Abstract
Restenosis after percutaneous transluminal coronary angioplasty is caused by neointimal hyperplasia, which involves impairment of nitric oxide (NO)-dependent pathways, and may be further exacerbated by a concomitant aging process. We compared the effects of NO-releasing-aspirin (NCX-4016) and aspirin (ASA) on experimental restenosis in both adult and elderly rats. Moreover, to ascertain the efficacy of NCX-4016 during vascular aging, we fully characterized the release of bioactive NO by the drug. Sprague–Dawley rats aged 6 and 24 months were treated with NO releasing-aspirin (55 mg/kg) or ASA (30 mg/kg) for 7 days before and 21 days after standard carotid balloon injury. Histological examination and immunohistochemical double-staining were used to evaluate restenosis. Plasma nitrite and nitrate and S-nitrosothiols were determined by a chemiluminescence-based assay. Electron spin resonance was used for determining nitrosylhemoglobin. Treatment of aged rats with NCX-4016 was associated with increased bioactive NO, compared with ASA. NO aspirin, but not ASA, reduced experimental restenosis in old rats, an effect associated with reduced vascular smooth muscle cell proliferation. NCX-4016, but not ASA, was well tolerated and virtually devoid of gastric damage in either adult or old rats. Thus, impairment of NO-dependent mechanisms may be involved in the development of restenosis in old rats. We suggest that an NCX-4016 derivative could be an effective drug in reducing restenosis, especially in the presence of aging and/or gastrointestinal damage.
Aging can be defined as a progressive deterioration of biological functions after the organism has attained its maximal reproductive competence, and is usually associated with a decrease in cellular proliferative ability. However, with aging, the diameter of arteries tends to increase, and thickening of intima and medial layers is observed (1). This impairment may be the result of a loss in replicative potential and telomere length decrease (2–4). Moreover, in atherosclerosis and restenosis, advancing age has been associated with a higher level of vascular smooth muscle cell (VSMC) proliferation and neointimal lesion formation. Indeed, VSMC proliferation after vascular injury is greater in older rats and rabbits (5, 6). Cultured VSMCs from older animals show higher percentage of their population in the S phase (7). After de-endothelialization in vivo, no substantial neointimal formation is observed in young rats, whereas the intimal thickness markedly increases in the older rats (8). This phenomenon could be related to a concurrent decrease in the bioavailability of the antiproliferative effect of nitric oxide (NO). Indeed, there is considerable growing experimental and clinical evidence that aging is also associated with a decline in the release of bioactive NO (9–13). Decreased free NO levels in aged rat aortas are associated with increased formation of the toxic peroxynitrite (14). Transient increased endothelial NO-synthase activity could be a compensatory, but eventually futile, mechanism to counter-regulate the age-related loss of NO (9–14). Therefore, arteries from aged living organs are predisposed to develop vascular lesions and have a reduced endogenous pathophysiological cascade of events leading to vascular protection.
Treatment of myocardial infarction in older patients remains problematic. Older patients have increased risk for adverse events, a result of the presence of more comorbid conditions and advanced multivessel coronary disease, and loss of ischemic preconditioning (15–17). Primary percutaneous transluminal coronary angioplasty (PTCA) does not seem to be more beneficial in older than in adult patients, and the incremental adverse effect of age does not vary by different treatment strategies (18). Older patients had a higher mortality and morbidity despite aspirin (ASA), beta-blockers, or thrombolytic therapy (19). A highly clinically relevant pathophysiological process that involves VSMC proliferation is restenosis. This complication is often addressed by using ASA (as well as other antiplatelet drugs) (20, 21), which is limited by a modest therapeutic action and by its common gastrointestinal adverse effects (22–25). Restenosis has also been proposed to involve impaired NO production by the damaged endothelium (26), and modulation of NO-dependent pathways may be useful to modify the development of restenosis (27–30). Thus, increased NO production at the site of balloon injury may potentially exert beneficial effects (31). NO-releasing-aspirin [2-acetoxybenzoic acid 3-(nitrooxymethyl) phenyl ester (NCX-4016); ref. 32] exhibits little or no ulcerogenic activity in several experimental models (23, 24, 32), inhibits caspase activation (24), and has antithrombotic properties (33). Because NCX-4016 could restore vascular NO deficiency with increasing age and has been shown to reduce restenosis in hypercholesterolemic mice (34), it could be also beneficial in the restenotic process of aged rats.
Hence, to investigate the relevance of NO-dependent pathways after balloon injury in vascular aging, the present study was designed to compare the effects of NCX-4016 and ASA on experimental restenosis in both adult and elderly rats, the most recognized experimental model of aging. Moreover, to ascertain the therapeutic efficacy of NO-aspirin during vascular aging, we fully characterized the release of bioactive NO.
Materials and Methods
Experimental Procedures.
Studies were carried out according to the guidelines of the American Heart Association for Accreditation of Laboratory Animal Care. Experiments were carried out in adult (6 months old, 403 ± 81 g of body weight) and elderly (24 months old, 710 ± 120 g of body weight) Sprague–Dawley male rats. The animals were individually housed in stainless steel cages under standard conditions (temperature 22 ± 2°C; 50 ± 10% relative humidity; artificial light from 06.00 to 20.00). After anesthesia with i.p. ketamine (80 mg/kg of body weight) and xylazine (5 mg/kg of body weight), the carotid artery was exposed (midline neck incision) under light microscope and ligated distally (6–0 silk ties). Standard balloon injury was induced by using a Fogarty 2F microcatheter into the common carotid artery. Animals were treated with NCX-4016 (55 mg/kg, a generous gift from NicOx, Nice, France) or ASA (30 mg/kg) following the preventive protocol (i.e., drugs were administered 7 days before and 21 after balloon injury) (34). Additional experiments were performed by using higher doses of NCX-4016 (up to 186 mg/kg) or an NO donor (S-nitroso-N-acetylpenicillamine, 500 μM). A control vehicle-treated (PEG 400) group was also included.
