Abstract
C75 is a potent inhibitor of fatty acid synthase that acts centrally to reduce food intake and body weight in mice; a single dose causes a rapid (>90%) decrease of food intake. These effects are associated with inhibition of fasting-induced up-regulation and down-regulation, respectively, of the expression of orexigenic (NPY and AgRP) and anorexigenic (POMC and CART) neuropeptide messages in the hypothalamus. Repeated administration of C75 at a submaximal level, however, differentially affected food intake of lean and obese mice. With lean mice, C75 suppressed food intake by ≈50% and, with obese mice (ob/ob and dietary-induced obesity), by 85–95% during the first day of treatment. Lean mice, however, became tolerant/resistant to C75 over the next 2–5 days of treatment, with food intake returning to near normal and rebound hyperphagia occurring on cessation of treatment. In contrast, ob/ob obese mice responded to C75 with a >90% suppression of food intake throughout the same period with incipient tolerance becoming evident only after substantial weight loss had occurred. Dietary-induced obese mice exhibited intermediate behavior. In all cases, a substantial loss of body weight resulted. Pair-fed controls lost 24–50% less body weight than C75-treated mice, indicating that, in addition to suppressing food intake, C75 may increase energy expenditure. The decrease in body weight by ob/ob mice was due primarily to loss of body fat. In contrast to the short-term effects of C75 on “fasting-induced” changes of hypothalamic orexigenic and anorexigenic neuropeptide mRNAs, repeated administration of C75 either had the inverse or no effect as tolerance developed.
Keywords: AgRP‖CART‖DIO‖NPY‖POMC
The hypothalamus plays an important role in the energy balance of higher animals. Neuronal centers within the hypothalamus monitor and integrate peripheral signals that reflect “energy status” and respond by releasing orexigenic and anorexigenic neuropeptides (1). Notable among these centers is the arcuate nucleus, which possesses NPY and AgRP (orexigenic) and POMC and CART (anorexigenic) expressing neurons that possess receptors for peptide hormones including insulin, leptin, and ciliary neurotrophic factor that are known to affect feeding behavior (1, 2). These neurons project to other centers in the hypothalamus that regulate energy intake and expenditure (1).
Increasing evidence (3) suggests that there is a link between anabolic energy metabolism and appetite control that is mediated by an intermediate in the pathway of fatty acid biosynthesis. It is well documented (4) that fatty acid synthesis in lipogenic tissues, e.g., liver and adipose tissue, occurs only during energy surplus and that excess physiological fuels are channeled into energy storage pathways, primarily lipogenesis and glycogenesis. Recent evidence (3, 5) suggests that a key regulatory intermediate in the fatty acid biosynthetic pathway, namely malonyl-CoA, may serve as a physiological link/mediator between fatty acid synthesis in the hypothalamus and the control of food intake. Cerulenin and C75 are potent inhibitors of fatty acid synthase and have been shown to cause accumulation of malonyl-CoA (substrate of the enzyme) in the liver, a tissue more accessible than the hypothalamus (3). It is now firmly established that in the liver and muscle, malonyl-CoA regulates fatty acid oxidation by controlling the entry of fatty acids into the mitochondrial matrix, the site of β-oxidation (6, 7). Thus, there is a precedent for the role of malonyl-CoA as a mediator of energy status (8, 9).
Increasing circumstantial evidence (3, 10, 11) supports the hypothesis that an increased malonyl-CoA level in the hypothalamus, caused by inhibition of fatty acid synthase, is responsible for blocking fasting-induced up-regulation of hypothalamic NPY. In addition to effects on the expression of NPY, we recently found (10) that the suppressive effect of C75 on food intake of lean mice seems to be mediated by reciprocal changes in the expression of NPY/AgRP and POMC/CART. In contrast, in obese (ob/ob) mice, the effect seems to be mediated only by changes in the expression of NPY and AgRP. Consistent with these differences, we found that a single injection of C75 to lean mice initially reduces food intake and body weight, but repeated administration over several days provokes tolerance. In contrast, ob/ob mice are more responsive to C75 and exhibit a continual reduction in food intake and loss of body weight throughout the study period, with incipient tolerance becoming evident only after a substantial decrease in adiposity.
Experimental Procedures
Feeding and Handling of Mice.
