Treatment of HIV infection has been revolutionized by the development of potent inhibitors of critical viral enzymes, particularly the HIV-1 reverse transcriptase and protease (1). Appropriate combinations of such drugs (referred to as highly active antiretroviral therapy or HAART) markedly suppress viral replication in most treated persons, leading to significant restoration of immune system function. In the developed world, HAART is responsible for dramatic reductions in HIV-associated morbidity and mortality. However, the quest for improved therapies continues, because of problems that seriously limit the current HAART regimens, including toxic side effects, viral persistence, difficulties in adhering to treatment, high cost, and the emergence of drug-resistant escape variants. The resistance problem is particularly challenging because of the extraordinarily high HIV-1 mutation rate, and the ability of viral variants harboring resistance mutations in both reverse transcriptase and protease to continue replicating in vivo. The viral mutability provides a rationale for developing anti-HIV drugs that target components of the host cell machinery essential for viral replication, because such molecules would not mutate in the face of drug pressure. The cellular receptors involved in HIV-1 entry are receiving special attention, with numerous candidate inhibitors at various stages of clinical development (2). However, HIV-1 finds ways to escape such inhibitors. In a recent issue of PNAS, Trkola et al. (3) analyze the mechanism by which HIV-1 escapes in vitro from a low molecular weight inhibitor targeted against the CCR5 coreceptor. The findings raise important questions not only about the clinical applications of this novel class of anti-HIV agents, but also about coreceptor usage in HIV-1 disease.
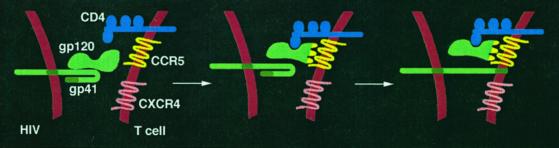
As shown in Fig. 1, HIV-1 enters CD4+ T cells by a cascade of molecular interactions between the virion envelope glycoprotein (Env) and two specific cell surface receptors (2). The external gp120 subunit of Env first binds to CD4 (the primary receptor); CD4 binding induces a conformational change in gp120 that exposes (or creates) determinants involved in binding to a specific chemokine receptor (the coreceptor, typically CCR5 or CXCR4); coreceptor binding then triggers conformational changes in the transmembrane gp41 subunit of Env, leading to insertion of its N-terminal fusion peptide into the target cell membrane. Fusion between the virion and cell membranes results, with the consequent release of the viral genome into the cytoplasm. The fusion/entry reaction thus presents multiple protein targets for therapeutic attack, on both the virus (gp120 and gp41) and the target cell (CD4 and coreceptors).
Figure 1.
Sequential receptor interactions involved in HIV-1 entry into T cells. In the report by Trkola et al. (3), the initial virus population used CCR5 but not CXCR4.
The coreceptors, which are members of the superfamily of G protein-coupled receptors, have provoked particular interest (4). Although more than a dozen chemokine receptors and related proteins have been described with coreceptor activity in vitro, CCR5 and/or CXCR4 are considered to be the major coreceptors operating in vivo, and are used by all HIV-1 HIV-1 strains. The HIV-1 viruses transmitted from one individual to another almost invariably use CCR5 but not CXCR4 (this phenotype is designated R5). R5 viruses predominate throughout the asymptomatic phase of infection, which can last several years, during which time the viral load in blood and lymph nodes remains relatively low, and the immune system functions adequately. Typically, however, the viral load eventually rises, concomitant with a dramatic loss of CD4+ T cells; the resulting immune system demise heralds the onset of AIDS (5). It is around the time of transition to the symptomatic phase that HIV-1 variants capable of using CXCR4 (R5X4 or X4 phenotypes) often appear among the population of R5 viruses. Indeed, the prevailing notion is that CXCR4 use contributes to the acceleration of CD4+ T cell depletion in late-stage disease (6). The chemokine ligands for these coreceptors (RANTES, MIP-1α, MIP-1β, and MCP-2 for CCR5; SDF-1 for CXCR4) also may influence HIV-1 transmission and pathogenesis (7).
