Abstract
Asymmetric distribution of phospholipids is ubiquitous in the plasma membranes of many eukaryotic cells. The majority of the aminophospholipids are located in the inner leaflet whereas the cholinephospholipids are localized predominantly in the outer leaflet. Several functional roles for asymmetric phospholipid distribution in plasma membranes have been suggested. Disruption of lipid asymmetry creates a procoagulant surface on platelets and serves as a trigger for macrophage recognition of apoptotic cells. Furthermore, the dynamic process of phospholipid translocation regulates important cellular events such as membrane budding and endocytosis. In the present study, we used the red cell membrane as the model system to explore the contribution of phospholipid asymmetry to the maintenance of membrane mechanical properties. We prepared two different types of membranes in terms of their phospholipid distribution, one in which phospholipids were scrambled and the other in which the asymmetric distribution of phospholipids was maintained and quantitated their mechanical properties. We documented that maintenance of asymmetric distribution of phospholipids resulted in improved membrane mechanical stability. The greater difficulty in extracting the spectrin–actin complex at low-ionic strength from the membranes with asymmetric phospholipid distribution further suggested the involvement of interactions between aminophospholipids in the inner leaflet and skeletal proteins in modulating mechanical stability of the red cell membrane. These findings have enabled us to document a functional role of lipid asymmetry in regulating membrane material properties.
Asymmetric distribution of phospholipids is ubiquitous in the plasma membranes of many eukaryotic cells. The majority of the aminophospholipids, phosphatidylserine (PS) and phosphatidylethanolamine (PE), are located in the inner leaflet whereas the cholinephospholipids, phosphatidylcholine and sphingomyelin, are localized predominantly in the outer leaflet (1, 2). At least three distinct activities are involved in the regulation of membrane lipid sidedness. The MgATP-dependent aminophospholipid translocase, (also known as flippase or ATPase II; ref. 3), is responsible for localization of PS and PE in the inner leaflet by rapidly translocating them from the outer to inner leaflet against the electrochemical gradient (4–6). PS is a better substrate than PE for the aminophospholipid translocase (7–9), and the stoichiometry between aminophospholipid translocation and ATP hydrolysis is close to one (10). The activity of the aminophospholipid translocase is inhibited by high concentrations of Ca2+ (11–13), by the pseudosubstrates of P-type ATPase such as vanadate (14), acetyl phosphate, and p-nitrophenyl phosphate (15), and by sulfhydryl reactive reagents such as N-ethylmaleimide (16) and pyridyldithioethylamine (PDA), a specific inhibitor of phospholipid translocase (16–19). The less specific MgATP-requiring phospholipid translocase, floppase, moves phospholipids from the inner to outer leaflet (20–23). This outward lipid movement is much slower than the inward movement of PS and PE by the aminophospholipid translocase. In conjunction with these two ATP-requiring transporters, the MgATP-independent Ca2+-dependent scramblase facilitates bidirectional transbilayer movement of all phospholipids, resulting in a collapse of phospholipid asymmetry (24–26). Indeed, a high Ca2+ concentration can disrupt phospholipid asymmetry by activation of scramblase and by inhibition of aminophospholipid translocase. It is generally accepted that, under physiological conditions (cytosolic MgATP concentration in the mM range and Ca2+ in the submicromolar range), phospholipid asymmetry of biological membranes is maintained through the activation of the aminophospholipid translocase and floppase and inactivation of the scramblase. Under these conditions, all phospholipids are slowly but continuously moved to the outer leaflet by floppase, whereas the aminophospholipids PS and PE are immediately shuttled back to the inner leaflet by aminophospholipid translocase.
Several functional roles for asymmetric phospholipid distribution in plasma membranes have been suggested. For instance, several regulatory and structural proteins including protein kinase C (27), annexin (28), and membrane skeletal proteins, such as spectrin (29, 30), appear to localize to the cytoplasmic face of the membrane through their interaction with PS. On the other hand, disruption of lipid asymmetry leading to exposure of PS on the outer surface of the plasma membrane, creates a procoagulant surface on platelets (2, 31–33) and serves as a trigger for macrophage recognition of apoptotic cells (34–36). Furthermore, the dynamic process of phospholipid translocation can play an important role in cellular events such as membrane budding and endocytosis (2).
In the present study, we used the well characterized red cell membrane as a model system to explore the contribution of phospholipid asymmetry to the maintenance of membrane mechanical properties. We chose the red cell membrane as the model system for two important reasons: (i) the mechanical properties of deformability and stability of the red cell membrane can be quantitatively assayed (37) and (ii) the asymmetric distribution of phospholipids in human red cells has been well documented. Red cell membrane mechanical properties have shown to be regulated primarily by the spectrin-based submembranous skeletal network (38) through lateral interactions among skeletal proteins and vertical interactions between skeletal components and transmembrane proteins in the bilayer (39, 40). Posttranslational modifications of these proteins, such as phosphorylation (41), and various intracellular constituents, such as Ca2+ and calmodulin (42), have also been shown to regulate membrane mechanical properties by modulating protein–protein interactions. However, a role for lipid–protein interactions in regulating membrane mechanical properties has not been defined. In the present study, we prepared two different types of membranes in terms of their phospholipid distribution, one in which phospholipids were scrambled and the other in which the asymmetric distribution of phospholipids was maintained, and quantitated their mechanical properties. We documented that maintenance of asymmetric distribution of phospholipids resulted in improved membrane mechanical stability. Moreover, the spectrin–actin complex was more difficult to extract at low-ionic strength from membranes with asymmetric phospholipid distribution, further suggesting a role for inner leaflet aminophospholipid/skeletal protein interactions in modulating red cell membrane mechanical stability. These findings have enabled us to show that association of the membrane skeletal network with the lipid bilayer contributes to red cell membrane function and thus enabled us to document a functional role of lipid asymmetry in modulating membrane material properties.
