Abstract
Simultaneous measurements of CO2 and O2 fluxes from wheat (Triticum aestivum) shoots indicated that short-term exposures to elevated CO2 concentrations diverted photosynthetic reductant from NO or NO
or NO reduction to CO2 fixation. With longer exposures to elevated CO2, wheat leaves showed a diminished capacity for NO
reduction to CO2 fixation. With longer exposures to elevated CO2, wheat leaves showed a diminished capacity for NO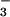 photoassimilation at any CO2 concentration. Moreover, high bicarbonate levels impeded NO
photoassimilation at any CO2 concentration. Moreover, high bicarbonate levels impeded NO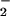 translocation into chloroplasts isolated from wheat or pea leaves. These results support the hypothesis that elevated CO2 inhibits NO
translocation into chloroplasts isolated from wheat or pea leaves. These results support the hypothesis that elevated CO2 inhibits NO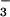 photoassimilation. Accordingly, when wheat plants received NO
photoassimilation. Accordingly, when wheat plants received NO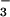 rather than NH
rather than NH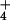 as a nitrogen source, CO2 enhancement of shoot growth halved and CO2 inhibition of shoot protein doubled. This result will likely have major implications for the ability of wheat to use NO
as a nitrogen source, CO2 enhancement of shoot growth halved and CO2 inhibition of shoot protein doubled. This result will likely have major implications for the ability of wheat to use NO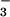 as a nitrogen source under elevated CO2.
as a nitrogen source under elevated CO2.
Atmospheric CO2 concentrations have increased from about 280 to 370 μmol mol−1 since 1800 (1, 2) and may reach 500–900 μmol mol−1 by the end of the century (3). Several responses of higher plants to such changes were not anticipated (4). For example, a doubling of CO2 level initially accelerates carbon fixation in C3 plants by about 30%, yet after days to weeks of exposure to high CO2 concentrations, depending on species, carbon fixation declines until it stabilizes at a rate that averages 12% above ambient controls (5). This general phenomenon, known as CO2 acclimation, is correlated with a decline in the activity of Rubisco and other enzymes in the Calvin cycle (6, 7). The change in Calvin cycle enzyme activities is not necessarily selective; rather, it often follows a decline in overall shoot protein and N contents (8). Shoot N contents diminish by an average of 14% with a doubling of CO2 (9), a difference that exceeds what would be expected if a given amount of N were diluted by additional biomass (8).
In wheat, CO2 acclimation varies with N supply (10, 11). Wheat shoots accumulate free NO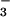 under elevated CO2 (12), and shoot protein declines (13) despite little change in total N (12, 14). Here, we present several lines of evidence that elevated CO2 concentrations inhibit NO
under elevated CO2 (12), and shoot protein declines (13) despite little change in total N (12, 14). Here, we present several lines of evidence that elevated CO2 concentrations inhibit NO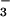 assimilation in wheat shoots and suggest two physiological mechanisms for this phenomenon.
assimilation in wheat shoots and suggest two physiological mechanisms for this phenomenon.
Materials and Methods
We surface-sterilized wheat (Triticum aestivum cv. Veery 10) seeds for 1 min in 2.6% NaClO, washed them thoroughly with water, and germinated them for several days on thick paper toweling (germination paper) saturated with 1 mM CaSO4. Twenty seedlings were transplanted to 19-liter opaque plastic containers filled with an aerated nutrient solution containing 0.2 mM NH4NO3, 1 mM CaSO4, 0.5 mM K2HPO4, 0.5 mM KH2PO4, 1 mM MgSO4, 0.2 g liter−1 Fe-NaEDTA, and micronutrients (15). The nutrient solution was replenished every 3 days.
The containers were placed in a controlled environment chamber [Conviron (Winnipeg, MB, Canada) PGV-36] equipped with a nondispersive infrared CO2 analyzer (Horiba, Kyoto, no. APBA-250E) and a Conviron process controller that added CO2 to maintain the chamber concentration at 360 μmol mol−1 for the photon flux density (PFD) response experiments (i.e., shoot photosynthesis as a function of photosynthetic PFD at plant height) and either 360 or 700 μmol mol−1 for the A–Ci response (i.e., shoot photosynthesis as a function of internal CO2 concentration) experiments. The growth/N relations experiments were also conducted at 360 or 700 μmol CO2 mol−1. The CO2 added was filtered through a KMnO4 column to remove contaminating hydrocarbons such as ethylene. A combination of high-pressure sodium, metal halide, and incandescent lamps provided a photosynthetic PFD of 700 μmol m−2 s−1 at plant height. The light/dark period was 16 h/8 h at 25°C and 15°C, respectively.
Gas-Exchange Measurements.
We transferred a seedling about 14 days old into a measurement system in which a split rubber stopper was fitted around the stem, sealing the root into an acrylic plastic and stainless steel cuvette (16) and the shoot into a gold-plated cuvette with a glass top (17). The leaves in the shoot cuvette were at their normal orientation; thus, the angle of incidence was 70–80°. Shoot gas fluxes were monitored with a commercial nondispersive infrared CO2 analyzer (Horiba VIA-500R), a custom O2 analyzer, and relative humidity sensors (Vaisala, Helsinki) (17). The custom O2 analyzer contains two cells of calcia-stabilized zirconium oxide ceramic similar to those found in an Applied Electrochemistry model N-37 M. When heated to 752.00 ± 0.01°C, these cells become selectively permeable to O2 and generate a 106-nV Nernst potential per μmol mol−1 difference in O2 partial pressures between the cells; in practice, this analyzer can resolve O2 concentration differences to within 2 μmol mol−1 on the normal background of 209,700 μmol mol−1 (17). Mass flow controllers (Tylan, Torrance, CA) prepared the various gas mixtures, and a pressure transducer (Validyne, North Ridge, CA) monitored gas flows through the cuvette. In the photosynthetic PFD experiments, a 1,000-W metal halide lamp with an adjustable ballast (Wide-Lite, San Marcos, TX) and neutral density filters controlled PFD at plant height between 0–1900 μmol m−2 s−1. Plants exhibited photoinhibition if the PFD was increased to levels where the response became light-saturated (3000 μmol m−2 s−1). In the A–Ci response experiments, the PFD was 1200 μmol m−2 s−1.
In the PFD experiments, a plant was exposed to 360 μmol mol−1 CO2 and received an aerated nutrient solution of 1 mM CaSO4, 0.5 μM K2HPO4, and either 0.2 mM KNO3 or 0.2 mM NH4Cl for 36 h before taking any measurements. In the A–Ci response experiments, a plant received an aerated nutrient solution of 0.2 mM NH4Cl, 1 mM CaSO4, and 0.5 μM K2HPO4 for 16 h before taking any measurements. A plant after such a pretreatment contained no detectable NO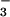 in its tissues (data not shown). This plant then received a nutrient solution containing 0.2 mM KNO3, 1 mM CaSO4, and 0.5 μM K2HPO4 for 16 h before measurements of the A–Ci response were repeated. We followed standard protocols to assess the response of photosynthesis to PFD or intercellular CO2 (18). The calculation of fluxes included an adjustment for changes in leaf area during the measurements.
in its tissues (data not shown). This plant then received a nutrient solution containing 0.2 mM KNO3, 1 mM CaSO4, and 0.5 μM K2HPO4 for 16 h before measurements of the A–Ci response were repeated. We followed standard protocols to assess the response of photosynthesis to PFD or intercellular CO2 (18). The calculation of fluxes included an adjustment for changes in leaf area during the measurements.