Six-week-old C57BL/6J and C57BL/6J-Lepob (ob/ob) mice from The Jackson Laboratory were individually housed in a light (12-h dark, 1800–0600 h/12-h light cycle, 0600–1800 h) and temperature (22°C) controlled chamber. Mice were fed a lab diet (Prolab RMH 1000) from PMI Feeds (St. Louis). To prepare diet-induced obese (DIO) mice, C57BL6/J mice were fed either a high-fat diet (D12492) or a low-fat diet (D12450B) (Research Diets, New Brunswick, NJ) for 7 weeks. The high-fat diet contained 60% of total calories from fat, predominantly in the form of lard, whereas the low-fat diet contained 10% of total calories from fat. Mice were weighed twice weekly. After 7 weeks, mice on the low-fat diet had an increase in body weight of 34 ± 4%, whereas those on the high-fat diet had an increase in body weight of >70% and were defined as DIO.
After a 7-day acclimation period, mice were randomized to one of three treatment groups: group 1, a control group injected i.p. daily with the vehicle for 5 days; group 2, a C75-treated group that was injected i.p. daily with 10 mg/kg body wt of C75 for 5 days; and group 3, a pair-fed group that was fed the same quantity of diet as consumed by animals receiving C75. Food intake and body weight were monitored several times daily (see below). Mice were injected with C75 or vehicle 3 h before lights off (1500 h). Food consumption was measured at various intervals after injection (interval 1, 1500–1800 h; interval 2, 1800–2100 h; interval 3, 2100–0900 h; interval 4, 0900–1500 h). On day 5 (end of the study period), mice received the last injection (of C75 or vehicle) 3 h before lights off and were killed at the beginning of the dark cycle (at 1800 h). Hypothalami (≈20 mg each) defined by the posterior margin of the optic chiasm and the anterior margin of the mammillary bodies to a depth of ≈2 mm were dissected, quickly frozen in liquid nitrogen, and stored at −80°C.
The fatty acid synthase inhibitor, C75 (molecular weight, 254.32), was custom synthesized and dissolved in RPMI media 1640 (GIBCO/BRL).
Preparation of Probes for RNase Protection Assay.
Partial cDNAs encoding fragments of CART, NPY, and POMC were obtained by reverse transcriptase (RT)-PCR (using OneStep RT-PCR, Qiagen, Chatsworth, CA) from the first-strand cDNA by using mouse hypothalamic total RNA as described (10). Each PCR product was cloned into pCRII-TOPO vector by using a TOPO TA cloning kit dual promoter (Invitrogen) and sequenced. A plasmid DNA with the correct sequence was prepared by using the Qiagen plasmid maxi kit, purified, linearized with HindIII or XbaI, extracted with phenol/chloroform, and stored at −80°C. pT7 blue plasmid (Novagen) containing a partial sequence of mouse AgRP cDNA (396 bp, 1–396 of U89494, gift from Tina M. Hahn, Univ. of California, Davis, CA) was linearized with EcoRI, amplified with T7 RNA polymerase to make antisense RNA, and used for RNase protection assay.
RNA Isolation.
Frozen hypothalamic tissue (paired hypothalami from each group) was pulverized with a BioPulverizer II (Research Products International) chilled in liquid nitrogen, and total RNA was extracted from the tissue by using Isogen (Nippon Gene, Toyama, Japan) according to the manufacturer's procedure. RNA concentration was determined from A260 nm and a fluorometric assay by using a RiboGreen RNA quantitation kit (Molecular Probes), adjusted to a constant concentration (ca. 100 ng/μl) with RNase-free 1 × TE buffer (10 mM TE/1 mM EDTA, pH 8.0), and stored at −80°C before analysis.
RNase Protection Assay.
