Abstract
Relaxin, a peptide hormone secreted by the corpus luteum during pregnancy, exerts actions on reproductive tissues such as the pubic symphysis, uterus, and cervix. It may also influence body fluid balance by actions on the brain to stimulate thirst and vasopressin secretion. We mapped the sites in the brain that are activated by i.v. infusion of a dipsogenic dose of relaxin (25 μg/h) by immunohistochemically detecting Fos expression. Relaxin administration resulted in increased Fos expression in the subfornical organ (SFO), organum vasculosum of the lamina terminalis (OVLT), median preoptic nucleus, and magnocellular neurons in the supraoptic and paraventricular nuclei. Ablation of the SFO abolished relaxin-induced water drinking, but did not prevent increased Fos expression in the OVLT, supraoptic or paraventricular nuclei. Although ablation of the OVLT did not inhibit relaxin-induced drinking, it did cause a large reduction in Fos expression in the supraoptic nucleus and posterior magnocellular subdivision of the paraventricular nucleus. In vitro single-unit recording of electrical activity of neurons in isolated slices of the SFO showed that relaxin (10−7 M) added to the perfusion medium caused marked and prolonged increase in neuronal activity. Most of these neurons also responded to 10−7 M angiotensin II. The data indicate that blood-borne relaxin can directly stimulate neurons in the SFO to initiate water drinking. It is likely that circulating relaxin also stimulates neurons in the OVLT that influence vasopressin secretion. These two circumventricular organs that lack a blood–brain barrier may have regulatory influences on fluid balance during pregnancy in rats.
Relaxin is a peptide hormone secreted by the corpus luteum of the ovary during pregnancy. Relaxin acts on reproductive tissues such as the pubic symphysis, uterus, and cervix (1, 2), but it may also influence the brain (3). Evidence of this influence is presented in reports that the intracerebroventricular (i.c.v.) (1) injection of relaxin results in stimulation of oxytocin and vasopressin secretion, water drinking, and a pressor response (3–8). i.c.v. administration of relaxin also stimulates increased expression of c-fos in groups of neurons in the supraoptic nucleus (SON) and hypothalamic paraventricular nucleus (PVN), as well as in the subfornical organ (SFO), median preoptic nucleus (MnPO), and organum vasculosum of the lamina terminalis (OVLT) (9). Circulating relaxin probably has endocrine actions on the brain, because high-affinity-binding sites for relaxin are present in the SFO and OVLT of the rat (10), two brain regions that are accessible to circulating relaxin because they lack a blood–brain barrier (11). Also, i.v. infusion of relaxin causes vasopressin secretion and water drinking in the rat, effects that may be mediated through brain angiotensinergic mechanisms (12, 13). Evidence that relaxin [together with other hormones such as angiotensin II (Ang II) and vasopressin] plays a physiological role in regulating body fluid homeostasis during pregnancy is that passive immunization with centrally administered antibodies raised against rat relaxin reduces water drinking in pregnant rats (14), and that chronic i.v. infusion of relaxin in ovariectomized rats results in hyponatremia, mimicking the hyponatremia that occurs in pregnant animals and humans (15). We have observed that circulating Ang II and relaxin have synergistic effects on water drinking in the rat (13), and the dipsogenic effect of circulating Ang II is known to be mediated through its receptors in the SFO (16).
Whereas it is known that intravenously infused relaxin induces water drinking, the central site at which circulating relaxin acts to stimulate this response is unknown. Because the SFO and OVLT exhibit relaxin-binding sites (10), are accessible to blood-borne relaxin (9, 11), and mediate water drinking in response to other stimuli (9, 11, 16), a major aim of our work was to determine whether either one or both of these circumventricular organs (CVO) mediate relaxin-induced drinking. At present, no electrophysiological investigation of the actions of relaxin on any brain region has been reported, so another aim was to study the actions of relaxin on the electrical discharges of neurons in the SFO and the relationship of these neurons to known angiotensin-sensitive neurons therein. In addition, we further characterized the actions of blood-borne relaxin on the brain by immunohistochemically identifying the full complement of neurons in the lamina terminalis and hypothalamus that respond to intravenously infused relaxin by increasing Fos production, and we studied the effects of ablation of the SFO and OVLT on these responses and on relaxin-induced drinking.
Materials and Methods
Animals.
Male (n = 86) and female (n = 5) rats (Sprague–Dawley strain, 280–400 g body weight) were housed in individual cages and had ad libitum access to pelleted food and water. Rats that were given i.v. infusions of relaxin or isotonic saline were prepared surgically 3 days before infusions. A polyethylene cannula was inserted into the femoral vein while rats were anesthetized with i.p equithesin (3 ml/kg). The heparinized cannula was brought s.c. to the surface at the back of the animal's neck. All experiments were approved by the Howard Florey Institute's Animal Ethics Committee, which adheres to the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes.