Chloroplast Isolation.
Wheat and pea (Pisum sativum cv. Progress 9) were grown for about 2 weeks at ambient CO2. The wheat received the nutrient solution described above, whereas the pea was in vermiculite that was watered daily. The following procedures were conducted at 0–4°C. The wheat (30–50 g) or pea (100–130 g) shoots were blended for 10 seconds in 0.2 liters of a grinding buffer (0.05 M K-Hepes, pH 7.3/0.33 M sorbitol/1 mM MgCl2/1 mM MnCl2/2 mM Na2EDTA/0.1% BSA). The homogenate was squeezed through two layers of miracloth and centrifuged at 2,900 × g for 5 min. The pellet was resuspended in 3 ml of a homogenization buffer (0.05 M K-Tricine, pH 8.0/0.33 M sorbitol) and layered onto a 30-ml Percoll gradient that was generated by centrifugation of 50% (vol/vol) Percoll in an equal volume of the grinding buffer at 37,000 × g for 30 min. After centrifugation of the overlayered gradients at 8,000 × g for 10 min, the intact chloroplasts formed a band near the bottom of the Percoll gradient. These were washed twice with 45 ml of the homogenization buffer and pelleted at 1,500 × g for 5 min. To test for the intactness of the chloroplasts, 15 μl of chloroplast material were layered on top of 100 μl of silicone oil that floated above 100 μl of a buffer solution (0.1 M K-Tricine, pH 8.0/0.66 M sorbitol) in a 4-ml Eppendorf tube. After centrifugation at maximum speed for 15 seconds (about 50,000 × g), broken chloroplasts floated on top of silicone oil, whereas intact chloroplasts passed through the silicone oil phase and formed a pellet at the bottom of the tube. To determine the amount of chlorophyll in the isolated chloroplasts, 10 μl of the chloroplast suspension were diluted into 5 ml of 80% acetone and filtered through Whatman no. 1 paper; the absorbance was read at 720, 663, and 645 nm, where Chl (mg liter−1) = 4.02 × (A663 − A720) + 10.14 × (A645 − A720) (19).
Chloroplast Nitrite Absorption.
We incubated intact chloroplasts containing about 1 g liter−1 chlorophyll in 50 mM K-Tricine (pH 8.0), 330 mM sorbitol, 0.3 mM KNO2, and 0, 0.3, 1.0, or 3.0 mM KHCO3 at about 22°C and at a PFD of 650 μmol quanta m−2 s−1. A concentration of 0.3 mM HCO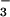 is probably higher than is present in vivo, but at lower concentrations, the relative depletion of HCO
is probably higher than is present in vivo, but at lower concentrations, the relative depletion of HCO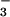 by carbon fixation during the incubation period was prohibitive. We took samples of the incubation mixture between 15 and 60 min, placed them immediately in the dark at 0–4°C, and centrifuged them at 3,000 × g for 3 min. To analyze NO
by carbon fixation during the incubation period was prohibitive. We took samples of the incubation mixture between 15 and 60 min, placed them immediately in the dark at 0–4°C, and centrifuged them at 3,000 × g for 3 min. To analyze NO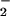 in the supernatant, we pipetted a 0.1-ml aliquot into 0.5 ml of a sulfanilamide solution [0.5 g sulfanilamide in 150 ml of 15% (vol/vol) CH3COOH], added after 5 min 0.5 ml of a NED solution [0.2 g of N-(1-naphthyl)ethylenediamide⋅2HCl in 150 ml of 15% (vol/vol) CH3COOH], allowed color to develop for 15 min, and measured absorbance at 540 nm (20). We fitted a cubic spline curve to the data for NO
in the supernatant, we pipetted a 0.1-ml aliquot into 0.5 ml of a sulfanilamide solution [0.5 g sulfanilamide in 150 ml of 15% (vol/vol) CH3COOH], added after 5 min 0.5 ml of a NED solution [0.2 g of N-(1-naphthyl)ethylenediamide⋅2HCl in 150 ml of 15% (vol/vol) CH3COOH], allowed color to develop for 15 min, and measured absorbance at 540 nm (20). We fitted a cubic spline curve to the data for NO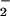 concentration as a function of time and derived net NO
concentration as a function of time and derived net NO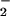 uptake from the slope of the cubic spline (mathcad, MathSoft, Cambridge, MA).
uptake from the slope of the cubic spline (mathcad, MathSoft, Cambridge, MA).
Growth and Nitrogen Parameters Under NH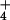 or NO
or NO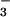 .
.
We germinated seeds as described above and, when they were 6 days old, transferred them to two controlled environmental chambers, one maintained at 360 μmol mol−1 CO2 and the other at 700 μmol mol−1 CO2. Each chamber had a continuous-flow nutrient solution system that supplied NH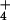 as the sole N source and one that supplied NO
as the sole N source and one that supplied NO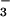 as the sole source. A solution system consisted of a 100-liter main reservoir, a centrifugal chemical pump, a distribution manifold with 6 4-liter hour−1 drip irrigation emitters, 6 opaque 4-liter polyethylene containers, and a manifold that returned the overflow from the 6 containers to the main reservoir. Each container held two plants. The nutrient solutions were composed of 0.1 mM (NH4)2SO4 or 0.2 mM KNO3, 2 mM CaCl2, 1 mM K2HPO4, 1 mM KH2PO4, 2 mM MgSO4, 0.2 g liter−1 Fe-NaEDTA, and micronutrients (15). To minimize nitrification and denitrification, we added ampicillin (20 mg liter−1) and cefotaxime (10 mg liter−1) (21). Every 2 days, we measured and adjusted the concentration of NH
as the sole source. A solution system consisted of a 100-liter main reservoir, a centrifugal chemical pump, a distribution manifold with 6 4-liter hour−1 drip irrigation emitters, 6 opaque 4-liter polyethylene containers, and a manifold that returned the overflow from the 6 containers to the main reservoir. Each container held two plants. The nutrient solutions were composed of 0.1 mM (NH4)2SO4 or 0.2 mM KNO3, 2 mM CaCl2, 1 mM K2HPO4, 1 mM KH2PO4, 2 mM MgSO4, 0.2 g liter−1 Fe-NaEDTA, and micronutrients (15). To minimize nitrification and denitrification, we added ampicillin (20 mg liter−1) and cefotaxime (10 mg liter−1) (21). Every 2 days, we measured and adjusted the concentration of NH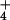 (22) or NO
(22) or NO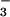 (23) in the solution systems. In another study, increasing the NO
(23) in the solution systems. In another study, increasing the NO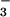 supply from 0.1 to 1.0 mM had little effect on wheat biomass production at 360 or 1000 μmol mol−1 CO2 (12). Here, the plants received 0.2 mM NH
supply from 0.1 to 1.0 mM had little effect on wheat biomass production at 360 or 1000 μmol mol−1 CO2 (12). Here, the plants received 0.2 mM NH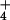 or NO
or NO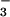 , and shoot N contents under all treatments were high at around 5%, indicating that N availability was not limiting growth. We conducted four replicate experiments in which we switched the ambient and elevated chambers and rotated the positions of the NH
, and shoot N contents under all treatments were high at around 5%, indicating that N availability was not limiting growth. We conducted four replicate experiments in which we switched the ambient and elevated chambers and rotated the positions of the NH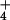 and NO
and NO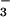 treatments in a chamber.
treatments in a chamber.
