Abstract
Antae-eum (ATE), a traditional herbal formula consisting of ten medicinal herbs, has been primarily used for fetal health and the alleviation of abdominal pain during pregnancy. However, comprehensive experimental studies on its pharmacological effects and mechanisms of action are lacking. Local inflammatory responses in the uterus play a crucial role in embryo implantation and early placental development, while excessive inflammation can lead to implantation failure, recurrent miscarriage, and preeclampsia. In the present study, we hypothesized that ATE may help promote pregnancy stability by modulating the inflammatory responses during pregnancy, and investigated the effect of ATE on the inflammatory response. First, the marker compounds in ATE were identified using a high-performance liquid chromatography system. Then, we assessed inflammatory cytokine production, expression changes related to inflammatory signaling, as well as the expression and flux of autophagic markers in RAW 264.7 macrophages stimulated by lipopolysaccharide (LPS). In ATE, the nineteen marker compounds including baicalin, hesperidin, paeoniflorin, and wogonoside were detected, and the most abundant was baicalin. The anti-inflammatory effect of ATE is achieved by reducing pro-inflammatory cytokine production and further downregulating the TLR4-mediated MAPK and NF-κB signalings. ATE suppressed autophagic flux by disrupting the accumulation of FOXO3a. In summary, our results indicate that ATE exerts anti-inflammatory effects by the blockade of autophagic flux. These findings suggest that ATE has potential as a therapeutic agent for various inflammatory diseases, including inflammatory-related reproductive disorders.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-04960-y.
Keywords: Antae-eum, Inflammatory response, Autophagy flux, Lipopolysaccharide, RAW 264.7 macrophage
Subject terms: Drug discovery, Immunology, Molecular biology
Introduction
Globally, the prevalence of chronic diseases and associated medical expenditure are gradually increasing, driven by an aging population. This trend has contributed to a growing interest in traditional medicine as an alternative treatment method1. In Korea, Antae-eum (ATE; An Tai Yin in Chinese) has been traditionally used to alleviate various pregnancy-related symptoms, including abdominal pain and bleeding during pregnancy, miscarriage, and premature labor2. ATE consists of ten herbs: Atractylodis Rhizoma Alba, Scutellariae Radix, Amomi Fructus, Angelicae Gigantis Radix, Citri Unshius Pericarpium, Paeoniae Radix, Rehmanniae Radix Preparata, Cnidii Rhizoma, Periilae Folium, and Glycyrrhizae Radix et Rhizoma (Table 1). During pregnancy, the uterine environment undergoes dynamic immunological changes, including an initial pro-inflammatory stage that facilitates embryo implantation, a subsequent anti-inflammation stage that supports fetal development, and a second pro-inflammatory stage that initiates parturition3. Regulation of inflammation within the uterine decidua plays an important role in normal implantation and pregnancy maintenance. In particular, macrophages are critical for modulating endometrial receptivity, embryo implantation, and maintaining immune homeostasis. An imbalance of M1 and M2 macrophages can cause an excessive inflammatory response, potentially leading to fetal resorption and miscarriage3. Several studies have reported that ATE does not cause adverse effects such as mutagenesis or hepatotoxicity4. In particular, when ATE was administered to mice from days 1 to 20 after mating, as part of a reproductive toxicity test, it had no adverse effects on mothers or fetuses5. Administration of ATE during early pregnancy (gestation phase 1) increased offspring birth weight in a mouse model6and ATE treatment assisted embryo implantation by promoting decidualization of uterine endometrial stromal cells7. Furthermore, a systematic review of randomized controlled trials (RCTs) evaluating the efficacy and safety of various herbal medicines, including ATE, suggested that it may be beneficial for the treatment and prevention of recurrent miscarriage8. Accumulating evidences suggest that ATE may have therapeutic potential in the treatment of reproductive disorders. However, the regulatory effects and underlying molecular mechanisms of ATE on macrophage inflammatory responses related to pregnancy stability remain elusive.
Table 1.
Herbal composition of Antae-um (ATE).
| Herbal name | Scientific name | Family | Using part | Origin | Amount (g) | Ratio (%) |
|---|---|---|---|---|---|---|
| Atractylodis Rhizoma Alba | Atractylodes japonica Koidz. | Compositae | Rhizome | Uljin, Korea | 952.5 | 19.1 |
| Scutellariae Radix | Scutellaria baicalensis Georgi | Lamiaceae | Root | Yeosu, Korea | 713.7 | 14.3 |
| Amomi Fructus | Amomum villosum Lour. | Zingiberaceae | Fruit | Myanmar | 476.3 | 9.5 |
| Angelicae Gigantis Radix | Angelica gigas Nakai | Apiaecae | Root | Pyeongchang, Korea | 476.3 | 9.5 |
| Citri Unshius Pericarpium | Citrus unshiu Marcow. | Rutaeceae | Peel | Jeju, Korea | 476.3 | 9.5 |
| Paeoniae Radix | Paeonia lactiflora Pall. | Paeoniaceae | Root | Uiseong, Korea | 476.3 | 9.5 |
| Rehmanniae Radix Preparata | Rehmannia glutinosa (Gaertn.) DC. | Plantaginaceae | Root | Jeongeup, Korea | 476.3 | 9.5 |
| Cnidium Rhizome | Cnidium officinale Makino | Umbelliferae | Rhizome | Yeongyang, Korea | 381.0 | 7.6 |
| Perillae Folium | Perilla frutescens Britton var. acuta Kudo | Labiatae | Leaf | Gurye, Korea | 381.0 | 7.6 |
| Glycyrrhizae Radix et Rhizoma | Glycyrrhiza uralensis Fisch. | Leguminosae | Root and rhizome | China | 190.3 | 3.9 |
| Total | 5000.0 | 100.0 |
Inflammation is the process through which immune cells protect the body against pathogens (e.g., viruses and bacterial toxins)9,10. Chronic inflammatory diseases are a major cause of death globally, with more than 50% of mortality being attributable to inflammation-related disorders11. Among immune cells, macrophages are central regulators of the inflammatory response, secreting a variety of pro-inflammatory cytokines12. Toll-like receptor 4 (TLR4) is a major recognizer for initiating the innate immune response and is activated by stimuli such as lipopolysaccharide (LPS). The interaction between TLR4 and LPS induces signaling pathways through myeloid differentiation factor 88 (MyD88), triggering the activation of mitogen-activated protein kinases (MAPKs), such as p38, extracellular signal-regulated kinase (ERK), and c-Jun N-terminal kinase (JNK), which leads to downstream events, including the nuclear translocation of nuclear factor kappa-light-chain-enhancer of activated B (NF-κB)13. A consequence of these signaling events is the production of cytokines interleukin (IL) 1 beta, IL-6, and tumor necrosis factor-alpha (TNF-α)14.