There are several reasons that the coreceptors are considered to be attractive targets for therapy. First, G protein-coupled receptors have proven especially amenable to drug development (8). Second, although HIV-1 variants have been obtained (at least in vitro) that circumvent the requirement for CD4 (9), none have ever been described that are coreceptor-independent. Finally, genetic analyses of human populations (10) reveal that CCR5 is dispensable for normal health. The only significant medical consequence yet noted for individuals lacking CCR5 because of homozygosity for a CCR5 “knockout” allele (CCR5 Δ-32) is their nearly complete resistance to HIV-1 infection; CCR5 Δ-32 heterozygosity has been associated with slower rates of HIV-1 disease progression. Thus CCR5 inhibitors might be expected to suppress HIV-1 replication in the body, without causing unacceptable side effects. Indeed numerous categories of CCR5-targeted approaches have been reported (4), including chemokine ligands and their derivatives, anti-CCR5 monoclonal antibodies, synthetic peptides, gene therapy approaches, and low molecular weight inhibitors. The last class of agents seem particularly promising because of their potential for favorable pharmacologic properties (oral delivery, bioavailabilty, half-life, etc.). However, a major concern for all of these CCR5-directed strategies is that they may unintentionally accelerate appearance of highly pathogenic CXCR4-using variants.
The potential for coreceptor switching is the central focus of the article by Trkola et al. (3). The authors analyzed the development of resistance of R5 viruses cultured in the presence of AD101, a small molecule antagonist of CCR5. This compound is chemically related to another agent developed by Schering-Plough called SCH-C (11), which is in clinical development. The article's major finding is that although AD101-resistant virus emerged during repeated passage, the escape mutants did not switch to CXCR4, or any alternative coreceptor; rather they persisted in using CCR5 as the obligate coreceptor. The study's in vitro system was designed to represent the in vivo environment with respect to several relevant parameters: the R5 virus was from an individual in whom CXCR4-using variants were undetectable at the time of virus isolation, but emerged within the next 13 months; the experimental inoculum was a heterogeneous swarm rather than a biological or molecular clone; the target cells were phytohemagglutinin-activated primary CD4+ T cells, which would be expected to express both CCR5 and CXCR4, as well as some alternative coreceptors. Thus there was seemingly ample potential for coreceptor swithing.
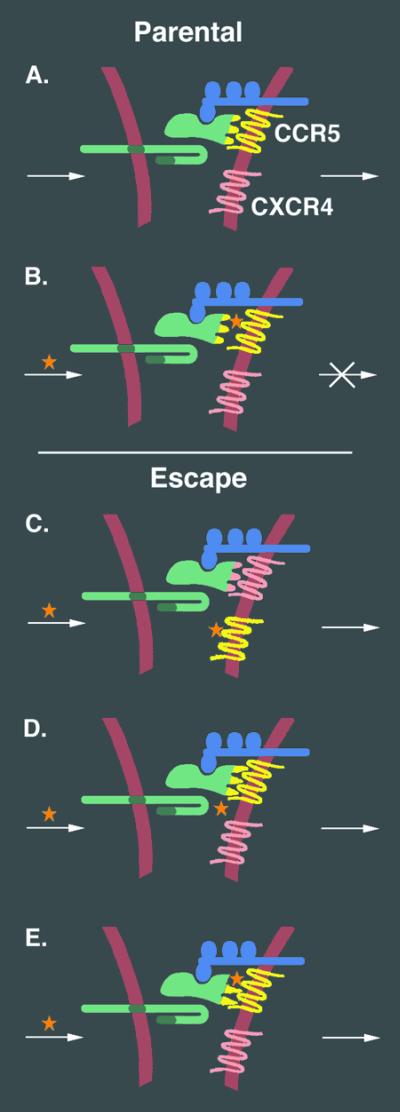
Selection was performed by exposing the cultures to incremental increases in AD101 concentration over a >10-fold range. Virus isolated after the sixth passage showed intermediate resistance (3-fold), and virus isolated after the 19th passage displayed high-level resistance (>20,000-fold). Both escape mutants were able to infect cells expressing low levels of CCR5 more efficiently than the parental virus. Experiments with chimeric viruses established that changes in Env accounted for the highly resistant phenotype. Sequence analysis suggested that the selection pressure operated primarily on gp120 rather than gp41; comparison of gp120 amino acid sequences from individual clones revealed that the highly resistant variant had greatly reduced diversity as compared with the parental virus. The authors propose a two-stage model for the selection of the highly resistant mutant (see Fig. 2). Initially, gp120 acquired the ability to use CCR5 more efficiently, i.e., by competing more effectively with the drug for binding to CCR5, or by increasing the likelihood that a given gp120/CCR5 interaction would trigger fusion. In the second step, the authors suggest that gp120 adapted to function with the drug-bound form of CCR5. This two-stage mechanism is reasonable given the selection protocol involving stepwise increases in drug concentration.