Morphometric and Immunohistochemical Analysis.
Rats were killed with a lethal dose of sodium pentobarbital 21 days after injury, and the stomach was excised. An observer unaware of the treatments the rats had received examined each stomach and measured the length (mm) of each hemorrhagic erosion. The gastric damage score was calculated for each stomach by summing the lengths of all erosions (23, 24, 32, 33). Carotid arteries were perfused with phosphate-buffered paraformaldehyde (4%, 0.1 mol/liter, pH 7.3) for histology or normal saline for immunohistochemistry assessed by computer-assisted imaging analysis (34–36). Carotid arteries were serially sectioned into 15–20 slices (10 μM) with a rotating diamond-coated knife, and sections were stained with toluidine blue. The area confined by the internal elastic lamina, the cross-sectional neointimal area, and the percent area stenosis was measured by morphometry. Vessel injury score was also determined (34). The number of proliferating VSMCs in the neointima was determined by immunohistochemical double-staining of serial sections with α-actin monoclonal mouse Ab (no. M0858, Dako) and proliferating cell nuclear antigen (PCNA) Ab against PCNA (1:250 dilution; clone PC10, Dako) (34, 37).
Ozone-Based Chemiluminescence Detection of Plasma Nitrite and Nitrate (NOx), and S-nitrosothiols (RS-NO).
NOx and RS-NO levels were determined by a chemiluminescence-based assay (Sievers Instruments 280, Boulder, CO) as described (38).
ESR Measurement of Nitrosylhemoglobin [HbFe(II)NO].
ESR spectra were recorded at 100 K with a Bruker (Billerica, MA) EMX spectrometer (X band) equipped with a high-sensitivity cylindrical cavity (ER4119HS) by using the following instrument conditions (39): microwave frequency, 9.316 GHz; microwave power, 20 mW; modulation frequency, 100 kHz; modulation amplitude, 5 G (1 G = 0.1 mT); number of scans, 20; resolution, 1,024 points; sweep time, 21 sec; and center field, 3,300 G with a sweep width of 1,200 G. Spectra were analyzed by using bruker system (version 2.11) software. The concentration of [HbFe(II)NO], expressed as μM, was determined by double integration of the signal, using CuSO4-EDTA in phosphate buffer (50 mM, pH 7.4).
HPLC Analysis of Salicylic Acid in Blood.
HPLC analyses were carried out on an HP1050 with a Merck Licrospher (250 × 4 mm; particle size, 5 μm) and a guard column (20 × 4 mm; particle size, 5 μm). The HPLC pump operated at a flow rate of 1.0 ml min−1 at 25 ± 1°C. The mobile phase was CH3CN/H2O/H3PO4 10:99:1 (vol/vol/vol) (solvent A) and CH3CN (solvent B); gradient elution was used starting at 0% B, increasing linearly to 28% in 5 min, then to 33% at 12 min, 43% at 17 min, 60% at 20 min until 25 min. Volumes of 50 μl were injected and the detector (UV-visible diode array detector) was set at 228 nm.
Chemicals.
Benzoic acid, ASA, PEG 400 (average molecular weight 400), copper(II) sulfate, sodium fluoride, vanadium(III) chloride, sodium nitrate, and EDTA were purchased from Sigma–Aldrich.
Statistical Analysis.
The gastric damage scores were compared by using a one-way ANOVA followed by the Student–Newman–Keul test. The inhibitory effects on VSMC proliferation and restenosis induced by treatment with NCX-4016 and ASA were compared by using the Kruskal–Wallis test. Regressions were calculated by the least-squares method.
Results
Gastric Damage.
Oral administration of ASA resulted in extensive hemorrhagic damage in the stomach of the rats, but such damage was not seen in rats given an equimolar dose of NCX-4016. The mean gastric damage score in adult rats given ASA was 22 ± 3, whereas that in adult rats given NO-aspirin was 2 ± 0.2 (P < 0.0001). In the old rats, ASA-treated rats had a mean gastric damage score of 51 ± 8, whereas that in the rats receiving NO-aspirin was 3 ± 0.8 (P < 0.0001). Even when a much higher dose of NCX-4016 (186 mg/kg) was given to adult or old rats, the mean gastric damage score remained very low (2.1 ± 0.5 and 3.6 ± 1.2, respectively, both P < 0.0001 vs. ASA). These data are consistent with several previous reports (23, 24, 31, 32) in which NO-aspirin, unlike ASA, was not shown to induce significant gastric damage.
Salicylate Levels.
