Abstract
Background
Neoadjuvant chemotherapy-immunotherapy is the new standard of care for high-risk early-stage triple negative breast cancer (TNBC). As anthracyclines, pembrolizumab, and radiotherapy may each contribute to an increased risk of cardiovascular events, real-world assessment of early cardiovascular changes is of clinical interest.
Methods
Retrospective analysis of 85 women with early-stage TNBC treated with chemotherapy-pembrolizumab between 2018 and 2023 and had ≥ 1 transthoracic echocardiogram (TTE) available. Grade ≥ 2 cardiac common terminology criteria for adverse events (CTCAE) cumulative incidence estimates and Fine-Gray regressions (accounting for non-cardiac death as a competing risk) were calculated. Electrocardiogram (ECG) and TTE parameters during/following systemic therapy (vs. baseline) were compared.
Results
The median follow-up from immunotherapy start was 18.7 months [interquartile range (IQR) 13.6–39.1]. The median age was 50 years (IQR 38–61), 19% had hypertension, most (82%) with no detectable coronary artery calcium (CAC = 0), and 0% known cardiovascular disease. 9/85 (11%) experienced a grade ≥2 cardiac event with a median onset of 7.3 months (IQR 4.0–8.0) and a one-year cumulative incidence of 9.6%. Most (7/9) were grade 2 (n = 5 ejection fraction [EF] decline, n = 1 heart failure, n = 1 pericarditis); 2/9 were grade ≥ 3 (myocarditis, urgent percutaneous coronary intervention); all occurred among those receiving carboplatin, paclitaxel, doxorubicin, and cyclophosphamide-based therapy. Adjusting for age and CAC, mean left anterior descending coronary artery radiation dose was associated with an increased risk of cardiac events (sub-distribution hazard ratio 1.16/Gy, 95% confidence interval 1.01–1.35; p = 0.041). QTc prolongation ≥450ms was more common during treatment vs. baseline (39% vs. 15%; p = 0.025). On assessment for recovery, early grade 2 EF decline recovered in 3/5 patients (2/5 with absence of follow-up). In those with baseline and post-treatment TTE, 5/20 (25%) developed new moderate diastolic dysfunction, that persisted in a later TTE in 2/5 patients, downgraded to mild in 1/5, and not reevaluated by TTE in 2/5.
Conclusion
Early cardiovascular toxicity was observed during multi-modality TNBC treatment, even in young patients with low cardiovascular risk profiles, highlighting the importance of diligent surveillance. Longer follow-up and further studies are warranted, given the degree of recovery and later effects of these treatments may not yet be fully observed.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40959-025-00361-2.
Keywords: Cardiac events, Pembrolizumab, Radiation therapy, Triple negative breast cancer
Background
In women, breast cancer is the most common malignancy and the leading cause of cancer death worldwide [1]. Triple negative breast cancer (TNBC) is an aggressive subtype, accounting for 10–15% of all breast cancer cases and is most commonly diagnosed in women less than 50 years of age, carrying a higher rate of distant recurrence and greater risk of mortality [2]. Neoadjuvant chemoimmunotherapy per KEYNOTE-522 with an immune checkpoint inhibitor (ICI; pembrolizumab) together with carboplatin and anthracycline-containing chemotherapy is the new standard of care for high-risk early-stage TNBC, associated with a higher rate of pathologic complete response (pCR) and a significant improvement in event-free survival (EFS) [3, 4].
Both anthracyclines and pembrolizumab can cause adverse cardiovascular (CV) events, and combination therapy may further increase real-world CV risk. Anthracyclines carry an important risk of early, dose-dependent, and irreversible left ventricular dysfunction and heart failure [5]. ICI therapy has been associated with a 1–2% incidence of adverse cardiac events, for which myocarditis carriers a high risk of mortality (30–50%) [6, 7, 8, 9, 10, 11]. More recent studies have associated ICI therapy with accelerated atherosclerosis, atherosclerotic CV events [12], and conduction/interval prolongation abnormalities [13]. Furthermore, radiation therapy (RT) triggers an inflammatory cascade that accelerates the natural history of atherosclerosis [14]. In breast cancer survivors, left anterior descending (LAD) coronary artery radiation exposure is correlated with coronary artery calcium (CAC) score [15] and the need for mid-LAD revascularization [16]. In patients requiring revascularization following RT, nearly 30% present within the first 2-years and the median onset of significant LAD stenosis occurs less than 4 years from RT—demonstrating a small, but high-risk subgroup of patients experiencing significant, early CV events [16, 17]. Importantly, the correlation between LAD RT dose and cardiac events persists in the modern treatment era with advanced technology [18], especially as ongoing trials do not typically include dose limits to the LAD. Together, these observations highlight the need to better understand the cumulative associated cardiac risks during and after intensified therapy for TNBC.
The present study examines the cumulative risk of early grade ≥ 2 cardiac common terminology criteria for adverse events (CTCAE v5.0) in patients with TNBC receiving chemoimmunotherapy with or without RT. Additional goals were to identify factors associated with increased risk of grade ≥ 2 cardiac CTCAE and to characterize dynamic, short-term electrocardiogram (ECG) and transthoracic echocardiogram (TTE) changes during and after therapy.
Methods
Study population
The study cohort consisted of women aged ≥18 with newly diagnosed invasive TNBC treated with ICI therapy (pembrolizumab) between February 2018 and August 2023 at Cedars-Sinai Medical Center. We used Deep 6 AI (Deep 6 AI Inc., Pasadena, CA), a natural language processing and machine learning platform, to query our institution’s electronic medical record for patient records with structured data elements including “triple negative breast cancer” or “estrogen receptor (ER)/progesterone receptor (PR)/ human epidermal growth factor receptor 2 (HER2) negative status”, “pembrolizumab”, and “echocardiogram” (Supplemental Fig. 1). Patients with at least one TTE were included. Patients were excluded if they had metastatic or recurrent disease. The present study was approved by the Cedars-Sinai Medical Center Institutional Review Board with a waiver of informed consent due to minimal risk nature (STUDY00003061).
Baseline comorbidities and cancer information
Data on demographic, comorbidities, body mass index (BMI), smoking status, CV medications, laboratory values, breast cancer laterality, cancer stage, and cancer treatment details were retrospectively reviewed and collected manually from the electronic medical record. CV comorbidities included hypertension, hyperlipidemia, diabetes mellitus, coronary artery disease, ischemic heart failure, coronary heart disease equivalents (abdominal aortic aneurysm or peripheral artery disease), arrhythmia, and valvular disease. Any prior cancer diagnosed before the current TNBC was recorded. Cancer stage was based on the 8th edition of the American Joint Committee on Cancer staging of breast cancer.