Immunohistochemical Detection of Fos in Rat Brain Resulting from i.v. Relaxin.
Polyethylene tubing filled with either relaxin (synthetic human gene 2 in all experiments except for seven rats, infused with synthetic rat relaxin) or 0.15 M NaCl was connected to the venous cannula and infusions of test substance made at 1 ml/h for 1 h into the femoral vein. Six rats (five male and one female) received an infusion of 0.15 M NaCl solution (vehicle), four male rats were given human relaxin at 10 μg/h for 1 h, five male rats were given human relaxin at 25 μg/h for 1 h, two rats were infused simultaneously with the separated A (9.9 μg/h) and B (15.1 μg/h) chains of human relaxin, whereas three male and four female rats were infused with synthetic rat relaxin at 28 μg/h. Rats were not permitted access to water during these infusions. Relaxins were synthesized by described methods (17). Rats were deeply anesthetized (sodium thiopentone, 100 ml/kg i.v.) 30 min after the infusions finished, and the rats were perfused transcardially with 60 ml of 0.15 M NaCl followed by 250 ml of 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.2). Their brains were removed and immersed for 1–2 h in 4% paraformaldehyde/phosphate buffer, then placed overnight in 20% sucrose/phosphate buffer. The brains were serially sectioned at 40 μm in the coronal plane with a freezing microtome. After exposure of the sections to 10% horse serum for 1 h, the free-floating sections were incubated overnight at 4°C with antiserum (diluted 1:2000–5000 depending on the batch) raised in rabbits against a synthetic residue of human Fos. The primary antibodies were then reacted with biotin-labeled anti-rabbit immunoglobulins and finally with an avidin–biotin–peroxidase complex (Vector Laboratories). The peroxidase was detected by using 3,3′-diaminobenzidine hydrochloride as chromogen intensified with nickel ammonium sulfate. Some sections through the SON and PVN were then immersed for 24 h further in rabbit antiserum directed against vasopressin, which was subsequently localized according to the procedures outlined above without the nickel enhancement, yielding a brown reaction product. After mounting on glass slides, the sections were counterstained with cresyl violet. Cell nuclei that exhibited Fos-like immunoreactivity (Fos-LI) in sections of the lamina terminalis and hypothalamus were counted with a graticule in the microscope's eyepiece to prevent repeat counts.
Ablation of the SFO or OVLT and Relaxin Responses.
Rats were anesthetized with sodium pentobarbitone (60 mg/kg, i.p.) and placed in a stereotaxic frame with the top of the skull held horizontal. After making a scalp incision, a burr-hole was drilled in the midline and an electrode (epoxylite-coated entomological pin, size 00, 38 mm long, with noninsulated tip) was lowered perpendicularly either into or near the SFO, with coordinates 0–0.3 mm posterior to bregma, and 4.9–5.0 mm ventral to the dura mater in the midline of the brain (18), or OVLT (midline coordinates 0.1 posterior to bregma, and 8.4 mm ventral to the dura mater). The animals received electrolytic ablation at either three different insertion points along the anterior–posterior axis relative to bregma for the SFO or once for the OVLT by passing an anodal direct current (1 mA, 10- to 20-sec duration between the electrode tip and an electrode in the skin) at each insertion point. Animals with sham lesions received the same procedure without any current being applied (n = 4). The electrode was then removed, the wound sutured, and the animal allowed to recover. Daily water and food intake were monitored and rats were allowed 1–2 weeks recovery before they were prepared with a cannula in the femoral vein as described above. After 2–3 days, they were given an i.v. infusion of human relaxin at 25 μg/h for 1 h, and the water intake during this hour and the subsequent 30 min was measured at 15-min intervals. Rats were anesthetized with sodium pentobarbitone 30 min after ending the infusions and their brains perfused with saline and fixative, and processed immunohistochemically to detect Fos as described above. In addition, normal rats were infused with either relaxin (25 μg/h, n = 12) or 0.15 M NaCl (vehicle, n = 6), their water intakes measured, and brains subjected to the same immunohistochemical procedures. The brain sections were examined by light microscopy and the lesions classified into groups. Rats with lesions in or near the SFO or OVLT were classified in six groups depending on how much of the SFO was ablated: SFOX group (90–100% SFO ablation, n = 5); SFO>50X (50–90% SFO ablation, n = 5); SFO<50X (10–50% SFO ablated, n = 5), SFOML (lesion missed SFO leaving it intact, but adjacent tissue ablated, n = 5), OVLTX (>90% of OVLT ablated, n = 5), OVLTML (lesion missed OVLT leaving it intact but adjacent tissue ablated, n = 6). The number of rats with a partial OVLT lesion was insufficient to make a group for analysis. The number of cells exhibiting Fos-LI in the SFO and OVLT was counted by image analysis (MCID, Imaging Research, St Catherine's, ON, Canada), whereas those in the ventral MnPO, SON (at the level just caudal to the SFO), and the posterior magnocellular subdivision of the PVN (PVNm) were counted by a different observer who used a microscope with a graticule in the eyepiece. This observer made all counts of these regions to ensure consistency of nuclear boundaries, and was unaware of the classification of the type of lesion in each rat brain to prevent bias in the counts. Statistical analysis of Fos-LI counts for each brain site was made by one-way analysis of variance followed by Dunnett's test for comparison of each group with the nonlesioned group infused with relaxin.
