechol O-methyltransferase is affecting our results (data not shown).
Figure 3.
Zn2+ enhances [3H]dopamine uptake in hDAT-Y335A. (A) The effect of Zn2+ on [3H]dopamine uptake in COS-7 cells transiently expressing hDAT-Y335A (●) or WT hDAT (○). Data are means ± SE in cpm of triplicate determinations from a representative experiment. Please note that the experiment was done by using a subsaturating concentration of [3H]dopamine, causing the uptake for Y335A to be relatively higher due to its lower KM as compared with the WT than when actual Vmaxs are calculated (Table 1). (B) Zn2+ effect on [3H]dopamine uptake in COS-7 cells transiently expressing hDAT-Y335A (●) or WT hDAT (○). Values are percent of control [3H]dopamine uptake in the absence of Zn2+ expressed as means ± SE of five (hDAT-Y335A) and three (hDAT) experiments performed in triplicate.
In agreement with previous data, Zn2+ caused a substantial decrease in [3H]dopamine uptake velocity (Vmax) in the WT with only a minor change in the KM value (Table 1) (6). In Y335A, however, Zn2+ caused a 24-fold increase in [3H]dopamine uptake velocity (Vmax) (Table 1 and Fig. 4). The KM value for [3H]dopamine uptake was 0.13 μM in Y335A and thus 20-fold lower in Y335A than in the WT (KM = 2.6). In the presence of Zn2+, the KM value for [3H]dopamine uptake increased 2–3-fold (Table 1).
Figure 4.
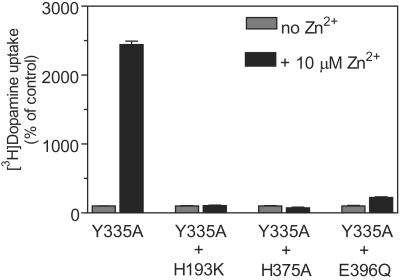
The endogenous Zn2+ binding site is responsible for the activating effect of Zn2+ on [3H]dopamine uptake in hDAT-Y335A. Specific [3H]dopamine uptake in COS-7 cells transiently expressing hDAT-Y335A, hDAT-Y335A-H193K, hDAT-Y335A-H375A, or hDAT-Y335A-E396Q in the absence (gray bar) or presence (black bar) of 10 μM Zn2+. Data are means ± SE of 3–11 experiments performed in triplicate.
The Endogenous Zn2+ Binding Site Is Responsible for the Activating Effect of Zn2+ on Y335A.
To address the question of whether the activating effect of micromolar concentrations of Zn2+ on Y335A was caused by interaction with the same tridentate site as that previously defined in the WT, the three coordinating residues of the endogenous Zn2+ binding site were mutated in the Y335A background, one at a time. In the absence of Zn2+, each of the three mutations (H193K-Y335A, H375A-Y335A, and E396Q-Y335A) displayed a phenotype identical to that of Y335A (Table 1 and Fig. 4). In the presence of Zn2+, however, we either observed no or only very weak potentiation of [3H]dopamine uptake in these three mutants (Table 1 and Fig. 4).
Mutation of Tyr-335 Impairs Inhibitor Binding Affinity but Increases Substrate Affinity.
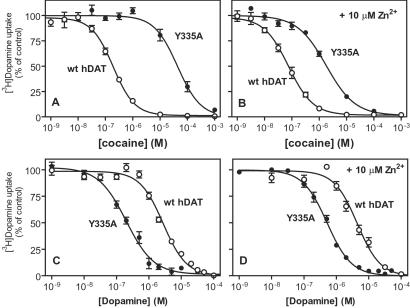
Attempts to measure direct binding of the cocaine analogue [125I]RTI-55 to Y335A were not successful either in the absence or presence of Zn2+ (data not shown). Because the transporter is expressed on the cell surface and capable of transport this finding suggested an impaired capability of the mutant to bind cocaine and related analogues caused by decreased affinity or inaccessibility to the binding site. To investigate this possibility in detail, the ability of cocaine to inhibit [3H]dopamine uptake mediated by Y335A both in the presence and absence of Zn2+ was examined. As shown in Fig. 5, the ability of cocaine to inhibit [3H]dopamine uptake in Y335A was impaired considerably, displaying an almost 150-fold increase in KI value (KI = 26,000 nM) as compared with that of the WT (KI = 180 nM) (Fig. 5 and Table 2). Addition of 10 μM Zn2+ increased the apparent cocaine affinity from a KI value of 26,000 nM to 1,600 nM (Fig. 5 and Table 2). However, this result still represented a significant decrease in apparent cocaine affinity as compared with that of the WT in the presence of Zn2+ (20-fold difference in KI values, Table 2) (Fig. 5).
