Abstract
DNA–protein crosslinks (DPCs) arise in biological systems as a result of exposure to a variety of chemical and physical agents, many of which are known or suspected carcinogens. The biochemical pathways for the recognition and repair of these lesions are not well understood in part because of methodological difficulties in creating site-specific DPCs. Here, a strategy for obtaining site-specific DPCs is presented, and in vitro interactions of the Escherichia coli nucleotide excision repair (NER) UvrABC nuclease at sites of DPCs are investigated. To create site-specific DPCs, the catalytic chemistry of the T4 pyrimidine dimer glycosylase/apurinic/apyrimidinic site lyase (T4-pdg) has been exploited, namely, its ability to be covalently trapped to apurinic/apyrimidinic sites within duplex DNA under reducing conditions. Incubation of the DPCs with UvrABC proteins resulted in DNA incision at the 8th phosphate 5′ and the 5th and 6th phosphates 3′ to the protein-adducted site, generating as a major product of the reaction a 12-mer DNA fragment crosslinked with the protein. The incision occurred only in the presence of all three protein subunits, and no incisions were observed in the nondamaged complementary strand. The UvrABC nuclease incises DPCs with a moderate efficiency. The proper assembly and catalytic function of the NER complex on DNA containing a covalently attached 16-kDa protein suggest that the NER pathway may be involved in DPC repair and that at least some subset of DPCs can be removed by this mechanism without prior proteolytic degradation.
In cells, DNA is tightly associated with a variety of proteins that serve both to maintain the structural organization of the genetic material and to coordinate cellular processes including replication, repair, recombination, and transcription. Many endogenous compounds (e.g., metabolites of lipid peroxidation) as well as environmental agents are reactive with both DNA and proteins and thus can produce covalent linkage between these two types of macromolecules (1–13). DNA–protein crosslinks (DPCs) represent a relatively abundant form of DNA damage as evidenced by data indicating that the background level of DPCs in human white blood cells ranged from 0.5 to 4.5 per 107 bases (1). An age-related accumulation of DPCs has also been observed in mouse organs (2), supporting the hypothesis that oxidative mechanisms contribute to the formation of these DNA damages (2, 3). DPC levels increase dramatically upon exposure to a variety of physical or chemical agents, including UV light (4), ionizing radiation (5), β-propiolactone (6), aldehydes (1, 7–9), arsenite (10), ferric nitrilotriacetate (11), chromate (12), nickel (13), and others. Chemotherapeutic agents, such as cisplatin (12, 14), bisplatinum (15), and neocarcinostatin (16), have also been shown to induce DPC formation. Exposure to several DPC-inducing agents gives rise to genotoxic and carcinogenic effects, and for some agents, such as formaldehyde, their primary mutagenic effects are believed to be mediated through the formation of DPCs (7, 9).
Despite the recognition of the biological significance of DPCs, there are very limited data concerning the repair of these lesions. Analyses of data generated in cell culture revealed the existence of mechanisms of active DPC removal and suggested that more than one repair pathway can be involved in the repair of these lesions (17–21). Because of its wide substrate specificity toward a variety of bulky DNA lesions, nucleotide excision repair (NER) (22, 23) has been proposed to be potentially responsible for DPC removal (17, 20, 21) that initially proceeds through a proteolytic degradation pathway (21). Indeed, trans-Pt(II) diamminedichloride-induced DPCs were found to be more persistent in NER-deficient xeroderma pigmentosum (XP) group A fibroblasts compared with normal cells (17). However, the active removal of formaldehyde-derived DPCs was not significantly affected in XP-A or XP-F cells, suggesting a limited role (if any) of NER in the repair of those DPCs (18, 20, 21). In the current study, we present a strategy for obtaining site-specific DPCs and investigate an ability of UvrABC nuclease, which initiates the NER in Escherichia coli (22, 24), to excise DPCs in vitro.
Materials and Methods
Reagents and Enzymes.