Antisense RNA was transcribed with [α-32P]UTP by using MAXIscript SP6/T7 kit (Ambion, Austin, TX). Briefly, a 20-μl in vitro transcription reaction contains 1 μg of linearized DNA template, 1 × transcription buffer, 0.5 mM each of ATP, CTP, and GTP, 6 μl of [α-32P]UTP (800 Ci/mmol, 20 mCi/ml) (1 μl of UTP for cyclophilin probe synthesis) and 0.5 μM unlabeled (“cold”) UTP for NPY and CART (40 μM cold UTP for cyclophilin, no addition for AgRP and POMC), and 1 μl of SP6 or T7 RNA polymerase (15 units/μl). At the end of reaction for 1 h, 1 μl of Dnase I (RNase-free, 2 units/μl) was added, incubated for 30 min, and extracted with phenol/chloroform. Each of the ethanol-precipitated probes was gel-purified according to the manufacturer's manual (Ambion), and radioactivity was measured. RNase protection assays were performed by using a HybSpeed RPA kit (Ambion). Aliquots of hypothalamic total RNA (5 μg) were mixed with 32P-labeled probe mixtures (7 × 104 cpm/each probe) for mouse AgRP, CART, NPY, POMC, and cyclophilin and ethanol precipitated with yeast RNA (50 μg) and GlycoBlue (50 μg/ml final) at −80°C for 20 min. Pellets were dissolved in a 10-μl HybSpeed buffer and denatured at 95°C for 5 min. A sample was then hybridized at 68°C for 10 min. After digestion with RNase A/T1, protected fragments corresponding to mouse AgRP (396 bp), CART (159 bp), NPY (205 bp), POMC (304 bp), and cyclophilin (103 bp) were separated on an 8 M urea/5% polyacrylamide gel. Gels were dried and exposed on Imaging Plate (BAS-IP MP 2040) (Fuji) for 24 h. Relative mRNA levels of neuropeptides were quantified with a Fuji BioImaging analyzer 1500, and the data were normalized with an intensity of signal of cyclophilin mRNA.
Body Composition Analysis.
Animals were killed and carcasses were stored at −80°C before analysis. Following the removal of the gastrointestinal tract, the remaining carcass was dried to constant mass at 60°C. Carcass water weight was calculated as the difference between wet and dry weights. Fat mass was determined in the dried carcass by lipid extraction in a Soxhlet apparatus by using petroleum ether (12). Fat-free dry mass was taken to be the mass remaining following the extraction. Fat-free mass was calculated as the eviscerated carcass mass minus fat mass.
Statistical Analysis.
All data are presented as means ± SE of multiple determinations. Data were analyzed by one-way or two-way ANOVA, with or without repeated measures, as applicable.
Results
Previous studies (3, 10) revealed that administration of a single, high dose (30 mg/kg body weight) of C75 to lean or ob/ob mice rapidly (within 1 h) blocked food intake and caused a dramatic decrease in body weight over the next 24 h. At lower levels of C75, the response was dose-dependent and rapidly reversed on withdrawal of the agent (3). It was of interest to ascertain whether the effects of C75 could be sustained with repeated administration and whether the weight loss of C75-treated mice is due entirely to reduced food/caloric intake.
Effect of Repeated Administration of C75 to Lean Mice.
Because lean mice will not survive the nearly complete restriction of food intake for 4–5 days caused by a high dose of C75, preliminary experiments were conducted to determine the level of C75 needed to cause an intermediate decrease in food intake. A dose of 10 mg of C75/kg body weight per day was found to suppress food intake of lean mice (C57BL/6J) by 50–60% (results not shown); therefore, this level of C75 was used in subsequent experiments.
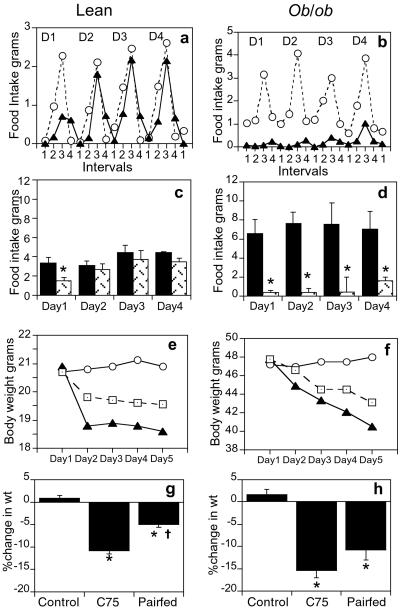
Fig. 1 a and c shows the changes in food intake of lean mice on repeated administration of C75 (10 mg/kg body weight daily) for the 5-day study period. There was an immediate reduction of food intake on treatment with C75, and by the end of day 1, the 24-h cumulative food intake of C75-treated mice was 56% lower than that of controls (P = 0.003). However, by day 2, C75-treated mice became “tolerant,” with their food intake returning to almost that of controls, where it remained for the remainder of the study despite continued treatment with C75. By the end of day 1, the C75-treated mice had lost 9.9 ± 1.7% of their initial body weight; however, there was no additional loss of weight over the next 4 days (Fig. 1e). Mice pair-fed the same quantity of food consumed by C75-treated mice lost only 4.1 ± 1.3% of their initial body weight by the end of day 1. The % change from initial body weight at the end of the study period was 10.7 ± 0.4% for C75-treated mice and 4.9 ± 0.4% for pair-fed mice (Fig. 1g, P < 0.001 by one-way ANOVA). Hence, weight loss of pair-fed mice was ≈50% less (P = 0.003) than that of C75-treated mice despite consumption of an equal quantity of food during the study (Fig. 1e).