Coronary calcification quantification
Coronary artery calcium (CAC) was manually measured on non-ECG gated, non-contrast-enhanced RT planning computed tomography (CT) scans using syngo.via (Siemens Healthineers, Malvern, PA) by a single investigator (KDS) with adjudication by a radiation oncologist experienced in cardiac anatomy (KMA). Helical RT planning CT scans were obtained with or without deep inspiration breath hold (DIBH), with slice thickness 2.5–5.0 mm (General Electric Medical Systems, Milwaukee, WI). If the patient did not undergo RT, the most recent diagnostic non-contrast enhanced chest CT or attenuation CT scan from positron emission tomography (PET)/CT studies with slice distances ≤ 5.0 mm acquired within six months before the initiation of chemoimmunotherapy was used to obtain a baseline CAC score. CAC was quantified using the Agatston method [19], with individual coronary vessel scores summed into a total CAC score and grouped into no atherosclerosis (0), minimal (1–10), mild (11–100), moderate (101–400), and high (> 400) risk.
Radiotherapy and dosimetry data
RT was planned with 3D conformal RT (3D-CRT) or intensity-modulated RT (IMRT) techniques using Varian Eclipse (Varian Medical Systems, Palo Alto, California). Dose fractionations were typically 50 Gy in 25 fractions or 42.6 Gy in 16 fractions, with or without a photon or electron tumor bed boost. The heart and the LAD coronary artery were delineated manually by the same radiation oncologist (JPN) and adjudicated by a radiation oncologist experienced in cardiac anatomy (KMA) according to a published cardiac contouring atlas [20]. The RT dose was converted to the equal dose in 2 Gy fractions (EQD2) and mean LAD and whole heart doses obtained.
Cardiac CTCAE, electrocardiogram, and echocardiogram endpoints
The primary outcome was grade ≥2 cardiac CTCAE (version 5.0) [21]. An in-depth manual medical record review was performed. Any cardiac event was examined, including CV death, myocardial infarction, coronary revascularization, unstable angina, heart failure/cardiomyopathy, ejection fraction (EF) decline, valvular disease, dysrhythmias/conduction abnormalities, and pericardial disease (e.g., pericarditis, pericardial effusion). Twelve-lead ECG and TTE data obtained within 6 months prior to pembrolizumab administration, and at any time after pembrolizumab was started, were extracted from patients’ records. The following automated ECG measurements were extracted from reports: heart rate, P-wave amplitude, QT interval, RR interval, corrected QT interval (QTc), QRS duration, PR interval, and QRS axis. ST-T changes were analyzed according to the AHA/ACCF/HRS (American Heart Association/American College of Cardiology Foundation/Heart Rhythm Society) recommendations [22]. The Cornell index and the Sokolow-Lyon index were evaluated as electrocardiographic LVH criteria [23, 24]. Any QTc prolongation, calculated by Bazett formula [25], was defined as ≥450 ms per CTCAE v5.0. TTEs were executed by dedicated sonographers. Evaluation of global function (left ventricular ejection fraction [LVEF]), structure (maximal wall thickness [MWT]), diastolic function (assessed and graded per the American Society of Echocardiography [ASE]/European Association of Cardiovascular Imaging (EACVI) guidelines) [26], left atrial volume index (LAVi), contractile function (global longitudinal strain [GLS]), pulmonary pressures (pulmonary artery systolic pressure [PASP]) and heart valves was performed. In patients with multiple ECGs and TTEs, the most abnormal (ECG: based on QTc interval and/or new arrythmia; TTE: based on EF and/or the presence of new/worsening diastolic dysfunction) and most recent studies during, and after pembrolizumab therapy were used for analysis. The index date was the start of pembrolizumab. All the patients were followed until loss to follow-up, death, or the end of January 2024, whichever came first.
Statistical analysis
Follow-up was calculated based on the start of ICI therapy using the reverse Kaplan Meier method [27]. Descriptive data are presented as number of patients (%) for categorical variables and mean (± standard deviation [SD]) or median (interquartile range [IQR]) for continuous variables. Cumulative incidence estimates of grade ≥ 2 and grade ≥ 3 cardiac events were estimated using non-CV death as a competing risk. Fine-Gray regression models were performed (accounting for non-cardiac death as a competing risk) [28], and results presented as sub-distribution hazard ratio (sHR) with 95% confidence interval (CI). Multivariable models included covariates with p ≤ 0.05 on univariable analysis and clinically pertinent variables. Comparisons between during and/or post-treatment ECG or TTE parameters versus baseline were calculated by a paired t-test or signed rank test for continuous variables, and McNemar’s test for categorical variables. Two-sided p-value ≤ 0.050 was considered statistically significant. All analyses were performed using StataSE, version 17.0 (StataCorp LLC) statistical software.
Results
Patient characteristics
The study cohort consisted of 85 patients (CONSORT diagram, Supplemental Fig. 1). The median follow-up from the start of ICI therapy was 18.7 months [interquartile range (IQR) 13.6–39.1 months]. Baseline patient characteristics are summarized in Table 1. The median age at TNBC diagnosis was 50 years (IQR 38–61), the median BMI was 24.0 (IQR 21.3–27.4), 31.8% were current or former smokers, 18.8% had hypertension, and 16.5% had hyperlipidemia. None had pre-existing coronary heart disease. The majority (81.9%) had baseline CAC of 0, while only 1.4% had CAC > 400. Most (96.5%) received neoadjuvant chemotherapy-pembrolizumab, with an anthracycline-based regimen in 78.8% of cases, though 3.5% received only adjuvant chemotherapy-pembrolizumab. Mastectomy was performed in 41 patients (48.2%). 67 patients (78.8%) received RT (61.2% left side; 47.5% with regional nodes (including internal mammary chain); 81.5% with boost; 69.5% standard fractionation; 81.4% with deep inspiration breath hold).
Table 1.