Oligodeoxynucleotides were synthesized by the University of Texas Medical Branch, National Institute on Environmental Health Sciences Center Molecular Biology Core. T4 polynucleotide kinase, terminal transferase, uracil DNA glycosylase, and SnaBI and HaeIII restriction endonucleases were obtained from New England Biolabs. T4 DNA ligase was obtained from GIBCO/BRL. [γ-32P]ATP and [α-32P]3′dATP were purchased from New England Nuclear. T4 pyrimidine dimer glycosylase/apurinic/apyrimidinic (AP) site lyase (T4-pdg) and UvrA, UvrB, and UvrC proteins were purified as described previously (refs. 25 and 26, respectively).
DNA Substrate Preparation.
Sequences and preparations of the 50-mer DNAs containing (+)-trans-benzo[a]pyrene diolepoxide and N-2-acetyl-aminofluorene (AAF) guanine have been described (refs. 26 and 27, respectively). To obtain uracil-, reduced AP site-, and DPC-containing DNA substrates, 60-mer oligodeoxynucleotide containing a centrally located uracil (Fig. 1A) was phosphorylated with T4 polynucleotide kinase by using [γ-32P]ATP or, alternatively, was 3′-terminally labeled with terminal transferase and [α-32P]3′dATP. To obtain internally labeled DNA substrates, two oligodeoxynucleotides, a 26-mer and a 34-mer, were designed so that the product of their ligation would have the same sequence as shown in Fig. 1A. The 34-mer oligodeoxynucleotide was phosphorylated by [γ-32P]ATP and ligated to the 26-mer by using T4 DNA ligase and 60-mer complementary strand. The single-stranded 60-mer product of the ligation was purified through 15% urea-PAGE. The 32P-labeled 60-mer oligodeoxynucleotides were annealed to the complementary strand, and 1 pmol of these DNA duplexes was reacted with uracil DNA glycosylase. An aliquot was probed by reaction with T4-pdg to verify that all uracils had been converted to AP sites. For AP site reduction, 10 mM NaBH4 was added to 5 pmol of the AP site containing DNA. For DNA–protein covalent complex formation, 100× molar excess of T4-pdg was added to 5 pmol of the AP site containing DNA in the presence of 10 mM NaBH4. To dissociate the noncovalently bound T4-pdg from DNA, 100 mM NaCl was added to the trapping reaction. Uracil-, reduced AP site-, and DPC-containing DNA duplexes were purified through 5% native PAGE in the presence of the 100 mM NaCl, and the bands of interest were excised and eluted with buffer consisting of 500 mM ammonium acetate/10 mM magnesium acetate/1 mM EDTA. DNA samples were dialyzed against 10 mM Tris-HCl, pH 7.6/1 mM EDTA overnight. Restriction enzyme analyses with SnaBI and HaeIII were performed according to manufacturer protocols.
Figure 1.
Preparation of site-specific DNA–protein crosslinks. (A) Sequence of the uracil-containing 60-mer oligodeoxynucleotide. (B) Urea-PAGE showing DNA substrate preparation. Lane 1, uracil-containing 60-mer; lane 2, uracil-containing 60-mer, digested with uracil DNA glycosylase and tested with T4-pdg (control of AP-site formation); reduced AP site-containing DNA before (lane 3) and after (lanes 4–6) purification; DPC-containing DNA before (lane 7) and after (lanes 8–10) purification. After purification, DNAs were subjected to the restriction endonuclease digestion with SnaBI (5, 9) or HaeIII (6, 10). (C) SDS/PAGE showing DPC-containing DNA substrates before (lane 1) and after (lane 2) HaeIII digestion.
DNA Incision by UvrABC.
UvrABC incision reactions were performed essentially as described (26). The DNA substrates (1 nM) were incubated with UvrA (10 nM), UvrB (250 nM), and UvrC (50 nM) in a 10-μl reaction buffer containing 50 mM Tris-HCl, pH 7.5/50 mM KCl/10 mM MgCl2/5 mM DTT/1 mM ATP at 37°C for 30 min or, in time course experiments, as indicated. Reactions were terminated by the addition of 50% formamide/10 mM EDTA and heating at 90°C for 2 min, and the products were separated through 15% urea-PAGE. Alternatively, reactions were terminated by the addition of a protein loading buffer containing 62.5 mM Tris-HCl, pH 6.8/2% SDS/10% glycerol (vol/vol)/100 mM DTT and boiling for 5 min. Samples were separated by electrophoresis through 12.5% SDS/PAGE. Bands were visualized by autoradiography of the gel by using Hyperfilm MP x-ray film (Amersham Pharmacia Biotech). Quantitative analyses were performed by using PhosphorImager screens and IMAGE QUANT 5.0 software (Molecular Dynamics).
