Abstract
Background
The glymphatic system is a recently discovered brain-wide clearance system that allows the cerebrospinal fluid (CSF) flow to clear metabolic waste, but the tools for the quantitative and non-invasive investigation of its function and activity especially in humans is lacking, hindering studies on glymphatic system physiology and therapeutic potential of glymphatic drug delivery and modulation. We postulated that albumin-binding radiotracers could be used to this end by binding to the endogenous protein in CSF, constituting a macromolecular, biological radiotracer, allowing for the visualization of CSF flow in the central nervous system non-invasively with positron emission tomography (PET).
Results
We prepared three albumin-binding tracers based on 4-(p-iodophenyl)butyric acid and truncated Evans Blue radiolabeled with gallium-68 using the NODAGA chelator for in vivo radiolabeling of CSF albumin, and an in vitro radiolabeled reference tracer Al[18F]F-RESCA-rat serum albumin (RSA) with high radiochemical yield and purity, and acceptable molar activity (Am). The biological evaluation of the tracers showed high radiolabel stability and rapid binding with albumin in vitro and in vivo with the biological half-life in Swiss mice after intravenous administration matching serum albumin (> 18 h). Dynamic PET imaging in female Sprague Dawley rats under ketamine/dexmedetomidine anesthesia after lumbar and intracisternal infusion showed distribution of the tracers towards intracranial space and along the spinal canal from the infusion site. However, the cervical lymph nodes were only visualized after the infusion of Al[18F]F-RESCA-RSA, characteristic for macromolecular tracers, indicating that the gallium-68-labeled tracers did not bind fully to endogenous CSF albumin in vivo, but were distributing to different brain areas according to their physicochemical properties.
Conclusions
While the relatively low molar activity (Am) of the [68Ga]Ga-NODAGA complex achieved in our setup combined with the limited amount of endogenous albumin at the infusion site (0.012–0.024 nmol) resulted in residual unbound tracer in the rat CSF in vivo, the tracers, especially the Al[18F]F-RESCA-RSA show promise for tracking CSF flow with PET, constituting the first tailored radiotracers to this end.
Supplementary Information
The online version contains supplementary material available at 10.1186/s41181-025-00365-4.
Keywords: Albumin-binding, Cerebrospinal fluid (CSF) flow, Glymphatic system, Gallium-68, Intrathecal, Positron emission tomography (PET)
Background
The glymphatic system is a cerebrospinal fluid (CSF) mediated waste clearance system in the brain (Iliff et al. 2012; Xie et al. 1979). Periarterial spaces surrounding the cerebral arteries allow the influx of CSF from the subarachnoid space into the brain, where the movement is facilitated by physiological pulsations. Perivascular spaces are surrounded by astrocytic end-feet occupied by aquaporin 4 (AQP4) water channels, contributing to the CSF influx (MacAulay 2021). In the brain parenchyma, CSF mixes with interstitial fluid (ISF), and flows through the brain parenchyma and is eventually drained to perivenous spaces and sinus-associated efflux routes (Rasmussen et al. 2022; Iliff et al. 2013; Hablitz and Nedergaard 2021; Nedergaard and Goldman 1979; Mestre et al. 1979).
Novel imaging tools are required to visualize the glymphatic function in vivo (Mestre et al. 2020; Eide and Ringstad 2019). Positron emission tomography (PET) provides minimally invasive, sensitive, and quantitative whole-body imaging with nanomolar concentrations, being suitable for low-volume intrathecal (i.t.) administration in rodents. PET imaging is also fully translational, which offers exciting opportunities for future clinical studies on the glymphatic system (Ziegler 2005; Schöder et al. 2003; Bolus et al. 2009). However, the use of PET for glymphatic CSF flow imaging has so far been limited, and PET tracer development for this application lacks behind (Benveniste et al. 2021).
Albumin is the dominant protein in plasma and CSF (Zorzi et al. 2019), and can serve as a carrier for radiotracers, making them macromolecular. Macromolecular tracers are needed to investigate the efflux routes and global changes of the CSF flow, when the diffusive properties of administered tracer to brain parenchyma is less significant. Small molecule albumin-binding tracers would reflect the behavior of the protein in CSF and potentially have greater translational potential than in vitro radiolabeled albumin, as the good manufacturing practice (GMP) production and related quality control (QC) is less complex than for biological molecules (Zorzi et al. 2019; Lau et al. 2019; Höltke et al. 2021).
In this study, we developed three radiotracers for the in vivo labeling of endogenous albumin in the CSF (Fig. 1), using two different albumin-binding moieties 4-(p-iodophenyl)butyric acid (IP), and truncated Evans Blue (EB) conjugated with 1,4,7-triazacyclononane-1-glutaric acid-4,7-acetic acid (NODAGA) chelator, suitable for radiolabeling with gallium-68 (t1/2 = 67.7 min, 100% ε, β+). Additionally, we developed a new in vitro fluorine-18-radiolabeled (t1/2 = 109.77 min, 96.7% β+) aluminum [18F]fluoride restrained complexing agent (RESCA) conjugated rat serum albumin (RSA) reference tracer.
Fig. 1.
(1) The intrathecally infused tracer binds to endogenous albumin in the CSF. (2) The tracer enters the brain with the CSF through periarterial spaces and enters the brain parenchyma through the gaps between astrocytic end-feet. (3) The tracer flows unidirectionally towards perivenous spaces, and (4) exits from the CNS through several outflow routes, such as lymphatic vessels. Figure modified from (Persson et al. 2022) under the CC BY 4.0 license. AQP4 = aquaporin 4, CSF = cerebrospinal fluid
Methods
Synthesis and radiolabeling
All synthetic and radiolabeling procedures, including reagents and instruments, are described in detail in the Supplementary Information (SI). The synthesis protocols, chemical structures of the synthesized 4-(p-iodophenyl)butyric acid (IP) and truncated Evans Blue (EB) precursors with the NODAGA chelator, the RESCA-RSA reference tracer, and the respective radiosynthesis methods are presented in Scheme 1.
Scheme 1.
Synthesis schemes, chemical structures, radiolabeling schemes, and yields of NODAGA-PEG3-IP (3), NODAGA-ethyl-IP (4), NODAGA-EB (6), and RESCA-RSA (7). RCY; radiochemical yield
In vitro evaluation
The logD values of the albumin-binders were determined with the shake-flask method to observe the distribution of radiotracer between aqueous (0.2 M PBS, pH 7.4) and organic (1-octanol) phases. The radiolabeled tracers [68 Ga]Ga-3, [68 Ga]Ga-4, and [68 Ga]Ga-6 were shaken mechanically at 500 rpm in 1:1 mixture of the aqueous and organic phases for 1 h and centrifuged for 15 min at 1000 rpm (n = 3 per tracer). Samples from each layer (200 μl) were measured with a gamma counter (Wallac Wizard 3″, PerkinElmer Life Sciences, Waltham, MA, USA). The LogD value was calculated as the ratio of radioactivity between the two layers.