Figure 1.
Effect of C75 on food intake and body weight in lean (C57BL/6J) and ob/ob mice. Mice were acclimated to 12-h dark (1800–0600 h)/12-h light (0600–1800 h) cycle. Groups of lean mice (n = 4) or ob/ob mice (n = 3) received a daily i.p. injection of vehicle (control) or C75 3 h before lights off (1500). (a and b) Food consumption was measured in lean and ob/ob mice treated with vehicle (○) or C75 (▴) during various intervals after injection (interval 1, 1500–1800 h; interval 2, 1800–2100 h; interval 3, 2100–0900 h; interval 4, 0900–1500 h) over 4 days (D1, D2, D3, and D4. (c and d) Twenty four-hour cumulative food intake in vehicle (black bars) and C75-treated (hatched bars) lean and ob/ob mice. (e and f) Body weight was measured daily in lean and ob/ob mice treated with vehicle (○), C75 (▴), or mice pair-fed (□) the same quantity of food consumed by C75-treated mice. (g and h) Percent change from initial body weight in vehicle-treated, C75-treated, and pair-fed lean and ob/ob mice. All data are mean ± SEM. *P < 0.05 versus corresponding vehicle control; 61 P < 0.05 vs. corresponding pair-fed controls.
Effect of Repeated Administration of C75 to ob/ob Mice.
Leptin is extremely effective in reducing food intake and body weight of ob/ob mice, although it is relatively ineffective in lean mice. To determine whether ob/ob mice are as responsive to C75 as lean mice, C75 (10 mg/kg body weight) was administered daily to ob/ob mice for 5 days. Similar to lean mice, food intake of C75-treated ob/ob mice was rapidly suppressed, with the 24-h cumulative food intake being suppressed by 95% compared with that of vehicle-injected controls (Fig. 1d, P < 0.005). Unlike lean mice, food intake of C75-treated ob/ob mice remained significantly suppressed (>90%) over the entire study period. However, there was an indication of incipient tolerance on day 4 when food intake of C75-treated mice increased to 25% that of vehicle-treated controls (compared with 5% that of controls during days 1–3; Fig. 1 b and d). Body weight of ob/ob mice decreased by 6.2 ± 0.3% during the first day of treatment with C75 and then continued to fall over the entire period (Fig. 1f). In contrast, the body weight of pair-fed ob/ob mice fell by only 2.5 ± 0.3% by the end of day 1. Like their C75-treated counterparts, pair-fed ob/ob mice continued to lose weight during the entire study period (Fig. 1f). The overall weight loss of C75-treated ob/ob mice was 15.4 ± 1.2% of their initial body weight, whereas that of pair-fed ob/ob mice was 10.7 ± 1.6% (Fig. 1h, P = 0.002 by one-way ANOVA). Thus, as with lean mice, weight loss of pair-fed ob/ob mice was significantly less than that of C75-treated ob/ob mice (Fig. 1h, P = 0.045).
The body composition of ob/ob mice was analyzed to determine whether weight loss caused by repetitive treatment with C75 was due primarily to the loss of fat. As shown in Table 1, there were marked differences in body fat mass both in C75-treated (−4.5 g) and pair-fed (−3.8 g) ob/ob mice relative to vehicle-treated controls during the course of the 5-day treatment period. These differences in body fat content accounted for most of the body weight differences between in C75-treated and pair-fed mice vs. control mice. C75 treatment had much smaller effects on fat-free mass and body water content.
Table 1.