Clinical and treatment characteristics
| Characteristics | No. (%) |
|---|---|
| Age, median (IQR, years) | 50 (38–61) |
| BMI (kg/m2), median (IQR) | 24.0 (21.3–27.4) |
| Tobacco | |
| Never | 58 (68.2%) |
| Former | 23 (27.1%) |
| Current | 4 (4.7%) |
| Medical history | |
| Hypertension | 16 (18.8%) |
| Hyperlipidemia | 14 (16.5%) |
| Diabetes | 2 (2.4%) |
| Heart disease | 0 (0%) |
| DVT | 3 (3.5%) |
| Prior cancer | 6 (7.1%) |
| CV medications | |
| ACE inhibitor | 1 (1.2%) |
| Angiotensin receptor blocker | 4 (4.7%) |
| Beta-blocker | 6 (7.1%) |
| Calcium Channel blocker | 6 (7.1%) |
| Statin | 13 (15.3%) |
| Oral antidiabetic agent | 2 (2.4%) |
| Insulin | 1 (1.2%) |
| Anti-platelet | 5 (5.9%) |
| CAC score† | |
| No atherosclerosis: 0 | 59 (81.9%) |
| Minimal: 1–10 | 4 (5.6%) |
| Mild: 11–100 | 6 (8.3%) |
| Moderate: 101–400 | 2 (2.8%) |
| High: >400 | 1 (1.4%) |
| TNBC clinical stage (anatomic) | |
| I | 5 (5.9%) |
| II | 62 (72.9%) |
| III | 16 (18.8%) |
| IV | 2 (2.4%) |
| TNBC post-neoadjuvant therapy pathological stage | |
| No residual disease | 52 (61.2%) |
| I | 16 (18.8%) |
| II | 8 (9.4%) |
| III | 4 (4.7%) |
| Cancer treatments | |
| Chemotherapy | 85 (100%) |
| Doxorubicin | 67 (78.8%) |
| Cyclophosphamide | 69 (81.2%) |
| Carboplatin | 71 (83.5%) |
| Taxane | 84 (98.8%) |
| Capecitabine | 20 (23.5%) |
| Pembrolizumab | 85 (100%) |
| Mastectomy | 41 (48.2%) |
| Radiotherapy | 67 (78.8%) |
| Left side | 41 (61.2%) |
| RNI (including IMN)‡ | 28 (47.5%) |
| Boost‡ | 53 (81.5%) |
| Standard fractionation‡ | 41 (69.5%) |
| DIBH‡ | 48 (81.4%) |
| Technique | |
| 3D-CRT | 60 (92.3%) |
| IMRT | 5 (7.7%) |
| Dose, median (IQR)‡ | |
| Heart mean, Gy | 1.5 (1.0–3.0) |
| LAD mean, Gy | 3.0 (0.6–5.6) |
Values represent n (%) unless otherwise specified. Abbreviations: TNBC, triple negative breast cancer; IQR, interquartile range; BMI, body mass index; SD, standard deviation; CV, cardiovascular; ACE, angiotensin-converting enzyme; CAC, coronary artery calcium; DIBH, deep inspiratory breath-hold; Gy, Gray; LAD, left anterior descending coronary artery; RNI, regional nodal irradiation; IMN, internal mammary node; 3D-CRT, 3-dimensional conformal radiotherapy; IMRT, intensity modulated radiotherapy
†Among n = 72 with baseline CAC available
‡Based on patients with available radiotherapy information
The median mean heart and LAD coronary artery doses were 1.5 Gy (IQR 1.0–3.0) and 3.0 Gy (IQR 0.6–5.6), respectively. The median mean heart dose (MHD) was significantly higher in left- vs. right-sided breast cancer (2.6 Gy vs. 1.3 Gy, respectively; p = 0.008). Similarly, the mean LAD dose was also significantly higher in left- vs. right-sided cancer (median 5.5 Gy vs. 0.9 Gy, respectively; p < 0.001).
Grade ≥ 2 cardiac events
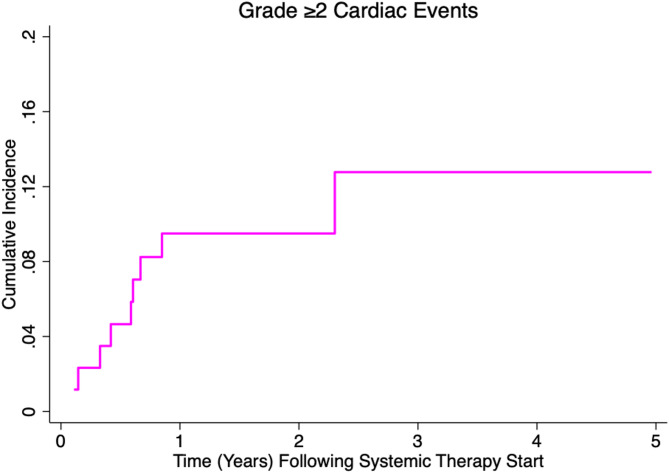
Following start of ICI therapy, 9 of 85 patients (10.6%) experienced a CTCAE grade ≥2 cardiac event with a median time to event of 7.3 months (IQR 4.0–8.0) and a one-year cumulative incidence of 9.6% (95% confidence interval [CI] 4.5–17.1%; Fig. 1). Most (7/9) were grade 2 (n = 5 EF decline, n = 1 heart failure, n = 1 pericarditis); 2/9 were grade ≥ 3 (myocarditis, cardiac chest pain requiring urgent percutaneous coronary intervention) (See Supplemental Table 1 for cardiac event details and patient risk factors). The myocarditis immune-related adverse event (irAE) occurred two months after initiation of ICI therapy and was delayed in diagnosis, in part due to transition of care from general cardiology to cardio-oncology. The myocarditis irAE occurred during the early time frame of this study, when awareness of ICI-related myocarditis amongst general cardiologists was less prevalent. Ultimately, the patient was treated with an angiotensin-converting-enzyme (ACE) inhibitor and beta blocker with resolution of symptoms (no glucocorticoids). The urgent percutaneous coronary intervention occurred in a 68-year-old woman with pre-existing hyperlipidemia one month after initiation of neoadjuvant ICI therapy, who required balloon angioplasty and placement of two stents in the LAD, who was later treated with adjuvant left-sided whole breast radiotherapy. Notably, all cardiac events (9/9) occurred in those receiving carboplatin, paclitaxel, doxorubicin, and cyclophosphamide chemotherapy. Among those receiving radiotherapy and dose volume histogram information available (n = 53), there were five cardiac events (9.4%). Adjusting for age and CAC score, mean LAD dose remained associated with an increased risk of cardiac events [sub-distribution hazard ratio (sHR) 1.16/Gy, 95% CI 1.01–1.35; p = 0.041] (Table 2). Among the 18 patients not treated with radiotherapy, there were four cardiac events (22.2%), a trend toward lower age versus those treated with radiotherapy (43 vs. 52 years, p = 0.084), and only one patient with CAC > 0. No other baseline cardiovascular or treatment factor was significantly associated with cardiac events on univariable analysis (p > 0.05) and was included in multivariable analysis.
Fig. 1.
