Abstract
2′,3′-Cyclic nucleotide-3′-phosphodiesterase (CNP) is firmly associated with tubulin from brain tissue and FRTL-5 thyroid cells as demonstrated by copolymerization with microtubules through several warm/cold cycles, the presence of CNP activity in purified tubulin preparations, and identical behavior during various extraction procedures. CNP acts as a microtubule-associated protein in promoting microtubule assembly at low mole ratios. This activity resides in the C terminus of the enzyme, which, by itself, promotes microtubule assembly at higher mole ratios. Phosphorylation of CNP interferes with its assembly-promoting activity, as does deletion of the C terminus, which leads to abnormal microtubule distribution in the cell. Submembranous colocalization of the proteins and CNP-dependent microtubule organization suggest that CNP is a membrane-bound microtubule-associated protein that can link tubulin to membranes and may regulate cytoplasmic microtubule distribution.
The bulk of cellular tubulin is cytoplasmic, but a significant fraction is embedded in, or firmly associated with, the plasma membrane and other membranes. This association is not an artifact of membrane isolation, as residence in the membrane changes some of the properties of tubulin, added labeled tubulin does not remain associated with membranes to which it has been added, and neutral detergent is required for release of the dimer from membranes (1–4). It has been a puzzle how this very polar protein can become so firmly membrane-associated (1–5); a hydrophobic interaction would seem to be required. Morphological evidence suggests that microtubules may attach to plasma or organelle membranes by linkers that have not, except for the motor proteins, been identified as such. Association could result from a bifunctional membrane anchor providing hydrophobic interaction with the membrane on the one hand, and a second, potentially but not necessarily more polar, interaction with tubulin on the other. Recently, we identified a possible linker protein for microtubules to the plasma membrane as 2′,3′-cyclic nucleotide-3′-phosphodiesterase (CNP) (6). The CNP is both prenylated and palmitoylated (7), providing hydrophobic domains for membrane intercalation and showing both cytoplasmic and membrane localization. In the present study with FRTL-5 thyroid cells and brain tissue, a stable association of this linker with tubulin is demonstrated, and we show the microtubule-associated protein (MAP)-like properties of a recombinant form of CNP and its polymerization-promoting domain. We suggest that CNP can serve as a regulator of tubulin polymerization and of microtubule distribution. Because a fraction of CNP is firmly embedded in membranes, it serves as a membrane anchor for microtubules.
Materials and Methods
Materials.
FRTL-5 cells (ATCC CRL 8305, a strain of rat thyroid cells) were grown as described previously (8). mAb to α-tubulin was from Amersham Pharmacia. mAbs to myelin basic protein (MBP) were from Boehringer Mannheim and human autoantibody against CNP1 was a generous gift of D. Jacobowitz (National Cancer Institute, Bethesda) (9). mAbs to Rab7 was kindly provided by C. Bucci (Universita “Federico II”, Naples). Phosphodiesterase reagents were from Sigma. Synthetic peptides were supplied by Primm (Milan).
Cell Fractionation.
Total cellular homogenate was centrifuged at 100,000 × g to obtain supernatant (S100) and pellet (P100) fractions. The P100 pellet (100 μg of protein) was resuspended in 0.2 ml of 10 mM Tris⋅HCl, pH 7.2, in the presence of different substances for 3 h at 4°C and recentrifuged at 100,000 × g for 30 min at 4°C to produce a soluble fraction (S) and a pellet (P).
Detergent extraction with 1% Triton X-100 in a microtubule-stabilizing buffer at 4°C (0.1 M Pipes/1 mM MgCl2/2 M glycerol/2 mM EGTA/1 mM PMSF/1 mM DTT, pH 6.9) yielded a detergent-insoluble cytoskeleton. For fractionation, cells were suspended in 0.3 M sucrose/1 mM EDTA/1 mM DTT, pH 7.4, sonicated on ice, and centrifuged at 900 × g for 5 min. Mitochondria and light mitochondria were sedimented at 8,700 × g for 10 min and then at 10,000 × g for 10 min at 4°C. The postmitochondrial supernatant was centrifuged for 30 min at 200,000 × g in an Airfuge at 4°C; it is referred to as microsomes.
Microtubule Purification.
Cells were homogenized and processed with 20 μM Taxol as described in ref. 10. For brain microtubule protein, total cellular lysate was carried through cycles of temperature-dependent assembly/disassembly (2). Rat brain tubulin was prepared from microtubule protein by sequential polymerization (11).
Electrophoresis and Immunoblotting.
Proteins were resolved by SDS/10% or 12% PAGE, transferred to nitrocellulose, and probed with antibodies. Immunoreactive proteins were detected by incubation with horseradish peroxidase-conjugated sheep anti-rabbit or anti-mouse IgG (Amersham Pharmacia) by using the enhanced chemiluminescence system (Amersham Pharmacia).
CNP Activity.
