Abstract
The prevalence of metabolic dysfunction-associated steatotic liver disease (MASLD) has rapidly increased world-wide to 30%, with increasing of type 2 diabetes (T2D) and obesity in last two decades. The spectrum of MASLD covers from simple hepatic steatosis to the progressive metabolic dysfunction-associated steatohepatitis (MASH) with or without fibrosis, cirrhosis and hepatocellular carcinoma. The MASLD symptoms include dyslipidemia, hyperglycemia, insulin resistance and obesity, the liver manifestations of metabolic syndrome. Treatment option for MASH fibrosis is limited. Since the discovery of bile acids as the endogenous ligands of farnesoid X receptor (FXR) in early 1990, bile acid and FXR based-drug therapies have been developed and tested in clinical trials for cholestatic liver diseases and MASH fibrosis. However, many of these drugs have unwanted side-effects and moderate efficacy in improving fibrosis. The US Food and Drug Administration has not approved any of bile acid- and FXR-based drugs for MASH fibrosis. Drug therapies alternative to bile acid derivatives for MASH have been in clinical trials. Recently, resmetirom, a liver-specific- and thyroid hormone receptor beta-selective agonist has been approved for MASH fibrosis. Glucagon-like peptide-1 receptor agonists also are in clinical trials for MASH. This review covers recent development of novel drug therapies for MASH fibrosis, T2D and obesity.
Keywords: Bile acids, Farnesoid X receptor (FXR), Takeda G protein-coupled receptor 5 (TGR5), Glucagon-like peptide-1 (GLP-1), Metabolic dysfunction-associated steatotic liver disease (MASLD), Metabolic dysfunction-associated steatohepatitis (MASH)
Graphical abstract
Highlights
-
•
Bile acid-activated FXR signaling has many metabolic benefits in the gastrointestinal tract.
-
•
Bile acid-activated TGR5 has diverse functions in many tissues.
-
•
Bile acid derivatives and FXR agonists have been developed, but none has been approved as drug therapy for MASH.
-
•
Resmetirom, a liver specific and thyroid hormone receptor β-selective agonist, is the only drug approved for MASH fibrosis.
-
•
A GLP-1 receptor agonist semaglutide is used for weight reduction and type 2 diabetes.
-
•
GLP-1 receptor agonists are in clinical trials for MASH fibrosis.
1. Introduction
Metabolic dysfunction-associated steatotic liver disease (MASLD) is a new recommended term for non-alcoholic fatty liver disease.1 MASLD has rapidly increased in recent years with an overall global prevenance of about 30%.2 The spectrum of MASLD covers from simple hepatic steatosis to the progressive metabolic dysfunction-associated steatohepatitis (MASH) with or without fibrosis, cirrhosis, and hepatocellular carcinoma (HCC), the end-stage liver disease.3 The MASLD symptoms include dyslipidemia, hyperglycemia, inflammations, insulin resistance and obesity. Adaptation of Western high-fat and high-calorie diets and the sedative lifestyle contribute to MASLD. Drug treatment option for MASH fibrosis is very limited.
Three decades ago, a nuclear receptor, farnesoid X receptor (FXR) was identified as a bile acid-activated receptor.4, 5, 6, 7 Bile acids are physiological detergent molecules derived from cholesterol. Bile acid synthesis is tightly regulated by a classic feedback mechanism: the end products of the cholesterol catabolic pathway, bile acids, inhibit the first and rate-limiting enzyme, cholesterol 7alpha-hydroxylase (CYP7A1) in the classic pathway of bile acid synthesis in the liver (Fig. 1).8 Cholesterol is converted to cholic acid (CA) and chenodeoxycholic acid (CDCA), two predominant primary bile acids synthesized in human liver. CDCA is the most potent endogenous FXR ligand. FXR plays a critical role in control of the circulating bile acid pool and protecting against liver injury by cytotoxic bile acids. Bile acids emulsify lipid-soluble vitamins and nutrients to form mixed micelles containing cholesteryl esters (CEs), monohydroxy glycerol, and free fatty acids. Chylomicrons (CM) and remnants (CMR) carry these cargos via the lymphatic drainage system to transport these nutrients to the liver. In hepatocytes, free fatty acids from diets, mobilized from adipose tissues or synthesized by de novo lipogenesis (DNL), are used for energy metabolism. Excessive free fatty acids are esterified to glycerol to form triglyceride (TG) for storage as lipid droplets. TG and CEs are assembled with ApoB100 to form very low-density lipoproteins (VLDLs), which are secreted to blood circulation to deliver TG to muscle and adipose tissues for storage or energy metabolism.9 Reduced VLDL assembly or secretion and impaired TG clearance contribute to fatty liver diseases.
Fig. 1.
The role of FXR signaling in liver, adipose tissue, pancreas and intestine. In the intestine, bile acids emulsify lipid-soluble vitamins and nutrients to form mixed micelles containing cholesteryl esters (CEs), mono-hydroxy glycerol, and free fatty acids (FFA). Chylomicrons (CM) and remnants (CMR) carry these cargos via the lymphatic drainage system to deliver nutrients to the liver. FFA from diets and adipose tissues are taken up into the liver via fatty acid transporter CD36. Fatty acids also are synthesized by de novo lipogenesis (DNL). FFA are oxidized by β-oxidation in mitochondria for energy metabolism. Excessive FFA are esterified to glycerol to form triglyceride (TG) for storage as lipid droplets in the liver. FXR inhibits CD36, DNL and VLDL assembly and secretion, and stimulates TG clearance by inducing lipoprotein lipase (LPL) and ApoCII. FXR induces ATP-binding cassette transporter A1 (ABCA1) for reverse cholesterol transport but increases serum LDL-cholesterol and reduces ApoA1 and serum HDL-cholesterol. In the liver, activation of FXR inhibits CYP7A1 in the classic pathway of bile acid synthesis via induction of SHP. FXR induces BSEP to secret bile acids into bile, and inhibits hepatic bile acid uptake transporter, NTCP to maintain low intrahepatic bile acid levels. In enterocytes, FXR stimulates apical sodium-dependent bile acid transporter (ASBT) in the ileum and induces organic solute transporter (OST)α/β for bile acids efflux into portal circulation. In the ileum, FXR induces fibroblast growth factor 19 (FGF19), which is secreted and transported via enterohepatic circulation to activate hepatic membrane FGFR4/β-Klotho signaling to inhibit CYP7A1 and bile acid synthesis. In intestinal L cells, FXR induces expression of TGR5, which stimulates GLP-1 secretion to pancreatic β cells to induce insulin secretion and insulin sensitivity. Activation of FXR reduces gluconeogenesis and glycolysis in fasting and DNL. In HSC and Kupffer cells, activation of FXR reduces inflammation via NLRP3 inflammasome and NF-κB-mediated production of pro-inflammatory cytokines. In adipose tissues, FXR stimulates energy metabolism by inducing peroxisome proliferator-activated receptor gamma (PPARγ) coactivator-1alpha (PGC-1α) and mitochondrial uncoupling protein-1 (UCP-1). Abbreviations: BSEP, bile salt export pump; BSH, bile salt hydrolase; CA, cholic acid; CDCA, chenodeoxycholic acid; CYP7A1, cholesterol 7alpha-hydroxylase; DCA, deoxycholic acid; FGFR4, fibroblast growth factor receptor 4; FXR, farnesoid X receptor; GLP-1, glucagon-like peptide-1; HDL, high-density lipoprotein; HSC, hepatic stellate cell; 7α-HSDH, 7alpha-hydroxysteroid dehydrogenase; LCA, lithocholic acid; LDL, low-density lipoprotein; MTTP, microsomal triglyceride transfer protein; NF-κB, nuclear factor-kappaB; NLRP3, NOD-, LRR- and pyrin domain-containing protein 3; NTCP, sodium taurocholate cotransporting polypeptide; SHP, small heterodimer partner; TCA, taurocholic acid; TCDCA, taurochenodeoxycholic acid; TGR5, Takeda G protein-coupled receptor 5; VLDL, very low-density lipoprotein. This Figure was modified and redrew from Fig. 3 in Chiang JYL, Ferrell JM, Bile acid receptors FXR and TGR5 signaling in fatty liver diseases and therapy, Am J Physiol Gastrointest Liver Physiol, 2020;318:G554-G573, https://doi.org/10.1152/ajpgi.00223.2019, with permission from the authors and the American Physiological Society.
Catabolism of cholesterol to bile acids is the predominant pathway for elimination of excessive cholesterol in the liver. Increasing bile acid synthesis can prevent hypercholesterolemia and cardiovascular diseases. However, increasing bile acid synthesis can cause cholestatic liver diseases and inflammation. Thus, maintaining bile acid homeostasis is critically important in preventing cholestasis, fatty liver diseases and atherosclerosis. Bile acids are conjugated to amino acids, glycine or taurine, excreted into bile and stored in the gallbladder. After meal intake, bile acids are released into the gastrointestinal tract for digestion and absorption of nutrients. Small amount (∼5%) of bile acids excreted into feces are replenished by de novo bile acid synthesis. Most conjugated-bile acids are reabsorbed in the terminal ileum and colon, where bacterial bile salt hydrolases (BSHs), de-conjugate taurine and glycine from conjugated-primary bile acids and subsequently bacterial 7alpha-hydroxysteroid dehydrogenases (7α-HSDHs) remove a 7α-hydroxy group from CA and CDCA to secondary bile acids, deoxycholic acid (DCA) and lithocholic acid (LCA), respectively (Fig. 1). Thus, gut bacteria play a critical role in control of circulating bile acid composition and pool size. The enterohepatic circulation of bile acids is an important physiological mechanism for control not only bile acid synthesis and transport but also whole-body glucose, lipid and energy metabolism.6 Targeting FXR as a drug therapy for cholestatic liver diseases, MASH fibrosis, diabetes and obesity became an emerging area of drug development.10, 11, 12
Bile acid-based drugs and non-steroidal FXR agonists have been in clinical trials for many years, but none of them has been approved for MASH fibrosis. In March 2024, the US Food and Drug Administration (FDA) approved Rezdiffra (resmetirom), a thyroid hormone receptor beta-specific agonist for MASH. Resmetirom is the first and only FDA approved drug for MASH fibrosis. Moreover, several glucagon-like peptide-1 receptor (GLP-1R) agonists have been approved for weight reduction and type 2 diabetes (T2D) and are currently in several clinical trials for MASH fibrosis. This review summarizes bile acid-activated receptors and their signaling in regulation of liver metabolism, the status of bile acid-based drugs for MASH fibrosis, then focuses on new therapeutic drugs for MASH fibrosis, which have not been included in recent reviews on drug therapies for MASH.