Results
To assess whether NER could participate in the initiation of DPC repair, it was necessary to obtain site-specific DPCs. To accomplish this, a precise understanding of the catalytic mechanism of T4-pdg was exploited. T4-pdg, a 16-kDa protein, initiates the repair of cis, syn-cyclobutane pyrimidine dimers within double-stranded DNA, proceeding through the formation of an imino (Schiff base) complex between its N-terminal α-amino group and the C1′ carbon of the 5′ sugar within the dimer (28). In the presence of a reducing agent such as sodium borohydride, this imino complex can be reduced and covalently trapped, thereby producing a stable, DNA–protein complex (29). Similarly, T4-pdg can also be covalently bound to the DNA backbone at an AP site, which is an intermediate product of the catalysis (30).
To create site-specific DPCs, the starting substrate DNA was a uracil-containing 60-mer oligodeoxynucleotide (Fig. 1A) that was 32P labeled either at the 5′ or 3′ end or, alternatively, at the 5th nucleotide position 5′ to the uracil. A protocol was developed (see Material and Methods) that allowed DNA–protein complexes to be isolated separately from both DNAs not containing the covalent linkage (Fig. 1B, lane 8, and Fig. 1C) and excess enzyme. To serve as positive incision controls for these experiments, reduced AP site- and AAF lesion-containing DNAs were purified, both known substrates for the well characterized NER system, UvrABC nuclease (27, 31). It was shown by urea-PAGE (Fig. 1B) that both reduced AP site- and DPC-containing oligodeoxynucleotides were cleaved by the restriction endonucleases, confirming the double-stranded character and purity of the substrate preparations. Because urea-PAGE does not permit the separation of the DNA–protein complexes composed of oligodeoxynucleotides of different size, the product of cleavage of DPC-DNAs by HaeIII was detected on SDS/PAGE (Fig. 1C).
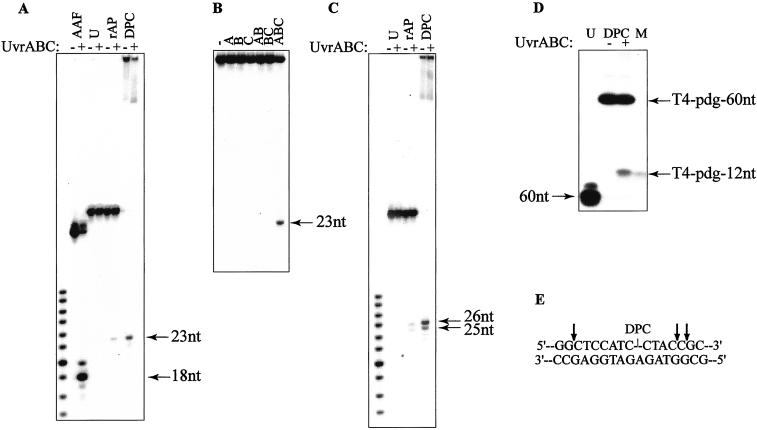
Incubation of the 5′-terminally labeled DPC-containing substrate in the presence of the UvrABC nuclease resulted in accumulation of a 23-mer product, indicating that incision occurred at the 8th phosphodiester bond 5′ to the adducted site (Fig. 2A). DNA incision at this position is specific for the UvrABC system (22, 24) and was also observed in reactions with two known substrates for NER, namely DNAs containing either an AAF-guanine lesion or a reduced AP site (27, 31). In contrast, no product was detected in a reaction on the uracil-containing 60-mer that was used as a negative control. On the DPC-adducted substrate, the 5′ incision occurred only in the presence of all three Uvr proteins (Fig. 2B), indicating that all three subunits were required for the reaction to proceed. To assay for a UvrABC-mediated 3′ incision, DPC substrates 3′-terminally labeled on the damaged strand were used. Two products, a major 26-mer and a minor 25-mer, were detected (Fig. 2C), revealing that these incisions took place predominantly at the 5th phosphate and additionally at the 6th phosphate 3′ to the adducted site. Multiple incision sites are typical for 3′ incision by UvrABC nuclease (22, 24) and were also observed in the control reaction with the reduced AP site-containing DNA (Fig. 2C).