CNP enzymatic activity was measured by a modification of the method of Stephon et al. (12) by using fluorescence at 464 nm with excitation at 344 nm. Results are reported as nmol of NADPH per min per mg of total protein.
Microscopy.
Cells were cultured on 1.2-cm glass coverslips, fixed for 20 min with 3.7% paraformaldehyde in PBS, washed twice with 50 mM ammonium chloride in PBS, permeabilized in 0.1% Triton X-100 for 5 min at room temperature, and blocked for 30 min with 5% FCS/2% BSA in 1 mM MgCl2 and 1 mM CaCl2. They then were incubated with monoclonal anti-α-tubulin antibody (Amersham Pharmacia) for 1 h, followed by tetramethylrhodamine isothiocyanate (TRITC)-labeled goat anti-mouse IgG (Sigma) for 1 h. Secondary antibodies for tubulin were either fluorescein- or rhodamine-labeled. Cells were examined in a Zeiss fluorescence microscope.
Vectors and Expression of Glutathione S-Transferase (GST) Fusion Protein.
The full-length cDNA of rat CNP1 (pCNP7) was generously provided by D. A. De Angelis and P. E. Braun (McGill Univ., Montreal). The cDNA deleted at the C terminus of 13 amino acid residues was obtained by PCR. The cDNA were fused to the C-terminal end of the Schistosoma japonicum GST gene by cloning into the BamHI/EcoRI sites of pGEX-2T (Amersham Pharmacia). The two recombinant vectors were introduced into JM83 Escherichia coli cells. Fusion protein was obtained by induction of expression with 0.1 mM isopropyl 1-thio-β-galactopyranoside and purified on glutathione-coupled Sepharose beads (Amersham Pharmacia). Beads were pelleted, and the supernatant was concentrated by using Centricon 10 tubes (Amicon), diluted 10-fold with a buffer containing 10 mM Tris⋅HCl (pH 7.6), 150 mM NaCl, 2 mM MgCl2, and 0.1 mM DTT, and concentrated again. The purified fusion proteins were divided into aliquots and stored at −80°C.
Vectors for Expression of the Green Fluorescent Protein (GFP) Fusion Protein and Transfection.
Full-length CNP and CNP lacking 13 C-terminal amino acids were cloned into the XhoI/EcoRI sites of a EGFP-C3 plasmid (CLONTECH). Constructs were made in COS-7 cells grown to ≈60% confluence on coverslips in 3-mm wells. Transfections were performed with 1 μg of DNA and 6 ml of liposomes (Fu-Gene, Roche, Mannheim). Cells were incubated at 37°C under 5% CO2/95% air in the presence of DNA—liposome complexes for 5 h in growth medium. Transfectants then were analyzed at 48 h for expression of exogenous protein. The coverslips were fixed and treated as above.
In Vitro Tubulin Polymerization Assay.
GST-CNP (5 μg) was incubated with or without pure rat brain tubulin (3.9 μg) at 37°C for 45 min in 1 mM GTP/1 mM MgCl2/60 mM Mes buffer, pH 6.9/2.6 M glycerol, followed by centrifugation at 44,000 rpm in a Beckman 70.1 Ti rotor for 45 min at 37°C. CNP and tubulin were detected with monoclonal anti-CNP and anti-α-tubulin antibodies. Samples of GST-CNP or peptides were incubated in 60 mM Mes buffer, pH 6.9, containing 1 mM GTP, 1 mM MgCl2, 1 mM EGTA, and 10% DMSO in masked, thermostated, 3-mm path-length cuvettes. Tubulin was added to start the reaction. OD350 was read 20 s after mixing in a Cary 300 spectrophotometer (13). For cold depolymerization, cuvettes containing polymerized tubulin were kept on ice for 5 min and read as the sample rewarmed.
GST Pull-Down Assay.
Binding of full-length GST-CNP (10 μg) and deleted GST-CNP (10 μg) to glutathione-agarose beads (Amersham Pharmacia) was measured for 1 h in PBS. The GST fusion proteins bound to the beads were incubated with tubulin (7.8 μg) in PBS plus 1% Triton X-100 and protease inhibitors for 3 h at 4°C. After washing five times with the same buffer, bound proteins were eluted in loading buffer, electrophoresed in a 12% polyacrylamide gel, transferred to nitrocellulose, and probed with antibodies.
Results
CNP Association with Cytoplasmic and Membrane Tubulin.