Cumulative incidence of common terminology criteria for adverse events grade ≥ 2 cardiac events in patients with triple negative breast cancer treated with chemoimmunotherapy
Table 2.
Competing risk regression model for CTCAE grade ≥ 2 cardiac events
| Variable | Univariable | Multivariable | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age | 1.05 (1.01–1.10) | 0.024 | 1.07 (0.99–1.15) | 0.051 |
| BMI | 1.09 (0.98–1.20) | 0.11 | ||
| Tobacco | ||||
| Never | 1.0 (Reference) | |||
| Ever | 0.98 (0.26–3.73) | 0.98 | ||
| Hypertension | 0.54 (0.07–4.01) | 0.55 | ||
| Hyperlipidemia | 1.46 (0.32–6.72) | 0.62 | ||
| CV medications | ||||
| Any antihypertensive | 0.54 (0.07–4.01) | 0.55 | ||
| Statin | 1.65 (0.36–7.54) | 0.52 | ||
| Anti-platelet | 1.67 (0.23–12.16) | 0.61 | ||
| CAC score | ||||
| CAC = 0 | 1.0 (Reference) | 1.0 (Reference) | ||
| CAC > 0 | 2.11 (0.40-11.03) | 0.38 | 1.43 (0.11–18.13) | 0.78 |
| Chemotherapy | ||||
| Doxorubicin | † | - | ||
| Cyclophosphamide | † | - | ||
| Carboplatin | † | - | ||
| Taxane | † | - | ||
| Capecitabine | 0.39 (0.05–3.22) | 0.38 | ||
| Mastectomy | 0.86 (0.24–3.16) | 0.82 | ||
| RT dose | ||||
| Mean heart dose (per Gy) | 1.16 (0.93–1.44) | 0.18 | ||
| Mean LAD dose (per Gy) | 1.16 (1.03–1.31) | 0.015 | 1.16 (1.01–1.35) | 0.041 |
Abbreviations: HR, hazard ratio; CI, confidence interval; BMI, body mass index; CV, cardiovascular; CAC, coronary artery calcium; RT, radiation therapy; LAD, left anterior descending coronary artery. †All failure events occurred in these groups, infinitely large hazard ratio, omitted from model
Dynamic electrocardiogram changes
There were 41 patients with ECGs obtained within 6 months prior to the initiation of pembrolizumab, 37 patients with ECGs during treatment, and 41 patients with ECGs after completion of treatment. The median time to latest post-treatment ECG was 14 months (IQR, 6–27 months). On ECG evaluation, there was increased frequency of QTc prolongation ≥450 ms on ECG obtained during treatment vs. baseline (38.9% vs. 14.6%; p = 0.025). The average heart rate was also significantly higher from 81±17 beats/min during pembrolizumab-based combination therapy vs. 72±12 beats/min at baseline (p = 0.036). Both observed overall differences resolved by the latest post-treatment ECG (Table 3). No additional statistically significant changes in conduction, rhythm, or other ECG parameters were observed in the overall cohort. Individually, among those that experienced grade ≥ 2 cardiac events, five patients additionally developed QTc prolongation during/after treatment (one resolved 3-years later, four did not have follow-up study to evaluate for resolution), one patient developed a type I AV block and LVH after treatment (no follow-up study), and one patient developed left posterior fascicular block during treatment (resolved 3-months later) and a right bundle branch block (not evaluated for resolution) (Supplemental Table 1).
Table 3.
Electrocardiogram characteristics before, during, and after pembrolizumab-based combination therapy
| ECG characteristics | Baseline (n = 41) |
†During treatment (n = 37) |
‡p-value (vs. baseline) |
†Post-treatment (n = 41) |
‡p-value (vs. baseline) |
|---|---|---|---|---|---|
| HR (beats/min), mean (+/-SD) | 72 (+/- 12) | 81 (+/- 17) | 0.036 | 76 (+/-17) | 0.91 |
| PR interval, median (IQR) | 147 (+/-23) | 150 (+/-22) | 0.91 | 146 (22.6) | 0.25 |
| QTc length, median (IQR) | 428 (+/-23) | 441 (+/-23) | 0.060 | 430 (+/-25) | 0.46 |
| QTc prolongation ≥ 450ms | 6 (14.6%) | 14 (38.9%) | 0.025 | 10 (24.4%) | 0.26 |
| Conduction disorder | 1 (2.4%) | 2 (5.4%) | > 0.99 | 3 (7.3%) | 0.25 |
| PVC/PAC | 0 | 2 (5.4%) | > 0.99 | 3 (7.3%) | 0.50 |
| Normal sinus rhythm | 32 (80.0%) | 25 (67.6%) | 0.41 | 28 (68.3%) | 0.48 |
| Sinus tachycardia | 1 (2.5%) | 7 (18.9%) | 0.16 | 3 (7.3%) | > 0.99 |
| Sinus bradycardia | 3 (7.5%) | 3 (8.1%) | 0.16 | 5 (12.2%) | 0.69 |
| Atrial fibrillation/flutter | 0 | 0 | - | 0 | - |
| LVH | 0 | 0 | - | 2 (4.9%) | > 0.99 |
| Low QRS voltage | 2 (4.9%) | 5 (13.5%) | 0.50 | 4 (9.8%) | > 0.99 |
| Abnormal P wave | 0 | 0 | - | 0 | - |
| Pathologic Q wave | 0 | 0 | - | 0 | - |
The most abnormal data obtained during treatment, and the most recent data obtained after treatment, are shown
Data are presented as number of patients (column %), mean (± SD), or median (IQR, interquartile range)
P-value is calculated by a paired t-test or signed rank test for continuous variables, and McNemar’s test for categorical variables
†Some variables may have smaller sample due to availability of reported parameter
‡Statistical analysis was done in patients with both baseline and post-treatment electrocardiogram
Abbreviations: ECG, electrocardiogram; HR, heart rate; SD, standard deviation; IQR, interquartile range; QTc, corrected QT interval; PVC, premature ventricular contractions; PAC, premature atrial contractions; LVH, left ventricular hypertrophy
Dynamic echocardiographic changes
Comparably, there were 47 patients with TTEs obtained prior to the initiation of pembrolizumab, 47 patients with TTEs during treatment, and 30 patients with TTEs after completion of treatment. The median time to latest post-treatment TTE was 23 months (IQR 12–33 months). After initiation of pembrolizumab, five patients developed a grade 2 EF decrease, defined as 40–50% resting EF or 10–19% drop from baseline (Supplemental Table 1). Of these five patients, three ultimately had recovery in EF, though 2/5 did not have further studies to evaluate recovery by the time of data collection. In addition, among the twenty individual patients with a TTE obtained both at baseline and after initiation of pembrolizumab, 5/20 (25%) developed new moderate diastolic dysfunction, with a trend towards statistical significance (p = 0.103). No significant differences were observed in other echocardiographic parameters (Table 4). Notably, among the five patients who developed moderate diastolic dysfunction, all had CAC 0, only two had any baseline CV risk factors (age > 65 years, one of whom had hypertension and hyperlipidemia), and by the latest post-treatment TTE—two still had moderate diastolic dysfunction, one had down-graded to mild, but two did not have any post-treatment TTE performed after abnormality was noted during treatment.