Experimental Procedures
Materials.
ATP and AMPPNP (Sigma) were dissolved in deionized water to a final concentration of 60 mM and the pH was adjusted to 7.4 with Tris. These solutions were mixed with an equal volume of 100 mM MgCl2 and kept at −80°C. Vanadate (Na3VO4) was dissolved in deionized water to a final concentration of 0.1 M. This stock solution of vanadate was diluted to 0.1 mM in 5 mM Tris⋅HCl (pH 7.4) containing 5 mM KCl (5T5K buffer). PDA was synthesized as previously described (14, 15). Ouabain, FITC-conjugated annexin V, and PS (di-lauroyl PS) were purchased from Sigma, MBL (Nagoya, Japan), and Avanti Polar-Lipids Inc., respectively. Diisopropyl fluorophosphate was obtained from Wako Pure Chemical, Osaka.
Preparation of Resealed Ghosts.
After obtaining informed consent, blood was obtained from healthy human volunteers and used in all of the studies outlined. Red cells were collected and washed three times with 10 mM Tris⋅HCl buffer (pH 7.4) containing 120 mM KCl. Intact cells were lysed and washed three times in 35 vol of 5T5K buffer with or without 0.6 mM MgATP plus 1.0 mM MgCl2 or 0.6 mM MgAMPPNP plus 1.0 mM MgCl2 at 4°C. To restore isotonicity, a small volume of a concentrated solution of KCl, MgCl2, and DTT was added to the membrane suspension, to obtain final concentrations of 150, 1.6, and 1 mM, respectively. In some experiments, MgATP at different concentrations (0–0.8 mM) and various ATPase inhibitors (vanadate, PDA, EGTA, and ouabain) were added to the suspension. The ghost suspension was incubated at 37°C for 40 min for membrane resealing. In the studies outlined, ghosts are designated according to the additive in the lysing and resealing buffers during ghost preparation. For example, MgATP-ghosts or MgAMPPNP-ghosts are those prepared in the presence of MgATP or MgAMPPNP throughout the process (in both lysing and resealing buffers), respectively. On the other hand, control-ghosts are prepared in the absence of MgATP. These ghosts were white, whereas the MgATP-ghosts and MgAMPPNP-ghosts retained a pink hue, indicative of a small amount of membrane-associated hemoglobin. The morphology of unfixed resealed ghosts was examined by dark-field light microscopy using a Nikon microscope (Optiphot-2).
Annexin V Binding to Resealed Ghosts.
The Ca2+-dependent binding of annexin V has been widely used to detect PS on the cell surface (43–45). Resealed ghosts bearing PS in the outer leaflet of the plasma membrane were identified as those binding FITC-labeled annexin V by using an Apoptosis Detection Kit (MBL). Briefly, 105 cells were washed once with PBS, resuspended in 85 μl of the binding buffer, and 10 μl of FITC-labeled annexin V solution was added. After incubation for 30 min at room temperature in the dark, the samples were transferred to ice and the sample volume brought to 0.5 ml with the binding buffer. Membrane-associated fluorescence was quantitated by using a EPICS XL/XL-MCL flow cytometer (Beckman Coulter), and the results were analyzed with system ii v3.0 software.
Measurements of Membrane Deformability and Mechanical Stability.
For assessing membrane deformability, resealed ghosts were suspended in stractan (viscosity of 22 centipoise, 290 mOsm) and examined by an ektacytometer as previously described (40). Briefly, the ghosts were subjected to linearly increasing shear stress, and changes in laser diffraction patterns were analyzed to derive the deformability index (DI). DI provides a measure of the ellipticity of deforming ghosts in the flow field. DI was measured as a function of applied shear stress up to 150 dynes/cm2. The rate of increase in DI is a measure of membrane deformability, and the maximal value of deformability index is a measure of the surface area of resealed ghosts.
To measure membrane mechanical stability, resealed ghosts were suspended in dextran (40,000 mol wt, 50% wt/vol) and examined by the ektacytometer as described previously (40). Briefly, ghosts were subjected to constant shear stress of 750 dynes/cm2, and changes in DI as function of time were recorded. As ghosts fragment and lose membrane surface, the DI value decreases with time. The rate at which DI decreases is a measure of the rate of membrane fragmentation, and hence, provides a quantitative measure of membrane mechanical stability.
Spectrin Extraction from Ghosts.
Erythrocyte ghosts were prepared by hypotonic lysis with either 5T5K buffer or the same buffer supplemented with 0.6 mM MgATP + 1.0 mM MgCl2, 0.6 mM MgATP + 1.0 mM MgCl2 + 10 μM vanadate or 1 mM MgAMPPNP + 1.0 mM MgCl2. After resealing, the ghosts were washed three times with the same original lysing buffer and subsequently incubated at 37°C for 30 min in 30-fold excess of 0.5 mM phosphate buffer (pH 8.0) with or without various additives. The membranes and the supernatant were separated by centrifugation at 40,000 × g at 4°C for 30 min. Proteins in the supernatant were analyzed by 9% SDS/PAGE and subsequently stained with Coomassie brilliant blue.