Figure 2.
Incision of DNA–protein crosslinks by UvrABC nuclease. (A) Urea-PAGE of 5′-terminally labeled DNAs incubated without (−) or with (+) UvrABC proteins. DNAs were AAF-guanine-containing 50-mer (AAF), uracil-containing 60-mer (U), reduced AP site-containing 60-mer (rAP), and DPC-containing 60-mer (DPC). (B) Urea-PAGE of 5′-terminally labeled DPC-containing 60-mer incubated without (−) or with (+) UvrA, UvrB, and UvrC proteins as indicated. (C) Urea-PAGE of 3′-terminally labeled DNAs incubated without (−) or with (+) UvrABC proteins. DNAs designated as in A. (D) SDS/PAGE of the internally labeled (at 5th position from 5′ side relative to the adducted site) DPC-containing 60-mer incubated without (−) and with (+) UvrABC nuclease. Left lane (U) represents uracil-containing 60-mer. Right lane (M, marker) contains 12-mer DNA with centrally positioned T4-pdg-DNA crosslink. (E) Positions of the 5′-side and 3′-side incisions by UvrABC nuclease.
Having demonstrated that UvrABC nuclease can make both 5′ and 3′ incisions on a DPC-containing substrate, we then examined whether these two events take place within the same DNA molecule. For this purpose, internally 32P-labeled substrates were created at the 5th position 5′ to the adducted site and reacted with UvrABC. When the products of the reaction were analyzed by SDS/PAGE, a new product band was detectable that had the same electrophoretic mobility as a 12-mer oligodeoxynucleotide covalently bound with T4-pdg (Fig. 2D). Because no other larger molecular weight bands appeared that could have suggested independent 3′ or 5′ incisions, these data confirmed a coordinated dual incision on the DPC substrate. Taken together, these data indicate that on a DPC-containing substrate, a major product of incision by UvrABC nuclease was the12-mer DNA fragment covalently bound with the protein (Fig. 2E). The possibility that the nondamaged complementary strand may be a substrate for UvrABC incision was also examined. No incision products were detected in reaction with the DPCs in which only the nondamaged strand was 5′-terminally labeled (data not shown).
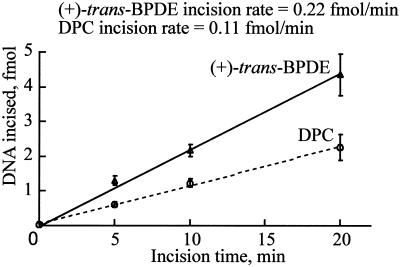
Analyses of the data in Fig. 2 suggested that the yield of the incision products was higher in reactions with DPCs compared with reduced AP site-containing DNAs. To better evaluate the efficiency of DPC removal, comparative studies were performed by using both DPC- and (+)-trans-benzo[a]pyrene diolepoxide-adducted DNA, a well established substrate for the UvrABC system (26). The relative rates of adduct removal were monitored by assaying the 5′ incision. As shown in Fig. 3, the kinetics of the incision on the DPC substrate indicated that the incision was about one-half efficient as that of the (+)- trans-benzo[a]pyrene diolepoxide-containing DNA, suggesting that UvrABC nuclease incises DPCs with a moderate efficiency.
Figure 3.
Kinetics of DNA incision by UvrABC nuclease on the 5′-terminally labeled DNA substrates containing (+)-trans-benzo[a]pyrene diolepoxide or DPC. Data are the means ± SD of three independent experiments. The incision rate was calculated as the slope of the line obtained from unweighted linear regression.