Figure 5.
Evidence for higher apparent affinity for dopamine and lower binding affinity for cocaine in hDAT-Y335A. (A and B) Cocaine inhibition of [3H]dopamine uptake in COS7 cells expressing hDAT-Y335A (●) or WT hDAT (○) in the absence (A) or the presence (B) of 10 μM Zn2+. (C and D) Inhibition of [3H]dopamine uptake in COS7 cells expressing hDAT-Y335A (●) or WT hDAT (○) by unlabeled dopamine in the absence (C) or the presence (D) of 10 μM Zn2+. Data are means ± SE of 6–11 experiments performed in triplicate.
Table 2.
Apparent affinities for several substrates and blockers at Y335A and hDAT WT
| hDATWT
|
Y335A
|
|||||||
|---|---|---|---|---|---|---|---|---|
| KI [SE interval] | n | KI [SE interval] + 10 μM Zn2+ | n | KI [SE interval] | n | KI [SE interval] + 10 μM Zn2+ | n | |
| Substrates | ||||||||
| Dopamine (KM) | 2.6 [2.3–2.9] | 11 | 4.5 [3.9–5.2] | 10 | 0.13 [0.11–0.17] | 11 | 0.46 [0.43–0.50] | 11 |
| 5-HT | 90 [77–104] | 4 | 82 [75–89] | 3 | 2.0 [1.1–3.5] | 4 | 5.2 [4.5–6.1] | 3 |
| Noradrenaline | 24 [20–29] | 3 | 29 [27–31] | 3 | 1.2 [1.1–1.4] | 3 | 2.8 [2.4–3.1] | 3 |
| Amphetamine | 0.19 [0.15–0.23] | 3 | 0.4 [0.3–0.5] | 3 | 0.05 [0.04–0.06] | 3 | 0.073 [0.062–0.081] | 3 |
| MPP+ | 17 [15–19] | 3 | 16 [13–21] | 3 | 1.9 [1.5–2.2] | 3 | 1.5 [1.4–1.7] | 3 |
| Blockers | ||||||||
| WIN 32,528 | 24 [22–26] | 5 | 9.0 [7.8–10.3] | 5 | 2,600 [2,300–3,100] | 5 | 200 [190–220] | 5 |
| Cocaine | 180 [160–200] | 6 | 77 [57–90] | 6 | 26,000 [21,000–33,000] | 7 | 1,640 [1,490–1,820] | 6 |
| GBR 12909 | 47 [44–51] | 5 | 37 [33–43] | 5 | 660 [540–810] | 4 | 230 [200–260] | 4 |
| Mazindol | 24 [19–30] | 4 | 9.1 [7.9–10.4] | 4 | 3,300 [2,600–4,200] | 4 | 150 [120–180] | 4 |
| RTI-55 | 7.4 [5.6–9.8] | 3 | 6.2 [5.9–6.5] | 3 | 510 [440–580] | 3 | 45 [37–55] | 3 |
The KI values were determined from experiments of [3H]dopamine uptake as described in Experimental Procedures. The mean KI value is calculated from means of pKI, and the SE interval from the pKI ± SE. For substrates values are in μM, and for blockers values are in nM. MPP+, 1-methyl-4-phenylpyridinium.