After 2 weeks, we evaluated plant growth and nitrogen parameters. Eight to 10 plants were dried in a forced-air oven at 70°C, weighed, and ground in a ball mill. We measured total N in one subsample via a carbon, hydrogen, and nitrogen (CHN) elemental analyzer (PDZ Europa, Cheshire, England, ANCA-SL), extracted another subsample with 1 mM KCl adjusted to pH 2 with H2SO4, and analyzed the extract for free NO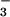 by means of HPLC (23). We analyzed two plants for total protein by using the Coomassie dye binding protein assay (Bio-Rad Bradford Protein Assay) and for in vitro activities of NO
by means of HPLC (23). We analyzed two plants for total protein by using the Coomassie dye binding protein assay (Bio-Rad Bradford Protein Assay) and for in vitro activities of NO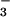 and NO
and NO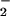 reductases based on the appearance or disappearance, respectively, of NO
reductases based on the appearance or disappearance, respectively, of NO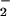 using a colorimetric assay (24). We used a general linear model (GLM procedure, SAS Institute, Cary, NC) to perform Tukey's Studentized Range and Bonferroni t tests on the ambient vs. elevated CO2 treatments under NH
using a colorimetric assay (24). We used a general linear model (GLM procedure, SAS Institute, Cary, NC) to perform Tukey's Studentized Range and Bonferroni t tests on the ambient vs. elevated CO2 treatments under NH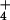 or NO
or NO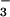 nutrition and designated probabilities of less than 5% as significant.
nutrition and designated probabilities of less than 5% as significant.
Results and Discussion
Gas-Exchange Measurements.
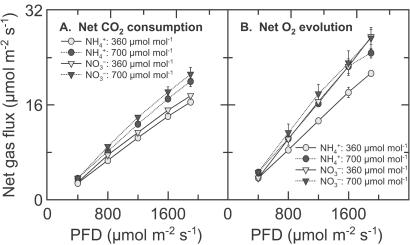
For wheat shoots grown at ambient CO2, net CO2 consumption at any given PFD was higher at elevated CO2 than ambient CO2 (Fig. 1A). Net O2 evolution was also higher at elevated CO2 than ambient CO2 under NH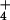 , but was insensitive to CO2 concentration under NO
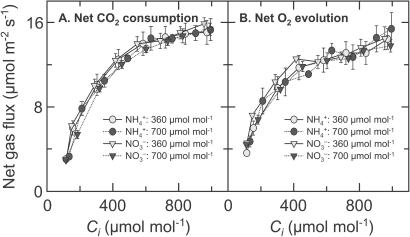
, but was insensitive to CO2 concentration under NO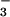 (Fig. 1B). The response of net CO2 consumption vs. Ci (shoot internal CO2 concentration) was similar among all treatments (Fig. 2), as is usually observed for C3 plants (25). Net O2 evolution, by contrast, was lower under NH
(Fig. 1B). The response of net CO2 consumption vs. Ci (shoot internal CO2 concentration) was similar among all treatments (Fig. 2), as is usually observed for C3 plants (25). Net O2 evolution, by contrast, was lower under NH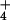 than NO
than NO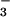 for the wheat grown under ambient CO2 and measured at the two lowest Cis (Fig. 2).
for the wheat grown under ambient CO2 and measured at the two lowest Cis (Fig. 2).
Figure 1.
Net CO2 consumption (A) and O2 evolution (B) by the shoot of a wheat seedling as a function of photosynthetic PFD at plant height. The plants had been grown in controlled environment chambers at 360 μmol mol−1 CO2 and measured at 360 (light symbols) or 700 (dark symbols) μmol mol−1 CO2. They received either NH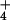 (circles) or NO
(circles) or NO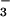 (triangles) as a sole N source during measurements. The leaves in the gas-exchange cuvette were at their natural orientation. Shown are mean ± SE for 5–8 replicate plants.
(triangles) as a sole N source during measurements. The leaves in the gas-exchange cuvette were at their natural orientation. Shown are mean ± SE for 5–8 replicate plants.
Figure 2.
Net CO2 consumption (A) and O2 evolution (B) by the shoot of a wheat seedling as a function of internal CO2 concentration (Ci), estimated from changes in CO2 and water vapor concentrations. The plants had been grown in controlled environment chambers at 360 (light symbols) or 700 (dark symbols) μmol mol−1 CO2. During measurements, the plants were exposed to NH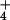 (circles) and then to NO
(circles) and then to NO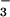 (triangles). The PFD at plant height was 1200 μmol quanta m−2 s−1. Shown are mean ± SE for 6 replicate plants.
(triangles). The PFD at plant height was 1200 μmol quanta m−2 s−1. Shown are mean ± SE for 6 replicate plants.
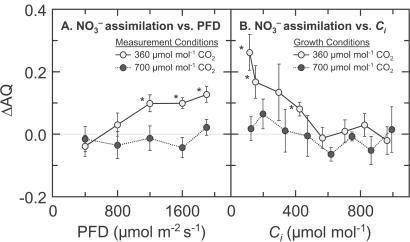
The assimilatory quotient (AQ), the ratio of net CO2 consumption to net O2 evolution, highlights these differences (Fig. 3). The AQ was verified as a nondestructive measure of in planta NO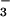 assimilation over 50 years ago for algae (26) and over a decade ago for higher plants by using barley mutants deficient in NO
assimilation over 50 years ago for algae (26) and over a decade ago for higher plants by using barley mutants deficient in NO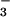 reductase activity (17). Transfer of electrons to NO
reductase activity (17). Transfer of electrons to NO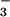 and NO
and NO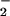 during photoassimilation increases O2 evolution from the light-dependent reactions of photosynthesis, while CO2 consumption by the light-independent reactions continues at similar rates. Therefore, plants that are photoassimilating NO
during photoassimilation increases O2 evolution from the light-dependent reactions of photosynthesis, while CO2 consumption by the light-independent reactions continues at similar rates. Therefore, plants that are photoassimilating NO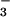 exhibit a lower AQ and the difference in the AQ with a shift from NO
exhibit a lower AQ and the difference in the AQ with a shift from NO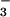 to NH
to NH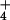 nutrition (ΔAQ) is proportional to NO
nutrition (ΔAQ) is proportional to NO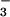 photoassimilation. The AQ may respond to other shoot processes—principally, photorespiration and the Mehler-peroxidase reaction—but these processes probably would not differ with N form in the root medium and, thus, would not influence ΔAQ.
photoassimilation. The AQ may respond to other shoot processes—principally, photorespiration and the Mehler-peroxidase reaction—but these processes probably would not differ with N form in the root medium and, thus, would not influence ΔAQ.
