Abstract
Although neural activity associated with emotion is becoming better understood, the influence of affective parameters on brain activity reflecting cognitive functioning in humans remains poorly characterized. We examined affective influences on working memory (WM) and tested the hypotheses that (i) dorsolateral prefrontal cortex (DLPFC) activity reflecting WM is influenced by the emotion-evoking qualities of task-relevant stimuli, but only when brought “on-line” by task demands, and (ii) DLPFC and orbitofrontal cortex (OFC) activities are inversely related as a function of emotional valence. Participants performed two tasks while event-related functional MRI measured brain activity; one task required active maintenance of stimulus representations in WM, and the other task required target detection responses with no demand for WM. Stimuli were standardized emotional (pleasant and unpleasant) and neutral pictures. Emotional stimuli differentially influenced DPFC and OFC activity during WM; DLPFC was influenced by emotional valence, enhanced by pleasant and reduced by unpleasant, compared to neutral stimuli, only when task conditions required WM. OFC was valence-sensitive during both tasks, greater to arousing than neutral stimuli when WM demand was low and in inverse relationship to DLPFC with high WM demand. Further, DLPFC and OFC activities are inversely related with respect to emotional valence during the WM task. The results are consistent with the hypothesis that the intrinsic valence of task-relevant stimuli maintained in WM modulates DLPFC activity but only when the DLPFC is required for task demands. Findings suggest a conceptualization of DLPFC and its involvement in WM that takes into account a role for affective parameters.
The prefrontal cortex (PFC) is critical to motivation, emotion, and higher cognitive functioning.‖ The primary functions of the PFC often have been dissociated into “emotional” and “cognitive” domains, with motivational/emotional processes attributed to the medial orbitofrontal region (1), and high-level cognitive processes to the phylogenetically newer dorsolateral region (2, 3). The orbitofrontal cortex (OFC), for example, mediates aspects of motivational functioning including emotional reactions and social behavior. OFC lesions alter functioning in these domains (1, 4) and aspects of motivationally-based decision making (5–7). Anatomical studies indicate that the OFC is interconnected extensively with the amygdala, ventral striatum, hypothalamus, and other areas implicated in emotional processing (8, 9). Neurophysiological studies indicate that the OFC is sensitive to the reward-predicting properties of stimuli (10) and the expectancy of appetitive and aversive outcomes (11). The OFC has been implicated also in affective and fear-related disorders, showing altered activity during pathological depression and induced sadness (12, 13) and during fear-symptom provocation (14).
The dorsolateral PFC (DLPFC), on the other hand, is critically involved in high-level cognition, most notably working memory (WM), the short-term retention and use of information to guide behavior (3, 15–17). The DLPFC is sparsely connected with classic limbic regions but is interconnected with paralimbic structures such as the hippocampus and anterior cingulate (3, 18). Lesion and single-unit recording studies in primates have shown that the DLPFC is involved in actively maintaining stimulus representations across a period of delay in order to guide task-relevant behavior (2, 3), and a host of human functional neuroimaging studies have supported such a role in humans (16, 17). Dysfunction of the DLPFC has been implicated in schizophrenia, which manifests a range of alterations in emotional and cognitive functioning such as selective attention (19) and WM (20). The DLPFC has been shown recently in nonhuman primates to be sensitive to the motivationally significant aspects of stimuli during a delay (11, 21).
Activity in regions of the DLPFC and OFC has been shown to be inversely related (12, 13, 22, 23), which is consistent with suggestions based on anatomical and single-unit recording studies that ventromedial PFC and limbic/paralimbic regions are reciprocally interactive with dorsal regions of the PFC (6, 24, 25). These findings suggest that although the DLPFC itself may not be involved in mediating motivational operations, it is well positioned to be influenced by the motivational state of the organism through connections with more ventromedial frontal cortex.
Importantly, little research in humans has examined whether motivational (i.e., affective) parameters influence DLPFC activity in the context of high-level cognitive processing or the relationship between DLFPC and OFC activity during cognitive task performance. In animals, the motivational context of task-relevant behavior has been shown to influence both OFC and DLPFC activity (21, 26–28). For example, acute stress impairs WM and concomitant delay-period activity in DLPFC neurons. In contrast, mild positive affect in humans enhances performance on tasks heavily dependent on PFC-mediated WM processes (29), whereas a negative affect impairs WM performance (30). Additionally, certain forms of psychopathology associated with prominent affective symptoms such as schizophrenia and depression are associated also with DLPFC-mediated WM dysfunction (20).