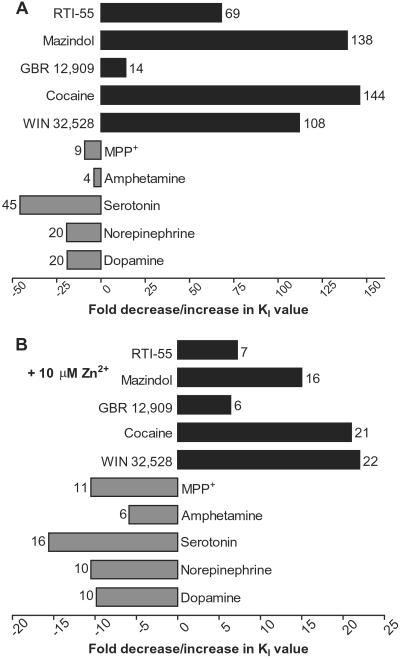
In contrast to cocaine, dopamine displayed an apparent affinity increase both in the absence and presence of Zn2+ in Y335A as compared with the WT (Table 2 and Fig. 5). To investigate this further, a series of different DAT substrates and inhibitors were tested for their ability to inhibit [3H]dopamine uptake in the absence and presence of Zn2+. The cocaine-like substances WIN 32,528 and RTI-55, as well as mazindol, exhibited apparent decreases in affinities of a magnitude similar to that of cocaine (Fig. 6 and Table 2). However, the DAT inhibitor GBR 12,909, which is structurally distinct from cocaine and mazindol, was much less affected by mutation of Tyr-335 (Fig. 6 and Table 2). For the substrates, all tested compounds showed, like dopamine, increases in apparent affinity (Fig. 6 and Table 2). The increases were either not or moderately reversed by Zn2+ (Fig. 6 and Table 2).
Figure 6.
Fold changes in KI values for inhibitors and substrates upon mutation of Tyr-335. (A and B) Bar diagram of the fold increases in KI values of indicated blockers (black bars) and fold decreases in KI of indicated substrates (gray bars) for hDAT-Y335A compared with WT DAT in the absence (A) or presence (B) of 10 μM Zn2+. The fold increases are calculated as KI(hDAT-Y335A)/KI(hDAT) and the fold decreases as −KI(hDAT)/KI(hDAT-Y335A). Data are calculated from the data in Table 2.
Discussion
It is a general prediction that sodium-coupled transporters, such as the DAT, in the presence of sodium but in the absence of substrate, exists primarily in a conformation with the substrate binding site exposed to the extracellular side (outward facing conformation). Substrate binding promotes translocation resulting in a change in the conformational equilibrium with an increased fraction of transporters in an inward facing conformation at any given time. Previously, we have obtained data indicating that binding of Zn2+ to the endogenous Zn2+ binding site in the WT hDAT likely stabilizes the transporter in the outward facing conformation, allowing dopamine to bind but inhibiting its translocation (6, 7). Our recent studies of sodium-dependent pre-steady-state currents in the γ-aminobutyric acid transporter-1 (GAT-1) containing artificial Zn2+ binding sites transferred from the DAT have provided further support for this hypothesis by showing evidence the Zn2+ is stabilizing the sodium-bound outward facing state of the transporter (24).
In this scenario, it can be envisioned that mutation of Tyr-335 spontaneously changes the conformational equilibrium of the transport cycle with accumulation of the transporter in conformational states subsequent to substrate binding and that Zn2+, at least in part, is capable of reversing this change. A changed conformational equilibrium is strongly supported by the substantial changes in apparent affinities for substrate and inhibitors (Table 2 and Fig. 6) as well as it can explain the reduced transport capacity of the Y335A mutant because only a small fraction of transporters would reside in the outward facing resting conformation and thus be available for transport. A possible explanation for the altered conformational equilibrium would be that mutation of Tyr-335 leads to disruption of intramolecular interactions involved in stabilizing the transporter in the outward facing conformation and that this results in an impaired ability of the transporter to return to this conformation. Consequently, the transporter may accumulate in the inward facing conformation and/or putative intermediate states between the inward and outward facing conformation. Thus, if Zn2+ stabilizes the outward facing conformation, it is conceivable that Zn2+ is capable of re-establishing a conformational equilibrium similar to the WT allowing substrate binding and translocation to occur. In this context it is important to note that occupancy of the endogenous high affinity Zn2+ binding site in the WT does not result in full inhibition of uptake but only in maximum around 75% inhibition (Fig. 2). Thus, the Zn2+-occupied transporter is still capable of translocating substrate albeit with reduced efficiency.