The in vitro radiolabel stability assays of radiotracers [68 Ga]Ga-3, [68 Ga]Ga-4, [68 Ga]Ga-6, and Al[18F]F-7 were done in 100% mouse serum (Fisher Scientific, Waltham, MA, USA), 100% rat CSF, 2 mM EDTA, and 0.2 mM FeCl3 solutions (n = 3). The rat CSF was collected from four female Sprague Dawley rats, according to the previously reported protocol by Nirogi et al (Nirogi et al. 2009) and stored in a − 80 °C freezer prior to the assay. The radiolabeled tracers were dispersed in corresponding media (1–2 MBq/250 μl) and incubated at 37 °C. Samples (2 μl) were drawn at predetermined time points until the last time point of 6 h. The samples were analyzed using the corresponding radio-TLC quality control method for each tracer. The release of free radionuclide over time was quantified using photostimulated luminescence autoradiography.
The albumin-binding affinities for the in vivo albumin-binding tracers were investigated by incubating the radiotracers (25 μl, 1–2 MBq, 37 °C) at physiological CSF albumin concentration in PBS buffer pH 7.4 (0.15–0.35 g/l). Samples (5 μl) were drawn from the mixture every 5 min until the last time point of 1 h and analyzed with radio-SEC-HPLC (Supplemental Table 1) (n = 3).
Animals
Animal experiments were carried out under project license numbers ESAVI/9782/2022, ESAVI/36258/2020, and ESAVI/4935/2024 approved by the Regional State Administrative Agency of Southern Finland (Hämeenlinna, Finland), and compliance with the respective Finnish and EU regulations and guidelines. This study used female RjOrl:SWISS mice (aged 8–12 weeks, weighing 18–25 g, Janvier laboratories, Le Genest-Saint-Isle, France, n = 21) and female Hsd:Sprague–Dawley rats (aged 10–14 weeks, weighing 180–240 g, Envigo, Horst, the Netherlands, n = 48) group housed (four rats per cage and six mice per cage) in conventional polysulfone cages with aspen bedding material (Tapvei®, Harjumaa, Estonia) and enrichments (e.g., disposable cardboard hut and nesting material). Food pellets (Envigo Teklad Global Diet 2016) and tap water were provided ad libitum. The housing conditions were maintained at 12:12 h light/dark cycle, 22 ± 1 °C, and 55 ± 15% relative humidity. The sample sizes were decided based on previous studies on molecular imaging of CSF flow. All in vivo data were included in this study and no animals were excluded. No animals were lost during the experiments. The experiments were done unblinded, but analyses of the collected data were conducted blinded. Tracers were administered in randomized order from three tracer batches during different experimental days and the animals were taken from different cages.
In vivo and ex vivo biological evaluation in mice
Each radiotracer (2.5–3.5 MBq, 100 μl in 5% EtOH–0.9% NaCl) was administered individually to awake mice via the lateral tail vein (n = 3/radiotracer). At predetermined time points (15, 30, 60, 90, 120 min), blood (5–10 μl) was collected from the tail vein for biological half-life determination. The biological half-lives were calculated according to previously published article by Fan and Lannoy (Fan and Lannoy 2014). At a predetermined time point (15 min or 120 min), the animals were euthanized with carbon dioxide (CO2) asphyxiation and cervical dislocation. The collected blood samples and selected organs and tissues were weighed and analyzed with a gamma counter for ex vivo biodistribution. A larger (50–100 µl) blood sample that was collected via cardiac puncture for in vivo albumin-binding affinity determination. Briefly, the blood sample was collected into a tube containing 25 μl of 1% heparin in saline. The sample was centrifuged (1000 g, 10 min, 4 °C) to separate the plasma and the blood cells. The plasma fraction was collected to a new tube, and ice-cold acetonitrile (100 μl) was added to precipitate the proteins, after which the tube was centrifuged (4 °C, 10,000 rpm, 5 min). The collected fractions, including the supernatant containing acetonitrile, were analyzed with a gamma counter. Additionally, plasma, urine, and bile samples were analyzed with radio-HPLC (Supplemental Table 2) and radio-TLC using the corresponding QC method for in vivo stability. A 15-min static PET/CT (Molecubes β-CUBE for PET and X-CUBE for CT, Ghent, Belgium) was done under 3.5–4.5% isoflurane anesthesia in medical oxygen carrier (1 l/min) 15 min and 120 min after intravenous injection of tracers [68 Ga]Ga-3, [68 Ga]Ga-4, and [68 Ga]Ga-6 (n = 3/radiotracer).
Dynamic PET/CT imaging after intrathecal administration in rats
Each radiotracer was infused i.t. (cisterna magna: 26 μl, 0.5–1.5 MBq, 1.6 μl/min, lumbar: 30 μl, 0.5–1.5 MBq, 2 μl/min) to treatment-naive female Sprague–Dawley rats (n = 6/infusion site/tracer) under ketamine (100 mg/kg)-dexmedetomidine (0.5 mg/kg) anesthesia. The cisterna magna and lumbar cannulations were done as described by Xavier et al. (Xavier et al. 2018) and Størkson et al. (Størkson et al. 1996) respectively, with small modifications described in detail in the SI. After the cannulation, the rats were transferred to the imaging bed, and a 100-μl Hamilton microsyringe (Hamilton Company) was placed on an infusion pump (Phd Ultra Harward Apparatus) was connected to the catheters via PE-10 tubing and a 30G dental needle (Sofic, France). Body temperature and respiration rates were maintained and monitored throughout the experiment. The rats were positioned in the bed so that their head was supported by the frame, front paws were on both sides of the head, and hind paws were pointing back. Fixation devices were not used apart from the head frame. The used PET system has a resolution of 850 mm, which is achieved through the 5-ring combination of monolithic scintillators and GPU-based event positioning, energy resolution of 12%, and a 13-cm field-of-view (FOV), which was placed from the head down, covering the brain and the upper spinal cord. Additionally, a 5-min static PET scan on the lumbar region was conducted after the 120-min dynamic imaging to cover the lumbar infusion site. A whole-body computed tomography (CT) scan was done after the PET imaging. PET/CT images were analyzed with the VivoQuant (InviCRO LLC, Needham, MA, USA, version 4.0.0). The 120-min dynamic PET scans were reconstructed to 24×5 min frames, resulting in a final voxel dimension of 0.4×0.4×0.4 mm. The injected dose for each administration was calculated by measuring the activity of the vial with a dose calibrator before and after the tracer injection, and subtracting the activity left inside the tubing after the scan. The time-activity-curves (TACs) were determined based on the chosen regions of interest (ROIs) including the liver, heart, spinal canal, intracranial space, striatum, cortex, hippocampus, cisterna magna, lymph nodes, and processed as standardized uptake values (SUVs). A magnetic resonance imaging (MRI)-derived rat brain template was co-registered to the reference CT to enable the localization of brain regions (cortex, hippocampus, and striatum).