On the basis of these considerations, the present study aimed to determine whether activity in PFC (dorsolateral and orbitofrontal) evoked during performance of a task requiring active maintenance in WM is influenced by the affective tone of foreground task stimuli. Measuring brain activity by using event-related functional MRI (fMRI), we predicted that active maintenance of stimulus representations in WM would enhance DLPFC activity, and the OFC would show activity decreases. OFC activity is decreased reliably during a variety of visual cognitive tasks (31, 32), and neurons in this region do not show sustained responses to object features of stimuli during delay periods (27). More importantly, we predicted that WM-related DLPFC activity would be differentially influenced by stimulus conditions that induce brief mild affective responses, enhanced to pleasant and reduced to unpleasant stimuli relative to neutral stimuli but only when task demands are placed on WM. These predictions are based on findings from animal studies pointing to reduced DLPFC activity during unpleasant affect (e.g., stress; ref. 26) and human studies showing enhanced and impaired WM performance, respectively, during mild pleasant (29) and unpleasant (30) affect as well as the absence of DLPFC activity during passive affective picture viewing (33, 34). We also predicted that OFC activity would be enhanced by emotionally arousing stimuli, consistent with previous findings of emotional picture viewing (33, 34). Finally, a basic premise of this study is that the influence of emotional pictures would be sustained during the WM task and thereby influence brain activity, reflecting the retention of stimulus representations in WM. As a check on this assumption, a second group of participants performed the experimental tasks while acoustic startle blinks were elicited during the delay period. Startle modulation is a powerful method for assessing emotional valence influences during affective picture viewing (35, 36).
Method
Participants.
Ten right-handed college-age students (four females) aged 21–31 years (mean = 24.6, SD = 3.8) participated in the research. After a complete description of the study, written informed consent was obtained from all subjects.
Stimuli.
Stimuli were gray-scale images derived from the International Affective Picture System (IAPS; ref. 37) taken from three affective valence categories: pleasant (embracing/erotic couples), neutral (household/nature scenes), and unpleasant (mutilations, attacking animals/humans). The mean (±SE) valence ratings from IAPS norms are pleasant = 6.67 ± 0.14, neutral = 4.87 ± 0.06, and unpleasant = 2.20 ± 0.14, and arousal ratings are pleasant = 6.16 ± 0.16, neutral = 2.62 ± 0.10, and unpleasant = 6.78 ± 0.07. These pictures have been shown to evoke differential autonomic and somatic responses, to vary systematically in subjective ratings of pleasure and arousal (35, 36), and during sustained picture viewing, to prompt brain activity that covaries with affective responses (33, 34). Gray-scale images were employed to reduce the possibility that color-related cues would aid in task performance, and a previous fMRI study revealed that occipitoparietal cortical activity to color and gray-scale IAPS pictures did not differ.**
Cognitive Tasks.
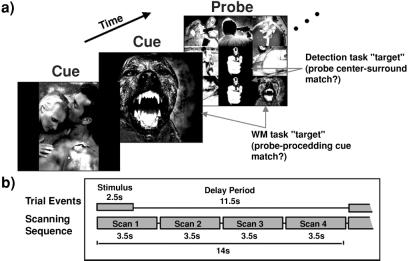
Picture stimuli, presented as cues and probes (Fig. 1a), were back-projected onto a screen mounted in the scanner bore. Cues were single pictures, and probes were matrices of nine pictures. All stimuli were presented for 2.5 s followed by an 11.5-s delay. Probes were presented unpredictably on 30% of the trials. Two tasks required right-hand button presses to stimuli on all trials: to cues, subjects pressed a button with their index finger, and to probes, subjects indicated target status with forced-choice button press (index finger = target, middle finger = nontarget). In the WM task, a target was a probe that contained within it the immediately preceding cue. In the detection (DET) task, a target was a probe in which the center picture of the matrix was identical to any of the surrounding matrix pictures. Thus, the two tasks were identical with respect to stimulus-encoding and response demands, but the WM task required constant maintenance of cue-stimulus representations across delay periods to respond correctly to the occasionally presented probes. Cue responses were required to ensure that subjects attended to and encoded these stimuli. The cue valence varied pseudo-randomly within the trial block, with the constraints that the valence of a cue was not repeated immediately, and at least two repetitions of each valence occurred during each trial block. Additional constraints were that (i) probes followed at least one cue of identical valence, (ii) the probability of a cue probe or center surround match was 70%, (iii) a probe, when presented, could not occur on the first trial of a block, and (iv) a probe always matched the valence of the immediately preceding cue. Subjects performed 10 blocks of 10–14-s trials per task, and task order alternated across blocks. Instructions provided prior to each block signified the subjects' task.
Figure 1.
Cognitive tasks, sample stimuli, and fMRI scanning sequence. (a) Stimuli (70% cues, 30% probes) were presented for 2.5 s followed by an 11.5-s delay. Pleasant, neutral, and unpleasant pictures were presented randomly within each trial block. Subjects indicated if an infrequently presented probe matrix contained the immediately preceding cue (WM) or an image in the surround that was identical to the image in the center (DET). (b) fMRI acquisition was synchronized to stimulus onset, and four 3.5-s scans were acquired during the course of each 14-s trial.
Image Acquisition.
Scanning took place in a conventional 1.5 Tesla General Electric Signa whole-body scanner with a standard head coil. Event-related functional images were acquired in the coronal plane using a two-interleave T2-weighted spiral-scan pulse sequence (ref. 38; 22 slices, voxels = 3.75 mm2 in-plane, 4.5-mm thick, repetition time = 1,750 ms per spiral, echo time = 35 ms, field of view = 24 cm, flip = 60°). Scans were positioned perpendicular to the intercommissural line, with the trailing edge of the most anterior scan beginning three slices anterior to the leading edge of the cingulate sulcus. Scan acquisition was synchronized to trial onset (Fig. 1b), and four 3.5-s acquisitions exactly filled the 14-s trial period. Prior to functional scanning, structural images were acquired using a standard T1-weighted pulse sequence.