2. Bile acid receptor signaling in metabolism
Bile acids activate several nuclear receptors, FXR, pregnane X receptor, vitamin D receptor as well as G protein-coupled receptors (GPCRs), Takeda G protein-coupled receptor 5 (TGR5, also known as G protein-coupled bile acid receptor 1) and sphingosine-1-phosphate receptor 2. These receptors play critical roles in regulation of bile acid, glucose and energy metabolism, and inflammation and immune responses. FXR and TGR5 are targets for drug therapies for liver diseases, diabetes and obesity. Recent development of drug therapies based on these two receptors will be briefly reviewed. Vitamin D receptor, pregnane X receptor and sphingosine-1-phosphate receptor 2 will not be reviewed here since few drugs targeted to these receptors have been developed for MASH treatment.
2.1. FXR
FXR is a nuclear hormone receptor. FXR forms a heterodimer with retinoid X receptor (RXR)α and binds to the hormone responsive element on its target gene promoter, the inverted-repeat of AGGTCA separated by one nucleotide. Without ligand binding, FXR/RXRα interacts with a co-repressor and is inactive. Upon ligand binding, FXR recruits a co-activator to replace a co-repressor and stimulates RNA polymerase II-mediated trans-activation of their target genes. FXR is activated by endogenous ligands, primary bile acids CDCA and CA the most officious, followed by secondary bile acids DCA, LCA and ursodeoxycholic acid (UDCA). FXR is highly expressed in the gastrointestinal tract and plays diverse functions in liver metabolism as follows: (i) it induces a negative nuclear receptor, small heterodimer partner (SHP), which inhibits the expression of CYP7A1 in bile acid synthesis; (ii) it induces biliary bile salt export pump (BSEP) to secret bile acids into bile; (iii) it inhibits sinusoidal sodium taurocholate cotransporting polypeptide (NTCP) for uptake bile acids from portal blood circulation; (iv) it reduces fatty acid uptake transporter CD36 in hepatocytes; (v) it inhibits DNL in hepatocytes; (vi) it reduces microsomal triglyceride transfer protein (MTTP) for VLDL assembly and secretion; (vii) it induces lipoprotein lipase (LPL) and ApoCII for TG hydrolysis; (viii) it inhibits glycolysis and gluconeogenesis in the post-prandial state to reduce serum glucose; (ix) it stimulates insulin synthesis and secretion in pancreatic β cells to increase insulin sensitivity; (x) it induces ATP-binding cassette transporter A1 (ABCA1) in macrophage to liver reverse cholesterol transport; (xi) it reduces ApoA1 and serum high-density lipoprotein (HDL)-cholesterol but increases serum low-density lipoprotein (LDL)-cholesterol (Fig. 1).12, 13, 14, 15, 16, 17 In addition, activation of FXR reduces oxidative stress, endoplasmic reticulum (ER) stress and HCC.18,19 FXR signaling also shapes the gut microbiota to control lipid metabolism.20
2.2. The gut-to-liver axis in enterohepatic circulation of bile acids
The underlying mechanisms of bile acid feedback regulation of bile acid synthesis are complicated and not completely understood. The enterohepatic circulation of bile acids is an important physiological system for control bile acid homeostasis. Pandak et al.21 first reported in 1995 that intraduodenal infusion, but not intravenous infusion of taurocholate, inhibited Cyp7a1 and bile acid synthesis in bile fistula rats, suggesting factors synthesized and released from the intestine must be involved in regulation of bile acid synthesis. Twenty years later, fibroblast growth factor 15 (FGF15, or human orthologue FGF19) was identified as the intestinal factor that is induced by FXR and circulated via enterohepatic circulation to activate hepatic membrane FGF receptor 4 (FGFR4)/β-Klotho receptor signaling to inhibit Cyp7a1 and bile acid synthesis in mice (Fig. 1).22 Activation of FXR-FGF19 signaling reduces hepatic and intestinal inflammation by inhibiting NOD-, LRR- and pyrin domain-containing protein 3 (NLRP3) inflammasomes and nuclear factor-kappaB (NF-κB)-mediated pro-inflammatory-cytokine production. In hepatic Kupffer cells (resident macrophages), FGF19 inhibits tumor growth factor (TGF)β1 signaling. Activation of FXR/FGF19 signaling inhibits activation of the quiescent hepatic stellate cells (HSCs) to myofibroblast and progression of MASH fibrosis.23
Intestine FXR antagonism has been shown to protect against diet-induced obesity in mice. Antioxidant tempol reduces Lactobacillus and BSH activity to increase tauro-beta-muricholic acid (T-β-MCA), which antagonizes intestinal FXR to protect against diet-induced obesity in mice.24 Deficiency of intestine Fxr protects mice from diet-induced obesity and diabetes by reducing ceramides, hepatic ER stress and reactive oxygen species (ROS) and improves insulin resistance.25,26 A T2D drug, metformin reduces Bacteroides fragilis and increases T/GUDCA to antagonize FXR and improves hepatic metabolism in T2D patients.27 However, intestine-restricted FXR agonist fexaramine activates intestine FXR/FGF15 to promote white adipose tissue browning and improve glucose and insulin sensitivity in diet-induced obese mice.28 This effect may be mainly through TGR5 since fexaramine increased gut bacteria Acetatifactor and Bacteroides to convert CDCA and UDCA to LCA, which activates TGR5 to stimulate GLP-1 secretion and improve insulin sensitivity.29 It appears that FXR agonists and antagonists may reshape the gut microbiome differently and both have metabolic benefits on obesity and diabetes. All these studies implicate the critical role of gut microbiota in host metabolism and pathogenesis of T2D, obesity and MASLD.
High-fat, high-cholesterol, and high-sugar/fructose diets can cause dysbiosis and contribute to progression of MASLD to MASH, diabetes and obesity. In white adipose tissue, activation of FXR/FGF19 signaling promotes adipose tissues beiging/browning by stimulating mitochondria energy metabolism via induction of peroxisome proliferator-activated receptor gamma (PPARγ) coactivator (PGC)-1α and uncoupling protein-1 (UCP-1) (Fig. 1).
2.3. TGR5
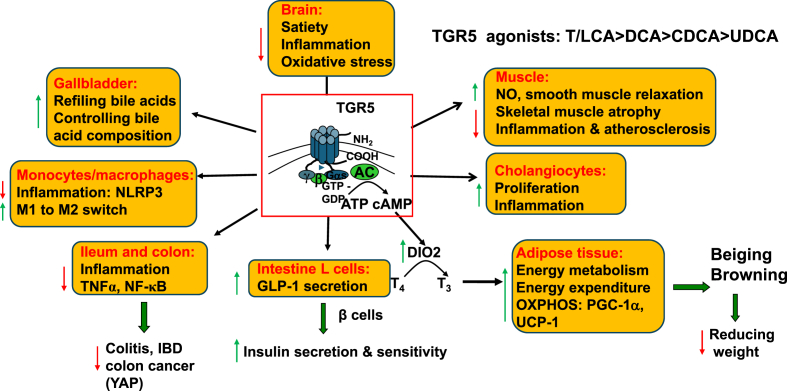
Secondary bile acids (tauro-conjugated or non-conjugated) are more efficacious than primary bile acids in activation of TGR5 (T/LCA > DCA > CDCA > UDCA). UDCA is a weak TGR5 agonist that can modulate FXR signaling.30 TGR5 is widely distributed and has diverse functions in many tissues including hepatic Kupffer cells, sinusoidal endothelial cells (SECs), stellate cells, gallbladder, cholangiocytes, intestinal enteroendocrine L cells, white adipose tissues, smooth muscle cells, hypothalamus of the brain, etc. (Fig. 2).31,32 As a GPCR, activation of TGR5 stimulates adenylyl cyclase to convert ATP to cAMP, a second messenger that phosphorylates protein kinase A (PKA) to activate target proteins or enzymes by phosphorylation. Many functions of TGR5 signaling overlap with FXR signaling. In adipose tissue, activation of TGR5 stimulates cAMP, which activates iodothyronine deiodinase 2 (DIO2), to convert thyroxine (T4) to triiodothyronine (T3) that stimulates mitochondrial energy metabolism. In white adipose tissue, TGR5 promotes adipose tissue beiging and browning and induces mitochondrial PGC-1α and UCP-1 to stimulate oxidative phosphorylation. FXR and TGR5 are co-expressed in intestinal L cells.33 Bile acid activation of TGR5 stimulates secretion of GLP-1 to induce insulin synthesis in pancreatic islets and increase insulin sensitivity. In monocytes and macrophages, TGR5 reduces NLRP3 inflammasomes. Activation of TGR5 stimulates switching of macrophages from pro-inflammatory M1 phenotype to anti-inflammatory M2 phenotype (Fig. 2).34, 35, 36, 37 Activation of TGR5 induces nitric oxide (NO), which relaxes smooth muscle and reduces skeletal muscle atrophy and inflammation and atherosclerosis.38, 39, 40, 41 Opposite to its anti-inflammatory function in most cells, activation of TGR5 stimulates cholangiocyte proliferation and inflammation.42 TGR5 is highly expressed in the gallbladder endothelia to stimulate bile acid refiling and control bile acid composition and pool. In ileum and colon, activation of TGR5 reduces tumor necrosis factor alpha (TNFα) and NF-κB mediated-inflammation. In the brain, activation of TGR5 stimulates satiety to reduce food intake and inflammation and oxidative stress (Fig. 2).32,37,43, 44, 45, 46, 47, 48 The gut produced signals GLP-1 and neuropeptide Y (NPY) are circulated to the hypothalamus of the brain to control appetite. The vagus nerve system connects the gastrointestinal tract to the hypothalamus and other regions of the central nervous system to control appetite by balancing NPY and agouti-related protein (AgRP) signals in orexigenic neurons and proopiomelanocortin (POMC) and cocaine- and amphetamine-regulated transcript peptide (CART) signals in anorexigenic neurons.48, 49, 50
Fig. 2.