Several studies have shown that autophagy controls a wide range of immune responses, including the regulation of cytokine levels, inflammasome activity, clearance of invading pathogens, and the T cell immune response15–18. Autophagy, referred to as “self-eating”, describes a process through which essential cellular components are enclosed by double-membrane vesicles (autophagosomes), and subsequently undergo lysosomal fusion (autolysosomes), eventually leading to cellular degradation19. Beyond its traditional role in cellular maintenance, autophagy serves as a critical modulator of inflammation by removing inflammasome activators, regulating NF-κB signaling, and controlling the secretion of pro-inflammatory cytokines such as IL-1β and TNF-α20,21. LPS-induced autophagy has been observed in various cell types, including macrophages22, keratinocytes23, and myoblasts24 highlighting its role in immune regulation during infection and inflammatory response. 3-methyladenine, a selective autophagy inhibitor, rescued LPS-induced acute lung injury by blocking inflammation and autophagy25, and punicalagin, an ingredient of pomegranates, reduced the LPS-induced inflammatory response by downregulating forkhead box class O 3a (FOXO3a)/autophagy signaling in macrophages26. These previous studies denote that inhibiting autophagy may suppress LPS-induced inflammatory responses in macrophages.
In the present study, we investigated the effects and underlying molecular mechanism of ATE on the inflammatory response in LPS-induced RAW 264.7 macrophages, with a particular focus on the suppression of the inflammatory response and the associated role of autophagy.
Results
Identification and quantification of ATE components
Nineteen marker compounds were identified using the established high-performance liquid chromatography (HPLC) analytical method within 65 min, with a resolution greater than 1.5 (Fig. 1). The detection wavelengths for quantitative analysis were as follows: 230 nm (albiflorin, paeoniflorin, and benzoic acid), 250 nm (glycyrrhizin), 260 nm (protocatechuic acid), 270 nm (gallic acid), 275 nm (baicalin, wogonoside, baicalein, and wogonin), 280 nm (5-hydroxymethylfurfural, narirutin, and hesperidin), 325 nm (chlorogenic acid and ferulic acid), 330 nm (rosmarinic acid, decursin, and decursinol angelate), and 335 nm (nodakenin), respectively. In ATE, these marker compounds were detected at concentrations ranging from 0.11 to 17.96 mg/g extract, with baicalin, the primary component of Scutellariae Radix, being the most abundant (Table 2).
Fig. 1.
Representative HPLC chromatogram of the standard solution (A) and the ATE sample solution (B) at 230 nm. The concentrations of each marker analyte in the standard solution (A) were as follows: 10 µg/mL (ferulic acid), 20 µg/mL (gallic acid, 5-hydroxymethylfurfural, protocatechuic acid, chlorogenic acid, nodakenin, benzoic acid, rosmarinic acid, baicalin, wogonoside, baicalein, and wogonin), 30 µg/mL (narirutin, decursin, and decursinol angelate), 50 µg/mL (albiflorin, paeoniflorin, and hesperidin), and 100 µg/mL (glycyrrhizin). Gallic acid (1), 5-hydroxymethylfurfural (2), protocatechuic acid (3), chlorogenic acid (4), albiflorin (5), paeoniflorin (6), ferulic acid (7), nodakenin (8), narirutin (9), hesperidin (10), benzoic acid (11), rosmarinic acid (12), baicalin (13), wogonoside (14), baicalein (15), glycyrrhizin (16), wogonin (17), decursin (18), and decursinol angelate (19).
Table 2.
Amounts of 19 marker analytes in ATE extract using HPLC (n = 3).
| Analyte | Amount (mg/g) | SD (× 10−2) | RSD (%)a |
|---|---|---|---|
| Gallic acid | 0.56 | 0.53 | 0.94 |
| 5-Hydroxymethylfurfural | 0.70 | 0.23 | 0.33 |
| Protocatechuic acid | 0.11 | 0.22 | 2.02 |
| Chlorogenic acid | 0.42 | 0.52 | 1.23 |
| Albiflorin | 0.76 | 0.21 | 0.27 |
| Paeoniflorin | 3.40 | 1.68 | 0.49 |
| Ferulic acid | 0.19 | 0.09 | 0.49 |
| Nodakenin | 0.69 | 0.29 | 0.43 |
| Narirutin | 1.72 | 1.25 | 0.73 |
| Hesperidin | 4.72 | 2.49 | 0.53 |
| Benzoic acid | 0.29 | 0.26 | 0.89 |
| Rosmarinic acid | 0.33 | 0.24 | 0.73 |
| Baicalin | 17.96 | 7.38 | 0.41 |
| Wogonoside | 3.38 | 0.17 | 0.05 |
| Baicalein | 0.22 | 0.24 | 1.12 |
| Glycyrrhizin | 1.29 | 0.27 | 0.21 |
| Wogonin | 0.25 | 0.04 | 0.15 |
| Decursin | 0.14 | 0.08 | 0.59 |
| Decursinol angelate | 0.11 | 0.09 | 0.88 |
aRSD: relative standard deviation.
ATE suppressed LPS-induced inflammatory cytokine production in RAW 264.7 cells
To assess the anti-inflammatory effects of ATE on RAW 264.7 macrophages, a cell proliferation assay was conducted. ATE treatment at 15.6 to 500 µg/mL showed no cytotoxicity in RAW 264.7 cells (Supplementary Fig. S1). Based on these results, the maximum concentration of ATE, up to 500 µg/mL, was used in subsequent experiments.
To elucidate the anti-inflammatory effects of ATE, RAW 264.7 cells were stimulated with LPS to induce the production of inflammatory cytokines (Fig. 2A and B). As expected, LPS significantly increased the release of pro-inflammatory cytokines, IL-1β and IL-6, triggering inflammatory responses. However, treatment with a high dose of ATE (500 µg/mL) significantly reduced IL-1β and IL-6 levels to 44.1% and 66.5% of LPS-only levels, respectively. These findings demonstrate that ATE exerts anti-inflammatory effects by suppressing cytokine production in LPS-stimulated RAW 264.7 cells.
Fig. 2.
ATE suppresses LPS-induced inflammatory cytokine production in RAW 264.7 cells. Cells were treated with LPS and/or ATE (62.5–500 µg/mL), as indicated, for 24 h. The levels of IL-1β (A) and IL-6 (B) were measured using ELISA kits. The significance of differences was assessed via the Student’s t-test. Data are represented as the mean ± SD (n = 3). ###p < 0.001 vs. control; *p < 0.05, **p < 0.01, and ***p < 0.001 vs. LPS.