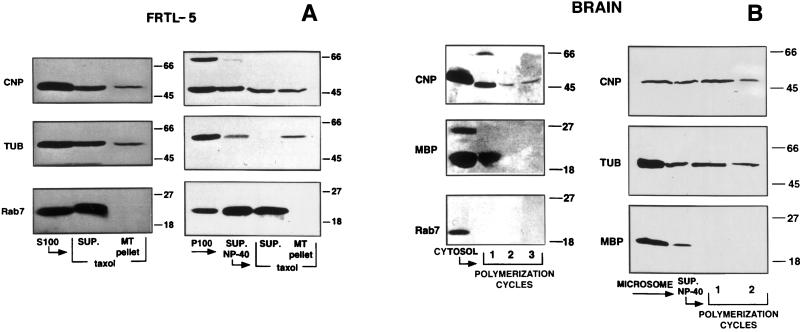
Both CNP and tubulin are readily identified in homogenates of FRTL-5 cells as well as in the 100,000 × g supernatant and in the crude pellet fractions by using immunoblot and size as criteria (Fig. 1A Left). Cytoskeletons were obtained in the presence of a microtubule-stabilizing buffer; both the soluble and the cytoskeletal fractions contain CNP and tubulin with an apparently higher concentration in the latter (Fig. 1A Right).
Figure 1.
Association of CNP with cytoplasmic and membrane tubulin in FRTL-5 cells (A) and in brain tissue (B). (A) Comparison of the presence of CNP, tubulin, and Rab7 in a Taxol–microtubule pellet (MT pellet) or a supernatant (SUP) obtained from S100 fractions (Left) and from P100 fractions extracted by Nonidet P-40 (SUP. Nonidet P-40) of FRTL-5 cells (Right). (B) Comparison of the persistence of CNP, MBP, and Rab7 during cycles of polymerization of rat brain supernatants (Left) and Nonidet P-40 extracts of brain microsomes (Right) by immunoblot analysis.
The tubulin/CNP association persisted during cycles of polymerization–depolymerization when brain tissue was used as a better source of CNP and tubulin. Cytosolic CNP remained with the brain microtubule pellet, whereas Rab7 and MBP were lost (Fig. 1B Left). Similarly, extracts from the crude microsome/membrane fraction prepared by 0.4% Nonidet P-40 showed tubulin/CNP association for at least two polymerization cycles, whereas MBP did not remain even in the first cycle (Fig. 1B Right).
CNP Enzymatic Activity in Microtubule Preparations.
Crude supernatant solutions from rat brain homogenates contain ample diesterase activity (5.56 nmol of NADPH per mg of protein per min). Surprisingly, partially purified brain microtubule preparations (≈90% tubulin) that still contained MAPs had almost the same specific activity of CNP (4.54 nmol of NADPH per mg per min). However, purification to a level of >99% tubulin (11, 13) removed essentially all of the diesterase activity (0.08 nmol of NADPH per mg per min). This persistence suggests a reasonably stable association of the two proteins.
Characteristics of Membrane-Bound CNP and Tubulin.
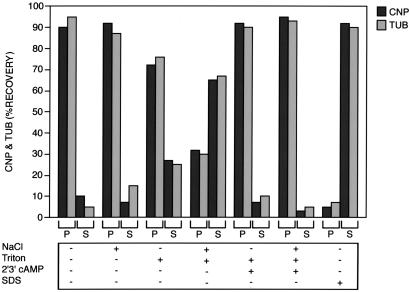
In all procedures listed in Fig. 2, CNP and tubulin behaved identically, whereas 1% SDS solubilized both proteins from the P100 fractions of brain. Neither 1.0 M NaCl nor 1% Triton X-100 alone led to a complete solubilization of the two proteins; however, when these were used together, substantial solubilization of CNP and tubulin was achieved. This observation suggests that both hydrophobic and ionic forces are involved in membrane attachment of these two proteins. They appear to form a complex that can associate with membranes. The substrate for CNP, 2′,3′-cAMP, almost completely prevented the solubilizing effect of 1% Triton X-100 alone and of NaCl + Triton. Thus, this nucleotide could regulate membrane localization of CNP, tubulin, or both proteins. Similar results were obtained in fractions derived from FRTL-5 cells.
Figure 2.
Solubilization characteristics of membrane-bound CNP and tubulin in brain tissue. The 100,000 × g crude membrane pellet from brain was resuspended and extracted under one of the following conditions: (i) 10 mM Tris⋅HCl buffer, pH 7.2 (control); (ii) 1 M NaCl; (iii) 1% (vol/vol) Triton X-100; (iv) 1% Triton X-100 + 1 M NaCl; (v) 1% Triton X-100 + 2′3′-cAMP (7.5 mM); (vi) 1% Triton X-100 + 1 M NaCl + 2′3′-cAMP; or (vii) 1% SDS. Percentages of CNP and tubulin immunoreactivity extracted from the pellet (P) into the soluble fraction (S) were determined by quantitative immunoblot analysis. The results are the means of three experiments.
The MAP-Like Nature of CNP.