Statistical methods
The statistical comparisons between the tracers for in vivo albumin binding affinity experiments in mice were done using repeated measures two-way ANOVA with Šídák's multiple comparisons test. For descriptive statistics and visualization of the time-activity curves (TAC), GraphPad Prism (version 10.3.1) was utilized. The standardized uptake values (SUVs) and in selected. Cases (intracranial space, hear) the percentages of injected dose (%ID) were plotted over time into the time activity curves (TACs) for analysis. The area under curves (AUC0-120), and maximum concentrations (Cmax) are presented as geometric means (GM) with coefficients of variation (CV), as recommended for pharmacokinetic data (Statistical approaches to establishing bioequivalence guidance for industry 2022; Statistical Guide for Clinical Pharmacology & Therapeutics 2010). The geometric mean ratios with 95% confidence interval (95% CI) were calculated, and two-tailed t-tests were performed on log-transformed variables. The data for time corresponding to Cmax (tmax) are presented with median and range and were compared using a two-tailed Mann–Whitney test. All the measurements are presented as mean ± standard deviation (SD). The p-values below 0.05 were considered significant.
Results
Synthesis and radiolabeling
All precursors were successfully synthesized in acceptable yields (Scheme 1). Precursors 3 and 4 were characterized with 1H NMR and 13C NMR (400 MHz Bruker Advance NEO NMR spectrometer, Karlsruhe, Germany) (Supplemental Fig. S1–S9), liquid chromatography-mass spectrometry (LC–MS; Supplemental Table 3), and high-resolution mass spectrometry (HR-MS; Agilent Technologies, Santa Clara, CA, USA). The characterization of precursor 6 was done with 1H NMR, 13C NMR, matrix-assister laser ionization desorption mass spectrometry (MALDI-MS; Axima Performance, Tokyo, Japan) and elemental analysis (Vario Microcube Analyzer, HANAU Elemental analyzing system GmbH, Germany) (Supplemental Fig. S8-S11, Supplemental Table 4). Precursor 7 was characterized with MALDI-MS (Supplemental Fig. S12).
Each precursor was successfully radiolabeled, and the decay-corrected radiochemical yields (RCY), radiochemical purities (RCP), and molar activities (Am) of each compound after radiosynthesis optimization are shown in Table 1. Representative radio-TLC and radio-HPLC results for the QC of each radiolabeled tracer are shown in Supplemental Table 5.
Table 1.
Radiolabeling results after radiosynthesis optimization and logD values
| Tracer [68 Ga]Ga-3 | Tracer [68 Ga]Ga-4 | Tracer [68 Ga]Ga-6 | Tracer Al[18F]F-7 | |
|---|---|---|---|---|
| RCY | 82 ± 1.5% | 80 ± 2.0% | 64 ± 2.2% | 96 ± 2.1% |
| RCP | 99.4 ± 0.5% | 99.5 ± 0.4% | 96.7 ± 2.5%* | 99.2 ± 0.4% |
| Am | 1.69 GBq/μmol ± 1.9% | 1.7 GBq/μmol ± 1.2% | 1.72 GBq/μmol ± 2.9% | 5.65 GBq/μmol ± 0.7% |
| LogD (octanol:PBS pH 7.4) | + 0.22 ± 0.08 | − 0.52 ± 0.05 | − 1.32 ± 0.03 | – |
*Only done with radio-TLC method
In vitro evaluation
The LogD values for each tracer are shown in Table 1. All radiolabeled tracers were stable in physiological media where the radiolabel complexes remained intact (> 97%) up to 6 h of incubation. Radio-SEC-HPLC for the albumin-binding affinity at physiological CSF albumin concentration (0.15–0.35 g/l) for the albumin-binders showed that all tracers were rapidly bound to albumin after 5 min of incubation, with no unbound tracer found within 1 h of incubation (Fig. 2).
Fig. 2.
The in vitro radiolabel stability assays of developed tracers up to 6 h of incubation in rat CSF, rat serum, 2 mM EDTA and 0.2 mM FeCl3. In vitro albumin-binding affinity assays in physiological rat CSF albumin concentration using radio-SEC. CSF = cerebrospinal fluid, RSA = rat serum albumin
In vivo and ex vivo biological evaluation in mice
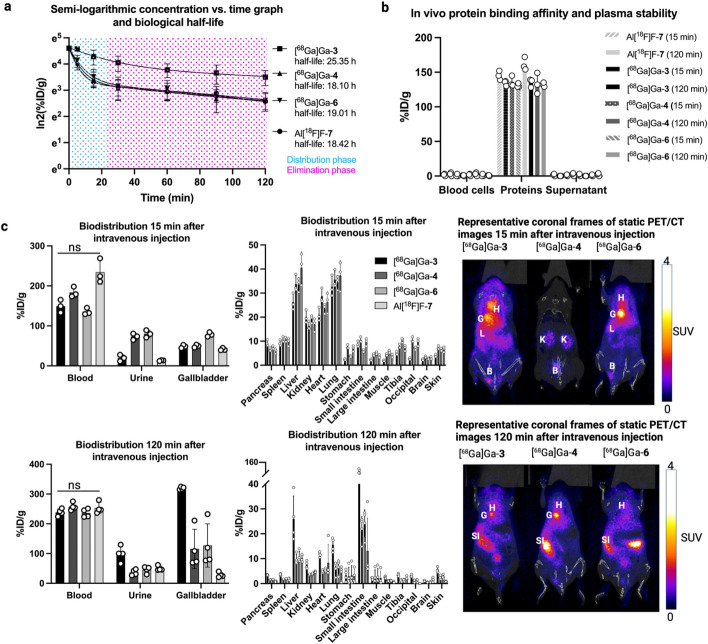
The biological half-lives of the albumin-binding tracers corresponded to that of albumin in mice (reported 18–24 h) (Nguyen et al. 2006) (Fig. 3a). The in vivo protein-binding assays showed that the activity was in the protein fraction when measured with a gamma counter (Fig. 3b). Ex vivo biodistribution studies showed that most of the activity stayed in blood after 120 min for all tracers with no statistical difference (p > 0.05). However, there was excretion to urine and bile (gallbladder) and uptake in major organs (liver, kidneys, heart, lung, small intestine), supported by the 15-min static PET/CT images (Fig. 3c). Radio-HPLC and radio-TLC analyses of plasma, urine, and bile showed that the metabolized activity was either the tracer or a metabolite with similar retention time. The activity in plasma was bound to protein (Supplemental Table 6), confirming a degree of rapid elimination of unbound tracer during the first 15 min post-injection and stable binding with serum albumin thereafter.
Fig. 3.