The role of TGR5 signaling in the brain, gallbladder, monocytes and macrophages, muscle, intestine and adipose tissues. TGR5 (or G protein-coupled bile acid receptor-1) is expressed in many tissues and activated by bile acids in the order of T/LCA > DCA > CDCA > UDCA. Activation of TGR5 converts ATP to cAMP. In central nervous system of the brain TGR5 controls satiety and reduces food intake, inflammation and oxidative stress. In the gallbladder, TGR5 stimulates gallbladder refiling and controls circulating bile acid pool and composition. In smooth muscles, activation of TGR5 induces nitric oxide (NO), relaxes smooth relaxation, and reduces skeletal muscle atrophy and inflammation and atherosclerosis. In adipose tissue, activation of TGR5 activates iodothyronine deiodinase 2 (DIO2) to active T4 to T3 and stimulate white adipose tissue beiging and browning and mitochondrial energy metabolism to reduce weight. In postprandial state, bile acids, fats and carbohydrates activate TGR5 to stimulate GLP-1 secretion to pancreatic β cells to induce insulin secretion and insulin sensitivity. In monocytes and macrophages, activation of TGR5 reduces inflammation, but in cholangiocytes, activation of TGR5 promotes proliferation and inflammation. Abbreviations: CDCA, chenodeoxycholic acid; DCA, deoxycholic acid; GLP-1, glucagon-like peptide-1; IBD, inflammatory bowel disease; LCA, lithocholic acid; NF-κB, nuclear factor-kappaB; NLRP3, NOD-, LRR- and pyrin domain-containing protein 3; OXPHOS, oxidative phosphorylation; PGC-1α, peroxisome proliferator-activated receptor gamma coactivator-1alpha; T3, triiodothyronine; T4, thyroxine; TGR5, Takeda G protein-coupled receptor 5; TNFα, tumor necrosis factor alpha; UCP-1, uncoupling protein-1; UDCA, ursodeoxycholic acid.
3. The gut microbiota, bile acids and bariatric bypass surgery on MASLD
The gut microbiota plays a critical role in MASLD.51, 52, 53 Diets, drugs, antibiotics, alcohol, circadian disruption, hormones, etc. can shape gut bacteria community (microbiome) to have negative effects on host metabolism (dysbiosis). Obese and T2D patients often have dysbiosis. Depletion of gut microbiota aggravates bile acid-induced liver diseases.54
The human gut microbiome consists of 3 trillion microorganisms in four phyla: Firmicutes (60%), Bacteroidetes (22%), Actinobacteria (17%) and Proteobacteria (1%). Firmicutes are butyrate-producing bacteria, while Bacteroidetes prefers carbohydrates. T2D and obese patients have increased ratio of Firmicutes to Bacteroidetes, which enables gut bacteria to extract energy more efficiently from fats and short-chain fatty acids, thus increases adiposity and obesity of the host.55,56 In metabolic diseases, dysbiosis reduces bacteria diversity and alters bile acid composition and pool size, gut microbiome and host metabolism. It has been reported that animal high saturated-fat diets rapidly altered human gut microbiota to increase bile-tolerate bacteria (Alistipes, Bilophila and Bacteroides), decrease Firmicutes to metabolize plant polysaccharides and increase Bilophila wadesworthia. This study supports a link of dietary fats, bile acids and overgrowing of gut microbacteria to promote pro-inflammatory responses and inflammatory bowel disease.57 Dietary saturated fats induce taurocholic acid to expand the low abundant, sulfur-reducing pathobiont Bilophila wadesworthia and pro-inflammatory T-helper type 1 (Th-1) immune response and colitis in interleukin-10 (Il-10) deficient mice.58 This study suggests that animal fats increase tauro-conjugated bile acids to alter host bile acid composition and result in dysbiosis and inflammatory diseases.
Many studies suggest that gut microbiota may play a role in improving glycemic control soon after bariatric bypass surgery for weight reduction.59,60 The abundance of a mucin-degrading bacteria and probiotics, Akkermanisia muciniphia is lower in severe obesity, but increased after bariatric surgery.61 It has been reported that T2D patients have lower serum GLP-1 levels. After bariatric surgery, circulating bile acids, FGF19, and GLP-1 levels are increased and may link them to improving insulin sensitivity.62 Both FXR and TGR5 may play a role in improving glycemic control soon after bariatric surgery.44,63,64
MASLD patients have increased CA and CDCA, bile acid synthesis, and the ratio of primary bile acids to secondary bile acids.55 MASH patients have increased circulating conjugated-primary bile acids and the ratio of conjugated CA to CDCA and decreased secondary bile acids.65 Increasing primary bile acids cause a shift of microbiome to increase Firmicutes (Clostridium cluster XIVa) and DCA production, increase LPS and inflammation, reduce bile acid synthesis, and induce the progression of liver cirrhosis.66 A study reported that Clostridium subclusters IV and XIVa are associated with fatty liver disease in a large population.67 Interestingly, liver transcriptome analysis of MASLD patients identified a cluster of genes involved in regulation of bile acid synthesis and secretion and FXR signaling, CYP7A1, ABCB1/C2/A3, SHP, FXR, and FGF19, which were increased in early state of MASLD but decreased with progression of MASH fibrosis stages from F1 to F4.68 In a cohort study, hyocholic acid, a 6α-hydroxylated bile acid, was shown to increase after bariatric bypass surgery and improving insulin resistance, and may be used to access the risk for metabolic abnormality in overweight patients.69 Another study reported bariatric surgery increased gut-produced cholic acid-7-sulfate in T2D and identified cholic acid-7-sulfate as a TGR5 agonist in both mice and humans.70,71
4. Bile acid-based therapies for MASH
For over 50 years, CDCA and CA have been used as bile acid replacement therapy to treat patients with bile acid synthesis enzyme deficiency and cerebrotendinous xanthomatosis (CTX) patients with sterol 27-hydroxylase (CYP27A1) mutations. UDCA (ursodiol), a soluble non-toxic bile acid has been used to treat cholestatic liver diseases by reducing bile acid toxicity and dissolving cholesterol gallstones. Bile acids and derivatives and non-steroidal FXR agonists have been developed for MASH in last twenty years.
4.1. FXR agonists
Based on the diverse functions of FXR signaling discovered in last three decades, FXR agonists have been developed to treat cholestatic liver disease and metabolic diseases. Bile acid replacement therapy has been used to treat inborne error of bile acid synthesis for several decades. Details of bile acids and non-steroid FXR agonists for MASH fibrosis have been reviewed extensively.6,10, 11, 12,72, 73, 74
A synthetic bile acid derivative, obeticholic acid (OCA, 6-ethylchenodeoxycholic acid) is a highly selective FXR agonist for the treatment of MASH.75,76 However, by virtue of its bile acid structure, OCA has significant adverse effects such as pruritus, lowering serum HDL and increasing LDL. The efficacy of OCA for reversing fibrosis stage without worsening MASH is quite modest (phase 3 clinical trial, REGENERATE). Pruritus is a major adverse effect in patients received 25 mg OCA (51% vs. 19% placebo). The mechanism that causes pruritus by bile acid drugs is not clear. It was reported that serum autotaxin is increased in pruritus of cholestasis.77 Another study reports that a human sensory neuron expressed Mas-related G protein-coupled receptor X4 (MRGPRX4) is activated by bile acids and may contribute to pruritus.78 Increasing LDL-cholesterol is another concern for adverse cardiovascular events. Some patients may be treated with statins to reduce serum LDL-cholesterol. OCA causes cholesterol gallstone disease in some patients treated with 25 mg OCA in REGENERATE clinical trials. In 2023, FDA denied the accelerated new drug application of OCA for MASH fibrosis citing the modest efficacy and considerable cardiovascular risk.12
4.2. Non-steroidal FXR agonists
Several non-bile acid-derived steroidal FXR agonist (EDP-305) and non-steroidal FXR agonists (cilofexor, tropifexor, etc.) have been developed and in phase 2 and phase 3 clinical trials.12,79, 80, 81, 82, 83, 84, 85 Some of non-bile acid-based FXR agonists also cause pruritus. It is imperative to develop drugs alternative to FXR agonists for treating MASH.
4.3. FGF19 and FGF21
FGF19 and FGF21 are growth factors that may cause cell proliferation. NGM282, a human FGF19 analog without the tumorigenic effect of growth factors has been in clinical trial for MASLD.59,86,87 NGM282 improved fibrosis by at least 1 stage without worsening of MASH in 25% and 42% patients received NGM282 1 mg or 3 mg, respectively.87
FGF21 belongs to the same subfamily as FGF19. FGF21 is induced by fasting or torpor, high carbohydrate diets, and ketogenic diets, and mainly produced in the liver but also in other tissues, such as adipose tissues and pancreas. FGF21 exerts several metabolic benefits including increasing energy expenditure, stimulating fatty acid β-oxidation, and increasing insulin sensitivity.88, 89, 90 In the central nervous system, FGF21 signaling determines dietary preference for carbohydrates but reduced preference for sweet and alcohol.90, 91, 92 Recently, a second phase clinical trial of PEGylated-FGF21 analogue, pegbelfermin, reported that twenty weeks pegbelfermin 20 mg daily treatment improved metabolic parameters and fibrosis biomarkers in patients with obesity and T2D with fatty liver disease. FGF21 analogues may be further explored as new drugs for MASH fibrosis.93, 94, 95
5. Thyroid hormone receptor β-selective agonist for MASH
Thyroid hormone is known to stimulate energy metabolism and reduce hepatic lipid levels. Thyroid hormone's action is through their receptors, thyroid hormone receptors α and β (THRα and THRβ). THRs are the type 1 classic endocrine receptors activated by T3 to bind to the thyroid hormone responsive element (direct repeats separated by 4 nucleotides) on their target gene promoters as a homodimer or heterodimer with RXRα. THR stimulates mitochondrial biogenesis and induces PGC-1α and energy metabolism. Hypothyroidism is higher in MASH patients and linked to several clinical symptoms of the metabolic syndrome, such as obesity and dyslipidemia.96 Hepatic THR signaling may be altered in MASLD, and drug targeting to liver THRβ may be used to treat MASLD.96
THRα is the major form of THRs in heart muscle and other tissues, but in liver THRβ predominates. Selective THRβ activation has been shown to reduce weight and lower serum cholesterol and lipoprotein (a) with reduced cardiovascular liability.97 Thyroid hormones reduce de novo lipogenesis by suppression of activation and transcription of sterol regulatory element-binding protein-1 (SREBP-1).