ATE suppressed iNOS and COX-2 in RAW 264.7 cells
To determine whether the anti-inflammatory effects of ATE were mediated through the modulation of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2), we measured the levels of nitric oxide (NO) and prostaglandin E2 (PGE2). ATE effectively reduced NO and PGE2 levels. Notably, even at the lowest concentration of ATE (62.5 µg/mL), PGE2 was completely suppressed to a level similar to the control. The positive controls, NG-methyl-L-arginine acetate (L-NNMA) and indomethacin (Indo), also significantly reduced NO and PGE2 release, respectively (Fig. 3A and B). To confirm whether the observed effects were linked to the downregulation of iNOS and COX-2, we assessed their expression levels (Fig. 3C). As expected, LPS treatment upregulated iNOS and COX-2 expressions, while ATE reversed these changes at doses of 125 µg/mL and 500 µg/mL. In particular, at 500 µg/mL, ATE decreased iNOS and COX-2 expression by 58.9% and 49.8%, respectively, compared to the LPS-treated cells.
Fig. 3.
ATE suppresses NO and PGE2 release by modulating iNOS and COX-2 expression in RAW 264.7 cells. (A, B) The levels of NO and PGE2 were measured using ELISA kits after 24 h of treatment with LPS and/or ATE (62.5–500 µg/mL). (C) Western blot analysis of iNOS and COX-2 expression in cells treated with LPS and/or ATE (62.5–500 µg/mL) for 24 h. Quantification of protein expression was performed using ImageJ software. The significance of differences was assessed via Student’s t-tests. Data are represented as the mean ± SD (n = 3). ###p < 0.001 vs. control; **p < 0.01 and ***p < 0.001 vs. LPS. L-NMMA and indomethacin (Indo) were used as positive controls.
ATE suppressed LPS-induced TLR4 signaling in RAW 264.7 cells
TLR4 recognizes LPS as the first responder in inflammatory signaling cascades. To confirm whether ATE mediates its effects through the regulation of TLR4 signaling, we evaluated the expression levels of TLR4 and MyD88. LPS induced the expression of both TLR4 and MyD88. Meanwhile, ATE suppressed their expression in a dose-dependent manner, with reductions of 77.7% and 81.3%, respectively, at a concentration of 500 µg/mL (Fig. 4A).
Fig. 4.
ATE suppresses LPS-induced TLR4, MAPKs, and NF-κB signaling pathways in RAW 264.7 cells. (A) Western blot analysis of TLR4 and MyD88 expression in cells treated with LPS and/or ATE (125 and 500 µg/mL) for 8 h. (B) Phosphorylated p38, ERK, and JNK expression levels were assessed by western blotting after 30 min of ATE treatment (125 and 500 µg/mL). (C) NF-κB and IκBα expression levels were analyzed by western blotting after 30 min of ATE treatment (125 and 500 µg/mL). Lamin B1 and GAPDH were used as loading controls for nuclear and cytosolic fractions, respectively. Quantification of protein expression was performed using ImageJ software. The significance of differences was assessed via the Student’s t-test. Data are represented as the mean ± SD (n = 3). ##p < 0.01 and ###p < 0.001 vs. control; *p < 0.05 and ***p < 0.001 vs. LPS.
To examine the effects of ATE on downstream signaling, we assessed the MAPK and NF-κB pathways. LPS significantly activated the MAPK pathway, as indicated by increased phosphorylation of p38, ERK, and JNK. Conversely, ATE suppressed their phosphorylation in a dose-dependent manner, with reductions to 69.2%, 83.5%, and 74.5%, respectively, at a high dose of 500 µg/mL (Fig. 4B). Additionally, we examined the expression of NF-κB and nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor alpha (IκBα) in both cytosolic and nuclear extracts. Our findings showed that LPS induced IκBα degradation in the cytosol, which resulted in the release of NF-κB and its translocation into the nucleus, leading to the functional activation of NF-κB. However, ATE dose-dependently inhibited LPS-induced degradation of cytosolic IκBα, thereby attenuating the nuclear translocation of NF-κB. At a concentration of 500 µg/mL, ATE significantly decreased NF-κB nuclear translocation by 57.4% compared to the LPS-treated cells (Fig. 4C).
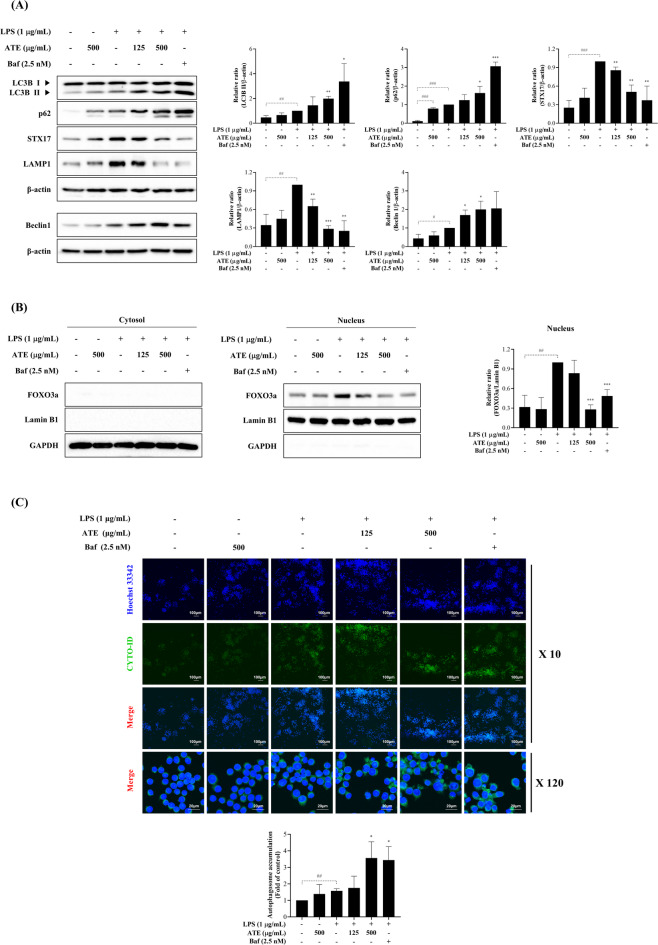
ATE suppresses LPS-induced autophagic flux in RAW 264.7 cells
LPS-induced autophagy plays a crucial role in the innate immune response and has been observed in macrophages. In the present study, we explored the effect of ATE on autophagy in LPS-stimulated cells. LPS treatment increased the protein expressions of autophagy-related factors, including Beclin 1 and microtubule-associated protein 1 A/1B-light chain 3 (LC3)B I/II, in a time-dependent manner, indicating autophagosome formation. Notably, p62 levels also increased over time, suggesting impaired autophagic degradation despite enhanced autophagosome formation. Additionally, LPS upregulated the expression of syntaxin 17 (STX17) and lysosomal-associated membrane protein (LAMP1), markers of autophagosome-lysosome fusion and lysosome function, respectively. However, the concurrent accumulation of p62 along with STX17 and LAMP1 expression suggest that although autophagosome-lysosome function may be promoted, lysosomal degradation capacity is compromised, resulting in impaired autophagic flux (Supplementary Fig. S2A). Based on these findings, an 18 h LPS stimulation was selected for further experiments.