Effect of C75 on body composition of ob/ob mice
| Treatment | Body weight
|
FFM† g | Water g | Fat
|
||
|---|---|---|---|---|---|---|
| Live g | Eviscerated g | Total g | Δ | |||
| Control | 48.0 (1.5) | 45.1 (0.9) | 17.8 (0.5) | 13.0 (0.4) | 26.5 (0.8) | — |
| C75 | 40.5 (1.7) | 38.6 (1.2) | 15.8 (0.4) | 11.1 (0.5) | 22.0 (1.3) | −4.5 |
| Pair-fed* | 42.6 (2.0) | 40.9 (1.5) | 17.1 (0.7) | 11.3 (0.1) | 23.7 (1.6) | −3.8 |
Food intake limited to that of C75-treated mice.
FFM indicates fat-free mass as outlined in Experimental Procedures. Data are presented as mean (±SEM).
Effect of Repeated Administration of C75 on DIO Mice.
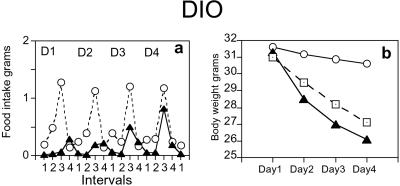
To assess the effect of C75 on a leptin-resistant obesity model (for comparison to the leptin-deficient ob/ob model), the same protocol as for ob/ob mice was used. Obesity was induced in C57BL6/J mice by feeding a high-fat diet in which 60% of total calories were derived from fat; control mice were fed a diet in which 10% of total calories were derived from fat. Over a 7-week period, mice fed the high-fat diet increased in body weight by 76 ± 7%, whereas controls (on low-fat diet) increased in body weight by only 34 ± 4%. Administration of C75 to DIO mice caused an 83% suppression of food intake during the first day (Fig. 2a). Although food intake remained suppressed relative to vehicle-treated DIO mice, there was a gradual development of tolerance to C75 over the study period. Whereas food intake on day 1 was suppressed by 83%, suppression was 77% on day 2, 59% on day 3, and 44% on day 4. Thus, in contrast to ob/ob mice (which developed only incipient tolerance on day 4), DIO mice became “partially” resistant, although the resistance did not set in immediately, nor was it as severe as in lean mice (Compare Figs. 1 a and b and 2a). Nevertheless, DIO mice continued to lose weight throughout the study period. The initial weight loss (after 1 day) of C75-treated DIO mice of 8.9 ± 1.0% was significantly greater than the weight loss, i.e., 4.7 ± 0.5% of pair-fed DIO controls (P < 0.0001, Fig. 2b). By the end of study period, C75-treated mice had lost 16.8 ± 1.2% of their initial body weight, whereas pair-fed mice lost 13.8 ± 1.2% (P < 0.001 vs. vehicle-treated controls); however, the difference in weight loss between C75-treated and pair-fed mice was not statistically significant (P = 0.081).
Figure 2.
Effect of C75 on food intake and body weight in DIO mice. Mice were acclimated to a 12-h dark (1800–0600 h)/12-h light (0600–1800 h) cycle. Groups of DIO mice (n = 4) received a daily i.p. injection of vehicle (control) or C75 3 h before lights off (1500 h). (a) Food consumption was measured in DIO mice during various intervals after injection (interval 1, 1500–1800 h; interval 2, 1800–2100 h; interval 3, 2100–0900 h; interval 4, 0900–1500 h). (b) Body weight was measured daily in DIO mice treated with vehicle (control), C75, or mice pair-fed the same quantity of food consumed by C75-treated mice. Other labels are the same as in Fig. 1 a and e.
Changes in the Expression of Hypothalamic Neuropeptide mRNAs in Lean and ob/ob Mice on Repeated Administration of C75.