Electrophysiological Studies.
Male rats were decapitated, and their brains were removed and superfused with ice-cold artificial cerebrospinal fluid (aCSF) having the following composition (in millimolar): NaCl, 124; KCl, 5; NaH2PO4, 1.2; MgSO4, 1.3; CaCl2, 1.2; NaHCO3, 26; glucose, 10; and equilibrated with 95% O2 and 5% CO2 yielding a pH of 7.4 and osmolality of 290 mosmol/kg. The brain was trimmed to a square block containing the entire hypothalamus, from which a coronal section was cut by hand at the level of the anterior commissure. A slice containing the entire SFO was cut by hand and preincubated in aCSF at 35°C for 2 h before recording started. The slice was then transferred to a chamber perfused with prewarmed aCSF at 1.6 ml/min. Temperature was held constant at 37°C by a Peltier element. Extracellular recordings were made from SFO neurons by using glass-coated platinum-iridium electrodes as described (19). The SFO could be identified easily by its protrusion into the third ventricle and the lateral blood vessels lining the organ on both sides. Ang II or relaxin was added to the aCSF perfusate shortly before their application. After a stable recording from a single spontaneously active neuron was obtained, its responsiveness to Ang II was tested by switching to a perfusion solution containing Ang II at 10−7 M. The recorded action potentials were amplified and displayed on a storage oscilloscope (Gould, Erlensee, Germany) and, after passing a window discriminator (World Precision Instruments, Sarasota, FL), were analyzed with custom-made software (SPIKE 2, Cambridge Electronic Design, Cambridge, U.K.) on a computer. The concentration of Ang II (10−7 M) was chosen according to published experiments (19), which showed that this concentration produced clearly visible responses with minimum desensitization. From the continuously recorded rate meter counts, the average discharge rate of each neuron was evaluated for 60 sec before the stimulus. This value, referred to as the control, was subtracted from all subsequent changes in firing rate, and the results were expressed as percent change from control. If the averaged change of discharge rate during the entire response time was larger than 20%, the neuron was considered sensitive to the applied substance. The Ang II-containing solution was perfused through the chamber for 3–6 min before the perfusion reverted to aCSF. After a further 10–15 min, a solution of relaxin was perfused through the chamber for 4–8 min and the activity of the same neuron recorded as described above.
Results
Effects of Relaxin on Fos-LI.
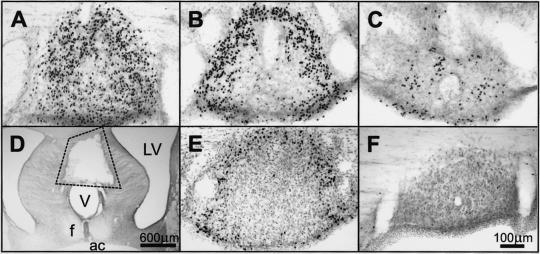
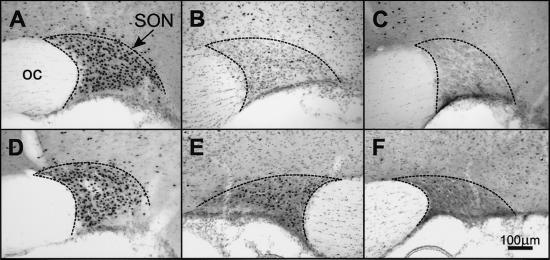
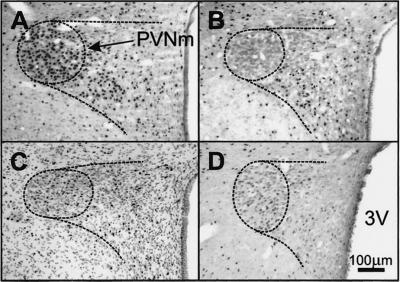
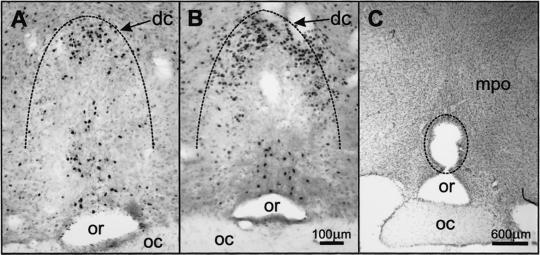
Intravenous infusion of human relaxin at 25 μg/h in male rats caused increased Fos-LI in the lamina terminalis, and in the SON and PVNm (counts per section: SFO, 340 ± 63; MnPO, 23 ± 2; OVLT, 145 ± 13; SON 89 ± 11; PVNm 64 ± 6), which did not occur in animals infused with isotonic saline (counts per section: SFO, 3 ± 1; MnPO, 10 ± 3; and OVLT, 14 ± 5; SON 3 ± 1; PVNm 3 ± 1; see Figs. 1F, 3C, and 4B). The Fos-LI resulting from i.v. infusion of rat relaxin was similar to that observed with human relaxin and was similar in males and females (data not shown). In the lamina terminalis, whereas many cells exhibited increased Fos-LI in their two CVO, the SFO (Fig. 1 A–C) and the OVLT (Fig. 2A), these cells