Resmetirom (MGL-3196) is a liver-specific- and THRβ-selective agonist.98 A recent clinical study found a significant negative correlation of liver organic anion transporting polypeptide (SLCO1C1) with THRβ and DIO1 and THRβ levels are negative correlated with serum TGs and hemoglobin A1C (HbA1C) and MASH scores.
Resmetirom ameliorated MASH fibrosis by inhibiting intestinal lipid absorption and reducing 12-hydroxylated bile acids (CA and DCA), which were elevated and have been linked to insulin resistance and dyslipidemia in mice and humans.99,100 Thus, selective agonists targeted to liver THRβ may be used to treat obesity and hypercholesterolemia without unwanted effects on the heart and other tissues.
In March 2024, FDA issued a conditional approval of resmetirom for MASH fibrosis treatment. Clinical trials of resmetirom for MASH (NCT03900429) reported a significant reduction of liver fat compared with placebo in phase 2b trial and was associated with higher rate of MASH resolution.96,101,102 The phase 3 trial of MASH (NCT04197479) reported that resmetirom at 80 and 100 mg for over 52 weeks was safe and well tolerated in adult MASH patients.103 An expert panel recommends monitoring resmetirom in patients with MASH with moderate to non-cirrhotic fibrosis.104 Several clinical trials of this drug for MASH are ongoing.105 A phase 3 trial of resmetirom showed MASH patients with fibrosis achieved fibrosis improvement at least one stage without worsening of fibrosis activity score in 24.2% patients at 80 mg, and 29.5% patients at 100 mg (NCT03900429).106
6. Incretin and glucagon receptor agonists for MASH
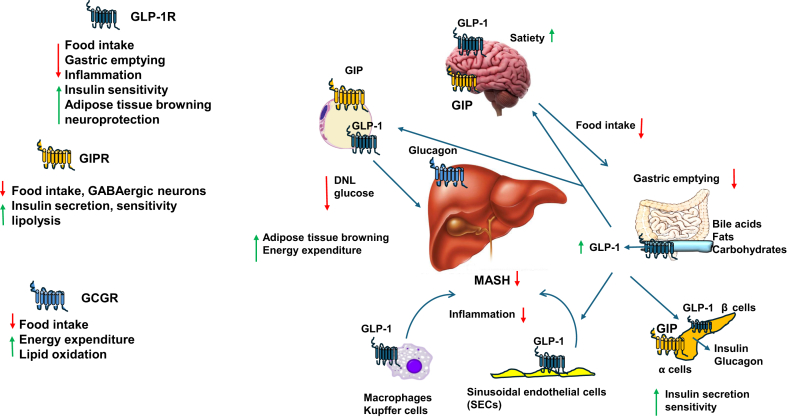
Incretin hormones, mainly glucagon, glucose-dependent insulinotropic polypeptide (GIP) and GLP-1, play a critical role in control of appetite, food intake, energy expenditure, insulin secretion and glucose levels (Fig. 3).107 Incretins activate a family of class B heterotrimeric (α,β,γ) GPCRs including glucagon receptor (GCGR), GLP-1R and GIP receptor (GIPR) (Fig. 3).
Fig. 3.
Glucagon receptor family in the brain, adipose tissues, gastrointestinal tract, pancreas and liver. Glucagon receptor (GCGR), GLP-1 receptor (GLP-1R) and GIPR are B type family of GPCR. GCGR is mainly expressed in the liver to control food intake, energy expenditure and lipid oxidation. GLP-1R is expressed in adipose tissues, intestinal L cells, pancreatic β cells, macrophages and Kupffer cells, and sinusoidal endothelial cells (SECs) to regulate various functions. GIPR is expressed in adipose tissue, pancreatic α and central nervous system to control various functions similar to TGR5 as illustrated and described in Fig. 2. Abbreviations: DNL, de novo lipogenesis; GIPR, glucose-dependent insulinotropic polypeptide receptor; GLP-1, glucagon-like peptide-1; GPCR, G protein-coupled receptor; MASH, metabolic dysfunction-associated steatohepatitis; TGR5, Takeda G protein-coupled receptor 5.
Pro-glucagon is cleaved to glucagon (27 amino acid peptide) by prohormone convertase 2 (PC2) in pancreatic α cells and β cells. In β cells, PC2/3 converts pro-glucagon to glucagon, while in the brain and intestinal enteroendocrine L cell PC2/3 converts pro-glucagon to GLP-1. GLP-1(7-36) is a biologically active GLP-1.
6.1. GCGR
GCGR is expressed mainly in the liver. GCGR is the first incretin receptor identified and cloned. Activation of incretin receptors by their ligands releases a second messenger cAMP, which activates a PKA cascade by phosphorylation. Glucagon activation of GCGR stimulates phosphorylation and activation of phosphoenolpyruvate carboxykinase (PEPCK) in gluconeogenesis in the liver and muscles, and PPARγ in lipid oxidation.
6.2. GIPR
GIPR is expressed in adipose tissue, pancreatic α cells and the brain. GIP stimulates lipolysis, insulin secretion and sensitivity and reduces serum glucose. In GABAergic neurons, GIP reduces food intake, gastric emptying, appetite and body weight.
6.3. GLP-1R
GLP-1R is expressed in the pancreatic β cells, the neuron of the central nervous system, adipose tissues, hepatic resident macrophages, Kupffer cells and SECs, but not in the hepatocytes (Fig. 3). In white adipose tissue, activation of GLP-1R promotes adipose tissue beiging and browning to stimulate mitochondrial energy metabolism and reduces weight. GLP-1 stimulates insulin secretion but inhibits glucagon release from islets to control blood sugars. In T cells, GLP-1 activates GLP-1R and directly or indirectly reduces inflammation and protects against neurodegenerative diseases such as Alzheimer's and Parkinson's diseases, and MASLD.107, 108, 109 A recent study reports GLP-1R agonists indirectly attenuate systemic inflammation, not through hematopoietic or endothelial GLP-1R, but through the central neuronal GLP-1R to inhibit TNFα induction by Toll-like receptor agonists.110 This study suggests that the brain immune networks in the gut-brain GLP-1R axis play a critical role in anti-inflammation. It was demonstrated that GLP-1(7-36 amide) was able to stimulate insulin secretion to the normal levels in type 2 diabetic patients and infusion of GLP-1 normalized fasting hyperglycemia in type 2 diabetic patients.111,112 These studies proofed the concept for the therapeutic treatment of T2D with GLP-1 agonists (Fig. 3).
Analysis of available clinical data showed high efficacy and safety of GLP-1 analogues for treating metabolic disease.113 A randomized, double-blind, placebo-controlled trial (NCT03357380) of subjects with liver steatosis with subcutaneous semaglutide 0.4 mg once-daily reported significant differences between semaglutide and placebo at week 48.114 Reduction of liver steatosis was significantly greater with semaglutide compared to placebo, and more subject achieved 30% reduction in liver fat content at 24, 48 and 72 weeks.114 Decreases in liver enzymes, HbA1C and body weight were also observed in semaglutide group.114 A double-blinded, randomized, placebo-controlled clinical trial of semaglutide 2.4 mg once weekly in biopsy-confirmed MASH-cirrhosis patients with body mass index of 27 kg/mm or more was shown to significantly improve liver metabolism and fibrosis by one stage or more without worsening of MASH compared to placebo-controlled patients after 48 weeks (NCT03987451). In 2017, FDA approved semaglutide (Ozempic) for T2D.107,115, 116, 117, 118 A systematic review of randomized controlled trials reported GLP-1 analogues were effective in suppression of appetite, delayed gastric emptying, and changes in taste and food preferences, and reducing weight by most studies.119 In 2021, FDA approved semaglutide (Wegovy) specifically for weight loss. FDA also expanded Wegovy's and Ozempic's labels to include reducing cardiovascular risk, heart attack, stroke and deaths. These once-every week injection drugs have become the blockbuster drugs for weight reduction and T2D.
Another GLP-1 analogue liraglutide 3 mg significantly reduced appetite, taste preference, total body, trunk, and upper and lower fat stores without reduction of lean body mass in obese patients.120 Liraglutide reduced primary bile acids and serotonin in the colon independent of feeding and reduced bile acid reabsorption by reducing apical sodium-dependent bile acid transporter (ASBT), bile acid binding protein and organic solute transporter (OST)α/β.121 This study suggests that liraglutide can be used as an inhibitor of primary bile acids and serotonin in the colon for treating bile acid diarrhea in addition to reducing weight and T2D. In a phase 2 clinical trial, liraglutide was shown to be safe with high efficacy for treating alcoholic steatohepatitis.122 GLP-1R agonists may be used as drugs for treating MASLD.28,30,109,123 GLP-1R agonists are currently in clinical trials for MASLD and MASH fibrosis.