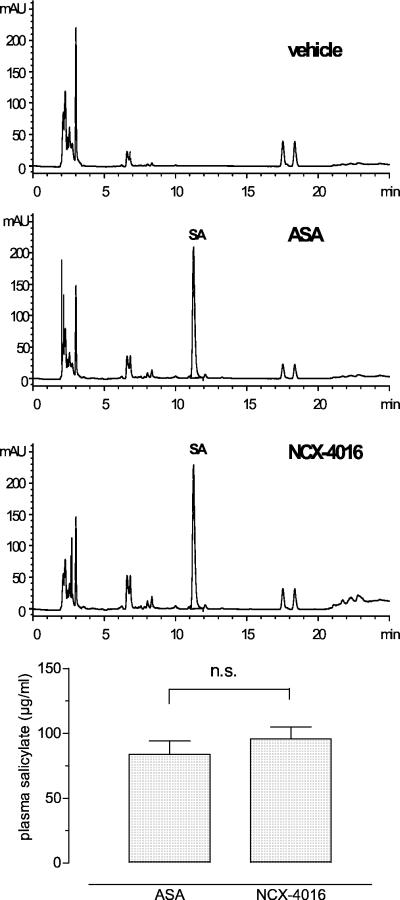
In Fig. 1 (Upper) are shown the representative HPLC profiles of blood samples relative to vehicle-, ASA-, and NO-aspirin-treated aged rats. Blood salicylate levels after oral administration of ASA or equimolar doses of NO-aspirin did not differ significantly (P = not significant), indicating similar salicylate release from the two drugs 4 h after last administration (Fig. 1 Lower). These data were similar to those obtained from adult rats (not shown).
Figure 1.
(Upper) Representative HPLC profiles of blood from vehicle-, ASA- (30 mg/kg), and NCX-4016-treated (55 mg/kg) old rats. SA, salicylic acid. (Lower) Plasma salicylates in ASA- and NCX-4016-treated old rats.
Plasma NOx.
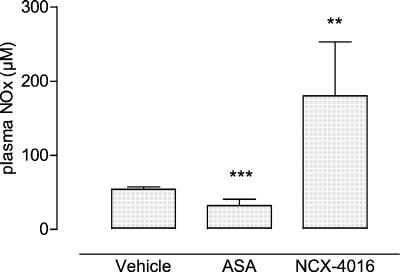
We determined by chemiluminescence detection the plasma levels of NOx. As shown in Fig. 2, the oral treatment of old rats with NCX-4016 induced more than a 3-fold increase of plasma NOx compared with the vehicle- (180 ± 73 μM vs. 54.14 ± 3.13 μM; P = 0.0048) and ASA-treated groups (31.98 ± 8.58 μM; P = 0.0019, t test). In addition, equimolar doses of ASA were found to induce a significant decrease of plasma NOx compared with vehicle-treated aged rats (P = 0.0006). Plasma NOx concentrations after drug administration (both ASA and NCX-4016) were also similar to those obtained from adult rats (not shown).
Figure 2.
Plasma NOx levels in vehicle-, ASA- (30 mg/kg), and NCX-4016-treated (55 mg/kg) old rats. Mean ± SEM of 5 animals (3 replicates). **, P = 0.0048 NCX-4016 vs. vehicle; ***, P = 0.0006 ASA vs. vehicle.
[HbFe(II)NO] in Blood.
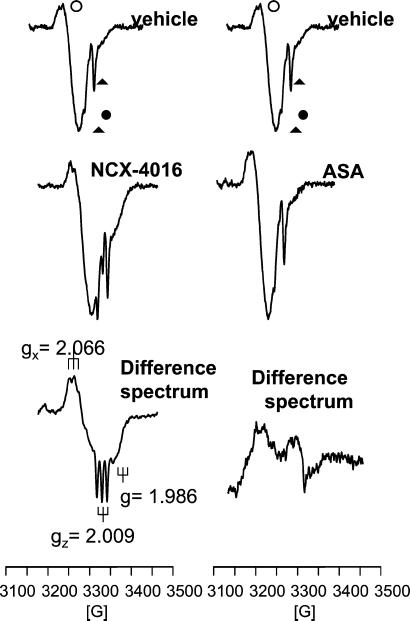
To further show the NO release by NO-aspirin, we measured [HbFe(II)NO], a bioactive storage form of NO formed by the reaction of NO addition to Fe(II)-Hb through a coordinate bond (40), by ESR spectroscopy. [HbFe(II)NO] is present in the blood as a pentacoordinate nitrosyl complex and characterized by ESR spectroscopy as a three-line hyperfine structure, because of the coupling of the unpaired electron in the 14N nucleus of NO (41). In Fig. 3 (Left) is shown a representative ESR spectrum of blood from vehicle-treated aged rats characterized by 2 main signals at g values 2.054 and 2.000, attributable to copper-ceruloplasmin and to a semiquinone radical. As reported (39), the 2 minor peaks at g = 2.019 and g = 2.009 are relative to 2 of the 3 lines characteristic of the endogenous [HbFe(II)NO] complex. The ESR spectra of blood samples from NCX-4016-treated aged rats were characterized by a significant increase of the 2 lines at g = 2.019 and g = 2.009, to demonstrate an increase of [HbFe(II)NO] compared with the vehicle-treated group. To better resolve and quantitate the [HbFe(II)NO] signal, to each ESR spectrum was subtracted the spectrum from vehicle-treated old rats. A representative ESR difference spectrum is shown in Fig. 3 (Left), which clearly shows the typical three-line hyperfine structure of the pentacoordinate complex [HbFe(II)NO] with coupling constants of Ax = Az = 17 G at gx = 2.066 and gz = 2.009. At g = 1.986, the signal relative to the hexacoordinated complex (R form) is also detectable. The double integration of the difference signals gives an increase of [HbFe(II)NO] content compared with the vehicle-treated group of 1.65 ± 0.62 nmol/ml. By contrast, the ESR spectra of blood samples from ASA-treated old rats were practically superimposable with those obtained from vehicle-treated animals (Fig. 3 Right). In particular, no differential increase of line intensity at g = 2.019 and g = 2.009 was observed and no signal of [HbFe(II)NO] (both in penta- and hexacoordinate state) was detectable.