Table 4.
Echocardiogram characteristics before, during, and after pembrolizumab-based combination therapy
| TTE characteristics | Baseline (n = 47) |
†During treatment (n = 47) |
‡p-value (vs. baseline) |
†Post-treatment (n = 30) |
‡p-value (vs. baseline) |
|---|---|---|---|---|---|
| E/E’, median (IQR) | 6.3 (5.1–7.7) | 6.8 (6.0-8.3) | 0.09 | 6.7 (5.2–7.8) | 0.82 |
| MWT (cm), mean (+/-SD) | 0.9 (0.2) | 0.9 (0.2) | 0.17 | 0.9 (0.2) | 0.82 |
| LVEF (%), median (IQR) | 64 (60–66) | 63 (58–66) | 0.10 | 63 (57–65) | 0.78 |
| LVEF ≤ 50% | 3 (5.9%) | 3 (6.4%) | > 0.99 | 2 (6.7%) | > 0.99 |
| Diastolic dysfunction | |||||
| Mild | 13 (26.0%) | 18 (43.9%) | > 0.99 | 11 (44.0%) | 0.50 |
| Moderate | 1 (2.0%) | 4 (9.8%) | > 0.99 | 3 (12.0%) | 0.25 |
| PASP (mmHg), mean (+/-SD) | 19.6 (5.4) | 20.2 (8.0) | 0.70 | 20.2 (5.1) | 0.73 |
| LAVI, median (IQR) | 17.7 (14.7–24.3) | 19.8 (15.6–24.4) | 0.85 | 24.4 (15.7–26.1) | 0.17 |
| GLS (%), mean (+/-SD) | -21.4 (2.3) | -19.2 (3.2) | 0.17 | -21.3 (3.2) | 0.16 |
| Valvular disease moderate | 1 (2.1%) | 3 (6.4%) | 0.25 | 2 (6.7%) | > 0.99 |
The most abnormal data obtained during treatment, and the most recent data obtained after treatment, are shown
Data are presented as number of patients (column %), mean (± SD), or median (IQR, interquartile range)
P-value is calculated by a paired t-test or signed rank test for continuous variables, and McNemar’s test for categorical variables
†Some variables may have smaller sample due to availability of reported parameter
‡Statistical analysis was done in patients with both baseline and post-treatment echocardiogram
Abbreviations: TTE, transthoracic echocardiogram; IQR, interquartile range; MWT, maximal wall thickness; SD, standard deviation; LVEF, left ventricular ejection fraction; PASP, pulmonary artery systolic pressure; LAVI, left atrial volume index; GLS, global longitudinal strain
Individually, among those that experienced grade ≥ 2 cardiac events, four patients additionally experienced mild diastolic dysfunction during/after treatment, (one resolved three months later, one resolved two years later, and two did not have follow-up studies), and one patient developed a grade 1 pericardial effusion after (without follow-up).
Discussion
CV morbidity is an important endpoint for survivors of cancer, because of its impact on quality of life and future oncologic treatment possibilities. In this retrospective study of TNBC patients treated with modern, intensified, multi-modality, chemotherapy-pembrolizumab based treatment, we observed a 10% 1-year cumulative incidence of grade ≥2 cardiac events. Among those treated with RT and dose information available, mean LAD dose was significantly associated with the increased risk of grade ≥ 2 cardiac events, accounting for baseline CV risk. Importantly, these cardiac changes were noted in a cohort of younger TNBC patients (median age of 50) with relatively low CV risk profiles and low CAC burden (88% with CAC < 10). Together, these findings highlight the importance of diligent CV surveillance. Longer follow-up and further studies are warranted, given the degree of recovery, potential cardiac remodeling changes, and/or later effects of treatments may not yet be fully observed.
ICI therapy has been associated with electrocardiogram changes, including heart block, low-voltage, pathological Q wave, and QTc prolongation, though this has predominantly been reported in the context of ICI-related myocarditis [29, 30, 31]. This study observed that QTc prolongation ≥ 450 ms was more common during treatment compared to baseline, and with recovery post-treatment, though the extent to which this is due to anti-emetics versus ICI therapy is not discernable in this cohort. QT interval prolongation is associated with a potential increase in the risk of ventricular arrhythmia, specifically torsades de pointes (TdP) [32]. While the risk of TdP is generally more significant with a QTc > 500 ms, grade 2 changes should still be monitored and attention should be given to additive QTc prolonging drugs (i.e., anti-emetics, antibiotics) as well as correction of underlying electrolyte abnormalities. Though not incorporated in current cardio-oncology consensus guidelines for monitoring [33], if these findings were validated in other cohorts, it could warrant consideration for inclusion. Furthermore, as prolonged ICI therapy is known to accelerate atherosclerosis [12], it is unclear how these risks may be impacted by natural or treatment-induced menopause and development of additional metabolic risk factors.
Our analysis of dynamic TTE changes revealed early grade 2 LVEF deterioration in five patients, with subsequent recovery in three patients, and absence of follow-up study in the other two. Notably, the 2022 European cardio-oncology consensus guidelines recommend close surveillance of anthracycline-treated patients with cardiac biomarkers and TTE, including a TTE every few cycles—even in low-risk patients [33], though these recommendations have not been fully implemented in routine clinical practice. In addition, among patients with both baseline and during/post treatment TTE, 25% (5/20) developed moderate diastolic dysfunction, that persisted in a later study in two patients, was downgraded to mild in one patient, and was not reevaluated in two patients. None of these patients had CAC identified on baseline CT. Importantly, diastolic dysfunction has not been routinely assessed on TTEs in prior studies and requires longer-term follow-up to determine if associated with heart failure with reduced or preserved ejection fraction (HFrEF, HFpEF). Further, diastolic dysfunction is an important predictor of all-cause mortality in large epidemiologic studies [34], often precedes the development of systolic dysfunction [35], and has been shown to play an essential role in the pathophysiology of other cardiac diseases [36]. Though notably, diastolic dysfunction is also relatively common and may occur due to factors like elevated blood pressure, and while it may be linked to a small risk of systolic dysfunction [37]—it may not necessarily portend an increased risk of reduced ejection fraction associated with treatment. Together, these findings support monitoring diastolic and other echocardiographic changes that might identify patients at risk for developing overt left ventricular systolic dysfunction.