were not uniformly distributed within these two regions. In the SFO, many cells exhibiting Fos-LI were observed in its most rostral (Fig. 1A), dorsal and lateral parts (Fig. 1B), but they were virtually absent in its inner core (Fig. 1B) and sparse in the most caudal region (Fig. 1C). In coronal sections, the distribution of cells exhibiting Fos-LI in the SFO gave the appearance of an annulus in the periphery of the organ (Fig. 1B). In the OVLT, Fos-LI was observed mainly in cells in its dorsal cap region (Fig. 2A), and in a small caudal, ventral periventricular group (Fig. 2A). Only a few Fos-positive cells were observed in its central and lateral zones. We observed a significant increase in the number of Fos-positive neurons in the other component of the lamina terminalis, the MnPO in response to i.v. relaxin, although they were far less numerous than in the SFO or OVLT. In the hypothalamus, extensive Fos-LI was observed in cells of the SON (Fig. 3A) and posterior magnocellular subdivision of the PVN (Fig. 4A), many of which also exhibited vasopressin immunoreactivity. Because considerable Fos-LI existed in several parvocellular subgroups in the PVN in rats infused with isotonic saline (Fig. 4B), we did not assess these in relaxin-treated animals. No increase in Fos-LI was observed in the area postrema, the other sensory CVO of the rat brain.
Figure 1.
(A–C) Fos-LI (black dots) in coronal sections of the SFO of a rat infused i.v. with relaxin (25 μg/h). (A) Rostral SFO. (B) Main body of the SFO. (C) Caudal SFO. (D) A typical lesion in the SFO (indicated). (E) Relaxin-induced Fos-LI in the SFO of a rat with the OVLT ablated. (F) SFO of a rat infused with vehicle. f, fornix; LV, lateral ventricle; V, third ventricle.
Figure 3.
Fos-LI (black dots) in coronal sections of the SON of rats infused with relaxin (25 μg/h, i.v.). (A) Normal rat. (B) Rat in which the OVLT had been totally ablated. (C) Rat infused with vehicle. (D) Rat with the SFO ablated. (E and F) The supraoptic nuclei from a rat with a unilateral right-sided lesion in the tissue lateral to the OVLT showing more Fos-LI in the left (E) than right (F) SON. Boundaries of the SON are indicated.
Figure 4.
Fos-LI (black dots) in coronal sections of the PVNm of rats infused with relaxin (25 μg/h, i.v.). (A) Normal rat. (B) Rat infused with vehicle. (C) Rat with the SFO ablated infused with relaxin (25 μg/h). (D) Rat in which the OVLT had been totally ablated. PVNm is enclosed by the interrupted line. 3V, third ventricle.
Figure 2.
(A) Fos-LI (black dots) in coronal sections of the OVLT of a rat infused with relaxin (25 μg/h, i.v.); (B) relaxin-induced Fos-LI in the OVLT of a rat that had the SFO totally ablated; (C) a typical lesion (indicated) in the OVLT. dc, dorsal cap of OVLT; mpo, medial preoptic region; oc, optic chiasm; or, optic recess of the third ventricle. The dorsal boundary of the OVLT is indicated by the interrupted line.
Very few neurons exhibited Fos-LI in the lamina terminalis, SON, or PVNm of rats infused with the reduced A and B chains of relaxin. In rats infused with human relaxin at 10 μg/h (i.v.), Fos-LI in the SFO, OVLT, and SON seemed to be greater than in control rats; however; the density of the immunoreactivity was quite pale and much less than those infused with relaxin at 25 μg/h, making it difficult to count reliably the cell nuclei exhibiting Fos-LI.
Effects of Ablation of the SFO or OVLT on Relaxin-Stimulated Water Drinking and Fos-LI.