Spectrin Binding to PS-Loaded Inside-Out Vesicles (IOVs).
Spectrin dimer and IOVs were prepared as described previously (45). PS-loaded IOVs were prepared by the addition of 0.5 mg of di-lauroyl PS in small volume of chloroform/methanol(1:3 vol/vol) solution to 3 mg protein of IOVs on ice. The mixture was gently stirred during a 5-min incubation, and PS-loaded IOVs were collected by centrifugation using a sucrose cushion. As assayed by TLC, the PS content of PS-loaded IOVs was approximately twice that of native IOVs. IOVs or PS-loaded IOVs were mixed with equal volume of various concentrations of spectrin dimer in the low ionic buffer (0.5 mM phosphate buffer, pH 8.0). The IOVs were incubated with spectrin dimer for 90 min on ice and then centrifuged at 50,000 × g for 25 min in a Microfuge (Beckman) through an equal volume of 20% sucrose solution in the same buffer. The supernatant was carefully removed, and the membrane pellet was isolated and analyzed by 9% SDS/PAGE. Similar experiments were performed under isotonic conditions by using the isotonic buffer (5 mM phosphate buffer, pH 7.4, containing 130 mM KCl, 20 mM NaCl, 1 mM DTT, 0.02% NaN3, and 1 mM MgCl2). Protein concentrations were determined by the method of Lowry (46) by using BSA as a standard.
Results
Morphology and PS Localization in the Variously Resealed Ghosts.
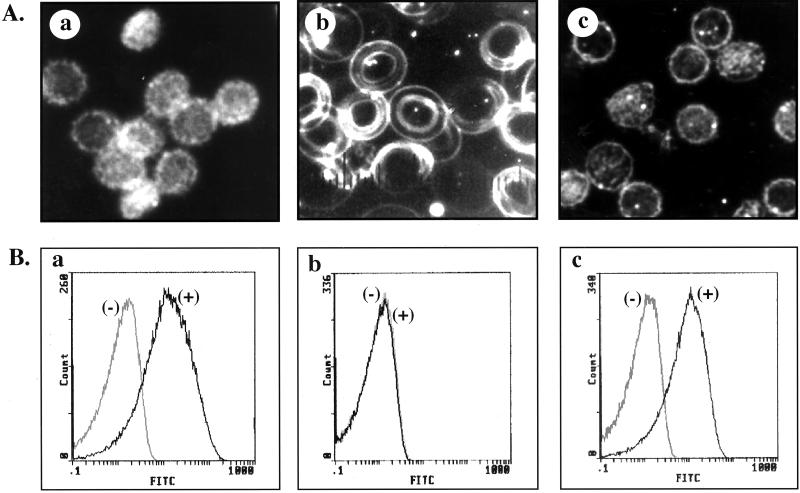
The morphology of control resealed ghosts prepared with no MgATP was echinocytic (Fig. 1Aa) whereas MgATP-ghosts exhibited discoid shapes (Fig. 1Ab). In contrast, MgATP-ghosts prepared in the presence of aminophospholipid translocase inhibitors, such as vanadate and PDA, displayed echinocytic morphology similar to that of control ghosts prepared in the absence of MgATP (data not shown). Importantly, MgAMPPNP-ghosts prepared by using the unhydrolyzable analogue of MgATP were also echincoytic (Fig. 1Ac). These findings imply that MgATP-dependent activation of aminophospholipid translocase is necessary for the maintenance of normal discoid shape.
Figure 1.
The morphology and the binding of FITC-labeled annexin V to various resealed membrane preparations. (A) Dark-field light microscopy of control ghosts (a), MgATP-ghosts (b), and MgAMPPNP-ghosts (c). Control and MgAMPPNP-ghosts are echinocytic whereas MgATP ghosts are discocytic. (B) Representative cytofluorograph histograms of various resealed membrane preparations after binding of FITC-labeled annexin V. Histograms in gray represent background fluorescence whereas histograms in black represent specific binding. Control ghosts (a) and MgAMPPNP-ghosts (c) exhibit significant binding of annexin V whereas there is no binding of annexin V to MgATP-ghosts (b).
To examine PS exposure on the outer surface of the various ghost preparations, the resealed membrane preparations were subjected to flow cytometric analysis by using FITC-labeled annexin V, which specifically binds to PS (Fig. 1B). The marked increase in membrane-associated fluorescence (shift to the right of fluorescence histograms) of control ghosts (Fig. 1Ba) and of MgAMPPNP-ghosts (Fig. 1Bc) compared with background fluorescence implies extensive binding of annexin V to the outer surface of both these resealed membrane preparations. In marked contrast, the fluorescence histogram for MgATP ghosts was indistinguishable from that of background fluorescence, implying no binding of annexin V to these membranes (Fig. 1Bb). These data demonstrate that significant amounts (approximately 50%) of PS translocates from the inner leaflet to the outer leaflet because of loss of aminophospholipid translocase activity in ghosts prepared in the absence of MgATP or in the presence of unhydrolyzable analogue of MgATP. Importantly, PS is restricted to the inner leaflet in the MgATP ghosts because of continual maintenance of aminophospholipid translocase activity during the membrane preparation. The involvement of aminophospholipid translocase in maintaining phospholipid asymmetry was further confirmed by the fact that PS translocated to the outer leaflet in MgATP ghosts in the presence of PDA (data not shown).