Bafilomycin (Baf) is used as a positive control in studies of autophagic flux, as it inhibits the vacuolar H+-ATPase (V-ATPase), thereby preventing lysosomal acidification. This inhibition disrupts lysosomal function and blocks the fusion of autophagosomes with lysosomes, leading to impaired autophagic degradation. Thus, Baf is useful for distinguishing between increased autophagy induction and blocked autophagic degradation. Under LPS stimulation, ATE dramatically elevated Beclin 1, LC3B II, and p62 expression, while significantly downregulating the expression of STX17 and LAMP1, mimicking the effects of Baf, a late-phase autophagy inhibitor (Fig. 5A). Our results indicate that ATE modulates LPS-induced autophagic flux by disrupting autophagosome-lysosome fusion and lysosome function. FOXO3a, an autophagy regulator27, was upregulated by LPS in a time-dependent manner (Supplementary Fig. S2B), while ATE decreased FOXO3a expression in LPS-stimulated macrophages, similar to Baf treatment (Fig. 5B). These results suggest that ATE modulates autophagic flux through FOXO3a suppression. Using the CYTO-ID green detection kit, we observed autophagosome accumulation in ATE-treated macrophages, indicating a blockade in autophagic flux (Fig. 5C).
Fig. 5.
ATE suppresses LPS-induced autophagic flux. (A) Western blot analysis of autophagic flux-related proteins, including LC3B, p62, STX17, and LAMP1, in cells treated with LPS and/or ATE (125 and 500 µg/mL). (B) FOXO3a expression levels were also assessed by western blotting. Lamin B1 and GAPDH were used as loading controls for nuclear and cytosolic fractions, respectively. (C) Autophagosome formation (green puncta) was visualized using an FSX100 all-in-one fluorescence microscope. Cells were treated with LPS and/or ATE (125 and 500 µg/mL) for 18 h. Bafilomycin (Baf) was used as a positive control. Quantification of expression levels was performed using ImageJ software. The significance of differences was assessed via the Student’s t-test. Data are represented as the mean ± SD (n = 3). #p < 0.05, ##p < 0.01, and ###p < 0.001 vs. control; *p < 0.05, **p < 0.01, and ***p < 0.001 vs. LPS.
Discussion
In a review of Korean medicine literature on herbal medicines used for pregnancy, maternal, and perinatal care, all ten herbal medicines comprising ATE were ranked in the top 15 in terms of frequency of use, and in particular, Atractylodis Rhizoma Alba (ARA), which constitutes 19.1% of ATE (Table 1), was the most frequently used28. ARA has been reported to exhibit anti-inflammatory effects through the inhibition of LPS-induced NO release in macrophages and to exert immunomodulatory effects by activating the secretion of G-CSF in colon epithelial cells29. Scutellariae Radix (SR), which makes up 14.3% of ATE (Table 1), has been traditionally used to prevent miscarriage and is known to have anti-inflammatory effects by suppressing the production of pro-inflammatory cytokines, such as TNF-α and IL-6, through the inhibition of the NF-κB signaling pathway in an LPS-induced inflammatory in vitro model30. Baicalin, the most abundant phytochemical in the ATE extract (17.96 mg/g, Table 2), has been identified as a main component of SR and exhibits potent anti-inflammatory, antioxidant, and anticancer activities as well as the ability to inhibit TLR4 signal transduction and autophagy expression in bacterial-infected macrophages31. The pregnancy-related pharmacological effects of ATE have been reported, yet its influence on the inflammatory response remains unclear. In this study, we characterized the anti-inflammatory effects of ATE in macrophages as well as its underlying mechanism, namely, the suppression of autophagic flux.
Macrophages activated by LPS overproduce inflammatory cytokines, which drive inflammation11. Cytokines are generally classified as pro- or anti-inflammatory based on their activity. The former act as regulators of the host response to infection and trauma, potentially exacerbating certain pathological conditions32. In our study, ATE significantly suppressed the LPS-induced production of pro-inflammatory cytokines, IL-1β and IL-6. iNOS and COX-2 are potential targets for the suppression of inflammation owing to their production of NO and PGE233,34. We observed that ATE inhibited iNOS and COX-2 expression in addition to reducing their enzymatic activity. Cell proliferation assays showed that ATE did not affect cell viability, indicating that its anti-inflammatory effects were not mediated via cytotoxicity. Further, ATE reduced TLR4-mediated inflammatory signaling in LPS-stimulated macrophages, including NF-κB signaling and phosphorylation of MAPKs (p38, ERK, and JNK). These results suggest that the suppression of LPS-induced TLR4 signaling by ATE led to a decrease in the production of NO, PGE2, IL-1β, and IL-6. In the absence of LPS, ATE slightly induced TLR4 expression (1.5-fold) compared to the untreated control, but the increase was not significant. Although the expressions of MyD88 and its downstream signal, p-p38, were increased in macrophages treated with ATE alone, their activation was unlikely to affect the anti-inflammatory effects of ATE because there was no change in the subsequent signaling.
Autophagy, the process of breaking down and recycling cellular components, plays an important role in regulating immune and inflammatory responses35. Autophagosome formation is regulated by three key steps: assembly of the PI3K complex (Beclin 1, VPS34, and ATG14), conjugation of the ATG5-ATG12-ATG16L1 and LC3 II protein complexes36,37, and autophagosome fusion with lysosomes via anterograde and retrograde transport, which involves SNAP receptors, tethering factors, and Rab GTPases38. Lysosomal damage may interfere with the degradation of autophagic cargo, subsequently leading to a blockade of autophagic flux39. LC3 is a representative marker of autophagy. It typically remains as LC3 I under normal cellular conditions, while otherwise being converted to LC3 II, which allows the latter to be utilized as an indicator of autophagosomes40,41. The p62/SQSTM1 primarily functions as a cargo adaptor protein that facilitates the sequestration of ubiquitinated substrates into autophagosomes. Additionally, it serves as a marker of impaired autophagic flux, with its accumulation indicating disrupted autophagic degradation42. The accumulation of p62 promotes the phosphorylation and degradation of IκBα, which thereby facilitating the nuclear translocation of NF-κB and the subsequent release of pro-inflammatory cytokines. Thus, p62 acts as a mediator linking impaired autophagy and enhanced inflammatory responses through NF-κB activation43. STX17 and LAMP1 are located on the membrane of autophagosomes and lysosomes, respectively, and play essential roles in the late stage of the autophagy process, during which autophagosomes fuse with lysosomes to form autolysosomes44. Dysfunction of STX17 and LAMP1 can disrupt autophagosome clearance, thereby promoting p62 accumulation and impairing autophagic flux45.