Figure 2.
Potential viral escape mechanisms from CCR5-targeted inhibitor AD101. The normal gp120/CCR5 interaction (A) is blocked when AD101 (*) binds specifically to CCR5, thereby preventing membrane fusion and virus entry (B). Several mutational variations in gp120 can be envisioned that would lead to viral escape from AD101, including: change (or expansion) of coreceptor usage to enable CXCR4 usage (C); increased affinity of gp120 for CCR5, thereby enabling more efficient competition with AD101 for binding to CCR5 (D); acquisition of the ability of gp120 to function with CCR5 bound to AD101. In the study by Trkola et al., no evidence for escape mechanism C was detected. The authors propose a sequential resistance pathway, involving first adaption to more efficient use of CCR5 (D) followed by selection for variants that can use the drug-bound form of CCR5 (E).
The finding that a population of primary R5 viruses was loath to switch to CXCR4 usage under pressure from a CCR5-directed inhibitor might at first seem counterintuitive. But would coreceptor switching be expected as a preferred escape pathway in the protocol used by Trkola et al.? In fact, the literature only documents experimentally induced transition of CCR5 to CXCR4 usage in restricted circumstances (12–14). Fortuitously or by design, the starting R5 virus in each of those reports required only minimal sequence change for acquition of CXCR4 usage. In the published in vitro studies (12, 13), selection was performed in CD4+ T cell lines expressing abundant CXCR4 but lacking CCR5, thereby forcing a coreceptor switch. In the in vivo study using hu-PBL-SCID mice (14), two CCR5-blocking RANTES derivatives were compared; only the more potent CCR5-targeting agent induced appearance of CXCR4-using variants. Thus, CCR5 to CXCR4 adaptation was achieved experimentally under deliberately favorable circumstances, i.e., starting with R5 strains that required only minimal change, and under conditions in which CCR5 was either absent or maximally blocked. Whether primary R5 viruses in the infected person can readily adapt to CXCR4 usage is unknown. Perhaps many more mutations are required, with transitional intermediates that are not readily generated in vitro.
In the selection protocol used by Trkola et al., an escape pathway involving sequential changes permitting continued use of the same coreceptor was preferred over a pathway demanding adaptation to an alternative coreceptor. Although the experimental system was designed for optimal relevance, some of its features may have inadvertently favored the former pathway over the latter. Despite the fact that the primary R5 virus swarm was from an individual who eventually developed CXCR4-using viruses, there was no direct demonstration that it had the potential to adapt to CXCR4 usage under these in vitro conditions. Also, although the stepwise increase in AD101 concentration was presumably required to obtain resistant variants, the associated early adaptation to more efficient CCR5 usage at low drug concentrations may have precluded a subsequent switch to CXCR4. It will be valuable to test the generalizability of the major conclusion by extending examination of coreceptor switching in this experimental system under different conditions, including: starting with an R5 virus that is known to require only a few amino acid changes for CXCR4 use (as in the published experiments described above); testing other classes of CCR5 blocking agents, e.g., chemokines and their derivatives that have the capacity to make CCR5 completely unavailable, both by blocking its use and down-modulating its surface expression.
In considering the relationships between experimentally induced coreceptor switching and the corresponding phenomenon in infected individuals, several aspects of the in vivo situation are worth noting. For one, the appearance of CXCR4-using variants occurs very inefficiently in infected persons, typically after several years or not at all. Secondly, the duration, quantity, and environmental complexity of in vivo viral replication vastly exceeds that which can be mimicked in any in vitro system. The existence of diverse tissue reservoirs for viral replication, the changing expression levels of CCR5 and CXCR4 and their respective ligands, the potential use of a broader coreceptor repertoire, and the viral and host reactions to an evolving immune response are factors that may contribute to this complexity. These features of in vivo HIV-1 replication presumably are permissive for multistep pathways that might be required for the coreceptor switch in the viral population. Whatever the mechanism(s), the widely held view is that the emergence of CXCR4-using variants drives an unfavorable clinical outcome (6).