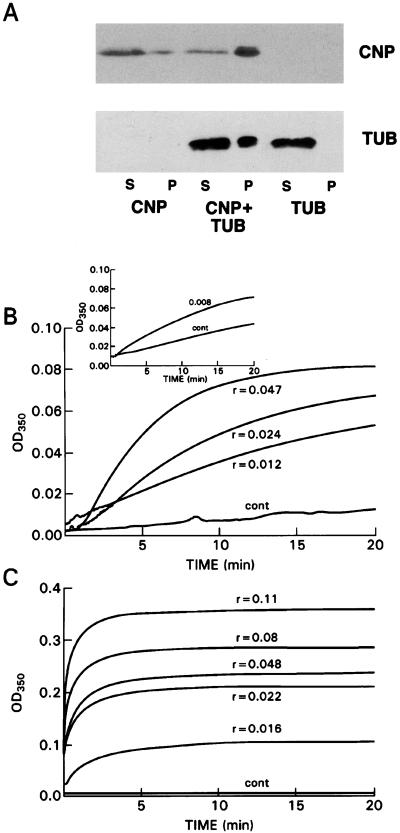
From the foregoing it seemed likely that CNP behaves like a MAP. Therefore, we investigated the potential MAP-like activity of CNP in binding and promoting tubulin polymerization in vitro by incubating rat recombinant CNP-fusion protein, with or without pure rat brain tubulin, followed by centrifugation and analysis of supernatant and microtubule-pellet fractions by immunoblotting. Fig. 3A shows that under limiting polymerization conditions, a microtubule pellet formed only when tubulin was incubated with CNP. Eighty percent of the CNP remained in the supernatant solution when tubulin was not added (Fig. 3A), whereas 80% of the CNP appeared in the pellet when incubated in the presence of tubulin. These data demonstrate the MAP-like character of CNP.
Figure 3.
The MAP-like character of CNP. (A) Binding of CNP to tubulin and promotion of tubulin polymerization in vitro. Shown are supernatants (S) and microtubule pellets (P) after incubation of CNP (5 μg) without or with pure rat brain tubulin (3.9 μg); also shown is tubulin alone (lanes 5 and 6) at 37°C for 45 min in 1 mM GTP/2.6 M glycerol, followed by centrifugation at 110,000 × g for 45 min at 37°C. CNP and tubulin were detected by immunoblot analysis. (B and C) Comparison of recombinant CNP and MAPs in promoting tubulin polymerization. (B) Recombinant CNP: 100 μl contained 1 mM GTP, 10% DMSO, CNP, pH 6.9 buffer, and 1.82 nmol of pure rat brain tubulin. Mole ratios shown above or below the curves were calculated on the basis of 70 kDa for the fusion protein. (Inset) No DMSO was used and tubulin was increased to 3.12 nmol. (C) MAPs containing ≈50% MAP2; mole ratios are calculated on the basis of 280 kDa. One hundred microliters of mixture contained 1 mM GTP, MAPs, assembly buffer at pH 6.9, and 3.12 nmol of pure rat brain tubulin, added last. All reactions were carried out at 37°C in 3-mm light-path-masked cuvettes.
A convenient measure of MAP-like activity of various proteins is the turbidimetric determination of microtubule assembly under conditions in which unaided polymerization is marginal. Here, we compare semipurified MAPs [generously provided by Ernest Hamel (14)] with recombinant CNP. To conserve the latter, we used lower concentrations of tubulin in the presence of 10% DMSO. The CNP-fusion protein exhibits the assembly-promoting effects as evidenced by a reduction in the latent period and an increase in the extent of polymerization at low mole ratios (Fig. 3B). The Inset in Fig. 3B shows that DMSO was not required to elicit the CNP effect. As shown in Fig. 3C, using authentic MAPs, microtubule assembly is promoted at similar mole ratios as with CNP, but the effect on the nucleation phase of polymerization appears to be greater than that seen with CNP. Precise comparison of CNP with MAPs cannot be calculated because of the multiple components of the MAP preparation and the use of different amounts of tubulin. These effects are not due to the GST portion of the fusion protein because it has been shown that GST alone does not bind to tubulin (15, 16).
Identification of the Tubulin-Binding Domain.