Figure 3.
(Left) Representative ESR spectra of blood samples from vehicle- and NCX-4016-treated (55 mg/kg) old rats and the corresponding difference spectrum (NCX-4016 after subtraction of vehicle). ○, ceruloplasmin; ●, semiquinone radical. The 2 peaks at g = 2.019 and g = 2.009 (arrows) are relative to [HbFe(II)NO]. (Right) Representative ESR spectra of blood from vehicle- and ASA-treated (30 mg/kg) old rats and the corresponding difference spectrum (ASA after subtraction of vehicle).
Plasma RS-NO Levels.
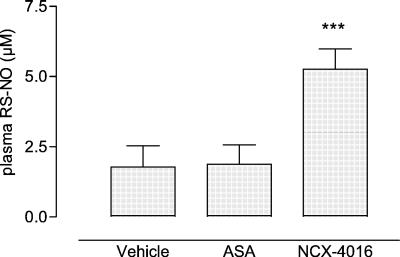
In parallel to the increase of [HbFe(II)NO], in NO-aspirin-treated old rats a significant rise (almost 3-fold) in plasma levels of RS-NO was observed in respect to both vehicle- (5.26 ± 0.72 μM vs. 1.78 ± 0.76 μM; P = 0.0006) and ASA-treated old rats (1.88 ± 0.69 μM; P = 0.002) (Fig. 4). Plasma RS-NO concentrations were similar to those obtained from adult rats (not shown). This phenomenon was in accordance with the transport of NO that, from erythrocyte HbFe(II), is shuttled intramolecularly to the cysteine residue β-93 and then exported to plasma thiol-containing molecules, which readily deliver NO in the presence of transition metals (42, 43).
Figure 4.
Plasma RS-NO levels in vehicle-, ASA- (30 mg/kg), and NCX-4016-treated (55 mg/kg) old rats; Mean ± SEM of 5 animals (3 replicates). ***, P < 0.005 NCX-4016 vs. vehicle and ASA.
Effects of NCX-4016 and ASA on Restenosis.
In agreement with previous studies (6, 8), the degree of restenosis after balloon angioplasty was higher in older than younger adult rats (Fig. 5 and Table 1). The intima/media ratio was 34 ± 6% greater than that of younger adult rats (P < 0.01). Most important, the balloon injury scores related to the treated groups were similar among different protocols (Table 1).
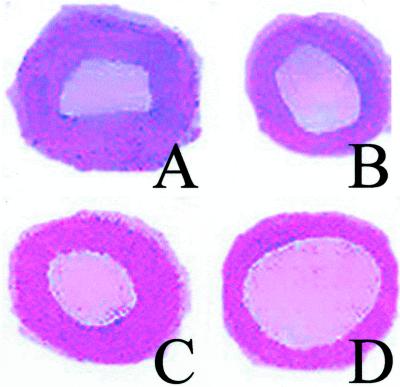
Figure 5.
Representative images of restenosis after balloon angioplasty and neointimal hyperplasia in carotid artery. (A) Control vehicle-treated old rat. (B) Vehicle-treated adult rat. Treatment with ASA (30 mg/kg) in an old rat (C) did not significantly reduce restenosis. In contrast, NCX-4016 (55 mg/kg) showed a significant reduction of neointimal hyperplasia and restenosis in an old rat (D).
Table 1.
Effects on restenosis of NCX-4016 vs. ASA in adult and old rats
| Injury score | Neointimal area, μm2 | % Lumen stenosis | % Proliferating cells (VSMC/PCNA) |
|---|---|---|---|
| Control adult rats (N = 8; balloon injury + vehicle) | |||
| 1.85 ± 0.3 | 31.8 ± 2.9 | 58.5 ± 5.6 | 23.6 ± 2.5 |
| Control old rats (N = 8; balloon injury + vehicle) | |||
| 1.86 ± 0.4 | 44.9 ± 3.6* | 78.9 ± 6.8* | 40.6 ± 3.4* |
| Adult rats (N = 8; balloon injury + 55 mg/kg of NCX-4016) | |||
| 1.83 ± 0.4 | 18.2 ± 1.8†‡ | 31.4 ± 2.3†‡ | 13.2 ± 0.5†‡ |
| Adult rats (N = 8; balloon injury + 30 mg/kg of ASA) | |||
| 1.84 ± 0.5 | 29.6 ± 2.5 | 53.1 ± 4.3 | 22.1 ± 2.1 |
| Old rats (N = 8; balloon injury + 55 mg/kg of NCX-4016) | |||
| 1.85 ± 0.4 | 24.1 ± 1.6§¶ | 37.2 ± 2.8§¶ | 15.1 ± 0.9§¶ |
| Old rats (N = 8; balloon injury + 30 mg/kg of ASA) | |||
| 1.86 ± 0.5 | 41.8 ± 4.5 | 73.2 ± 5.9 | 38.3 ± 3.5 |
Drugs or vehicle was administered 7 days before and 21 days after balloon injury. Data are expressed as the mean ± SEM.
, P < 0.05 vs. vehicle-treated control adult rats.
†, P < 0.01 vs. vehicle-treated control adult rats.