With these complexities in mind, a CCR5-blocking agent may have varying effects depending on the status of the infected person. At an early stage of infection when viruses with the R5 phenotye vastly predominate and those along the pathway to CXCR4 usage may be extremely rare, it might be desirable to prevent CCR5 usage as completely as possible. At a later stage when many viruses may have progressed along the CXCR4-using pathway, blocking CCR5 presumably would have a beneficial effect of inhibiting the predominant R5 population, but also might provide a selective advantage to viruses that have progressed to CXCR4 usage. Various classes of CCR5-blocking agents therefore might produce different outcomes depending on the situation. For example, an agent with the capacity to prevent CCR5 usage totally (e.g., chemokines and their derivatives) also might have the greatest potential to accelerate coreceptor switching; this might be problematic late in infection. Alternatively, an agent that can select for use of the drug-bound form of CCR5 (i.e., AD101 and related compounds like SCH-C) provides the potential for escape without inducing the coreceptor switch, and therefore may be more suitable late in infection. Further development of coreceptor blocking agents and their evaluation in clinical trials is certain to continue in the coming years. Hopefully, this class of inhibitors will become new weapons in the armamentarium for combination therapy against HIV.
Acknowledgments
We gratefully acknowledge the thoughtful insights of Leonid Margolis and Keith Peden.
Footnotes
See companion article on page 395 in issue 1 of volume 99.
References
- 1.Richman D D. Nature (London) 2001;410:995–1001. doi: 10.1038/35073673. [DOI] [PubMed] [Google Scholar]
- 2.Eckert D M, Kim P S. Annu Rev Biochem. 2001;70:777–810. doi: 10.1146/annurev.biochem.70.1.777. [DOI] [PubMed] [Google Scholar]
- 3.Trkola A, Kuhmann S E, Strizki J M, Maxwell E, Ketas T, Morgan T, Pugach P, Xu S, Wojcik L, Tagat J, et al. Proc Natl Acad Sci USA. 2002;99:395–400. doi: 10.1073/pnas.012519099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berger E A, Murphy P M, Farber J M. Annu Rev Immunol. 1999;17:657–700. doi: 10.1146/annurev.immunol.17.1.657. [DOI] [PubMed] [Google Scholar]
- 5.Fauci A S. Nature (London) 1996;384:529–534. doi: 10.1038/384529a0. [DOI] [PubMed] [Google Scholar]
- 6.Husman A M D, Schuitemaker H. Trends Microbiol. 1998;6:244–249. doi: 10.1016/s0966-842x(98)01249-9. [DOI] [PubMed] [Google Scholar]
- 7.Lusso P. Virology. 2000;273:228–240. doi: 10.1006/viro.2000.0443. [DOI] [PubMed] [Google Scholar]
- 8.Sautel M, Milligan G. Curr Med Chem. 2000;7:889–896. doi: 10.2174/0929867003374570. [DOI] [PubMed] [Google Scholar]
- 9.Hoxie J A, Labranche C C, Endres M J, Turner J D, Berson J F, Doms R W, Matthews T J. J Reprod Immunol. 1998;41:197–211. doi: 10.1016/s0165-0378(98)00059-x. [DOI] [PubMed] [Google Scholar]
- 10.O'Brien S J, Moore J P. Immunol Rev. 2000;177:99–111. doi: 10.1034/j.1600-065x.2000.17710.x. [DOI] [PubMed] [Google Scholar]
- 11.Strizki J M, Xu S, Wagner N E, Wojcik L, Liu J, Hou Y, Endres M, Palani A, Shapiro S, Clader J W, et al. Proc Natl Acad Sci USA. 2001;98:12718–12723. doi: 10.1073/pnas.221375398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng-Mayer C, Liu R, Landau N R, Stamatos L. J Virol. 1997;71:1657–1661. doi: 10.1128/jvi.71.2.1657-1661.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dejucq N, Simmons G, Clapham P R. J Gen Virol. 2000;81:2899–2904. doi: 10.1099/0022-1317-81-12-2899. [DOI] [PubMed] [Google Scholar]
- 14.Mosier D E, Picchio G R, Gulizia R J, Sabbe R, Poignard P, Picard L, Offord R E, Thompson D A, Wilken J. J Virol. 1999;73:3544–3550. doi: 10.1128/jvi.73.5.3544-3550.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]