We compared the amino acid sequence of CNP with the tubulin-binding domain of MAP2. The last 13 amino acids at the C terminus of CNP show significant homology with 13 amino acids present in the tubulin-binding domain of MAP2:
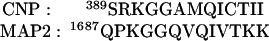 |
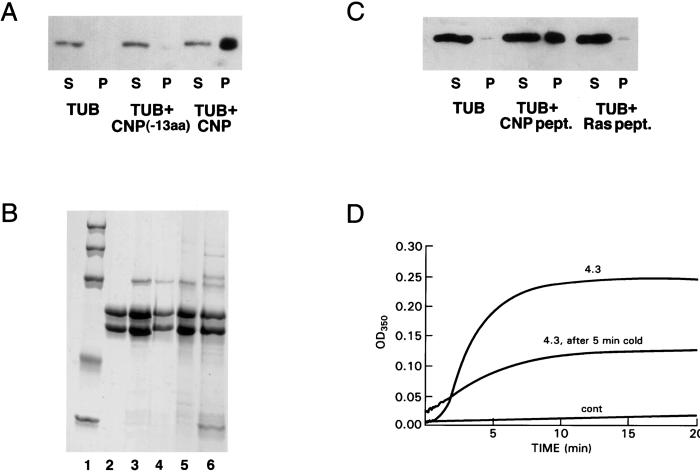
We have cloned and produced a recombinant CNP-fusion protein lacking the C-terminal 13 residues (CNP-13aa). This truncated CNP has lost most of its ability to promote tubulin polymerization (Fig. 4A, pellet (P) fractions), and we have confirmed this by light scattering (data not shown). However, binding of this construct to preformed microtubules still occurred. The bulk of the CNP was pelleted with the microtubule fraction (compare lane 3 with lane 4 of Fig. 4B). When the truncated CNP was exposed to Taxol-stabilized microtubules, almost as much of the truncated protein copolymerized with microtubules (lane 5) as did the intact CNP (lane 3). Note that the microtubules selectively excluded the contaminants present in the preparation, confirming the specificity of the binding (lane 6). The data suggest that the 13 C-terminal amino acids are likely to be required for assembly but not for binding to tubulin.
Figure 4.
Comparison of CNP and truncated CNP in promoting tubulin polymerization and microtubule association. (A) Truncated CNP does not promote assembly; conditions are similar to those of Fig. 3A. (B) Rat brain tubulin (750 μg) was incubated in Mes assembly buffer containing 1 mM GTP and 22 μM Taxol at 37°C for 28 min. Microtubules were pelleted, washed once with Taxol-containing buffer, and resuspended in 300 μl of the same buffer. Twenty microliters was incubated with full-length CNP, truncated CNP, or buffer for 30 min at 37°C, and sedimented, and pellet and supernatant solution were applied to a 10% acrylamide/SDS gel. Lanes: 1, molecular weight standards (200,000, 116,250, 97,400, 66,300, 45,000, 31,000); 2, rat brain tubulin; 3, pellet with full-length CNP; 4, supernatant with full-length CNP; 5, pellet with truncated CNP; 6, supernatant with truncated CNP. (C) Comparison of CNP peptide (SRKGGAMQICTII) and H-ras peptide (SGLGCMSCKCVLS) in promoting tubulin polymerization; S, supernatant; P, pellet. (D) Stimulation of tubulin polymerization by the C-terminal peptide of mouse CNP. One hundred microliters of sample contained 1.82 nmol of pure rat brain tubulin, 1 mM GTP, and 10% DMSO in Mes assembly buffer at 37°C. Numbers on curves show mole ratios of peptide to tubulin. Cold reversibility of peptide-induced polymerization also is shown. Conditions are as in the control. After 20 min, the sample was cooled on ice for 5 min and allowed to repolymerize. Repolymerization was incomplete by 20 min; the rate includes a temperature component.
To confirm this hypothesis, we synthesized a 13-aa peptide corresponding to a mouse C-terminal CNP—SRKGGAMQICTII. The rat congener, lacking one of the basic amino acids, was insufficiently soluble. It is apparent from Fig. 4C that only the CNP peptide promoted microtubule assembly. As a negative control, we used a 13-aa peptide from the C terminus of H-Ras (SGLGCMSCKCVLS). Although the peptide contains a CAAX box as does CNP, it was not able to induce tubulin polymerization [Fig. 4C, pellet (P) fractions]. Furthermore, the CNP peptide promoted cold-sensitive microtubule assembly (Fig. 4D). The mole ratio required to promote polymerization was much higher than that required for the intact fusion protein; at mole ratios < 1, little or no polymerization occurred with the peptide. This observation suggests that important contributions to binding are provided by the remainder of the CNP molecule.
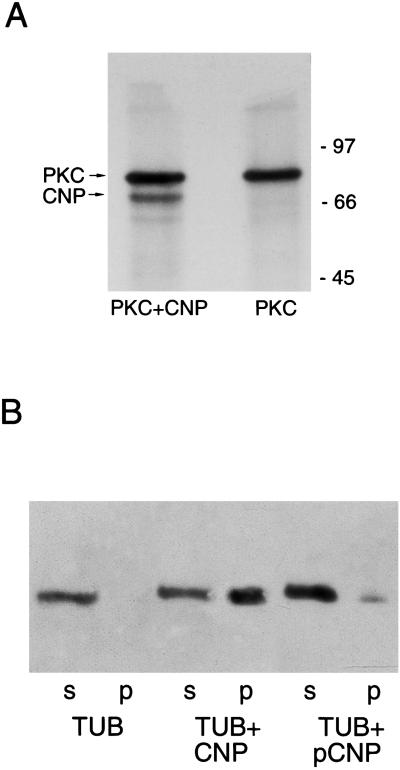
A specific characteristic of the MAPs is the loss of tubulin binding and promotion of microtubule polymerization after phosphorylation. Consequently, we tested the influence of the phosphorylation state of CNP on its MAP-like activity. CNP is phosphorylated in vivo by protein kinase C (PKC) (17), and we show here that recombinant PKC is able to phosphorylate recombinant CNP (Fig. 5A). Phosphorylation of CNP significantly reduces its capacity to bind tubulin and promote polymerization in vitro as shown by the tubulin pellet (Fig. 5B).