Image Reduction and Analysis.
After reconstruction, images were normalized to a common mean and movement-corrected using six-parameter rigid body translation (39). To facilitate pooled-subject analyses (see below), each subject's images were coregistered to a common reference using a 12-parameter algorithm and then smoothed using a three-dimensional Gaussian filter (5-mm full-width half-maximum). The anatomic localization of suprathreshold activity was determined by overlaying statistical maps onto the reference structural image and transforming the data into standard reporting coordinates (40) by using afni software (41).
To examine the task-related prefrontal regions that are modulated by affective valence, the fMRI data were analyzed using voxel-wise paired t tests with task as the independent variable (subject = random factor). Only activity during cues and subsequent delay periods was analyzed, because maintenance activity would not be reflected in response to probes. A significance threshold of P < .0025 (two-tailed) and a five-voxel three-dimensional contiguity were used to create statistical maps. Image preprocessing and voxel-wise tests were conducted using nis software (kraepelin.wpic.pitt.edu/nis/). Planned contrasts were conducted on the mean signal intensity across all contiguous suprathreshold voxels, following within-subject range correction ([xi − minimum]/[maximum − minimum]), testing for main effects of task, valence, scan in trial, and their interaction. Range correction was applied to equate the raw signal intensities across the two frontal areas that on average were greater in the OFC (mean = 3,453) than DLPFC (mean = 3,386). Follow-up analyses included 1-df polynomial trends on valence for each task separately. Analyses with more than two levels of a factor employed an ɛ adjustment to correct for the use of repeated measures (42).
Because it is possible that the different task and valence conditions could systematically influence subjects' movement in the scanner and contribute to differences in brain activity, we evaluated the translational (mm x, y, and z) and rotational (degrees pitch, roll, and yaw) estimated-movement parameters derived from the movement-correction algorithm (39) using task × valence ANOVAs. Scan-to-scan movement did not differ in either dimension as a function of task, valence, or their interaction (Ps > .32), indicating that movement did not systematically differ as a function of experimental manipulations and likely did not contribute to the observed differences in signal intensity.
Affective Manipulation Check.
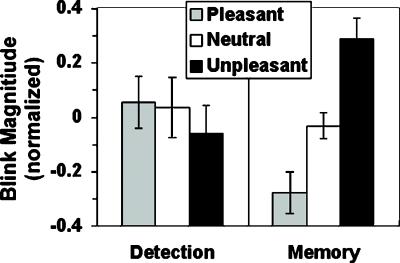
Acoustic startle blink (indexed via rectified and integrated m. orbicularis oculi electromyogram, time constant = 200 ms) was obtained while nine college-age subjects performed the WM and DET tasks. Stimuli were presented for 2.5 s at a stimulus onset asynchrony of 10 s. Acoustic startle was elicited after 33% of cue trials by a 40-ms-duration white noise (95 dB sound pressure level; near-instantaneous rise time) delivered binaurally through headphones 2.5 or 6 s after cue offset. We predicted that the impact of emotional pictures would persist during the delay interval in the maintenance task, producing the expected linear valence effect on blink magnitude (unpleasant > neutral > pleasant) typically obtained during sustained viewing of IAPS pictures (35, 36). Blink magnitude was scored by using a previously described algorithm (43) and analyzed by using a 1-df test of task × linear trend over valence.
Results
Affective Manipulation Check.
Startle blink (Fig. 2) showed the expected emotion-modulation effect after cues during the WM task, potentiated after unpleasant cues and inhibited after pleasant cues relative to neutral cues, but not during the DET task (task × linear valence interaction: F[1,8] = 9.47, P = .015). This pattern of blink modulation indicates that the chosen stimuli were evocative emotionally and that the intended affective impact was sustained during the delay period of the WM task.
Figure 2.
Mean normalized magnitude of startle blink (n = 9) evoked during the delay period after the presentation of cues in the DET and WM tasks. (Standard error bars are shown.)
fMRI Data.
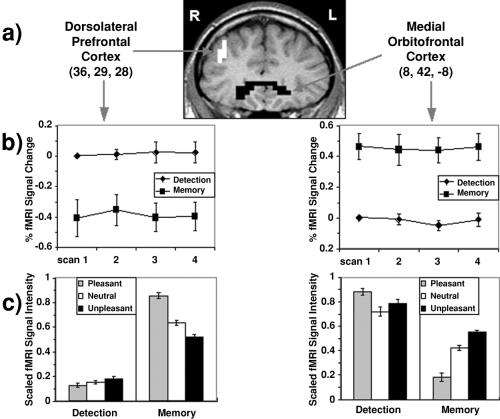
The effects of task were pronounced and statistically supported for both the right DLPFC [middle frontal gyrus, Broadmann area (BA) 46/9] and the OFC (BA 10/11; Table 1; Fig. 3a). In both regions, task effects were sustained across the trial period and did not interact with scan in trial (Fig. 3b). For the right DLPFC, the effects of valence (Fig. 3c) differed dramatically by task because of a task × linear valence trend interaction; in the WM task, activity was greater to pleasant and less to unpleasant compared to neutral cues, and valence did not affect DLPFC activity during the DET task. A valence main effect also reflected the overall linear trend with greater activity to pleasant and less activity to unpleasant compared to neutral cues.