Figure 3.
The change in AQ (AQ = CO2 consumed/O2 evolved) with a shift in N source from NO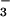 to NH
to NH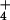 as a function of either photosynthetic flux density (A) or internal CO2 concentration (B), based on the data presented in Figs. 1 and 2. The plants had been measured (A) or grown (B) in controlled environment chambers at 360 (light symbols) or 700 (dark symbols) μmol mol−1 CO2. Shown are means ± SE for 5–8 replicate wheat plants. Asterisks mark the means that were significantly different from zero (P < 0.05, a Student's t test).
as a function of either photosynthetic flux density (A) or internal CO2 concentration (B), based on the data presented in Figs. 1 and 2. The plants had been measured (A) or grown (B) in controlled environment chambers at 360 (light symbols) or 700 (dark symbols) μmol mol−1 CO2. Shown are means ± SE for 5–8 replicate wheat plants. Asterisks mark the means that were significantly different from zero (P < 0.05, a Student's t test).
Here, the ΔAQs measured at elevated CO2 concentrations did not differ significantly from zero over a range of light levels, indicating little NO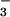 photoassimilation (Fig. 3A). This finding is consistent with a tight coupling between the light-dependent and light-independent reactions of photosynthesis. Net O2 evolution under NO
photoassimilation (Fig. 3A). This finding is consistent with a tight coupling between the light-dependent and light-independent reactions of photosynthesis. Net O2 evolution under NO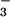 remained high at both CO2 levels (Fig. 1B), suggesting that the rate of photosynthetic electron transport and the amount of photosynthetic reductant generated were independent of CO2 level. In contrast, the ΔAQs measured at ambient CO2 increased with PFD (Fig. 3A), indicating that NO
remained high at both CO2 levels (Fig. 1B), suggesting that the rate of photosynthetic electron transport and the amount of photosynthetic reductant generated were independent of CO2 level. In contrast, the ΔAQs measured at ambient CO2 increased with PFD (Fig. 3A), indicating that NO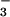 photoassimilation increased with light intensity. Thus, the shoots seemed to conduct NO
photoassimilation increased with light intensity. Thus, the shoots seemed to conduct NO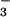 photoassimilation only to the extent that carbon fixation was CO2-limited and surplus photosynthetic reductant became available. Giving priority to carbon fixation seems an appropriate strategy in that plants can store moderate levels of NO
photoassimilation only to the extent that carbon fixation was CO2-limited and surplus photosynthetic reductant became available. Giving priority to carbon fixation seems an appropriate strategy in that plants can store moderate levels of NO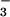 with little difficulty until reductant becomes available, but cannot directly store significant amounts of CO2.
with little difficulty until reductant becomes available, but cannot directly store significant amounts of CO2.
The response of ΔAQ as a function of internal CO2 concentration (Ci) supports this interpretation. Wheat grown under ambient CO2 and measured at the lower Cis exhibited ΔAQs greater than zero (Fig. 3B). These results indicate that exposure to elevated CO2 concentrations, either in the short term (hours) or long term (weeks), diminished NO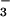 photoassimilation. The same mechanism could account for both these responses: short-term inhibition of NO
photoassimilation. The same mechanism could account for both these responses: short-term inhibition of NO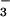 assimilation caused a specific down-regulation of shoot NO
assimilation caused a specific down-regulation of shoot NO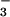 and NO
and NO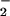 reductase activities (Fig. 6) and, therefore, a long-term decline in the capacity of the shoot to assimilate NO
reductase activities (Fig. 6) and, therefore, a long-term decline in the capacity of the shoot to assimilate NO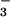 even under ambient CO2 conditions.
even under ambient CO2 conditions.
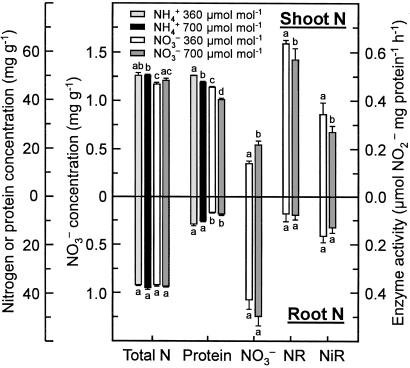
Figure 6.
Total N concentration (mg g−1 dry mass), protein concentration (mg g−1 fresh mass), NO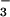 concentration (mg g−1 fresh mass), NO
concentration (mg g−1 fresh mass), NO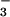 reductase activity (μmol NO
reductase activity (μmol NO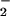 generated mg−1 protein h−1), and NO
generated mg−1 protein h−1), and NO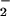 reductase activity (μmol NO
reductase activity (μmol NO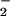 consumed mg−1 protein h−1) in the shoot (Top) or root (Bottom) of wheat grown for 14 days in controlled environment chambers at 360 or 700 μmol mol−1 CO2 and under NH
consumed mg−1 protein h−1) in the shoot (Top) or root (Bottom) of wheat grown for 14 days in controlled environment chambers at 360 or 700 μmol mol−1 CO2 and under NH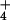 or NO
or NO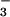 nutrition. Shown are means ± SE for four replicate experiments, each with 8–10 plants per treatment. Treatments labeled with different letters differ significantly (P < 0.05). Chlorophyll concentrations were 0.32 ± 0.02 and 0.34 ± 0.07 g liter−1 (mean ± SE, n = 2) for the NH
nutrition. Shown are means ± SE for four replicate experiments, each with 8–10 plants per treatment. Treatments labeled with different letters differ significantly (P < 0.05). Chlorophyll concentrations were 0.32 ± 0.02 and 0.34 ± 0.07 g liter−1 (mean ± SE, n = 2) for the NH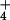 treatment at 360 and 700 μmol mol−1, respectively, and 0.30 ± 0.01 g liter−1 and 0.26 ± 0.04 g liter−1 (mean ± SE, n = 2) for the NO
treatment at 360 and 700 μmol mol−1, respectively, and 0.30 ± 0.01 g liter−1 and 0.26 ± 0.04 g liter−1 (mean ± SE, n = 2) for the NO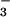 treatment at 360 and 700 μmol mol−1, respectively.
treatment at 360 and 700 μmol mol−1, respectively.