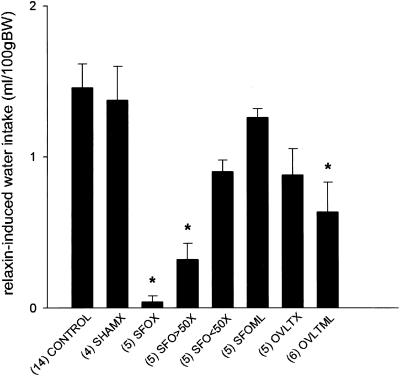
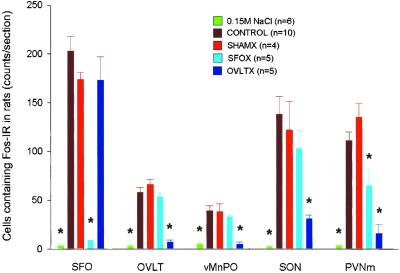
In nonlesioned rats, i.v. infusion of relaxin at 25 μg/h for 1 h caused water drinking within 10–20 min of the commencement of infusion, whereas i.v. infusion of isotonic saline was ineffective. Water intake in response to i.v. relaxin was almost abolished in the SFOX group and significantly inhibited in the group with 50–90% of the SFO ablated, whereas ablation of less than 50% of the SFO, or tissue adjacent to the SFO, did not significantly reduce relaxin-induced drinking (Fig. 5). Representative lesions of the SFO and OVLT are shown in Figs. 1D and 2C, respectively. Ablation of the OVLT, missed SFO lesions, or sham lesions, had little effect on relaxin-induced drinking (Fig. 5). In rats in which the SFO had been totally ablated, i.v. infusion of relaxin still resulted in Fos expression in the OVLT (Fig. 2B), SON (Fig. 3D), and PVN (Fig. 4C), although it was significantly reduced in the PVNm compared with nonlesioned rats (Fig. 6). Expression of Fos in response to i.v. relaxin in neurons in the MnPO (Fig. 6), SON (Figs. 3B and 6), and PVNm (Figs. 4D and 6), SFO was considerably less in rats in which the OVLT had been ablated than in control or sham-lesioned rats infused with relaxin. Considerable Fos-LI was still observed in the SFO of OVLT-lesioned rats infused with relaxin (Figs. 1E and 6). In regard to the SFO lesion groups (Fig. 1D), the dorsal part of the MnPO often incurred some damage, but it never extended below the level of the anterior commissure, so that the anteroventral third ventricle (AV3V) region always remained intact. Only minor damage to the MnPO immediately adjacent to the OVLT was observed in the OVLTX group, but it was damaged in three rats from the OVLTML group. In three rats that incurred unilateral damage to tissue immediately lateral to the OVLT, it was noticeable that Fos-LI was considerably less (<10%) in the SON ipsilateral to the lesion (Fig. 3F) than in the contralateral SON (Fig. 3E).
Figure 5.
Water intake during 90 min after the start of an i.v. infusion of human relaxin at 25 μg/h for 1 h in normal rats or rats with ablation of the SFO, OVLT, parts of the SFO, or tissue adjacent to these regions, or sham lesions. Mean and SEM are shown, and number of animals in each group are given in parentheses. Control, normal rats; SHAMX, sham lesions; SFOX, >90% of SFO ablated; SFO>50×, 50–90% of SFO ablated; SFO<50×, <50% of SFO ablated; SFOML, lesion missed SFO and damaged adjacent tissue; OVLTX, >90% of OVLT ablated; OVLTMX, lesion missed OVLT and ablated tissue in the adjacent regions. * indicates a significant difference (P < 0.05, Dunnett's test) from the control group infused with relaxin.
Figure 6.
Number of cells showing Fos-LI (counts per section) in SFO, OVLT, vMnPO, PVNm, and SON in normal rats (CONTROL), and rats with brain lesions (SFO ablated, SFOX; OVLT ablated, OVLTX; sham lesions, SHAMX) infused with relaxin (25 μg/h, i.v.). Normal rats infused with isotonic 0.15 M NaCl are also shown. * indicates a significant difference (P < 0.05, Dunnett's test) from the control group infused with relaxin.
In Vitro Electrophysiological Recordings.
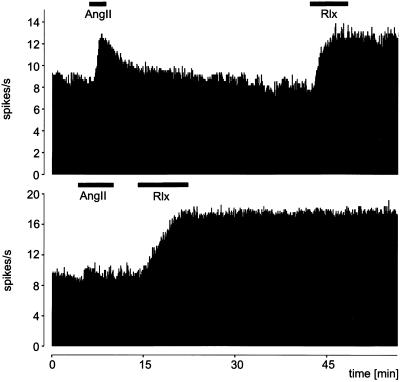
Data were obtained from 14 SFOs. Recordings were made from spontaneously active neurons in the periphery of the SFO. By using a dissecting microscope, it was possible to lower accurately a recording electrode into this region and test spontaneously active neurons for responsiveness to Ang II added to the perfusion medium. As expected, several neurons showed a rapid and marked increase in firing frequency when tested with Ang II (Fig. 7). On washing out the Ang II from the organ bath, the activity subsided within 5 min. Of 12 Ang II-responsive neurons found, all exhibited large and prolonged increases in firing frequency with the introduction of relaxin (Fig. 7). Two neurons were not responsive to Ang II, but increased their firing rate in response to relaxin (Fig. 7). Unlike the rapidly reversible response obtained with Ang II, the increase in activity of all 14 relaxin-responsive neurons continued despite the continuous perfusion of relaxin-free aCSF for many minutes which would have washed any residual relaxin from the tissue chamber. Because the response to relaxin usually lasted more than 45 min, further tests were not made.