‡, P < 0.05 vs. adult rats treated with ASA.
§, P < 0.01 vs. vehicle-treated control old rats.
¶, P < 0.05 vs. old rats treated with ASA.
Pretreatment with NO-aspirin for 7 days before and 21 days after balloon injury significantly reduced the degree of restenosis in adult and elderly rats (Table 1). Restenosis after balloon dilation of carotid arteries is reflected as migration and proliferation of VSMCs. Neointimal proliferation was reduced by NCX-4016 but not ASA. Accordingly, the residual stenosis was significantly reduced in animals treated with NCX-4016. Double-label immunohistochemistry in serial carotid sections for VSMCs indicated that the number of VSMCs was reduced by NO-aspirin, but not ASA, at the lesion site (Table 1). These beneficial effects clearly demonstrate the efficacy of NO-aspirin on the development of restenosis in aged rats. Data obtained in this experimental setting with a typical NO donor agent, S-nitroso-N-acetylpenicillamine (500 μM) (44), showed only a 13 ± 4% reduction in proliferating VSMCs from old rats (n = 3, P = 0.342 vs. untreated controls).
Discussion
The reduction of restenosis in adult rats elicited by NCX-4016, but not ASA, was observed also in old rats. This phenomenon was the result of reduced VSMC proliferation and cell density indicating that this was likely caused by NO-mediated effects on VSMCs. Moreover, administration of NO-aspirin did not cause any gastric damage in either adult or old rats. The efficacy of NCX-4016 in aged rats was studied first through an estimate of plasma NOx content and then through the more specific measurements of bioactive forms ([HbFe(II)NO] and RS-NO) of NO. The rise in both of these markers unequivocally demonstrate that, after the pretreatment period, NO-aspirin enhanced the systemic and tissue release of bioactive NO in aged animals. Importantly, the oral administration of equimolar doses of ASA was found to reduce the endogenous production of NO, because a significant decrease in plasma NOx levels was observed.
VSMC proliferation is involved in the pathogenesis of restenosis after PTCA (45). NO inhibits VSMC growth in vitro and experimental neointimal hyperplasia in vivo, indicating a role for NO as a regulator of VSMC proliferation (10, 46–48). NO is also involved in the control of other pathophysiological responses including platelet function and inflammatory cells, vascular reactivity, and endothelial permeability (10, 46–48). The administration of l-arginine, the precursor to NO, reduces restenotic lesion formation in cholesterol-fed rabbits (27, 28), whereas l-NAME, a nonselective inhibitor of NO-synthase, stimulates neointimal hyperplasia (29). Moreover, chronic inhalation of NO may reduce neointimal thickening in rats (30). Thus, a large body of data indicates that NO-release or delivery could be useful in restenosis after PTCA. Indeed, reduction of neointimal proliferation observed in the present study seems to agree very well with the potent inhibitory properties of NCX-4016 on rat aortic SMC proliferation (49).
The therapeutic effects of NO-aspirin were obtained with a 1-week pretreatment prior to balloon injury following the same protocol that reduced restenosis in hypercholesterolemic mice (34). Although ASA-like drugs have little or no effect on restenosis in humans (20, 21), NO-releasing drugs have not been evaluated in human restenosis. Here, we showed that during treatment with NO-releasing-aspirin, NO molecules released from the drug enter into the physiological pathways involved in the transport and targeted delivery of endogenous NO in damaged arteries. The efficacy of NO-aspirin in reversing restenosis may be also the result of additional NO-mediated effects on vascular inflammation after PTCA. Our findings are in line with several studies where NO has been shown to serve as vasoprotector, including inhibition of platelet aggregation and leukocyte adherence to the site of injury (47, 50). Further studies at the molecular level are needed to better understand which are the main signaling pathways involved in the protective action of NCX-4016 and its long-term effects on vascular remodeling after balloon injury. In the present study, using S-nitroso-N-acetylpenicillamine, a typical NO donor, we observed a modest reduction of restenosis in aged rats indicating that transient administration of NO alone may be not sufficient to exert protective effects in aged rats. To develop vascular protection, a chronic intervention would be necessary to counterbalance NO deficiency in arteries from aged animals. S-nitrosoglutathione, an NO donor with preferential action on platelets, has been shown to inhibit platelet aggregation after PTCA in patients under ASA treatment without altering blood pressure (51). However, routine clinical management using pure NO donors could be difficult because of systemic effects on blood pressure.
The mechanisms underlying restenosis are further complicated by vascular aging (5–14). Decreased vasorelaxation caused by a decline in NO and endothelium-derived hyperpolarizing factor, as well as increased vasoconstriction mediated by cyclooxygenase products such as thromboxane A2, and concomitantly increased sensitivity of apoptosis, are likely to occur in aging (5–14, 52). More importantly, endothelium-dependent vasodilation declines with aging in humans and the inhibitory effect of NG-monomethyl-l-arginine (l-NMMA) on responses to acetylcholine decreases in parallel with advancing age, whereas vitamin C increases vasodilation to acetylcholine in only the oldest group (age > 60 years) (53). Moreover, in older hypertensive patients, vitamin C enhances endothelium-dependent vasodilation and restores the inhibitory effect of l-NMMA on responses to acetylcholine (53). Thus, in normotensive individuals, an earlier primary dysfunction of the NO pathway and a later production of oxidative stress cause age-related reduction in endothelium-dependent vasodilation; these alterations are similar but earlier in hypertensive patients compared with normotensive subjects (53). Furthermore, enhanced oxidative stress plays a critical role in the deleterious effect of aging on the endothelium through acceleration of NO breakdown by reactive oxygen species (8–14). Increased oxidative stress also occurs during vascular response to PTCA (54, 55). Thus, increased lipid peroxidation and oxidative stress with aging (56) together with vascular changes related to NO bioavailability may contribute to the higher severity of restenosis in elderly patients. In this regard, NCX-4016 also exhibited antioxidant properties during myocardial ischemia-reperfusion injury, in which a large amount of oxygen radicals are formed during reperfusion (57). It was also demonstrated that reduced expression of thymosin β-10 may contribute to senescence of vascular endothelium by reducing endothelial cell plasticity (58). Moreover, there is an age-related increase in c-fos activity that contributes to augmented cyclin A expression and VSMC proliferation in old rabbits (59). These signaling pathways can be also influenced by age-related NO deficiency that was restored by NCX-4016 in plasma and tissue.