6.4. GLP-1R and GIPR or GCGR dual agonists for MASH
The rationale for using GLP-1R and GIPR or GCGR dual agonists for treating MASH is that GLP-1R is the main driver for treating MASH and dual agonists will counterbalance glucagon-driving glucose production by GIPR or GCGR. The dual agonists should be more efficacious than GLP-1R agonists alone.124, 125, 126 A randomized crossover study of obese patients showed GLP-1 infusion alone significantly reduced energy intake compared with GIP or GIP plus GLP-1 infusion.127 GLP-1 and GIP receptor agonists, tirzepatide and survodutide are in clinical trials for MASH fibrosis.128, 129, 130 Tirzepatide is effective for weight loss in adult.131, 132, 133 A recent report of phase 2 trial (NCT04166773), a multi-center, double-blinded, randomized and placebo controlled, tirzepatide dosage study showed that 30% of control, 55% of 5 mg group, 51% of 10 mg, and 51% of 15 mg group had an improvement of fibrosis without worsening of at least one fibrosis stage at 52 weeks of treatment.128 The adverse effects are gastrointestinal events, which were mild or moderate in severity. The phase 2 survodutide trial (NCT04771273) reported that 47% in 2.4 mg group, 62% in the 4.8 mg group, and 43% in the 6.0 mg group, as compared to 14% of placebo control group, had improvement of MASH without worthening of fibrosis.130 The side effects include nausea, diarrhea, and vomiting. These phase 2 trials results are encouraging and further study of the long-term efficacy and safety of GIP and GLP-1 receptor dual agonists for MASH fibrosis are ongoing.
A randomized double-blind placebo-controlled PROXYMO phase 2 clinical trial (NCT04019561) of cotadutide, a dual agonist for GLP-1R and GCGR (specificity 5 to 1, respectively) found that at 300 μg and 600 μg dosages are safe and efficacious for biopsy proven non-cirrhotic MASH with fibrosis patients compared to placebo-control. Cotadutide at 600 μg significantly improved alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels in MASH patients. The majority adverse effects are gastrointestinal events.134 However, this study did not compare the efficacy of this dual agonist vs. a single agonist for GLP-1R.
7. Conclusions and future perspectives
Bile acid- and FXR-based drugs for cholestatic liver diseases have been approved for treating cholestatic diseases. However, their uses for fatty liver diseases and inflammatory bowel diseases have not been approved. The approval of a liver-specific and THRβ-selective agonist for MASH fibrosis is encouraging for further development of drugs alternative to bile acid receptor FXR for MASH. It is important to point out the critical role of gut microbiota plays in host metabolism, T2D, obesity and MASLD, and different gut microbiota in different patients may affect the outcome of drug treatments. Individualized and precision medicine may be needed for treating MASLD and MASH fibrosis. Monitoring gut microbiome in clinical trials is recommended. Many recent studies have identified more than 200 gut derived-bile acid metabolites in mice and humans.135 Several studies have identified 3-oxo-LCA and isoallo-LCA as T cell regulators: 3-oxo-LCA directly binded to retinoid-related orphan receptor γ to inhibit the differentiation of T helper 17 cells, and isoallo-LCA increased the differentiation of regulatory T cells through production of mitochondrial ROS.136 A TGR5-specific agonist, hyocholic acid has been shown to improve glucose metabolism in mice and humans suggesting that hyocholic acid may be developed as a drug for glycemic control.41,59,137 Interestingly, a recent study reported that iso-, 3-oxo-, allo-, 3-oxoallo and isoallo-LCA were increased in Japanese centenarians and suggested these bile acid metabolites may have potent antimicrobial effects against Gram-positive pathogens including Clostridiodes difficile and Enterococcus faecium.138 Although one can argue that these gut-derived bile acid metabolites are very low abundance and may not have significant physiological effect on host metabolism, but these bile acid derivatives may change in abundance in dysbiosis or by drugs to a level that may have pathophysiology effects on disease pathogenesis. It is anticipated that these microbial-derived bile acid metabolites maybe developed as new drugs for diabetes, obesity, longevity, and MASH fibrosis in the future.
Author’s contributions
John Y. L. Chiang: Conceptualization, Writing-original draft, Writing-review & editing, Funding acquisition.
Declaration of competing interest
John Y. L. Chiang is an executive associate editor for liver research and was not involved in the editorial review or the decision to publish this article. The author declares that there is no conflicts of interest.
Acknowledgements
This work was supported by National Institutes of Health (NIH) grants DK44442 and DK58379.
Footnotes
Peer review under the responsibility of Editorial Office of Liver Research.
References
- 1.Rinella M.E., Lazarus J.V., Ratziu V., et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J Hepatol. 2023;79:1542–1556. doi: 10.1016/j.jhep.2023.06.003. [DOI] [PubMed] [Google Scholar]
- 2.Younossi Z.M., Golabi P., Paik J.M., Henry A., Van Dongen C., Henry L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology. 2023;77:1335–1347. doi: 10.1097/HEP.0000000000000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kucukoglu O., Sowa J.P., Mazzolini G.D., Syn W.K., Canbay A. Hepatokines and adipokines in NASH-related hepatocellular carcinoma. J Hepatol. 2021;74:442–457. doi: 10.1016/j.jhep.2020.10.030. [DOI] [PubMed] [Google Scholar]
- 4.Makishima M., Okamoto A.Y., Repa J.J., et al. Identification of a nuclear receptor for bile acids. Science. 1999;284:1362–1365. doi: 10.1126/science.284.5418.1362. [DOI] [PubMed] [Google Scholar]
- 5.Chiang J.Y.L. Bile acid metabolism and signaling in liver disease and therapy. Liver Res. 2017;1:3–9. doi: 10.1016/j.livres.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiang J.Y.L., Ferrell J.M. Bile acids as metabolic regulators and nutrient sensors. Annu Rev Nutr. 2019;39:175–200. doi: 10.1146/annurev-nutr-082018-124344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiang J.Y.L., Ferrell J.M. Discovery of farnesoid X receptor and its role in bile acid metabolism. Mol Cell Endocrinol. 2022;548 doi: 10.1016/j.mce.2022.111618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiang J.Y.L., Ferrell J.M. Up to date on cholesterol 7 alpha-hydroxylase (CYP7A1) in bile acid synthesis. Liver Res. 2020;4:47–63. doi: 10.1016/j.livres.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiang J. In: Pathobiology of Human Disease. McManus L.M., Mitchell R.N., editors. Elsevier; 2014. Liver physiology: metabolism and detoxification; pp. 1770–1782. [DOI] [Google Scholar]
- 10.Parlati L., Régnier M., Guillou H., Postic C. New targets for NAFLD. JHEP Rep. 2021;3 doi: 10.1016/j.jhepr.2021.100346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tilg H., Adolph T.E., Trauner M. Gut-liver axis: pathophysiological concepts and clinical implications. Cell Metab. 2022;34:1700–1718. doi: 10.1016/j.cmet.2022.09.017. [DOI] [PubMed] [Google Scholar]
- 12.Adorini L., Trauner M. FXR agonists in NASH treatment. J Hepatol. 2023;79:1317–1331. doi: 10.1016/j.jhep.2023.07.034. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez F.J., Jiang C., Patterson A.D. An intestinal microbiota-farnesoid X receptor axis modulates metabolic disease. Gastroenterology. 2016;151:845–859. doi: 10.1053/j.gastro.2016.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu Y., Li F., Zalzala M., et al. Farnesoid X receptor activation increases reverse cholesterol transport by modulating bile acid composition and cholesterol absorption in mice. Hepatology. 2016;64:1072–1085. doi: 10.1002/hep.28712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xie C., Jiang C., Shi J., et al. An intestinal farnesoid X receptor-ceramide signaling axis modulates hepatic gluconeogenesis in mice. Diabetes. 2017;66:613–626. doi: 10.2337/db16-0663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo C., Xie S., Chi Z., et al. Bile acids control inflammation and metabolic disorder through inhibition of NLRP3 inflammasome. Immunity. 2016;45:802–816. doi: 10.1016/j.immuni.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 17.Gong Z., Zhou J., Zhao S., et al. Chenodeoxycholic acid activates NLRP3 inflammasome and contributes to cholestatic liver fibrosis. Oncotarget. 2016;7:83951–83963. doi: 10.18632/oncotarget.13796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takahashi S., Tanaka N., Fukami T., et al. Role of farnesoid X receptor and bile acids in hepatic tumor development. Hepatol Commun. 2018;2:1567–1582. doi: 10.1002/hep4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun L., Cai J., Gonzalez F.J. The role of farnesoid X receptor in metabolic diseases, and gastrointestinal and liver cancer. Nat Rev Gastroenterol Hepatol. 2021;18:335–347. doi: 10.1038/s41575-020-00404-2. [DOI] [PubMed] [Google Scholar]
- 20.Zhang L., Xie C., Nichols R.G., et al. Farnesoid X receptor signaling shapes the gut microbiota and controls hepatic lipid metabolism. mSystems. 2016;1 doi: 10.1128/mSystems.00070-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pandak W.M., Heuman D.M., Hylemon P.B., Chiang J.Y., Vlahcevic Z.R. Failure of intravenous infusion of taurocholate to down-regulate cholesterol 7 alpha-hydroxylase in rats with biliary fistulas. Gastroenterology. 1995;108:533–544. doi: 10.1016/0016-5085(95)90083-7. [DOI] [PubMed] [Google Scholar]