Figure 5.
The role of phosphorylation of CNP. (A) CNP was phosphorylated in vitro with recombinant PKC, and products were analyzed on a 12% polyacrylamide gel followed by radioautography. (B) Comparison of CNP and phosphorylated CNP in promoting tubulin polymerization. Phosphorylated GST-CNP was purified on glutathione agarose and tested for MAP-like activity.
Subcellular Distribution of CNP.
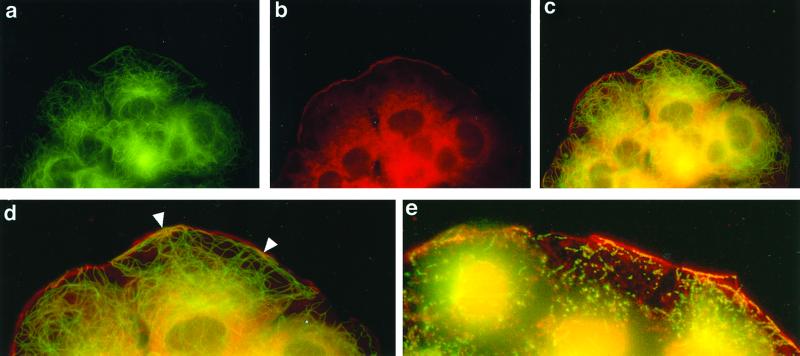
To confirm the biochemical data on association of CNP and tubulin in membrane and cytoskeletal compartments, we performed immunofluorescence studies. As shown in Fig. 6 a–c, fluorescent labeling of microtubules (green) and CNP (red) of FRTL-5 cells reveals patches of colocalization (yellow) of the two proteins at the membrane and subplasmalemmal region. An enlarged version of the membrane area is shown in Fig. 6d. The patches are interrupted by zones containing only CNP. Microtubules extending toward the membrane show no association with CNP, but the perinuclear region again shows abundant overlap of the two proteins. Cytoskeletons produced from these cells also show clear colocalization at the subplasmalemmal level (Fig. 6e).
Figure 6.
Colocalization of tubulin and CNP in FRTL-5 cells. Cells were grown on coverslips, fixed, and stained with a monoclonal anti-α-tubulin antibody [fluorescein (a)] or an antibody to CNP1 [rhodamine (b)]. (c) Double immunofluorescence (superimposition of a and b staining). (d) Enlarged membrane region from c. (e) Overlap of CNP and tubulin in a cytoskeleton prepared from a COS cell.
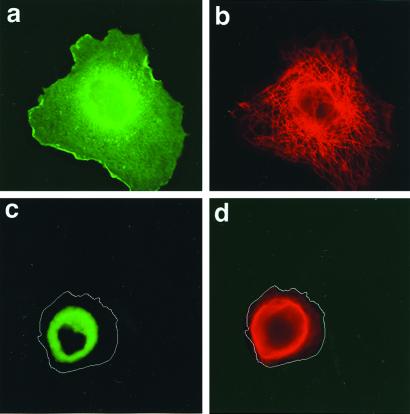
The functional significance of the CNP/tubulin association is shown by transient expression of fusions of GFP-CNP and GFP-C-terminal-deleted CNP in COS cells. Localization of fusion proteins 24 h after introduction is shown in Fig. 7; note that colors are reversed compared with Fig. 6. GFP fluorescence of full-length CNP is widely distributed through the cell, with patches clearly visible at the plasma membrane region (Fig. 7a), whereas microtubule distribution (red) appears to be normal (Fig. 7b). By contrast, the distribution of the C-terminal-deleted CNP was limited to the perinuclear area (Fig. 7c); this coincided with a contraction of the microtubule network around the nucleus of the cell (Fig. 7d). It is clear that the normal distribution of these two proteins is linked and depends on a functional CNP.
Figure 7.
Effect of CNP truncation on CNP and tubulin distribution. Note that because GFP is used, the secondary antibody for tubulin uses rhodamine. Localization of GFP-CNP fusion protein (a) and GFP-C-terminal-deleted CNP fusion protein (c) expressed in COS cells compared with the corresponding microtubule distribution (b and d) by fluorescence microscopy (red). The cell borders are outlined in c and d.