Our ability to monitor shoot O2 fluxes simultaneously with CO2 fluxes under normal physiological conditions provided a unique perspective. Previous studies of photosynthetic responses to PFD and Ci have monitored primarily CO2 exchange, which is relatively insensitive to N source (Figs. 1A and 2A). Measurements of photosynthetic O2 exchange are generally conducted by using polarographic O2 electrodes at saturating CO2 concentrations. Under these conditions, measurements of CO2 exchange are not feasible, and N source would have little effect on O2 evolution (Figs. 1B and 2B). For example (27), oxygen evolution monitored with a polarographic O2 electrode at saturating CO2 did not differ between detached barley leaves given NH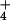 and those given NO
and those given NO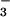 at levels similar to the xylem NO
at levels similar to the xylem NO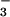 concentrations measured here (15.4 ± 0.7 mM; mean ± SE, n = 55). Another technique, chlorophyll fluorescence, in contrast with O2 fluxes, does not provide an accurate measure for electron transport rates of an entire wheat shoot (28).
concentrations measured here (15.4 ± 0.7 mM; mean ± SE, n = 55). Another technique, chlorophyll fluorescence, in contrast with O2 fluxes, does not provide an accurate measure for electron transport rates of an entire wheat shoot (28).
Carbon fixation may interfere with NO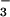 photoassimilation at several junctures. First, reduction of NO
photoassimilation at several junctures. First, reduction of NO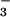 to NO
to NO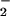 occurs in the cytosol (29, 30) and requires NADH generated from malate that is shuttled from the chloroplast (31). The demands of carbon fixation for reductant might limit this malate shuttle. Second, the reduction of NO
occurs in the cytosol (29, 30) and requires NADH generated from malate that is shuttled from the chloroplast (31). The demands of carbon fixation for reductant might limit this malate shuttle. Second, the reduction of NO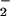 to NH
to NH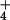 , the incorporation of NH
, the incorporation of NH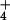 into amino acids, and the Calvin cycle all occur in the stroma of a chloroplast (32) and require ferredoxin that is reduced via photosynthetic electron transport (33). Elevated CO2 stimulates the Calvin cycle and, under light-limited conditions, can diminish the amount of reduced ferredoxin available for NO
into amino acids, and the Calvin cycle all occur in the stroma of a chloroplast (32) and require ferredoxin that is reduced via photosynthetic electron transport (33). Elevated CO2 stimulates the Calvin cycle and, under light-limited conditions, can diminish the amount of reduced ferredoxin available for NO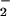 reduction or NH
reduction or NH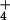 assimilation (34, 35). Our finding that ΔAQ declined to zero in low light or elevated CO2 (Fig. 3) is consistent with a diminished availability of NADH or reduced ferredoxin for NO
assimilation (34, 35). Our finding that ΔAQ declined to zero in low light or elevated CO2 (Fig. 3) is consistent with a diminished availability of NADH or reduced ferredoxin for NO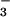 assimilation.
assimilation.
Chloroplast Nitrite Absorption.
NO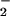 transport from the cytosol into the chloroplast involves the diffusion of HNO2 across chloroplast membranes and, therefore, requires the stroma to be more alkaline than the cytosol (36). Carbon dioxide at elevated concentrations can dissipate this pH gradient because additional CO2 movement into the chloroplast acidifies the stroma (37) and because enhanced carbon fixation hydrolyzes ATP faster and requires supplementary proton exchange across the thylakoid membrane to regenerate this ATP. The addition of 0.3, 1.0, or 3.0 mM HCO
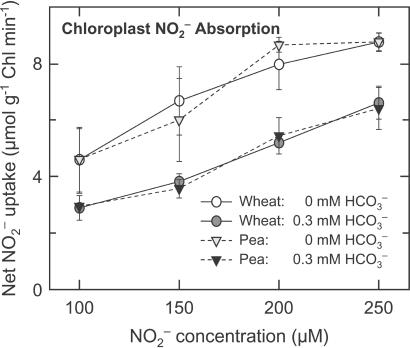
transport from the cytosol into the chloroplast involves the diffusion of HNO2 across chloroplast membranes and, therefore, requires the stroma to be more alkaline than the cytosol (36). Carbon dioxide at elevated concentrations can dissipate this pH gradient because additional CO2 movement into the chloroplast acidifies the stroma (37) and because enhanced carbon fixation hydrolyzes ATP faster and requires supplementary proton exchange across the thylakoid membrane to regenerate this ATP. The addition of 0.3, 1.0, or 3.0 mM HCO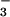 decreased chloroplast NO
decreased chloroplast NO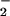 absorption by an average of 38, 45, or 61% in wheat and 32, 48, or 60% in pea (Fig. 4 shows the 0 and 0.3 mM data). These results confirm that high CO2 levels can interfere with NO
absorption by an average of 38, 45, or 61% in wheat and 32, 48, or 60% in pea (Fig. 4 shows the 0 and 0.3 mM data). These results confirm that high CO2 levels can interfere with NO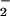 transport into the chloroplast and thereby provide another mechanism through which elevated CO2 might inhibit shoot NO
transport into the chloroplast and thereby provide another mechanism through which elevated CO2 might inhibit shoot NO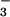 assimilation.
assimilation.
Figure 4.
Net NO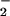 uptake (μmol mg−1 chlorophyll min−1) by isolated chloroplasts as a function of NO
uptake (μmol mg−1 chlorophyll min−1) by isolated chloroplasts as a function of NO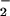 concentration when the medium contained 0 (light symbols) or 0.3 (dark symbols) mM HCO
concentration when the medium contained 0 (light symbols) or 0.3 (dark symbols) mM HCO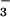 . Shown are the mean ± SE (n = 3) for wheat (circles) and pea (inverted triangles).
. Shown are the mean ± SE (n = 3) for wheat (circles) and pea (inverted triangles).
Growth and Nitrogen Parameters under NH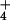 and NO
and NO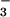 .
.
If CO2 at elevated concentrations inhibits NO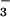 photoassimilation, then plants receiving NH
photoassimilation, then plants receiving NH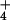 as a N source should prove more responsive to CO2 enrichment. To test this prediction, we grew wheat seedlings in controlled environment chambers where CO2 was controlled at ambient or elevated levels (360 or 700 μmol mol−1) and the plants received either 0.2 mM NH
as a N source should prove more responsive to CO2 enrichment. To test this prediction, we grew wheat seedlings in controlled environment chambers where CO2 was controlled at ambient or elevated levels (360 or 700 μmol mol−1) and the plants received either 0.2 mM NH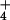 or 0.2 mM NO
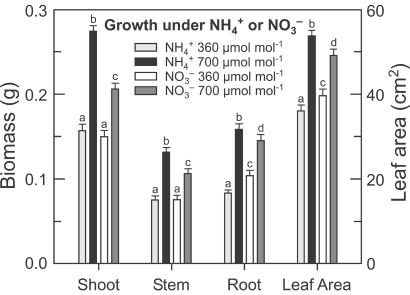
or 0.2 mM NO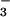 as the sole N source. The form in which N was supplied did not influence plant growth at 360 μmol mol−1 (ambient) CO2, but had a dramatic effect at 700 μmol mol−1 (elevated) CO2 (Fig. 5). Leaf area in the elevated CO2 treatment relative to the ambient CO2 treatment increased 49% under NH
as the sole N source. The form in which N was supplied did not influence plant growth at 360 μmol mol−1 (ambient) CO2, but had a dramatic effect at 700 μmol mol−1 (elevated) CO2 (Fig. 5). Leaf area in the elevated CO2 treatment relative to the ambient CO2 treatment increased 49% under NH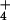 nutrition but only 24% under NO
nutrition but only 24% under NO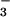 nutrition (Fig. 5). Total plant biomass increased 78% under NH
nutrition (Fig. 5). Total plant biomass increased 78% under NH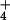 nutrition but only 44% under NO
nutrition but only 44% under NO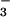 nutrition (Fig. 5). Thus, the plants receiving NH
nutrition (Fig. 5). Thus, the plants receiving NH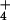 were more responsive to CO2 enrichment than those receiving NO
were more responsive to CO2 enrichment than those receiving NO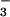 .