- 22.Inagaki T., Choi M., Moschetta A., et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2:217–225. doi: 10.1016/j.cmet.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 23.Vavassori P., Mencarelli A., Renga B., Distrutti E., Fiorucci S. The bile acid receptor FXR is a modulator of intestinal innate immunity. J Immunol. 2009;183:6251–6261. doi: 10.4049/jimmunol.0803978. [DOI] [PubMed] [Google Scholar]
- 24.Li F., Jiang C., Krausz K.W., et al. Microbiome remodelling leads to inhibition of intestinal farnesoid X receptor signalling and decreased obesity. Nat Commun. 2013;4:2384. doi: 10.1038/ncomms3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang C., Xie C., Li F., et al. Intestinal farnesoid X receptor signaling promotes nonalcoholic fatty liver disease. J Clin Invest. 2015;125:386–402. doi: 10.1172/JCI76738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang C., Xie C., Lv Y., et al. Intestine-selective farnesoid X receptor inhibition improves obesity-related metabolic dysfunction. Nat Commun. 2015;6 doi: 10.1038/ncomms10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun L., Xie C., Wang G., et al. Gut microbiota and intestinal FXR mediate the clinical benefits of metformin. Nat Med. 2018;24:1919–1929. doi: 10.1038/s41591-018-0222-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fang S., Suh J.M., Reilly S.M., et al. Intestinal FXR agonism promotes adipose tissue browning and reduces obesity and insulin resistance. Nat Med. 2015;21:159–165. doi: 10.1038/nm.3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pathak P., Xie C., Nichols R.G., et al. Intestine farnesoid X receptor agonist and the gut microbiota activate G-protein bile acid receptor-1 signaling to improve metabolism. Hepatology. 2018;68:1574–1588. doi: 10.1002/hep.29857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carino A., Biagioli M., Marchianò S., et al. Ursodeoxycholic acid is a GPBAR1 agonist and resets liver/intestinal FXR signaling in a model of diet-induced dysbiosis and NASH. Biochim Biophys Acta Mol Cell Biol Lipids. 2019;1864:1422–1437. doi: 10.1016/j.bbalip.2019.07.006. [DOI] [PubMed] [Google Scholar]
- 31.Keitel V., Cupisti K., Ullmer C., Knoefel W.T., Kubitz R., Häussinger D. The membrane-bound bile acid receptor TGR5 is localized in the epithelium of human gallbladders. Hepatology. 2009;50:861–870. doi: 10.1002/hep.23032. [DOI] [PubMed] [Google Scholar]
- 32.Keitel V., Häussinger D. Role of TGR5 (GPBAR1) in liver disease. Semin Liver Dis. 2018;38:333–339. doi: 10.1055/s-0038-1669940. [DOI] [PubMed] [Google Scholar]
- 33.Pathak P., Liu H., Boehme S., et al. Farnesoid X receptor induces Takeda G-protein receptor 5 cross-talk to regulate bile acid synthesis and hepatic metabolism. J Biol Chem. 2017;292:11055–11069. doi: 10.1074/jbc.M117.784322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Katsuma S., Hirasawa A., Tsujimoto G. Bile acids promote glucagon-like peptide-1 secretion through TGR5 in a murine enteroendocrine cell line STC-1. Biochem Biophys Res Commun. 2005;329:386–390. doi: 10.1016/j.bbrc.2005.01.139. [DOI] [PubMed] [Google Scholar]
- 35.Fiorucci S., Mencarelli A., Palladino G., Cipriani S. Bile-acid-activated receptors: targeting TGR5 and farnesoid-X-receptor in lipid and glucose disorders. Trends Pharmacol Sci. 2009;30:570–580. doi: 10.1016/j.tips.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 36.Thomas C., Gioiello A., Noriega L., et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009;10:167–177. doi: 10.1016/j.cmet.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shi Y., Su W., Zhang L., et al. TGR5 regulates macrophage inflammation in nonalcoholic steatohepatitis by modulating NLRP3 inflammasome activation. Front Immunol. 2021;11 doi: 10.3389/fimmu.2020.609060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Nierop F.S., Scheltema M.J., Eggink H.M., et al. Clinical relevance of the bile acid receptor TGR5 in metabolism. Lancet Diabetes Endocrinol. 2017;5:224–233. doi: 10.1016/S2213-8587(16)30155-3. [DOI] [PubMed] [Google Scholar]
- 39.Deutschmann K., Reich M., Klindt C., et al. Bile acid receptors in the biliary tree: TGR5 in physiology and disease. Biochim Biophys Acta Mol Basis Dis. 2018;1864:1319–1325. doi: 10.1016/j.bbadis.2017.08.021. [DOI] [PubMed] [Google Scholar]
- 40.Keitel V., Stindt J., Häussinger D. Bile acid-activated receptors: GPBAR1 (TGR5) and other G protein-coupled receptors. Handb Exp Pharmacol. 2019;256:19–49. doi: 10.1007/164_2019_230. [DOI] [PubMed] [Google Scholar]
- 41.Makki K., Brolin H., Petersen N., et al. 6α-hydroxylated bile acids mediate TGR5 signalling to improve glucose metabolism upon dietary fiber supplementation in mice. Gut. 2023;72:314–324. doi: 10.1136/gutjnl-2021-326541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reich M., Deutschmann K., Sommerfeld A., et al. TGR5 is essential for bile acid-dependent cholangiocyte proliferation in vivo and in vitro. Gut. 2016;65:487–501. doi: 10.1136/gutjnl-2015-309458. [DOI] [PubMed] [Google Scholar]
- 43.Flint A., Raben A., Astrup A., Holst J.J. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest. 1998;101:515–520. doi: 10.1172/JCI990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McGavigan A.K., Garibay D., Henseler Z.M., et al. TGR5 contributes to glucoregulatory improvements after vertical sleeve gastrectomy in mice. Gut. 2017;66:226–234. doi: 10.1136/gutjnl-2015-309871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klindt C., Reich M., Hellwig B., et al. The G protein-coupled bile acid receptor TGR5 (Gpbar1) modulates endothelin-1 signaling in liver. Cells. 2019;8:1467. doi: 10.3390/cells8111467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Holter M.M., Chirikjian M.K., Govani V.N., Cummings B.P. TGR5 signaling in hepatic metabolic health. Nutrients. 2020;12:2598. doi: 10.3390/nu12092598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Castellanos-Jankiewicz A., Guzmán-Quevedo O., Fénelon V.S., et al. Hypothalamic bile acid-TGR5 signaling protects from obesity. Cell Metab. 2021;33:1483–1492 (e10). doi: 10.1016/j.cmet.2021.04.009. [DOI] [PubMed] [Google Scholar]
- 48.Schwartz M.W., Woods S.C., Porte D Jr., Seeley R.J., Baskin D.G. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 49.Alcantara I.C., Tapia A.P.M., Aponte Y., Krashes M.J. Acts of appetite: neural circuits governing the appetitive, consummatory, and terminating phases of feeding. Nat Metab. 2022;4:836–847. doi: 10.1038/s42255-022-00611-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ahn B.H., Kim M., Kim S.Y. Brain circuits for promoting homeostatic and non-homeostatic appetites. Exp Mol Med. 2022;54:349–357. doi: 10.1038/s12276-022-00758-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aron-Wisnewsky J., Gaborit B., Dutour A., Clement K. Gut microbiota and non-alcoholic fatty liver disease: new insights. Clin Microbiol Infect. 2013;19:338–348. doi: 10.1111/1469-0691.12140. [DOI] [PubMed] [Google Scholar]
- 52.Joyce S.A., Gahan C.G. The gut microbiota and the metabolic health of the host. Curr Opin Gastroenterol. 2014;30:120–127. doi: 10.1097/MOG.0000000000000039. [DOI] [PubMed] [Google Scholar]
- 53.Aron-Wisnewsky J., Vigliotti C., Witjes J., et al. Gut microbiota and human NAFLD: disentangling microbial signatures from metabolic disorders. Nat Rev Gastroenterol Hepatol. 2020;17:279–297. doi: 10.1038/s41575-020-0269-9. [DOI] [PubMed] [Google Scholar]
- 54.Verkade E., Shen W., Hovingh M.V., et al. Gut microbiota depletion aggravates bile acid-induced liver pathology in mice with a human-like bile acid composition. Clin Sci (Lond) 2023;137:1637–1650. doi: 10.1042/CS20230812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mouzaki M., Wang A.Y., Bandsma R., et al. Bile acids and dysbiosis in non-alcoholic fatty liver disease. PLoS One. 2016;11 doi: 10.1371/journal.pone.0151829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shen F., Zheng R.D., Sun X.Q., Ding W.J., Wang X.Y., Fan J.G. Gut microbiota dysbiosis in patients with non-alcoholic fatty liver disease. Hepatobiliary Pancreat Dis Int. 2017;16:375–381. doi: 10.1016/S1499-3872(17)60019-5. [DOI] [PubMed] [Google Scholar]
- 57.David L.A., Maurice C.F., Carmody R.N., et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Devkota S., Wang Y., Musch M.W., et al. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10-/- mice. Nature. 2012;487:104–108. doi: 10.1038/nature11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bozadjieva N., Heppner K.M., Seeley R.J. Targeting FXR and FGF19 to treat metabolic diseases-lessons learned from bariatric surgery. Diabetes. 2018;67:1720–1728. doi: 10.2337/dbi17-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee Y., Doumouras A.G., Yu J., et al. Complete resolution of nonalcoholic fatty liver disease after bariatric surgery: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2019;17:1040–1060 (e11). doi: 10.1016/j.cgh.2018.10.017. [DOI] [PubMed] [Google Scholar]
- 61.Dao M.C., Belda E., Prifti E., et al. Akkermansia muciniphila abundance is lower in severe obesity, but its increased level after bariatric surgery is not associated with metabolic health improvement. Am J Physiol Endocrinol Metab. 2019;317:E446–E459. doi: 10.1152/ajpendo.00140.2019. [DOI] [PubMed] [Google Scholar]
- 62.Albaugh V.L., Banan B., Antoun J., et al. Role of bile acids and GLP-1 in mediating the metabolic improvements of bariatric surgery. Gastroenterology. 2019;156:1041–1051 (e4). doi: 10.1053/j.gastro.2018.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ryan K.K., Tremaroli V., Clemmensen C., et al. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature. 2014;509:183–188. doi: 10.1038/nature13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Risstad H., Kristinsson J.A., Fagerland M.W., et al. Bile acid profiles over 5 years after gastric bypass and duodenal switch: results from a randomized clinical trial. Surg Obes Relat Dis. 2017;13:1544–1553. doi: 10.1016/j.soard.2017.05.024. [DOI] [PubMed] [Google Scholar]