Table 1.
F ratios and P values for planned analyses of fMRI signal intensity in the right DLPFC and medial OFC
| Source | Right DLPFC (Broadmann's area 46/9)
|
Medial OFC (Broadmann's area 10/11)
|
||
|---|---|---|---|---|
| F | P | F | P | |
| Task (T)* | 489.57 | <.0001 | 20.66 | <.001 |
| Valence (V)† | 9.57 | .003 | 8.11 | .015 |
| V (linear)* | 18.15 | .002 | 10.63 | .01 |
| V (quadratic)* | 2.03 | — | 1.63 | — |
| Scan (S)‡ | <1 | — | <1 | — |
| TxV† | 20.59 | <.0001 | 65.11 | <.0001 |
| TxV (linear)* | 26.67 | .001 | 82.39 | <.0001 |
| TxV (quadratic)* | 3.95 | — | 29.41 | <.0001 |
| TxS‡ | 2.00 | — | 2.54 | — |
| VxS§ | 1.32 | — | <1 | — |
| TxVxS | 2.01 | — | 1.37 | — |
| Detection task | ||||
| Valence (V)† | 1.62 | — | 10.72 | .006 |
| V (linear)* | 2.84 | — | 6.99 | .027 |
| V (quadratic)* | <1 | — | 14.91 | .004 |
| Memory task | ||||
| Valence (V)† | 19.74 | <.0001 | 31.45 | <.0001 |
| V (linear)* | 30.47 | <.0001 | 38.10 | <.0001 |
| V (quadratic)* | 1.17 | — | 4.49 | — |
Values are taken from the voxel showing the maximal effect in each cluster.
df = 1, 9.
df = 2, 18.
df = 3, 27.
df = 6, 54.
Figure 3.
Task- and valence-related effects on prefrontal cortex activity (n = 10). (a) Slice image illustrates regions of the right DPFC (BA 46/9) and medial OFC (BA 10/11) that exhibited significant task-related changes in signal intensity (P < .0025; white, memory > detection; black, detection > memory). Maximum z values for dorsolateral and medial frontal clusters were 4.61 and 4.01, respectively; cluster sizes were 95 and 102 voxels, respectively. Numbers in parentheses show the standard reporting coordinates (ref. 40; x, y, z) for voxels exhibiting the most significant P value. R, right; L, left. (b) Task-related signal intensity change as a function of scan-in-trial shown as the percentage change in signal intensity from scan 1 in the DET task. (c) Mean range-corrected (xi − minimum/maximum − minimum) signal intensity as a function of task and valence for the right dorsolateral (left) and medial orbitofrontal (right) clusters. Signal intensity was averaged across all significant voxels in each cluster. (Standard error bars are shown.)
The expected task- and valence-related inverse pattern was observed in the dorsal portion of the OFC (Fig. 3 b and c), as confirmed by planned contrasts on mean signal intensity within this cluster (Table 1). The region of activity extended from the dorsal portion of the OFC (BA 10/11) to the anterior portion of the subgenual anterior cingulate (BA 25/32). Significant effects of valence reflected a linear trend on valence (unpleasant > pleasant), and a task × valence interaction reflected interactions of task with both linear (unpleasant > pleasant) and quadratic (pleasant and unpleasant > neutral) trends on valence. Follow-up tests on valence for each task separately revealed for the WM task a significant linear trend on valence, indicating that activity was greater to unpleasant than to pleasant stimuli. Examination of task-related effects for each valence separately using voxel-wise t test revealed that the observed effects of valence were not caused by activation of different regions of the DLPFC and OFC.
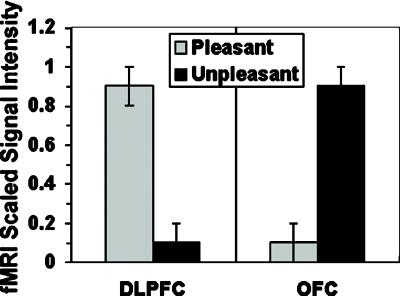
Activity in the DLPFC and OFC was highly inversely related as a function of valence during the WM task: 9 of 10 subjects showed greater activity to pleasant than unpleasant stimuli in the DLPFC, and the opposite effect was shown in the OFC. Analysis of signal intensity, with the minimum/maximum scaled within the region for pleasant and unpleasant stimuli (Fig. 4), revealed a significant region (DLPFC, OFC) × valence (pleasant, unpleasant) interaction [F(1,9) = 16.00, P = .0031].
Figure 4.