.
Figure 5.
Biomass (g of dry mass) and leaf area (cm2) per plant of wheat seedlings grown for 14 days in controlled environment chambers at 360 or 700 μmol mol−1 CO2 and under NH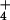 or NO
or NO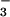 nutrition. Shown are means ± SE for four replicate experiments, each with 8–10 plants per treatment. Treatments labeled with different letters differ significantly (P < 0.05).
nutrition. Shown are means ± SE for four replicate experiments, each with 8–10 plants per treatment. Treatments labeled with different letters differ significantly (P < 0.05).
Shoot and root N concentrations were similar under the two CO2 regimes, indicating that N absorption per unit plant mass remained unchanged (Fig. 6). The fate of N after it was absorbed, however, differed under ambient and elevated CO2 as demonstrated by the balance between inorganic and organic N (Fig. 6). In the elevated CO2 treatment relative to the ambient CO2 treatment, shoot protein concentrations decreased 6% under NH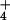 nutrition, as might be expected given the dilution by additional biomass, but decreased 13% under NO
nutrition, as might be expected given the dilution by additional biomass, but decreased 13% under NO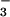 nutrition despite less additional biomass (Fig. 6). Thus, shoot protein per plant increased 73% and 32% under NH
nutrition despite less additional biomass (Fig. 6). Thus, shoot protein per plant increased 73% and 32% under NH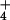 and NO
and NO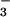 , respectively. Shoot NO
, respectively. Shoot NO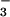 concentrations were undetectable in plants receiving NH
concentrations were undetectable in plants receiving NH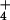 , but increased 62% at elevated CO2 in those receiving NO
, but increased 62% at elevated CO2 in those receiving NO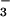 (Fig. 6). In vitro shoot activities of NO
(Fig. 6). In vitro shoot activities of NO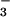 reductase and NO
reductase and NO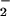 reductase decreased 12% and 27% from ambient to elevated CO2, respectively, on a total protein basis (Fig. 6) and decreased 33% and 30%, respectively, on a fresh mass basis. Root protein, NO
reductase decreased 12% and 27% from ambient to elevated CO2, respectively, on a total protein basis (Fig. 6) and decreased 33% and 30%, respectively, on a fresh mass basis. Root protein, NO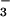 , and enzyme activities were similar under both CO2 treatments (Fig. 6).
, and enzyme activities were similar under both CO2 treatments (Fig. 6).
These results support a hypothesis that elevated CO2 inhibits NO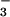 photoassimilation (12). Although the plants received the various CO2 and N treatments only from day 6 through day 20, the differences were substantial. Elevated CO2 concentrations stimulated shoot growth of the plants receiving NO
photoassimilation (12). Although the plants received the various CO2 and N treatments only from day 6 through day 20, the differences were substantial. Elevated CO2 concentrations stimulated shoot growth of the plants receiving NO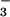 to only half the extent of those receiving NH
to only half the extent of those receiving NH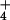 . Shoot protein concentrations at elevated CO2 concentrations declined more than twice as much under NO
. Shoot protein concentrations at elevated CO2 concentrations declined more than twice as much under NO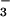 than under NH
than under NH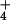 (Fig. 6). Shoot activities of NO
(Fig. 6). Shoot activities of NO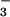 assimilatory enzymes declined even more than the overall protein concentrations (Fig. 6), suggesting that they were selectively inhibited. Studies on Plantago major (38), Nicotiana tabacum (39), Nicotiana plumbaginifolia (40), and spinach (41) have also found that longer exposures (4 h to over 2 weeks) to elevated CO2 inhibited shoot NO
assimilatory enzymes declined even more than the overall protein concentrations (Fig. 6), suggesting that they were selectively inhibited. Studies on Plantago major (38), Nicotiana tabacum (39), Nicotiana plumbaginifolia (40), and spinach (41) have also found that longer exposures (4 h to over 2 weeks) to elevated CO2 inhibited shoot NO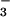 reductase activity. Selective inhibition of NO
reductase activity. Selective inhibition of NO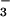 assimilation led to the accumulation of NO
assimilation led to the accumulation of NO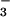 in the shoots of N. plumbaginifolia (40) and wheat (12).
in the shoots of N. plumbaginifolia (40) and wheat (12).
Nitrogen parameters from the growth analysis were consistent with the gas-exchange measurements. Changes in O2 evolution after exposure to NO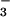 indicated that the NO
indicated that the NO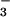 photoassimilation rate for plants grown and measured under ambient CO2 concentrations was about 0.3 μmol NO
photoassimilation rate for plants grown and measured under ambient CO2 concentrations was about 0.3 μmol NO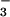 m−2 s−1.§ This rate was sufficient to account for the organic N that accumulated in the plants receiving NO
m−2 s−1.§ This rate was sufficient to account for the organic N that accumulated in the plants receiving NO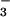 nutrition under ambient CO2 during the current experiment.¶ Moreover, maximum NO
nutrition under ambient CO2 during the current experiment.¶ Moreover, maximum NO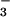 reductase activity in the shoots of these plants was 1.9 μmol NO
reductase activity in the shoots of these plants was 1.9 μmol NO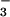 m−2 s−1, a value consistent with a NO
m−2 s−1, a value consistent with a NO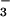 photoassimilation rate of 0.3 μmol NO
photoassimilation rate of 0.3 μmol NO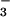 m−2 s−1 considering that maximum NO
m−2 s−1 considering that maximum NO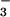 reductase activity may exceed the actual rate of NO
reductase activity may exceed the actual rate of NO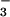 assimilation by a factor of 6 (41).
assimilation by a factor of 6 (41).
Despite extensive evidence on the importance of N availability for determining plant responses to CO2 enrichment (4, 14, 25, 39, 42–45), few other studies have considered the form of N. The two major N forms, NH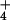 and NO
and NO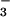 , have distinct physiological effects on plant growth and development (46), yet this may be the first study to examine CO2 responses under controlled levels of NH
, have distinct physiological effects on plant growth and development (46), yet this may be the first study to examine CO2 responses under controlled levels of NH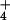 vs. NO
vs. NO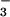 as sole N sources. Periodic watering of pots with solutions containing various N forms may not provide adequate control because N transformations are both rapid in nonsterile cultures (47) and sensitive to atmospheric CO2 (48). In the present study, we compared NH
as sole N sources. Periodic watering of pots with solutions containing various N forms may not provide adequate control because N transformations are both rapid in nonsterile cultures (47) and sensitive to atmospheric CO2 (48). In the present study, we compared NH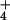 and NO
and NO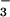 as sole N sources by using a continuous flow system to maintain constant nonlimiting levels of nutrients and a mixture of antibiotics to minimize conversion among N forms (21).
as sole N sources by using a continuous flow system to maintain constant nonlimiting levels of nutrients and a mixture of antibiotics to minimize conversion among N forms (21).