Discussion
The data within this report demonstrate the extreme versatility of the NER damage recognition and incision proteins. The UvrABC nuclease was able to form a catalytically competent complex on DPC-containing DNA in which incisions were made at the exact same 5′ and 3′ positions as compared with a reduced AP site-containing substrate. Direct evidence is presented for the nucleotide excision repair of DNA–protein crosslinks. These data are significant because they not only expand the understanding of the diversity and flexibility of the protein–DNA adduct interactions associated with NER, they also reveal a new role of NER in the biological pathways processing DNA–protein crosslinks.
The dual catalytic incision performed by the UvrABC nuclease results from a complex process that involves several sequential steps. The process is initiated with the formation of a UvrA2B complex and its binding to DNA at the damaged site, followed by a localized strand separation. Concomitant with the formation of this “open” complex is a dissociation of the UvrA dimer and formation of a stable preincision UvrB–DNA complex. Next, recruitment of UvrC by interactions with UvrB leads to formation of the UvrBC–DNA intermediate structure that triggers 3′ and 5′ endonuclease activities of UvrC (22, 24, 32). Despite these complexities in catalysis and some controversies regarding the mechanisms of the damage recognition by UvrABC, great progress has been made recently in understanding many important aspects of this process. What is widely accepted is that both DNA modification and distortions in duplex structure must be present in the DNA for efficient incision by NER (22, 24, 33, 34). The first structural feature recognized by the UvrA2B complex upon its loading onto DNA is a local distortion of the DNA helix caused by the damage (34). In some cases, e.g., when damage is localized in a bubble region (34, 35), incision can be achieved in the absence of UvrA, suggesting that the requirement of UvrA can be circumvented by designing the DNA substrate with a preformed, stable bubble structure. Within the preincision complex, hydrophobic forces were reported to be involved in the interactions between UvrB and the DNA substrate (36). The crystal structures of the UvrB proteins revealed the presence of a unique structural element, a flexible β-hairpin that is rich in conserved hydrophobic residues (37–39). Two recent studies on the mutant E. coli and Bacillus caldotenax UvrB suggested that the β-hairpin motif of the protein is directly involved in the recognition of DNA adducts probably through its insertion or stacking into the DNA helix (40, 41). In the case of E. coli NER, the Tyr-95 and Tyr-96 residues at the base of the hairpin were shown to be crucial for damage-specific binding (41).
The cocrystal structure of a catalytically incompetent T4-pdg with DNA containing a cis, syn-cyclobutane pyrimidine dimer (42) as well as the molecular footprint of T4-pdg bound to damage-containing DNA (43) revealed that the enzyme makes numerous contacts with both damaged and nondamaged strands. According to the cocrystal structure, although the enzyme did not undergo major structural alterations, the DNA was kinked at a ≈60° bend with the nucleotide pairing partner to the 5′ pyrimidine of the dimer flipped to an extrahelical position on a surface cleft of the enzyme. The stability of the DNA bend has not been experimentally confirmed under the conditions used to form the covalent complex, but fluorescence data measuring the local environment and extrahelical extrusion of a 2-aminopurine opposite a damaged site have revealed the formation of a stably flipped complex (44). Based on assumptions made concerning these data, it is predicted that the starting DPC substrate for incision by UvrABC is a highly distorted, kinked complex containing an extrahelical nucleotide, in which the enzymes' overall molecular footprint is ≈10 nucleotides.
Because the linkage of this 16-kDa enzyme represents a covalent bond, and there was no evidence for degradation of the protein, it is hypothesized that the sequential loading of the UvrABC complex must sufficiently remodel the original complex to correctly position UvrC for incision. UvrA was a necessary component to the DPC incision reactions. Thus, these data imply that despite the severity of the structural DNA distortion in the DPC complex (42, 44), an even more distorted, bubble-like structure forms as a result of the initial damage recognition by UvrA2B. These, as well as sequential local structural alterations of the UvrA2B–DNA complex, may lead to the disruption of contacts between the DNA and T4-pdg. This remodeling is necessary because the site of the 3′ UvrC incision was previously masked with the molecular footprint of T4-pdg (43). We hypothesize that within the preincision complex, the major part of T4-pdg is pointing out of the UvrB–DNA interaction interface. Such a hypothetical structure would allow for the interaction of Tyr-95 and Tyr-96 from the UvrB β-hairpin with the damaged-containing strand that may help to stabilize the UvrB–DPC complex at a hydrophobic interface.