Mean range-corrected (xi − minimum/maximum − minimum) signal intensity as a function of pleasant and unpleasant valence for the right dorsolateral and orbitofrontal regions in the WM task. Nine of ten subjects showed the inverse DLPFC-OFC valence pattern.
Task Performance.
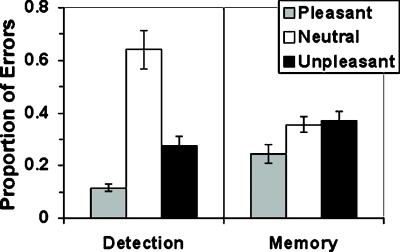
Behaviorally, the two tasks were equally difficult: Correct responding to infrequently presented probes averaged 68 and 71% on the DET and WM tasks, respectively, with no significant effects of task or valence, although accuracy generally was poorer to pleasant (65%) and unpleasant (65%) than to neutral (81%) probes. This response pattern suggests that task-difficulty effects do not underlie the task- and valence-related PFC activation differences. Because the tasks proved to be so difficult, with accuracy to valence-laden stimuli near 65%, an additional group of 23 unscanned subjects performed a modified version of the task to provide a more sensitive test of valence effects on behavioral performance during the WM task. The probe matrix was modified to include two rather than nine pictures to minimize the possibility of floor effects. Analysis of error rates (Fig. 5) revealed that the tasks did not differ [F(2,22) = 0.42, P > .50]. However, in the WM task, the effect of valence on error rates was highly significant [F(2,44) = 8.10, P < .002] and exhibited a pattern that paralleled the signal intensity pattern in the DLPFC.
Figure 5.
Task- and valence-related effects on behavioral performance in 23 subjects who performed a modified version of the task wherein probe stimuli contained only two rather than nine pictures.
Discussion
Consistent with our predictions and previous WM studies using either letter stimuli (16) or complex images (17), right DLPFC activity was enhanced while stimulus representations were maintained actively in WM. Moreover, WM-related DLPFC activity was modulated by the intrinsic emotional valence of the maintained picture stimuli (greater to pleasant and lesser to unpleasant, compared to neutral stimuli) even when subjects were not requested explicitly to maintain valence information during the delay period. In contrast, the DLPFC was insensitive to valence during the non-WM-dependent task, suggesting that modulation of DLPFC activity by the emotional valence of stimuli requires that it be brought “on-line” for affective influences to be expressed. The absence of emotion effects on DLPFC during the DET task also suggests that, in normal subjects, the affective valence of task-relevant stimuli does not obligatorily influence DLPFC activity. If engaged, the DLPFC seems to reflect activity in an appetitive system. In contrast but in line with previous observations, the OFC is involved in the processing of unpleasant information (44), reflecting activity in a defensive system. Consequently, activity in the DLPFC and OFC was reciprocally related as a function of valence during the WM task. Finally, the overall pattern of activity during WM indicates that generalized “arousal” effects associated with pleasant and unpleasant stimuli are unlikely to account for the valence modulation of PFC activity, because both pleasant and unpleasant stimuli are emotionally arousing and evoked directionally opposite effects.
The adjunct behavioral study, which used modified probes to minimize floor effects on task performance, revealed a pattern of behavioral performance that paralleled that of the WM-related DLPFC activity. That is, performance was best under conditions of pleasant valence and worst under conditions of unpleasant valence. Although these data are from a subject group that differed from the subjects that participated in the scanning session, the performance pattern suggests that increased DLPFC activity in the pleasant WM condition may be associated with improved WM performance.
The present research demonstrates a functional dissociation between medial PFC and DLPFC activity when actively maintaining emotionally arousing information in WM. The observed inverse relationship is consistent with suggestions that one region may modulate activity in the other either directly or indirectly (11, 12). Because the amygdala has been shown to be responsive to IAPS picture stimuli (33, 34) and is highly interconnected to the OFC, it is likely that amygdala input is important to the processing of motivational stimuli in the OFC (6, 24, 45), which in turn may modulate DLPFC activity.‡‡ As suggested by Hikosaka and Watanabe (11), the OFC may play a role in assigning motivational/affective qualities to relevant stimuli, information that is then “transmitted to the [DLPFC], where integration of motivational and cognitive operations would be achieved.”
The finding of reciprocal valence-related activity in the DLPFC and OFC also points to a potentially important principle in the organization of brain systems engaged by motivational stimuli, namely, that macroscopic differences in the functional representation of appetitive (approach) and defensive (avoidance) response modes engage different pools of neuronal systems during appetitive and defensive responding. This finding is consistent with the view that these two ends of the valence dimension prepare for very different output patterns (35, 36). The current findings also bear upon interpretation of the reciprocal nature of task- and valence-related activity in medial and lateral frontal cortex. Drevets and Raichle (23), for example, suggest that the relationship between “cognition” and “emotion” maps onto the reciprocity between limbic/paralimbic and neocortical regions. The present results, however, point to a modulatory role on cognition of emotion or motivational factors and additionally suggest that the reciprocal relationship may map more onto defining emotional valence (pleasant vs. unpleasant) than onto an emotion-cognition dichotomy.