Mechanical Properties of the Variously Resealed Ghosts.
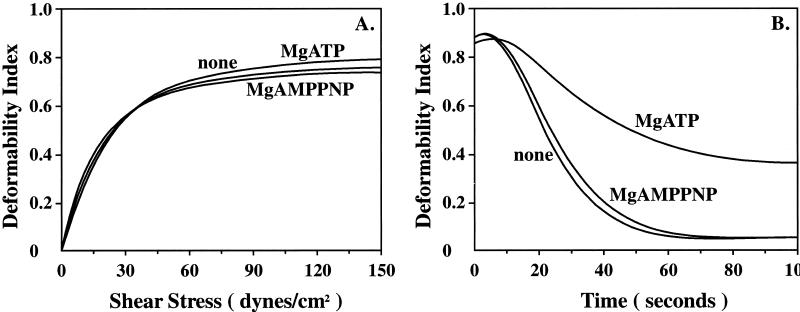
Representative membrane deformability profiles of control-, MgAMPPNP-, and MgATP-ghosts are shown in Fig. 2A. When control ghosts were subjected to linearly increasing values of applied shear stress, the deformability index value increased, reaching a maximum value of 0.8. The deformability profiles of MgATP-ghosts and MgAMPPNP-ghosts were indistinguishable from that of control ghosts. Because the initial slope of the deformability profile is a measure of membrane deformability and the maximum value of deformability index attained is a measure of membrane surface area, these findings imply that the three membrane preparations are indistinguishable in terms of their membrane deformability and their membrane surface area.
Figure 2.
Membrane mechanical properties of various resealed membrane preparations. Membrane deformability (A) and mechanical stability (B) were measured by using an ektacytometer. (A) The membrane deformability profile of MgATP-ghosts is virtually the same as that of control-ghosts and MgAMPPNP-ghosts. (B) The rate of decrease in deformability index of MgATP-ghosts was slower than that for control ghosts, implying increased membrane mechanical stability of MgATP ghosts. In marked contrast, fragmentation pattern of MgAMPPNP-ghosts was the same as that of control ghosts.
Representative membrane mechanical stability profiles of control-, MgAMPPNP-, and MgATP-ghosts are shown in Fig. 2B. When control ghosts were subjected to a constant high value of applied shear stress (750 dynes/cm2), the membranes were rapidly maximally deformed but underwent fragmentation as a function of time resulting in a time-dependent decrease in the deformability index value. The rate of deformability index decrease is a direct measure of membrane mechanical stability. Whereas MgAMPPNP-ghosts fragmented at a rate very similar to that of control ghosts, MgATP-ghosts prepared with 0.6 mM MgATP throughout membrane preparation fragmented at a slower rate. The time required for the deformability index value to reach half of its maximal intial value (t1/2), a quantitative measure of membrane mechanical stability, was increased by two fold for MgATP-ghosts compared with control ghosts. These findings imply that ATP hydrolysis plays a role in modulating membrane mechanical stability. It is important to note that maintenance of membrane stability requires continuous hydrolysis of ATP because removal of MgATP from the lysing buffer at any stage during the washing of the membranes eliminated the beneficial effect of MgATP on membrane mechanical stability (data not shown). These findings imply an important role for aminophospholipid translocase in modulation of membrane stability. To examine the contribution of Ca2+-activated scramblase to the observed membrane changes, ghosts were prepared with MgATP in the presence of 0.1 mM EGTA to chelate Ca2+ during both the lysing and resealing steps. Membrane stability of these ghosts was identical to that of membranes prepared without the chelator (data not shown), implying that scramblase does not contribute to the observed changes in membrane stability.
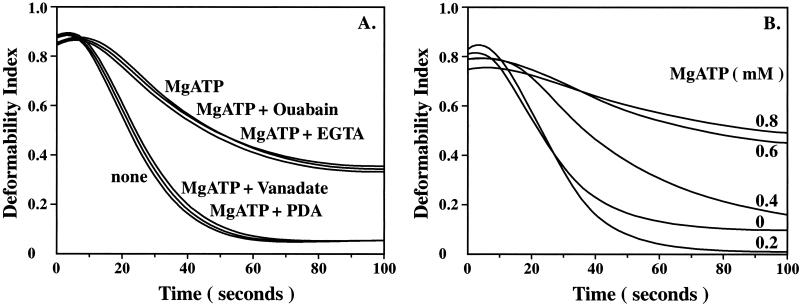
The effect of ATPase inhibitors in resealing buffer on membrane stability is shown in Fig. 3A. EGTA, an inhibitor of Ca2+-ATPase, and ouabain, an inhibitor of Na+K+-ATPase, had no effect on MgATP induced changes in membrane stability (Fig. 3A). In marked contrast, vanadate and PDA had significant effects on MgATP induced changes in membrane stability (Fig. 3A). Whereas MgATP increased the mechanical stability of red cell membranes, the stability of MgATP-ghosts prepared in the presence of 10 μM vanadate was similar to that of control ghosts. Vanadate by itself had no effect on the mechanical stability of control ghosts (data not shown). Importantly, PDA, a specific inhibitor of aminophospholipid translocase (14–16), also abrogated the beneficial effect of MgATP on membrane stability. These findings imply an important role for asymmetric distribution of aminophospholipids maintained by translocase in stabilizing red cell membranes.