A summary of the in vivo and ex vivo results in mice after intravenous administration demonstrating the albumin-binding ability of the tracers. A The biological half-life of radiotracers in semi-logarithmic activity concentration versus time graph. B In vivo protein binding affinity and plasma stability of radiotracers determined from fractionated blood samples. C Ex vivo biodistribution at 15 min and 120 min after intravenous injection of radiotracers, and static PET/CT images of the albumin-binding tracers corresponding to the same time points. B = bladder, G = gallbladder, H = heart, K = kidney, L = liver, SI = small intestine
Dynamic PET/CT imaging after intrathecal administration in rats
Dynamic PET imaging after i.t. infusion (Figs. 4a and 5a) showed distribution of activity towards the ICS and spinal canal (Figs. 4c and 5c, Supplemental Fig. S13-S20). Liver uptake was observed with all the tracers (Fig. 4d: TAC 6, Fig. 5d: TAC 6) and less than 1%ID (Supplemental Fig. 21) was seen in the heart at 120 min, suggesting slow tracer elimination from CNS to circulation during the imaging. Lymph nodes could only be visualized with the reference tracer (Fig. 4d: TAC 4, and Fig. 5d: TAC 7). Due to limited FOV of the PET scanner, kidneys and the bladder were not included in from the images. In addition, the lumbar infusion site was not included to the FOV of dynamic imaging (Figs. 4b and 5b). The dynamic PET/CT movies are provided in Supplemental Movies 1–8.
Fig. 4.
CNS delivery of the tracers after cisterna magna infusion. A The experimental setup used for PET/CT imaging; B The regions of interest (ROI) used for the PET/CT analysis; C representative sagittal PET/CT frames representing 60 and 120 min timepoints combined with a transverse PET/CT slice from the brain. D The time-activity curves of chosen ROIs. CM = cisterna magna, CT = computed tomography, FOV = field of view, PET = positron emission tomography, TAC = time-activity curve. Created in BioRender, Peltoniemi, M. (2025) https://BioRender.com/rve3g4p
Fig. 5.
CNS delivery of the tracers after lumbar infusion. A The experimental setup used for PET/CT imaging; B The regions of interest (ROIs) used in the PET/CT data analysis; C Representative sagittal PET/CT frames for 60 min and 120 min timepoints; D The time-activity curves of chosen ROIs. CT = computed tomography, FOV = field of view, PET = positron emission tomography, TAC = time-activity curve. Created in BioRender. Peltoniemi, M. (2025) https://BioRender.com/7pb7yms
The reference tracer (Al[18F]F-7) was observed to leave the cisterna magna and lumbar infusion site faster compared to the albumin-binding tracers (cisterna magna: tmax − 7 = 20(0) min, tmax-3 = 32.50(15) min, tmax-4 = 30(10) min, tmax-6 = 30(10) min, Fig. 4d: TAC 1, and Supplemental Table 7) (lumbar: tmax−7 = 25(35) min, tmax−3 = 75(0) min, tmax−4 = 75(0) min, tmax−6 = 75(0) min, Fig. 5d: TAC 1, and Supplemental Table 8). After intracisternal infusion, the reference tracer reached higher concentrations in the spinal canal than the albumin-binding tracers (Cmax − 7 = 4.5(17%), Cmax−3 = 2.9(19%), Cmax−4 = 2.4(44%), Cmax−6 = 3.2(30%)) (Fig. 4d: TAC 3, and Supplemental Table 7). Similar behavior was observed after lumbar infusion, as larger concentration of the reference tracer reached the thoracic vertebrae (AUC0-120–7 = 710(11%), AUC0-120–3 = 360(11%), AUC0-120–4 = 354(9.2%), AUC0-120–6 = 370(20%)), cervical vertebrae (AUC0-120–7 = 37(47%), AUC0-120–3 = 2.1(16%), AUC0-120–4 = 4.4(21%), AUC0-120–6 = 4.2(18%)), and ICS (Cmax = 0.27%ID, Supplemental Fig. 21b) (AUC0-120–7 = 3.2(13%), AUC0-120–3 = 1.5(18%), AUC0-120–4 = 2.8(12%), AUC0-120–6 = 2.6(20%)) (Fig. 5d: TACs 2, 3, and 4, and Supplemental Table 8). Within the ICS, TACs showed distribution of all tracers towards the deeper brain areas, including cortex (Fig. 4d: TAC 7), hippocampus (Fig. 4d: TAC 8) and striatum (Fig. 4d: TAC 9). AUCs of [68 Ga]Ga-3 had significantly higher distribution to striatum compared to other tracers (AUC0-120–3 = 223(25%), AUC0-120–4 = 60(20%), AUC0-120–6 = 98(20%), AUC0-120–7 = 68(31%)) (Fig. 4d: TAC 9, Supplemental Table 7).
Discussion
In this work, we developed and evaluated three endogenous albumin-binding radiotracers and a rat serum albumin-based reference radiotracer for PET imaging of the glymphatic CSF flow. The IP-conjugated precursors were synthesized using two different linkers to evaluate the effects on the physiological properties of the tracer. The in vitro RSA-conjugated tracer Al[18F]F-7 was developed to be used as a reference to model the pharmacokinetics of albumin after i.t. administration since neither in vivo albumin-binding tracers nor PET imaging have been previously used to study the glymphatic flow.
The albumin-binding strategy and the employed albumin-binding moieties were chosen for several reasons. Albumin is the dominant protein in plasma, lymph, and CSF, and has been widely used as a carrier for small molecule drugs after intravenous administration due to its long biological half-life (19 days in humans), stability, non-toxicity, and lack of immunogenicity (Larsen et al. 2016). We postulated that using radiotracers binding to endogenous albumin could present several advantages over in vitro radiolabeled albumin conjugates (Kratz et al. 2000). First, as the use of commercial lyophilized isolated albumin is avoided, the albumin-binding imaging probe would truly reflect the biological distribution of the protein present in the CSF at physiologically relevant concentrations. Second, small-molecule radiotracers can be easy and inexpensive to synthesize with high Am and activity concentration, permitting the ultra-low volume (~30 μl) for i.t. infusion in rats, and the related QC can be simple and fast. Finally, the small-molecule radiotracers have greater translational potential, as the GMP production is well established and less complex compared to radiolabeled macromolecules (Vāvere and Scott 2017). In this study, the synthesis and characterization of IP-conjugated precursors 3 and 4 were more accessible and less complex than the EB-conjugated tracer 6, since only fast coupling reactions with high yields were employed. The precursor 6 was achieved with a lower yield, and the procedure included a diazonium salt intermediate, which is highly explosive and decomposes when heated. Additionally, the characterization was complex, and no MS or analytical HPLC results were obtained during this study despite multiple trials and adjustments.