Thus, we show that NCX-4016, but not ASA, reduces restenosis in aged rats. Although experimental laboratory animal models may not entirely represent the complexity of the human disease (60), from a clinical point of view, these results could be extended to the common clinical conditions of both primary and elective PTCA performed in elderly patients and in patients with gastrointestinal restrictions to the use of ASA (as well as other antiplatelet drugs) (22, 25). Indeed, the benefits of primary PTCA are more impressive for the aging patient (61). The survival gain and reduction in intracranial hemorrhage may combine to magnify the advantages of performing PTCA on aged patients. Emerging evidence concerning the aging population validates continued examination of this invasive reperfusion approach (61). Thus, the safety of NO-aspirin at the doses tested may constitute a favorable pharmacological and clinical safety profile in elderly patients in the presence of more comorbid conditions.
Acknowledgments
This study was supported by National Institutes of Health Grants HL-58433, HL-63282, and HL-66999, and by the Mayo Foundation.
Abbreviations
- PTCA
percutaneous transluminal coronary angioplasty
- NCX-4016
nitric oxide releasing-aspirin derivative
- ASA
aspirin
- VSMC
vascular smooth muscle cell
- NOx
plasma nitrite and nitrate
- RS-NO
S-nitrosothiols
- [HbFe(II)NO]
nitrosylhemoglobin
References
- 1.Bilato C, Crow M T. Aging Clin Exp Res. 1996;8:221–234. doi: 10.1007/BF03339572. [DOI] [PubMed] [Google Scholar]
- 2.Lundblad V, Szostack J W. Cell. 1989;57:633–643. doi: 10.1016/0092-8674(89)90132-3. [DOI] [PubMed] [Google Scholar]
- 3.Harley C B, Futcher A B, Geider C W. Nature (London) 1990;345:458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 4.Chang E, Harley C B. Proc Natl Acad Sci USA. 1995;92:11190–11124. doi: 10.1073/pnas.92.24.11190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stemerman M B, Weinstein R, Rowe J W, Maciag T, Fuhro R, Gardner R. Proc Natl Acad Sci USA. 1982;79:3863–3866. doi: 10.1073/pnas.79.12.3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwartz S M, de Blois D, O'Brien E R M. Circ Res. 1995;77:445–465. doi: 10.1161/01.res.77.3.445. [DOI] [PubMed] [Google Scholar]
- 7.Hariri R J, Hajjar D P, Colett D, Alonso D R, Weksler M E, Rabellino E. Am J Pathol. 1988;131:132–136. [PMC free article] [PubMed] [Google Scholar]
- 8.Hariri R J, Alonso D R, Hajjar D P, Coletti P, Wekser M E. J Exp Med. 1986;164:1171–1178. doi: 10.1084/jem.164.4.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luscher T F, Tanner F C. Am J Hypertens. 1993;6:283S–293S. doi: 10.1093/ajh/6.7.283s. [DOI] [PubMed] [Google Scholar]
- 10.Ignarro L J, Cirino G, Casini A, Napoli C. J Cardiovasc Pharmacol. 1999;34:879–886. doi: 10.1097/00005344-199912000-00016. [DOI] [PubMed] [Google Scholar]
- 11.Matz R L, Schott C, Stoclet J C, Andriantsitohaina R. Physiol Rev. 2000;49:11–18. [PubMed] [Google Scholar]
- 12.Toprakci M, Ozmen D, Mutaf I, Turgan N, Parildar Z, Habif S, Guner I, Bayindir O. Int J Clin Lab Res. 2000;30:3–5. doi: 10.1007/BF02874163. [DOI] [PubMed] [Google Scholar]
- 13.Imaoka Y, Osanai T, Kamada T, Mio Y, Satoh K, Okumura K. J Cardiovasc Pharmacol. 1999;33:756–761. doi: 10.1097/00005344-199905000-00012. [DOI] [PubMed] [Google Scholar]
- 14.Van der Loo B, Labugger R, Skepper J N, Bachschmid M, Kilo J, Powell J, Palacios-Callender M, Erusalimsky J D, Quaschning T, Malinski T, et al. J Exp Med. 2000;192:1731–1744. doi: 10.1084/jem.192.12.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weaver W D, Litwin P E, Martin J S, Kudenchuk P J, Maynard C, Eisenberg M S, Ho M T, Cobb L A, Kennedy J W, Wirkus M S. J Am Coll Cardiol. 1991;18:657–662. doi: 10.1016/0735-1097(91)90784-7. [DOI] [PubMed] [Google Scholar]