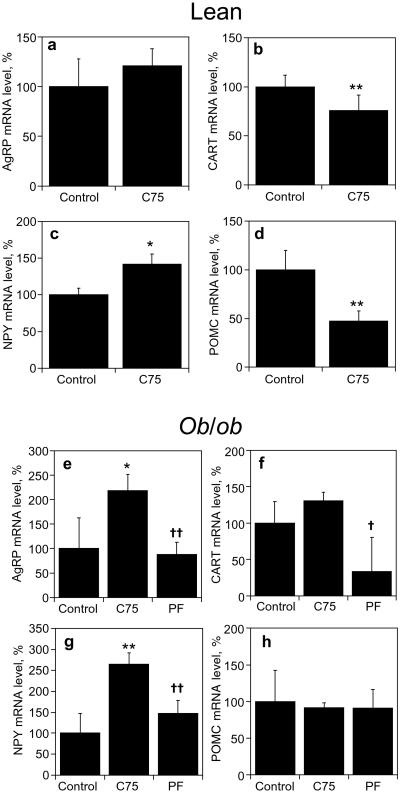
Previous studies (10) showed that administration of C75 prevented the fasting-induced up-regulation of key orexigenic neuropeptide (NPY and AgRP) mRNAs and down-regulation of anorexigenic neuropeptides (POMC and CART) in the hypothalami of lean mice. These changes occurred within 24 h after C75 treatment, i.e., before the development of tolerance observed in the present experiments (see Fig. 1a). To determine whether reciprocal changes in the expression of these neuropeptides might explain the full-blown acquisition of tolerance in lean mice or the incipient appearance of tolerance in ob/ob obese mice, AgRP, CART, NPY, and POMC mRNA levels were quantitated in hypothalami from control and C75-treated lean and ob/ob mice at the end of the treatment period (Fig. 3).
Figure 3.
Effect of C75 on hypothalamic neuropeptide mRNA levels in lean and ob/ob mice. Groups of mice (n = 4 for lean and n = 3 for ob/ob) received a daily injection of vehicle (control) or C75 (10 mg/kg bw) 3 h before lights off (at 1500) for 5 days. Mice were killed 3 h after the last injection (at 1800 on day 5) and hypothalami were harvested. Total RNA (5 μg) was probed with a probe mixture containing [32P]UTP-labeled cRNAs for a and e (AgRP), b and f (CART), c and g (NPY), and d and h (POMC) and subjected to RNase protection protocol as described in Experimental Procedures. mRNA levels are expressed as % relative to mRNA level in corresponding control mice. All data are means ± SE. *, P < 0.05; **, P < 0.01 vs. control group; †, P < 0.01 vs. C75-treated group.
With C75-treated lean mice, tolerance/resistance was associated with suppressed expression of POMC mRNA (−Δ52.6 ± 10.0% relative to controls, P = 0.03; Fig. 3d) and CART mRNA levels (−Δ25%, Fig. 3b) and increased expression of NPY mRNA (+Δ41.4 ± 14.1% relative to controls, P = 0.02; Fig. 3c) and of AgRP mRNA (+Δ21%, Fig. 3a). These results suggest that acquisition of tolerance to C75 by lean mice is prompted by compensatory changes in the expression of these key hypothalamic neuropeptides.
Although C75 effectively suppresses food intake by ob/ob mice when administered for extended periods of time, i.e., for 4 (Fig. 1b) to 8 (ref. 3) days, the extent of suppression is diminished somewhat by day 4 (−Δ95% on day 1 to −Δ75% on day 5; Fig. 1d), suggesting the onset of tolerance, albeit minimal. Changes in the expression of hypothalamic neuropeptides were evident, however, by the end of the study period apparently presaging fully developed tolerance. Expression of both orexigenic neuropeptide messages increased significantly, i.e., AgRP (+Δ2.2 ± 0.3-fold, P < 0.05 vs. controls; Fig. 3e) and NPY (+Δ2.6 ± 0.2-fold, P < 0.01 vs. controls; Fig. 3g). In pair-fed ob/ob mice, no significant changes in the AgRP or NPY message levels were observed, indicating that the changes observed with C75 treatment were not merely because of changes in food intake. CART and POMC mRNA levels were unaltered in C75-treated and ob/ob mice (Fig. 3 f and h).
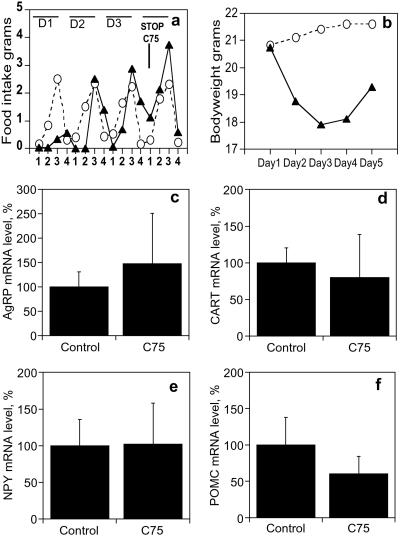
Rebound Hyperphagia in Lean Mice After Cessation of C75 Treatment.