Figure 7.
Electrical activity (spikes per second) recorded from two neurons in slices of the rat SFO in vitro. Extracellular recordings were made and Ang II and relaxin (Rlx) perfused into the organ bath at the times indicated by the black bar. Upper shows a neuron that was sensitive to both Ang II and relaxin, and Lower shows a neuron that was sensitive to relaxin but not Ang II.
The 14 neurons responsive to relaxin had a spontaneous frequency of 5.8 ± 0.7 Hz. This frequency increased by 3.6 ± 0.5 Hz in response to 10−7 M relaxin. The latency of the response was 121 ± 19 sec. The duration was >1,434 ± 19 sec, with only two neurons showing a reversible response after 825 and 920 sec, respectively.
Discussion
Our results show that relaxin directly stimulates neurons in the rat SFO. In the absence of evidence of relaxin crossing the blood–brain barrier, the SFO is the likely site of blood-borne relaxin's dipsogenic effect because ablation of the SFO, but not the OVLT (the other of the sensory CVO stimulated by circulating relaxin), abolished relaxin-induced drinking. Evidence that i.v. infusion of relaxin increases vasopressin release has been reported (12, 13). Ablation of the OVLT significantly reduced the number of magnocellular neurons expressing Fos in the PVNm and SON (sites of vasopressin-containing neurons) in response to i.v. infusion of relaxin, suggesting that the OVLT may mediate vasopressin release in response to i.v. relaxin.
It is unlikely that the lack of relaxin-induced drinking in rats with SFO lesions is caused by generalized behavioral debilitation, because these animals exhibited normal daily intake of food and water on recovery from lesion surgery. Others have shown that behaviors such as feeding and osmotically stimulated drinking are not affected by ablation of the SFO (16, 20), and the inhibitory effects of SFO lesions on drinking apparently are specific for dipsogenic agents that act on specific receptors located on neurons in the SFO to stimulate drinking.
In these experiments, we used two different techniques (electrophysiology and immunohistochemistry to detect Fos-LI) to identify activated neurons, and showed that many neurons responsive to circulating relaxin are found in the rostral and peripheral parts of the SFO, and most likely in the dorsal cap region of the OVLT. Although human relaxin was infused in most experiments to male rats, administration of rat relaxin stimulated the same pattern of Fos-LI in the brain, and this stimulation occurred regardless of the sex of the rat. Fos expression has occurred in several different neuronal pathways when they have been activated by synaptic transmission, injection of neurotransmitter, or hormonal stimulation (21–23). A study of Fos-LI in rat brain, monitoring i.c.v. administration (rather than systemic infusion) of relaxin, also showed that neurons were activated in the outer part of the SFO and dorsal cap of the OVLT; in addition, many cells exhibited Fos-LI in the MnPO (9). The rich reciprocal interconnections between these three components of the lamina terminalis made it impossible to determine the site of primary neuronal stimulation by relaxin in that experiment. The present experiment showing marked Fos-LI in the SFO and OVLT, but much less in the MnPO after systemic infusion of relaxin, also supports the view that, with circulating relaxin, the primary stimulus is in one or both of these CVOs, which, unlike the MnPO and nearly all other brain regions, lack a blood–brain barrier, and therefore are accessible to the circulating peptide. Ablation of the OVLT resulted in a marked reduction of Fos-LI in the MnPO in response to i.v. infusion of relaxin, suggesting that relaxin-sensitive neurons in the OVLT may provide excitatory input to the MnPO, which is consistent with neuroanatomical evidence of an efferent pathway from the OVLT to the MnPO (24).
The electrophysiological recordings made from neurons in the isolated SFO in which connections to other brain regions had been severed show that neurons in the outer parts of the SFO respond directly to relaxin added to their environment. Several investigators have shown that Ang II can directly stimulate neurons in the SFO (19, 25), and we have observed that the great majority of these neurons were also stimulated by relaxin in the perfusion medium. We have not made such recordings from the isolated OVLT, but in view of the relaxin binding present in this site (10), to which circulating relaxin should have access, it seems likely that relaxin also acts directly on neurons in the OVLT to stimulate neuronal activity there. Most of the activation of neurons in the OVLT by i.v. infusion of relaxin is unlikely to be secondary to stimulation of neurons in the SFO known to have efferent neural connections to the OVLT (9), because i.v. relaxin stimulated increased Fos-LI in the OVLT of rats in which the SFO had been ablated.