A question can be raised as to whether all DPCs will be processed by the NER complex. Current literature would suggest that this would not be the case as rates of human cell repair of formaldehyde-induced DPCs are not dramatically different when wild-type, XP-A, and XP-F cells are compared (18, 20, 21), whereas removal of trans-Pt(II) diamminedichloride-induced DPCs were found to persist in XP-A cells relative to wild-type cells (17). A partial determinant of the accessibility of a DPC to the NER incision complex may lie in the degree to which the protein lesion distorts the DNA to which it is bound. In the case of T4-pdg, this complex is severely distorted, and this may have served to prepay part of the energetic penalty of forming the incision complex. Thus, the relative ease with which the Michaelis complex can be formed may determine whether efficient catalysis can occur. This model is currently being tested by creating site-specific covalent histone–DNA complexes in which DNA stability should be enhanced rather than diminished.
Recent data suggest that ubiquitin-dependent proteolytic degradation is involved in DPC removal in mammalian cells (21), although it has not been established whether this process is coupled with NER or with some other repair mechanism. The data presented here suggest that at least some subset of DPCs can be removed by NER mechanisms without an obligatory prior proteolytic degradation. A mechanism of repair that includes degradation of proteins covalently bound to DNA is an attractive model, as this would presumably lead to greater accessibility to incision sites for NER nucleases. Alternatively, the extent of DNA distortion may be diminished following proteolytic degradation at the site of a DPC, thereby decreasing the efficiency of lesion removal by interfering with damage recognition.
An additional parameter that may influence the catalytic efficiency and accessibility of the NER complex is the site of addition. Currently, we are investigating strategies in which the covalent linkage can be moved from the abasic sugar moiety to either the minor groove accessible site (N2-guanine) or the major groove (N6-adenine). Detailed analyses of these potential substrates may reveal the fundamental principles of recognition of covalent DPCs.
Although all of the data presented here describe in vitro reactions with purified UvrABC proteins, we have preliminary data from in vivo studies that are in agreement with what was reported here. Plasmid DNAs were crosslinked with T4-pdg and transfected into control and several repair-deficient E. coli strains. After selection on antibiotic-containing media, viability of uvrA− mutants was noticeably diminished in comparison with wild-type cells (unpublished data). However, it was not evident whether DPCs composed of T4-pdg were a substrate for NER in vivo or proteolytic degradation was involved in the process.
Acknowledgments
We thank Dr. Amanda K. McCullough and Andrew J. Kurtz for helpful discussions, and Ritche Jabil for T4-pdg purification. This work was supported by National Institutes of Health, National Institute for Environmental Health Sciences Grants ES04091, ES06676, and ES07955 (to R.S.L.) and National Institutes of Health National Cancer Institute Grant CA86927 (to Y.Z.). R.S.L. holds the Mary Gibbs Jones Distinguished Chair in Environmental Toxicology from the Houston Endowment.