Both IP and EB have shown high albumin-binding affinity in previous reports (Davis et al. 2022; Ganguly et al. 2021; Hausner et al. 2020; Wen et al. 2022; Chen et al. 2016), supported by the present study. Several albumin-binding experiments were done after intravenous injection, although the ultimate purpose was i.t. administration. This approach was justified, as the biological mechanism of action for albumin-binding remains the same, but blood is easier to sample than CSF. The high albumin-binding affinity seen in the in vitro studies was corroborated in in vivo studies, with the biological half-life of each tracer after the distribution phase correlating with that of albumin in mice (> 18 h)(Nguyen et al. 2006), which was confirmed by the analysis of blood fractions (blood cells, proteins, supernatant). Tracers [68 Ga]Ga-4 and [68 Ga]Ga-6 had similar behavior during biological half-life studies than reference tracer Al[18F]F-7, but tracer [68 Ga]Ga-3 had a significantly less steep distribution phase (Fig. 3a). Tracer [68 Ga]Ga-3 is the only tracer with a slightly positive logD value (0.22 ± 0.08), which can lead to a lipophilic nature and tracer interaction with cell membranes. All tracers were observed to follow two-compartment plasma concentration/time profiles and were plotted on a semi-logarithmic plot and appeared as a bi-phase decline with a straight line during the terminal-phase (Fan and Lannoy 2014; Jeong and Jusko 2022).
Radio-HPLC analysis revealed unbound tracer in urine and gallbladder 15 min after intravenous injection the tracers. Prior to our work, Niu et al. (Niu et al. 2014) developed a series of radiolabeled NOTA-EB (NEB) tracers and an in vitro radiolabeled mouse serum albumin (MSA) based tracer and reported rapid NEB clearance to urine immediately after intravenous injection before binding to albumin. Analogous to our findings, they reported activity in the circulation that stayed considerably higher during 120 min, and accumulation of the radiotracers to large blood volume organs such as the liver, kidneys, and spleen. In our research, the in vivo albumin-binding tracers behaved similarly to the in vitro radiolabeled RSA in circulation, aligning with the previously published results with NEB. In contrast, Niu and co-workers reported higher liver and spleen uptake with in vitro radiolabeled MSA (Niu et al. 2014), which was not observed in our study with the RSA based reference tracer. However, in their discussion, they laid out the possibility that the in vitro labeling process might have compromised the structure of albumin, leading to the differing results. In our study, MALDI-MS analysis revealed the average molecular weight of 70 787 g/mol (Supplemental Fig. 12) for Al[18F]F-7, translating to an average of 8 chelators conjugated with the RSA (MW of RESCA 584.52 g/mol).
The use of dynamic in vivo PET imaging enables direct quantification of the whole-body tracer distribution, and due to its high sensitivity, translationality, and extensive tracer selection, it is a fascinating approach for future research on the glymphatic system, especially in humans. Previous in vivo data of the biodistribution of i.t. infused drugs have mainly been collected using optical microscopy (Iliff et al. 2012; Xie et al. 1979; Mestre et al. 2018) and contrast-enhanced magnetic resonance imaging (MRI) (Iliff et al. 2013). While these methods have provided some groundbreaking results at high spatial detail, they also have limitations, such as limited FOV, lower sensitivity, and limited selection of contrast agents.(Lilius et al. 2022; Bohr et al. 2022) To date, research on nuclear imaging of the glymphatic system is scarce, and only a small number of studies have reported findings using either PET (Benveniste et al. 2021) or single photon emission computed tomography (SPECT) (Lilius et al. 2022, 2023; Sigurdsson et al. 2023; Persson et al. 2024). Despite the advantages, PET imaging also has some limitations, such as insufficient spatial resolution to achieve detailed information on whether the infused tracer is in the perivascular space or blood vessels within the ICS. Furthermore, preclinical dynamic whole-body imaging typically requires anesthesia, affecting the glymphatic fluid flow (Hablitz et al. 2019). In this work, we used ketamine/dexmedetomidine anesthesia as it has been previously reported to improve the glymphatic flow into the rodent brain and provide sufficient anesthesia for dynamic imaging (Lilius et al. 2019; Benveniste et al. 2017).
Recently, Sigurdsson et al. (Sigurdsson et al. 2023) used [99mTc]Tc-human serum albumin nanocolloid to study the glymphatic system under different anesthetics with SPECT/CT and reported a clear visualization of cervical lymph nodes during 140-min dynamic scan. In our study, lymph nodes could only be visualized with the in vitro radiolabeled RSA on the PET images, suggesting that the albumin binding of the gallium-68-radiotracers in the CSF after intrathecal administration remained incomplete. Despite their high albumin-binding affinity, the relatively low Am of the gallium-68-labeled albumin-binding tracers resulted in administered tracer concentrations that were too high relative to the physiological albumin concentration in the CSF. The albumin concentration in the rat CSF is approximately 0.15–0.35 g/l, and the volume of CSF in the cisterna magna of an adult rat is 10–20 ml (Barthel et al. 2021), meaning that the amount of albumin at the infusion site is approximately 0.012–0.024 nmol while the amount of infused tracer was 0.7–0.9 nmol, potentially hindering the generation of the macromolecular tracer in vivo. The excess unbound tracer could distribute freely with the CSF flow to deeper brain areas and interact with brain tissues and possibly other proteins in the CNS based on its physicochemical properties or continue to bind albumin it encounters through the CSF flow or be eliminated as a free compound to the systemic side. The pharmacokinetic parameters show that the albumin-binding tracers reach higher Cmax values in the deeper brain areas (hippocampus, striatum and cortex), while the reference tracer reached higher Cmax values in the spinal canal after intracisternal infusion. This aligns with the previously described hypothesis of incomplete albumin binding in the CSF, as with the macromolecular reference tracer the distribution to deeper brain tissues is slower and it distributes more rapidly within the larger CSF-filled spaces and the spinal canal. Based on the TACs for liver and heart, the hepatobiliary excretion of the albumin-binding tracers trumps retention in circulation, suggesting that the radioactive species that enters the systemic side from the CNS is not a macromolecule. Minor pharmacokinetic differences were also observed among the albumin-binding tracers. After intracisternal infusion, the significantly larger Cmax and AUC0-120 of [68 Ga]Ga-3 in the striatum correlates with the differences seen in the LogD values. [68 Ga]Ga-3 is the only tracer with a slightly positive LogD value (0.22 ± 0.08), potentially making the tracer more likely to adhere to cell membranes, also supporting the hypothesis of unbound tracer interacting with the brain tissues and proteins according to its own physiochemical properties. After lumbar infusion, [68 Ga]Ga-3 was found to have slower distribution towards intracranial space via the CSF flow than the other two albumin-binders and is more likely to diffuse from the infusion site towards surrounding tissues.