Because lean mice develop tolerance to C75 after several days of treatment, it was of interest to determine the effect of withdrawal of treatment on food intake. Thus, lean mice were treated daily with C75 (10 mg/kg body weight) for 3 days, at which point administration of C75 was stopped. A substantial decrease in food intake occurred during the first day of treatment, but intake returned to the level of the controls over the next 2–3 days of treatment (Fig. 4a). On cessation of C75 treatment, rebound hyperphagia occurred, food intake increased dramatically (Fig. 4a) to a level of 7.5 ± 0.5 g/day (compared with 4.6 ± 0.3 g/day for controls, P = 0.008), and body weight began to rise (Fig. 4b). Thus, an increase in weight gain accompanied rebound hyperphagia. Whereas changes in the levels of hypothalamic neuropeptide mRNAs 26 h after cessation of C75 treatment were not statistically significant, there seemed to be decreases in the anorexigenic neuropeptide (CART and POMC) levels and an increase in the level of the orexigenic neuropeptide AgRP (Fig. 4 c–f).
Figure 4.
Rebound hyperphagia and hypothalamic neuropeptide levels in lean mice after cessation of C75. Groups of lean mice (n = 4) received daily i.p. injections of vehicle (control) or C75 3 h before lights off (1500 h) for 3 days, after which all treatment was stopped. (a) Food consumption was measured in lean mice treated with vehicle (○) or C75 (▴) during various intervals after injection (interval 1, 1500–1800 h; interval 2, 1800–2100 h; interval 3, 2100–0900 h; interval 4, 0900–1500 h) over 4 days (D1, D2, D3, D4). (b) Body weight was measured daily in lean mice treated with vehicle (○) or C75 (▴). Mice were killed 26 h after cessation of C75 (at 1800 on day 5), and hypothalami were harvested. Total RNA (5 μg) was probed with a probe mixture containing [32P]UTP-labeled cRNAs for c (AgRP), d (CART), e (NPY), and f (POMC) and subjected to RNase protection protocol as described in Experimental Procedures. mRNA levels are expressed as % relative to mRNA level in control mice. All data are means ± SE.
Discussion
Previous studies (refs. 3 and 10 and results not shown) showed that a single, relatively high dose of C75 administered i.p. to lean or ob/ob obese mice rapidly (<1 h) blocked food intake over the next 24 h. Associated with these effects were changes in the levels of hypothalamic neuropeptides known to affect feeding behavior (10). These and other results (not shown) suggested that the anorectic effect of C75 on lean and obese (ob/ob) mice is because of prevention of the fasting-induced up-regulation of the orexigenic hypothalamic neuropeptides, NPY and AgRP, in lean and ob/ob obese mice along with down-regulation of the anorexigenic neuropeptides, POMC and CART, in lean mice (10). It was also shown (3) that at lower levels of C75 the response was dose-dependent and readily reversible on withdrawal of C75 (3). In the present study, we found, quite unexpectedly, that repeated administration of C75 to lean mice for 2–3 days provoked resistance to the inhibitor (Fig. 1a). In contrast, similarly treated obese mice (ob/ob or DIO) developed little or far less resistance to C75. After 3 days of treatment, ob/ob mice exhibited virtually no resistance to C75 and, by day 5, only incipient tolerance. DIO mice exhibited intermediate tolerance to repeated administration of C75 (Fig. 2) most likely because their obesity was not as severe as ob/ob mice; thus, the associated side-effects were less severe, e.g., elevated blood insulin levels, hyperglycemia, and steatosis. We speculate that as obese animals lose adipose tissue because of C75 treatment and become leaner, tolerance and/or resistance to C75 increases. At present, the basis for these differences in responsiveness to tolerance of C75 is not known. However, several possibilities come to mind including differences in insulin resistance and the consequences of hyperglycemia and fatty liver.