Abbreviations
- DPC
DNA–protein crosslink
- NER
nucleotide excision repair
- T4-pdg
T4 pyrimidine dimer glycosylase/apurinic/apyrimidinic site lyase
- AP
apurinic/apyrimidinic
- XP
xeroderma pigmentosum
- AAF
N-2-acetyl-aminofluorene
References
- 1.Voitkun V, Zhitkovich A. Mutat Res. 1999;424:97–106. doi: 10.1016/s0027-5107(99)00011-1. [DOI] [PubMed] [Google Scholar]
- 2.Izzotti A, Cartiglia C, Taningher M, De Flora S, Balansky R. Mutat Res. 1999;446:215–223. doi: 10.1016/s1383-5718(99)00189-8. [DOI] [PubMed] [Google Scholar]
- 3.Kulcharyk P A, Heinecke J W. Biochemistry. 2001;40:3648–3656. doi: 10.1021/bi001962l. [DOI] [PubMed] [Google Scholar]
- 4.Shetlar M D. Photochem Photobiol Rev. 1980;5:105–197. [Google Scholar]
- 5.Oleinick N L, Chiu S-M, Ramakrishnan N, Xue L-Y. Br J Cancer. 1987;55:135–140. [PMC free article] [PubMed] [Google Scholar]
- 6.Nietert C, Kellicutt L M, Kubinski H. Cancer Res. 1974;34:859–864. [PubMed] [Google Scholar]
- 7.Casanova M, Morgan K T, Steinhagen W H, Everitt J I, Popp J A, Heck H D. Fundam Appl Toxicol. 1991;17:409–428. doi: 10.1016/0272-0590(91)90230-2. [DOI] [PubMed] [Google Scholar]
- 8.Kuykendall J R, Bogdanffy M S. Mutat Res. 1992;283:131–136. doi: 10.1016/0165-7992(92)90145-8. [DOI] [PubMed] [Google Scholar]
- 9.Conaway C C, Whysner J, Verna L K, Williams G M. Pharmacol Ther. 1996;71:29–55. doi: 10.1016/0163-7258(96)00061-7. [DOI] [PubMed] [Google Scholar]
- 10.Ramírez P, Del Razo L M, Gutierrez-Ruíz M C, Gonsebatt M E. Carcinogenesis. 2000;21:701–706. doi: 10.1093/carcin/21.4.701. [DOI] [PubMed] [Google Scholar]
- 11.Toyokuni S, Mori T, Hiai H, Dizdaroglu M. Int J Cancer. 1995;62:309–313. doi: 10.1002/ijc.2910620313. [DOI] [PubMed] [Google Scholar]
- 12.Miller C A, III, Cohen M D, Costa M. Carcinogenesis. 1991;12:269–276. doi: 10.1093/carcin/12.2.269. [DOI] [PubMed] [Google Scholar]
- 13.Chakrabarti S K, Bai C, Subramanian K S. Toxicol Appl Pharmacol. 2001;170:153–165. doi: 10.1006/taap.2000.9097. [DOI] [PubMed] [Google Scholar]
- 14.Pinto A L, Lippard S J. Biochim Biophys Acta. 1985;780:167–180. doi: 10.1016/0304-419x(85)90001-0. [DOI] [PubMed] [Google Scholar]
- 15.Van Houten B, Illenye S, Qu Y, Farrell N. Biochemistry. 1993;32:11794–11801. doi: 10.1021/bi00095a007. [DOI] [PubMed] [Google Scholar]
- 16.Hashimoto M, Greenberg M M, Kow Y W, Hwang J-T, Cunningham R P. J Am Chem Soc. 2001;123:3161–3162. doi: 10.1021/ja003354z. [DOI] [PubMed] [Google Scholar]
- 17.Fornace A J, Jr, Seres D S. Mutat Res. 1982;94:277–284. doi: 10.1016/0027-5107(82)90291-3. [DOI] [PubMed] [Google Scholar]
- 18.Grafstrom R C, Fornace A, Jr, Harris C C. Cancer Res. 1984;44:4323–4327. [PubMed] [Google Scholar]
- 19.Gantt R. Mutat Res. 1987;183:75–87. doi: 10.1016/0167-8817(87)90048-4. [DOI] [PubMed] [Google Scholar]
- 20.Speit G, Schütz P, Merk O. Mutagenesis. 2000;15:85–90. doi: 10.1093/mutage/15.1.85. [DOI] [PubMed] [Google Scholar]
- 21.Quievryn G, Zhitkovich A. Carcinogenesis. 2000;21:1573–1580. [PubMed] [Google Scholar]
- 22.Sancar A. Annu Rev Biochem. 1996;65:43–81. doi: 10.1146/annurev.bi.65.070196.000355. [DOI] [PubMed] [Google Scholar]