Figure 3.
Effects of various ATPase inhibitors and of different MgATP concentrations on membrane mechanical stability of MgATP-ghosts. (A) The rate of decrease in the deformability index of MgATP-ghosts treated with either Ouabain (10 μM) or EGTA (0.1 mM) was the same as MgATP ghosts, implying that these two ATPase inhibitors had no effect on mechanical stability. In marked contrast, MgATP-ghosts treated with either Vanadate (10 μM) or PDA (10 mM) fragmented at the same rate as control ghosts and at a much faster rate than untreated MgATP ghosts, implying that these two ATPase inhibitors abolished the effect of MgATP on membrane mechanical stability. (B) Effect of various MgATP concentrations (0.0 to 0.8 mM) on membrane mechanical stability. The rate of decrease in the deformability index decreased with increasing concentrations of MgATP.
The effect of MgATP concentration (0 to 0.8 mM) on membrane mechanical stability is illustrated in Fig. 3B. A concentration-dependent increase in membrane stability, reflected by decreased rates of decline in deformability index, was evident. Maximal effect on mechanical stability was noted at MgATP concentration of 0.6 mM. These findings imply that membrane stability is modulated by MgATP at a physiologically relevant concentration.
Association of Spectrin with the Inner Leaflet of the Lipid Bilayer in the Various Membrane Preparations.
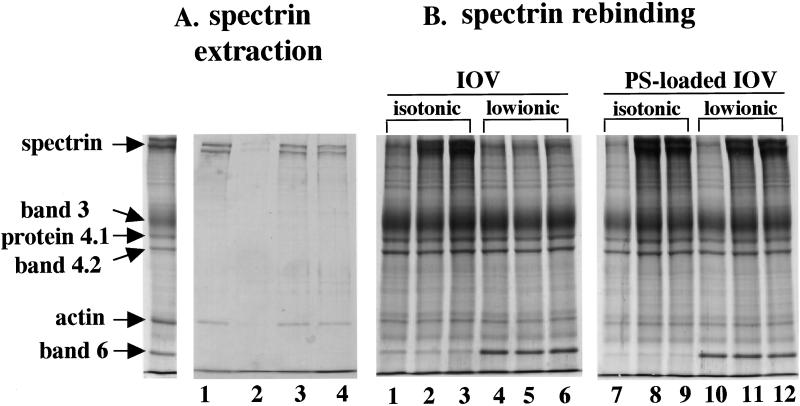
To obtain insights into the molecular mechanism by which asymmetric distribution of aminophospholipids can regulate membrane stability, we explored the interactions between the spectrin–actin complex and the inner leaflet of the bilayer in various membrane preparations. The spectrin–actin complex could be readily extracted by low-ionic strength buffer from either control- or MgATP-ghosts treated with vanadate (Fig. 4A). In marked contrast, the protein complex was inextractable from MgATP ghosts, implying altered association of the spectrin–actin complex with the inner monolayer of MgATP ghosts.
Figure 4.
Interaction of spectrin with membranes in various ghost preparations (A) Protein composition of low ionic buffer (0.5 mM phosphate buffer, pH 8.0) extracts from control ghosts (lane 1), MgATP-ghosts (lane 2), MgATP-ghosts with vanadate (lane 3), and MgAMPPNP-ghosts (lane 4). The far left lane shows the protein composition of native red cell membranes. Note spectrin and actin are extracted from the membranes of control, MgATP ghosts treated with vanadate and MgAMPPNP ghosts but not from MgATP ghosts. (B) Rebinding of purified spectrin dimer to IOVs and PS-loaded IOVs. Spectrin dimer at concentrations of 0.75 mg/ml (lanes 2, 5, 8, and 11) or 1.13 mg/ml (lanes 3, 6, 9, and 12) was added to 0.5 mg of IOVs or PS-loaded IOVs. Spectrin bound to IOVs in isotonic buffer (lanes 2 and 3) but not in low ionic buffer (lanes 5 and 6). However, spectrin bound to PS-loaded IOVs under both isotonic (lanes 8 and 9) and low ionic (lanes 11 and 12) conditions. The protein composition of native IOVs (lanes 1 and 4) and PS-loaded IOVs (lanes 7 and 10) before addition of spectrin are also shown. Note that band 6 remains bound to IOVs under low ionic conditions but is released from IOVs under isotonic conditions.
To further explore, the nature of an altered interaction between spectrin and PS in the inner monolayer of MgATP-ghost membranes, rebinding of spectrin to native IOVs and IOVs loaded with PS was examined (Fig. 4B). Spectrin bound to native IOVs under isotonic conditions but did not bind under hypotonic conditions. Surprisingly, spectrin bound to PS-loaded IOVs even under hypotonic conditions, indicating differences in the nature of spectrin binding to native IOVs mediated by protein–protein interactions and of spectrin binding to aminophospholipids in PS-loaded IOVs.