On a clinical level, the present findings may have relevance to fear-related disorders such as specific phobias and posttraumatic stress disorder, to affective psychopathology more generally, and to drug addiction. Concerning affective and fear-related disorders, several authors have proposed that stress may function to take the PFC “off-line” and “shift control to subcortical regulation of behavior” (ref. 46; refs. 26 and 45); that is, a “functional disconnect” between motivationally critical structures (e.g., amygdala) and structures critical to high-level cognition (e.g., DLPFC). Arnsten and coworkers, for example, have suggested that the amygdala may orchestrate the stress-related response in PFC (46, 47) on the basis of its connectivity and capacity to regulate monoamine levels in the PFC (48). The observed reduction in DLPFC activity while maintaining unpleasant stimuli relative to neutral and pleasant stimuli during the WM task is consistent with the hypothesis that strong aversive affect indeed may decrease, decouple, or alter interactions between the DLPFC and motivationally relevant subcortical structures and promote “more automatic, reflexive or habitual responses dependent on the environment to control … behavior” (ref. 46), as suggested by the poorer behavioral performance while maintaining unpleasant rather than pleasant cues. Finally, with respect to drug addiction, several studies have pointed to abnormal activity in both the DLPFC and OFC and have related these abnormalities to dopaminergic activity, expectancy, and motivationally-based decision making (49, 50).
The pattern of DLPFC response to affective stimulation during WM prompts speculation about possible mechanisms underlying the observed effects, namely, catecholaminergic modulation by midbrain afferents. Mesocortical dopamine and norepinephrine are critical to WM, and there may be an optimal level of their turnover in PFC above and below which DLPFC-mediated WM processes are disrupted (refs. 46 and 51; i.e., an inverted-U relationship with WM). Unpleasant affect (e.g., stress) impairs DLPFC activity and WM function and preferentially increases dopamine release in the DLPFC, perhaps to a level that exceeds optimal (26), whereas other cortical and subcortical terminal fields show little effects (52, 53). Stress-related norepinephrine increases similarly impair WM performance (46, 47). In contrast, pleasant affect, within normal limits, may enhance dopamine turnover in the DLPFC but not above optimal levels, resulting in increased WM-related activity. Moderate positive affect increases cognitive flexibility and problem solving, leading to speculation that this enhanced PFC-mediated cognitive functioning results from increased mesocortical dopamine turnover in the DLPFC induced by positive affect (29). The pattern of findings in this study is consistent with both lines: WM demand combined with maintenance of appetitive cues may enhance dopamine activity in DLPFC but not beyond levels that would lead to DLPFC dysfunction, and maintenance of unpleasant cues associated with a modest decrease in DLPFC activity, in contrast, may reflect a greater than optimal increase in dopamine release in the DLPFC, leading to decreased WM-related DLPFC activity relative to neutral and pleasant cues. The influence of dopamine on OFC activity, however, is less certain, although this region receives denser dopaminergic projections than the DLPFC, albeit from somewhat different input sources (54).
Additional studies are required to better understand the neuroanatomical and neuromodulatory bases of emotion-modulated PFC activity. However, these findings clearly highlight the role of affect in PFC functioning and suggest a conceptualization of the DLPFC and its involvement in WM that takes into consideration motivational parameters.
Acknowledgments
We thank Peter Lang, Margaret Bradley, Amy Arnsten, and Robert Simons for their helpful comments and Brittany Lourea, Candice Mills, and Christopher May for their assistance in data collection. This research was supported by the National Alliance for Research in Schizophrenia and Depression (NARSAD), the Scottish Rite Foundation Schizophrenia Research Program, the University of Florida McKnight Brain Research Grant Program, and National Institute of Mental Health Mentored Research Scientist Development Award K01 MH01857 (to W.M.P.).
Abbreviations
- PFC
prefrontal cortex
- DLPFC
dorsolateral PFC
- OFC
orbitofrontal cortex
- fMRI
functional MRI
- WM
working memory
- BA
Broadmann's area
- DET
detection
- IAPS
International Affective Picture System
Footnotes
The results of this article were presented in preliminary form at the Sixth Annual Meeting of the Organization for Human Brain Mapping, June 16–20, 2000, San Antonio, TX.
Bradley, M. M., Lang, P. J., Fitzsimmons, J. R., Sabatinelli, D., King, W., Desai, P., Perlstein, W. M. & Cuthbert, B. N. (1999) Soc. Neurosci. Abstr. 25, 2148.
Amygdala activity was not observed because of significant signal intensity decreases in most subjects and reflecting magnetic susceptibility effects present in this region.