The increased expression of LC3B II and p62 by Baf, which was used as a positive control, indicates that autophagosomes accumulate because they fail to fuse with lysosomes, thereby blocking autophagic flux at the degradation stage. In contrast, the increase in Beclin 1 suggests enhanced autophagy initiation rather than a blockade46–48. Thus, Baf suppressed the late stages of autophagy by inhibiting lysosomal acidification, thereby inhibiting autophagosome degradation.
As expected, ATE treatment increased the expression of LC3B II, p62, and Beclin 1 to a greater extent than LPS, while STX17 and LAMP1 were decreased. Additionally, ATE downregulated FOXO3a in LPS-stimulated macrophages. FOXO3a is a crucial transcription factor that regulates cellular homeostasis, survival, and autophagy by inducing autophagy-related genes and promoting autophagosome formation38,49–51. It also plays a role in inflammation by modulating NF-κB signaling and pro-inflammatory cytokine expression. While increased p62 expression and suppressed FOXO3a expression contribute to the inhibition of NF-κB activation and cytokine secretion, they do not independently induce an anti-inflammatory effect but rather function within a broader regulatory network that modulates inflammation52. Our findings demonstrate that ATE blocks autophagic flux by causing the accumulation of LC3B II, p62, and Beclin 1, but not STX17 and LAMP1, which are associated with the late stage of autophagy. This suggests that ATE disrupts autophagosome-lysosome fusion, consequently leading to the blockade of autophagic flux and the accumulation of undegraded autophagosomes.
In the present study, we demonstrated that ATE inhibits autophagic flux, thereby contributing to its anti-inflammatory effects in LPS-stimulated macrophages. However, it is important to recognize that autophagy exerts dual roles in the regulation of inflammation. Under normal physiological conditions, autophagy acts to maintain cellular homeostasis by removing damaged organelles and regulating excessive inflammatory responses53. In pathological conditions such as rheumatoid arthritis, dysregulated or excessive autophagy has been implicated in promoting the survival of inflammatory cells, thereby sustaining chronic inflammation54. While, in diseases where efficient clearance of aggregated proteins is crucial, such as neurodegenerative disorders, suppression of autophagy could potentially exacerbate disease progression55. Although the inhibitory effect of ATE on autophagic flux appears beneficial in macrophage-driven inflammatory responses, it is essential to carefully evaluate its effects in other disease models. The present study did not investigate the broader implications of ATE’s effects on other immune cell types or inflammatory diseases beyond its impact on RAW 264.7 macrophages. These cells provide a useful in vitro model, but they may not fully replicate the complexity of inflammatory responses in vivo. To confirm the therapeutic potential of ATE under specific pathological conditions, further studies are needed both in vitro and in vivo, including investigations of inflammatory responses in endometrial cells and in animal models of recurrent miscarriage, arthritis, and dermatitis. Although the present study has identified the key molecular mechanisms involved in the inhibition of TLR4 signaling and autophagic flux, the precise interactions between the active compounds of ATE and these pathways remain unclear. Further research should focus on identifying individual bioactive components and evaluating their specific contributions to ATE’s anti-inflammatory effects. The HPLC-based phytochemical analysis method established in this study can be used for standardization and quality control of ATE, with a focusing on bioactive components. These additional studies are expected to enhance the clinical applications of ATE.
In conclusion, our findings collectively suggest that ATE inhibits the TLR4-mediated NF-κB and MAPK signaling pathways, leading to reduced production of key inflammatory mediators and pro-inflammatory cytokines. Moreover, the study reveals that ATE disrupts autophagic flux by blocking autophagosome-lysosome fusion, which further contributes to its anti-inflammatory effects (Fig. 6). These findings not only reinforce the traditional use of ATE in pregnancy-related conditions but also suggest its potential as a novel therapeutic agent for inflammation-associated diseases.
Fig. 6.
Summary of the anti-inflammatory mechanism of ATE in LPS-stimulated macrophages. A diagram depicting the anti-inflammatory effects of ATE by regulating autophagy flux in LPS-induced RAW 264.7 cells. ATE inhibits TLR4 signaling, suppressing MAPK phosphorylation and NF-κB activation, leading to reduced inflammatory mediator production. Additionally, ATE disrupts autophagy flux by blocking autophagosome-lysosome fusion and regulating FOXO3a.
Methods
Materials
Reagents and materials used in the present study are listed in the Supplementary information (Table S1).
Preparation of ATE extract
The ten raw herbal medicines constituting the ATE formula were purchased from a traditional herb market, Kwangmyungdang Medicinal Herbs (Ulsan, Republic of Korea). The voucher specimens were deposited in the herbarium of the KM Science Research Division of the Korea Institute of Oriental Medicine (No. KE91-1 to KE91-10). To obtain a water decoction of ATE, the ten herbal medicines were mixed as shown in Table 1 (total = 5.0 kg, approximately 127 times the amount in a single dose) and extracted in distilled water at 100 °C for 2 h using an electric extractor (COSMOS-660, Kyungseo E&P, Incheon, Korea). The ATE extract was filtered using a standard test sieve (mesh size: 53 μm) and lyophilized to obtain a powdered form. The yield of ATE extract was 27.4% (1,371.0 g, voucher number KE91).
Phytochemical analysis of ATE via HPLC
Phytochemical analysis of ATE was conducted using a Shimadzu Prominence LC-20 A series system (Shimadzu, Kyoto, Japan) controlled using LabSolutions software (version 5.53, SP3). All analytes were separated on a Waters SunFire™ C18 analytical column (length: 250 mm, inner diameter: 4.6 mm, particle size: 5 μm, Milford, MA, USA) maintained at 40 °C. The mobile phase consisted of 0.1% (v/v) formic acid in distilled water (A) and 0.1% (v/v) formic acid in acetonitrile (B) and was run under the following gradient elution conditions: 5% B (0 min), 60% B (60 min), 95% B (70 min, held for 5 min), and 5% B (80 min, held for 10 min). The flow rate was 1.0 mL/min, and the injection volume was 10 µL. For the phytochemical analysis of ATE, a photodiode array detector was used, which scanned simultaneously in the 190–800 nm region. The amounts of the following 19 compounds in the ATE extract were determined: albiflorin, baicalein, baicalin, benzoic acid, chlorogenic acid, decursin, decursinol angelate, ferulic acid, gallic acid, glycyrrhizin, hesperidin, 5-hydroxymethylfurfural, narirutin, nodakenin, paeoniflorin, protocatechuic acid, rosmarinic acid, wogonin, and wogonoside.