An alternative explanation for our data could be that mutation of Tyr-335 is inducing an aberrant, structural state of the transporter and that Zn2+ upon binding to this state is capable of restoring a “normal” functional state. Although we cannot exclude it, we find this possibility unlikely. Both the highly efficient targeting of Y335A to the cell surface and the ability of Zn2+ to rescue transporter function with a potency similar to that observed for inhibition of the WT support that the overall structure is preserved in Y335A. Because of the very strict structural requirements for binding of the small Zn2+ ion (25), even a minor structural change would be expected to be accompanied by reduced Zn2+ affinity. The increases in apparent affinities for substrates in Y335A and the only slight change in apparent GBR-12,909 binding affinity support furthermore that the mutant transporter is in a functionally relevant conformation. We should also note that we find it unlikely that the Y335A phenotype is caused by altered sodium dependence because the mutant displayed a sodium dependency similar to the WT both in absence and presence of Zn2+ (data not shown). Moreover, it is unlikely that the mutant is capable of working in an exchange mode as found recently for a γ-aminobutyric acid transporter-1 (GAT-1) mutant (26) because preloading of Y335A expressing cells with dopamine only caused a negligible increase in [3H]dopamine uptake (data not shown).
Interestingly, our interpretation of the Y335A phenotype is similar in several ways to constitutive receptor activation of G protein-coupled receptors (GPCRs) (11, 12). Substantial experimental evidence suggests that such mutations promote the release of intramolecular constraining interactions in the receptor molecule, leading to spontaneous formation of the active receptor state and, thus, a change in the conformational equilibrium between inactive and active receptor conformations (27, 28). Importantly, constitutively activating mutations in GPCRs have proven to be highly valuable tools for gaining insight into the molecular function of this family of membrane proteins (12).
Given the location of Tyr-335 in the third intracellular loop, which has a highly conserved amino acid sequence, it is interesting to consider the possibility that Tyr-335 is a direct part of an intracellular “gating” domain. Interestingly, previous data also support that the third intracellular loop is conformationally active. Access of the sulfhydryl-reactive compound MTSEA (2-aminoethyl methanethiosulfonate) to the Cys-342 is increased by the presence of substrate and cocaine is capable of protecting access of MTSEA to this cysteine (5, 29). Mutation of Cys-342 to alanine also causes a lowered KM for [3H]dopamine uptake similar to Y335A (5, 29) and similar data have now been obtained for the corresponding mutation in the serotonin transporter (30). Furthermore, mutation of the nearby Arg-345 results in some of the same affinity changes that we find in Y335A (31).
Y335A is, in addition to the altered effect of Zn2+, characterized by a dramatic decrease in the apparent affinity for cocaine-like inhibitors and mazindol (Fig. 6 and Table 2). In contrast, the apparent affinities for several DAT substrates are increased (Table 2 and Fig. 6). Likely, these observations are pertinent to a changed conformational equilibrium; hence, a changed conformational equilibrium may expose conformations with apparent affinities markedly different from those exposed in the WT. Clearly, the prevailing conformation of Y335A is characterized by low affinity for cocaine-like inhibitors and mazindol. In the case of cocaine, the KI value increased almost 150-fold in the absence of Zn2+ (Table 2 and Fig. 6). To the best of our knowledge, this represents the largest effect reported for cocaine upon mutation of a single residue in the hDAT and clearly supports the notion that inhibitors and substrates are preferentially interacting with distinct transporter conformations. Interestingly, the role of Tyr-335 seems specific for cocaine-like compounds and mazindol because binding of the structurally distinct inhibitor GBR 12,909 is affected much less in Y335A (Fig. 6). It is possible that GBR 12,909 is binding to a different site on the transporter. However, it is also possible that the interaction between GBR 12,909 and the transporter is less conformationally dependent; hence, the compound may bind to both inward and outward facing conformations with similar affinities in contrast to cocaine-like compounds, which would be predicted to highly depend on the conformational state of the transporter. GBR 12,909 could then be considered analogous to a “neutral” receptor antagonist, characterized by binding with similar affinity to the “active” and “inactive” receptor conformations (12, 32, 33), whereas cocaine and related inhibitors could be analogous to “inverse agonists,” which preferentially bind to and actively promote formation of the inactive state (12, 32, 33). In support of this view, cocaine is in fact capable of altering the conformation of the DAT (5). It is tempting to speculate that the distinct effect of the Tyr-335 mutation on cocaine and GBR 12,909 may relate to the previously reported divergent reinforcing and neuroadaptive effects of these compounds (34). This observation has the interesting implication that classification of a transporter inhibitor based on its action at the transporter in vitro could allow prediction of its addictive properties in vivo.