References
- 1.Stuss D T, Benson D F. The Frontal Lobes. New York: Raven; 1986. [Google Scholar]
- 2.Fuster J M. The Prefrontal Cortex: Anatomy, Physiology and Neuropsychology of the Frontal Lobe. 3rd Ed. New York: Raven; 1997. [Google Scholar]
- 3.Goldman-Rakic P S. In: Handbook of Physiology: The Nervous System V. Plum F, Mountcastle V, editors. Bethesda: Am. Physiol. Soc.; 1987. pp. 373–414. [Google Scholar]
- 4.Butter C M, Snyder D R. Acta Neurobiol Exp. 1972;32:525–565. [PubMed] [Google Scholar]
- 5.Bechera A, Damasio H, Tranel D, Anderson S W. J Neurosci. 1998;18:428–437. doi: 10.1523/JNEUROSCI.18-01-00428.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baxter M G, Parker A, Lindner C C C, Izquierdo A D, Murray E A. J Neurosci. 2000;20:4311–4319. doi: 10.1523/JNEUROSCI.20-11-04311.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rolls E T, Hornak J, Wade D, McGrath J. J Neurol Neurosurg Psychiatr. 1994;57:1518–1524. doi: 10.1136/jnnp.57.12.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cavada C, Compañy T, Tejedor J, Cruz-Rizzolo R J, Reinoso-Suárez F. Cereb Cortex. 2000;10:220–242. doi: 10.1093/cercor/10.3.220. [DOI] [PubMed] [Google Scholar]
- 9.Öngür D, Price J L. Cereb Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- 10.Schultz W, Tremblay L, Hollerman J R. Cereb Cortex. 2000;10:272–284. doi: 10.1093/cercor/10.3.272. [DOI] [PubMed] [Google Scholar]
- 11.Hikosaka K, Watanabe M. Cereb Cortex. 2000;10:263–271. doi: 10.1093/cercor/10.3.263. [DOI] [PubMed] [Google Scholar]
- 12.Mayberg H S, Liotti M, Brannan S K, McGinnis S, Mahwin R K, Jerabek P A, Silva J A, Tekell J L, Martin C C, Lancaster J L, Fox P T. Am J Psychiatry. 1999;156:675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- 13.Mayberg H S. J Neuropsychiatry Clin Neurosci. 1997;9:471–481. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- 14.Tillfors M, Furmark T, Marteinsdottir I, Fischer H, Pissiota A, Langstrom B, Fredrikson M. Am J Psychiatry. 2001;158:1220–1226. doi: 10.1176/appi.ajp.158.8.1220. [DOI] [PubMed] [Google Scholar]
- 15.Baddeley A D. Working Memory. Oxford: Oxford Univ. Press; 1986. [Google Scholar]
- 16.Cohen J D, Perlstein W M, Braver T S, Nystrom L E, Noll D C, Jonides J, Smith E E. Nature (London) 1997;386:604–608. doi: 10.1038/386604a0. [DOI] [PubMed] [Google Scholar]
- 17.Courtney S M, Ungerleider L G, Keil K, Haxby J V. Nature (London) 1997;386:608–611. doi: 10.1038/386608a0. [DOI] [PubMed] [Google Scholar]
- 18.Morris R, Pandya D N, Petrides M. J Comp Neurol. 1999;407:183–192. doi: 10.1002/(sici)1096-9861(19990503)407:2<183::aid-cne3>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 19.Perlstein W M, Carter C S, Barch D M, Baird J. Neuropsychology. 1998;12:414–425. doi: 10.1037//0894-4105.12.3.414. [DOI] [PubMed] [Google Scholar]
- 20.Perlstein W M, Carter C S, Noll D C, Cohen J D. Am J Psychiatry. 2001;158:1105–1113. doi: 10.1176/appi.ajp.158.7.1105. [DOI] [PubMed] [Google Scholar]
- 21.Watanabe M. Nature (London) 1996;382:629–632. doi: 10.1038/382629a0. [DOI] [PubMed] [Google Scholar]
- 22.Northoff G, Richter A, Gessner M, Schlagenhauf F, Fell J, Baumgart F, Kaulisch T, Kotter R, Stephan K E, Leschinger A, et al. Cereb Cortex. 2000;10:93–107. doi: 10.1093/cercor/10.1.93. [DOI] [PubMed] [Google Scholar]
- 23.Drevets W C, Raichle M E. Cognition Emotion. 1998;12:353–385. [Google Scholar]
- 24.Morgan M, LeDoux J E. Behav Neurosci. 1995;109:681–688. doi: 10.1037//0735-7044.109.4.681. [DOI] [PubMed] [Google Scholar]
- 25.Selemon L D, Goldman-Rakic P S. J Neurosci. 1998;8:4049–4068. doi: 10.1523/JNEUROSCI.08-11-04049.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arnsten A F T, Goldman-Rakic P S. Arch Gen Psychiatry. 1998;55:362–368. doi: 10.1001/archpsyc.55.4.362. [DOI] [PubMed] [Google Scholar]
- 27.Tremblay L, Schultz W. Nature (London) 1999;398:704–708. doi: 10.1038/19525. [DOI] [PubMed] [Google Scholar]