Cell culture
Murine macrophages, RAW 264.7 (American Type Culture Collection, Rockville, MD, USA), were incubated in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 5.5% fetal bovine serum and 1% penicillin/streptomycin, and grown in a humidified atmosphere containing 5% CO2 at 37 °C. Subculture was performed when cell confluency reached 80–90%, and cells at passage numbers 5 to 10 after purchase were used for experiments.
Cell proliferation assay
Cell proliferation was measured using the CCK-8 reagent. In brief, the cells (1 × 104 cells/well) were incubated for 18 h in a 96-well plate, followed by treatment with varying concentrations of ATE (15.6–500 µg/mL). After 24 h incubation, CCK-8 reagent (10 µL) was added to each well, and the plate was incubated at 37 °C for 4 h. The absorbance of each well was measured at 450 nm using a microplate reader (Bio-Rad, Hercules, CA, USA). The rate of cell proliferation was calculated using the following method based on absorbance (Abs):
Measurement of inflammatory mediators and cytokines
Cells (7.5 × 104 cells/well) were seeded into 48-well plates. After overnight incubation, the cells were treated with ATE (62.5–500 µg/mL), L-NMMA (100 µM), and Indo (2 nM). LPS (1 µg/mL) was used as a stimulator to induce an inflammatory response. Following another 24 h of incubation, the supernatants were collected. NO levels were measured using the Griess reagent, following the manufacturer’s instructions. PGE2, IL-1β, and IL-6 levels in the supernatants were quantified using the respective ELISA kits, according to the provided protocols. L-NMMA and Indo were used as positive controls for the inhibition of NO and PGE2 release, respectively.
Analysis of expressions for inflammatory response- and autophagy-related factors
Cells (2–4 × 106 cells/dish) were seeded into 100-mm dishes and incubated for 24 h. The cells were treated with LPS (1 µg/mL) and/or ATE (125 and 500 µg/mL), L-NMMA (100 µM), Indo (10 nM), and Baf (2.5 nM) for appropriate time periods to detect specific factors (e.g., iNOS, COX-2). Whole cells were lysed in ice-cold radioimmunoprecipitation assay (RIPA) buffer containing 1 mM PMSF and 1× protease and phosphatase inhibitor cocktail for 30 min, followed by centrifugation at 14,000 g for 30 min. Cytosolic (CE) and nuclear extracts (NE) were prepared using NE-PER nuclear and cytoplasmic extraction reagent kits. Briefly, cells were lysed in 100 µL of CER I, vortexed for 10 s, and kept on ice for 10 min. Then, 5.5 µL of CER II was added, vortexed for 5 s, and kept on ice for 1 min. After centrifugation at 21,000 g for 15 min, the cytosolic supernatants (CE) were collected. The remaining pellets were washed three times with PBS, lysed in 50 µL of NER, sonicated for 20 s on ice, centrifuged at 21,000 g for 10 min, and the nuclear supernatants (NE) were collected. Protein concentrations in whole cell extracts, CE, and NE were quantified using a BCA protein assay kit.
A total of 20 µg of protein lysate was separated via SDS-PAGE and transferred to PVDF membranes. The membranes were blocked with 5% skim milk and incubated with specific primary antibodies. The antibody-antigen complexes were detected with HRP-conjugated secondary antibodies and visualized using ECL. Protein bands were analyzed using Chemi-Doc (Bio-Rad) and quantified with ImageJ 1.53 software (National Institutes of Health, Bethesda, MD, USA).
Autophagy staining
To assess autophagic activity at the cellular level, a CYTO-ID autophagy detection kit was used. Briefly, cells (2 × 104 cells/well) were seeded into 8-well chamber slides. After 24 h of incubation, the cells were treated with LPS (1 µg/mL), ATE (125 and 500 µg/mL), and Baf (2.5 nM). The treated cells were stained with CYTO-ID Green dye, which containis a cationic amphiphilic tracer (CAT) dye (diluted 1:500), and Hoechst 33,342 nuclear staining dye (diluted 1:1,000) at 37 °C for 30 min. The CYTO-ID Green dye specifically labels spherical vacuoles, typically accumulating in cytoplasmic foci, indicating a blockade in autophagic flux56. Images were obtained using an all-in-one fluorescence microscope FSX100 (OLYMPUS, Tokyo, Japan) at 10× and 120× magnification. Pixel intensity was quantified using ImageJ 1.53 software. To quantify autophagosome accumulation, we measured the fluorescence intensity (FI) of CYTO-ID and expressed it using the following equation:
Statistical analysis
All data were independently analyzed at least 3 times to ensure consistency. For experiments involving two groups, we conducted Student’s t-tests to assess statistical significance. Results are presented as the mean ± standard deviation (SD) using statistical software GaphPad Prism 9 (GraphPad Prism, CA, USA). A p-value of less than 0.05 was considered statistically significant.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This study was supported by the Korea Institute of Oriental Medicine [grant number: KSN2022310 and KSN2224011].
Abbreviations
- ATE
Antae-eum
- ATG5
Autophagy related 5
- ATG12
Autophagy related 12
- ATG14
Autophagy related 14
- ATG16L1
Autophagy related 16 like 1
- Baf
Bafilomycin
- COX-2
Cyclooxygenase 2
- ERK
Extracellular signal-regulated kinase
- FOXO3a
Forkhead box class O 3a
- GAPDH
Glyceraldehyde 3-phosphate dehydrogenase
- G-CSF
Granulocyte-colony stimulating factor
- IgG
Immunoglobulin G
- IκBα
Nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor alpha
- IL-1β
Interleukin 1 beta
- IL-6
Interleukin 6
- iNOS
Inducible nitric oxide synthase
- JNK
C-Jun N-terminal kinase
- LAMP1
Lysosomal-associated membrane protein
- LC3
Microtubule-associated protein 1A/1B-light chain 3
- L-NMMA
NG-methyl-L-arginine acetate
- LPS
Lipopolysaccharide
- MAPK
Mitogen-activated protein kinase
- MyD88
Myeloid differentiation factor 88
- NF-κB
Nuclear factor kappa-light-chain-enhancer of activated B
- NO
Nitric oxide
- PGE2
Prostaglandin E2
- PI3K
Phosphoinositide 3-kinase
- SQSTM1
Sequestosome 1
- SNAP
Soluble N-ethylmaleimide-sensitive factor attachment protein
- STX17
Syntaxin 17
- TLR4
Toll-like receptor 4
- TNF-α
Tumor necrosis factor-alpha
- VPS34
Vascular protein sorting 34
Author contributions
Y.J.O.: Investigation, Methodology, Formal analysis, Writing - original draft, Writing - review & editing. C.S.S.: Investigation, Formal analysis. S.E.J.: Investigation, Methodology, Formal analysis. H.K.S.: Conceptualization, Project administration. H.H.: Investigation, Formal analysis, Methodology, Writing – review & editing, Supervision. All authors reviewed the manuscript.