Discussion
In the present study,we identified a functional role for lipid asymmetry in modulating membrane biophysical properties. Specifically, we have shown that distribution of phospholipids can regulate mechanical stability of the red cell membrane. Furthermore, we have obtained biochemical evidence suggesting that this regulation of membrane properties is mediated by the interaction of skeletal proteins with aminophospholipids in the inner leaflet.
The ability to prepare red cell membranes in which the phospholipid asymmetry was either maintained or lost was key to the success of the present study. Asymmetric distribution of phospholipids in the membrane bilayer was maintained by using MgATP through the entire process of lysing red cells and of washing and resealing membranes. Whereas all phospholipids are slowly but continuously moved to the outer leaflet by floppase in these membranes, the aminophospholipids PS and PE are rapidly shuttled back to the inner leaflet by the aminophospholipid translocase, enabling the maintenance of asymmetric phospholipid distribution. The importance of aminophospholipid translocase was established in our studies by documenting loss of asymmetry in MgATP membranes after treatment with PDA, a specific inhibitor of aminophospholipid translocase. Membranes with scrambled phospholipid distribution were prepared by not providing MgATP to drive the aminophospholipid translocase during membrane preparation. In ghosts prepared in the absence of MgATP, phospholipids are scrambled during hypotonic lysis through direct connection between the inner and outer leaflets around the ruptured membrane, and the lack of aminophospholipid translocase activity prevents rapid redistribution of aminophospholipids back to the inner monolayer. This result is consistent with the previous report in which spin-labeled phospholipids were used to document loss of lipid asymmetry during hemolysis and subsequent resealing in the absence of MgATP (47, 48). The validity of the aminophospholipid distributions in the presence and absence of MgATP was confirmed in the current study by monitoring the localization of PS by using FITC-conjugated annexin V.
Assessment of the morphology and membrane material properties of the different resealed ghost preparations enabled us to show that asymmetric distribution of aminophospholipids has significant effects on cell shape and on membrane mechanical stability but not on membrane deformability. Ghosts that maintained their asymmetric lipid distribution had normal discoid morphology whereas ghosts in which asymmetric lipid distribution was lost exhibited echinocytic morphology. These findings are consistent with the earlier finding regarding the importance of MgATP in maintaining normal discoid shape (49). The mechanistic basis for the differences in the morphology of MgATP- and control-ghosts remains to be defined. Based on the documented differences in the nature of association of the skeletal network with the lipid bilayer in MgATP- and control-ghosts, we suggest that this differential association modulates the curvature of the lipid bilayer and hence regulate cell shape. In contrast to the differences in cell morphology, the membrane deformability of various resealed ghosts preparations was identical, implying that neither phospholipid asymmetry or cell shape influence the ability of red cell membranes to undergo extensive deformation. Membrane deformation thus appears to be primarily determined by the ability of the spectrin-based membrane skeleton to undergo extensive rearrangements.
The major finding of the present study is that membrane mechanical stability is modulated by the asymmetric distribution of phospholipids in the bilayer. The presence of MgATP throughout ghost preparation resulted in membranes that exhibited significantly increased resistance to deformation-induced fragmentation. Mg2+ by itself at mM concentrations has previously been proposed to stabilize red cell membranes (50). We also observed a small effect of MgCl2 on stability of ghost membranes, but the stablization was about an order of magnitude less than that seen with MgATP (data not shown). Furthermore, our finding that vanadate inhibited MgATP-induced membrane stabilization but had no effect on Mg2+-induced stabilization implies that the effect of MgATP on membrane stability that we documented is indeed due to MgATP and not Mg2+ alone.
The spectrin–actin network is linked to the bilayer through protein–protein interactions (spectrin-ankyrin-band 3 and spectrin-protein 4.1-glycophorin C interactions) as well as through direct association of spectrin with PS in the inner leaflet. We showed that, whereas significant amounts of the spectrin–actin complex could be extracted from control-ghost membranes into low ionic strength medium, only small amounts of the protein complex are extracted from MgATP-ghosts. This finding raises the possibility that the differential localization of PS in these two membrane preparations may account for the observed differences in spectrin–actin extractability. Because only spectrin–actin complex linked to the membrane through protein–protein interactions is likely to be dissociated by low ionic strength, we suggest that the differential extraction of spectrin–actin from control and MgATP ghosts can be accounted for by the binding of spectrin to PS in the MgATP ghosts. Strong support for this hypothesis comes from our finding of the tight association between spectrin and PS in PS-loaded IOV membranes under the conditions of low ionic strength.
For the red cell to fragment under applied fluid shear stress, the spectrin-based skeletal network has to be fragmented and dissociated from the lipid bilayer. We suggest that additional interactions of the skeletal network with the membrane through spectrin–PS interaction in MgATP ghosts enables these membranes to withstand high levels of applied shear stress by providing an additional stabilizing interaction between the skeletal network and the bilayer. Recently, we have shown that association of spectrin with chlorpromazine intercalated into the membrane lipid bilayer can increase membrane mechanical stability of red cell ghosts (51). This finding in conjunction with the findings of the present study lend strong support to our thesis that the interaction of skeletal proteins with the inner leaflet of the lipid bilayer can modulate membrane mechanical stability. In addition, the present findings have enabled us to document a functional role of lipids in regulating membrane material properties.