Acknowledgments
Dr. Jonathan Javitch, Dr. Erika Adkins, and Søren Rasmussen are thanked for helpful comments. RTI-55 was kindly provided by Dr. F. Ivy Carroll. The study was supported by the Danish Natural Science Research Council, National Institutes of Health Grant P01 DA 12408, the Lundbeck Foundation, and the NOVO Nordisk Foundation.
Abbreviations
- DAT
dopamine transporter
- NET
norepinephrine transporter
- hDAT
human DAT
- WT
wild type
- EGFP
enhanced green fluorescent protein
- HEK
human embryonic kidney
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Amara S G, Kuhar M J. Annu Rev Neurosci. 1993;16:73–93. doi: 10.1146/annurev.ne.16.030193.000445. [DOI] [PubMed] [Google Scholar]
- 2.Giros B, Caron M G. Trends Pharmacol Sci. 1993;14:43–49. doi: 10.1016/0165-6147(93)90029-j. [DOI] [PubMed] [Google Scholar]
- 3.Blakely R D, Bauman A L. Curr Opin Neurobiol. 2000;10:328–336. doi: 10.1016/s0959-4388(00)00088-x. [DOI] [PubMed] [Google Scholar]
- 4.Chen J G, Liu-Chen S, Rudnick G. J Biol Chem. 1998;273:12675–12681. doi: 10.1074/jbc.273.20.12675. [DOI] [PubMed] [Google Scholar]
- 5.Ferrer J, Javitch J A. Proc Natl Acad Sci USA. 1998;95:9238–9243. doi: 10.1073/pnas.95.16.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Norregaard L, Frederiksen D, Nielsen E O, Gether U. EMBO J. 1998;17:4266–4273. doi: 10.1093/emboj/17.15.4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loland C J, Norregaard L, Gether U. J Biol Chem. 1999;274:36928–36934. doi: 10.1074/jbc.274.52.36928. [DOI] [PubMed] [Google Scholar]
- 8.Norregaard L, Visiers I, Loland C J, Ballesteros J, Weinstein H, Gether U. Biochemistry. 2000;39:15836–15846. doi: 10.1021/bi0018335. [DOI] [PubMed] [Google Scholar]
- 9.Rudnick G. In: Neurotransmitter Transporters: Structure, Function, and Regulation. Reith M E A, editor. Totowa, NJ: Humana; 1997. pp. 73–100. [Google Scholar]
- 10.Chen J G, Rudnick G. Proc Natl Acad Sci USA. 2000;97:1044–1049. doi: 10.1073/pnas.97.3.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lefkowitz R J, Cotecchia S, Samama P, Costa T. Trends Pharmacol Sci. 1993;14:303–307. doi: 10.1016/0165-6147(93)90048-O. [DOI] [PubMed] [Google Scholar]
- 12.Gether U. Endocr Rev. 2000;21:90–113. doi: 10.1210/edrv.21.1.0390. [DOI] [PubMed] [Google Scholar]
- 13.Giros B, el Mestikawy S, Godinot N, Zheng K, Han H, Yang-Feng T, Caron M G. Mol Pharmacol. 1992;42:383–390. [PubMed] [Google Scholar]
- 14.Pifl C, Giros B, Caron M G. J Neurosci. 1993;13:4246–4253. doi: 10.1523/JNEUROSCI.13-10-04246.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rees S, Coote J, Stables J, Goodson S, Harris S, Lee M G. BioTechniques. 1996;20:102–110. doi: 10.2144/96201st05. [DOI] [PubMed] [Google Scholar]
- 16.Johansen T E, Scholler M S, Tolstoy S, Schwartz T W. FEBS Lett. 1990;267:289–294. doi: 10.1016/0014-5793(90)80947-h. [DOI] [PubMed] [Google Scholar]