Initially, gallium-68 was chosen as the label for the albumin-binding tracers due to its facile labeling chemistry and cost-effective on-site production with a 68Ge/68 Ga-generator (Green and Welch 1989). However, as shown in this study, the low albumin concentration in the rat CSF requires higher Am than what were achieved with gallium-68 in our setup, warranting the development of higher Am tracers, achieved with, for example, nucleophilic fluorination with fluorine-18 (Halder and Ritter 2021). The images obtained in this study also clearly illustrate that the intermediate positron energy (635 keV) of fluorine-18 is advantageous for the glymphatic system imaging where high spatial resolution is needed. Alternatively, the Am might not be an issue in larger animal models, such as pigs and primates and in humans, as more volume could be infused. For example, the amount of albumin in the cisterna magna of a piglet can be up to 20 nmol (Sakka et al. 2011), and with an adult human up to 50 nmol (Romagnoli et al. 2014). Nevertheless, since rodent models are the backbone of neuroscience studies, it is important to develop imaging technologies that are tailored for rats and mice to propel investigation into the glymphatic system function and pharmacology in these organisms. Interestingly, the reference tracer Al[18F]F-7, seems to be a highly potential candidate to this end.
Conclusions
This research is at the forefront of glymphatic PET tracer development with the evaluation of new gallium-68-radiolabeled albumin-binding tracers, and a fluorine-18-labeled RSA-based macromolecular reference tracer. The albumin-binding tracers were successfully synthesized, characterized, and radiolabeled, and then evaluated in vitro and in vivo to show that they bind to endogenous albumin with high rate and stability. However, due to the relatively low Am of gallium-68-NODAGA complex achieved in this study and the low albumin concentration in rat CSF, the i.t. infused tracer concentration did not correlate with the albumin concentration at the infusion site, suggesting that a portion of the infused tracer remained free in the CSF and interacted with the brain tissues according to its own physiochemical properties. The RSA-based reference tracer proved out to be a potential candidate for future PET studies on the glymphatic system in a rodent model, but the greater clinical relevance prompts further optimization of the albumin-binding tracers as well. In conclusion, our studies corroborate the feasibility of PET imaging with albumin-based radiotracers to study the glymphatic fluid flow in rodent models.
Supplementary Information
Supplementary video 1. Dynamic PET/CT movie of cisterna magna infused [68Ga]Ga-3.
Supplementary video 2. Dynamic PET/CT movie of cisterna magna infused [68Ga]Ga-4.
Supplementary video 3. Dynamic PET/CT movie of cisterna magna infused [68Ga]Ga-6.
Supplementary video 4. Dynamic PET/CT movie of cisterna magna infused Al[18F]F-7.
Supplementary video 5. Dynamic PET/CT movie of lumbar infused [68Ga]Ga-3.
Supplementary video 6 Dynamic PET/CT movie of lumbar infused [68Ga]Ga-4.
Supplementary video 7. Dynamic PET/CT movie of lumbar infused [68Ga]Ga-6.
Supplementary video 8. Dynamic PET/CT movie of lumbar infused Al[18F]F-7.
Acknowledgements
The authors would like to thank M.Sc. Anisa Biti and M.Sc. Alessia Centanni for all the advice during tracer development, and senior engineer Markus Nyman for helping with instrumentation. The Helsinki In Vivo Imaging Platform (HiLIFE) is acknowledged for the PET/CT imaging.
Abbreviations
- Am
Molar activity
- AUC
Area under curve
- CI
Confidence interval
- Cmax
Maximum concentration
- CM
Cisterna magna
- CNS
Central nervous system
- CSF
Cerebrospinal fluid
- CT
Computed tomography
- CV
Coefficient of variation
- EB
Evans blue
- FOV
Field of view
- GM
Geometric mean
- GMP
Good manufacturing practice
- GPU
Graphics processing unit
- HPLC
High performance liquid chromatography
- HR-MS
High resolution mass spectrometry
- IP
4-(P-iodophenyl)butyric acid
- ICS
Intracranial space
- ISF
Interstitial fluid
- i.t.
Intrathecal
- LC-MS
Liquid chromatography mass spectrometry
- MALDI-MS
Matrix assisted laser ionization desorption mass spectrometry
- MRI
Magnetic resonance imaging
- NMR
Nuclear magnetic resonance
- NODAGA
1,4,7-Triazacyclononane-1-glutaric acid-4,7-acetic acid
- PET
Positron emission tomography
- PBS
Phosphate buffer saline
- QC
Quality control
- RCP
Radiochemical purity
- RCY
Radiochemical yield
- RESCA
Restained complexing agent
- ROI
Region of interest
- RSA
Rat serum albumin
- SD
Standard deviation
- SEC
Size exclusion chromatography
- SPECT
Single photon emission computed tomography
- SUV
Standardized uptake value
- TAC
Time activity curve
- TLC
Thin layer chromatography
- Tmax
Time corresponding to maximum concentration
- t1/2
Half-life
Author contributions
MP: Writing—Original Draft, Writing—Review & Editing, Investigation, Visualization, Formal analysis SI: Methodology, Writing -Original Draft, Writing—Review & Editing, Investigation, Formal analysis SCJ: Investigation, Writing—Review & Editing NDÅP: Investigation, Writing—Review & Editing TJL: Writing—Review & Editing, Formal analysis TOL: Conceptualization, Methodology, Writing—Original Draft, Writing—Review & Editing, Supervision, Project administration, Funding acquisition, Formal analysis MS: Conceptualization, Methodology, Writing—Original draft, Writing—Review & Editing, Supervision, Project administration, Funding acquisition, Formal analysis. All authors read and approved the final manuscript.
Funding
Open Access funding provided by University of Helsinki (including Helsinki University Central Hospital). This research received funding from the Academy of Finland (decision numbers 318422, 346122 and 350371), the Sigrid Jusélius Foundation, the Paulo Foundation, the Orion Research Foundation sr, Helsinki One Health organization, the Finnish Cultural Foundation, Finnish Medical Foundation, the Emil Aaltonen Foundation, the Alfred Kordelin Foundation (grant no. 220147) and the University of Helsinki Research Funds. Open access to the article is funded by the Helsinki University Library.
Availability of data and materials
The study data is available upon reasonable request from corresponding authors.
Declarations
Ethics approval and consent to participate
Animal experiments were carried out under project license numbers ESAVI/9782/2022, ESAVI/36258/2020, and ESAVI/4935/2024 approved by the Regional State Administrative Agency of Southern Finland (Hämeenlinna, Finland), and compliance with the respective Finnish and EU regulations and guidelines. We used the ARRIVE checklist when writing our report (Percie du Sert N, Hurst V, Ahluwalia A et al. The ARRIVE Guidelines 2.0: updated guidelines for reporting animal research).
Consent for publication
Not applicable.
Competing interests
Tuomas Lilius is listed in a patent (patent number 20220280423) describing glymphatic-assisted drug delivery.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Tuomas O. Lilius and Mirkka Sarparanta have contributed equally to this work.