Prior studies have demonstrated an additive or synergistic effect of breast cancer therapies on CV risk. In a study by Kim et al., the investigators showed that left-side irradiation was associated with increased risk of CV events only in patients who also received a cumulative doxorubicin-equivalent dose ≥250 mg/m2 [38]. Another study examining 55 patients with non-metastatic TNBC found a 13% rate of grade 3 myocarditis after neoadjuvant chemo-immunotherapy, but no additional myocarditis risk in the setting of adjuvant RT [39]. In the current study, we observed a 1% rate of grade 2 myocarditis, though non-myocarditis events were common (10%), and all occurred among those receiving carboplatin, paclitaxel, doxorubicin, and cyclophosphamide chemotherapy. Alternative anthracycline-free chemotherapy in the preoperative setting has been recently tested in early-stage TNBC. The phase 2 NeoPACT trial evaluated preoperative docetaxel with carboplatin for 6 cycles in addition to pembrolizumab and yielded a 58% pCR rate, as well as a 98% 3-year EFS rate for those achieving pCR, and 68% for those not achieving pCR, mirroring the EFS results from the KEYNOTE-522 trial [40]. The phase 3 OptimICE-PCR trial is examining omission of adjuvant pembrolizumab in TNBC patients with pCR after neoadjuvant chemo-immunotherapy [41]. Another consideration of emerging interest is the contribution of genetic underpinnings associated with increased cancer therapy associated cardiac toxicity. A study by Garcia-Pavia et al. reported that 7% of patients with anthracycline-induced cardiac dysfunction carried a likely pathologic variant within known cardiomyopathy genes (vs. <1% identified in matched control cohort) [42], suggestive that a certain subset of patients may be at even greater risk of toxicity. Together, these findings underscore the potential cumulative CV risk of multimodal cancer therapy, the need for close CV surveillance, and to optimize CV risk mitigation strategies. Improved adoption of existing cardio-oncology consensus guidelines-based risk assessment and monitoring [33] is warranted. Future, larger studies are needed to identify subgroup(s) that may be particularly vulnerable to combination chemotherapy-immunotherapy, to investigate novel blood-based and imaging biomarkers, and who may warrant closer surveillance with periodic ECGs and echocardiograms.
Given initial studies establishing the link between cardiac radiation dose and coronary heart disease in breast cancer survivors [43], significant efforts have been made to reduce heart dose exposure. Such strategies include deep inspiration breath hold (DIBH) technique to optimize distance between the heart and target [44], prone positioning (though results for cardiac dose sparing are mixed) [45, 46], target volume reduction (i.e., partial breast irradiation) [47, 48], and/or advanced planning techniques such as intensity-modulated RT or proton RT [49, 50, 51]. While heart doses have significantly reduced in the modern breast RT treatment era [52], recent data continue to support a correlation between LAD dose and adverse cardiac events [18]. Similarly, we observed an association between mean LAD dose and CTCAE grade ≥2 cardiac events (but not MHD), consistent with data supporting LAD as a better predictor (vs. MHD) of coronary events and MHD being a poor surrogate for LAD dose exposure during breast cancer RT [18, 53, 54]. Therefore, continued efforts are needed to optimize cardiac radiation sparing techniques in clinical practice to transform LAD dose into a modifiable cardiac risk factor, particularly given the potential interaction between RT and systemic therapies such as ICIs. It should additionally be noted that heart/LAD radiation dose is not incorporated in modern risk prediction models for cardio-toxicity [55] and further work is needed to account for cardiac radiation exposure in these models.
Limitations of our study include the retrospective nature with a relatively small sample size and limited follow-up that may be insufficient for drawing significant conclusions. Further, the shorter-term follow up may not fully capture the degree of recovery and/or cardiac remodeling or later effects of therapies. Specifically, radiation-induced heart disease can have a latency of several years, and while early events (within 2–5 years) occur [16, 17]—this shorter timeframe study may under-capture the extent of these risks. For instance, the patient who required acute coronary intervention following initiation of ICI was subsequently treated with left-breast radiotherapy, for which the added risk multi-modal therapy is likely insufficient to evaluate in this timeframe. In addition, we did not analyze cardiac events from patients who did not have echocardiograms available, which might have introduced a selection bias. Notably, only 47 patients had baseline echocardiographic data available, which limits our understanding of the extent to which observed ejection fraction changes were new or pre-existing, particularly given the known cardiotoxic potential of the commonly used chemotherapies. Lastly, the observed grade ≥ 2 cardiac event rate of 10% may be an under- or over-estimate given the incomplete nature of the echocardiogram and electrocardiogram surveillance.
Conclusion
Early CV toxicity related to multimodal treatment of TNBC was observed, even in young patients with low CV risk profiles. This highlights the importance of early CV surveillance, including improved cardiac monitoring during treatment, and continued efforts at cardiac toxicity risk mitigation strategies for breast cancer survivors. Longer follow-up and further studies are warranted, given degree of recovery and later effects of treatment may not yet be fully accounted for.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Author contributions
Conception: JPN, KMA, APN, RHM; methodology: JPN, KMA, APN, RHM; data acquisition: JPN, KDS, OP, AS, PB; data analysis: JPN, KMA, KDS; writing - original draft preparation: JPN, KMA; writing - review and editing: KMA, APN, RHM, MRK, JKJ, SLS, ACK, CR; supervision: KMA. All authors read and approved the final manuscript.