- 65.Puri P., Daita K., Joyce A., et al. The presence and severity of nonalcoholic steatohepatitis is associated with specific changes in circulating bile acids. Hepatology. 2018;67:534–548. doi: 10.1002/hep.29359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ridlon J.M., Kang D.J., Hylemon P.B., Bajaj J.S. Bile acids and the gut microbiome. Curr Opin Gastroenterol. 2014;30:332–338. doi: 10.1097/MOG.0000000000000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ruuskanen M.O., Åberg F., Männistö V., et al. Links between gut microbiome composition and fatty liver disease in a large population sample. Gut Microbes. 2021;13:1–22. doi: 10.1080/19490976.2021.1888673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Govaere O., Cockell S., Tiniakos D., et al. Transcriptomic profiling across the nonalcoholic fatty liver disease spectrum reveals gene signatures for steatohepatitis and fibrosis. Sci Transl Med. 2020;12 doi: 10.1126/scitranslmed.aba4448. [DOI] [PubMed] [Google Scholar]
- 69.Zheng X., Chen T., Zhao A., et al. Hyocholic acid species as novel biomarkers for metabolic disorders. Nat Commun. 2021;12:1487. doi: 10.1038/s41467-021-21744-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chaudhari S.N., Luo J.N., Harris D.A., et al. A microbial metabolite remodels the gut-liver axis following bariatric surgery. Cell Host Microbe. 2021;29:408–424 (e7). doi: 10.1016/j.chom.2020.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chaudhari S.N., Harris D.A., Aliakbarian H., et al. Bariatric surgery reveals a gut-restricted TGR5 agonist with anti-diabetic effects. Nat Chem Biol. 2021;17:20–29. doi: 10.1038/s41589-020-0604-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cai J., Rimal B., Jiang C., Chiang J.Y.L., Patterson A.D. Bile acid metabolism and signaling, the microbiota, and metabolic disease. Pharmacol Ther. 2022;237 doi: 10.1016/j.pharmthera.2022.108238. [DOI] [PubMed] [Google Scholar]
- 73.Chiang J.Y.L., Ferrell J.M. Bile acid biology, pathophysiology, and therapeutics. Clin Liver Dis (Hoboken) 2020;15:91–94. doi: 10.1002/cld.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chiang J.Y.L., Ferrell J.M. Bile acid receptors FXR and TGR5 signaling in fatty liver diseases and therapy. Am J Physiol Gastrointest Liver Physiol. 2020;318:G554–G573. doi: 10.1152/ajpgi.00223.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mudaliar S., Henry R.R., Sanyal A.J., et al. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology. 2013;145:574–582 (e1). doi: 10.1053/j.gastro.2013.05.042. [DOI] [PubMed] [Google Scholar]
- 76.Neuschwander-Tetri B.A., Loomba R., Sanyal A.J., et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:956–965. doi: 10.1016/S0140-6736(14)61933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Huesmann M., Huesmann T., Osada N., Phan N.Q., Kremer A.E., Ständer S. Cholestatic pruritus: a retrospective analysis on clinical characteristics and treatment response. J Dtsch Dermatol Ges. 2013;11:158–168. doi: 10.1111/j.1610-0387.2012.08028.x. [DOI] [PubMed] [Google Scholar]
- 78.Meixiong J., Vasavda C., Snyder S.H., Dong X. MRGPRX4 is a G protein-coupled receptor activated by bile acids that may contribute to cholestatic pruritus. Proc Natl Acad Sci U S A. 2019;116:10525–10530. doi: 10.1073/pnas.1903316116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Patel K., Harrison S.A., Elkhashab M., et al. Cilofexor, a nonsteroidal FXR agonist, in patients with noncirrhotic NASH: a phase 2 randomized controlled trial. Hepatology. 2020;72:58–71. doi: 10.1002/hep.31205. [DOI] [PubMed] [Google Scholar]
- 80.Hollenback D., Hambruch E., Fink G., et al. Development of cilofexor, an intestinally-biased farnesoid X receptor agonist, for the treatment of fatty liver disease. J Pharmacol Exp Ther. 2024;389:61–75. doi: 10.1124/jpet.123.001900. [DOI] [PubMed] [Google Scholar]
- 81.Trauner M., Gulamhusein A., Hameed B., et al. The nonsteroidal farnesoid X receptor agonist cilofexor (GS-9674) improves markers of cholestasis and liver injury in patients with primary sclerosing cholangitis. Hepatology. 2019;70:788–801. doi: 10.1002/hep.30509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Camilleri M., Nord S.L., Burton D., et al. Randomised clinical trial: significant biochemical and colonic transit effects of the farnesoid X receptor agonist tropifexor in patients with primary bile acid diarrhoea. Aliment Pharmacol Ther. 2020;52:808–820. doi: 10.1111/apt.15967. [DOI] [PubMed] [Google Scholar]
- 83.Loomba R., Noureddin M., Kowdley K.V., et al. Combination therapies including cilofexor and firsocostat for bridging fibrosis and cirrhosis attributable to NASH. Hepatology. 2021;73:625–643. doi: 10.1002/hep.31622. [DOI] [PubMed] [Google Scholar]
- 84.Anstee Q.M., Lucas K.J., Francque S., et al. Tropifexor plus cenicriviroc combination versus monotherapy in nonalcoholic steatohepatitis: results from the phase 2b TANDEM study. Hepatology. 2023;78:1223–1239. doi: 10.1097/HEP.0000000000000439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sanyal A.J., Lopez P., Lawitz E.J., et al. Tropifexor for nonalcoholic steatohepatitis: an adaptive, randomized, placebo-controlled phase 2a/b trial. Nat Med. 2023;29:392–400. doi: 10.1038/s41591-022-02200-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhou M., Wang X., Phung V., et al. Separating tumorigenicity from bile acid regulatory activity for endocrine hormone FGF19. Cancer Res. 2014;74:3306–3316. doi: 10.1158/0008-5472.CAN-14-0208. [DOI] [PubMed] [Google Scholar]
- 87.Harrison S.A., Rossi S.J., Paredes A.H., et al. NGM282 improves liver fibrosis and histology in 12 weeks in patients with nonalcoholic steatohepatitis. Hepatology. 2020;71:1198–1212. doi: 10.1002/hep.30590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Potthoff M.J., Inagaki T., Satapati S., et al. FGF21 induces PGC-1alpha and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proc Natl Acad Sci U S A. 2009;106:10853–10858. doi: 10.1073/pnas.0904187106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bookout A.L., de Groot M.H., Owen B.M., et al. FGF21 regulates metabolism and circadian behavior by acting on the nervous system. Nat Med. 2013;19:1147–1152. doi: 10.1038/nm.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Talukdar S., Owen B.M., Song P., et al. FGF21 regulates sweet and alcohol preference. Cell Metab. 2016;23:344–349. doi: 10.1016/j.cmet.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Talukdar S., Zhou Y., Li D., et al. A long-acting FGF21 molecule, PF-05231023, decreases body weight and improves lipid profile in non-human primates and type 2 diabetic subjects. Cell Metab. 2016;23:427–440. doi: 10.1016/j.cmet.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 92.Flippo K.H., Trammell S.A.J., Gillum M.P., et al. FGF21 suppresses alcohol consumption through an amygdalo-striatal circuit. Cell Metab. 2022;34:317–328 (e6). doi: 10.1016/j.cmet.2021.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Charles E.D., Neuschwander-Tetri B.A., Pablo Frias J., et al. Pegbelfermin (BMS-986036), PEGylated FGF21, in patients with obesity and type 2 diabetes: results from a randomized phase 2 study. Obesity (Silver Spring) 2019;27:41–49. doi: 10.1002/oby.22344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Loomba R., Sanyal A.J., Kowdley K.V., et al. Randomized, controlled trial of the FGF21 analogue pegozafermin in NASH. N Engl J Med. 2023;389:998–1008. doi: 10.1056/NEJMoa2304286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Harrison S.A., Rolph T., Knott K., Dubourg J. FGF21 agonists: an emerging therapeutic for metabolic dysfunction-associated steatohepatitis and beyond. J Hepatol. 2024;81:562–576. doi: 10.1016/j.jhep.2024.04.034. [DOI] [PubMed] [Google Scholar]
- 96.Alkhouri N. Thyromimetics as emerging therapeutic agents for nonalcoholic steatohepatitis: rationale for the development of resmetirom (MGL-3196) Expert Opin Investig Drugs. 2020;29:99–101. doi: 10.1080/13543784.2020.1708899. [DOI] [PubMed] [Google Scholar]
- 97.Grover G.J., Mellström K., Ye L., et al. Selective thyroid hormone receptor-beta activation: a strategy for reduction of weight, cholesterol, and lipoprotein (a) with reduced cardiovascular liability. Proc Natl Acad Sci U S A. 2003;100:10067–10072. doi: 10.1073/pnas.1633737100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kelly M.J., Pietranico-Cole S., Larigan J.D., et al. Discovery of 2-[3,5-dichloro-4-(5-isopropyl-6-oxo-1,6-dihydropyridazin-3-yloxy)phenyl]-3,5-dioxo-2,3,4,5-tetrahydro[1,2,4]triazine-6-carbonitrile (MGL-3196), a highly selective thyroid hormone receptor β agonist in clinical trials for the treatment of dyslipidemia. J Med Chem. 2014;57:3912–3923. doi: 10.1021/jm4019299. [DOI] [PubMed] [Google Scholar]
- 99.Haeusler R.A., Pratt-Hyatt M., Welch C.L., Klaassen C.D., Accili D. Impaired generation of 12-hydroxylated bile acids links hepatic insulin signaling with dyslipidemia. Cell Metab. 2012;15:65–74. doi: 10.1016/j.cmet.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sun K., Zhu N.L., Huang S.L., et al. A new mechanism of thyroid hormone receptor β agonists ameliorating nonalcoholic steatohepatitis by inhibiting intestinal lipid absorption via remodeling bile acid profiles. Acta Pharmacol Sin. 2024;45:2134–2148. doi: 10.1038/s41401-024-01303-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Harrison S.A., Bashir M.R., Guy C.D., et al. Resmetirom (MGL-3196) for the treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2019;394:2012–2024. doi: 10.1016/S0140-6736(19)32517-6. [DOI] [PubMed] [Google Scholar]