- 28.Watanabe M, Kodama T, Hikosaka K. J Neurophysiol. 1997;78:2795–2798. doi: 10.1152/jn.1997.78.5.2795. [DOI] [PubMed] [Google Scholar]
- 29.Ashby F G, Isen A M, Turken A U. Psychol Rev. 1999;106:529–550. doi: 10.1037/0033-295x.106.3.529. [DOI] [PubMed] [Google Scholar]
- 30.Linnenbrink E A, Ryan A M, Pintrich P R. Learn Individ Dif. 2000;11:213–230. [Google Scholar]
- 31.Shulman G L, Fiez J A, Corbetta M, Buckner R L, Miezin F M, Raichle M E, Petersen S E. J Cognit Neurosci. 1997;9:648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- 32.Simpson J R, Oenguer D, Akbudak E, Conturo T E, Ollinger J M, Snyder A Z, Gusnard D A, Raichle M E. J Cognit Neurosci. 2000;12:157–170. doi: 10.1162/089892900564019. [DOI] [PubMed] [Google Scholar]
- 33.Lane R D, Reiman E M, Bradley M M, Lang P J, Ahern G L, Davidson R J, Schwartz G E. Neuropsychologia. 1997;35:1437–1444. doi: 10.1016/s0028-3932(97)00070-5. [DOI] [PubMed] [Google Scholar]
- 34.Paradiso S, Johnson D L, Andreasen N C, O'Leary D S, Watkins G L, Ponto L L, Hichwa R D. Am J Psychiatry. 1999;156:1618–1629. doi: 10.1176/ajp.156.10.1618. [DOI] [PubMed] [Google Scholar]
- 35.Lang P J, Bradley M M, Cuthbert B N. In: Attention and Orienting. Lang P J, Simons R F, Balaban M T, editors. Mahwah, NJ: Lawrence Erlbaum Associates; 1997. pp. 97–136. [Google Scholar]
- 36.Bradley M M. In: Handbook of Psychophysiology. 2nd Ed. Cacioppo J T, Tassinary L G, Berntson G G, editors. Cambridge, U.K.: Cambridge Univ. Press; 2000. pp. 602–644. [Google Scholar]
- 37.Lang P J, Bradley M M, Cuthbert B N. International Affective Picture System (IAPS): Technical Manual and Affective Ratings. Gainesville, FL: Univ. of Florida Center for Research in Psychophysiology; 1998. [Google Scholar]
- 38.Noll D C, Cohen J D, Meyer C H, Schneider W. J Magn Reson Imaging. 1995;5:49–56. doi: 10.1002/jmri.1880050112. [DOI] [PubMed] [Google Scholar]
- 39.Woods R P, Cherry S R, Maziotta J C. J Comput Assist Tomogr. 1992;16:620–633. doi: 10.1097/00004728-199207000-00024. [DOI] [PubMed] [Google Scholar]
- 40.Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. New York: Thieme; 1988. [Google Scholar]
- 41.Cox R W. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 42.Greenhouse S W, Geisser S. Psychometrika. 1959;24:95–112. [Google Scholar]
- 43.Balaban M T, Losito B, Simons R F, Graham F K. Psychophysiology. 1986;23:612. [Google Scholar]
- 44.Frey S, Kostopoulos P, Petrides M. Eur J Neurosci. 2000;12:3709–3712. doi: 10.1046/j.1460-9568.2000.00227.x. [DOI] [PubMed] [Google Scholar]
- 45.Garcia R, Vouimba R M, Baudry M, Thompson R F. Nature (London) 1999;402:294–296. doi: 10.1038/46286. [DOI] [PubMed] [Google Scholar]
- 46.Arnsten A F T. Trends Cogn Sci. 1998;11:435–447. doi: 10.1016/s1364-6613(98)01240-6. [DOI] [PubMed] [Google Scholar]
- 47.Birnbaum S, Gobeske K T, Auerbach J, Taylor J R, Arnsten A F T. Biol Psychiatry. 1999;46:1266–1274. doi: 10.1016/s0006-3223(99)00138-9. [DOI] [PubMed] [Google Scholar]
- 48.Wallace D M, Magnuson D J, Gray T S. Brain Res Bull. 1992;28:447–454. doi: 10.1016/0361-9230(92)90046-z. [DOI] [PubMed] [Google Scholar]
- 49.London E D, Ernst M, Grant S, Bonson K, Weinstein A. Cereb Cortex. 2000;10:334–342. doi: 10.1093/cercor/10.3.334. [DOI] [PubMed] [Google Scholar]
- 50.Volkow N D, Fowler J S. Cereb Cortex. 2000;10:318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- 51.Williams G V, Goldman-Rakic P S. Nature (London) 1995;376:572–575. doi: 10.1038/376572a0. [DOI] [PubMed] [Google Scholar]
- 52.Deutch A Y, Roth R H. Prog Brain Res. 1990;85:367–403. doi: 10.1016/s0079-6123(08)62691-6. [DOI] [PubMed] [Google Scholar]
- 53.Thiery A M, Tassin J P, Blanc G, Glowinski J. Nature (London) 1976;263:242–244. doi: 10.1038/263242a0. [DOI] [PubMed] [Google Scholar]
- 54.Williams S M, Goldman-Rakic P S. Cereb Cortex. 1998;8:321–345. doi: 10.1093/cercor/8.4.321. [DOI] [PubMed] [Google Scholar]