Most relaxin-sensitive neurons in the outer parts of the SFO were also sensitive to Ang II in vitro, raising the possibility that the same neurons mediate drinking and vasopressin secretion induced by i.v. infusion of relaxin and Ang II. Although we did not test effects of angiotensin AT1 antagonists on the relaxin-induced stimulation of neurons in the SFO, it seems unlikely that relaxin exerts its effects through angiotensin receptors in the SFO, because i.c.v. injection of the Ang II antagonist losartan does not block the increase in Fos-LI that occurs in the SFO and OVLT in response to centrally administered relaxin (M.J.M. and P. Sinnayah, unpublished observations). Consistent with this study is our observation that 2 of the 14 relaxin-stimulated neurons from which we obtained recordings were insensitive to Ang II, suggesting the independence of the Ang II and relaxin receptors.
Neurons in the SON and PVNm, which contained vasopressin, also showed increased Fos-LI and therefore neuronal activation after i.v. relaxin, consistent with previous studies (26, 27). Although binding sites for relaxin have also been reported to be present in these two nuclei (10), it seems likely that the blood–brain barrier would prevent circulating relaxin from gaining access to these sites. In view of our recent evidence that many of the neurons in the SFO and OVLT that were activated (as shown by Fos-LI) by blood-borne relaxin have direct neural connections with the SON and PVN, it seemed probable that vasopressin (and oxytocin) secretion resulting from systemic infusion of relaxin is driven by relaxin-sensitive neurons in the SFO and OVLT (27). However, only ablation of the OVLT significantly reduced the Fos-LI in both the SON and PVNm, suggesting it may play the major role in mediating relaxin-induced vasopressin secretion. An interesting observation made in three animals, in which the tissue just lateral and caudal to the OVLT was ablated, resulted in a pronounced asymmetry in the number of Fos-LI neurons in the SON after relaxin treatment (Fig. 3 E and F). The neural pathways from the lamina terminalis to the SON proceed through the region caudal and lateral to the OVLT (28, 29), and it seems likely that this effect was caused by interruption of a relaxin-activated neural pathway from the lamina terminalis to the SON.
The concentrations of relaxin that stimulated drinking, Fos-LI, or increased spike frequency in vitro in the present experiments in rats have been supraphysiological. However, we have observed water drinking in rats at infusion rates that have contrived physiological blood levels (13). The fact that ablation of the SFO abolished water drinking at a maximal dose of relaxin indicates that drinking stimulated by more physiological blood levels is also likely to be mediated by the SFO. Because blood levels of Ang II and of relaxin are elevated in the pregnant rat, and these two circulating dipsogenic peptides can act synergistically in stimulating drinking (13), it seems likely that they both act on the SFO and OVLT to influence body fluid homeostasis during pregnancy in rats.
Acknowledgments
This work was supported by Institute Block Grant 983001 from the National Health and Medical Research Council of Australia, the Robert J. Jr. and Helen C. Kleberg Foundation, and the G. Harold and Leila Y. Mathers Charitable Foundation. M.E. holds a fellowship from the Swiss National Science Foundation. T.C.D.B. holds a National Health and Medical Research Council Peter Doherty Postdoctoral Fellowship.
Abbreviations
- Ang II
angiotensin II
- Fos-LI
Fos-like immunoreactivity
- i.c.v.
intracerebroventricular
- MnPO
median preoptic nucleus
- OVLT
organum vasculosum of the lamina terminalis
- PVN
hypothalamic paraventricular nucleus
- PVNm
posterior magnocellular division of the paraventricular nucleus
- SFO
subfornical organ
- SON
supraoptic nucleus
- CVO
circumventricular organ
- aCSF
artificial cerebrospinal fluid
References
- 1.Sherwood O D. In: The Physiology of Reproduction. Knobil E, Neill J D, editors. New York: Raven; 1994. pp. 861–1009. [Google Scholar]