- 102.Harrison S.A., Bashir M., Moussa S.E., et al. Effects of resmetirom on noninvasive endpoints in a 36-week phase 2 active treatment extension study in patients with NASH. Hepatol Commun. 2021;5:573–588. doi: 10.1002/hep4.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Harrison S.A., Taub R., Neff G.W., et al. Resmetirom for nonalcoholic fatty liver disease: a randomized, double-blind, placebo-controlled phase 3 trial. Nat Med. 2023;29:2919–2928. doi: 10.1038/s41591-023-02603-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Noureddin M., Charlton M.R., Harrison S.A., et al. Expert panel recommendations: practical clinical applications for initiating and monitoring resmetirom in patients with MASH/NASH and moderate to noncirrhotic advanced fibrosis. Clin Gastroenterol Hepatol. 2024;22:2367–2377. doi: 10.1016/j.cgh.2024.07.003. [DOI] [PubMed] [Google Scholar]
- 105.Kokkorakis M., Boutari C., Hill M.A., et al. Resmetirom, the first approved drug for the management of metabolic dysfunction-associated steatohepatitis: trials, opportunities, and challenges. Metabolism. 2024;154 doi: 10.1016/j.metabol.2024.155835. [DOI] [PubMed] [Google Scholar]
- 106.Harrison S.A., Bedossa P., Guy C.D., et al. A phase 3, randomized, controlled trial of resmetirom in NASH with liver fibrosis. N Engl J Med. 2024;390:497–509. doi: 10.1056/NEJMoa2309000. [DOI] [PubMed] [Google Scholar]
- 107.Drucker D.J., Holst J.J. The expanding incretin universe: from basic biology to clinical translation. Diabetologia. 2023;66:1765–1779. doi: 10.1007/s00125-023-05906-7. [DOI] [PubMed] [Google Scholar]
- 108.Kopp K.O., Glotfelty E.J., Li Y., Greig N.H. Glucagon-like peptide-1 (GLP-1) receptor agonists and neuroinflammation: implications for neurodegenerative disease treatment. Pharmacol Res. 2022;186 doi: 10.1016/j.phrs.2022.106550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yabut J.M., Drucker D.J. Glucagon-like peptide-1 receptor-based therapeutics for metabolic liver disease. Endocr Rev. 2023;44:14–32. doi: 10.1210/endrev/bnac018. [DOI] [PubMed] [Google Scholar]
- 110.Wong C.K., McLean B.A., Baggio L.L., et al. Central glucagon-like peptide 1 receptor activation inhibits Toll-like receptor agonist-induced inflammation. Cell Metab. 2024;36:130–143 (e5). doi: 10.1016/j.cmet.2023.11.009. [DOI] [PubMed] [Google Scholar]
- 111.Nauck M.A., Heimesaat M.M., Orskov C., Holst J.J., Ebert R., Creutzfeldt W. Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest. 1993;91:301–307. doi: 10.1172/JCI116186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nauck M.A., Kleine N., Orskov C., Holst J.J., Willms B., Creutzfeldt W. Normalization of fasting hyperglycaemia by exogenous glucagon-like peptide 1 (7-36 amide) in type 2 (non-insulin-dependent) diabetic patients. Diabetologia. 1993;36:741–744. doi: 10.1007/BF00401145. [DOI] [PubMed] [Google Scholar]
- 113.Baggio L.L., Drucker D.J. Glucagon-like peptide-1 receptor co-agonists for treating metabolic disease. Mol Metab. 2021;46 doi: 10.1016/j.molmet.2020.101090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Flint A., Andersen G., Hockings P., et al. Randomised clinical trial: semaglutide versus placebo reduced liver steatosis but not liver stiffness in subjects with non-alcoholic fatty liver disease assessed by magnetic resonance imaging. Aliment Pharmacol Ther. 2021;54:1150–1161. doi: 10.1111/apt.16608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Campbell J.E., Drucker D.J. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013;17:819–837. doi: 10.1016/j.cmet.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 116.Htike Z.Z., Zaccardi F., Papamargaritis D., Webb D.R., Khunti K., Davies M.J. Efficacy and safety of glucagon-like peptide-1 receptor agonists in type 2 diabetes: a systematic review and mixed-treatment comparison analysis. Diabetes Obes Metab. 2017;19:524–536. doi: 10.1111/dom.12849. [DOI] [PubMed] [Google Scholar]
- 117.Nauck M.A., Quast D.R., Wefers J., Meier J.J. GLP-1 receptor agonists in the treatment of type 2 diabetes - state-of-the-art. Mol Metab. 2021;46 doi: 10.1016/j.molmet.2020.101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Drucker D.J. GLP-1 physiology informs the pharmacotherapy of obesity. Mol Metab. 2022;57 doi: 10.1016/j.molmet.2021.101351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Aldawsari M., Almadani F.A., Almuhammadi N., Algabsani S., Alamro Y., Aldhwayan M. The efficacy of GLP-1 analogues on appetite parameters, gastric emptying, food preference and taste among adults with obesity: systematic review of randomized controlled trials. Diabetes Metab Syndr Obes. 2023;16:575–595. doi: 10.2147/DMSO.S387116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kadouh H., Chedid V., Halawi H., et al. GLP-1 analog modulates appetite, taste preference, gut hormones, and regional body fat stores in adults with obesity. J Clin Endocrinol Metab. 2020;105:1552–1563. doi: 10.1210/clinem/dgz140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nonogaki K., Kaji T. The GLP-1 receptor agonist liraglutide decreases primary bile acids and serotonin in the colon independently of feeding in mice. Int J Mol Sci. 2024;25:7784. doi: 10.3390/ijms25147784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Armstrong M.J., Gaunt P., Aithal G.P., et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387:679–690. doi: 10.1016/S0140-6736(15)00803-X. [DOI] [PubMed] [Google Scholar]
- 123.Carino A., Cipriani S., Marchianò S., et al. BAR502, a dual FXR and GPBAR1 agonist, promotes browning of white adipose tissue and reverses liver steatosis and fibrosis. Sci Rep. 2017;7 doi: 10.1038/srep42801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Day J.W., Ottaway N., Patterson J.T., et al. A new glucagon and GLP-1 co-agonist eliminates obesity in rodents. Nat Chem Biol. 2009;5:749–757. doi: 10.1038/nchembio.209. [DOI] [PubMed] [Google Scholar]
- 125.Day J.W., Gelfanov V., Smiley D., et al. Optimization of co-agonism at GLP-1 and glucagon receptors to safely maximize weight reduction in DIO-rodents. Biopolymers. 2012;98:443–450. doi: 10.1002/bip.22072. [DOI] [PubMed] [Google Scholar]
- 126.Scheen A.J. Dual GIP/GLP-1 receptor agonists: new advances for treating type-2 diabetes. Ann Endocrinol (Paris) 2023;84:316–321. doi: 10.1016/j.ando.2022.12.423. [DOI] [PubMed] [Google Scholar]
- 127.Bergmann N.C., Lund A., Gasbjerg L.S., et al. Effects of combined GIP and GLP-1 infusion on energy intake, appetite and energy expenditure in overweight/obese individuals: a randomised, crossover study. Diabetologia. 2019;62:665–675. doi: 10.1007/s00125-018-4810-0. [DOI] [PubMed] [Google Scholar]
- 128.Loomba R., Hartman M.L., Lawitz E.J., et al. Tirzepatide for metabolic dysfunction-associated steatohepatitis with liver fibrosis. N Engl J Med. 2024;391:299–310. doi: 10.1056/NEJMoa2401943. [DOI] [PubMed] [Google Scholar]
- 129.Vuppalanchi R., Loomba R., Sanyal A.J., et al. Randomised clinical trial: design of the SYNERGY-NASH phase 2b trial to evaluate tirzepatide as a treatment for metabolic dysfunction-associated steatohepatitis and modification of screening strategy to reduce screen failures. Aliment Pharmacol Ther. 2024;60:17–32. doi: 10.1111/apt.18042. [DOI] [PubMed] [Google Scholar]
- 130.Sanyal A.J., Bedossa P., Fraessdorf M., et al. A phase 2 randomized trial of survodutide in MASH and fibrosis. N Engl J Med. 2024;391:311–319. doi: 10.1056/NEJMoa2401755. [DOI] [PubMed] [Google Scholar]
- 131.Sidhu J.K., Singh S. A new drug for obesity: tirzepatide. Endocr Metab Immune Disord Drug Targets. 2025;25:267-270 doi: 10.2174/0118715303319530240703111013. [DOI] [PubMed] [Google Scholar]
- 132.Corrao S., Pollicino C., Maggio D., Torres A., Argano C. Tirzepatide against obesity and insulin-resistance: pathophysiological aspects and clinical evidence. Front Endocrinol (Lausanne) 2024;15 doi: 10.3389/fendo.2024.1402583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rodriguez P.J., Goodwin Cartwright B.M., Gratzl S., et al. Semaglutide vs Tirzepatide for weight loss in adults with overweight or obesity. JAMA Intern Med. 2024;184:1056–1064. doi: 10.1001/jamainternmed.2024.2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shankar S.S., Daniels S.J., Robertson D., et al. Safety and efficacy of novel incretin co-agonist cotadutide in biopsy-proven noncirrhotic MASH with fibrosis. Clin Gastroenterol Hepatol. 2024;22:1847–1857 (e11). doi: 10.1016/j.cgh.2024.04.017. [DOI] [PubMed] [Google Scholar]
- 135.Mohanty I., Mannochio-Russo H., Schweer J.V., et al. The underappreciated diversity of bile acid modifications. Cell. 2024;187:1801–1818 (e20). doi: 10.1016/j.cell.2024.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hang S., Paik D., Yao L., et al. Bile acid metabolites control TH17 and Treg cell differentiation. Nature. 2019;576:143–148. doi: 10.1038/s41586-019-1785-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zheng X., Chen T., Jiang R., et al. Hyocholic acid species improve glucose homeostasis through a distinct TGR5 and FXR signaling mechanism. Cell Metab. 2021;33:791–803 (e7). doi: 10.1016/j.cmet.2020.11.017. [DOI] [PubMed] [Google Scholar]
- 138.Sato Y., Atarashi K., Plichta D.R., et al. Novel bile acid biosynthetic pathways are enriched in the microbiome of centenarians. Nature. 2021;599:458–464. doi: 10.1038/s41586-021-03832-5. [DOI] [PubMed] [Google Scholar]