Contributor Information
Tuomas O. Lilius, Email: tuomas.lilius@helsinki.fi
Mirkka Sarparanta, Email: mirkka.sarparanta@helsinki.fi.
References
- Barthel L, Engler H, Hadamitzky M, Lückemann L, Sure U, Schedlowski M, et al. A step-by-step guide for microsurgical collection of uncontaminated cerebrospinal fluid from rat cisterna magna. J Neurosci Methods. 2021;352: 109085. 10.1016/j.jneumeth.2021.109085. [DOI] [PubMed] [Google Scholar]
- Benveniste H, Lee H, Ding F, Sun Q, Al-Bizri E, Makaryus R, et al. Anesthesia with dexmedetomidine and low-dose isoflurane increases solute transport via the glymphatic pathway in rat brain when compared with high-dose isoflurane. Anesthesiology. 2017;127:976–88. 10.1097/ALN.0000000000001888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste H, Lee H, Ozturk B, Chen X, Koundal S, Vaska P, et al. Glymphatic cerebrospinal fluid and solute transport quantified by MRI and PET imaging. Neuroscience. 2021;474:63–79. 10.1016/j.neuroscience.2020.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohr T, Hjorth PG, Holst SC, Hrabětová S, Kiviniemi V, Lilius T, et al. The glymphatic system: Current understanding and modeling. iScience. 2022;25:104987. 10.1016/j.isci.2022.104987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolus NE, George R, Newcomer BR. PET/MRI: The blended-modality choice of the future? J Nucl Med Technol. 2009;37:63–71. 10.2967/jnmt.108.060848. [DOI] [PubMed] [Google Scholar]
- Chen H, Wang G, Lang L, Jacobson O, Kiesewetter DO, Liu Y, et al. Chemical conjugation of evans blue derivative: a strategy to develop long-acting therapeutics through albumin binding. Theranostics. 2016;6:243–53. 10.7150/thno.14322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RA, Hausner SH, Harris R, Sutcliffe JL. A comparison of evans blue and 4-( p-Iodophenyl)butyryl albumin binding moieties on an integrin αvβ6 binding peptide. Pharmaceutics. 2022;14:745. 10.3390/pharmaceutics14040745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eide PK, Ringstad G. Delayed clearance of cerebrospinal fluid tracer from entorhinal cortex in idiopathic normal pressure hydrocephalus: A glymphatic magnetic resonance imaging study. J Cereb Blood Flow Metab. 2019;39:1355–68. 10.1177/0271678X18760974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, de Lannoy IAM. Pharmacokinetics. Biochem Pharmacol. 2014;87:93–120. 10.1016/j.bcp.2013.09.007. [DOI] [PubMed] [Google Scholar]
- Ganguly T, Bauer N, Davis RA, Hausner SH, Tang SY, Sutcliffe JL. Evaluation of copper-64-Labeled αvβ6-targeting peptides: addition of an albumin binding moiety to improve pharmacokinetics. Mol Pharm. 2021;18:4437–47. 10.1021/acs.molpharmaceut.1c00632. [DOI] [PubMed] [Google Scholar]
- Green MA, Welch MJ. Gallium radiopharmaceutical chemistry. Int J Radiation Appl Instrum. 1989;16:435–48. 10.1016/0883-2897(89)90053-6. [DOI] [PubMed] [Google Scholar]
- Hablitz LM, Nedergaard M. The glymphatic system: A novel component of fundamental neurobiology. J Neurosci. 2021;41:7698–711. 10.1523/JNEUROSCI.0619-21.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hablitz LM, Vinitsky HS, Sun Q, Stæger FF, Sigurdsson B, Mortensen KN, et al. Increased glymphatic influx is correlated with high EEG delta power and low heart rate in mice under anesthesia. Sci Adv. 2019. 10.1126/sciadv.aav5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halder R, Ritter T. 18F-fluorination: challenge and opportunity for organic chemists. J Org Chem. 2021;86:13873–84. 10.1021/acs.joc.1c01474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausner SH, Bauer N, Davis RA, Ganguly T, Tang SYC, Sutcliffe JL. The effects of an albumin binding moiety on the targeting and pharmacokinetics of an integrin αvβ6-selective peptide labeled with aluminum [18F]fluoride. Mol Imag Biol. 2020;22:1543–52. 10.1007/s11307-020-01500-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höltke C, Grewer M, Stölting M, Geyer C, Wildgruber M, Helfen A. Exploring the influence of different albumin binders on molecular imaging probe distribution. Mol Pharm. 2021;18:2574–85. 10.1021/acs.molpharmaceut.1c00064. [DOI] [PubMed] [Google Scholar]
- Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med. 2012;4:147. 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliff JJ, Wang M, Zeppenfeld DM, Venkataraman A, Plog BA, Liao Y, et al. Cerebral arterial pulsation drives paravascular CSF-Interstitial fluid exchange in the murine brain. J Neurosci. 2013;33:18190–9. 10.1523/JNEUROSCI.1592-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong Y-S, Jusko WJ. Determinants of biological half-lives and terminal slopes in physiologically based pharmacokinetic systems: assessment of limiting conditions. AAPS J. 2022;24:96. 10.1208/s12248-022-007395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratz F, Müller-Driver R, Hofmann I, Drevs J, Unger C. A novel macromolecular prodrug concept exploiting endogenous serum albumin as a drug carrier for cancer chemotherapy. J Med Chem. 2000;43:1253–6. 10.1021/jm9905864. [DOI] [PubMed] [Google Scholar]
- Larsen MT, Kuhlmann M, Hvam ML, Howard KA. Albumin-based drug delivery: harnessing nature to cure disease. Mol Cell Ther. 2016;4:3. 10.1186/s40591-016-0048-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau J, Jacobson O, Niu G, Lin K-S, Ois F, Bénard B, et al. Bench to bedside: albumin binders for improved cancer radioligand therapies. Bioconjugate Chem. 2019;30:487–502. 10.1021/acs.bioconjchem.8b00919. [DOI] [PubMed] [Google Scholar]
- Lilius TO, Blomqvist K, Hauglund NL, Liu G, Stæger FF, Bærentzen S, et al. Dexmedetomidine enhances glymphatic brain delivery of intrathecally administered drugs. J Control Release. 2019;304:29–38. 10.1016/j.jconrel.2019.05.005. [DOI] [PubMed] [Google Scholar]
- Lilius TO, Rosenholm M, Klinger L, Skytte M, Nielsen N, Rantamä T. SPECT/CT imaging reveals CNS-wide modulation of glymphatic cerebrospinal fluid flow by systemic hypertonic saline. iScience. 2022;25:105250. 10.1016/j.isci.2022.105250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilius TO, Mortensen KN, Deville C, Lohela TJ, Stæger FF, Sigurdsson B, et al. Glymphatic-assisted perivascular brain delivery of intrathecal small gold nanoparticles. J Control Release. 2023;355:135–48. 10.1016/j.jconrel.2023.01.054. [DOI] [PubMed] [Google Scholar]