- 2.Hwang J-J, Sherwood O D. Endocrinology. 1988;123:2486–2490. doi: 10.1210/endo-123-5-2486. [DOI] [PubMed] [Google Scholar]
- 3.Geddes B J, Summerlee A J S. J Neuroendocrinol. 1995;7:411–417. doi: 10.1111/j.1365-2826.1995.tb00777.x. [DOI] [PubMed] [Google Scholar]
- 4.Mumford A D, Parry L J, Summerlee A J S. J Endocrinol. 1989;123:747–755. doi: 10.1677/joe.0.1220747. [DOI] [PubMed] [Google Scholar]
- 5.Parry L J, Poterski R S, Summerlee A J S. Biol Reprod. 1994;50:622–628. doi: 10.1095/biolreprod50.3.622. [DOI] [PubMed] [Google Scholar]
- 6.Thornton S N, Fitzsimons J T. J Neuroendocrinol. 1995;7:165–170. doi: 10.1111/j.1365-2826.1995.tb00743.x. [DOI] [PubMed] [Google Scholar]
- 7.Summerlee A J S, Robertson G F. Endocrine. 1995;3:377–381. doi: 10.1007/BF03021422. [DOI] [PubMed] [Google Scholar]
- 8.McKinley M J, Burns P, Colvill L M, Oldfield B J, Wade J D, Weisinger R S, Tregear G W. J Neuroendocrinol. 1997;9:431–437. doi: 10.1046/j.1365-2826.1997.00600.x. [DOI] [PubMed] [Google Scholar]
- 9.Johnson A K, Gross P M. FASEB J. 1993;7:678–686. doi: 10.1096/fasebj.7.8.8500693. [DOI] [PubMed] [Google Scholar]
- 10.Osheroff P L, Phillips H S. Proc Natl Acad Sci USA. 1991;88:6413–6417. doi: 10.1073/pnas.88.15.6413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McKinley M J, McAllen R M, Mendelsohn F A O, Allen A M, Chai S Y, Oldfield B J. Front Neuroendocrinol. 1990;11:91–127. [Google Scholar]
- 12.Geddes B J, Parry L J, Summerlee A J S. Endocrinology. 1994;134:1188–1192. doi: 10.1210/endo.134.3.8119158. [DOI] [PubMed] [Google Scholar]
- 13.Sinnayah P, Burns P, Wade J D, Weisinger R S, McKinley M J. Endocrinology. 1999;140:5082–5086. doi: 10.1210/endo.140.11.7091. [DOI] [PubMed] [Google Scholar]
- 14.Zhao S, Malmgren C H, Shanks R D, Sherwood O D. Endocrinology. 1995;136:1892–1897. doi: 10.1210/endo.136.5.7720635. [DOI] [PubMed] [Google Scholar]
- 15.Weisinger R S, Burns P, Eddie L W, Wintour E M. J Endocrinol. 1993;137:505–510. doi: 10.1677/joe.0.1370505. [DOI] [PubMed] [Google Scholar]
- 16.Simpson J B, Epstein A N, Camardo J S. J Comp Physiol Psychol. 1978;92:581–601. doi: 10.1037/h0077503. [DOI] [PubMed] [Google Scholar]
- 17.Tregear G W, Wade J D. In: Progress in Relaxin Research. MacLennan A H, Tregear G W, Bryant-Greenwood G, editors. Singapore: World Scientific; 1994. pp. 31–34. [Google Scholar]
- 18.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. North Ryde, Australia: Academic; 1986. [Google Scholar]
- 19.Rauch M, Schmid H A, de Vente J, Simon E. J Neurosci. 1997;17:363–371. doi: 10.1523/JNEUROSCI.17-01-00363.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weisinger R S, Denton D A, Di Nicolantonio R, Hards D K, McKinley M J, Oldfield B J, Osborne P G. Brain Res. 1990;526:23–30. doi: 10.1016/0006-8993(90)90245-7. [DOI] [PubMed] [Google Scholar]
- 21.Sagar S M, Sharp F R, Curran T. Science. 1988;40:1328–1331. doi: 10.1126/science.3131879. [DOI] [PubMed] [Google Scholar]
- 22.Dragunow M, Faull R. J Neurosci Methods. 1989;29:261–265. doi: 10.1016/0165-0270(89)90150-7. [DOI] [PubMed] [Google Scholar]
- 23.Oldfield B J, Badoer E, Hards D K, McKinley M J. Neuroscience. 1994;60:255–262. doi: 10.1016/0306-4522(94)90219-4. [DOI] [PubMed] [Google Scholar]
- 24.Saper C B, Levisohn D. Brain Res. 1983;288:21–31. doi: 10.1016/0006-8993(83)90078-1. [DOI] [PubMed] [Google Scholar]
- 25.Phillips M I, Felix D. Brain Res. 1976;109:531–540. doi: 10.1016/0006-8993(76)90032-9. [DOI] [PubMed] [Google Scholar]
- 26.Heine P A, Di S, Ross L R, Anderson L L, Jacobson C D. Neuroendocrinology. 1997;66:38–46. doi: 10.1159/000127217. [DOI] [PubMed] [Google Scholar]
- 27.Sunn N, McKinley M J, Oldfield B J. J Neuroendocrinol. 2001;13:432–437. doi: 10.1046/j.1365-2826.2001.00650.x. [DOI] [PubMed] [Google Scholar]
- 28.Miselis R R. Brain Res. 1981;230:1–23. doi: 10.1016/0006-8993(81)90388-7. [DOI] [PubMed] [Google Scholar]
- 29.Lind R W, Van Hoesen G W, Johnson A K. J Comp Neurol. 1982;210:265–277. doi: 10.1002/cne.902100306. [DOI] [PubMed] [Google Scholar]