- 16.Napoli C, Pinto A, Cirino G. Pharmacol Ther. 2000;88:311–331. doi: 10.1016/s0163-7258(00)00093-0. [DOI] [PubMed] [Google Scholar]
- 17.Gurwitz J H, Goldberg R J, Chen Z, Gore J M, Alpert J S. Arch Intern Med. 1994;154:2202–2208. [PubMed] [Google Scholar]
- 18.Holmes D R, White H D, Pieper K S, Ellis S G, Califf R M, Topol E J. J Am Coll Cardiol. 1999;33:412–419. doi: 10.1016/s0735-1097(98)00579-8. [DOI] [PubMed] [Google Scholar]
- 19.White H D, Barbash G I, Califf R M, Simes R J, Granger C B, Weaver W D, Kleiman N S, Aylward P E, Gore J M, Vahanian A, et al. Circulation. 1996;94:1826–1833. doi: 10.1161/01.cir.94.8.1826. [DOI] [PubMed] [Google Scholar]
- 20.Folts J D, Schafer A I, Loscalzo J, Willerson J T, Muller J E. J Am Coll Cardiol. 1999;33:295–303. doi: 10.1016/s0735-1097(98)00601-9. [DOI] [PubMed] [Google Scholar]
- 21.Lefkovits J, Topol E J. Prog Cardiovasc Dis. 1997;40:141–158. doi: 10.1016/s0033-0620(97)80006-0. [DOI] [PubMed] [Google Scholar]
- 22.Roderick P J, Wilkes H C, Meade P W. Br J Clin Pharmacol. 1993;35:219–226. doi: 10.1111/j.1365-2125.1993.tb05689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wallace J L, McNight W, Del Soldato P, Baydoun A, Cirino G. J Clin Invest. 1995;96:2711–2718. doi: 10.1172/JCI118338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fiorucci S, Antonelli E, Santucci L, Morelli O, Miglietti M, Federici B, Mannucci R, Del Soldato P, Morelli A. Gastroenterology. 1999;116:1089–1106. doi: 10.1016/s0016-5085(99)70012-0. [DOI] [PubMed] [Google Scholar]
- 25.Awtry E H, Loscalzo J. Circulation. 2000;101:1206–1218. doi: 10.1161/01.cir.101.10.1206. [DOI] [PubMed] [Google Scholar]
- 26.Myers P R, Webel R, Thondapu V, Xu X P, Amann J, Tanner M A, Jenkins J S, Pollock J, Laughlin M H. Int J Cardiol. 1996;55:183–191. doi: 10.1016/0167-5273(96)02684-8. [DOI] [PubMed] [Google Scholar]
- 27.Schwarzacher S P, Lim T T, Wang B, Kernoff R S, Niebauer J, Cooke J P, Yeung A C L. Circulation. 1997;95:1863–1869. doi: 10.1161/01.cir.95.7.1863. [DOI] [PubMed] [Google Scholar]
- 28.Niebauer J, Schwarzacher S P, Hayase M, Wang B, Kernoff R S, Cooke J P, Yeung A C. Circulation. 1999;100:1830–1835. doi: 10.1161/01.cir.100.17.1830. [DOI] [PubMed] [Google Scholar]
- 29.Le Tourneau T, Van Belle E, Corseaux D, Vallet B, Lebuffe G, Dupuis B, Lablanche J M, McFadden E, Bauters C, Bertrand M E. J Am Coll Cardiol. 1999;33:876–882. doi: 10.1016/s0735-1097(98)00621-4. [DOI] [PubMed] [Google Scholar]
- 30.Lee J S, Adrie C, Jacob H J, Roberts J D, Jr, Zapol W M, Bloch K D. Circ Res. 1996;78:337–342. doi: 10.1161/01.res.78.2.337. [DOI] [PubMed] [Google Scholar]
- 31.Sato J, Nair K, Hiddinga J, Eberhardt N L, Fitzpatrick L A, Katusic Z S, O'Brien T. Cardiovasc Res. 2000;47:697–706. doi: 10.1016/s0008-6363(00)00137-1. [DOI] [PubMed] [Google Scholar]
- 32.Del Soldato P, Sorrentino R, Pinto A. Trends Pharmacol Sci. 1999;20:319–323. doi: 10.1016/s0165-6147(99)01353-x. [DOI] [PubMed] [Google Scholar]
- 33.Wallace J L, Muscara M N, McKnight W, Dicay M, Del Soldato P, Cirino G. Thromb Res. 1999;93:43–50. doi: 10.1016/s0049-3848(98)00134-0. [DOI] [PubMed] [Google Scholar]
- 34.Napoli C, Cirino G, Del Soldato P, Sorrentino R, Sica V, Condorelli M, Pinto A, Ignarro L J. Proc Natl Acad Sci USA. 2001;98:2860–2864. doi: 10.1073/pnas.041602898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Napoli C, Witztum J L, de Nigris F, Palumbo G, D'Armiento F P, Palinski W. Circulation. 1999;99:2003–2010. doi: 10.1161/01.cir.99.15.2003. [DOI] [PubMed] [Google Scholar]
- 36.Napoli C, Salomone S, Godfraind T, Palinski W, Capuzzi D M, Palumbo G, D'Armiento F P, Donzelli R, de Nigris F, Capizzi R L, et al. Stroke. 1999;30:1907–1915. doi: 10.1161/01.str.30.9.1907. [DOI] [PubMed] [Google Scholar]