Data availability
Data is provided within the manuscript or supplementary information files.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization. WHO Traditional Medicine Strategy 2014–2023. https://www.who.int/publications/i/item/9789241506096 (2013).
- 2.Heo, J. Donguibogam: Principle and Pratice of Eastern Medicine (Ministry of Health & Welfare, 2012). [Google Scholar]
- 3.Zhang, D., Yu, Y., Duan, T. & Zhou, Q. The role of macrophages in reproductive-related diseases. Heliyon8, e11686. 10.1016/j.heliyon.2022.e11686 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee, D. N. et al. Studies on the mutagenicity and hepatotoxicity of antaeum. Korean J. Pharmacogn.28, 149–155 (1997). [Google Scholar]
- 5.Kim, C. et al. The effects of the administration on Oriental medicine, antaeeum, in the pregnant rat and their fetuses. J. Environ. Health Sci.33, 306–316 (2007). [Google Scholar]
- 6.Chung, H. et al. Changes of reproductive functions in pregnant mice administrated Kyoaekungkue-tang, Bojungykki-tang, Kungso-san, Antae-eum, Antaegumchul-tang. J. Korean Orient. Med.21, 166–173 (2000). [Google Scholar]
- 7.Park, S., Noh, E., Seo, C., Lee, S. & Shin, H. A study on the regulation of endometrial and placental cell function by water extract of 3 types of herbal medicines and ethanol extract on scutellariae Radix. J. Korean Obstet. Gynecol.34, 1–14 (2021). [Google Scholar]
- 8.Yang, G. Y., Luo, H., Liao, X. & Liu, J. P. Chinese herbal medicine for the treatment of recurrent miscarriage: A systematic review of randomized clinical trials. BMC Complement. Altern. Med.13, 320. 10.1186/1472-6882-13-320 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Netea, M. G. et al. A guiding map for inflammation. Nat. Immunol.18, 826–831. 10.1038/ni.3790 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kotas, M. E. & Medzhitov, R. Homeostasis, inflammation, and disease susceptibility. Cell160, 816–827. 10.1016/j.cell.2015.02.010 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jin, B. & Jin, H. Oxymatrine attenuates lipopolysaccharide-induced acute lung injury by activating the epithelial sodium channel and suppressing the JNK signaling pathway. Exp. Anim.67, 337–347. 10.1538/expanim.17-0121 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van den Bossche, J., O’Neill, L. A. & Menon, D. Macrophage immunometabolism: Where are we (Going)? Trends Immunol.38, 395–406. 10.1016/j.it.2017.03.001 (2017). [DOI] [PubMed] [Google Scholar]
- 13.Ariyadi, B., Isobe, N. & Yoshimura, Y. Toll-like receptor signaling for the induction of mucin expression by lipopolysaccharide in the Hen vagina. Poult. Sci.93, 673–679. 10.3382/ps.2013-03667 (2014). [DOI] [PubMed] [Google Scholar]
- 14.Gilmore, T. D. Introduction to NF-kappaB: Players, pathways, perspectives. Oncogene25, 6680–6684. 10.1038/sj.onc.1209954 (2006). [DOI] [PubMed] [Google Scholar]
- 15.Cadwell, K. Crosstalk between autophagy and inflammatory signalling pathways: Balancing defence and homeostasis. Nat. Rev. Immunol.16, 661–675. 10.1038/nri.2016.100 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris, J. Autophagy and cytokines. Cytokine56, 140–144. 10.1016/j.cyto.2011.08.022 (2011). [DOI] [PubMed] [Google Scholar]
- 17.Münz, C. Autophagy beyond intracellular MHC class II antigen presentation. Trends Immunol.37, 755–763. 10.1016/j.it.2016.08.017 (2016). [DOI] [PubMed] [Google Scholar]
- 18.Nakahira, K. et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat. Immunol.12, 222–230. 10.1038/ni.1980 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deretic, V., Saitoh, T. & Akira, S. Autophagy in infection, inflammation and immunity. Nat. Rev. Immunol.13, 722–737. 10.1038/nri3532 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee, J. et al. Autophagy suppresses interleukin-1β (IL-1β) signaling by activation of p62 degradation via lysosomal and proteasomal pathways. J. Biol. Chem.287, 4033–4040. 10.1074/jbc.M111.280065 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi, C. S. et al. Activation of autophagy by inflammatory signals limits IL-1β production by targeting ubiquitinated inflammasomes for destruction. Nat. Immunol.13, 255–263. 10.1038/ni.2215 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davenport, A. Peritonitis remains the major clinical complication of peritoneal dialysis: The london, UK, peritonitis audit 2002–2003. Perit. Dial. Int.29, 297–302 (2009). [PubMed] [Google Scholar]
- 23.Lee, H. M. et al. Autophagy negatively regulates keratinocyte inflammatory responses via scaffolding protein p62/SQSTM1. J. Immunol.186, 1248–1258. 10.4049/jimmunol.1001954 (2011). [DOI] [PubMed] [Google Scholar]
- 24.Doyle, A., Zhang, G., Abdel Fattah, E. A., Eissa, N. T. & Li, Y. P. Toll-like receptor 4 mediates lipopolysaccharide-induced muscle catabolism via coordinate activation of ubiquitin-proteasome and autophagy-lysosome pathways. FASEB J.25, 99–110. 10.1096/fj.10-164152 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ding, D. et al. 3-Methyladenine and Dexmedetomidine reverse lipopolysaccharide-induced acute lung injury through the Inhibition of inflammation and autophagy. Exp. Ther. Med.15, 3516–3522. 10.3892/etm.2018.5832 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cao, Y. et al. Punicalagin prevents inflammation in LPS-Induced RAW264.7 macrophages by inhibiting FoxO3a/Autophagy signaling pathway. Nutrients. 10.3390/nu11112794 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li, C., Wang, Z., Wang, C., Ma, Q. & Zhao, Y. Perivascular adipose tissue-derived adiponectin inhibits collar-induced carotid atherosclerosis by promoting macrophage autophagy. PLoS One10, e0124031. 10.1371/journal.pone.0124031 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jo, J. et al. Use and safety of Korean herbal medicine during pregnancy: A Korean medicine literature review. Eur. J. Integr. Med.8, 4–11. 10.1016/j.eujim.2015.10.008 (2016). https://doi.org:. [Google Scholar]