Repeated administration of C75 to lean and obese mice led to compensatory changes in the expression of mRNAs encoding key orexigenic (i.e., AgRP and NPY) and anorexigenic (POMC and CART) hypothalamic neuropeptides. These effects were distinctly different from the effects caused by a single dose of C75. Lean mice became almost completely resistant to C75 after 1–2 days of C75 treatment, whereas ob/ob mice developed only slight resistance by day 5 of C75 treatment. Nevertheless, both lean and ob/ob mice expressed increased hypothalamic levels of AgRP and NPY messages (Fig. 3). In contrast to this response, in a previous study we found that a single dose of C75 prevented the normal fasting-induced increase in expression of the NPY and AgRP messages. Adjustment, i.e., down-regulation, of the expression of the anorexigenic neuropeptides to repetitive C75 treatment seems to be limited to lean mice (Fig. 3 b and d). The lack of response of ob/ob (leptin-deficient) mice (Fig. 3 f and h) is consistent with their inability to alter POMC expression when fasted (13) or when treated with a single dose of C75 (10). Thus, it seems that lean and ob/ob mice compensate for the long-term administration of C75 by up-regulating hypothalamic orexigenic neuropeptide expression and, in the case of lean mice, by also down-regulating the expression of anorexigenic neuropeptides.
C75 led to a marked reduction of body weight in lean, ob/ob, and DIO mice. Whereas ob/ob and DIO mice continued to lose weight throughout the 5-day treatment period (Figs. 1f and 2b), lean mice exhibited no further loss of body weight after day 1 (after day 1, food intake had normalized). However, despite normalization of food intake, the lean mice maintained their lower body weight after day 1. Pair-fed lean, ob/ob, and DIO mice all lost less body weight than the corresponding C75-treated mice over the study period. These observations suggest that the mechanism of action of C75 is more complex and may involve mechanisms in addition to suppression of food intake, possibly through enhanced energy expenditure. Experiments are underway to test this possibility.
Acknowledgments
This research was supported by a research grant from the National Institutes of Health and the Yamanouchi USA Foundation (2000) and by National Institute of Diabetes and Digestive and Kidney Diseases Research Grant DK56336 (to T.R.N.).
Abbreviations
- AgRP
agouti-related protein
- CART
cocaine–amphetamine-regulated transcript
- NPY
neuropeptide Y
- POMC
pro-opiomelanocortin
- DIO
dietary-induced obese
References
- 1.Schwartz M W, Woods S C, Porte D, Jr, Seeley R J, Baskin D G. Nature (London) 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 2.Beck B. Nutrition. 2000;16:916–923. doi: 10.1016/s0899-9007(00)00410-x. [DOI] [PubMed] [Google Scholar]
- 3.Loftus T M, Jaworsky D E, Frehywot G L, Townsend C A, Ronnett G V, Lane M D, Kuhajda F P. Science. 2000;288:2379–2381. doi: 10.1126/science.288.5475.2379. [DOI] [PubMed] [Google Scholar]
- 4.Wakil S. Biochemistry. 1989;28:4523–4530. doi: 10.1021/bi00437a001. [DOI] [PubMed] [Google Scholar]
- 5.Bouchard C. N Engl J Med. 2000;343:1888–1889. doi: 10.1056/NEJM200012213432511. [DOI] [PubMed] [Google Scholar]
- 6.McGarry J, Foster D. J Biol Chem. 1979;254:8163–8168. [PubMed] [Google Scholar]
- 7.Chien C, Dean D, Saha A, Flatt J P, Ruderman N. Am J Physiol Endocrinol Metab. 2000;279:259–265. doi: 10.1152/ajpendo.2000.279.2.E259. [DOI] [PubMed] [Google Scholar]
- 8.Ruderman N, Saha A, Vavvas D, Witters L. Am J Physiol. 1999;276:E1–E18. doi: 10.1152/ajpendo.1999.276.1.E1. [DOI] [PubMed] [Google Scholar]
- 9.McGarry J D, Foster D W. J Biol Chem. 1979;254:8163–8168. [PubMed] [Google Scholar]
- 10.Shimokawa T, Kumar M, Lane M D. Proc Natl Acad Sci USA. 2002;99:66–71. doi: 10.1073/pnas.012606199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Makimura H, Mizuno T M, Yand X J, Silverstein J, Beasley Mobbs V N. Diabetes. 2001;50:733–739. doi: 10.2337/diabetes.50.4.733. [DOI] [PubMed] [Google Scholar]
- 12.Dobush G R, Ankey C D, Krementz D G. Can J Zool. 1985;63:1917–1920. [Google Scholar]
- 13.Campfield L A, Smith J H, Guisez Y, Devos R, Burn P. Science. 1995;269:546–549. doi: 10.1126/science.7624778. [DOI] [PubMed] [Google Scholar]