Acknowledgments
We are grateful to Ms. Miyama N. and Ms. Okubo M. for technical assistant and Dr. Horikawa H. for preparation of PDA. This work was supported in part by Grants-in-Aid for Scientific Research 10877022 and 10671388 from the Ministry of Education of JAPAN, and by National Institutes of Health Grant DK26263.
Abbreviations
- PS
phosphatidylserine
- PE
phosphatidylethanolamine
- PDA
pyridyldithioethylamine
- DI
deformability index
- IOV
inside-out vesicle
References
- 1.Devaux PF. Biochemistry. 1991;30:1163–1173. doi: 10.1021/bi00219a001. [DOI] [PubMed] [Google Scholar]
- 2.Williamson P, Schlegel R A. Mol Membr Biol. 1994;11:199–216. doi: 10.3109/09687689409160430. [DOI] [PubMed] [Google Scholar]
- 3.Tang X, Halleck M S, Schlegel R A, Williamson P. Science. 1996;272:1495–1497. doi: 10.1126/science.272.5267.1495. [DOI] [PubMed] [Google Scholar]
- 4.Devaux PF. Annu Rev Biophys Biomol Struct. 1992;21:417–439. doi: 10.1146/annurev.bb.21.060192.002221. [DOI] [PubMed] [Google Scholar]
- 5.Zwaal R F A, Schroit A J. Blood. 1997;89:1121–1132. [PubMed] [Google Scholar]
- 6.Bevers E M, Comfurius P, Dekkers D W C, Harmsma M, Zwaal R F A. Biol Chem. 1998;379:973–986. [PubMed] [Google Scholar]
- 7.Zachowski A, Favre E, Cribier S, Herve P, Devaux P F. Biochemistry. 1986;25:2585–2590. doi: 10.1021/bi00357a046. [DOI] [PubMed] [Google Scholar]
- 8.Auland M E, Roufogalis B D, Devaux P F, Zachowski A. Proc Natl Acad Sci USA. 1994;91:10938–10942. doi: 10.1073/pnas.91.23.10938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ding J, Wu Z, Crider B P, Ma Y, Li X, Slaughter C, Gong L, Xie X-S. J Biol Chem. 2000;275:23378–23386. doi: 10.1074/jbc.M910319199. [DOI] [PubMed] [Google Scholar]
- 10.Beleznay Z, Zachowski A, Devaux P F, Navazo P, Ott P. Biochemistry. 1993;32:3146–3152. doi: 10.1021/bi00063a029. [DOI] [PubMed] [Google Scholar]
- 11.Williamson P, Algarin L, Bateman J, Cho H-R, Schlegel R A. J Cell Physiol. 1985;123:209–214. doi: 10.1002/jcp.1041230209. [DOI] [PubMed] [Google Scholar]
- 12.Bitbol M, Fellman P, Zachowski A, Devaux P F. Biochim Biophys Acta. 1987;904:268–282. doi: 10.1016/0005-2736(87)90376-2. [DOI] [PubMed] [Google Scholar]
- 13.Williamson P, Kulick A, Zachowski A, Schlegel R A, Devaux P F. Biochemistry. 1992;31:6355–6360. doi: 10.1021/bi00142a027. [DOI] [PubMed] [Google Scholar]
- 14.Seigneuret M, Devaux P F. Proc Natl Acad Sci USA. 1984;81:3751–3755. doi: 10.1073/pnas.81.12.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beleznay Z, Zachowski A, Devaux P F, Navazo P, Ott P. Eur J Biochem. 1997;243:58–65. doi: 10.1111/j.1432-1033.1997.58_1a.x. [DOI] [PubMed] [Google Scholar]
- 16.Conner J, Schroit A J. Biochemistry. 1988;27:848–851. doi: 10.1021/bi00403a002. [DOI] [PubMed] [Google Scholar]
- 17.Daleke D L, Huestis W H. Biochemistry. 1985;24:5406–5416. doi: 10.1021/bi00341a019. [DOI] [PubMed] [Google Scholar]
- 18.Conner J, Schroit A J. Biochemistry. 1990;29:37–43. doi: 10.1021/bi00453a005. [DOI] [PubMed] [Google Scholar]
- 19.Conner J, Schroit A J. Biochim Biophys Acta. 1991;1066:37–42. doi: 10.1016/0005-2736(91)90247-6. [DOI] [PubMed] [Google Scholar]
- 20.Bitbol M, Devaux PF. Proc Natl Acad Sci USA. 1988;85:6783–6787. doi: 10.1073/pnas.85.18.6783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Conner J, Pak C H, Zwaal R F A, Schroit A J. J Biol Chem. 1992;267:19412–19417. [PubMed] [Google Scholar]
- 22.Conner J, Gillum K, Schroit A J. Biochim Biophys Acta. 1990;1025:82–86. doi: 10.1016/0005-2736(90)90193-r. [DOI] [PubMed] [Google Scholar]
- 23.Andrick C, Broring K, Deuticke B, Haest C W M. Biochim Biophys Acta. 1991;1064:235–241. doi: 10.1016/0005-2736(91)90307-t. [DOI] [PubMed] [Google Scholar]
- 24.Zhao J, Zhou Q, Wiedmer T, Sims P J. Biochemistry. 1998;37:6361–6366. doi: 10.1021/bi980218m. [DOI] [PubMed] [Google Scholar]