- 37.Condorelli G L, Aycock J, Frati G, Napoli C. FASEB J. 2001;15:2162–2170. doi: 10.1096/fj.01-0032com. [DOI] [PubMed] [Google Scholar]
- 38.Fang K, Ragsdale N V, Carey R M, McDonald T, Gaston B. Biochem Biophys Res Commun. 1998;252:535–540. doi: 10.1006/bbrc.1998.9688. [DOI] [PubMed] [Google Scholar]
- 39.Carini M, Aldini G, Furlanetto S, Stefani R, Maffei R. J Pharm Biomed Anal. 2001;24:517–526. doi: 10.1016/s0731-7085(00)00431-3. [DOI] [PubMed] [Google Scholar]
- 40.Gow A J, Luchsinger B P, Pawloski J R, Singel D J, Stamler J S. Proc Natl Acad Sci USA. 1999;96:9027–9032. doi: 10.1073/pnas.96.16.9027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kosaka H. Biochim Biophys Acta. 1999;141:370–377. doi: 10.1016/s0005-2728(99)00026-2. [DOI] [PubMed] [Google Scholar]
- 42.Pawloski J R, Douglas T H, Stamler J S. Nature (London) 2001;409:622–626. doi: 10.1038/35054560. [DOI] [PubMed] [Google Scholar]
- 43.Gross S S. Nature (London) 2001;409:577–578. doi: 10.1038/35054661. [DOI] [PubMed] [Google Scholar]
- 44.Ferrero R, Rodriguez-Pascual F, Miras-Portugal M T, Torres M. Br J Pharmacol. 1999;127:779–787. doi: 10.1038/sj.bjp.0702607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu M W, Roubin G S, King S B. Circulation. 1989;79:1374–1387. doi: 10.1161/01.cir.79.6.1374. [DOI] [PubMed] [Google Scholar]
- 46.Napoli C, Ignarro L J. Nitric Oxide. 2001;5:88–97. doi: 10.1006/niox.2001.0337. [DOI] [PubMed] [Google Scholar]
- 47.George S E. Coron Artery Dis. 1999;10:295–300. doi: 10.1097/00019501-199907000-00004. [DOI] [PubMed] [Google Scholar]
- 48.Sarkar R, Webb R C. J Vasc Res. 1998;35:135–142. doi: 10.1159/000025576. [DOI] [PubMed] [Google Scholar]
- 49.Ignarro L J, Buga G M, Wei L H, Bauer P M, Wu G, Del Soldato P. Proc Natl Acad Sci USA. 2001;98:4202–4228. doi: 10.1073/pnas.071054698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kibbe M, Billiar T, Tzeng E. Cardiovasc Res. 1999;43:650–657. doi: 10.1016/s0008-6363(99)00130-3. [DOI] [PubMed] [Google Scholar]
- 51.Langford E J, Brown A S, Wainwright R J, de Belder A J, Thomas M R, Smith R E, Radomski M W, Martin J F, Moncada S. Lancet. 1994;344:1458–1460. doi: 10.1016/s0140-6736(94)90287-9. [DOI] [PubMed] [Google Scholar]
- 52.Hoffmann J, Haendeler J, Aicher A, Rossig L, Vasa M, Zeiher A M, Dimmeler S. Circ Res. 2001;89:709–715. doi: 10.1161/hh2001.097796. [DOI] [PubMed] [Google Scholar]
- 53.Taddei S, Virdis A, Ghiadoni L, Salvetti G, Bernini G, Magagna A, Salvetti A. Hypertension. 2001;38:274–279. doi: 10.1161/01.hyp.38.2.274. [DOI] [PubMed] [Google Scholar]
- 54.Iuliano L, Pratico D, Greco C, Mangieri E, Scibilia G, FitzGerald G A, Violi F. J Am Coll Cardiol. 2001;37:76–80. doi: 10.1016/s0735-1097(00)01040-8. [DOI] [PubMed] [Google Scholar]
- 55.Azevedo L C, Pedro M A, Souza L C, de Souza H P, Janiszewski M, da Luz P L, Laurindo F R. Cardiovasc Res. 2000;47:436–445. doi: 10.1016/s0008-6363(00)00091-2. [DOI] [PubMed] [Google Scholar]
- 56.Napoli C, Abete P, Corso G, Malorni A, Ambrosio G, Cacciatore F, Rengo F, Palumbo G. Coron Artery Dis. 1997;8:129–136. doi: 10.1097/00019501-199703000-00002. [DOI] [PubMed] [Google Scholar]
- 57.Rossoni G, Manfredi B, Colonna V D, Bernareggi M, Berti F. J Pharmacol Exp Ther. 2001;297:380–387. [PubMed] [Google Scholar]
- 58.Vasile E, Tomita Y, Brown L F, Kocher O, Dvorak H F. FASEB J. 2001;15:458–466. doi: 10.1096/fj.00-0051com. [DOI] [PubMed] [Google Scholar]
- 59.Rivard A, Principe N, Andres V. Cardiovasc Res. 2000;45:1026–1034. doi: 10.1016/s0008-6363(99)00385-5. [DOI] [PubMed] [Google Scholar]
- 60.Johnson G J, Griggs T R, Badimon L. Thromb Haemostasis. 1999;81:835–843. [PubMed] [Google Scholar]
- 61.Lane G E, Holmes D R., Jr Coron Artery Dis. 2000;11:305–313. doi: 10.1097/00019501-200006000-00003. [DOI] [PubMed] [Google Scholar]