Funding
Not applicable.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
This study obtained ethical approval from the Cedars-Sinai Medical Center Institutional Review Board with a waiver of informed consent due to minimal risk nature (STUDY00003061).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2.Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res Off J Am Assoc Cancer Res. 2007;13:4429–34. [DOI] [PubMed] [Google Scholar]
- 3.Schmid P, Cortes J, Pusztai L, McArthur H, Kümmel S, Bergh J, et al. Pembrolizumab for early Triple-Negative breast Cancer. N Engl J Med. 2020;382:810–21. [DOI] [PubMed] [Google Scholar]
- 4.Schmid P, Cortes J, Dent R, Pusztai L, McArthur H, Kümmel S, et al. Event-free survival with pembrolizumab in early Triple-Negative breast Cancer. N Engl J Med. 2022;386:556–67. [DOI] [PubMed] [Google Scholar]
- 5.Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer. 2003;97:2869–79. [DOI] [PubMed] [Google Scholar]
- 6.Mahmood SS, Fradley MG, Cohen JV, Nohria A, Reynolds KL, Heinzerling LM, et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol. 2018;71:1755–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nielsen DL, Juhl CB, Nielsen OH, Chen IM, Herrmann J. Immune checkpoint Inhibitor-Induced cardiotoxicity: A systematic review and Meta-Analysis. JAMA Oncol. 2024;10:1390–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scard C, Nguyen J-M, Varey E, Moustaghfir I, Khammari A, Dreno B. Cardiac adverse events associated with anti-PD-1 therapy in patients treated for advanced melanoma: relevance of dosing troponin T levels. Eur J Dermatol EJD. 2021;31:205–12. [DOI] [PubMed] [Google Scholar]
- 9.Liu M, Cheng X, Ni R, Zheng B, Huang S, Yang J. Cardiotoxicity of immune checkpoint inhibitors: A frequency network meta-analysis. Front Immunol. 2022;13:1006860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang DY, Salem J-E, Cohen JV, Chandra S, Menzer C, Ye F, et al. Fatal toxic effects associated with immune checkpoint inhibitors: A systematic review and Meta-analysis. JAMA Oncol. 2018;4:1721–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lyon AR, Yousaf N, Battisti NML, Moslehi J, Larkin J. Immune checkpoint inhibitors and cardiovascular toxicity. Lancet Oncol. 2018;19:e447–58. [DOI] [PubMed] [Google Scholar]
- 12.Suero-Abreu GA, Zanni MV, Neilan TG. Atherosclerosis with immune checkpoint inhibitor therapy: evidence, diagnosis, and management: JACC: cardiooncology State-of-the-Art review. JACC CardioOncology. 2022;4:598–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song W, Zheng Y, Dong M, Zhong L, Bazoukis G, Perone F, et al. Electrocardiographic features of immune checkpoint Inhibitor-Associated myocarditis. Curr Probl Cardiol. 2023;48:101478. [DOI] [PubMed] [Google Scholar]
- 14.Bergom C, Bradley JA, Ng AK, Samson P, Robinson C, Lopez-Mattei J, et al. Past, present, and future of Radiation-Induced cardiotoxicity: refinements in targeting, surveillance, and risk stratification. JACC CardioOncology. 2021;3:343–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Honaryar MK, Allodji R, Ferrières J, Panh L, Locquet M, Jimenez G, et al. Early coronary artery calcification progression over two years in breast Cancer patients treated with radiation therapy: association with cardiac exposure (BACCARAT Study). Cancers. 2022;14:5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wennstig A-K, Garmo H, Isacsson U, Gagliardi G, Rintelä N, Lagerqvist B, et al. The relationship between radiation doses to coronary arteries and location of coronary stenosis requiring intervention in breast cancer survivors. Radiat Oncol Lond Engl. 2019;14:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van den Bogaard VAB, Spoor DS, van der Schaaf A, van Dijk LV, Schuit E, Sijtsema NM, et al. The importance of radiation dose to the atherosclerotic plaque in the left anterior descending coronary artery for radiation-Induced cardiac toxicity of breast Cancer patients?? Int J Radiat Oncol Biol Phys. 2021;110:1350–9. [DOI] [PubMed] [Google Scholar]
- 18.Zureick AH, Grzywacz VP, Almahariq MF, Silverman BR, Vayntraub A, Chen PY, et al. Dose to the left anterior descending artery correlates with cardiac events after irradiation for breast Cancer. Int J Radiat Oncol Biol Phys. 2022;114:130–9. [DOI] [PubMed] [Google Scholar]
- 19.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–32. [DOI] [PubMed] [Google Scholar]
- 20.Feng M, Moran JM, Koelling T, Chughtai A, Chan JL, Freedman L, et al. Development and validation of a heart atlas to study cardiac exposure to radiation following treatment for breast cancer. Int J Radiat Oncol Biol Phys. 2011;79:10–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Common Terminology. Criteria for Adverse Events (CTCAE). 2017.
- 22.Rautaharju PM, Surawicz B, Gettes LS, Bailey JJ, Childers R, Deal BJ, et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part IV: the ST segment, T and U waves, and the QT interval: a scientific statement from the American heart association electrocardiography and arrhythmias committee, Council on clinical cardiology; the American college of cardiology foundation; and the heart rhythm society. Endorsed by the international society.for computerized electrocardiology. J Am Coll Cardiol. 2009;53:982–91. [DOI] [PubMed] [Google Scholar]
- 23.Molloy TJ, Okin PM, Devereux RB, Kligfield P. Electrocardiographic detection of left ventricular hypertrophy by the simple QRS voltage-duration product. J Am Coll Cardiol. 1992;20:1180–6. [DOI] [PubMed] [Google Scholar]
- 24.Sokolow M, Lyon TP. The ventricular complex in left ventricular hypertrophy as obtained by unipolar precordial and limb leads. Am Heart J. 1949;37:161–86. [DOI] [PubMed] [Google Scholar]
- 25.Bazett HC. An analysis of the Time-Relations of electrocardiograms. Ann Noninvasive Electrocardiol. 1997;2:177–94. [Google Scholar]
- 26.Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, Dokainish H, Edvardsen T, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr Off Publ Am Soc Echocardiogr. 2016;29:277–314. [DOI] [PubMed] [Google Scholar]
- 27.Shuster JJ. Median follow-up in clinical trials. J Clin Oncol Off J Am Soc Clin Oncol. 1991;9:191–2. [DOI] [PubMed] [Google Scholar]
- 28.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 29.Power JR, Alexandre J, Choudhary A, Ozbay B, Hayek S, Asnani A, et al. Electrocardiographic manifestations of immune checkpoint inhibitor myocarditis. Circulation. 2021;144:1521–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ganatra S, Neilan TG. Immune checkpoint Inhibitor-Associated myocarditis. Oncologist. 2018;23:879–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pohl J, Mincu R-I, Mrotzek SM, Hinrichs L, Michel L, Livingstone E, et al. ECG changes in melanoma patients undergoing Cancer Therapy-Data from the ECoR registry. J Clin Med. 2020;9:2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moss AJ, Schwartz PJ, Crampton RS, Tzivoni D, Locati EH, MacCluer J, et al. The long QT syndrome. Prospective longitudinal study of 328 families. Circulation. 1991;84:1136–44. [DOI] [PubMed] [Google Scholar]