- 25.Zhou Q, Zhao J, Stout J G, Luhm R A, Wiedmer T, Sims P J. J Biol Chem. 1997;272:18240–18244. doi: 10.1074/jbc.272.29.18240. [DOI] [PubMed] [Google Scholar]
- 26.Woon L A, Holland J W, Kable E P W, Roufogalis B D. Cell Calcium. 1999;25:313–320. doi: 10.1054/ceca.1999.0029. [DOI] [PubMed] [Google Scholar]
- 27.Palfrey H C, Wasrrm A. J Biol Chem. 1985;260:16021–16029. [PubMed] [Google Scholar]
- 28.Meers P, Mealy T. Biochemistry. 1993;32:11711–11721. doi: 10.1021/bi00094a030. [DOI] [PubMed] [Google Scholar]
- 29.O'Toole P J, Wolfe C, Ladha S, Cherry R J. Biochim Biophys Acta. 1999;1419:64–70. doi: 10.1016/s0005-2736(99)00048-6. [DOI] [PubMed] [Google Scholar]
- 30.MacDonald RI. Biochemistry. 1993;32:6957–6964. doi: 10.1021/bi00078a021. [DOI] [PubMed] [Google Scholar]
- 31.Schroit A J, Zwaal R F A. Biochim Biophys Acta. 1991;1071:313–329. doi: 10.1016/0304-4157(91)90019-s. [DOI] [PubMed] [Google Scholar]
- 32.McEvoy L, Williamson P, Schlegel R A. Proc Natl Acad Sci USA. 1986;83:3311–3315. doi: 10.1073/pnas.83.10.3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pradhan D, Williamson P, Schlegel R A. Mol Membr Biol. 1994;11:181–188. doi: 10.3109/09687689409162237. [DOI] [PubMed] [Google Scholar]
- 34.Fadok V A, Voelker D R, Campbell P A, Cohen J J, Bratton D L, Henson P M. J Immunol. 1992;148:2207–2216. [PubMed] [Google Scholar]
- 35.Bratton D L, Fadok V A, Richter D A, Kailey J M, Guthrie L A, Henson P M. J Biol Chem. 1997;272:26159–26165. doi: 10.1074/jbc.272.42.26159. [DOI] [PubMed] [Google Scholar]
- 36.Shiratsuchi A, Umeda M, Ohba Y, Nakanishi Y. J Biol Chem. 1997;272:2354–2358. doi: 10.1074/jbc.272.4.2354. [DOI] [PubMed] [Google Scholar]
- 37.Chasis J A, Mohandas N. J Cell Biol. 1986;103:343–350. doi: 10.1083/jcb.103.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mohandas N, Chasis J A. Semin Hematol. 1993;30:171–192. [PubMed] [Google Scholar]
- 39.Low P S, Willardson B M, Mohandas N, Rossi M, Shohet S. Blood. 1991;77:1581–1586. [PubMed] [Google Scholar]
- 40.An X-L, Takakuwa Y, Nunomura W, Manno S, Mohandas N. J Biol Chem. 1996;271:33187–33191. doi: 10.1074/jbc.271.52.33187. [DOI] [PubMed] [Google Scholar]
- 41.Manno S, Takakuwa Y, Nagao K, Mohandas N. J Biol Chem. 1995;193:265–275. doi: 10.1074/jbc.270.10.5659. [DOI] [PubMed] [Google Scholar]
- 42.Takakuwa Y, Mohandas N. J Clin Invest. 1988;82:394–400. doi: 10.1172/JCI113611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Megli F M, Selvaggi M, Liemann S, Quagliariello E, Huber R. Biochemistry. 1998;37:10540–10546. doi: 10.1021/bi9801255. [DOI] [PubMed] [Google Scholar]
- 44.Geldwerth D, Helly D, de Jong K, Sabolovic D, Sestier C, Roger J, Pons J-N, Freyssinet J-M, Devaux P F, Kuypers F A. Biochem Biophys Res Commun. 1999;258:199–203. doi: 10.1006/bbrc.1999.0620. [DOI] [PubMed] [Google Scholar]
- 45.Tyler J M, Reinhardt B N, Branton D. J Biol Chem. 1980;255:7034–7039. [PubMed] [Google Scholar]
- 46.Lowry O H, Rosebrough N J, Farr A L, Randal R J. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 47.Schrier S L, Zachowski A, Herve P, Kader J-C, Devaux P F. Biochim Biophys Acta. 1992;1105:170–176. doi: 10.1016/0005-2736(92)90176-m. [DOI] [PubMed] [Google Scholar]
- 48.Verhoven B, Schlegel R A, Williamson P. Biochim Biophys Acta. 1992;1104:15–23. doi: 10.1016/0005-2736(92)90126-7. [DOI] [PubMed] [Google Scholar]
- 49.Nakao M, Nakao T, Yamazoe S. Nature (London) 1960;187:945–946. doi: 10.1038/187945a0. [DOI] [PubMed] [Google Scholar]
- 50.Beaven G H, Parmar J, Nash G B, Bennett P M, Gratzer W B. J Membr Biol. 1990;118:254–257. doi: 10.1007/BF01868609. [DOI] [PubMed] [Google Scholar]
- 51.Enomoto A, Takakuwa Y, Manno S, Tanaka A, Mohandas N. Biochim Biophys Acta. 2001;1512:285–290. doi: 10.1016/s0005-2736(01)00329-7. [DOI] [PubMed] [Google Scholar]