Our results may explain several responses of wheat to elevated CO2. In a multiyear Free Air CO2 Enrichment (FACE) experiment conducted at Maricopa, Arizona, wheat received either moderate (about 200 kg N ha−1) or high (about 500 kg N ha−1) N as a mix of NH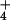 and NO
and NO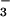 and was exposed to 360 or 550 μmol mol−1 CO2 (14). Grain yields did not vary with CO2 level in the moderate N treatment, but were 15% higher at elevated vs. ambient CO2 in the high N treatment (13). Leaf N concentrations and grain protein declined by more than 10% at elevated vs. ambient CO2 in the moderate N treatment, whereas these parameters varied only slightly with CO2 level in the high N treatment (13, 14). In the moderate N treatment, NO
and was exposed to 360 or 550 μmol mol−1 CO2 (14). Grain yields did not vary with CO2 level in the moderate N treatment, but were 15% higher at elevated vs. ambient CO2 in the high N treatment (13). Leaf N concentrations and grain protein declined by more than 10% at elevated vs. ambient CO2 in the moderate N treatment, whereas these parameters varied only slightly with CO2 level in the high N treatment (13, 14). In the moderate N treatment, NO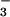 was the predominate N form (14); thus, CO2 inhibition of NO
was the predominate N form (14); thus, CO2 inhibition of NO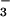 photoassimilation might account for the decline in leaf N and grain quality at elevated CO2 in this treatment. Plants in the high N treatment could compensate for CO2 inhibition of shoot NO
photoassimilation might account for the decline in leaf N and grain quality at elevated CO2 in this treatment. Plants in the high N treatment could compensate for CO2 inhibition of shoot NO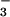 assimilation because they received additional NH
assimilation because they received additional NH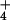 . A treatment of about 500 kg N ha−1, however, exceeds the average fertilizer recommendations for wheat by a factor of 3 or 4 (49, 50) and would exacerbate NO
. A treatment of about 500 kg N ha−1, however, exceeds the average fertilizer recommendations for wheat by a factor of 3 or 4 (49, 50) and would exacerbate NO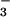 leaching, NH
leaching, NH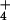 volatilization, and N2O release (51). Consequently, addition of such high N levels to compensate for CO2 inhibition of shoot NO
volatilization, and N2O release (51). Consequently, addition of such high N levels to compensate for CO2 inhibition of shoot NO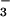 assimilation is unlikely for both economic and environmental reasons.
assimilation is unlikely for both economic and environmental reasons.
We feel that our laboratory results have implications for the real world of crop production. Wheat is grown on over 200 million hectares worldwide (50) and receives 18 million metric tons of N annually (49) or 20% of the world's production (50). In the well drained soils generally devoted to wheat cultivation, NO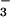 is a major N form. Were CO2 inhibition of NO
is a major N form. Were CO2 inhibition of NO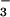 photoassimilation common among wheat cultivars, rising atmospheric CO2 levels would probably require major changes in fertilizer practices associated with wheat production.
photoassimilation common among wheat cultivars, rising atmospheric CO2 levels would probably require major changes in fertilizer practices associated with wheat production.
Acknowledgments
We thank Teena Stockert for her technical assistance; Steven Theg for his assistance in chloroplast preparation; and Jeffrey Amthor, Werner Kaiser, Robert Pearcy, and the anonymous reviewers for their comments on the manuscript. This work was supported by Department of Energy under Grant 95ER62128 TECO and National Science Foundation under Grant IBN-99-74927.
Abbreviations
- PFD
photon flux density
- AQ
assimilatory quotient
Footnotes
When plants grown under ambient CO2 were exposed to an atmospheric concentration of 360 μmol mol−1, their Cis averaged 295 μmol mol−1. At this Ci, net CO2 uptake did not differ with N source (Fig. 2A), but net O2 evolution increased by 0.6 μmol O2 m−2 s−1 with the shift from NH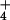 to NO
to NO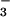 nutrition (Fig. 2B). Assuming that all of the extra O2 evolution was associated with generating reductant for NO
nutrition (Fig. 2B). Assuming that all of the extra O2 evolution was associated with generating reductant for NO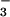 photoassimilation, (0.6 μmol O2 m−2 s−1 × 4 electrons per O2)/(8 electrons per NO
photoassimilation, (0.6 μmol O2 m−2 s−1 × 4 electrons per O2)/(8 electrons per NO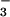 converted to NH
converted to NH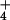 ) = 0.3 μmol NO
) = 0.3 μmol NO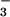 m−2 s−1 would be the potential NO
m−2 s−1 would be the potential NO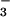 photoassimilation rate.
photoassimilation rate.
(7200 μg of organic N plant−1)/(0.3 μmol m−2 s−1 × 0.0014 m2 average leaf area plant−1 × 14 μg of N μmol−1 × 3600 s h−1 × 16 h light d−1) = 21 days, the age of the plant.