- MacAulay N. Molecular mechanisms of brain water transport. Nat Rev Neurosci. 2021;22:326–44. 10.1038/s41583-021-00454-8. [DOI] [PubMed] [Google Scholar]
- Mestre H, Tithof J, Du T, Song W, Peng W, Sweeney AM, et al. Flow of cerebrospinal fluid is driven by arterial pulsations and is reduced in hypertension. Nat Commun. 2018;9:4878. 10.1038/s41467-018-07318-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestre H, Mori Y, Nedergaard M. The brain’s glymphatic system: current controversies. Trends Neurosci. 2020;43:458–66. 10.1016/j.tins.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestre H, Du T, Sweeney AM, Liu G, Samson AJ, Peng W, et al. Cerebrospinal fluid influx drives acute ischemic tissue swelling. Science (1979). 2020;367:6483. 10.1126/science.aax7171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedergaard M, Goldman SA. Glymphatic failure as a final common pathway to dementia. Science. 1979;2020(370):50–6. 10.1126/science.abb8739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen A, Reyes AE, Zhang M, McDonald P, Wong WLT, Damico LA, et al. The pharmacokinetics of an albumin-binding Fab (AB.Fab) can be modulated as a function of affinity for albumin. Protein Eng Design Select. 2006;19:291–7. 10.1093/protein/gzl011. [DOI] [PubMed] [Google Scholar]
- Nirogi R, Kandikere V, Mudigonda K, Bhyrapuneni G, Muddana N, Saralaya R, et al. A simple and rapid method to collect the cerebrospinal fluid of rats and its application for the assessment of drug penetration into the central nervous system. J Neurosci Methods. 2009;178:116–9. 10.1016/j.jneumeth.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Niu G, Lang L, Kiesewetter DO, Ma Y, Sun Z, Guo N, et al. In vivo labeling of serum albumin for PET. J Nucl Med. 2014;55:1150–6. 10.2967/jnumed.114.139642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson NDÅ, Uusalo P, Nedergaard M, Lohela TJ, Lilius TO. Could dexmedetomidine be repurposed as a glymphatic enhancer? Trends Pharmacol Sci. 2022;43:1030–40. 10.1016/j.tips.2022.09.007. [DOI] [PubMed] [Google Scholar]
- Persson NDÅ, Lohela TJ, Mortensen KN, Rosenholm M, Li Q, Weikop P, et al. Anesthesia blunts carbon dioxide effects on glymphatic cerebrospinal fluid dynamics in mechanically ventilated rats. Anesthesiology. 2024;141:338–52. 10.1097/ALN.0000000000005039. [DOI] [PubMed] [Google Scholar]
- Rasmussen MK, Mestre H, Nedergaard M. Fluid transport in the brain. Physiol Rev. 2022;102:1025–151. 10.1152/physrev.00031.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagnoli N, Ventrella D, Giunti M, Dondi F, Sorrentino NC, Fraldi A, et al. Access to cerebrospinal fluid in piglets via the cisterna magna: optimization and description of the technique. Lab Anim. 2014;48:345–8. 10.1177/0023677214540881. [DOI] [PubMed] [Google Scholar]
- Sakka L, Coll G, Chazal J. Anatomy and physiology of cerebrospinal fluid. Eur Ann Otorhinolaryngol Head Neck Dis. 2011;128:309–16. 10.1016/j.anorl.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Schöder H, Erdi Y, Larson S, Yeung H. PET/CT: a new imaging technology in nuclear medicine. Eur J Nucl Med Mol Imaging. 2003;30:1419–37. 10.1007/s00259-003-1299-6. [DOI] [PubMed] [Google Scholar]
- Sigurdsson B, Hauglund NL, Lilius TO, Mogensen FL-H, Mortensen KN, Beschorner N, et al. A SPECT-based method for dynamic imaging of the glymphatic system in rats. J Cerebral Blood Flow Metabol. 2023;43:1153–65. 10.1177/0271678X231156982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statistical approaches to establishing bioequivalence guidance for industry. Rockville, MD, U.S. Dept. of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, 2022.
- Statistical Guide for Clinical Pharmacology & Therapeutics. Clin Pharmacol Ther. 2010;88:150–2. [DOI] [PubMed] [Google Scholar]
- Størkson RV, Kjørsvik A, Tjølsen A, Hole K. Lumbar catheterization of the spinal subarachnoid space in the rat. J Neurosci Methods. 1996;65:167–72. 10.1016/0165-0270(95)00164-6. [DOI] [PubMed] [Google Scholar]
- Vāvere AL, Scott PJH. Clinical applications of small-molecule PET radiotracers: current progress and future outlook. Semin Nucl Med. 2017;47:429–53. 10.1053/j.semnuclmed.2017.05.001. [DOI] [PubMed] [Google Scholar]
- Wen X, Xu P, Shi M, Liu J, Zeng X, Zhang Y, et al. Evans blue-modified radiolabeled fibroblast activation protein inhibitor as long-acting cancer therapeutics. Theranostics. 2022;2022:422–33. 10.7150/thno.68182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xavier ALR, Hauglund NL, von Holstein-Rathlou S, Li Q, Sanggaard S, Lou N, et al. Cannula Implantation into the Cisterna Magna of Rodents. J vis Exp. 2018;135:573–8. 10.3791/57378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, et al. Sleep drives metabolite clearance from the adult brain. Science. 1979;2013:342. 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler SI. Positron emission tomography: principles, technology, and recent developments. Nucl Phys A. 2005;752:679–87. 10.1016/j.nuclphysa.2005.02.067. [Google Scholar]
- Zorzi A, Linciano S, Angelini A. MedChemComm Non-covalent albumin-binding ligands for extending the circulating half-life of small biotherapeutics. Medchemcomm. 2019;10:1068–81. 10.1039/c9md00018f. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary video 1. Dynamic PET/CT movie of cisterna magna infused [68Ga]Ga-3.
Supplementary video 2. Dynamic PET/CT movie of cisterna magna infused [68Ga]Ga-4.
Supplementary video 3. Dynamic PET/CT movie of cisterna magna infused [68Ga]Ga-6.
Supplementary video 4. Dynamic PET/CT movie of cisterna magna infused Al[18F]F-7.
Supplementary video 5. Dynamic PET/CT movie of lumbar infused [68Ga]Ga-3.
Supplementary video 6 Dynamic PET/CT movie of lumbar infused [68Ga]Ga-4.
Supplementary video 7. Dynamic PET/CT movie of lumbar infused [68Ga]Ga-6.
Supplementary video 8. Dynamic PET/CT movie of lumbar infused Al[18F]F-7.
Data Availability Statement
The study data is available upon reasonable request from corresponding authors.