- 23.Wood R D. Biochimie. 1999;81:39–44. doi: 10.1016/s0300-9084(99)80036-4. [DOI] [PubMed] [Google Scholar]
- 24.Van Houten B. Microbiol Rev. 1990;54:18–51. doi: 10.1128/mr.54.1.18-51.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prince M A, Friedman B, Gruskin E A, Schrock R D, III, Lloyd R S. J Biol Chem. 1991;266:10686–10693. [PubMed] [Google Scholar]
- 26.Zou Y, Liu T-M, Geacintov N E, Van Houten B. Biochemistry. 1995;34:13582–13593. doi: 10.1021/bi00041a038. [DOI] [PubMed] [Google Scholar]
- 27.Luo C, Krishnasamy R, Basu A K, Zou Y. Nucl Acids Res. 2000;28:3719–3724. doi: 10.1093/nar/28.19.3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schrock R D, III, Lloyd R S. J Biol Chem. 1991;226:17631–17639. [PubMed] [Google Scholar]
- 29.Dodson M L, Schrock R D, III, Lloyd R S. Biochemistry. 1993;32:8284–8290. doi: 10.1021/bi00083a032. [DOI] [PubMed] [Google Scholar]
- 30.McCullough A K, Sanchez A, Dodson M L, Marapaka P, Taylor J-S, Lloyd R S. Biochemistry. 2001;40:561–568. doi: 10.1021/bi002404+. [DOI] [PubMed] [Google Scholar]
- 31.Snowden A, Kow Y W, Van Houten B. Biochemistry. 1990;29:7251–7259. doi: 10.1021/bi00483a013. [DOI] [PubMed] [Google Scholar]
- 32.Verhoeven E E A, van Kesteren M, Moolenaar G F, Visse R, Goosen N. J Biol Chem. 2000;275:5120–5123. doi: 10.1074/jbc.275.7.5120. [DOI] [PubMed] [Google Scholar]
- 33.Hess M T, Schwitter U, Petretta M, Giese B, Naegeli H. Proc Natl Acad Sci USA. 1997;94:6664–6669. doi: 10.1073/pnas.94.13.6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zou Y, Luo C, Geacintov N E. Biochemistry. 2001;40:2923–2931. doi: 10.1021/bi001504c. [DOI] [PubMed] [Google Scholar]
- 35.Zou Y, Van Houten B. EMBO J. 1999;18:4889–4901. doi: 10.1093/emboj/18.17.4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hsu D S, Kim S-T, Sun Q, Sancar A. J Biol Chem. 1995;270:8319–8327. doi: 10.1074/jbc.270.14.8319. [DOI] [PubMed] [Google Scholar]
- 37.Theis K, Chen P J, Skorvaga M, Van Houten B, Kisker C. EMBO J. 1999;18:6899–6907. doi: 10.1093/emboj/18.24.6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Machius M, Henry L, Palnitkar M, Deisenhofer J. Proc Natl Acad Sci USA. 1999;96:11717–11722. doi: 10.1073/pnas.96.21.11717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakagawa N, Sugahara M, Masui R, Kato R, Fukuyama K, Kuramitsu S. J Biochem (Tokyo) 1999;126:986–990. doi: 10.1093/oxfordjournals.jbchem.a022566. [DOI] [PubMed] [Google Scholar]
- 40.Skorvaga M, Theis K, Mandavilli B S, Kisker C, Van Houten B. J Biol Chem. 2002;277:1553–1559. doi: 10.1074/jbc.M108847200. [DOI] [PubMed] [Google Scholar]
- 41.Moolenaar G F, Höglund L, Goosen N. EMBO J. 2001;20:6140–6149. doi: 10.1093/emboj/20.21.6140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vassylyev D G, Kashiwagi T, Mikami Y, Ariyoshi M, Iwai S, Ohtsuka E, Morikawa K. Cell. 1995;83:773–782. doi: 10.1016/0092-8674(95)90190-6. [DOI] [PubMed] [Google Scholar]
- 43.Latham K A, Taylor J-S, Lloyd R S. J Biol Chem. 1995;270:3765–3771. doi: 10.1074/jbc.270.8.3765. [DOI] [PubMed] [Google Scholar]
- 44.McCullough A K, Dodson M L, Schärer O D, Lloyd R S. J Biol Chem. 1997;272:27210–27217. doi: 10.1074/jbc.272.43.27210. [DOI] [PubMed] [Google Scholar]