- 29.Zhang, W. J. et al. Atractylodis rhizoma: A review of its traditional uses, phytochemistry, pharmacology, toxicology and quality control. J. Ethnopharmacol.266, 113415. 10.1016/j.jep.2020.113415 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma, W. et al. Recent advances in scutellariae radix: A comprehensive review on ethnobotanical uses, processing, phytochemistry, Pharmacological effects, quality control and influence factors of biosynthesis. Heliyon10, e36146. 10.1016/j.heliyon.2024.e36146 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang, L. et al. Baicalin inhibits Salmonella typhimurium-induced inflammation and mediates autophagy through TLR4/MAPK/NF-κB signalling pathway. Basic. Clin. Pharmacol. Toxicol.128, 241–255. 10.1111/bcpt.13497 (2021). [DOI] [PubMed] [Google Scholar]
- 32.Gao, Y. et al. Inflammation negatively regulates FOXP3 and regulatory T-cell function via DBC1. Proc. Natl. Acad. Sci. U. S. A.112, E3246–E3254. 10.1073/pnas.1421463112 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mitchell, J. A., Larkin, S. & Williams, T. J. Cyclooxygenase-2: Regulation and relevance in inflammation. Biochem. Pharmacol.50, 1535–1542. 10.1016/0006-2952(95)00212-x (1995). [DOI] [PubMed] [Google Scholar]
- 34.Folino, A., Losano, G. & Rastaldo, R. Balance of nitric oxide and reactive oxygen species in myocardial reperfusion injury and protection. J. Cardiovasc. Pharmacol.62, 567–575. 10.1097/FJC.0b013e3182a50c45 (2013). [DOI] [PubMed] [Google Scholar]
- 35.Denton, D. & Kumar, S. Autophagy-dependent cell death. Cell. Death Differ.26, 605–616. 10.1038/s41418-018-0252-y (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tan, Y. Q., Zhang, J. & Zhou, G. Autophagy and its implication in human oral diseases. Autophagy13, 225–236. 10.1080/15548627.2016.1234563 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Devis-Jauregui, L., Eritja, N., Davis, M. L., Matias-Guiu, X. & Llobet-Navàs, D. Autophagy in the physiological endometrium and cancer. Autophagy17, 1077–1095. 10.1080/15548627.2020.1752548 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tilija Pun, N., Jang, W. J. & Jeong, C. H. Role of autophagy in regulation of cancer cell death/apoptosis during anti-cancer therapy: Focus on autophagy flux Blockade. Arch. Pharm. Res.43, 475–488. 10.1007/s12272-020-01239-w (2020). [DOI] [PubMed] [Google Scholar]
- 39.He, C. & Klionsky, D. J. Regulation mechanisms and signaling pathways of autophagy. Annu. Rev. Genet.43, 67–93. 10.1146/annurev-genet-102808-114910 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen, C., Kapoor, A. & Iozzo, R. V. Methods for monitoring matrix-induced autophagy. Methods Mol. Biol.1952, 157–191. 10.1007/978-1-4939-9133-4_14 (2019). [DOI] [PubMed]
- 41.Kabeya, Y. et al. LC3, GABARAP and GATE16 localize to autophagosomal membrane depending on form-II formation. J. Cell. Sci.117, 2805–2812. 10.1242/jcs.01131 (2004). [DOI] [PubMed] [Google Scholar]
- 42.Vadlamudi, R. K., Joung, I., Strominger, J. L. & Shin, J. p62, a phosphotyrosine-independent ligand of the SH2 domain of p56lck, belongs to a new class of ubiquitin-binding proteins. J. Biol. Chem.271, 20235–20237. 10.1074/jbc.271.34.20235 (1996). [DOI] [PubMed] [Google Scholar]
- 43.Wu, Y. et al. p62/SQSTM1 accumulation due to degradation Inhibition and transcriptional activation plays a critical role in silica nanoparticle-induced airway inflammation via NF-κB activation. J. Nanobiotechnol.18, 77. 10.1186/s12951-020-00634-1 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xia, Y. et al. The macrophage-specific V-ATPase subunit ATP6V0D2 restricts inflammasome activation and bacterial infection by facilitating autophagosome-lysosome fusion. Autophagy15, 960–975. 10.1080/15548627.2019.1569916 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang, D. et al. Hyperoxia reduces STX17 expression and inhibits the autophagic flux in alveolar type II epithelial cells in newborn rats. Int. J. Mol. Med.46, 773–781. 10.3892/ijmm.2020.4617 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xie, Z. et al. Bafilomycin A1 inhibits autophagy and induces apoptosis in MG63 osteosarcoma cells. Mol. Med. Rep.10, 1103–1107. 10.3892/mmr.2014.2281 (2014). [DOI] [PubMed] [Google Scholar]
- 47.Rahman, M. A., Hwang, H., Nah, S. Y. & Rhim, H. Gintonin stimulates autophagic flux in primary cortical astrocytes. J. Ginseng Res.44, 67–78. 10.1016/j.jgr.2018.08.004 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stempels, F. C. et al. Novel and conventional inhibitors of canonical autophagy differently affect LC3-associated phagocytosis. FEBS Lett.596, 491–509. 10.1002/1873-3468.14280 (2022). [DOI] [PubMed] [Google Scholar]
- 49.Salih, D. A. & Brunet, A. FoxO transcription factors in the maintenance of cellular homeostasis during aging. Curr. Opin. Cell. Biol.20, 126–136. 10.1016/j.ceb.2008.02.005 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martins, R., Lithgow, G. J. & Link, W. Long live FOXO: Unraveling the role of FOXO proteins in aging and longevity. Aging Cell.15, 196–207. 10.1111/acel.12427 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang, M. et al. Forkhead box o3a suppresses lipopolysaccharide-stimulated proliferation and inflammation in fibroblast-like synoviocytes through regulating tripartite motif-containing protein 3. J. Cell. Physiol.234, 20139–20148. 10.1002/jcp.28615 (2019). [DOI] [PubMed] [Google Scholar]
- 52.Lin, L., Hron, J. D. & Peng, S. L. Regulation of NF-kappaB, Th activation, and autoinflammation by the forkhead transcription factor Foxo3a. Immunity21, 203–213. 10.1016/j.immuni.2004.06.016 (2004). [DOI] [PubMed] [Google Scholar]
- 53.Levine, B., Mizushima, N. & Virgin, H. W. Autophagy in immunity and inflammation. Nature469, 323–335. 10.1038/nature09782 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vomero, M. et al. Autophagy and rheumatoid arthritis: Current knowledges and future perspectives. Front. Immunol.9, 1577. 10.3389/fimmu.2018.01577 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Menzies, F. M., Fleming, A. & Rubinsztein, D. C. Compromised autophagy and neurodegenerative diseases. Nat. Rev. Neurosci.16, 345–357. 10.1038/nrn3961 (2015). [DOI] [PubMed] [Google Scholar]
- 56.Lee, J. H. et al. Advanced maternal age deteriorates the developmental competence of vitrified oocytes in mice. Cells. 10.3390/cells10061563 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is provided within the manuscript or supplementary information files.