- 17.Gether U, Marray T, Schwartz T W, Johansen T E. FEBS Lett. 1992;296:241–244. doi: 10.1016/0014-5793(92)80295-r. [DOI] [PubMed] [Google Scholar]
- 18.Javitch J A, Fu D, Liapakis G, Chen J. J Biol Chem. 1997;272:18546–18549. doi: 10.1074/jbc.272.30.18546. [DOI] [PubMed] [Google Scholar]
- 19.Qian Y, Galli A, Ramamoorthy S, Risso S, DeFelice L J, Blakely R D. J Neurosci. 1997;17:45–57. doi: 10.1523/JNEUROSCI.17-01-00045.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng Y, Prusoff W H. Biochem Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- 21.Marks M S, Ohno H, Kirchhausen T, Bonifacino J S. Trends Cell Biol. 1997;7:124–128. doi: 10.1016/S0962-8924(96)10057-X. [DOI] [PubMed] [Google Scholar]
- 22.Daniels G M, Amara S G. J Biol Chem. 1999;274:35794–35801. doi: 10.1074/jbc.274.50.35794. [DOI] [PubMed] [Google Scholar]
- 23.Percy E, Kaye D M, Lambert G W, Gruskin S, Esler M D, Du X J. Br J Pharmacol. 1999;128:774–780. doi: 10.1038/sj.bjp.0702831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.MacAulay N, Bendahan A, Loland C J, Zeuthen T, Kanner B I, Gether U. J Biol Chem. 2001;276:40476–40485. doi: 10.1074/jbc.M105578200. [DOI] [PubMed] [Google Scholar]
- 25.Alberts I L, Nadassy K, Wodak S J. Protein Sci. 1998;7:1700–1716. doi: 10.1002/pro.5560070805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bennett E R, Su H, Kanner B I. J Biol Chem. 2000;275:34106–34113. doi: 10.1074/jbc.M004229200. [DOI] [PubMed] [Google Scholar]
- 27.Kjelsberg M A, Cotecchia S, Ostrowski J, Caron M G, Lefkowitz R J. J Biol Chem. 1992;267:1430–1433. [PubMed] [Google Scholar]
- 28.Gether U, Ballesteros J A, Seifert R, Sanders-Bush E, Weinstein H, Kobilka B K. J Biol Chem. 1997;272:2587–2590. doi: 10.1074/jbc.272.5.2587. [DOI] [PubMed] [Google Scholar]
- 29.Chen N, Ferrer J V, Javitch J A, Justice J B., Jr J Biol Chem. 2000;275:1608–1614. doi: 10.1074/jbc.275.3.1608. [DOI] [PubMed] [Google Scholar]
- 30.Androutsellis-Theotokis A, Ghassemi F, Rudnick G. J Biol Chem. 2001;276:45933–45938. doi: 10.1074/jbc.M107462200. [DOI] [PubMed] [Google Scholar]
- 31.Chen N, Vaughan R A, Reith M E. J Neurochem. 2001;77:1116–1127. doi: 10.1046/j.1471-4159.2001.00312.x. [DOI] [PubMed] [Google Scholar]
- 32.Chidiac P, Hebert T E, Valiquette M, Dennis M, Bouvier M. Mol Pharmacol. 1994;45:490–499. [PubMed] [Google Scholar]
- 33.Bond R A, Leff P, Johnson T D, Milano C A, Rockman H A, McMinn T R, Apparsundaram S, Hyek M F, Kenakin T P, Allen L F, Lefkowitz R J. Nature (London) 1995;374:272–276. doi: 10.1038/374272a0. [DOI] [PubMed] [Google Scholar]
- 34.Tella S R, Ladenheim B, Andrews A M, Goldberg S R, Cadet J L. J Neurosci. 1996;16:7416–7427. doi: 10.1523/JNEUROSCI.16-23-07416.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]