Discussion
Interference in protein prenylation (by use of lovastatin) blocks membrane reattachment of microtubules in FRTL-5 thyroid cells (18). Because tubulin has no C-terminal CAAX box for prenylation, we proposed that this blockade resulted from a lack of prenylation of a linker protein. A candidate for such a protein was found to be CNP (6), a MAP that is both prenylated and palmitoylated (19, 20). This protein is found attached to the plasma membrane of various cells as long as both lipid modifications have occurred (21–23), and association of CNP with the cytoskeleton was suggested. We now have obtained convincing, direct evidence that such an association occurs and confers MAP-like properties on CNP.
The strong association in brain and FRTL-5 cells occurs in two different locations: cytoplasm and membranes. Evidence for the former locus is the following: (i) isolated microtubules have CNP enzymatic activity; (ii) CNP has MAP-like activity in binding and promoting polymerization of tubulin at low mole ratios; and (iii) both proteins persist through repeated cycles of polymerization—depolymerization. Evidence for membrane association is as follows: (i) tubulin and CNP have been shown independently to be present in cell membranes; (ii) the two proteins behave identically in various membrane-extraction procedures; and (iii) when extracted from membranes, the two proteins subsequently cosediment in the microtubule pellet through several polymerization cycles.
CNP promotes polymerization of pure rat brain tubulin at low mole ratios, as measured by both protein pelleting and light scattering. The bulk of this activity appears to reside in the C-terminal 13-mer of CNP, a peptide that has substantial sequence similarity with a tubulin-binding repeat of MAP2 and a number of other tubulin-binding domains that promote tubulin polymerization; its absence from CNP abolishes this MAP-like activity and interferes with normal cellular distribution of tubulin and CNP. CNP thus is a dual-function protein, diesterase and membrane-bound MAP, that can act as a linker protein for microtubule association with cellular membranes.
Microtubules often are seen in contact with mitochondria (24–26), synaptic vesicles (27), endoplasmic reticulum (28), lysosomes (29), cilia (30), and plasma membranes (31). Such association via linker proteins may be more common than thought heretofore. Thus, although CNP can direct or promote microtubule attachment to plasma and, perhaps, internal membranes, linkers to endosomes (CLIP 170) and to rough endoplasmic reticulum (p63) exist (32, 33). The tubulin-binding domains of both of these proteins are basic, as is the C terminus of CNP, and it is likely that electrostatic interactions contribute to their association with tubulin.
In an earlier study (18), it was shown that after prenylation of proteins had been blocked with lovastatin in FRTL-5 thyroid cells, the microtubules become disconnected from the plasma membrane region and retracted toward the cytosol. Moreover, when microtubules are depolymerized with colchicine for 3 h and then are allowed to regrow after removal of the drug in the presence of lovastatin, assembly proceeds normally until the region near the plasma membrane is reached and then appears to stop without establishing contact with the membrane (data not shown). Similarly, the failure of normal microtubule extension in cells transfected with defective CNP-GFP fusion protein (Fig. 7) suggests that normal microtubule distribution requires the participation of CNP. Finally, the role of prenylated CNP as a membrane MAP may provide additional insight as to how compounds such as farnesyltransferase inhibitors and/or lovastatin could, by affecting microtubules, act as anticancer agents.
Acknowledgments
We thank Leslie Knipling for generous supplies of tubulin and Dr. P. Formisano for helpful discussions. This work was supported by the Associazione Italiana per la Ricerca sul Cancro, and Associazone Partenopea Ricerche Oncologiche. We dedicate this paper to the memory of Prof. Gaetano Salvatore.
Abbreviations
- CNP
2′,3′-cyclic nucleotide 3′-phosphodiesterase
- GFP
green fluorescent protein
- GST
glutathione S-transferase
- MAP
microtubule-associated protein
- PKC
protein kinase C
- MBP
myelin basic protein
References