- 33.Lyon AR, López-Fernández T, Couch LS, Asteggiano R, Aznar MC, Bergler-Klein J, et al. 2022 ESC guidelines on cardio-oncology developed in collaboration with the European hematology association (EHA), the European society for therapeutic radiology and oncology (ESTRO) and the international Cardio-Oncology society (IC-OS). Eur Heart J. 2022;43:4229–361. [DOI] [PubMed] [Google Scholar]
- 34.Redfield MM, Jacobsen SJ, Burnett JC, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. [DOI] [PubMed] [Google Scholar]
- 35.Stoddard MF, Seeger J, Liddell NE, Hadley TJ, Sullivan DM, Kupersmith J. Prolongation of isovolumetric relaxation time as assessed by doppler echocardiography predicts doxorubicin-induced systolic dysfunction in humans. J Am Coll Cardiol. 1992;20:62–9. [DOI] [PubMed] [Google Scholar]
- 36.Møller JE, Pellikka PA, Hillis GS, Oh JK. Prognostic importance of diastolic function and filling pressure in patients with acute myocardial infarction. Circulation. 2006;114:438–44. [DOI] [PubMed] [Google Scholar]
- 37.Upshaw JN, Finkelman B, Hubbard RA, Smith AM, Narayan HK, Arndt L, et al. Comprehensive assessment of changes in left ventricular diastolic function with contemporary breast Cancer therapy. JACC Cardiovasc Imaging. 2020;13:198–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim DY, Youn J-C, Park M-S, Lee S, Choi S-W, Ryu K-H, et al. Cardiovascular outcome of breast cancer patients with concomitant radiotherapy and chemotherapy: A 10-year multicenter cohort study. J Cardiol. 2019;74:175–81. [DOI] [PubMed] [Google Scholar]
- 39.Tison T, Loap P, Arnaud E, Cao K, Bringer S, Kissel M, et al. Tolerance of concurrent adjuvant radiation therapy and pembrolizumab for triple negative breast cancer: real life experience. Adv Radiat Oncol. 2024;9:101384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharma P, Stecklein SR, Yoder R, Staley JM, Schwensen K, O’Dea A, et al. Clinical and biomarker findings of neoadjuvant pembrolizumab and carboplatin plus docetaxel in Triple-Negative breast cancer: NeoPACT phase 2 clinical trial. JAMA Oncol. 2024;10:227–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pembrolizumab versus Observation in Patients with Early Stage Triple-Negative. Breast Cancer who had a Pathologic Complete Response after Chemotherapy plus Pembrolizumab, OptimICE-PCR Trial - NCI [Internet]. 2016 [cited 2024 Oct 11]. Available from: https://www.cancer.gov/about-cancer/treatment/clinical-trials/search/v?id=NCI-2022-07859
- 42.Garcia-Pavia P, Kim Y, Restrepo-Cordoba MA, Lunde IG, Wakimoto H, Smith AM, et al. Genetic variants associated with Cancer Therapy-Induced cardiomyopathy. Circulation. 2019;140:31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Brønnum D, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987–98. [DOI] [PubMed] [Google Scholar]
- 44.Stowe HB, Andruska ND, Reynoso F, Thomas M, Bergom C. Heart sparing radiotherapy techniques in breast cancer: A focus on deep inspiration breath hold. Breast Cancer Dove Med Press. 2022;14:175–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gerber NK, Yan SX, Levinson BA, Perez CA, Das IJ, Maisonet OG, et al. A prospective trial to compare deep inspiratory breath hold with prone breast irradiation. Pract Radiat Oncol. 2020;10:330–8. [DOI] [PubMed] [Google Scholar]
- 46.Saini AS, Hwang CS, Biagioli MC, Das IJ. Evaluation of sparing organs at risk (OARs) in left-breast irradiation in the supine and prone positions and with deep inspiration breath-hold. J Appl Clin Med Phys. 2018;19:195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Coles CE, Griffin CL, Kirby AM, Titley J, Agrawal RK, Alhasso A, et al. Partial-breast radiotherapy after breast conservation surgery for patients with early breast cancer (UK IMPORT LOW trial): 5-year results from a multicentre, randomised, controlled, phase 3, non-inferiority trial. Lancet Lond Engl. 2017;390:1048–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meattini I, Marrazzo L, Saieva C, Desideri I, Scotti V, Simontacchi G, et al. Accelerated Partial-Breast irradiation compared with Whole-Breast irradiation for early breast cancer: Long-Term results of the randomized phase III APBI-IMRT-Florence trial. J Clin Oncol Off J Am Soc Clin Oncol. 2020;38:4175–83. [DOI] [PubMed] [Google Scholar]
- 49.Mondal D, Jhawar SR, Millevoi R, Haffty BG, Parikh RR. Proton versus photon Breath-Hold radiation for Left-Sided breast Cancer after breast-Conserving surgery: A dosimetric comparison. Int J Part Ther. 2021;7:24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cartechini G, Fracchiolla F, Menegotti L, Scifoni E, La Tessa C, Schwarz M, et al. Proton pencil beam scanning reduces secondary cancer risk in breast cancer patients with internal mammary chain involvement compared to photon radiotherapy. Radiat Oncol Lond Engl. 2020;15:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garda AE, Hunzeker AE, Michel AK, Fattahi S, Shiraishi S, Remmes NB, et al. Intensity modulated proton therapy for bilateral breast or chest wall and comprehensive nodal irradiation for synchronous bilateral breast cancer: initial clinical experience and dosimetric comparison. Adv Radiat Oncol. 2022;7:100901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Quirk S, Grendarova P, Phan T, Conroy L, Burke B, Long K, et al. A retrospective analysis to demonstrate achievable dosimetry for the left anterior descending artery in left-sided breast cancer patients treated with radiotherapy. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2020;148:167–73. [DOI] [PubMed] [Google Scholar]
- 53.Atkins KM, Chaunzwa TL, Lamba N, Bitterman DS, Rawal B, Bredfeldt J, et al. Association of left anterior descending coronary artery radiation dose with major adverse cardiac events and mortality in patients with Non-Small cell lung Cancer. JAMA Oncol. 2021;7:206–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jacob S, Pathak A, Franck D, Latorzeff I, Jimenez G, Fondard O, et al. Early detection and prediction of cardiotoxicity after radiation therapy for breast cancer: the BACCARAT prospective cohort study. Radiat Oncol Lond Engl. 2016;11:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaboré EG, Macdonald C, Kaboré A, Didier R, Arveux P, Meda N, et al. Risk prediction models for cardiotoxicity of chemotherapy among patients with breast cancer: A systematic review. JAMA Netw Open. 2023;6:e230569. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.