References
- 1.Etheridge D M, Steele L P, Langenfelds R L, Francey R J, Barnola J M, Morgan V I. J Geophys Res Atmos. 1996;101:4115–4128. [Google Scholar]
- 2.Whorf T, Keeling C D. New Sci. 1998;157:54–54. [Google Scholar]
- 3.Joos F, Plattner G K, Stocker T F, Marchal O, Schmittner A. Science. 1999;284:464–467. doi: 10.1126/science.284.5413.464. [DOI] [PubMed] [Google Scholar]
- 4.Bazzaz F A. Annu Rev Ecol Syst. 1990;21:167–196. [Google Scholar]
- 5.Curtis P S. Plant Cell Environ. 1996;19:127–137. [Google Scholar]
- 6.Bowes G. Annu Rev Plant Physiol Plant Mol Biol. 1993;44:309–332. [Google Scholar]
- 7.Moore B D, Cheng S H, Rice J, Seemann J R. Plant Cell Environ. 1998;21:905–915. [Google Scholar]
- 8.Makino A, Mae T. Plant Cell Physiol. 1999;40:999–1006. [Google Scholar]
- 9.Cotrufo M F, Ineson P, Scott A. Glob Change Biol. 1998;4:43–54. [Google Scholar]
- 10.Farage P K, McKee I F, Long S P. Plant Physiol. 1998;118:573–580. doi: 10.1104/pp.118.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adam N R, Wall G W, Kimball B A, Pinter P J, LaMorte R L, Hunsaker D J, Adamsen F J, Thompson T, Matthias A D, Leavitt S W, Webber A N. Photosynth Res. 2000;66:65–77. doi: 10.1023/A:1010629407970. [DOI] [PubMed] [Google Scholar]
- 12.Smart D R, Ritchie K, Bloom A J, Bugbee B B. Plant Cell Environ. 1998;21:753–764. doi: 10.1046/j.1365-3040.1998.00315.x. [DOI] [PubMed] [Google Scholar]
- 13.Kimball B A, Morris C F, Pinter P J, Wall G W, Hunsaker D J, Adamsen F J, LaMorte R L, Leavitt S W, Thompson T L, Matthias A D, Brooks T J. New Phytol. 2001;150:295–303. [Google Scholar]
- 14.Sinclair T R, Pinter P J, Kimball B A, Adamsen F J, LaMorte R L, Wall G W, Hunsaker D J, Adam N, Brooks T J, Garcia R L, et al. Agric Ecosyst Environ. 2000;79:53–60. [Google Scholar]
- 15.Epstein E. Mineral Nutrition of Plants: Principles and Perspectives. New York: Wiley; 1972. [Google Scholar]
- 16.Bloom A J. In: Application of Continuous and Steady State Methods to Root Biology. Torrey J G, Winship L J, editors. Dordrecht, The Netherlands: Kluwer Academic; 1989. pp. 147–163. [Google Scholar]
- 17.Bloom A J, Caldwell R M, Finazzo J, Warner R L, Weissbart J. Plant Physiol. 1989;91:352–356. doi: 10.1104/pp.91.1.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bloom A J, Mooney H A, Bjorkman O, Berry J. Plant Cell Environ. 1980;3:371–376. [Google Scholar]
- 19.Walker D A, Cerovic Z G, Robinson S P. Methods Enzymol. 1987;148:145–157. [Google Scholar]
- 20.Cunniff P, editor. Association of Analytical Chemists: Official Methods of Analysis of AOAC International. Arlington, VA: AOAC Int.; 1997. , March suppl., Ch. 39, p. 8. [Google Scholar]
- 21.Smart D R, Ferro A, Ritchie K, Bugbee B B. Physiol Plant. 1995;95:533–540. [PubMed] [Google Scholar]
- 22.Goyal S S, Rains D W, Huffaker R C. Anal Chem. 1988;60:175–179. doi: 10.1021/ac00153a016. [DOI] [PubMed] [Google Scholar]
- 23.Thayer J R, Huffaker R C. Anal Biochem. 1980;102:110–119. doi: 10.1016/0003-2697(80)90325-5. [DOI] [PubMed] [Google Scholar]
- 24.Aslam M, Rosichan J L, Huffaker R C. Plant Physiol. 1987;83:579–584. doi: 10.1104/pp.83.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sage R F. Photosynth Res. 1994;39:351–368. doi: 10.1007/BF00014591. [DOI] [PubMed] [Google Scholar]
- 26.Myers J. In: Photosynthesis in Plants. Franck J, Loomis W E, editors. Ames, Iowa: Iowa State College Press; 1949. pp. 349–364. [Google Scholar]
- 27.De la Torre A, Delgado B, Lara C. Plant Physiol. 1991;96:898–901. doi: 10.1104/pp.96.3.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Biehler K, Fock H. J Plant Physiol. 1995;145:422–426. [Google Scholar]
- 29.Rufty T W, Thomas J F, Remmler J L, Campbell W H, Volk R J. Plant Physiol. 1986;82:675–680. doi: 10.1104/pp.82.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vaughn K C, Campbell Wilbur H. Plant Physiol. 1988;88:1354–1357. doi: 10.1104/pp.88.4.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robinson J M. In: Models in Plant Physiology and Biochemistry. Newman D W, Stuart K G, editors. Vol. 1. Boca Raton, FL: CRC; 1987. pp. 25–35. [Google Scholar]
- 32.Suess K H, Prokhorenko I, Adler K. Plant Physiol. 1995;107:1387–1397. doi: 10.1104/pp.107.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sivasankar S, Oaks A. Plant Physiol Biochem. 1996;34:609–620. [Google Scholar]
- 34.Baysdorfer C, Robinson M J. Plant Physiol. 1985;77:318–320. doi: 10.1104/pp.77.2.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peirson D R, Elliott J R. J Plant Physiol. 1988;133:425–429. [Google Scholar]
- 36.Shingles R, Roh M H, McCarty R E. Plant Physiol. 1996;112:1375–1381. doi: 10.1104/pp.112.3.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shingles R, Moroney J V, McCarty R E. Plant Physiol. 1997;114:198. [Google Scholar]
- 38.Fonseca F, Bowsher C G, Stulen I. Physiol Plant. 1997;100:940–948. [Google Scholar]
- 39.Geiger M, Haake V, Ludewig F, Sonnewald U, Stitt M. Plant Cell Environ. 1999;22:1177–1199. [Google Scholar]
- 40.Ferrario-Mèry S, Thibaud M C, Betsche T, Valadier M H, Foyer C H. Planta. 1997;202:510–521. [Google Scholar]
- 41.Kaiser W M, Kandlbinder A, Stoimenova M, Glaab J. Planta. 2000;210:801–807. doi: 10.1007/s004250050682. [DOI] [PubMed] [Google Scholar]
- 42.Eamus D, Jarvis P G. Adv Ecol Res. 1989;19:1–55. [Google Scholar]
- 43.McGuire A D, Melillo J M, Joyce L A. Annu Rev Ecol Syst. 1995;26:473–503. [Google Scholar]
- 44.Drake B G, Gonzalez-Meler M A, Long S P. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:609–639. doi: 10.1146/annurev.arplant.48.1.609. [DOI] [PubMed] [Google Scholar]
- 45.Oren R, Ellsworth D S, Johnsen K H, Phillips N, Ewers B E, Maier C, Schafer K V R, McCarthy H, Hendrey G, McNulty S G, Katul G G. Nature (London) 2001;411:469–472. doi: 10.1038/35078064. [DOI] [PubMed] [Google Scholar]
- 46.Bloom A J. In: Ecology in Agriculture. Jackson L E, editor. San Diego: Academic; 1997. pp. 145–172. [Google Scholar]
- 47.Padgett P E, Leonard R T. Plant Physiol. 1993;101:141–146. doi: 10.1104/pp.101.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smart D R, Ritchie K, Stark J M, Bugbee B. Appl Environ Microbiol. 1997;63:4621–4624. doi: 10.1128/aem.63.11.4621-4624.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.International Maize and Wheat Improvement Center. CIMMYT 1995/96 World Wheat Facts and Trends: Understanding Global Trends in the Use of Wheat Diversity and International Flows of Wheat Genetic Resources. 1996. http://www.cimmyt.org/Research/economics/map/facts_trends/wft9596/htm/wft9596sheet13.htm Available at http://www.cimmyt.org/Research/economics/map/facts_trends/wft9596/htm/wft9596sheet13.htm. Accessed December 13, 2001. . Accessed December 13, 2001. [Google Scholar]
- 50.FAO Statistical Databases. Agricultural Data. 2001. http://apps.fao.org/page/collections?subset=agriculture Available at http://apps.fao.org/page/collections?subset=agriculture. Accessed December 13, 2001. . Accessed December 13, 2001. [Google Scholar]
- 51.Matson P A, Naylor R, Ortiz-Monasterio I. Science. 1998;280:112–115. doi: 10.1126/science.280.5360.112. [DOI] [PubMed] [Google Scholar]