- 1.Bhattacharyya B, Wolff J. J Biol Chem. 1975;250:7639–7646. [PubMed] [Google Scholar]
- 2.Bhattacharyya B, Wolff J. Nature (London) 1976;264:576–577. doi: 10.1038/264576a0. [DOI] [PubMed] [Google Scholar]
- 3.Babitch J A. J Neurochem. 1981;37:1394–1400. doi: 10.1111/j.1471-4159.1981.tb06307.x. [DOI] [PubMed] [Google Scholar]
- 4.Kelly W G, Passaniti A, Woods J W, Daiss J L, Roth T F. J Cell Biol. 1983;97:1191–1199. doi: 10.1083/jcb.97.4.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stephens R E. Biol Cell. 1986;57:95–109. doi: 10.1111/j.1768-322x.1986.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 6.Laezza C, Wolff J, Bifulco M. FEBS Lett. 1997;413:260–264. doi: 10.1016/s0014-5793(97)00924-1. [DOI] [PubMed] [Google Scholar]
- 7.Braun P E, De Angelis D A, Shtybel W W, Bernier L. J Neurosci Res. 1991;30:540–544. doi: 10.1002/jnr.490300311. [DOI] [PubMed] [Google Scholar]
- 8.Ambesi-Impiombato F S, Parks L A M, Coon H G. Proc Natl Acad Sci USA. 1980;77:3455–3459. doi: 10.1073/pnas.77.6.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Möller J R, Ramaswamy S G, Jacobowitz D M, Quarles R H. J Neurochem. 1992;58:1829–1835. doi: 10.1111/j.1471-4159.1992.tb10059.x. [DOI] [PubMed] [Google Scholar]
- 10.Vallee R B, Collins C A. Methods Enzymol. 1986;134:116–127. doi: 10.1016/0076-6879(86)34080-1. [DOI] [PubMed] [Google Scholar]
- 11.Sackett D L, Knipling L, Wolff J. Protein Expression Purif. 1991;2:390–393. doi: 10.1016/1046-5928(91)90099-5. [DOI] [PubMed] [Google Scholar]
- 12.Stephon R L, Niedbala R S, Schray K J, Heindel N D. Anal Biochem. 1992;202:6–9. doi: 10.1016/0003-2697(92)90197-f. [DOI] [PubMed] [Google Scholar]
- 13.Wolff J. Biochemistry. 1998;37:10722–10729. doi: 10.1021/bi980400n. [DOI] [PubMed] [Google Scholar]
- 14.Hamel E, Lin C M. Biochemistry. 1984;23:4173–4184. doi: 10.1021/bi00313a026. [DOI] [PubMed] [Google Scholar]
- 15.Van Rossum D, Kuhse J, Betz H. J Neurochem. 1999;72:962–973. doi: 10.1046/j.1471-4159.1999.0720962.x. [DOI] [PubMed] [Google Scholar]
- 16.Gachet Y, Tournier S, Lee M, Lazaris-Karatzas A, Poulton T, Bommer U-A. J Cell Sci. 1999;112:1257–1271. doi: 10.1242/jcs.112.8.1257. [DOI] [PubMed] [Google Scholar]
- 17.Agrawal H C, Sprinkle T J, Agrawal D. Neurochem Res. 1994;19:721–728. doi: 10.1007/BF00967712. [DOI] [PubMed] [Google Scholar]
- 18.Bifulco M, Laezza C, Aloj S M, Garbi C. J Cell Physiol. 1993;155:340–348. doi: 10.1002/jcp.1041550215. [DOI] [PubMed] [Google Scholar]
- 19.Agrawal H C, Sprinkle T J, Agrawal D. J Biol Chem. 1990;265:11849–11853. [PubMed] [Google Scholar]
- 20.De Angelis D A, Braun P E. J Neurosci Res. 1994;39:386–397. doi: 10.1002/jnr.490390405. [DOI] [PubMed] [Google Scholar]
- 21.Dyer C A, Benjamins J A. J Neurosci Res. 1989;24:201–211. doi: 10.1002/jnr.490240211. [DOI] [PubMed] [Google Scholar]
- 22.Gillespie C S, Wilson R, Davidson A, Brophy P J. Biochem J. 1989;260:689–696. doi: 10.1042/bj2600689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson R, Brophy P J. J Neurosci Res. 1989;22:439–448. doi: 10.1002/jnr.490220409. [DOI] [PubMed] [Google Scholar]
- 24.Bernier-Valentin F, Aunis D, Rousset B. J Cell Biol. 1983;97:209–216. doi: 10.1083/jcb.97.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith S D, Jarlfors U, Cayer L M. J Cell Sci. 1977;27:255–272. doi: 10.1242/jcs.27.1.255. [DOI] [PubMed] [Google Scholar]
- 26.Heggeness M H, Simon M, Singer S J. Proc Natl Acad Sci USA. 1978;75:3863–3866. doi: 10.1073/pnas.75.8.3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gray E G. Proc R Soc London Ser B. 1975;190:369–372. [PubMed] [Google Scholar]
- 28.Vogl A W. Cell Motil Cytoskeleton. 1996;14:491–500. [Google Scholar]
- 29.Mithieux G, Audebet C, Rousset B. Biochim Biophys Acta. 1988;969:121–130. doi: 10.1016/0167-4889(88)90067-5. [DOI] [PubMed] [Google Scholar]
- 30.Dentler W L. Int Rev Cytol. 1981;72:1–47. doi: 10.1016/s0074-7696(08)61193-6. [DOI] [PubMed] [Google Scholar]
- 31.Hardham A R, Gunning B E S. J Cell Biol. 1978;77:14–34. doi: 10.1083/jcb.77.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pierre P, Scheel J, Rickard J E, Kreis T E. Cell. 1992;70:887–900. doi: 10.1016/0092-8674(92)90240-d. [DOI] [PubMed] [Google Scholar]
- 33.Klopfenstein D R C, Kappeler F, Hauri H-P. EMBO J. 1998;17:6168–6177. doi: 10.1093/emboj/17.21.6168. [DOI] [PMC free article] [PubMed] [Google Scholar]